Abstract
Background
Nephrogenic systemic fibrosis (NSF) is a systemic fibrotic disorder occurring in some patients with renal insufficiency after exposure to gadolinium-based contrast agents (GdBCA).
Objectives
To examine cultured NSF dermal fibroblast production and expression of collagens I and III, fibronectin, hyaluronic acid and α-smooth muscle actin (α-SMA) during serial passages and the effects of two GdBCA on collagen gene expression and production by normal dermal fibroblasts.
Methods
NSF fibroblasts were analysed for expression and production of types I and III collagen, fibronectin, hyaluronic acid and α-SMA. Collagen, type I, α1 (COL1A1) promoter transcription was examined in transient transfections. Nuclear extracts were assayed for binding activity of 108 transcription factors, and specific transcription factor binding was examined by electrophoretic gel mobility assays. Normal fibroblasts were cultured with GdBCA, and collagen expression assessed by real-time PCR and western blots.
Results
NSF fibroblasts displayed a marked increase in collagens I and III, fibronectin and hyaluronic acid production, which was maintained for 9–11 subpassages in vitro. NSF fibroblasts also showed a marked increase in α-SMA expression, twofold higher transcriptional activity of the COL1A1 promoter and increased cREL binding in nuclear extracts compared with normal fibroblasts. GdBCA induced a dose-dependent stimulation of COL1A1 expression and production of type I collagen in normal fibroblasts.
Conclusions
Fibroblasts from patients with NSF displayed a markedly profibrotic phenotype, which was maintained for several passages in culture. Elevated COL1A1 expression was mediated by transcriptional activation of its promoter associated with increased cREL binding activity. GdBCA stimulated cultured normal fibroblasts to produce increased amounts of collagen.
INTRODUCTION
Nephrogenic systemic fibrosis (NSF) is a serious systemic fibrosing disease occurring in patients with renal insufficiency.1,2 Typically, patients manifest intense cutaneous induration and fibrosis in feet, legs, thighs, hands and forearms, frequently preceded by swelling and pruritus and burning sensation. The skin often displays puckering, dimpling and deep furrowing. Musculoskeletal symptoms affecting multiple joints often occur and lead to disabling joint flexion contractures, thickening of tendons and woody induration of affected muscles.3–7 Skin histopathology demonstrates marked dermal and subdermal fibrosis, mucin deposition and proliferation of elongated, spindle-shaped fibroblasts.1,2 NSF-affected tissues also exhibit accumulation of macrophages and activated fibroblasts, and markedly increased expression of transforming growth factor β (TGFβ).6 Although the disorder was initially believed to solely affect the skin, NSF can involve the liver, lungs, muscles, heart, thyroid and even the dura mater.6–10
Numerous risk factors have been associated with the development of NSF in addition to renal failure2, 11; however, following a report describing that NSF became clinically apparent shortly after the administration of gadolinium-based contrast agents (GdBCA),12 numerous reports confirmed this association.13–22 Furthermore, deposition of gadolinium (Gd) in NSF skin and other tissues has been demonstrated.10,23–26 However, the mechanisms responsible for the remarkable fibrotic process remain unknown and only a few studies have examined the molecular and biochemical abnormalities in fibroblasts or tissues from patients with NSF. Here, we describe profound phenotypic and gene expression alterations in dermal fibroblasts cultured from affected skin from patients with GdBCA-associated NSF and examine the molecular mechanisms responsible for the exaggerated fibrotic response that is ultimately responsible for the clinical features of the disease. We also examined GdBCA induction of a profibrotic phenotype in normal fibroblasts.
METHODS
Cell culture
Dermal fibroblasts were obtained from full-depth surgical skin biopsy specimens from three previously reported6, 7 patients with NSF. These patients had been exposed to GdBCA shortly before the development of skin induration; however, the medical records available to us did not document which GdBCA or doses the patients received since these patients were evaluated before publication of the first report suggesting a role of GdBCA in the pathogenesis of NSF. Normal fibroblasts were obtained from the Thomas Jefferson University Scleroderma Center Tissue Bank. Normal and NSF fibroblasts were cultured at 37°C in a 5% CO2 humidified atmosphere in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, antibiotics, 50 mM HEPES and glutamine until confluent as described previously.27,28 For studies of collagen production and gene expression, cells were supplemented for 24 h with 40 μg/ml L-ascorbic acid phosphate magnesium salt n-hydrate (Waco Biochemical, Osaka, Japan).
Effects of gadolinium compounds on normal fibroblasts
Gd-Diethylenetriaminepenta-acetate (Gd-DTPA) was purchased from Sigma-Aldrich (St Louis, Missouri, USA) and was dissolved in sterile saline. Omniscan (provided by GE Healthcare, Chalfont St. Giles, UK) was supplied as a sterile, clear aqueous solution. The Omniscan preparation contained 287 mg/ml (500 mM) of gadodiamide plus 12 mg/ml of caldiamide sodium in water. Three normal dermal fibroblasts cell lines were seeded in six-well plates until confluence. After preincubation for 24 h with 40 μg/ml of ascorbic acid, cells were treated with 0, 5, 10, 25 and 50 mM of Gd-DTPA or Omniscan for 24 h. In control experiments the effects of caldiamide alone were also examined. Media were harvested and saved at −20°C until use.
