Abstract
Purpose of review:
Mutations in genes encoding proteins critical for the production and function of pulmonary surfactant cause diffuse lung disease. Timely recognition and diagnosis of affected individuals is important for proper counseling concerning prognosis and recurrence risk.
Recent findings:
Involved genes include those encoding for surfactant proteins A, B and C (SP-A, SP-B, SP- C), member A3 of the Adenosine-Triphosphate Binding Cassette family (ABCA3), and for Thyroid Transcription Factor 1 (TTF-1). Clinical presentations overlap and range from severe and rapidly fatal neonatal lung disease to development of pulmonary fibrosis well into adult life. The inheritance patterns, course and prognosis differ depending upon the gene involved, and in some cases the specific mutation. Treatment options are currently limited, with lung transplantation an option for patients with end-stage pulmonary fibrosis. Additional genetic disorders with overlapping pulmonary phenotypes are being identified through newer methods, although these disorders often involve other organ systems.
Summary:
Genetic disorders of surfactant production are rare but associated with significant morbidity and mortality. Diagnosis can be made invasively through clinically available genetic testing. Improved treatment options are needed and better understanding of the molecular pathophysiology may provide insights into treatments for other lung disorders causing fibrosis.
Keywords: Neonatal respiratory distress syndrome, interstitial lung disease, pulmonary fibrosis, alveolar proteinosis
Introduction: Overview of Surfactant Metabolism
Pulmonary surfactant is a mixture of lipids and proteins that coats the distal airspaces and reduces surface tension at end expiration. Surfactant is synthesized in alveolar type 2 cells (AEC2s), where it is first stored in specialized organelles derived from lysosomes called lamellar bodies before it is secreted by exocytosis. The main lipid in surfactant that helps lower surface tension is disaturated phosphatidyl choline (DSPC). Two small, very hydrophobic proteins called surfactant proteins (SP) B and C are essential in order for surfactant lipids to transition the thin layer of fluid coating the distal alveolus and spread at the air-liquid interface to form a monolayer and lower surface tension. Surfactant also contains two larger hydrophilic related proteins, SP-A and SP-D, which have important roles in innate immunity and local immune regulation. An inability to produce sufficient amounts of surfactant due to immaturity is the major cause of the respiratory distress syndrome (RDS) observed in prematurely born infants. Defects in the genes encoding the surfactant proteins can result in insufficient surfactant production, disrupted surfactant metabolism, and secondary injury to AEC2s. These changes cause severe respiratory distress in full-term newborns, or chronic interstitial lung disease in older children and adults. There is considerable overlap in the clinical presentations and lung pathology findings of these disorders, but the mechanisms, inheritance patterns and outcomes differ depending upon the gene involved. This article will review the clinical and laboratory features associated with genetic abnormalities of surfactant protein production.
ABCA3
The adenosine triphosphate (ATP) binding cassette transporters are transmembrane proteins that utilize energy from the hydrolysis of ATP to move substances across biological membranes. Member A3 (ABCA3) is highly expressed in AEC2s where it is located on the limiting membrane of lamellar bodies. ABCA3 transports essential surfactant lipids, specifically DSPC and phosphatidylglycerol (PG) into lamellar bodies, and may have a role in intracellular cholesterol metabolism1–3. ABCA3 is encoded by a large gene (ABCA3) on chromosome 16, which directs the synthesis of a 1704 amino acid protein with two membrane-spanning domains and two nucleotide (ATP) binding domains. ABCA3 is also expressed in brain, kidney and platelets, although its role in those organs is unclear.
Complete loss-of-function mutations on both alleles (biallelic) of ABCA3 result in a phenotype of severe surfactant deficiency and neonatal RDS4,5. Most ABCA3 deficient infants are born at full-term. A mutation on only one allele (monoalleleic) of ABCA3 may predispose prematurely born infants to RDS, primarily in late preterm and near-term infants6. In extremely preterm infants other genetic and environmental factors influencing maturation of the surfactant system and lung development may influence the phenotype to a greater extent.
Some infants have milder disease, with either transient neonatal disease or onset of symptoms after the neonatal period. Survival into the 5th decade of life has been recognized, although there is still substantial early mortality5,7,8. The ABCA3 genotype influences the phenotypic severity of disease. Infants with mutations on both alleles predicted to preclude ABCA3 expression have invariably had severe disease at birth and either died within the first year of life, or received a lung transplant. Children with onset of disease after the newborn period and more prolonged survival have at least one missense or small insertion or deletion mutation. Presumably, such mutations reduce, but do not fully eliminate, ABCA3 function. In support of this hypothesis, in vitro studies of the ABCA3 c.875 A>T (p.Glu292Val) mutation, which has generally been associated with milder disease, demonstrated reduced ATP hydrolysis compared to wild-type ABCA39. Currently over 400 mutations have been reported in ABCA3, but only a handful have been studied in vitro to determine their specific effects on ABCA3 expression, intracellular routing, and/or function10.
While animal and human studies demonstrate that complete loss-of-function mutations result in severe surfactant deficiency accounting for the neonatal RDS phenotype, the mechanisms underlying chronic disease are less clear. Inadequate surfactant production could lead to recurrent atelectasis, recurrent hypoxemia and subsequent chronic inflammation. Abnormal intracellular surfactant metabolism could also result in chronic injury to AEC2s through unidentified mechanisms. One possible such mechanism could involve a role of ABCA3 in sequestering cholesterol for example, and protecting it from its potentially toxic effects on the cell3.
