Abstract
Introduction
A large group of patients with chronic obstructive pulmonary disease (COPD) are exposed to an overload of oral corticosteroids (OCS) due to repeated exacerbations. This is associated with potential serious adverse effects. Therefore, we evaluated the impact of a recommended reduction of OCS duration in 2014 on the risk of pneumonia hospitalisation and all-cause mortality in patients with acute exacerbation of COPD (AECOPD).
Methods
This was a nationwide observational cohort study that was based on linked administrative registry data between 1 January 2010 and 31 October 2017. 10 152 outpatients with COPD (median age 70 years) treated with either a short (≤250 mg) or long course (>250 mg) of OCS for AECOPD were included in the study. Cox proportional hazards regression models were used to derive an estimation of multivariable adjusted HRs (aHRs) for pneumonia hospitalisation or all-cause mortality combined and pneumonia hospitalisation and all-cause mortality, separately.
Results
The long course of OCS treatment for AECOPD was associated with an increased 1-year risk of pneumonia hospitalisation or all-cause mortality (aHR 1.3, 95% CI 1.1 to 1.4; p<0.0001), pneumonia hospitalisation (aHR 1.2, 95% CI 1.0 to 1.3; p=0.0110) and all-cause mortality (aHR 1.8, 95% CI 1.5 to 2.2; p<0.0001) as compared with the short course of OCS treatment. These results were confirmed in several sensitivity analyses.
Conclusion
The change of recommendations from long courses to short courses of OCS for AECOPD in 2014 was strongly associated with a decrease in pneumonia admissions and all-cause mortality, in favour of short courses of OCS.
Keywords: copd exacerbations, copd pharmacology, pneumonia, copd epidemiology, clinical epidemiology
Key messages.
This nationwide cohort study reports a strong association between the long course (10 days) of oral corticosteroid (OCS) treatment and the sustained increase in risk of admission with pneumonia and all-cause mortality—up to 1 year after exposure—in outpatients with chronic obstructive pulmonary disease as compared with a short course (5 days) of OCS.
There was a time-dependent change in the magnitude of risk: those with current exposure had a higher vulnerability to pneumonia and all-cause mortality.
Understanding how risk depends on patterns of OCS will contribute to improved benefits and harm assessment.
Introduction
Exacerbations of chronic obstructive pulmonary disease (COPD) contribute to high morbidity and mortality.1 Patients with COPD are also at greater risk of developing pneumonia, mainly due to the impairment of lung defence mechanisms and use of inhaled corticosteroids (ICS).2 In addition, patients hospitalised for COPD and pneumonia exhibit substantially higher rates of intensive care admission, mortality and longer length of hospital stay compared with persons without COPD.3
Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendation for treatment duration of systemic corticosteroids (oral corticosteroids, OCS) for acute exacerbations of COPD (AECOPD) has changed over the last decades, ranging from treatment durations of 10–14 days,4 7–10 days5 and is currently 5–7 days. The last reduction was based on a robust randomised controlled trial.6
OCS are recommended to patients with COPD during acute exacerbations, and most COPD exacerbations can safely be managed in the outpatient setting.7 OCS have been found to shorten the length of hospital stays, improve lung function, and reduce the risk of early relapse and treatment failure in patients with non-pneumonia exacerbation. No effect has been observed regarding longer-term lung function, length of stay at intensive care unit and 30-day mortality.8 Most clinical trials investigating OCS for exacerbations were performed on patients who require hospitalisation, and only a few have described the effect of OCS in the outpatient setting.
OCS use is associated with a number of adverse effects including hyperglycaemia, fluid retention, weight gain, hypertension, diabetes mellitus, adrenal suppression, deep vein thrombosis, osteoporosis and increased fracture risk.8 9 Even short courses of OCS (median days=6) have been associated with adverse effects, and they increase with increased number of OCS courses.10 11 In addition, OCS have been shown to cause severe infections due to their immunosuppressive activity, which impairs phagocyte function and suppresses cell-mediated immunity.12 This was confirmed in a meta-analysis of 71 pooled controlled trials that showed a relative risk of 1.6 for infectious events in the prednisolone group (n=2111) compared with the controls (n=2087).13 However, the risk of severe infections and death following the use of OCS is in general unknown and mainly short-term follow-up endpoints have been reported in patients with COPD. Therefore, we conducted a nationwide, observational cohort study to determine the association between duration of OCS treatment in outpatients with AECOPD and the risk of pneumonia hospitalisation and all-cause mortality during a 1-year study period and to explore how the timing of the exposure affects the risk estimates.