Persistence of phenotype during serial subculturing
Primary cultures of NSF fibroblasts (three strains) and normal fibroblasts (two strains) were subcultured for several passages. The culture conditions were exactly as described above. Fibroblast cultures were harvested and analysed for their patterns of gene expression at passages 4–6 and 9–11. The pattern of expression of collagen type I and α-smooth muscle actin (α-SMA) was analysed in confluent cultures (passage 4) of dermal fibroblasts cultured from clinically affected (lower thigh) and non-affected (upper thigh) NSF skin.
Western blots
Culture media were removed and equal-volume aliquots were boiled in sodium dodecyl sulphate 1% β-mercaptoethanol for 5 min, loaded into 8% Tris-glycine polyacrylamide gels (Invitrogen, Carlsbad, California, USA) and electrophoresed. The separated proteins were transferred to nitrocellulose membranes (Bio-Rad, Hercules, California, USA), and the membranes incubated in 5% non-fat dry milk/Tris-buffered saline with Tween (TBST) for 1 h. The membranes were incubated with rabbit polyclonal anti-collagen type I antibodies (from A Fertala, Thomas Jefferson University or from Southern Biotechnologies, Birmingham, Alabama, USA), a polyclonal anti-collagen type III antibody (Rockland, Gilbertsville, Pennsylvania, USA) or a mouse monoclonal anti-fibronectin antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA) in 3% non-fat dry milk/TBST at 4°C overnight. After TBST washes the membranes were incubated for 1.5 h with horseradish peroxidase-conjugated anti-rabbit IgG (Amersham Biosciences, Piscataway, New Jersey, USA). The immunoreactive bands were visualised by electrochemiluminescence (Thermo Scientific, Rockford, Illinois, USA).
Northern hybridisations
Fibroblasts were grown to confluency and total RNA isolated employing the RNeasy mini-kit (Qiagen, Valencia, California, USA). Aliquots of total RNA (5–10 μg/well) were electrophoresed on 1.0% formaldehyde agarose gels. The RNA was then transferred to Hybond N+ membranes (Amersham Biosciences) and the filters hybridised to 32P-radiolabelled human cDNA for either collagen, type I, α1 (COL1A1) COL3A1 or fibronectin, as described.27 Equivalent amounts of RNA were loaded and RNA loading and transfer were evaluated by probing with glyceraldehyde phosphate dehydrogenase (GAPDH) cDNA. The filters were analysed using storage phosphor technology (Image-Quant V.5.1 software; Amersham Biosciences).
Reverse transcription (RT) PCR
Fibroblasts were grown to confluency and total RNA isolated with TRIzol (Invitrogen). cDNA was synthesised using 1 μg of RNA template with SuperScript II Reverse Transcriptase kit (Invitrogen). Subsequently, PCR was performed with the following primers: for collagen I: forward, 5′-CGCTACTACCGGGCTGATGATGC-3′ and reverse, 5′-CAGGCGGGAGGTCTTGGTGGTTTT-3′; and for GAPDH: forward, 5′-GCGGGGCTCTCCAGAACATCAT-3′ and reverse, 5′-CCAGCCCCAGCGTCAAAGGTG-3′. The PCR products were electrophoresed in 2% agarose gels. The densitometric analysis of DNA bands was performed using EZQuant-Gel program (EZQuant, Tel Aviv, Israel).
Assessment of transcriptional regulation of COL1A1
Transient transfections of normal and NSF fibroblasts were performed employing the FuGene 6 Kit (Roche, Indianapolis, Indiana, USA) as described.29 Transfections were performed in passage 5 fibroblasts at 80% confluence with 2.5 μg of each plasmid. COL1A1 transcription was assessed employing deletion constructs of the COL1A1 promoter fused to the chloramphenicol acetyl transferase reporter gene.30 The constructs examined end at nucleotide +42 to assure transcription in a proper reading frame and their 5’ ends are at −174 and −804 bp. Fresh medium was added 4 h later. Cells were harvested 72 h after transfection and chloramphenicol acetyl transferase activity determined. Transfection efficiency was normalised by co-transfecting 0.2 μg Escherichia coli β-galactosidase cDNA (pCMV β-galactosidase; Clontech, Palo Alto, California, USA) followed by assays of β-galactosidase activity.
Protein DNA arrays for transcriptional factors
Nuclear extracts were subjected to the TranSignal Protein/DNA arrays I and II (Panomics, Redwood City, California, USA) as described.31 This assay enables the parallel detection and semi-quantitative comparison of the DNA-binding activity of 108 different transcription factors from nuclear extracts from two different cell types. Briefly, biotin-labelled DNA-binding oligo-nucleotides (TranSignal Probe Mix) were incubated with 10 μg of nuclear extracts at 15°C for 30 min to allow the formation of transcription factor/DNA complexes. The transcription factor/DNA complexes were separated from the free probes by 2% agarose gel electrophoresis in 0.5×Tris/borate/EDTA at 120 V for 15 min. The probes in the complexes were then extracted, ethanol precipitated and hybridised to the TranSignal protein/DNA array. Signals were detected using chemiluminescence imaging.