The precise incidence of ABCA3 deficiency is unknown, but can be estimated from data on the population frequency of disease-causing mutations. The carrier frequency may be as high as 1 in 33 in individuals of European descent, which would translate into a disease frequency of 1 in 3500 for a recessive disorder6. This incidence seems high compared to case ascertainment. An explanation for this discrepancy may be related to the critical level of ABCA3 function needed to prevent lung disease. Several of the mutations that contribute to this relatively high population frequency (p.Arg288Lys, p.Glu292Val) reduce but do not eliminate ABCA3 function9. A genotype consisting of one of these variants and a mutation completely precluding ABCA3 function could be below the critical level and lead to lung disease. However a genotype consisting of two reduced function variants might exceed the threshold needed for normal lung function, or be close to the critical level and confer susceptibility to lung disease should other factors impair ABCA3 function11. ABCA3 expression is developmentally regulated and thus a prematurely born infant with a monoallelelic (single, heterozygous) mutation may fall below the critical threshold of ABCA3 expression necessary for proper lung function and have more severe RDS or protracted disease than would otherwise be expected from the child’s gestational age6.
SP-B
SP-B is a small, extremely hydrophobic protein encoded by a single gene on chromosome 2 (SFTPB) that directs the synthesis of a larger proprotein, which undergoes endoproteolytic cleavage to generate the mature SP-B protein which is secreted into the airspaces along with surfactant lipids and SP-C12. SP-B helps organize surfactant lipids within lamellar bodies, and the AEC2s of genetically engineered mice and human infants with loss-of-function SFTPB mutations do not contain normal appearing lamellar bodies, but instead have organelles with multiple vacuoles and disorganized appearing lipid membranes13,14. These observations are consistent with SP-B having an intracellular role in membrane fusion and promoting the organization of the lamellar body.
Biallelic loss-of-function mutations in SFTPB result in SP-B deficiency and a phenotype of severe RDS, usually in full-term infants that clinically and radiographically mimics RDS seen in preterm infants15,16. This phenotype is not surprising given the important role of SP-B in enhancing the function of surfactant lipids and its intracellular role in the biogenesis of lamellar bodies. The lack of normally developed lamellar bodies also leads to deficiency of secreted surfactant phospholipids. Additionally, SP-C is unable to be completely processed from its precursor form, leading to both a deficiency of mature SP-C and accumulation of partially processed intermediates that are not surface active and can further inhibit surfactant function17,18. Incompletely processed proSP-C can be detected in tracheal aspirate or bronchoalveolar lavage (BAL) fluid by protein blotting (currently available only on a research basis), or in lung tissue by immunohistochemical staining with antibodies directed against the amino-terminus of proSP-C. Extracellular staining for these peptides or their presence in lung fluid or tissue is an excellent biomarker for SP-B deficiency16.
Over 50 different disease-causing SFTPB mutations have been identified, with a specific frameshift mutation has accounted for approximately 70% of known-disease causing alleles due to a founder effect. This mutation was originally termed 121in2 as it resulted in a net 2 base insertion in codon 121 of the SP-B transcript19, but as the reference sequence has changed the current nomenclature is c.397delCinsGAA (or p.Pro133GlnfsTer95). The SP-B transcript from this mutation is unstable causing a complete absence of SP-B mRNA and protein20. Other disease-causing mutations either preclude production of proSP-B, or result in proSP-B that is unable to be processed to mature SP-B16. The disease is inherited in a recessive fashion, and adult carriers of mutations are generally asymptomatic, although may be predisposed to lung disease later in life, particularly if they smoke tobacco21.
The vast majority of SP-B deficient infants develop symptoms at or shortly after birth and have progressive disease, resulting in death or need for lung transplantation within the first few months of life22. Rarely affected infants may survive well beyond infancy – these children usually have at least one SFTPB allele that allows for some SP-B production, and are thus partially deficient23,24. While the exact incidence of SP-B deficiency is unknown, it appears to be extremely rare based upon population frequencies of the incidence of disease-causing mutations, estimated at less than one in 1,000,000 live births25.
SP-C
SP-C is a small, extremely hydrophobic protein encoded by a single gene (SFTPC) on chromosome 8, which directs the synthesis of a larger proprotein (proSP-C). Endoproteolytic processing of proSP-C generates the 35 amino acid mature SP-C protein, encoded in the second exon of the gene12. Mature SP-C is post-translationally palymitoylated and is thus a proteolipid. The last 100 amino acids of proSP-C have homology to a group of proteins that are mutated in familial dementia syndromes, known as the BRICHOS domain26. This domain may fold over the mature SP-C domain and thus protect the cell from its extreme hydrophobicity, and help chaperone proSP-C through the secretory pathway27. The final processing steps occur in lamellar bodies, with mature SP-C secreted along with SP-B and surfactant lipids. While SP-C enhances adsorption and spreading of lipids at the air-liquid interface, the precise role of mature SP-C in lung biology remains unclear. Mice genetically engineered to be unable to make SP-C do not develop neonatal lung disease and survive into adulthood, although may develop lung disease in a strain-specific fashion28.
Monoallelic mutations in SFTPC result in lung disease with a highly variable age of onset and severity, ranging from a neonatal RDS presentation (although unusual) to the development of pulmonary fibrosis in the fifth to sixth decade of life. The most common presentation is in infancy with symptoms including cough, and findings including retractions, digital clubbing and failure to thrive. Hypoxemia in room air is common, and diffuse abnormalities are present on chest imaging. There does not appear to be correlation between the location and nature of the mutation (genotype) and phenotype, and individuals with the same mutation in a family may have very different clinical presentations and courses29,30. A mutation in the domain between the mature peptide and BRICHOS domains, p.Ile73Thr, has accounted for ~ 30 – 40% of the reported cases to date22,31. This mutation has been identified in unrelated individuals of different ancestral backgrounds, associated with both familial and sporadic cases, and may thus result from a “hot-spot” for mutations31. Multiple identified mutations in the BRICHOS domain may reflect the importance of this region in stabilizing the structure of proSP-C.
