Abstract
Background:
Objective adherence metrics for tenofovir (TFV) disoproxil fumarate/emtricitabine(FTC)-based PrEP were critical for interpretation of efficacy in PrEP clinical trials, and there is increasing interest in using drug levels to tailor interventions for reengagement and adherence. Point-of-care immunoassays (POC) for TFV, which examine short-term adherence, are in development. However the ability of poor short-term and long-term adherence to predict future PrEP non-retention is unknown.
Setting:
Secondary data analysis of a large, prospective multi-site U.S. PrEP demonstration project
Methods:
An adjusted Cox-proportional hazards model examined the relationship of dried blood spot (DBS) levels of FTC-triphosphate (FTC-TP) or TFV-diphosphate (TFV-DP), measures of short-term and long-term PrEP adherence, respectively, with future study non-retention.
Results:
Overall, 294 individuals (median age 33 years) contributed drug levels within the U.S. PrEP demonstration project. By study end, 27% were lost to follow-up, 25% had at least one undetectable FTC-TP level indicating poor short-term adherence, and 29% had a drug level indicating sub-optimal long-term adherence (TFV-DP<700 fmol/punch). The strongest factor associated with future study non-retention using a binary drug-level cut-off was an undetectable DBS FTC-TP level [adjusted hazard ratio (AHR) 6.3; 95% confidence interval (CI) 3.8-10.2]. Sub-optimal long-term adherence based on low DBS TFV-DP levels was also associated with non-retention (AHR 4.3; 95% CI 2.4-7.6).
Conclusions:
Both short and long-term metrics of PrEP adherence are strongly associated with future loss to follow-up in a U.S. demonstration project study. Short-term metrics of adherence, once available at the point-of-care, could be used to direct real-time tailored retention and adherence interventions.
Keywords: Pre-exposure prophylaxis, PrEP, adherence, retention in care, persistence, dried blood spot
INTRODUCTION:
Retention in care has been called the next critical issue in pre-exposure prophylaxis (PrEP) implementation.1 Retention in diverse real-world PrEP cohorts has not matched open-label studies, with only 38–58% of adult men who have sex with men (MSM) and transwomen remaining on PrEP at 6 months and 43–63% at 12 months across U.S.-based cohorts.2–8 In a study of cis-gender women on PrEP in the Bronx, only 38% remained on PrEP at 6 months.9 Many individuals who stop PrEP remain at risk of HIV, with one study from Los Angeles demonstrating almost a four-fold higher HIV incidence in those stopping PrEP vs. those who remained on PrEP.8 Another study in Montreal revealed an HIV incidence of 3.9 per 100 person-years after stopping PrEP, the same HIV incidence rate seen in the placebo group of the iPrEx randomized clinical trial.10
Objective metrics of adherence, such as tenofovir-diphosphate (TFV-DP) in dried blood spots (DBS) and tenofovir (TFV) levels in hair, have proven crucial for the interpretation of PrEP studies, with self-reported adherence performing poorly.11,12 PrEP adherence, which was low in initial trials, has been higher in demonstration projects by objective metrics, with only 14–35% of individuals demonstrating sub-optimal adherence (<4 doses per week estimated by TFV-DP levels in DBS <700 fmol/punch)12,13 in two U.S.-based projects.5,14 However, adherence has been much lower in young MSM, particularly Latino and Black young MSM,14–16 with 66% of young MSM and 78% of adolescents having sub-optimal adherence by objective metrics at week 48 in two U.S.-based studies.17,18
Although prior research has examined both retention and adherence on PrEP, little is known about their relationship.5,16,19 Demonstration projects that incorporated drug level monitoring have combined adherence and retention into a single outcome; however the predictive ability of adherence for future retention has not been evaluated.5,14,15,17,18 Availability of point of care (POC) PrEP adherence testing using immunoassays is on the horizon, to be implemented as a rapid strip test analogous to a urine pregnancy test, allowing for real-time PrEP adherence feedback on dosing over the last 4 days to patients and providers.14,20 Emtricitabine-triphosphate (FTC-TP) in dried blood spots similarly identifies recent adherence over the previous 2–4 days,21 and has been collected in prior demonstration projects. If patients first develop poor adherence prior to disengaging in PrEP care, a POC urine test could be useful to target adherence interventions towards those at highest risk of stopping PrEP in the future. Knowledge of the predictive ability of adherence metrics for future PrEP outcomes in real-world studies is needed prior to broad implementation of these tests.