Electrophoretic mobility shift assay for transcription factor binding to the COL1A1 promoter
Normal and NSF cells at passage 5 were cultured in 175 mm flasks until confluence. Nuclear extracts were prepared and an electrophoretic mobility shift assay (EMSA) performed as described.29 A cREL consensus oligonucleotide (Santa Cruz Biotechnology) radioactive probe was prepared by 5′ phosphorylation with polynucleotide kinase (Promega, Madison, Winconsin, USA) and α32-ATP (Amersham Biosciences). Nuclear extracts (10 μg), 4 μg of poly (dI-dC) and 5×104 cpm of radiolabelled probe were incubated for 20 min in a buffer containing 40 mM KCl, 15 mM HEPES, pH 7.9, 1 mM EDTA, 0.5 mM dithiothreitol, 1 mM MgCl2 and 5% glycerol. EMSA was performed in 5% non-denaturing polyacrylamide gels as described.29 To test the specificity of binding, competition experiments using 100-fold molar excess of an unlabelled consensus oligonucleotide (Santa Cruz Biotechnology) were performed. The results were quantified by densitometry (Image-Quant, V.5.1; Molecular Dynamics, Sunnyvale, California, USA).
α-SMA indirect immunofluorescence
Normal and NSF fibroblasts at passage 9 were seeded on eight-well glass slides, cultured in Dulbecco’s modified Eagle’s medium, 10% fetal bovine serum until confluence, then washed three times in phosphate-buffered saline (PBS) and fixed with 2% paraformaldehyde in PBS for 30 min at room temperature. After fixation, cells were washed with PBS and permeabilised with PBS containing 0.2% bovine serum albumin (BSA) and 0.1% Triton X-100 at room temperature for 10 min. The slides were then incubated with primary anti-α-SMA antibodies (NeoMarker, Fremont, California, USA) diluted 1:200 in PBS containing 0.2% BSA, 0.1% Triton X-100 for 90 min at room temperature. After three 10 min washes with PBS the slides were incubated with secondary sheep anti-mouse IgG F(ab’) Cy3 conjugate (Sigma-Aldrich) diluted 1:200 in PBS containing 0.2% BSA for 60 min at room temperature. After 60 min incubation with secondary antibody, the slides were washed three times for 10 min with PBS, then mounted with Vectashield mounting medium containing 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, California, USA) and visualised by confocal microscopy.
Hyaluronic acid assay
Hyaluronic acid was assayed as described by Homer et al.32 Stains-all solution was prepared according to a modification of the method of Benchetrit et al33 employing the stains-all dye (Sigma-Aldrich). Phenol red in culture supernatants was removed by addition of 1 mg activated charcoal followed by centrifugation at 14 000 rpm for 10 min at room temperature. Culture media from one normal and three NSF cell lines at passages 4–6 and 9–11 were assayed in triplicate.
Statistical analysis
Data are shown as means±SEM. An unpaired Student t test was employed for comparing two groups of data with the GraphPad Software. Values of p<0.05 were considered significant.
RESULTS
Treatment of normal fibroblasts with GdBCA
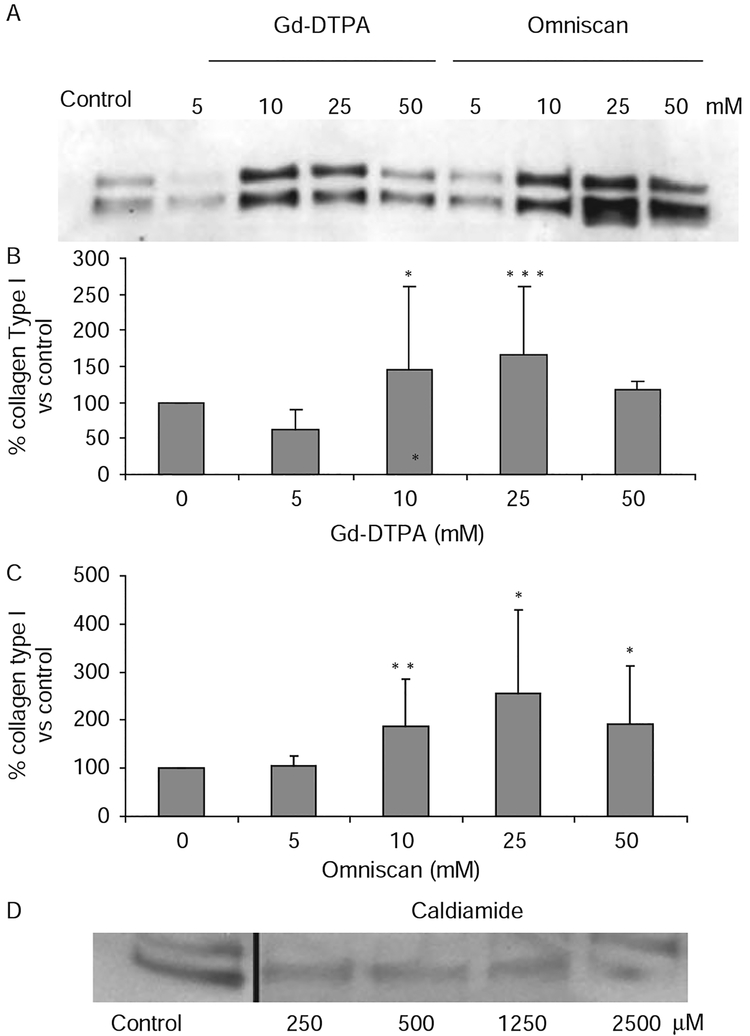
Culture of three cell lines of normal dermal fibroblasts with 5–50 mM Gd-DTPA or Omniscan for 48 h was well tolerated and there was no evidence of cell detachment or cytotoxicity. Assessment of type I collagen production showed that both GdBCA caused an increase in collagen I production. A representative experiment is shown in figure 1A and the averages of results for the three cell lines are shown in figure 1B,C. There was a ~50% increase in type I collagen production with 10 and 25 mM Gd-DTPA and a 100–150% increase with 10 and 25 mM Omniscan. These results are most remarkable since the cultures were preincubated with ascorbic acid supplementation to optimise their levels of collagen production before being exposed to GdBCA. The effects of the highest concentrations (50 mM) were less pronounced with both compounds, probably reflecting global inhibition of protein synthesis. To exclude any effects of the excess chelate, caldiamide, present in the preparation of Omniscan examined, similar experiments were performed with caldiamide alone. The results shown in figure 1D demonstrated that caldiamide did not cause any stimulation of collagen gene expression in these cells.