Methods
Data sources and covariates
The following nationwide registers were used to link on an individual level:
The Danish Register of Chronic Obstructive Pulmonary Disease (DrCOPD) is a nationwide database that contains information on the quality of treatment of all patients with COPD in Denmark.14 All Danish hospitals, since 2008, that treat patients with COPD report to the register. Covariates included in this study were age, lung function—assessed as FEV1% predicted, body mass index—assessed as kilograms per square metre, dyspnoea—assessed using the Medical Research Council (MRC) Dyspnoea Scale, smoking status, ICS, and long-acting β2-agonist (LABA) or long-acting muscarinic antagonist use.
The Danish National Patient Registry (DNPR), which holds information on all admissions to Danish hospitals, since 1977, and hospital outpatient clinic visits, since 1995. Each hospital visit is coded by physicians with one primary diagnosis and one or more secondary diagnoses, according to the International Classification of Diseases, eighth revision (ICD-8) codes until 1994 and ICD-10 thereafter.15 Covariates included exacerbations in the previous year, essential hypertension, diabetes mellitus, myocardial infarction, atrial fibrillation, heart failure, peripheral vascular disease, cerebrovascular disease, renal failure and depression (table 1 and online supplementary table s1).
The Danish National Health Service Prescription Database (DNHSPD) holds information on all prescriptions that have been dispensed in Danish pharmacies, since 2004 (coded according to the Anatomical Therapeutic Chemical (ATC) classification system), including the following information in terms of OCS: the date of dispensation, the quantity dispensed as well as the strength and formulation of all prescriptions that have been dispensed from Danish Pharmacies. All pharmacies are required by Danish legislation to provide information that ensures complete and accurate registration.16
The Danish Civil Registration System (CRS) includes individual information on the unique personal identification number, name, sex, date of birth and vital status.17
Table 1.
Baseline characteristics of outpatients with COPD who were treated with short vs long course of OCS during the period of January 2010 to October 2017
| Characteristics | All (n=10 152) | Short course (n=6002) | Long course (n=4150) |
| Demographics | |||
| Age, median (IQR) | 70 (63–77) | 70 (62–76) | 70 (63–77) |
| Age≤62 | 2456 (24.2) | 1528 (25.5) | 928 (22.4) |
| 63–70 | 2835 (27.9) | 1670 (27.8) | 1165 (28.1) |
| 71–77 | 2586 (25.5) | 1523 (25.4) | 1063 (25.6) |
| ≥78 | 2275 (22.4) | 1281 (21.3) | 994 (23.9) |
| Male | 5095 (50.2) | 2962 (49.4) | 2133 (51.4) |
| MRC, n/median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) |
| FEV1≥80% | 451 (5.3) | 251 (4.9) | 200 (5.8) |
| 50%≤FEV1<80% | 3523 (41.1) | 2176 (42.3) | 1347 (39.3) |
| 30%<FEV1<50% | 3310 (38.6) | 2220 (39.3) | 1290 (37.6) |
| FEV≤30% | 1289 (15.0) | 698 (13.5) | 593 (17.3) |
| Exacerbations within the past year | 0 | 4601 (76.7) | 3176 (76.5) |
| 1 | 1011 (16.8) | 669 (16.1) | |
| ≥2 | 390 (6.5) | 305 (7.4) | |
| Inhaled corticosteroids | 7320 (72.1) | 4361 (72.7) | 2959 (71.3) |
| Inhaled LABA or LAMA | 8781 (86.5) | 5233 (87.2) | 3548 (85.5) |
| BMI, median (IQR) | 25 (21.3–29) | 25 (21.1–29) | 25 (21.5–29) |
| BMI (kg/m2) | |||
| 10.0–18.4 | 706 (8.2) | 416 (8.1) | 290 (8.4) |
| 18.5–24.9 | 3400 (39.7) | 2003 (39.2) | 1397 (40.4) |
| 25.0–29.9 | 4464 (52.1) | 2693 (52.7) | 1771 (51.2) |
| Total dose OCS (mg), median (IQR) | 250 (250–500) | 250 (250–250) | 500 (500–1000) |
| Smoking | |||
| Current smokers | 3264 (32.2) | 2030 (33.8) | 1234 (29.7) |
| Ex-smokers/non-smokers | 5253 (51.7) | 3075 (51.2) | 2178 (52.5) |
| Missing data | 1635 (16.1) | 897 (15.0) | 738 (17.8) |
| Comorbidities | |||