The objective of our study was to examine the ability of both short and long-term adherence metrics to serve as early proxies of future non-retention in PrEP care.
Methods:
Participants included in this Analysis
The U.S. PrEP Demo Project provided TDF/FTC-based PrEP to 557 participants across study sites in 3 cities (Miami, San Francisco, and Washington D.C.) from October 2012 to February 2015. DBS were collected for the analysis of TFV-DP concentrations as a metric of long-term, average PrEP adherence over the previous 4–6 weeks, and for the analysis of emtricitabine-triphosphate (FTC-TP) concentrations as a metric of short-term adherence, reflecting a dose in the last 2–4 days.21 All participants consented to drug-level testing, but it was analyzed in all African-American MSM and transgender women participants, given that few individuals were recruited in these key populations,5 and in a random sample of remaining participants at week 4, 12, and then every 12 weeks until week 48 (n=294 total). Only individuals who attended at least one follow-up visit to provide DBS samples were included in the analysis. Participants were reimbursed $25 for each visit attended. The study and procedures of the U.S. PrEP Demo Project have been published previously5 and the protocol was approved by the instiutional review board at each site; procedures were followed in accordance with the Declaration of Helsinki.
PrEP drug level measurement in DBS
A quantifiable FTC-TP level served as the primary adherence metric in this analysis, representing a dose in the last 2–4 days, due to its similar assessment of recent adherence when compared to urine TFV detectability, which assess dosing within the previous 96 hours.22 For this substudy, FTC-TP levels in DBS were categorized as quantifiable vs. below the limit of quantification (BLQ) (FTC-TP <0.1 pmol/sample). To examine longer-term PrEP adherence, we used validated dosing cut-offs for TFV-DP levels in DBS to categorize participants into 5 estimated weekly pill-taking categories on average: daily drug-taking (>1250 TFV-DP fmol/punch), 4–6x weekly (700–1250 fmol/punch), 2–3x weekly (350–700 fmol/punch), <2x weekly (>BLQ-350 fmol/punch), and BLQ (<25 fmol/sample).12,13 We categorized sub-optimal pill-taking as <700 fmol/punch.12,13
Primary Outcome
Study non-retention/loss to follow-up in this analysis was defined as attending at least one follow-up visit after enrollment, and then not returning for a clinical visit at any point. Participants with missed follow-up visits who eventually returned were counted as retained. Participants who were lost to follow-up after the enrollment visit were excluded since no DBS samples were collected.
Statistical Analysis
We performed Cox-proportional hazards models examining time to non-retention as the primary outcome. The primary predictor was DBS FTC-TP levels examined as a cut-off (BLQ vs. quantifiable) at the week 4, 12, 24, 36 visits as a time-dependent predictor. In additional models, we substituted a DBS TFV-DP cut-off indicating sub-optimal dosing (700 fmol/punch). Finally, we examined the impact of examining FTC-TP or TFV-DP levels at only the initial (week 4) follow-up visit. We tested for violations of the proportional hazards assumption by examining correlation between time and scaled Schoenfeld residuals. In multivariable models, we adjusted for age per ten years, gender identity, race/ethnicity, and study site.
Results:
Overall, 294 individuals had DBS samples analyzed within the U.S. PrEP Demo Project over 503 person-years of follow-up. Young MSM, age<25, comprised 20% of the sample, 13% were Black, 32% were Latino, and 3% identified as transwomen (Table). By study end, over a quarter of individuals in the sub-analysis (27%, N=79) were lost to follow-up after a median of 24 weeks, with a crude rate of 0.3 study discontinuations per 1 person-year; and 9% of the sample (n=26) had a missed follow-up visit. Overall, 75% of non-retained individuals reported at least 2 condomless anal sex partners at their last visit compared to 83% of retained individuals at the final, week 48 visit (p=0.09).