Figure 1.
Effects of gadolinium-based contrast agents on production of type I collagen by normal dermal fibroblasts in vitro. Normal dermal fibroblast cultures were incubated with the indicated concentrations of Gd-diethylenetriaminepenta-acetate (Gd-DTPA), Omniscan, or caldiamide for 24 h and samples of culture media were processed for western blot assessment of type I collagen production. (A) Western blot of media from one of the cell lines. (B, C) Measurement of immunoreactive type I collagen. The amounts of immunoreactive type I collagen in the western blot were quantified using ImageJ software. Values are the mean of the results from the treatment of the three cell lines in triplicate and the percentage expression relative to the saline control, which was arbitrarily set at 100%. *p<0.02; **p<0.001; ***p<0.005. (D) Western blot of media from the same cell line shown in (A) after exposure to caldiamide alone.
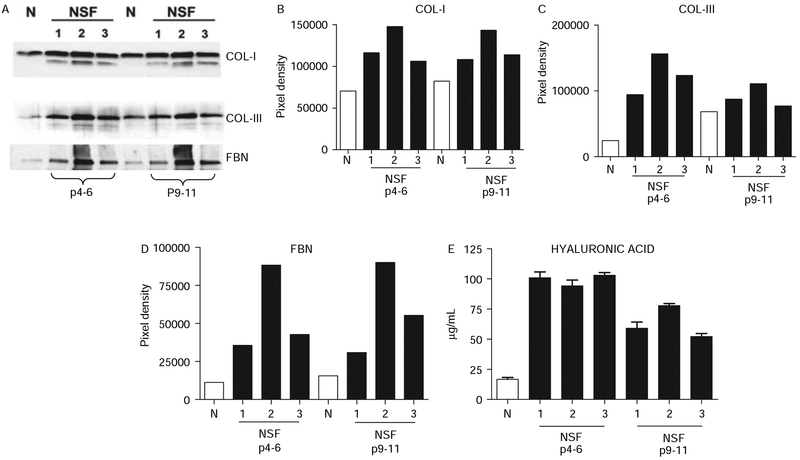
Collagen, fibronectin and hyaluronic acid production in cultured normal and NSF fibroblasts
Dermal fibroblasts from patients with NSF at early passages (4–6) displayed a marked increase in type I and type III collagen, fibronectin and hyaluronic acid production. The levels of collagen I were about two- to threefold higher, those of collagen III were 3.5–6-fold higher, those of fibronectin were three- to sevenfold higher and those of hyaluronic acid were four- to fivefold higher compared with normal fibroblasts (figure 2). To examine whether the activated phenotype was maintained after serial subpassages, levels of collagen types I and III, fibronectin and hyaluronic acid were examined in the same cell lines at passages 9–11. The results showed that the increased production of all these molecules by NSF dermal fibroblasts was maintained for at least 9–11 passages in vitro when the experiment was terminated (figure 2).
Figure 2.
Production of types I and III collagens, fibronectin (FBN) and hyaluronic acid by normal and nephrogenic systemic fibrosis (NSF) cultured dermal fibroblasts at early passage (passages 4–6) and at late passage (passages 9–11). (A) Western blot of media from NSF and normal (N) fibroblasts with specific antibodies for collagen I, collagen III and fibronectin. (B–D) Densitometric assessment of the intensity of western blot bands shown in A. (E) Quantitative assay for hyaluronic acid content.