As a mutation on only one allele is sufficient to cause disease, SFTPC mutations cause familial lung disease in an autosomal dominant pattern, or cause sporadic disease from de novo mutations. SP-C related lung disease result does not appear to result from a loss-of function mutation on one allele (haploinsufficieny). Instead, lung disease results from the adverse effects of the mutated protein on SP-C and AEC2 metabolism (gain-of-toxic function), with different cellular mechanisms depending upon the mutation32,33. Mutations in the proSP-C BRICHOS domain cause protein misfolding and endoplasmic reticulum stress, triggering the unfolded protein response, and protein aggregates may accumulate if degradation pathways are overwhelmed34. Alternatively, some mutations (such as p.Ile73Thr) result in abnormal trafficking of proSP-C. Instead of routing to the lamellar body, mutant protein is first trafficked to the plasma membrane and re-enters the cell through the endocytic pathway33,35,36. The abnormally processed protein can result in a block an autophagy36. The ultimate result of each of these different mechanisms is AEC2 apoptosis32. As AEC2 cells serve as progenitor cells for alveolar repair, a depletion of this AEC2 pool may eventually lead to fibrosis. Finally, proSP-C self-associates in the secretory pathway37. Normal proSP-C may be degraded along with misfolded, mutant protein causing a lack of SP-C through a dominant negative mechanism. Whether reduced or absent mature SP-C either through a dominant negative mechanism or due to impaired processing of proSP-C contributes to the pathogenesis of lung disease is unknown. Individuals with SP-C deficiency due to biallelic loss-of-function mutations have not been identified.
NKX2–1
NKX2–1 is a small gene on chromosome 14 that encodes a member of the homeobox transcription factor family, thyroid transcription factor 1 (TTF-1). TTF-1 has critical roles in thyroid gland and early lung development and is important for the transcription of the surfactant proteins, ABCA3, and many other proteins in the lung. NKX2–1 is also expressed in the basal ganglia and mutations in NKX2–1 were first identified in adults with the movement disorder benign familial chorea. Mutations in and deletions of NKX2–1 were subsequently found in individuals with manifestations of lung disease, neurological findings, and hypothyroidism, known as “Brain-Thyroid-Lung” syndrome”38–40. ~40% of individuals with NKX2–1 mutations have manifestations in all three organ system. Isolated hypothyroidism is rare (<2%); ~30% of subjects have two organ systems involved, and ~6% have solely or primarily pulmonary manifestations. These categorizations are not precise, as there is ascertainment bias in identification of subjects, not all reported subjects have been formally evaluated for deficits in all organ systems, and young infants may not have yet developed neurological symptoms or had non-specific symptoms (such as hypotonia) attributed to the severity of illness due to lung disease.
The lung disease resulting from NKX2–1 mutations or deletions is highly variable in onset and severity41. Many infants present with a neonatal RDS phenotype and the majority of such infants (>80%) have biochemical evidence of hypothyroidism. Delayed expression of ABCA3 and SFTPB likely contributes to severe lung disease40,41. As NKX2–1 is important in early lung development, impaired lung development may also contribute to early onset disease42. Recurrent infections or respiratory failure after a viral infection may reflect impaired expression of the pulmonary collectins, SP-A and SP-D. A plausible hypothesis for the variable pulmonary phenotypes is that the clinical phenotype reflects those target genes most severely impacted by the mutation. Other genetic factors (interacting genes, variations in regulatory regions) as well as environmental factors can contribute to the variable expression.
Mutations on a single NKX2–1 allele are sufficient to cause disease. Many arise de novo and cause sporadic disease, but can also be inherited and cause familial disease in an autosomal dominant pattern with variable penetrance. No predominant mutation has been recognized, and there does not appear to be a genotype-phenotype correlation. Haploinsufficiency due to reduced NKX2–1 expression or TTF-1 function appears to be the primary mechanism for disease, although some mutations could also cause disease through a gain-of-function mechanism38,40,43.
SP-A and SP-D
SP-A and SP-D are structurally related multimeric proteins encoded by a multigene family on chromosome 10, with two genes for SP-A (SFTPA1, SFTP2)44. The primary roles for SP-A and SP-D appears to be in innate immunity and immune regulation45. Monoallelic mutations in the genes encoding SFTPA2 or SFTPA1 have been identified in adults with the phenotype of pulmonary fibrosis and lung adenocarcinoma through a gain-of-toxic function mechanism46–49. Disease causing mutations in the gene encoding SP-D (SFTPD) have yet to be identified50.
Lung Histology and Ultrastructural findings associated with surfactant dysfunction
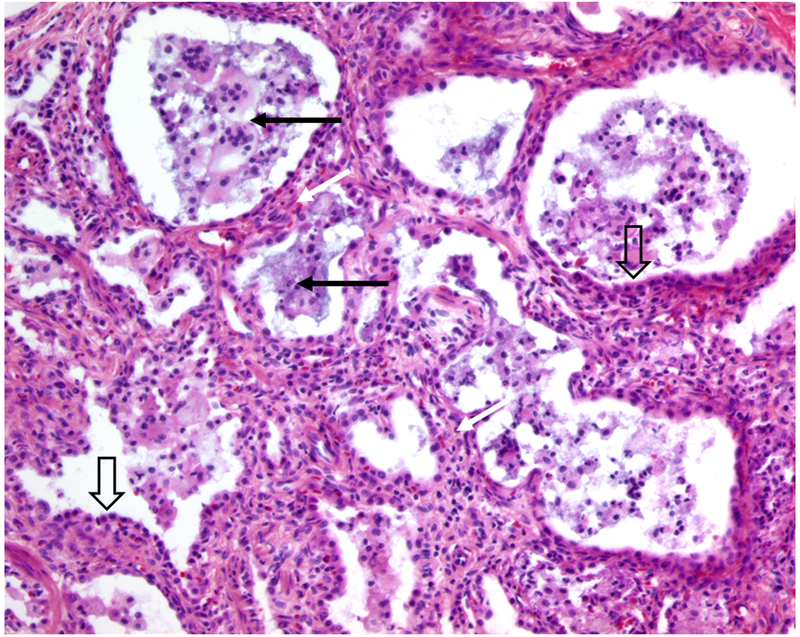
Characteristic histologic features observed in the lung of children with mutations in surfactant related genes include prominent AEC2 hyperplasia, widening of the interstitium with mesenchymal cells, and variable amounts of granular appearing, proteinaceous material in the distal airspaces mixed with macrophages (Figure). The term “surfactant dysfunction” is used to describe these findings, although other histopathology terms may be used. The amount of proteinaceous material might be so prominent that infants are labeled as having alveolar proteinsosis, although it is important to recognize that the syndrome of alveolar proteinosis in adults and older children results from different mechanisms and has a different clinical course and approach to diagnosis and treatment51. Prominent aggregations of macrophages has led to the description of desquamative interstitial pneumonitis7. The majority of children with the histologic diagnosis referred to as chronic pneumonitis of infancy52 have been found to have a mutation in a surfactant related gene, most often SFTPC. Similar lung pathology has been observed in infants without any identifiable mutation7. Whether these children have mutations in untranslated regions in known genes that escaped detection, had mutations in other genes yet to be identified, or have acquired disorders whose lung pathology mimics those of surfactant dysfunction is unknown. It is important to recognize that the lung histology findings are not specific for a given genetic cause, and identification of the specific gene is necessary for proper counseling concerning prognosis and recurrence risk.