| Heart failure | 2075 (20.4) | 1194 (19.9) | 881 (21.2) |
| Atrial fibrillation | 2341 (23.1) | 1350 (22.5) | 991 (23.9) |
| Myocardial infarction | 1197 (11.8) | 701 (11.7) | 496 (11.9) |
| Hypertension | 4215 (41.5) | 2473 (41.2) | 1742 (42.0) |
| Diabetes mellitus | 1391 (13.7) | 789 (13.2) | 602 (14.5) |
| Peripheral vascular disease | 1783 (17.6) | 1046 (17.4) | 737 (17.8) |
| Cerebrovascular disease | 1592 (15.7) | 900 (15.0) | 692 (16.7) |
| Renal failure | 866 (8.5) | 484 (8.1) | 382 (9.2) |
| Depression | 669 (6.6) | 395 (6.6) | 274 (6.6) |
Data are presented as n (%) unless otherwise stated.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; MRC, Medical Research Council Dyspnoea Scale; OCS, oral corticosteroids.
bmjresp-2019-000407supp001.pdf (220.7KB, pdf)
Study design
Danish residents who were registered with a diagnosis of COPD between 1 January 2010 and 31 October 2017 were identified in the DrCOPD. We formed the base cohort by linking these individuals with prednisolone prescriptions (ATC code H02AB06) for the treatment of AECOPD in the outpatient clinic in the DNHSPD. We chose not to include prednisone (ATC code H02AB07) as they were given for other indications. Patients were excluded if they did not receive prednisolone prescription, ever had an asthma diagnosis or had received prednisolone prescription above 2500 mg as these have been prescribed as maintenance treatment for stable patients with COPD. The study cohort was followed from prescription date to the occurrence of hospitalisation for pneumonia, death or 1 year from OCS prescription (figure 1 and online supplementary figure s2).
Figure 1.
Study flowchart. AECOPD, acute exacerbations of chronic obstructive pulmonary disease; DNHSPD, Danish National Health Service Prescription Database; DrCOPD, Danish Register of Chronic Obstructive Pulmonary Disease; OCS, oral corticosteroids.
Patients were stratified into two groups: (i) Prednisolone prescriptions below or equal to 250 mg, correspond to a short course of OCS (a package of 10 pieces of 25 mg given for a 5-day treatment with 37.5 mg daily). (ii) Prednisolone prescriptions above 250 mg, corresponding to a long course of OCS (two or more packages of 10 pieces of 25 mg given for a 10-day treatment with 37.5 mg daily). This definition of long and short courses was based on Danish guidelines and prednisolone prescription practice. The change of OCS duration recommendation in 2014 made it possible for us to compare the treatments short course versus long course in the periods before (2010-2013) versus after (2014-2017) recommendation change (supplementary figure s3). Both treatments were given during their respective periods as first line treatments – providing a comparison that mitigates the possibility that long course was given for the most severity of COPD patients. The study was approved by the Danish Data Protection Agency (journal number: HGH-2017-091, with I-Suite no.: 05884).
Patient and public involvement
Patients were not involved in the design of this study.
Outcomes
The outcomes of interest were admission with pneumonia and all-cause mortality. These were investigated both as a combined endpoint (pneumonia hospitalisation or all-cause mortality) and individual endpoints. The combined endpoint was chosen as the primary endpoint. Admissions with pneumonia were identified by DNPR registry data (primary diagnosis of pneumonia and no requirement for a secondary diagnosis) (figure 1). The diagnosis of pneumonia was based on ICD codes (DJ12 to DJ18) from hospitalisation. In Denmark, the National guideline on pneumonia states that such a diagnosis includes (obligatory) a novel infiltrate on a chest X-ray. All the participating departments refer to this definition. The mortality date was specified in the CRS. The date of the first OCS exposure was defined as the index date.
