Abstract
Hypothesis:
Electrocochleography (ECochG) recorded during cochlear implant (CI) insertion from the apical electrode in conjunction with post-insertion ECochG can identify electrophysiologic differences that exist between groups with and without a translocation of the array from the scala tympani (ST) into the scala vestibuli (SV).
Background:
Translocation of the CI electrode from ST into SV, can limit performance post-operatively. ECochG markers of trauma may be able to aid in the ability to detect electrode array-induced trauma/scalar translocation intraoperatively.
Methods:
Twenty-one adult CI patients were included. Subjects were post-operatively parsed into two groups based on analysis of post-operative imaging: (1) ST (n=14) insertion; (2) SV (n=7) insertion, indicating translocation of the electrode. The ECochG response elicited from a 500 Hz acoustic stimulus was recorded from the lead electrode during insertion when the distal electrode marker was at the round window, and was compared to the response recorded from a basal electrode (e13) after complete insertion.
Results:
No statistically significant change in mean ECochG magnitude was found in either group between recording intervals. There was a mean loss of pre-operative pure tone average of 52% for the non-translocation group and 94% for the translocation group.
Conclusions:
Intraoperative intracochlear ECochG through the CI array provides a unique opportunity to explore the impact of the CI electrode on the inner ear. Specifically, a translocation of the array from ST to SV does not appear to change the biomechanics of the cochlear region that lies basal to the area of translocation in the acute period.
Keywords: intracochlear electrocochleography, cochlear implants, trauma, scalar translocation
INTRODUCTION
Cochlear implant (CI) surgical techniques and technologies continue to advance as the goal of minimizing trauma to inner ear structures and, thus, preserve residual hearing becomes increasingly important. This is evident by significant effort dedicated towards improvements in electrode design, surgical approaches (round window vs. cochleostomy), and techniques that promote atraumatic insertions (1,2). These technical refinements do appear to help preserve the delicate intracochlear microanatomy based on incremental improvements in hearing preservation (3–5). However, in order to achieve more consistent structural preservation and achieve better residual hearing outcomes, some form of real-time feedback is imperative. Furthermore, given the known detriment on CI speech perception performance when cochlear structures are not preserved (1,6,7), an objective tool that reflects the physiologic integrity of intracochlear structures is essential as we move into an era of cochlear implantation where many CI candidates have functional low frequency hearing potentially amendable to electro-acoustic stimulation. With this new population of CI candidates in mind, the current practice of inferring intracochlear trauma from secondary indicators (e.g., impedances, radiographs) appears inadequate.
Recently, electrocochleography (ECochG) has proven to be an objective method of monitoring cochlear physiology (e.g., cochlear microphonic) in near real-time during cochlear implantation (8,9). Multiple studies have successfully recorded ECochG prior to, actively during, and/or immediately following insertion from extracochlear locations during electrode insertion (8–12). Advances in recording technique have furthered this technology by allowing recording of ECochG through the implant itself during insertion (13–16). Recent data collected with this technique has shown it to be a reliable method of determining audiometric thresholds when conducted in the post-operative period (16,17). Thus, ECochG appears to hold significant promise for real-time detection of adverse events, such as trauma, that alters cochlear physiology. Therefore, this technique could provide for unique evaluation of the biomechanics of the inner ear in patients with CIs.
The primary objective of this study was to correlate ECochG responses during insertion to responses obtained immediately post-CI insertion by utilizing the standard (blue) markers on the electrode to identify discrete points in time where the recording electrode was located during the insertion process as well as post-insertion. The goal was to test whether a disruption/trauma to a portion of the basilar membrane (i.e., translocation of the electrode from scala tympani to scala vestibuli) at a distant location to the site of recording would significantly influence the cochlear response at the recording site which was proximal to the site of translocation. Our overarching hypothesis was that the change/decrease in the ECochG response magnitude (or lack thereof) between the two recording intervals (before and after likelihood of a translocation) would be larger in the ears where the electrode transgressed the basilar membrane, disrupting basilar membrane motion, and entered scala vestibuli compared to ears where the electrode remained completely within the scala tympani.