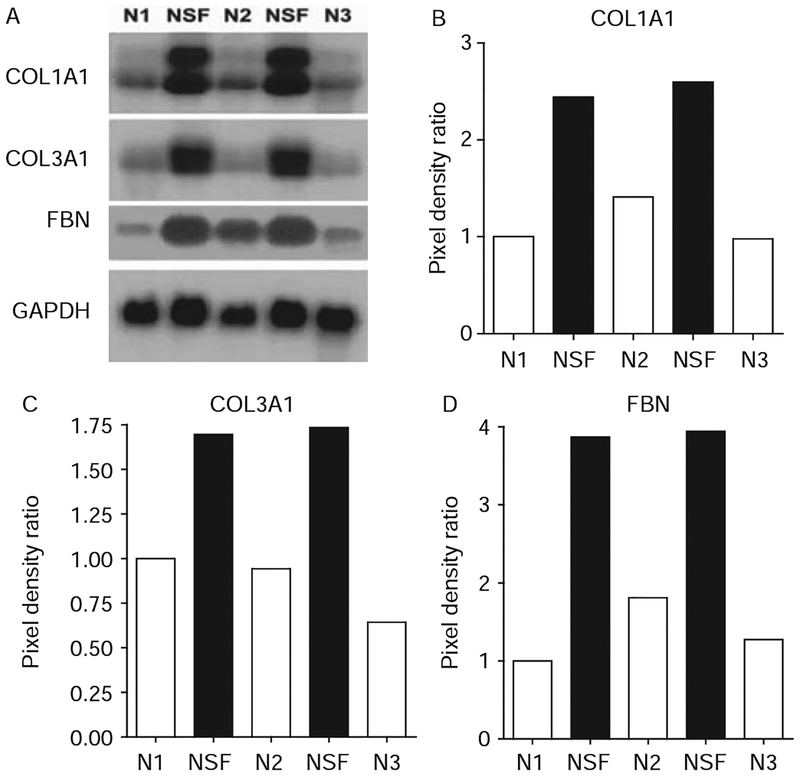
Steady-state mRNA levels and transcriptional regulation of COL1A1
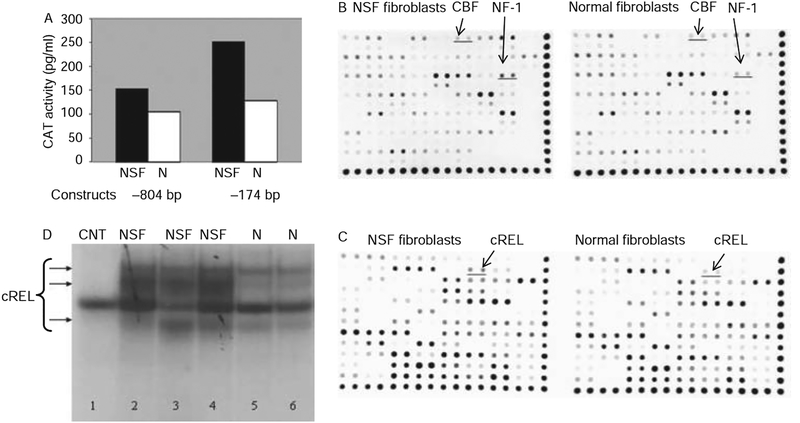
Northern hybridisations showed a marked increase in type I and III collagen and fibronectin steady-state mRNA levels in NSF cells (figure 3). To examine whether the increased type I collagen mRNA in the NSF cells was at the transcriptional level we studied the transcriptional activity of two COL1A1 promoter constructs encompassing −804 and −174 bp in transient transfection assays. The results demonstrated significantly higher transcriptional activity driven by both the −804 bp (~50%) and the −174 bp (~250%) constructs in NSF fibroblasts compared with normal fibroblasts (figure 4A).
Figure 3.
Steady state mRNA levels for types I and III collagens and fibronectin (FBN) in normal and nephrogenic systemic fibrosis (NSF) fibroblasts. (A) Northern hybridisations of total RNA from cultured NSF and normal (N) fibroblasts. (B–D) Densitometric assessment of the intensity of mRNA bands in the northern blots shown in (A). Pixel density was measured by Image J software. Each band was quantified and divided by the corresponding housekeeping band density value. The ratio was set to 1 for the first control sample and all others values were calculated as a multiple thereof. GAPDH, glyceraldehyde phosphate dehydrogenase.
Figure 4.
Transcriptional activity of the collagen, type I, α1 (COL1A1) proximal promoter in normal and nephrogenic systemic fibrosis (NSF) fibroblasts. (A) Two COL1A1 promoter constructs (−804 and −174 bp) ligated to the bacterial chloramphenicol acetyl transferase (CAT) gene were transiently transfected into subconfluent monolayer cultures of normal (N) or NSF dermal fibroblasts. CAT activity was assayed after transfection in TranSignal whole cell lysates. The data are the average of duplicate assays. (B, C) Protein/DNA binding activities identified using the protein/DNA arrays and assessment of cREL binding activity in normal and NSF fibroblasts. (B) Array 1; (C) Array 2. Differential binding activity (cREL, NF1 and CBF) is underlined. The results shown are representative of two separate experiments, each performed in duplicate. (D) Electrophoretic mobility shift assay of DNA/protein complexes obtained with nuclear extracts from NSF or N fibroblasts incubated with cREL oligonucleotide probes. Lane 1: control (CNT; 100-fold unlabelled cREL oligonucleotide). Lanes 2–4: nuclear extracts from each of the NSF cell lines. Lanes 5 and 6: Nuclear extracts from each of the normal cell lines.
Protein/DNA array and EMSA analysis of nuclear extracts from normal and NSF cells
To identify the transcription factors that may be responsible for the increased transcriptional activity of the COL1A1 promoter we profiled the binding activities of 108 transcription factors from normal and NSF fibroblasts. Figure 4B,C shows one illustrative experiment. We identified increased cREL, NF1 and CBF (NB. not beta) transcription factor binding (2.6-, 1.9- and 1.4-fold, respectively) in NSF fibroblasts compared with normal fibroblasts. The results were reproduced in two separate array experiments. To confirm the array data, EMSA analysis was performed with cREL consensus oligonucleotides as probes. A marked increase in cREL binding activity was noted in nuclear extracts from NSF fibro-blasts compared with normal fibroblasts (figure 4D).