Statistical analysis
For descriptive statistics, continuous variables were presented as median values and IQRs, and categorical variables as frequencies and proportions. For comparison, we used the Mann-Whitney U test for continuous variables and the χ2 test for dichotomous variables. Using the Aalen-Johansen estimator, we performed a cumulative incidence plot of the first pneumonia hospitalisation and all-cause mortality stratified on short versus long course of OCS. We used the log-rank test for the equivalence of the cumulative incidence curves. Cox proportional hazards regression analyses were fitted to assess the risk of pneumonia hospitalisation or all-cause mortality at 1, 3, 6 months and 1 year, respectively, after a long course of OCS treatment as compared with the short course of OCS treatment. An adjusted analysis including age, sex, smoking, calendar year, MRC dyspnoea score, BMI, FEV1% predicted, ICS therapy and all the nine comorbidities was performed to ensure that the results were not due to these potential confounding factors (tables 2 and 3). The results were presented as HRs with 95% confidence limits (CI).
Table 2.
Pneumonia hospitalisation or all-cause mortality (combined endpoint)
| Adjusted HR (95% CI) | |
| Time from exposure | |
| 1 month | 1.6 (1.1 to 2.2) |
| 3 months | 1.3 (1.1 to 1.7) |
| 6 months | 1.3 (1.2 to 1.6) |
| 1 year | 1.3 (1.1 to 1.4) |
| Time from exposure above day 30 | |
| 1 months | – |
| 3 months | 1.2 (1.0 to 1.6) |
| 6 months | 1.3 (1.2 to 1.6) |
| 1 year | 1.3 (1.1 to 1.4) |
First part: Adjusted HRs (aHRs) for pneumonia hospitalisation or all-cause mortality for outpatients with acute exacerbations of chronic obstructive pulmonary disease who were treated with a long-course oral corticosteroid (OCS) treatment during the 1-year follow-up. Second part: aHRs where we are not counting for any events before day 30. The reference group was the short-course OCS treatment. All analyses were adjusted for age, sex, inhaled corticosteroids, smoking status, calendar year, FEV1% predicted, Medical Research Council Dyspnoea Scale, body mass index, heart failure, atrial fibrillation, myocardial infarction, hypertension, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, renal failure and depression.
Table 3.
Pneumonia hospitalisation and all-cause mortality (separate endpoints)
| Time from exposure | Adjusted HR (95% CI) |
| Pneumonia hospitalisation | |
| 1 month | 1.4 (1.0 to 2.2) |
| 3 months | 1.2 (1.0 to 1.6) |
| 6 months | 1.2 (1.0 to 1.4) |
| 1 year | 1.2 (1.0 to 1.3) |
| All-cause mortality | |
| 1 month | 5.1 (1.7 to 15.6) |
| 3 months | 2.5 (1.5 to 4.3) |
| 6 months | 2.0 (1.5 to 2.6) |
| 1 year | 1.8 (1.5 to 2.2) |
Secondary endpoints: Adjusted hazard ratios (aHR) of pneumonia hospitalisation and all-cause mortality for outpatients with acute exacerbations of chronic obstructive pulmonary disease who were treated with a long-course oral corticosteroid (OCS) treatment during the 1-year follow-up. The reference group was the short-course OCS treatment. All analyses were adjusted for age, sex, inhaled corticosteroids, smoking status, calendar year, FEV1% predicted, Medical Research Council Dyspnoea Scale, body mass index, heart failure, atrial fibrillation, myocardial infarction, hypertension, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, renal failure and depression.
In another sensitivity analyses, in order to reduce potential effects of confounding in the case of a greater number of prescriptions in one group, patients were censored prior to receiving the second OCS. The purpose of this was to avoid the effect of an accumulated dose of prednisolone on the outcome.
Because exposure to OCS varied over time in the cohort, a nested case–control model was used to calculate the OR for pneumonia hospitalisation. The date of the first admission for pneumonia during follow-up was considered as the date of the event (index date). Cases with a pneumonia hospitalisation were matched, one control each at the time of pneumonia by age, sex, FEV1% predicted and smoking (current smoker vs ex-smoker/non-smoker), which was measured 1 year before the index date by using risk set sampling to assure that the match occurred before death and pneumonia hospitalisation in the controls. Neither the cases nor the controls were reused in the analysis. OCS exposure was defined as binary variable: short course (≤250 mg) versus long course (>250 mg). The same analysis was performed for all-cause mortality. Matching was performed with some loss in statistical power. Statistical analyses were performed using SAS statistical software V.9.4 (SAS Institute, Cary, North Carolina, USA) and R statistical software (V.3.4.3). SAS and R codes were reviewed by an independent statistician.