Representative histology of surfactant dysfunction in a patient with a mutation located in the SFTPC
BRICHOS domain.
Wide arrows point to hyperplastic AEC2s, dark arrows indicated proteinaceous material and macrophages in distal airspaces, and white arrows indicate the thickened insterstitium. Photomicrograph courtesy of Susan Wert, Ph.D., Cincinnati Children’s Hospital Medical Center.
Immunohistochemical staining has been used to further evaluate the lung pathology, but is not widely available and of limited utility in discriminating between the different genetic disorders. Absent staining for mature SP-B is usually found in children with SP-B deficiency, but may also be observed in children with ABCA3 mutations7,16. The most specific finding is staining of extracellular material with antibodies directed against the amino-terminus of proSP-C, which is indicative of SP-B deficiency due to the misprocessing of proSP-C in this disorder. Analysis of tracheal aspirate or BAL fluid for surfactant proteins has been studied on a research basis, but there is sufficient overlap in findings between the different genetic disorders that it is not helpful for diagnosis53.
Electron microscopy may be helpful for providing a specific diagnosis, but requires special handling and fixation. Disorganized lamellar bodies are seen in SP-B deficient infants14,54. Absent lamellar bodies or small dense bodies with a peripheral electron dense core giving them a “fried-egg” appearance have been typical observed in newborns with the severe form of ABCA3 deficiency4,54. Older infants with relatively milder disease may have more variable findings, although this has not been systemically evaluated. Variable electron microscopy findings have been reported in association with SFTPC mutations, but no characteristic pattern has been found to date. Children with NKX2–1 mutations have variable EM findings41.
Diagnosis of Surfactant Dysfunction Disorders
Knowledge of the typical presentations and inheritance of each of the different disorders is essential to suspecting the diagnosis and key features are summarized in table 1. The diagnosis should be suspected in a full-term infant who develops diffuse lung disease that clinically and radiographically resembles RDS, particularly if there are no risk factors for lung disease such as elective operative delivery without labor, maternal diabetes, or reasons to suspect an infectious etiology. SP-B and ABCA3 deficiencies are more likely than SP-C dysfunction in newborns with severe disease; congenital hypothyroidism should prompt suspicion for a NKX2–1 mutation. Older infants who present with diffuse lung disease on chest imaging, along with hypoxemia and failure-to thrive should be suspected of having an SFTPC mutation if other more common causes of lung disease are first excluded, although children with ABCA3 deficiency and NKX2–1 mutations can present similarly. A positive family history of diffuse lung disease or pulmonary fibrosis in an autosomal dominant pattern can be a clue to SFTPC mutations, although also seen in patients with NKX2–1 mutations. Detailed approaches to the evaluation of children suspected of these disorders have been published55,56.
Table 1:
Key features of genetic causes of surfactant dysfunction
| Locus | ABCA3 | SFTPB | SFTPC | NKX2–1 | SFTPA1, SFTPA2 |
|---|---|---|---|---|---|
| Protein | ABCA3 | SP-B | SP-C | TTF1 | SP-A |
| Function | Imports surfactant lipids (DSPC, PG) into lamellar bodies Lamellar body formation |
Enhances adsorption and spreading of surfactant phospholipids Lamellar body formation |
Enhances adsorption and spreading of surfactant phospholipids | Transcription Factor; Important for expression of SP-B, SP-C, ABCA3, CCSP and multiple other proteins in lung, thyroid and CNS |
Innate Immunity |
| Inheritance | Recessive | Recessive | Dominant or Sporadic (de novo) |
Sporadic (de novo) or Dominant |
Dominant or Sporadic (de novo) |
| Relatively Frequent Mutation |
c.875 A>T (p.Glu292Val) - < 10% alleles |
c.397delCinsGAA (p.Pro133Glnfs*)- ~70% of alleles |
c.218 T>C (p.Ile73Thr) ~30 – 40% |
None | None |
| Mechanism | Loss-of-function | Loss-of-function | Gain of toxic function | Loss-of-function (Haploinsufficiency) | Gain of toxic function |
| Pulmonary Phenotype | Neonatal RDS Childhood ILD |
Neonatal RDS |
Childhood ILD Adult PF Neonatal RDS |
Neonatal RDS Childhood ILD None |
Adult PF Lung Cancer |
| Extrapulmonary Phenotype |
No | No | No | Hypothyroidism Chorea, Ataxia, other movement disorder |
None |
| Outcome | Fatal within 3 months (~60%) with genotype indicating complete loss-of-function; Prolonged survival possible |
Usually fatal within 3 months | Variable. May be asymptomatic for decades. Critically ill infants may improve with time |
Variable | Variable |
CCSP: Club Cell Secretory Protein; RDS: Respiratory Distress Syndrome; ILD: Interstitial Lung Disease; PF: Pulmonary Fibrosis
The diagnosis of a disorder of the surfactant protein genes is made through DNA analysis, which identifies the specific gene and provides information on prognosis and recurrence risk. Multiple commercial, certified diagnostic laboratories offer next generation sequencing panels that include these genes, as well as others that may have overlapping phenotypes (www.ncbi.nlm.nih.gov/gtr). SFTPB, ABCA3, and especially NKX2–1 genic and intragenic deletions exist, so it is important that the assay be sensitive to deletion and duplications (del/dup)41,57,58. Knowledge of the inheritance pattern and analysis of parental samples is important in the interpretation of genetic studies. A finding of a single known or likely pathogenic mutation in SFTPC or NKX2–1 is diagnostic of those disorders. Finding of an apparent de novo mutation in SFTPC or NKX2–1 often supports that the mutation is likely to be pathogenic, although non-paternity must also be considered. Diagnosis of SP-B or ABCA3 deficiency requires the finding of a known or likely pathogenic mutation on both alleles. ABCA3 alleles with more than one mutation have been recognized, so it cannot be assumed if two mutations are found in a child that they are on opposite alleles5,59. ABCA3 and SP-B deficiency have both resulted from uniparental disomy, which alters the recurrence risk. Finally, testing of extremely premature infants with severe lung disease may be problematic, as the likelihood of finding a single ABCA3 variant and difficulty in interpreting its significance is far higher than obtaining a definitive result, given the population frequency of ABCA3 variants.