METHODS
The patients in this study were prospectively enrolled as part of a multi-institutional collaboration. Adults (≥18 years) with normal outer and middle ear structures and without history of middle ear disease and/or surgery, who were English speaking, undergoing cochlear implant surgery with an Advance Bionics (Valencia, CA) HiRes90K internal receiver and a HiFocus Mid Scala electrode were eligible and provided written consent for this study. Level of pre-operative audiometric thresholds was not an inclusion criterion. Institutional Review Board (IRB) approval was obtained at all participating institutions.
Intraoperative Recording Technique
Surgical and electrophysiologic recording setups have been described in detail in recent reports (15,17). In brief, the process begins with placement of a foam ear applicator in the external auditory canal. The pinna is then draped out of the sterile field. Following a cortical mastoidectomy and creation of a facial recess, a universal head piece is connected to the internal receiver-stimulator. The ECochG response is recorded from the lead contact (electrode 1) repeatedly during the electrode insertion. A 500 Hz tone burst of 50 ms with a rise/fall time of 5 ms is applied at a 110 dB SPL at a rate of 5 averages per second with the stimulus alternating in polarity (rarefaction and condensation). During insertion, the implanting surgeon audibly reported to the audiologist managing the evoked potential equipment three discrete points pertaining to how the insertion process was progressing (please see Electrode Markers below for further description of the implant and the corresponding blue lines): 1) “Round window”- lead electrode at/immediately within the cochlea, 2) “first blue line”- when the first blue line marker on the electrode at the round window, 3) “second blue line”- when the second blue line marker on the electrode at the round window, indicating complete electrode insertion. These three surgeon reported comments are logged into the recording computer and time stamped into the record with the corresponding ECochG trial.
Following complete electrode insertion, the computer software is adjusted to carry out ECochG recordings from multiple electrode (E) contacts (E1 (apical location), E5, E9, E13 (basal location) using a 500 Hz acoustic tone burst stimulus. The amplitude of the first harmonic of the ECochG response to the 500 Hz tone burst was logged for both recording intervals (insertion; post-insertion).
Electrode Markers
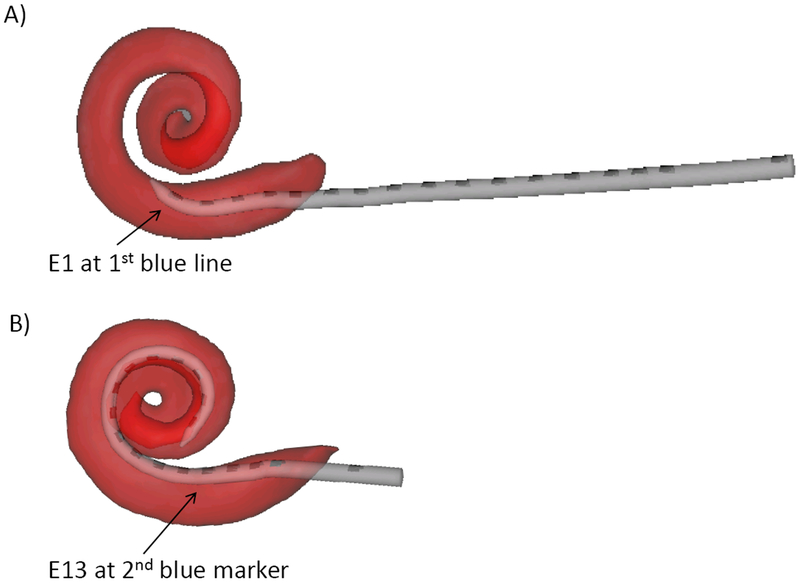
The HiFocus Mid Scala electrode array is a pre-curved stylet electrode that is designed to achieve an insertion depth of approximately 420 degrees and reside in the middle of scala tympani (18). The array contains 16 electrode contacts that are spaced 0.975 mm apart. Two blue lines are marked on the electrode array to provide the surgeon visual identification of approximate insertion depth. The first blue marker is located mid-way (.4875 mm) between electrodes five and six. Utilizing these measurements, it can be calculated that the apical electrode (E1) is 4.39 mm ((.975*4) + .4875) from the first blue marker. Therefore, when the surgeon reports that the “first blue line” is at the round window, the recording (active) electrode is approximately 4.4 mm into the cochlea (see Figure 1A). The second blue line marker on the electrode array (indicating full electrode insertion when at the round window) is located 3.0 mm distal to the basal most electrode contact (E16). Given that each contact is spaced 0.975 mm apart, electrode 13 is calculated to be approximately 2.925 mm proximal to electrode 16 and therefore 5.925 mm (3.0 + 2.925) away from the round window/second blue line marker (see Figure 1B). It can then be concluded that the ECochG data point collected when the surgeon reports “first blue line” should be in close proximity to the region in the cochlea where the data point obtained from electrode 13 was obtained once full insertion had been reached.