Results
The baseline characteristics of the two groups are presented in table 1. A total of 10 152 patients with COPD (median age 70 years; 50.2% male) who were treated with OCS were identified from the 67 208 patients with COPD in the sampling cohort between 1 January 2010 and 31 October 2017 (figure 1). The number of patients who received short and long course of OCS was 6002 (59.1%) and 4150 (40.9%), respectively. The patients in the short-course and long-course groups were similar, overall, with no clinically significant differences in their characteristics (table 1).
Main analyses
Risk associated with the use of long versus short courses of OCS
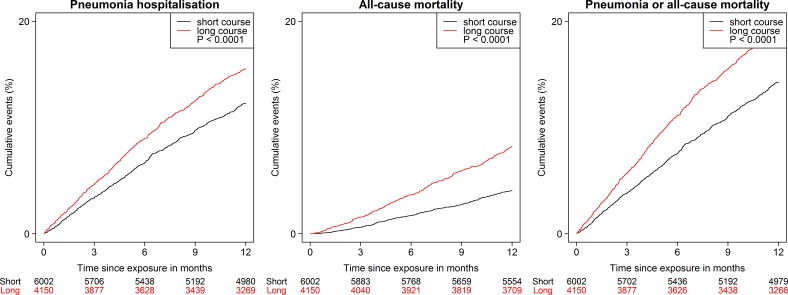
Cumulative incidence curves for estimating the probability of pneumonia hospitalisation and all-cause mortality, over time, are presented in figure 2. Significant differences were found between the two groups (p<0.0001, using log-rank test). After 1-year follow-up, the cumulative incidence of pneumonia hospitalisation and all-cause mortality was higher in the long-course group than in the short-course group, both as combined and separate endpoints.
Figure 2.
Cumulative incidence curves for the three outcomes among COPD outpatients treated with long vs short course of OCS, 2010–2017 (P<0.0001).
During the 1-year follow-up, long-course OCS was associated with a significantly increased risk of the composite of pneumonia hospitalisation or all-cause mortality (adjusted HR [aHR] 1.3, 95% CI 1.1 to 1.4; p<0.0001), pneumonia hospitalisation (aHR 1.2, 95% CI 1.0 to 1.3; p=0.0110) and all-cause mortality (aHR 1.8, 95% CI 1.5 to 2.2; p<0.0001) as compared with the short-course group (tables 2 and 3).
Sensitivity analyses
As part of the sensitivity analyses, all pneumonia and death events were removed up to day 30 and all analyses for the primary endpoint were repeated, which showed consistent results (table 2). With this sensitivity analysis, we wanted to exclude the possibility that it was not the increased risk the first month that was responsible for the continued risk of pneumonia or all-cause mortality 1 year after, that is, remove an ‘acute illness factor’ as a possible driver of events. In a second sensitivity analysis, censoring patients prior to the second OCS prescription showed consistent results for pneumonia hospitalisation, from OCS exposure to 1-year follow-up (aHR 1.1, 95% CI 1.0 to 1.3; p=0.1102), using the short course as a reference (table 4).
Table 4.
Sensitivity analyses: censoring and nested case–control design
| Time from exposure | Adjusted HR ()/OR (95% CI) |
| Pneumonia (aHR)* | 1.1 (1.0 to 1.3) |
| Pneumonia (OR)† | 1.4 (1.2 to 1.7) |
| All-cause mortality (OR)‡ | 2.4 (1.9 to 2.9) |
*Analyses of long vs short oral corticosteroid (OCS) courses in all patients, censored prior to the second OCS course, 1-year follow-up. Analyses were adjusted for age, sex, inhaled corticosteroids, smoking status, calendar year, FEV1% predicted, Medical Research Council Dyspnoea Scale, body mass index, heart failure, atrial fibrillation, myocardial infarction, hypertension, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, renal failure and depression.
†Estimated OR for pneumonia hospitalisation in a nested case–control design, 1 year before the index date. Matched on age, sex, FEV1% predicted and smoking.
‡Estimated OR for all-cause mortality in a nested case–control design, 1 year before the index date. Matched on age, sex, FEV1% predicted and smoking.