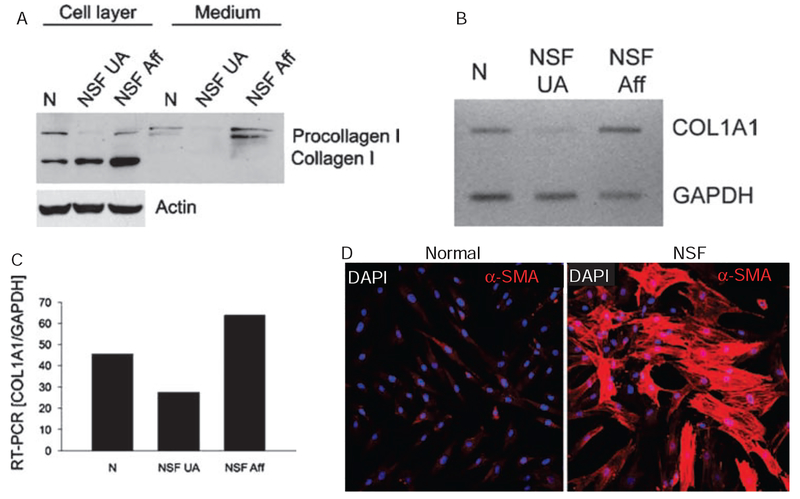
Collagen gene expression in fibroblasts from affected and non-affected NSF skin and α-SMA expression in NSF fibroblasts
Analysis of type I collagen gene expression and production of the corresponding protein in dermal fibroblasts cultured from clinically affected or clinically non-affected skin from one patient with NSF showed that fibroblasts from clinically affected skin produced much greater amounts of type I collagen and displayed a more than twofold increase in the expression of the corresponding gene (figure 5A–C). To examine whether fibroblasts cultured from NSF-affected skin displayed an activated myofibroblast phenotype we performed indirect immunofluorescence for α-SMA in confluent cultures at passage 9. We observed a marked increase in α-SMA containing cells in the monolayers from NSF dermal fibroblasts compared with normal cells (figure 5D). The intracellular organisation of α-SMA showed abundant filaments and stress fibres in the majority of cells in the NSF cultures, indicating that these cells were fully differentiated myofibroblasts.
Figure 5.
Comparison of collagen I production and gene expression by dermal fibroblasts isolated from clinically unaffected (NSF UA) and affected (NSF Aff) skin and immunofluorescence for α-smooth muscle actin (α-SMA) in normal and NSF fibroblasts. (A) Passage 4 fibroblasts obtained from samples of clinically affected and non-affected skin from the same patient were grown to confluency and then the medium was collected. Subsequently the cell layers were extracted. Proteins from the cell layers and culture media were separated in polyacrylamide gels, and protein bands corresponding to the pro-α1(I) and α1(I) chains were detected by western blots with specific antibodies to type I collagen. Actin was used as a loading control. Samples of media and cell extracts from fibroblasts from a healthy donor (N) are also included as controls. (B) Analysis of collagen, type I, α1 expression in dermal fibroblasts derived from unaffected (NSF UA) and affected (NSF Aff) skin. Total RNA was isolated from the cultured cells and RT-PCR was performed as described in materials and methods. (C) Densitometric analysis of the RT-PCR bands after normalisation to glyceraldehyde phosphate dehydrogenase (GAPDH) transcripts. A sample from normal human dermal fibroblasts (N) is included as a control. (D) Confocal microscopy of immunofluorescence for α-SMA in cultured normal and NSF dermal fibroblasts. NSF dermal fibroblasts display prominent α-SMA expression and intracellular filament organisation. α-SMA fluorescence is in red. 4’,6-Diamidino-2-phenylindole staining of all nuclei appears in blue/purple. NSF, nephrogenic systemic fibrosis.
DISCUSSION
NSF is a severe systemic fibrosing disease occurring in patients with renal insufficiency in association with the administration of GdBCA.1,2,10–21 The mechanisms by which GdBCA may result in tissue fibrosis in NSF are not known.
Here we have shown that fibroblasts cultured from affected skin from patients with Gd-associated NSF display a remarkable activation of their synthetic properties with a marked increase in the production of several extracellular matrix macromolecules. We have also shown that many of these cells are fully differentiated myofibroblasts, as indicated by the profound stimulation of expression of α-SMA and the presence of abundant stress fibres in their cytoplasms. The most remarkable observation was that the activated phenotype was maintained for at least 9–11 sequential passages in culture (when the experiments were terminated) and it is possible that the acquisition of this activated phenotype was a stable phenotypic change. The mechanisms responsible for the acquisition and maintenance of this activated phenotype probably include epigenetic modifications caused either directly, by the exposure of fibroblasts to GdBCA, or indirectly, by the action of profibrotic products released from macrophages, lymphocytes, or other inflammatory cell populations as a result of their interactions with the Gd compounds. The direct fibroblast activation by GdBCA is supported by the studies of Edward et al,34 who showed that cultures of normal dermal fibroblasts increased their proliferative capacity and displayed increased production and accumulation of hyaluronic acid when cultured with Gd compounds, and by our results showing stimulation of collagen production in normal dermal fibroblasts after in vitro culture with two GdBCA. The possibility of indirect effects of inflammatory cell products on fibroblasts is supported by our recent studies examining the effects of one GdBCA on human peripheral blood monocytes and the demonstration of potent stimulation of normal human fibroblasts by soluble products released from these cells upon contact with the GdBCA.35
Although increased production and deposition of collagen in fibrotic diseases can be caused by numerous mechanisms including pre- and post-transcriptional events, the results described here demonstrate that increased transcription of collagen genes is responsible for the fibrotic process in NSF. Numerous well-characterised transcription factors have been shown to interact with collagen gene promoters and are implicated in basal as well as cytokine- and growth factor-modulated collagen gene expression.36, 37 In previous studies we demonstrated a marked increase in CBF and Sp1 binding activity in systemic sclerosis fibroblasts compared with normal cells, indicating that these two transcription factors have a role in the development of tissue fibrosis in this disease.38, 39 The studies reported here demonstrate that the transcription factors displaying the highest binding activity in NSF fibroblasts were cREL and NF1. These results are important because they indicate that the molecular mechanisms involved in collagen gene activation in NSF may be distinct from those in systemic sclerosis.