Figure 1:
A) Graphical representation of approximate location within cochlea where the ECochG recording interval “first blue line” is recorded from during insertion. B) Graphical representation of approximate location within the cochlear where the ECochG recording interval “E13” is recorded from post- electrode insertion.
Data Analysis
First harmonic spectral amplitudes of the ECochG response, thought to primarily represent the cochlear microphonic, during insertion and immediately post-insertion were obtained using fast Fourier transforms (FFT) using custom routines in Matlab (Mathworks, Natick, MA). The ECochG amplitudes in microvolts (uV) were then converted to dB relative to 1 uV. The study results were divided into two groups based on whether there was a translocation: 1) complete scala tympani (ST) insertion; 2) translocation of a portion of the electrode array into scala vestibuli. Trans-scalar locations were confirmed via post-operative computed tomography (CT). ECochG responses obtained at “first blue line” marker were compared to responses obtained intraoperatively after full insertion recorded from electrode 13. Statistical analysis was performed using SPSS v.24.0 (IBM Corp., Armonk, NY, USA). Pearson correlation analysis and two tailed paired t-tests were used to assess the ECochG results of the two groups separately at the two recording intervals.
Computed Tomography Scan and Electrode Position
Conventional CT scans were obtained on all patients prior to CI surgery. Patients underwent a second scan following electrode insertion either in the operating room using a portable flat panel volumetric CT scanner or at the initial post-operative visit using traditional CT methods. Both scans were then co-registered and analyzed for electrode scalar placement using semi- and fully-automated processing strategies (see previously described protocols for complete details (19,20)).
Audiometry
All patients underwent a pre-operative CI evaluation as well as post-operative audiometric evaluation at the first post-operatively (4 weeks)and were evaluated to rule out middle ear effusion via otoscopy by the surgeon at that time. Low frequency pure tone average (LF-PTA) was calculated using .25, .5, 1 kHz for each participant. The relative change in LF-PTA from pre- to post-CI surgery was calculated using the equation described by Skarzynski et al, (2013) (21) where relative change = ((PTApost-PTApre)/(PTAmax-PTApre)).PTApost and PTApre are the pure tone averages measured at the corresponding testing interval and PTAmax is the value used to describe the limits of the audiometer.
RESULTS
ECochG responses from 30 patients were analyzed. Due to clinical restraints and/or patient factors, post-operative CT was not obtained on some participants and were therefore excluded from analyses (n=2). Also, due to severity of hearing loss (i.e., poor/low amplitude ECochG signal), multiple participants were excluded as responses were not above noise floor (n=7). After exclusion, 21 patients were sufficient for inclusion in analyses. Fourteen patients comprised the ST group (mean pre-operative LF-PTA: 70 dB HL (+/−21 standard deviation (sd))) and seven were included in the SV group (mean pre-operative LF-PTA: 70 dB HL (+/−21 sd). Patient characteristics can be seen in Table 1.
Table 1:
Patient characteristics. Low frequency pure-tone average (LF-PTA), scala tympani (ST), scala vestibuli (SV), round window (RW), extended round window (ERW).