Barriers to genetic testing include the high cost for such studies, which may not be covered by insurance, and that the time to have results reported (turn-around-time) may be weeks and unacceptably long in a critically ill child. An additional limitation to genetic testing involves the findings of genetic variants of unknown significance (VUS), usually missense mutations. While a variety of approaches can be employed to help determine the potential significance VUS, including programs to predict pathogenecity (in silico) and determining their frequency to large publically available databases of genetic variants), such methods are imperfect and it may not be able to determine with certainty the clinical significance of a VUS.
Increasing whole-exome or whole-genome sequencing is being used in the intensive care setting to diagnose rare genetic conditions60. As technology improves and costs for such studies decrease, this approach is likely to become the preferred one, as genes continue to be identified associated with phenotypes that overlap with surfactant genetic disorders (Summarized in Table 2). In many cases, next generation sequencing panels are available through diagnostic laboratories that include the surfactant proteins and other genes. An important limitation is that the more genes studied, the higher the likelihood of finding a VUS in one or more candidate genes, which may result in diagnostic confusion rather than clarity.
Table 2:
Other genetic causes of neonatal and childhood diffuse lung disease
| Gene | Gene function | Inheritance Pattern |
Age of Onset | Pulmonary Phenotype |
Extrapulmonary Involvement |
||||
|---|---|---|---|---|---|---|---|---|---|
| Lung Developmental Disorders | |||||||||
| FOXF1 | Transcription Factor |
Sporadic / Dominant with variable penetrance |
Neonatal | Hypoxemic respiratory failure; PPHN | Cardiac, Gastrointestinal, Genitourinary |
||||
| TBX4 | Transcription Factor |
Sporadic | Neonatal, Childhood | Hypoxemic respiratory failure; Pulmonary Hypertension |
Skeletal | ||||
| Structural genes | |||||||||
| FLNA | Intracellular scaffolding |
X-linked dominant; can occur in males | Neonatal Infancy |
BPD, cystic lung disease | Cardiac, Skeletal, CNS (periventricular Heterotopias) |
||||
| ITGA3 | Transmembrane receptor | Recessive | Neonatal | RDS Growth Disorder (BPD) |
Skin, Renal | ||||
| Primary Ciliary Dyskinesia – 40 known genes | 80% + with neonatal | Sinus, Ear, Situs | |||||||
| Pulmonary Alveolar Proteinosis Genes | |||||||||
| SLC7A7 | Solute Transporter |
Recessive | Infancy | PAP | Lysinuric Protein Intolerance | ||||
| CSF2RA | Membrane Receptor for GM-CSF |
Recessive | Infancy to adult | PAP | None | ||||
| CSF2RB | Membrane Receptor for GM-CSF |
Recessive | Infancy to adult | PAP | None | ||||
| MARS | tRNA synthetase (methionine) |
Recessive | Infancy to adult | PAP | Liver, Anemia, Thyroid | ||||
| GATA2 | Transcription Factor |
Sporadic or Dominant |
Childhood to adult | PAP | Bone marrow, Immune | ||||
| Storage Diseases | |||||||||
| NPC2 NPC1 |
Enzyme | Recessive | Neonatal to infancy | DLD | Neurologic Liver (Cholestasis) |
||||
| IDUA (MPS type I) |
Enzyme | Recessive | Neonatal to infancy | DLD | Neurologic Skeletal Visceromegaly |
||||
| Immune Dysfunction Disorders with prominent pulmonary pathology | |||||||||
| COPA | Intracellular Transport |
Dominant or Sporadic |
Infancy to adult | Pulmonary Hemorrhage; DLD | Joint, renal, immune | ||||
| TMEM173 | Intracellular Signaling |
Sporadic | Newborn to infancy | DLD | Skin (vasculitis), immune STING Associated Vasculitis of Infancy (SAVI) |
||||
RDS: Respiratory Distress Syndrome; BPD: Bronchopulmonary Dysplasia; DLD: Diffuse Lung Disease; GM-CSF: Granulocyte-Macrophage Colony Stimulating Factor; PAP: Pulmonary Alveolar Proteinosis; STING: Stimulator of Interferon Genes.