Our previous studies showing a remarkable increase in the expression of TGFβ in affected skin from patients with NSF6 coupled with the marked increase in binding activity of the cREL component of the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) complex observed here suggest an important interaction between the NF-κB and TGFβ pathways in the development of pathological fibrosis. A previous report showing that NF-κB is a potent inhibitor of TGFβ-induced SMAD7 (inhibitory SMAD) gene expression and promoter activity supports this hypothesis.40 Of further relevance is the recent evidence indicating that cREL is involved in maturation and normal function of dendritic cells,41 a population of cells which we and others have shown to be markedly increased in affected NSF tissues.6 Furthermore, these results suggest that activation of the NF-κB pathway may be involved in the pathogenesis of NSF.
We also demonstrated directly that GdBCA can induce a profibrotic pattern of gene expression in normal fibroblasts and that NSF fibroblasts display a persistently activated phenotype which is ultimately responsible for the severe fibrotic process characteristic of the disease. The activation of fibroblasts represents an acquired event since the elevated collagen production is only seen in fibroblasts cultured from affected skin. This phenotypic change is a stable modification of their cellular behaviour as it is maintained through several subpassages in vitro and is probably mediated by epigenetic mechanisms. Our observations also suggest novel pathways such as the NF-κB pathway by which environmental exposures may be involved in the pathogenesis of idiopathic fibrotic diseases such as systemic sclerosis.
Acknowledgements
The expert assistance of Susan V Castro, PhD, in the preparation of this manuscript is gratefully acknowledged.
Funding The contributions of SAJ, SP-V, NL and JF were supported by NIH/NIAMS grant RO1 AR019616 to SAJ. FDG was supported by a Dermatology Foundation Career Development Award and a Scleroderma Foundation grant. PJW was supported by NIAMS training grant T-32 AR007583–15.
Footnotes
Ethics approval This study was conducted with the approval of the Thomas Jefferson University Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000;356:1000–1. [DOI] [PubMed] [Google Scholar]
- 2.Cowper SE. Nephrogenic systemic fibrosis: an overview. J Am Coll Radiol 2008;5:23–8. [DOI] [PubMed] [Google Scholar]
- 3.Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med 2003;114:563–72. [DOI] [PubMed] [Google Scholar]
- 4.Mackay-Wiggan JM, Cohen DJ, Hardy MA, et al. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease). J Am Acad Dermatol 2003;48:55–60. [DOI] [PubMed] [Google Scholar]
- 5.Jiménez SA, Artlett CM, Sandorfi N, et al. Dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy): study of inflammatory cells and transforming growth factor beta1 expression in affected skin. Arthritis Rheum 2004;50:2660–6. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza FA, Artlett CM, Sandorfi N, et al. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum 2006;35:238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kay J Nephrogenic systemic fibrosis: a gadolinium-associated fibrosing disorder in patients with renal dysfunction. Ann Rheum Dis 2008;67(Suppl 3):iii66–9. [DOI] [PubMed] [Google Scholar]
- 8.Ting WW, Stone MS, Madison KC, et al. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol 2003;139:903–6. [DOI] [PubMed] [Google Scholar]
- 9.Levine JM, Taylor RA, Elman LB, et al. Involvement of skeletal muscle in dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy). Muscle Nerve 2004;30:569–77. [DOI] [PubMed] [Google Scholar]
- 10.Koreishi AF, Nazarian RM, Saenz AJ, et al. Nephrogenic systemic fibrosis: a pathologic study of autopsy cases. Arch Pathol Lab Med 2009;133:1943–8. [DOI] [PubMed] [Google Scholar]
- 11.Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007;243:148–57. [DOI] [PubMed] [Google Scholar]
- 12.Grobner T Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21:1104–8. [DOI] [PubMed] [Google Scholar]
- 13.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006;17:2359–62. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen HS. Nephrogenic systemic fibrosis: a serious late adverse reaction to gadodiamide. Eur Radiol 2006;16:2619–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collidge TA, Thomson PC, Mark PB, et al. Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology 2007;245:168–75. [DOI] [PubMed] [Google Scholar]
- 16.Marckmann P, Skov L, Rossen K, et al. Case-control study of gadodiamide-related nephrogenic systemic fibrosis. Nephrol Dial Transplant 2007;22:3174–8. [DOI] [PubMed] [Google Scholar]
- 17.Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol 2007;2:264–7. [DOI] [PubMed] [Google Scholar]