| Patient | Group | Approach | Pattern | LF-PTA Loss (%) |
|---|---|---|---|---|
| 1 | ST | ERW | A | 0 |
| 2 | ST | RW | A | 50 |
| 3 | ST | RW | A | 90 |
| 4 | ST | RW | A | 28 |
| 5 | ST | RW | A | 100 |
| 6 | ST | ERW | A | 38 |
| 7 | ST | RW | A | 83 |
| 8 | ST | RW | A | 53 |
| 9 | ST | RW | A | 82 |
| 10 | ST | RW | A | 41 |
| 11 | ST | RW | A | 0 |
| 12 | ST | ERW | C | 46 |
| 13 | ST | RW | A | 69 |
| 14 | ST | RW | C | 50 |
| 15 | SV | RW | A | 55 |
| 16 | SV | RW | C | 100 |
| 17 | SV | RW | A | 100 |
| 18 | SV | RW | B | 100 |
| 19 | SV | RW | A | 100 |
| 20 | SV | RW | C | 100 |
| 21 | SV | RW | C | 100 |
First Blue Line and Electrode 13
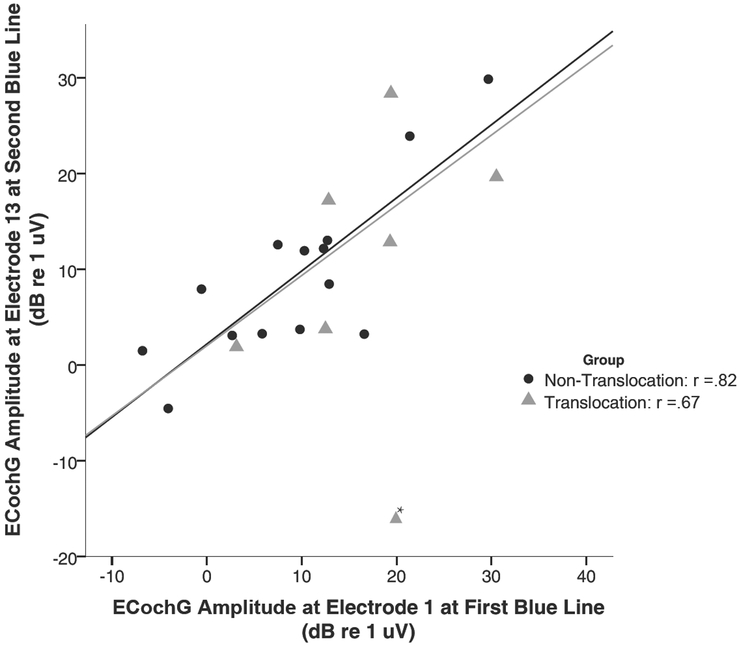
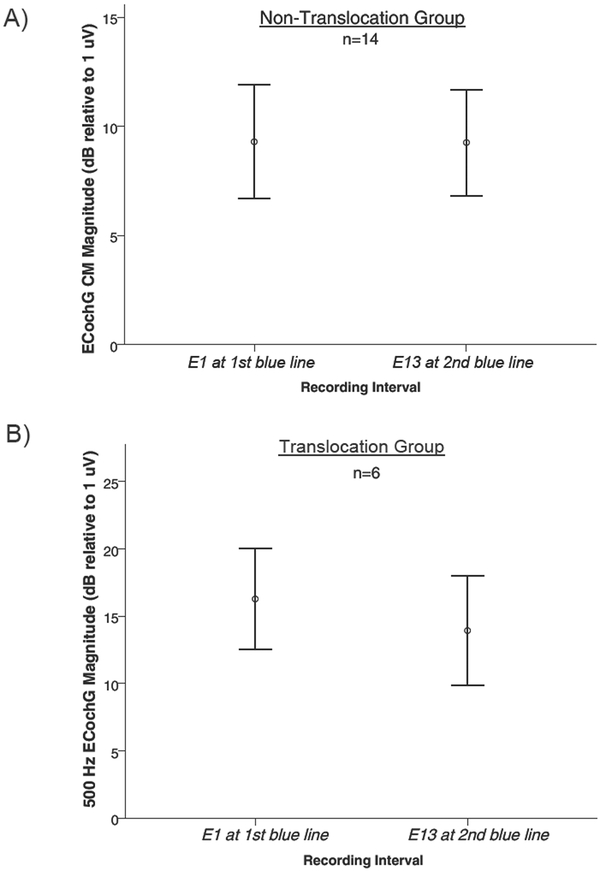
Magnitude for the ECochG response obtained from electrode 1 when the first blue marker was at the round window is plotted as a function of the ECochG response obtained from electrode 13 immediately post-electrode insertion (Figure 2). Bivariate correlation revealed a strong correlation between the response magnitude at the two recording intervals for the patients without a translocation (r =.82, p<.05), as well as for the patients with a translocation (r= .67, p<.05), indicating a similar amplitude of the ECochG response at the two recording intervals.. Note, in the SV group, patient #18 was identified as an outlier (change in response magnitude was greater than 2 standard deviations away from the mean) and was removed from correlation analyses, but was still plotted in Figure 2 for visualization. Mean magnitude for the “first blue marker” response of the ST group was 9.3 dB relative to 1 μV (+/− 9.78 sd) and immediately following complete insertion when recorded from E13 was 9.3 dB (+/− 9.1 sd) (Figure 3A). For the SV group the mean magnitudes were 16.2 dB relative to 1 μV (+/− 9.1 sd) and 14.0 dB (+/−10.0 sd) respectively (Figure 3B with outlier case #18 excluded). Neither group had a significant change in the ECochG magnitude between the two recording intervals (ST group 0.0 dB: p=.98; SV group 2.2 dB: p=.5).