Table 1:
Characteristics of the Sample and Predictors of Non-Retention in Cox-Proportional Hazards Models
| Overall % N=294 |
Unadjusted HR: 95% CI |
DBS FTC-TP Model AHR: 95% CI |
DBS TFV-DP Model AHR: 95% CI |
|
|---|---|---|---|---|
| Age at study entry, median (IQR)1 | 33 (27-42) | 0.79: 0.66-0.962 | 0.90: 0.71-1.152 | 0.82: 0.64-1.042 |
| Race: White | 45 | Ref. | Ref. | Ref. |
| Asian | 5 | 1.31: 0.56-3.05 | 1.42: 0.52-3.87 | 1.91: 0.71-5.12 |
| Black | 13 | 1.73: 0.93-3.25 | 1.09: 0.49-2.44 | 0.89: 0.39-2.02 |
| Latino | 32 | 1.78: 1.21-2.63 | 1.29: 0.65-2.53 | 1.36: 0.69-2.67 |
| Mixed/other | 4 | 0.94: 0.34-2.60 | 1.62: 0.55-4.80 | 1.41: 0.48-4.20 |
| Transwoman (vs. MSM) | 3 | 1.12: 0.28-4.54 | 0.87: 0.20-3.67 | 0.99: 0.24-4.11 |
| Site: San Francisco | 36 | Ref. | Ref. | Ref. |
| Miami | 31 | 2.37: 1.63-3.45 | 1.13: 0.59-2.18 | 1.15: 0.59-2.23 |
| Washington D.C. | 32 | 0.92: 0.52-1.58 | 0.73: 0.38-1.40 | 0.78: 0.41-1.51 |
| Sub-optimal adherence by TFV-DP (<700 fmol/punch) at any visit3 | 292 | 4.21: 2.57-6.903 | - | 4.31: 2.43-7.623 |
| Undetectable DBS FTC-TP at any visit3 | 252 | 7.53: 4.81-11.783 | 6.27: 3.83-10.223 | - |
AHR: Adjusted hazard ratio; DBS: Dried Blood Spot; FTC-TP: emtricitabine-triphosphate; TFV-DP: tenofovir-diphosphate; CI: Confidence interval; IQR: inter-quartile range; MSM: Men who have sex with men.
Scaled per ten years
Lowest documented adherence during course of the demo project
Treated as a time-dependent covariate in survival analysis
One-quarter (25%) of this sample had at least one BLQ DBS FTC-TP level, representing poor short-term adherence. When measuring TFV-DP in DBS, 29% of individuals had at least one drug level indicating sub-optimal long-term adherence (TFV-DP<700 fmol/punch). In unadjusted analyses, Latino ethnicity vs. White race [Hazard Ratio (HR) 1.78 95% Confidence Interval (CI):1.21–2.63; p=0.003], being at the Miami vs. San Francisco site (HR 2.37 95% CI:1.63–3.45; p<0.001), and having a BLQ FTC-TP level (HR 7.53 95% CI: 4.81–11.78; p<0.001) or suboptimal TFV-DP level (HR 4.21; 95% CI:2.57–6.90; p<0.001) were associated with a higher hazard of non-retention, while older age per ten years was inversely associated with non-retention (HR 0.79 95% CI:0.66–0.96; p=0.02) (Table). The p-value for the relationship of missing a study follow-up visit with future non-retention was 0.08 (HR 1.86; 95% CI: 0.94–3.71). In the two adjusted analyses, only BLQ FTC-TP or sub-optimal TFV-DP respectively predicted non-retention. The factor with the strongest association with future non-retention was the short-term marker of adherence: BLQ DBS FTC-TP levels [AHR 6.27; 95% CI:3.83–10.22; p<0.001]. A majority (51%) of individuals eventually lost to follow-up had a prior BLQ FTC-TP over the course of the study. Sub-optimal adherence based on DBS TFV-DP levels consistent with <4 doses per week dosing collected at every visit was also associated with non-retention (AHR 4.31; 95% CI:2.43–7.62; p<0.001), and sub-optimal DBS TFV-DP levels detected a similar number of individuals eventually lost to follow-up (47%) (Table). Using an alternate TFV-DP cut-off of very low adherence consistent with <2 doses per week (<350 fmol/punch) had minimal impact on the association with non-retention (AHR 3.92; 95% CI:2.16–7.14; p<0.001), but only identified 22% of individuals eventually lost to follow-up. In a model using all strata of estimated weekly adherence patterns based on DBS TFV-DP levels, there was a monotonic relationship between decreasing strata of DBS TFV-DP levels and higher hazard of non-retention (p<0.001).