In a third sensitivity analysis, a nested case–control model was applied to calculate the OR for pneumonia hospitalisation. The analysis comprised 4429 patients (2695 cases of pneumonia and 1734 controls without pneumonia). The sample size was less than the initial cohort due to the matching of age, sex, FEV1% predicted, smoking and index date. The estimated OR for the pneumonia hospitalisation was 1.4 (95% CI 1.2 to 1.7; p=0.0004) for the long-course group, using the short-course group as a reference. The same analysis for all-cause mortality (n=4476) was 2.4 (95% CI 1.9 to 2.9; p<0.0001) for the long-course group. These results were similar to the main analyses.
Discussion
The main findings of our study are that the use of a long course of OCS in outpatients with AECOPD increased the risk of pneumonia hospitalisation and all-cause mortality within a 1-year period from the start of treatment compared with the short course of OCS. Past use (up to 1 year before the index date) in the nested case–control model was also associated with an increased risk in the long-course group for both hospitalisation with pneumonia and all-cause mortality. There was a time-dependent change in the magnitude of risk: those with current exposure demonstrated a higher vulnerability to pneumonia and all-cause mortality. Several sensitivity analyses confirmed these results. These findings are important given the global burden of COPD, the frequent use of OCS in patients with COPD exacerbations and the many adverse effects to this medication. These observations suggest a more restrictive use of OCS for outpatients with AECOPD.
Because of their known efficacy in COPD, OCS are likely to be used frequently, and it is important for clinicians to know the risks associated with this therapy. Although the association between OCS and admission with pneumonia and all-cause mortality in COPD is poorly understood, the association has been investigated in other chronic diseases like rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). A study reported that OCS predisposes elderly patients with IBD to bacterial, fungal and protozoal infections. The rate of serious infections was significantly higher in patients with IBD who were exposed to OCS any time during the 6-month period before the index date as compared with non-users (adjusted rate ratio [aRR] 2.3, 95% CI 1.8 to 2.9). Those currently exposed (within 45 days) had a higher risk (aRR 2.8, 95% CI 2.1 to 3.7).18 For patients with Crohn’s disease who are enrolled in the TREAT registry, which is a prospective observational research programme, OCS significantly increased the subsequent risk of serious infections and any infection that required hospitalisation in the 6-month period before the event (aHR 1.6, 95% CI 1.2 to 2.1) and death (aHR 1.8, 95% CI 1.3 to 2.5).19 Another population-based study showed an increased risk for admission with pneumonia for a prednisone dose of 5 mg daily (HR 1.4, 95% CI 1.1 to 1.6) with a higher risk associated with doses greater than 10 mg, daily (HR 2.3, 95% CI 1.6 to 3.2) in patients with RA.20 Most of these results are consistent with our findings.
A recent observational study (n=327 452) found that the short-term use of OCS (median days=6) was associated with an increased risk of sepsis (incidence ratio [IR] 5.3, 95% CI 3.8 to 7.4), venous thromboembolism (IR 3.3, 95% CI 2.8 to 3.9) and fractures (IR 1.9, 95% CI 1.7 to 2.1) within 30 days of initiation, even at relatively low doses.21
Regular long-term use of OCS in COPD has been linked to mortality.22 A recent study showed that patients with COPD (N=444) that had been randomised to the conservative treatment arm in the National Emphysema Trial had an increased risk for death (HR 1.73, 95% CI 1.25 to 2.41; p=0.001) in the long-term corticosteroid group.23 However, data regarding the correlation between the short course of OCS on mortality in patients with COPD are sparse.
In an attempt to minimise confounding by indication, we selected the short-course OCS as the comparator group. This was only possible because the GOLD recommendations for the duration of OCS treatment changed from 7 to 10 days to 5–7 days in 2014. Thus, we could examine a cohort of patients receiving a long-course or short-course treatment, but the reason for choosing long or short OCS treatment was by recommendation and not by disease severity.