Figure 2:
The 500 Hz ECochG magnitude recorded during electrode insertion from electrode 1 when the first blue marker was at the round window plotted as a function of the 500 Hz ECochG magnitude recorded from electrode 13 after complete insertion. Note, * indicates outlier (>2 sd away from mean) which was not included in line of best fit.
Figure 3:
A) Magnitude of the ECochG response following a 500 Hz tone burst at two recording intervals: “first blue line” and “electrode 13” for those without a translocation (ST) Note: Error bars represent +/− 1 standard error of the mean, circles and squares represent mean magnitude for each group. B) Magnitude of the ECochG response following a 500 Hz tone burst at two recording intervals: “first blue line” and “electrode 13” for, those with a translocation (SV). Note: Error bars represent +/− 1 standard error of the mean, circles and squares represent mean magnitude for each group.
Insertion Tract Patterns By Group & Post-Operative Hearing Preservation
Based on previous work describing the insertion patterns of ECochG amplitude that are found when elicited by a 500 Hz tone burst as the electrode courses from the round window to full electrode insertion (A: low-high; B: high-low; C: low-high-low) (14), the pattern observed for the ECochG insertion tract was logged for each case (refer to Table 1). Of the fourteen cases in the ST group twelve (86%) were type A and two (14%) were type C. In the SV group three (43%) of the insertion tracts were type A, three (43%) were type C and only 1 was type B (14%).
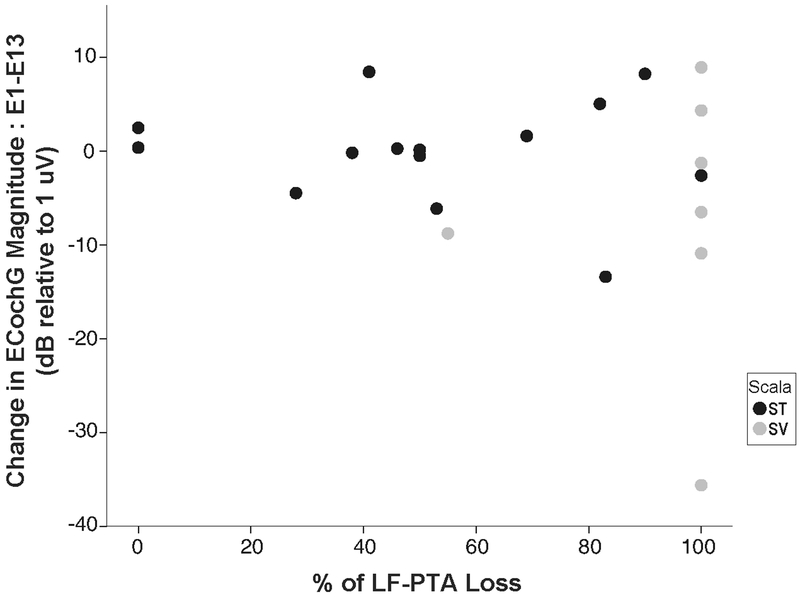
Patients underwent audiometric testing 4 weeks following CI surgery of the implanted ear. The average relative loss of pre-operative air conducted low frequency (.25, .5, 1 kHz PTA was 52% for the non-translocation group and 94% for the translocation group. Note, six of the seven patients comprising the SV group experienced complete loss of residual hearing (100%), while patient #15 revealed only a 55% loss of pre-operative LF-PTA. This patient’s CT analysis revealed a small but present breach of the electrode through the basilar membrane with minimal protrusion to SV, which is possibly why this patient did have measureable thresholds at the one month audiogram testing interval. To identify if there was a relationship between change in ECochG amplitude and percentage of pre-operative hearing lost, the difference in ECochG magnitude at the two recording intervals was plotted as a function of loss of LF-PTA (Figure 4). Considering the group without a translocation, there was no significant correlation (r= −0.083, p>.05), indicating that the change in response magnitude between E1 and E13 does not predict the amount of pre-operative LF-PTA that is lost when the electrode resides completely in ST.