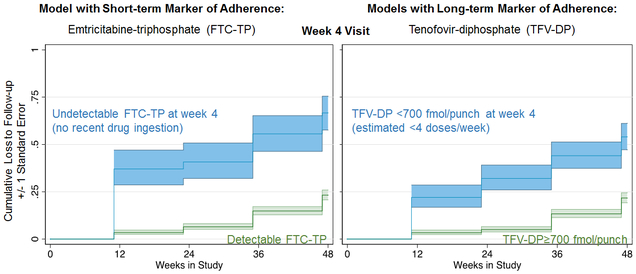
When examining the short-term adherence measure at the week 4 visit instead of at every visit, BLQ FTC-TP remained the only factor associated with non-retention (AHR 3.05; 95% CI: 1.75–7.31; p<0.001). Overall, 23% of individuals who were eventually lost to follow-up had a BLQ FTC-TP at the week 4 visit. When measuring TFV-DP levels at week 4, sub-optimal adherence remained the only factor associated with loss to follow-up (AHR 2.40; 95% CI: 1.40–4.10; p=0.001); 32% of participants eventually lost to follow-up had sub-optimal adherence as measured by TFV-DP levels at week 4 (Figure).
Figure:
Short and Long-Term Markers of Adherence at the Week 4 Visit Predict Loss to Follow-Up in the U.S. PrEP Demo Project
DISCUSSION:
In a large multi-site PrEP demonstration project, a short-term objective adherence metric had the strongest assocition with future loss to follow-up. Although Latino ethnicity, younger age, and the Miami site were predictors of non-retention in unadjusted analysis, only objective adherence metrics predicted retention in adjusted analyses. Three-fourths of participants lost to follow-up reported having condomless receptive anal sex with at least 2 partners at their last visit, indicating potential ongoing HIV risk.
Although some patients may discontinue PrEP due to decreased HIV risk, many stop PrEP for other reasons, including difficulty attending in-person visits and receiving laboratory monitoring, concerns about taking a daily pill, toxicity, or side effects.23,24 A prior demonstration project in Los Angeles showed success of plasma TFV drug level feedback in motivating later PrEP adherence in spite of a 1–3 week turnaround time.14 Metrics of adherence based on PrEP drug-levels including short-term metrics, particularly if readily available as a POC test, could permit tailored PrEP re-engagement interventions in real-time.
Re-engagement and adherence interventions tailored to PrEP drug level results were acceptable to patients in initial studies examining this approach,25–27 and can improve future adherence.14 A POC TFV urine test, which reflects short-term adherence, can be implemented outside of research settings at the time of a routine clinic visit, with results available in <5 minutes.20,28 Our analysis of data from a large, multi-site, U.S. demonstration project suggests that a short-term metric of adherence strongly predicts future poor PrEP retention in care in diverse patient populations. A recent analysis in people living with HIV found that FTC-TP, even when adjusting for TFV-DP, predicts viral suppression.29 It is possible that FTC-TP, as a short-term adherence metric, may be more sensitive to changes in adherence than TFV-DP, a long-term/cumulative metric. Therefore, a real-time short-term PrEP adherence metric may be useful to trigger retention and adherence interventions in PrEP clinics. For instance, some patients struggling with adherence may need two-way, automated text message reminders; some may prefer switching to intermittent pill-taking regimens to address pill fatigue; while others may need their PrEP care integrated with substance use treatment, transgender care, or case management.23,30,31
Limitations of this analysis include a smaller sample size, with few African-Americans and transwomen recruited into the study. It is unknown what impact knowledge that real-time feedback will be provided at the clinical visit will have on patient behavior at future visits, such as increasing adherence just prior to visits (e.g. “white coat adherence”).28 A combination of short-term and long-term metrics of adherence, such as TFV levels in urine (short-term) and DBS TFV-DP levels or TFV levels in hair (both long-term), may better tease out intermittent vs. sustained adherence patterns.11 The magnitude of study non-retention in U.S. PrEP Demo may have been impacted by patients having less incentive to attend the week 48 visit given that future study drug would not be available. In addition, demonstration project non-retention may not accurately reflect non-retention in care in a real-world setting, and patients in real-world settings may be less likely to attend clinic visits if they are not adherent to PrEP, compared with those in a study who were reimbursed $25 for study visits. Finally, we were unable to assess what proportion of individuals who were lost to follow-up remained at risk of HIV.