Treatment
There are no proven effective drug therapies for surfactant dysfunction disorders. High dose corticosteroids, hydroxychloroquine, and azithromycin have been used off-label with variable responses observed29,61–63. Lung transplantation is an option when end stage pulmonary fibrosis develops64. Determination of timing of transplant is difficult for children with ABCA3 and NKX2–1 mutations and relatively mild disease, and even more so for those with SFTPC mutations, as even infants with severe lung disease due to SFTPC mutations may spontaneously improve, and the natural history of the disease is poorly understood and difficult to predict. Gene replacement or editing strategies may be needed to effectively treat SP-B deficiency, but are at early stages65,66. In the future, treatment with compounds to improve cell trafficking or function of ABCA3, similar to approaches currently being used for cystic fibrosis, may be an option, especially given the related nature of ABCA3 and CFTR67.
Conclusion
Genetic surfactant dysfunction disorders are rare but important causes of respiratory morbidity and mortality. Knowledge of the typical clinical presentations of these disorders is important to recognize and diagnose affected children in a timely manner. Lung biopsy may help categorize the lung disease, but determining the specific gene involved is critical to provide accurate information on prognosis and recurrence risk. Diagnosis can be established non-invasively through clinically available genetic testing, but limited by cost, turn-around-time and difficulties in interpreting results. Therapeutic options are currently limited, but gene replacement or correction strategies may be future options.
Key points:
Single gene disorders of surfactant metabolism have been identified that result in acute and/or chronic lung disease, with onset ranging from the neonatal period to adulthood.
There is considerable overlap in the clinical presentations and lung pathology findings of the disorders, which differ in their inheritance and clinical courses.
Identification of the specific gene involved is essential in order to provide appropriate counseling regarding prognosis and recurrence risk.
Non-invasive diagnosis can be made through genetic testing, which may prevent the need for biopsy in an unstable patient.
Acknowledgements:
The author gratefully acknowledge the collaboration of Drs. Jeffrey Whitsett and Susan Wert (Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine), Aaron Hamvas (Lurie Children’s Hospital of Chicago and Northwestern University Feinberg School of Medicine), and Jennifer Wambach, and F. Sessions Cole (St. Louis Children’s Hospital and Washington University in St. Louis). Supported by U.S. National Institutes of Health, 1UO1HL134745, and the Eudowood Foundation. Dr. Nogee receives royalties from Wolters Kluwer Health for co-authoring a contribution to UpToDate, and is a site co-investigator on clinical trial for neonatal pulmonary hypertension funded by United Therapeutics.
Funding: Supported by U.S. National Institutes of Health, 1UO1HL134745, and the Eudowood Foundation.
References
- 1.Ban N, Matsumura Y, Sakai H, et al. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282(13):9628–9634. [DOI] [PubMed] [Google Scholar]
- 2.Garmany TH, Moxley MA, White FV, et al. Surfactant composition and function in patients with ABCA3 mutations. Pediatr Res. 2006;59(6):801–805. [DOI] [PubMed] [Google Scholar]
- 3.Zarbock R, Kaltenborn E, Frixel S, et al. ABCA3 protects alveolar epithelial cells against free cholesterol induced cell death. Biochim Biophys Acta. 2015;1851(7):987–995. [DOI] [PubMed] [Google Scholar]
- 4.Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350(13):1296–1303. [DOI] [PubMed] [Google Scholar]
- *5.Wambach JA, Casey AM, Fishman MP, et al. Genotype-phenotype correlations for infants and children with ABCA3 deficiency. Am J Respir Crit Care Med. 2014;189(12):1538–1543.All children in this study of a large (N = 185) number of ABCA3 deficient patients who had mutations on both ABCA3 alleles predicted to preclude ABCA3 expression (“null alleles”) had severe neonatal RDS and died in the first year of life. These data are important for counseling families and in making a decision whether to pursue lung transplantation.
- 6.Wambach JA, Wegner DJ, Depass K, et al. Single ABCA3 mutations increase risk for neonatal respiratory distress syndrome. Pediatrics. 2012;130(6):e1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullard JE, Wert SE, Whitsett JA, Dean M, Nogee LM. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172(8):1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campo I, Zorzetto M, Mariani F, et al. A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant. Respiratory research. 2014;15:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wambach JA, Yang P, Wegner DJ, et al. Functional Characterization of ATP-Binding Cassette Transporter A3 Mutations from Infants with Respiratory Distress Syndrome. Am J Respir Cell Mol Biol. 2016;55(5):716–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Schindlbeck U, Wittmann T, Hoppner S, et al. ABCA3 missense mutations causing surfactant dysfunction disorders have distinct cellular phenotypes. Human mutation. 2018;39(6):841–850.Different mechanisms (impaired intracellular transport, inability to bind or hydrolyze ATP) underly the pathophysiology of different ABCA3 mutations, which has implications for treatment strategies.