- 18.Broome DR, Girguis MS, Baron PW, et al. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 2007;188:586–92. [DOI] [PubMed] [Google Scholar]
- 19.Moreno-Romero JA, Segura S, Mascaró JM Jr, et al. Nephrogenic systemic fibrosis: a case series suggesting gadolinium as a possible aetiological factor. Br J Dermatol 2007;157:783–7. [DOI] [PubMed] [Google Scholar]
- 20.Todd DJ, Kagan A, Chibnik LB, et al. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis Rheum 2007;56:3433–41. [DOI] [PubMed] [Google Scholar]
- 21.Cowper SE, Kuo PH, Bucala R. Nephrogenic systemic fibrosis and gadolinium exposure: association and lessons for idiopathic fibrosing disorders. Arthritis Rheum 2007;56:3173–5. [DOI] [PubMed] [Google Scholar]
- 22.Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (omniscan). Invest Radiol 2007;42:139–45. [DOI] [PubMed] [Google Scholar]
- 23.High WA, Ayers RA, Chandler J, et al. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007;56:21–6. [DOI] [PubMed] [Google Scholar]
- 24.High WA, Ayers RA, Cowper SE. Gadolinium is quantifiable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007;56:710–12. [DOI] [PubMed] [Google Scholar]
- 25.Thakral C, Alhariri J, Abraham JL. Long-term retention of gadolinium in tissues from nephrogenic systemic fibrosis patient after multiple gadolinium-enhanced MRI scans: case report and implications. Contrast Media Mol Imaging 2007;2:199–205. [DOI] [PubMed] [Google Scholar]
- 26.Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 2007;56:27–30. [DOI] [PubMed] [Google Scholar]
- 27.Jimenez SA, Gaidarova S, Saitta B, et al. Role of protein kinase C-delta in the regulation of collagen gene expression in scleroderma fibroblasts. J Clin Invest 2001;108:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Galdo F, Jiménez SA. T cells expressing allograft inflammatory factor 1 display increased chemotaxis and induce a profibrotic phenotype in normal fibroblasts in vitro. Arthritis Rheum 2007;56:3478–88. [DOI] [PubMed] [Google Scholar]
- 29.Louneva N, Huaman G, Fertala J, et al. Inhibition of systemic sclerosis dermal fibroblast type I collagen production and gene expression by simvastatin. Arthritis Rheum 2006;54:1298–308. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez SA, Varga J, Olsen A, et al. Functional analysis of human alpha 1(I) procollagen gene promoter. Differential activity in collagen-producing and -nonproducing cells and response to transforming growth factor beta 1. J Biol Chem 1994;269:12684–91. [PubMed] [Google Scholar]
- 31.Roman-Blas JA, Stokes DG, Jimenez SA. Modulation of TGF-beta signaling by proinflammatory cytokines in articular chondrocytes. Osteoarthr Cartil 2007;15:1367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Homer KA, Denbow L, Beighton D. Spectrophotometric method for the assay of glycosaminoglycans and glycosaminoglycan-depolymerizing enzymes. Anal Biochem 1993;214:435–41. [DOI] [PubMed] [Google Scholar]
- 33.Benchetrit LC, Pahuja SL, Gray ED, et al. A sensitive method for the assay of hyaluronidase activity. Anal Biochem 1977;79:431–7. [DOI] [PubMed] [Google Scholar]
- 34.Edward M, Quinn JA, Mukherjee S, et al. Gadodiamide contrast agent ‘activates’ fibroblasts: a possible cause of nephrogenic systemic fibrosis. J Pathol 2008;214:584–93. [DOI] [PubMed] [Google Scholar]
- 35.Wermuth PJ, Del Galdo F, Jiménez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum 2009;60:1508–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez SA, Saitta B. Alterations in the regulation of expression of the alpha 1(I) collagen gene (COL1A1) in systemic sclerosis (scleroderma). Springer Semin Immunopathol 1999;21:397–414. [DOI] [PubMed] [Google Scholar]
- 37.Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Exp Biol Med (Maywood) 2002;227:301–14. [DOI] [PubMed] [Google Scholar]
- 38.Hitraya EG, Varga J, Artlett CM, et al. Identification of elements in the promoter region of the alpha1(I) procollagen gene involved in its up-regulated expression in systemic sclerosis. Arthritis Rheum 1998;41:2048–58. [DOI] [PubMed] [Google Scholar]
- 39.Saitta B, Gaidarova S, Cicchillitti L, et al. CCAAT binding transcription factor binds and regulates human COL1A1 promoter activity in human dermal fibroblasts: demonstration of increased binding in systemic sclerosis fibroblasts. Arthritis Rheum 2000;43:2219–29. [DOI] [PubMed] [Google Scholar]
- 40.Nagarajan RP, Chen F, Li W, et al. Repression of transforming-growth-factor-beta-mediated transcription by nuclear factor kappaB. Biochem J 2000;348 (Pt 3):591–6. [PMC free article] [PubMed] [Google Scholar]
- 41.Boffa DJ, Feng B, Sharma V, et al. Selective loss of c-Rel compromises dendritic cell activation of T lymphocytes. Cell Immunol 2003;222:105–15. [DOI] [PubMed] [Google Scholar]