In conclusion, we have demonstrated a strong independent association between short and long-term adherence to PrEP and future retention. Given that PrEP retention in care is poor in real-world settings and point-of-care short-term adherence metrics to PrEP via urine-based immunoassays are imminent, this study suggests that measuring short-term adherence at the POC could identify patients at increased risk for being lost of follow-up to deliver targeted, real-time adherence feedback counseling or other evidence-based interventions.
Acknowledgments
Conflicts of Interest and Sources of Funding: This work was supported by National Insitute of Allergy and Infectious Diseases at the National Institute of Health [grant number 5T32AI060530 to M.A.S., R03AI120819 to D.G.; R01AI143340 and 2R01AI098472 to M.G.; U01AI106499 and U01AI084735 to P.L.A.; U01AI069451 to A.Y.L.]. D.G. and P.L.A. have received grants from Gilead Sciences, paid to their institutions. P.L.A., S.P.B., R.M.G. and A.Y.L. have led studies in which Gilead Sciences donated study drug. The other authors have no conflicts of interest to declare.
References:
- 1.Golub SA, Enemchukwu CU. The critical importance of retention in HIV prevention. Lancet HIV. 2018;5(9):e475–e476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan PA, Mena L, Patel R, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19(1):20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rusie LK, Orengo C, Burrell D, et al. PrEP Initiation and Retention in care over five years, 2012–2017: Are quarterly visits too much? Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A. HIV Pre-exposure Prophylaxis (PrEP) Uptake and Retention Among Men Who Have Sex with Men in a Community-Based Sexual Health Clinic. AIDS Behav. 2018;22(4):1096–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016;176(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montgomery MC, Oldenburg CE, Nunn AS, et al. Adherence to Pre-Exposure Prophylaxis for HIV Prevention in a Clinical Setting. PLoS One. 2016;11(6):e0157742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinelli MA, Scott H, Vittinghoff E, et al. Factors impacting appropriate HIV/STI screening and PrEP persistence in primary care [#1028]. Paper presented at: Conference on Retroviruses and Opportunistic Infections 2018; Boston. [Google Scholar]
- 8.Shover C, Javanbakht M, Shoptaw S, Bolan R, Gorbach P. High Discontinuation of Pre-exposure Prophylaxis within Six Months of Initiation [#1009]. Paper presented at: Conference on Retroviruses and Opportunistic Infections2018; Boston. [Google Scholar]
- 9.Blackstock OJ, Patel VV, Felsen U, Park C, Jain S. Pre-exposure prophylaxis prescribing and retention in care among heterosexual women at a community-based comprehensive sexual health clinic. AIDS Care. 2017;29(7):866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwald Z, Beuachemin M, Benomar K, et al. High seroconversion rates following PrEP discontinuance in a Montreal clinic [#1038]. Paper presented at: Conference on Retroviruses and Opportunistic Infections 2018; Boston. [Google Scholar]
- 11.Koss CA, Hosek SG, Bacchetti P, et al. Comparison of Measures of Adherence to Human Immunodeficiency Virus Preexposure Prophylaxis Among Adolescent and Young Men Who Have Sex With Men in the United States. Clin Infect Dis. 2018;66(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular Tenofovir-Diphosphate and Emtricitabine-Triphosphate in Dried Blood Spots following Directly Observed Therapy. Antimicrob Agents Chemother. 2018;62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landovitz RJ, Beymer M, Kofron R, et al. Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. J Acquir Immune Defic Syndr. 2017;76(5):501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu AY, Vittinghoff E, von Felten P, et al. Randomized Controlled Trial of a Mobile Health Intervention to Promote Retention and Adherence to Pre-exposure Prophylaxis among Young People at Risk for Human Immunodeficiency Virus: The EPIC Study. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doblecki-Lewis S, Liu AY, Feaster DJ, et al. Patterns and Correlates of Participant Retention in a Multi-City Pre-Exposure Prophylaxis Demonstration Project. J Acquir Immune Defic Syndr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosek SG, Rudy B, Landovitz R, et al. An HIV Preexposure Prophylaxis Demonstration Project and Safety Study for Young MSM. J Acquir Immune Defic Syndr. 2017;74(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosek SG, Landovitz RJ, Kapogiannis B, et al. Safety and Feasibility of Antiretroviral Preexposure Prophylaxis for Adolescent Men Who Have Sex With Men Aged 15 to 17 Years in the United States. JAMA Pediatr. 2017;171(11):1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonacci RA, Frasca K, Swift L, et al. Antiretroviral Refill Adherence Correlates with, But Poorly Predicts Retention in HIV Care. AIDS Behav. 2016;20(5):1060–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi M, Bacchetti P, Rodrigues WC, et al. Development and Validation of an Immunoassay for Tenofovir in Urine as a Real-Time Metric of Antiretroviral Adherence. EClinicalMedicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo-Mancilla J, Seifert S, Campbell K, et al. Emtricitabine-Triphosphate in Dried Blood Spots as a Marker of Recent Dosing. Antimicrob Agents Chemother. 2016;60(11):6692–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koenig HC, Mounzer K, Daughtridge GW, et al. Urine assay for tenofovir to monitor adherence in real time to tenofovir disoproxil fumarate/emtricitabine as pre-exposure prophylaxis. HIV Med. 2017;18(6):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spinelli MA, Scott HM, Vittinghoff E, et al. Examining PrEP interruptions in a safety-net primary care network: missed opportunities to reengage PrEP users accessing non-PrEP services [#OA19.02]. Paper presented at: HIV Research For Prevention2018; Madrid. [Google Scholar]
- 24.Shover C, Flores D, Berymer M, et al. Online Survey about Reasons for Stopping PrEP is an Opportunity to Re-engage Inactive PrEP Clients [#P14.01]. Paper presented at: HIV Research For Prevention2018; Madrid. [Google Scholar]
- 25.Celum CL, Delany-Moretlwe S, Hosek S, et al. Risk Behavior, Perception and Reasons for PrEP among Young African Women in HPTN 082 [Abstract 1049]. Conference on Retroviruses and Opportunistic Infections; 2018; Boston. [Google Scholar]
- 26.Husnik MJ, Brown ER, Marzinke M, et al. Implementation of a Novel Adherence Monitoring Strategy in a Phase III, Blinded, Placebo-Controlled, HIV-1 Prevention Clinical Trial. J Acquir Immune Defic Syndr. 2017;76(3):330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lalley-Chareczko L, Clark D, Conyngham C, et al. Delivery of TDF/FTC for Pre-exposure Prophylaxis to Prevent HIV-1 Acquisition in Young Adult Men Who Have Sex With Men and Transgender Women of Color Using a Urine Adherence Assay. J Acquir Immune Defic Syndr. 2018;79(2):173–178. [DOI] [PubMed] [Google Scholar]
- 28.Anderson PL. What Can Urine Tell Us About Medication Adherence? EClinicalMedicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frasca K, Morrow M, Coyle RP, et al. Emtricitabine triphosphate in dried blood spots is a predictor of viral suppression in HIV infection and reflects short-term adherence to antiretroviral therapy. J Antimicrob Chemother. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegler AJ, Mayer KH, Liu AY, et al. Developing and assessing the feasibility of a home-based PrEP monitoring and support program. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stekler JD, McMahan V, Ballinger L, et al. HIV Pre-exposure Prophylaxis Prescribing Through Telehealth. J Acquir Immune Defic Syndr. 2018;77(5):e40–e42. [DOI] [PubMed] [Google Scholar]