Figure 4:
The difference in the ECochG magnitudes at the two recording intervals (E1-E13) plotted as a function with the change in low frequency-pure tone average (LF-PTA) percentage from pre- to post-surgery. The black circles represent the patients of the group without a translocation and the gray circles represent the patients in the group with a translocation
DISCUSSION
The aim of the current study was to better understand the effects scalar location has on intracochlear ECochG between CI electrode insertions into scala tympani and scala vestibuli. The current results show that recipients with a translocation of the electrode array from scala tympani to scala vestibuli do not reveal a statistically significant larger decrement in the ECochG response when recorded basal to the site of translocation after electrode insertion than those with complete scala tympani insertions.
First Blue Line and Electrode 13
This study took a novel approach of evaluating the impact translocation may have on the ECochG response by utilizing two discrete time points during CI electrode insertion. The basis of these location measurements is the blue contact marker on the electrode itself that gives the surgeon a visual estimation of the current depth of insertion. Due to the location of the first blue line marker, it is highly unlikely at this point that a translocation has occurred in the cochlea, as the electrode is less than approximately 6 mm into the basal turn. Therefore, using a round window or extended round window approach, this location should provide an “atraumatic” recording point, as opposed to the later time interval when the ECochG response is recorded from E13 after the possibility of a translocation having occurred. Thus, differences in ECochG responses between these points were hypothesized to be much larger when a translocation had occurred.
While differences between the two groups were detectable (0.0 dB for the ST only group and a 2.2 dB decrement for the translocation group), these differences were not found to be statistically significant. Our results are in contrast to both human and animal studies in this regard, as our results do not show a decrease in the CM amplitude before and after a translocation (13,22,23). We offer two potential reasons why this unexpected finding occurred. First, these previous studies using intracochlear ECochG have found that when recording from the lead electrode actively during electrode insertion and trauma occurs to the inner ear (i.e. translocation from scala tympani to scala vestibuli), a corresponding decrease in CM amplitude is observed when recording from that same recording electrode following the translocation. However, it must be pointed out that our technique utilized CM measurements that were recorded basal to the region of translocation (E13), whereas the decline in CM amplitude in the above mentioned studies was observed when recording from the lead electrode after a translocation occurred and apical to the region of the trauma. This suggests that it is possible that the location of the recording electrode is likely an important factor in detecting changes in the CM amplitude that are caused by trauma.
Therefore, perhaps our original hypothesis was incorrect and that translocation does not disrupt the cochlear response when it is recorded basal to the site of translocation in the acute period while using a low frequency stimulus. Such a finding was not necessarily intuitive to the authors given that trauma caused by the electrode array, such as translocation, is known to be a strong predictor of loss of residual hearing (24,25). However, in further examination of previous literature, this finding is consistent with the established theory initially raised by Helmholtz and then later demonstrated by von Bekesy and colleagues that the basilar membrane is composed of independent lightly coupled resonators which move in response to fluid movement through the perilymphatic space of the cochlea. This fluid movement in turn acts on each part of the basilar membrane individually, hence the traveling wave theory (as opposed to the BM conducting/passing the energy) (26). Further, the findings of early post-mortem studies by Guild and others identified various anomalies of basilar membrane fixation which affected hearing sensitivity (elevated threshold) of the fixated region but not of other areas of the cochlea (27,28) additionally support our current results. Therefore, it is possible that long-term changes following CI insertion likely take effect, leading to loss of residual hearing, and our technique was able to avoid those contributions by evaluating in the immediate intraoperative period following electrode insertion. However, further studies assessing the E13 response magnitude over time would be warranted to identify long-term effects secondary to basilar membrane disruption.