This is the first study with sufficient power that has assessed the impact of the OCS recommendation change on the risk of pneumonia hospitalisation and all-cause mortality in outpatients with AECOPD. By adjusting for several potential confounders, we were able to provide results that may better represent the magnitude of the association between OCS and the risk of pneumonia hospitalisation and all-cause mortality in a large unselected COPD population. Another strength of this study is that the OCS prescriptions issued in Denmark have high levels of completeness and accuracy.16
Some limitations should be considered while interpreting our findings. The primary limitation of this study is its observational design. Therefore, we cannot provide evidence of causation. However, several aspects of our findings support a potential causal association between long course of OCS and risk of pneumonia hospitalisation and all-cause mortality. While the baseline characteristics of the groups were similar, it is impossible to account for other possible confounders that could have affected the results. Second, the OCS data were based on prescriptions and check of prednisolone adherence was not possible. Thus, the true use of the drug is unknown and may result in some bias. Third, physician decisions regarding the choice of long course and short course may have been influenced by initial illness severity, as OCS use itself may be a marker of disease severity. However, time trends and the change in guidelines diminish this concern. The ratio of short to long treatment courses increased progressively after 2014 due to the new GOLD recommendation (online supplementary figure s3). However, already between 2011 and 2013, the number of short courses was increased. A possible explanation could be an increased awareness about the side effects of OCS and limited effect of OCS on long-term outcomes (death and lung function) among physicians prior to guideline change. Finally, our analyses were limited to outpatients and our results should not be generalised to more severe patients. To our knowledge, this is the first time long-term consequences of high doses of OCS have been investigated in a large-scale study with a complete long-term follow-up. Long courses of OCS in patients with COPD seem to have serious long-term adverse effects including a prolonged risk of pneumonia and even an increased risk of death. This supports increasing cautiousness by physicians when administering OCS to these patients and, whenever possible, to choose short courses rather than long courses. Additionally, our findings may prompt the development of personalised medicine strategies like eosinophil-guided OCS therapy.24 25
Future studies should focus on testing our results and investigating the possible causes of the increased all-cause mortality that is associated with long courses of OCS. Additionally, randomised trials should test strategies for safely administering OCS at the lowest possible accumulated dosage to achieve the documented positive short-term effects of OCS in COPD exacerbations without increasing the risk of serious long-term consequences. This could include blood eosinophils at presentation.24 So far, no data are available with regards to the minimal necessary OCS dose in an outpatient treatment setting. The development of several biological inhibitors of inflammatory mediators in COPD for targeting the cytokine/chemokine-mediated inflammation in COPD have provided little or no evidence of therapeutic effectiveness with regards to clinical outcomes in COPD.26
Conclusion
In conclusion, long course of OCS was associated with increased risk of pneumonia hospitalisation and all-cause mortality compared with the short course of OCS. Those with current exposure demonstrated a higher vulnerability to pneumonia and all-cause mortality. Understanding how risk depends on patterns of OCS will contribute to improved benefits and harm assessment. Importantly, causal inference cannot be drawn from these observational data.
Acknowledgments
We thank the Danish Register of Chronic Obstructive Pulmonary Disease, the Danish National Patient Registry and the Danish National Health Service Prescription Database for allowing us to use their data. Thanks to statistician Tobias Wirenfeldt Klausen for reviewing the SAS and R codes.
Footnotes
Contributors: All authors contributed to the conception and design of the work. Statistical analyses were done by PS with help from DBR, RS and J-USJ. All authors contributed to the interpretation of data. PS wrote the first draft of the manuscript with input from J-USJ, JV, RS and TSI. All authors critically revised the manuscript and approved the submitted version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work.
Funding: The study was financed by a grant from The Danish Regions Medical Fund and The Danish Council for Independent Research. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. JV is supported by the National Institute of Health Manchester Biomedical Research Centre.