- 11.Wittmann T, Frixel S, Hoppner S, et al. Increased Risk of Interstitial Lung Disease in Children with a Single R288K Variant of ABCA3. Mol Med. 2016;22:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver TE. Synthesis, processing and secretion of surfactant proteins B and C. Biochim Biophys Acta. 1998;1408(2–3):173–179. [DOI] [PubMed] [Google Scholar]
- 13.Clark JC, Wert SE, Bachurski CJ, et al. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci U S A. 1995;92(17):7794–7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.deMello DE, Heyman S, Phelps DS, et al. Ultrastructure of lung in surfactant protein B deficiency. Am J Respir Cell Mol Biol. 1994;11(2):230–239. [DOI] [PubMed] [Google Scholar]
- 15.Nogee LM, de Mello DE, Dehner LP, Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993;328(6):406–410. [DOI] [PubMed] [Google Scholar]
- 16.Nogee LM, Wert SE, Proffit SA, Hull WM, Whitsett JA. Allelic heterogeneity in hereditary surfactant protein B (SP-B) deficiency. Am J Respir Crit Care Med. 2000;161(3 Pt 1):973–981. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Ikegami M, Na CL, et al. N-terminally extended surfactant protein (SP) C isolated from SP-B-deficient children has reduced surface activity and inhibited lipopolysaccharide binding. Biochemistry. 2004;43(13):3891–3898. [DOI] [PubMed] [Google Scholar]
- 18.Vorbroker DK, Profitt SA, Nogee LM, Whitsett JA. Aberrant processing of surfactant protein C in hereditary SP-B deficiency. Am J Physiol. 1995;268(4 Pt 1):L647–656. [DOI] [PubMed] [Google Scholar]
- 19.Nogee LM, Garnier G, Dietz HC, et al. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest. 1994;93(4):1860–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beers MF, Hamvas A, Moxley MA, et al. Pulmonary surfactant metabolism in infants lacking surfactant protein B. Am J Respir Cell Mol Biol. 2000;22(3):380–391. [DOI] [PubMed] [Google Scholar]
- 21.Baekvad-Hansen M, Dahl M, Tybjaerg-Hansen A, Nordestgaard BG. Surfactant protein-B 121ins2 heterozygosity, reduced pulmonary function, and chronic obstructive pulmonary disease in smokers. Am J Respir Crit Care Med. 2010;181(1):17–20. [DOI] [PubMed] [Google Scholar]
- 22.Gower WA, Nogee LM. Surfactant dysfunction. Paediatr Respir Rev. 2011;12(4):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunbar AE 3rd, Wert SE, Ikegami M, et al. Prolonged survival in hereditary surfactant protein B (SP-B) deficiency associated with a novel splicing mutation. Pediatr Res. 2000;48(3):275–282. [DOI] [PubMed] [Google Scholar]
- *24.Lopez-Andreu JA, Hidalgo-Santos AD, Fuentes-Castello MA, et al. Delayed Presentation and Prolonged Survival of a Child with Surfactant Protein B Deficiency. J Pediatr. 2017;190:268–270 e261.Rare example of a child with SP-B deficiency due to a mutation known to cause partial deficiency presenting well after the newborn period.
- 25.Garmany TH, Wambach JA, Heins HB, et al. Population and disease-based prevalence of the common mutations associated with surfactant deficiency. Pediatr Res. 2008;63(6):645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Pulido L, Devos D, Valencia A. BRICHOS: a conserved domain in proteins associated with dementia, respiratory distress and cancer. Trends Biochem Sci. 2002;27(7):329–332. [DOI] [PubMed] [Google Scholar]
- 27.Johansson H, Eriksson M, Nordling K, Presto J, Johansson J. The Brichos domain of prosurfactant protein C can hold and fold a transmembrane segment. Protein Sci. 2009;18(6):1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasser SW, Detmer EA, Ikegami M, Na CL, Stahlman MT, Whitsett JA. Pneumonitis and emphysema in sp-C gene targeted mice. J Biol Chem. 2003;278(16):14291–14298. [DOI] [PubMed] [Google Scholar]
- 29.Kroner C, Reu S, Teusch V, et al. Genotype alone does not predict the clinical course of SFTPC deficiency in paediatric patients. The European respiratory journal. 2015;46(1):197–206. [DOI] [PubMed] [Google Scholar]
- *30.Litao MK, Hayes D Jr., Chiwane S, Nogee LM, Kurland G, Guglani L. A novel surfactant protein C gene mutation associated with progressive respiratory failure in infancy. Pediatr Pulmonol. 2017;52(1):57–68.Includes a nice review of published cases of patients with SP-C mutations.
- 31.Cameron HS, Somaschini M, Carrera P, et al. A common mutation in the surfactant protein C gene associated with lung disease. J Pediatr. 2005;146(3):370–375. [DOI] [PubMed] [Google Scholar]
- 32.Maguire JA, Mulugeta S, Beers MF. Multiple ways to die: delineation of the unfolded protein response and apoptosis induced by Surfactant Protein C BRICHOS mutants. Int J Biochem Cell Biol. 2012;44(1):101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **33.Stewart GA, Ridsdale R, Martin EP, et al. 4-Phenylbutyric acid treatment rescues trafficking and processing of a mutant surfactant protein-C. Am J Respir Cell Mol Biol. 2012;47(3):324–331.Demonstration of different cellular mechanisms from SFTPC mutations and suggestion that there may be drugs that can help improve normal intracellular metabolism of the misfolded protein.
- 34.Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol. 2005;32(6):521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beers MF, Hawkins A, Maguire JA, et al. A nonaggregating surfactant protein C mutant is misdirected to early endosomes and disrupts phospholipid recycling. Traffic. 2011;12(9):1196–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawkins A, Guttentag SH, Deterding R, et al. A non-BRICHOS SFTPC mutant (SP-CI73T) linked to interstitial lung disease promotes a late block in macroautophagy disrupting cellular proteostasis and mitophagy. Am J Physiol Lung Cell Mol Physiol. 2015;308(1):L33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conkright JJ, Bridges JP, Na CL, et al. Secretion of surfactant protein C, an integral membrane protein, requires the N-terminal propeptide. J Biol Chem. 2001;276(18):14658–14664. [DOI] [PubMed] [Google Scholar]
- 38.Krude H, Schutz B, Biebermann H, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2–1 haploinsufficiency. J Clin Invest. 2002;109(4):475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohlenz J, Dumitrescu A, Zundel D, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109(4):469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guillot L, Carre A, Szinnai G, et al. NKX2–1 mutations leading to surfactant protein promoter dysregulation cause interstitial lung disease in “Brain-Lung-Thyroid Syndrome”. Human mutation. 2010;31(2):E1146–1162. [DOI] [PubMed] [Google Scholar]
- 41.Hamvas A, Deterding RR, Wert SE, et al. Heterogeneous pulmonary phenotypes associated with mutations in the thyroid transcription factor gene NKX2–1. Chest. 2013;144(3):794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galambos C, Levy H, Cannon CL, et al. Pulmonary pathology in thyroid transcription factor-1 deficiency syndrome. Am J Respir Crit Care Med. 2010;182(4):549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Attarian SJ, Leibel SL, Yang P, et al. Mutations in the thyroid transcription factor gene NKX2–1 result in decreased expression of SFTPB and SFTPC. Pediatr Res. 2018;84(3):419–425.Demonstrates mechanism for two NKX2–1 mutations results in loss-of-expression of target genes important for normal lung function.