The second possibility of our results being in contrast to previous works could be due to the small sample size of 21 overall patients with 14 in the ST only group and 7 in the translocation (SV) group of which one, case #18, was excluded from further analysis as an outlier. Because this was the first time sequential ECochG recordings were made based on intracochlear position, we had no a priori basis for which to estimate sample size necessary to demonstrate a statistically significant effect. With the preliminary data herein (difference of 2.2 +/− 6.8 sd), a much larger study of n=150 within each group (n = 300 overall) would be necessary likely requiring more participating centers.
Patterns of ECochG and Hearing Preservation
This work has also built upon and complemented our understanding of previously described patterns of intracochlear ECochG (14). Interestingly, the group with a translocation exhibited more variation in insertion pattern type than the non-translocation group. In the non-translocation group, 86% (12/14) were of pattern A with only two being pattern C and none of pattern B. Conversely, only 43% (3/7) of cases in the translocation group exhibited a type A pattern and this group also had one outlier which was of pattern B.
Additionally, hearing preservation was far better in the non-translocation group, where there was on average a 52% loss of pre-operative LF-PTA, while 6/7 patients with a translocation had complete loss of residual hearing. The one patient with partial residual hearing in the SV group did have a breach to the basilar membrane by the electrode but had minimal protrusion into SV. While the change in ECochG amplitude between E1 and E13 did not predict the amount of hearing that was preserved, the findings of this study support the contention that final scalar location of the electrode is a sensitive marker in the preservation of residual hearing—namely, that long term hearing preservation is only possible when translocation does not occur.
Finally, limitations of the current study must be kept in mind. The sample size in general was relatively small and there were considerably fewer number of participants in the SV group compared to the ST group, which limits the generalization of these findings. Also, pre-operative hearing levels were not an inclusion criterion, nor were they controlled for amongst the groups, despite both groups having a mean pre-operative LF-PTA of 70 dB HL. Finally, it should be pointed out that there were seven patients that were excluded from analysis due to severity of preoperative hearing, indicating that this technique may not be useful in all patients undergoing CI surgery. Future work would benefit from accounting for these differences as well as establishing how these results may differ when using a lateral wall electrode opposed to the pre-curved electrode used in the current study.
CONCLUSIONS
Intraoperative intracochlear ECochG through the CI array provides a unique opportunity to explore the effects of the CI electrode on the inner ear. In this preliminary report, we found that translocation of the array from scala tympani to scala vestibuli may not change the biomechanics of the cochlear region which lies basal to the area of translocation in the acute period. Therefore, using post-insertion ECochG recorded from that basal region of the cochlea in conjunction with ECochG data recorded during insertion does not appear to be beneficial in helping to identify traumatic electrode insertions.
ACKNOWLEDGEMENTS
The authors would like to thank all of the subjects who participated in the study. Research reported in this publication was supported by the National Institute of Deafness and Other Communication Disorders within the National Institutes of Health (NIH, R01DC008408, R01DC014462, R01DC014037, and the Development of Clinician/Researchers in Academic ENT training grant, award number T32DC000022). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: KK and LL are employees of Advanced Bionics Corp. OFA, CAB, RFL are consultants for Advanced Bionics Corp, Med-El Corp, Cochlear Corporation and MSH is a consultant with Advanced Bionics Corp. OFA and CAB have an ownership interest in Advanced Cochlear Diagnostics, LLC.