Disclaimer: The funders had no role in the study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
Competing interests: JV reports personal fees from GlaxoSmithKline, personal fees from Chiesi Pharmaceuticals, personal fees from Boehringer-Ingelheim, personal fees from Novartis, personal fees from AstraZeneca, outside the submitted work. TSI reports personal fees from AstraZeneca, outside the submitted work.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Danish Data Protection Agency (journal no. HGH-2017-091, with I-Suite no. 05884). As this study did not involve contact with patients or an intervention, it was not necessary to obtain permission from the Danish scientific ethical committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786–96. 10.1016/S0140-6736(07)61382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Restrepo MI, Mortensen EM, Pugh JA, et al. . COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J 2006;28:346–51. 10.1183/09031936.06.00131905 [DOI] [PubMed] [Google Scholar]
- 4. Pauwels RA, Buist AS, Calverley PM, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256–76. 10.1164/ajrccm.163.5.2101039 [DOI] [PubMed] [Google Scholar]
- 5. Rabe KF, Hurd S, Anzueto A, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–55. 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 6. Leuppi JD, Schuetz P, Bingisser R, et al. . Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013;309:2223–31. 10.1001/jama.2013.5023 [DOI] [PubMed] [Google Scholar]
- 7. Wedzicha JA, Miravitlles M, Hurst JR, et al. . Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2017;49 10.1183/13993003.00791-2016 [DOI] [PubMed] [Google Scholar]
- 8. Walters JA, Tan DJ, White CJ, et al. . Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McEvoy CE, Niewoehner DE. Corticosteroids in chronic obstructive pulmonary disease. Clinical benefits and risks. Clin Chest Med 2000;21:739–52. [DOI] [PubMed] [Google Scholar]
- 10. Ernst P, Coulombe J, Brassard P, et al. . The risk of sepsis with inhaled and oral corticosteroids in patients with COPD. COPD 2017;14:137–42. 10.1080/15412555.2016.1238450 [DOI] [PubMed] [Google Scholar]
- 11. Bénard-Laribière A, Pariente A, Pambrun E, et al. . Prevalence and prescription patterns of oral glucocorticoids in adults: a retrospective cross-sectional and cohort analysis in France. BMJ Open 2017;7:e015905 10.1136/bmjopen-2017-015905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cutolo M, Seriolo B, Pizzorni C, et al. . Use of glucocorticoids and risk of infections. Autoimmun Rev 2008;8:153–5. 10.1016/j.autrev.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 13. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis 1989;11:954–63. 10.1093/clinids/11.6.954 [DOI] [PubMed] [Google Scholar]
- 14. Lange P, Tøttenborg SS, Sorknæs AD, et al. . Danish Register of chronic obstructive pulmonary disease. Clin Epidemiol 2016;8:673–8. 10.2147/CLEP.S99489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt M, Schmidt SAJ, Sandegaard JL, et al. . The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–90. 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ehrenstein V, Antonsen S, Pedersen L. Existing data sources for clinical epidemiology: Aarhus University Prescription Database. Clin Epidemiol 2010;2:273–9. 10.2147/CLEP.S13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014;29:541–9. 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 18. Brassard P, Bitton A, Suissa A, et al. . Oral corticosteroids and the risk of serious infections in patients with elderly-onset inflammatory bowel diseases. Am J Gastroenterol 2014;109:1795–802. quiz 803 10.1038/ajg.2014.313 [DOI] [PubMed] [Google Scholar]
- 19. Lichtenstein GR, Feagan BG, Cohen RD, et al. . Serious infection and mortality in patients with Crohn's disease: more than 5 years of follow-up in the TREAT™ registry. Am J Gastroenterol 2012;107:1409–22. 10.1038/ajg.2012.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum 2006;54:628–34. 10.1002/art.21568 [DOI] [PubMed] [Google Scholar]
- 21. Waljee AK, Rogers MAM, Lin P, et al. . Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017;357 10.1136/bmj.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groenewegen KH, Schols AMWJ, Wouters EFM. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003;124:459–67. 10.1378/chest.124.2.459 [DOI] [PubMed] [Google Scholar]
- 23. Horita N, Miyazawa N, Morita S, et al. . Evidence suggesting that oral corticosteroids increase mortality in stable chronic obstructive pulmonary disease. Respir Res 2014;15 10.1186/1465-9921-15-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bafadhel M, McKenna S, Terry S, et al. . Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med 2012;186:48–55. 10.1164/rccm.201108-1553OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sivapalan P, Moberg M, Eklöf J, et al. . A multi-center randomized, controlled, open-label trial evaluating the effects of eosinophil-guided corticosteroid-sparing therapy in hospitalised patients with COPD exacerbations—the cortico steroid reduction in COPD (CORTICO-COP) study protocol. BMC Pulm Med 2017;17 10.1186/s12890-017-0458-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rogliani P, Matera MG, Puxeddu E, et al. . Emerging biological therapies for treating chronic obstructive pulmonary disease: a pairwise and network meta-analysis. Pulm Pharmacol Ther 2018;50:28–37. 10.1016/j.pupt.2018.03.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2019-000407supp001.pdf (220.7KB, pdf)