- 44.Vieira F, Kung JW, Bhatti F. Structure, genetics and function of the pulmonary associated surfactant proteins A and D: The extra-pulmonary role of these C type lectins. Ann Anat. 2017;211:184–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ujma S, Horsnell WG, Katz AA, Clark HW, Schafer G. Non-Pulmonary Immune Functions of Surfactant Proteins A and D. J Innate Immun. 2017;9(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maitra M, Cano CA, Garcia CK. Mutant surfactant A2 proteins associated with familial pulmonary fibrosis and lung cancer induce TGF-beta1 secretion. Proc Natl Acad Sci U S A. 2012;109(51):21064–21069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Maitra M, Wang Y, Gerard RD, Mendelson CR, Garcia CK. Surfactant protein A2 mutations associated with pulmonary fibrosis lead to protein instability and endoplasmic reticulum stress. J Biol Chem. 2010;285(29):22103–22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Moorsel CH, Ten Klooster L, van Oosterhout MF, et al. SFTPA2 Mutations in Familial and Sporadic Idiopathic Interstitial Pneumonia. Am J Respir Crit Care Med. 2015;192(10):1249–1252. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gower WA, Nogee LM. Candidate gene analysis of the surfactant protein D gene in pediatric diffuse lung disease. J Pediatr. 2013;163(6):1778–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki T, Trapnell BC. Pulmonary Alveolar Proteinosis Syndrome. Clin Chest Med. 2016;37(3):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katzenstein AL, Gordon LP, Oliphant M, Swender PT. Chronic pneumonitis of infancy. A unique form of interstitial lung disease occurring in early childhood. Am J Surg Pathol. 1995;19(4):439–447. [PubMed] [Google Scholar]
- 53.Griese M, Lorenz E, Hengst M, et al. Surfactant proteins in pediatric interstitial lung disease. Pediatr Res. 2016;79(1–1):34–41. [DOI] [PubMed] [Google Scholar]
- 54.Edwards V, Cutz E, Viero S, Moore AM, Nogee L. Ultrastructure of lamellar bodies in congenital surfactant deficiency. Ultrastruct Pathol. 2005;29(6):503–509. [DOI] [PubMed] [Google Scholar]
- 55.Kurland G, Deterding RR, Hagood JS, et al. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188(3):376–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nogee LM. Interstitial lung disease in newborns. Semin Fetal Neonatal Med. 2017;22(4):227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henderson LB, Melton K, Wert S, et al. Large ABCA3 and SFTPC deletions resulting in lung disease. Ann Am Thorac Soc. 2013;10(6):602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wegner DJ, Hertzberg T, Heins HB, et al. A major deletion in the surfactant protein-B gene causing lethal respiratory distress. Acta Paediatr. 2007;96(4):516–520. [DOI] [PubMed] [Google Scholar]
- 59.Jackson T, Wegner DJ, White FV, Hamvas A, Cole FS, Wambach JA. Respiratory failure in a term infant with cis and trans mutations in ABCA3. J Perinatol. 2015;35(3):231–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **60.Meng L, Pammi M, Saronwala A, et al. Use of Exome Sequencing for Infants in Intensive Care Units: Ascertainment of Severe Single-Gene Disorders and Effect on Medical Management. JAMA Pediatr. 2017;171(12):e173438.Exome-sequencing in an intensive care setting, particularly trio sequencing where the proband and parents’ genomes were sequenced, had a high diagnostic yield and resulted in affected management in the majority of cases.
- 61.Braun S, Ferner M, Kronfeld K, Griese M. Hydroxychloroquine in children with interstitial (diffuse parenchymal) lung diseases. Pediatr Pulmonol. 2015;50(4):410–419. [DOI] [PubMed] [Google Scholar]
- *62.Kroner C, Wittmann T, Reu S, et al. Lung disease caused by ABCA3 mutations. Thorax. 2017;72(3):213–220.Comprehensive data from relatively large number of subjects (40) with this rare disease.
- 63.Avital A, Hevroni A, Godfrey S, et al. Natural history of five children with surfactant protein C mutations and interstitial lung disease. Pediatr Pulmonol. 2014;49(11):1097–1105. [DOI] [PubMed] [Google Scholar]
- **64.Eldridge WB, Zhang Q, Faro A, et al. Outcomes of Lung Transplantation for Infants and Children with Genetic Disorders of Surfactant Metabolism. J Pediatr. 2017;184:157–164 e152.Current experience with lung transplantation for these disorders. Five year survival was roughly 55% for infants, and 75–80% in older children, and these were subjects who would have died far sooner without transplantation.
- **65.Mahiny AJ, Dewerth A, Mays LE, et al. In vivo genome editing using nuclease-encoding mRNA corrects SP-B deficiency. Nat Biotechnol. 2015;33(6):584–586.Proof of concept study in mouse model of SP-B deficiency that gene correction strategies could work.
- **66.Jacob A, Morley M, Hawkins F, et al. Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell. 2017;21(4):472–488 e410.Several important advances. The investigators were able to derive induced pluripotent stem cells from subjects with disease, then drive these cells forward into a system that recapitulates the features of AEC2s. The lack of such a system has been limiting for in vitro studies that aim to use drugs or small molecules to try to correct the intracellular metabolic defects resulting from genetic surfactant dysfunction disorders. Finally, in vitro gene editing of cells from an SP-B deficient patient corrected the defects in SP-B synthesis and intracellular metabolism, an important proof-of-concept experiment.
- **67.Kinting S, Hoppner S, Schindlbeck U, et al. Functional rescue of misfolding ABCA3 mutations by small molecular correctors. Human molecular genetics. 2018;27(6):943–953.While the specific molecules were not identified, provides proof-of-concept that there may be “correctors” for ABCA3 mutations as have been developed for cystic fibrosis.