REFERENCES
- 1.Adunka O, Unkelbach MH, Mack M et al. Cochlear implantation via the round window membrane minimizes trauma to cochlear structures: a histologically controlled insertion study. Acta Otolaryngol 2004;124:807–12. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen Y, Mosnier I, Borel S et al. Evolution of electrode array diameter for hearing preservation in cochlear implantation. Acta Otolaryngol 2013;133:116–22. [DOI] [PubMed] [Google Scholar]
- 3.Aschendorff A, Kromeier J, Klenzner T et al. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear 2007;28:75S–9S. [DOI] [PubMed] [Google Scholar]
- 4.Carlson ML, Driscoll CL, Gifford RH et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol 2011;32:962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gantz BJ, Turner C, Gfeller KE et al. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope 2005;115:796–802. [DOI] [PubMed] [Google Scholar]
- 6.Finley CC, Holden TA, Holden LK et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol 2008;29:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanna GB, Noble JH, Carlson ML et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adunka O, Roush P, Grose J et al. Monitoring of cochlear function during cochlear implantation. Laryngoscope 2006;116:1017–20. [DOI] [PubMed] [Google Scholar]
- 9.Choudhury B, Fitzpatrick DC, Buchman CA et al. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol 2012;33:1507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalbert A, Pfiffner F, Roosli C et al. Extra- and Intracochlear Electrocochleography in Cochlear Implant Recipients . Audiol Neurootol 2015;20:339–48. [DOI] [PubMed] [Google Scholar]
- 11.Adunka OF, Giardina CK, Formeister EJ et al. Round window electrocochleography before and after cochlear implant electrode insertion. Laryngoscope 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick DC, Campbell AT, Choudhury B et al. Round window electrocochleography just before cochlear implantation: relationship to word recognition outcomes in adults. Otol Neurotol 2014;35:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell L, Kaicer A, Sly D et al. Intraoperative Real-time Cochlear Response Telemetry Predicts Hearing Preservation in Cochlear Implantation. Otol Neurotol 2016;37:332–8. [DOI] [PubMed] [Google Scholar]
- 14.Harris MS, Riggs WJ, Giardina CK et al. Patterns Seen During Electrode Insertion Using Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant. Otol Neurotol 2017;38:1415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris MS, Riggs WJ, Koka K et al. Real-Time Intracochlear Electrocochleography Obtained Directly Through a Cochlear Implant. Otol Neurotol 2017;38:e107–e13. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell BP, Holder JT, Dwyer RT et al. Intra- and Postoperative Electrocochleography May Be Predictive of Final Electrode Position and Postoperative Hearing Preservation. Front Neurosci 2017;11:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koka K, Saoji AA, Litvak LM. Electrocochleography in Cochlear Implant Recipients With Residual Hearing: Comparison With Audiometric Thresholds. Ear Hear 2017;38:e161–e7. [DOI] [PubMed] [Google Scholar]
- 18.Boyle PJ. The rationale for a mid-scala electrode array. Eur Ann Otorhinolary 2016;133:S61–S2. [DOI] [PubMed] [Google Scholar]
- 19.Schuman TA, Noble JH, Wright CG et al. Anatomic verification of a novel method for precise intrascalar localization of cochlear implant electrodes in adult temporal bones using clinically available computed tomography. Laryngoscope 2010;120:2277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teymouri J, Hullar TE, Holden TA et al. Verification of computed tomographic estimates of cochlear implant array position: a micro-CT and histologic analysis. Otol Neurotol 2011;32:980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skarzynski H, van de Heyning P, Agrawal S et al. Towards a consensus on a hearing preservation classification system. Acta Otolaryngol Suppl 2013:3–13. [DOI] [PubMed] [Google Scholar]
- 22.Campbell AP, Suberman TA, Buchman CA et al. Correlation of early auditory potentials and intracochlear electrode insertion properties: an animal model featuring near real-time monitoring. Otol Neurotol 2010;31:1391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhury B, Adunka OF, Demason CE et al. Detection of intracochlear damage with cochlear implantation in a gerbil model of hearing loss. Otol Neurotol 2011;32:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanna GB, Noble JH, Gifford RH et al. Impact of Intrascalar Electrode Location, Electrode Type, and Angular Insertion Depth on Residual Hearing in Cochlear Implant Patients: Preliminary Results. Otol Neurotol 2015;36:1343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connell BP, Hunter JB, Wanna GB. The importance of electrode location in cochlear implantation. Laryngoscope Investig Otolaryngol 2016;1:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wever EG, Lawrence M, Von Bekesy G. A Note on Recent Developments in Auditory Theory. Proc Natl Acad Sci U S A 1954;40:508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowe SJ, Guild SR, Polvogt LM. Observations on the pathology of high-tone deafness. B Johns Hopkins Hosp 1934;54:315–79. [Google Scholar]
- 28.Guild SR. Correlations of histologic observations and the acuity of hearing. Acta Otolaryngol 1932;17:207–49. [Google Scholar]