Abstract
Purpose
To investigate ocular and systemic diurnal rhythms in emmetropic and myopic adults and examine relationships with light exposure.
Methods
Adult subjects (n = 42, 22–41 years) underwent measurements every 4 hours for 24 hours, including blood pressure, heart rate, body temperature, intraocular pressure (IOP), ocular biometry, and optical coherence tomography imaging. Mean ocular perfusion pressure (MOPP) was calculated. Saliva was collected for melatonin and cortisol analysis. Acrophase and amplitude for each parameter were compared between refractive error groups. Subjects wore a light, sleep, and activity monitor for 1 week before measurements.
Results
All parameters exhibited significant diurnal rhythm (ANOVA, P < 0.05 for all). Choroidal thickness peaked at 2.42 hours, with a diurnal variation of 25.8 ± 13.44 μm. Axial length peaked at 12.96 hours, with a variation of 35.71 ± 6.6 μm. Melatonin peaked at 3.19 hours during the dark period, while cortisol peaked after light onset at 8.86 hours. IOP peaked at 11.24 hours, with a variation of 4.92 ± 1.57 mm Hg, in antiphase with MOPP, which peaked at 22.02 hours. Amplitudes of daily variations were not correlated with light exposure, and rhythms were not significantly different between emmetropes and myopes, except for body temperature and MOPP.
Conclusions
Diurnal variations in ocular and systemic parameters were observed in young adults; however, these variations were not associated with habitual light exposure. Emmetropic and myopic refractive error groups showed small but significant differences in body temperature and MOPP, while other ocular and systemic patterns were similar.
Keywords: circadian rhythm, choroid, intraocular pressure, myopia, melatonin
Diurnal rhythms are variations in physiological parameters that are synchronized to the solar day. Diurnal rhythms in the eye have been observed in chickens,1 rodents,2 nonhuman primates,3 and humans,4,5 and include variations in axial length,4 intraocular pressure (IOP),6 and choroidal thickness.4,7 In both animals and humans, eyes exposed to regular light/dark patterns undergo a decrease in axial length and increase in choroidal thickness at night, with the highest daily IOP exhibited in the morning.4,8,9 Several other physiological parameters in the body also show diurnal variations, including systemic melatonin secretion,10–12 core body temperature,13 and heart rate.14 These rhythms are mediated by light exposure and endogenous clock signals, which influence the amplitude and phase in the underlying circadian oscillator.15,16
The intrinsically photosensitive retinal ganglion cells (ipRGCs) are inner retinal cells involved in light-mediated circadian entrainment.17,18 The ipRGCs are known to influence systemic melatonin concentration in a pathway that leads from the retinohypothalamic tract to the suprachiasmatic nucleus, and, ultimately, to the pineal gland.17,19,20 A previous study21 has shown that light exposure is associated with the ipRGC-driven pupil response. Additionally, known relationships exist between light exposure and diurnal rhythms of melatonin synthesis,22 and these rhythms might differ between myopic and nonmyopic individuals. Kearney et al.23 have shown that young adult myopes have significantly higher morning serum melatonin concentration than nonmyopes.
Findings in animal models suggest that light exposure patterns affect eye development and refraction. When chicks are reared in constant light or less than 4 hours of darkness per day, altered refractive errors are observed.24–27 Light exposure patterns might also differ between myopic and nonmyopic young adults. For example, a study that investigated young adult third-year law students reports an association between myopia and less daily exposure to darkness, leading investigators to suggest that perturbations in the daily light/dark cycle may also lead to refractive errors in humans.28
Accumulating evidence suggests that light exposure may influence refractive development in children. A meta-analysis29 has concluded that increased outdoor time is effective in preventing the onset of myopia. Recent longitudinal interventional studies in Eastern Asia have shown that children with increased time outdoors have reduced incidence rate of myopia30 and decreased axial growth.31,32 The underlying mechanism of protective effects of time outdoors has yet to be elucidated. Potential contributors include changes in optical factors that occur outdoors compared to indoors, such as a smaller pupil and flatter dioptric space,33 or changes in biochemical pathways, such as an increase in retinal dopamine with increased light exposure, as demonstrated in animal eyes undergoing experimental myopia.34,35 Speculation exists whether the protective effects of bright light exposure in myopia may be associated with light-induced alterations in diurnal rhythms.
Evidence suggests that diurnal variations in IOP differ with axial length36 and refraction.37 In young adults, diurnal IOP fluctuation has been shown to negatively correlate to axial length, with shorter eyes demonstrating larger IOP variation over a 24-hour period.36 Another study in adults37 reports a significant difference in the timing and amplitude of diurnal IOP variations between emmetropes and moderate to severe myopes. However, studies assessing the relationship of IOP and refraction have been conflicting, with some studies finding that myopes have higher daytime IOP than nonmyopes,38,39 and others finding no difference in IOP between refractive error groups.40
This study addressed diurnal changes in young adults with and without myopia. Studying diurnal patterns in this age group is valuable for a number of reasons. Firstly, myopia carries with it a significant disease burden, including increased risks of retinal detachment41 and glaucoma.42 Understanding the diurnal changes in these parameters and their interactions, especially IOP and ocular perfusion, may improve understanding of the disease risks. Secondly, such study may provide understanding of myopia itself. Differences in diurnal changes in perfusion, IOP, light exposure profiles, and associated hormone levels may be present in this age group, as suggested in previous studies,23,37 either as a cause or consequence of myopia. Therefore, in light of reports that systemic melatonin concentration and IOP diurnal rhythms vary between myopic and nonmyopic adults, and evidence that outdoor time influences refractive development in children, we hypothesized that objectively measured light exposure is associated with ocular and systemic diurnal rhythms in young adults, and these rhythms may vary with refractive status. We measured diurnal changes in ocular and systemic parameters over a 24-hour period in emmetropic and myopic adults. Analysis of ocular biometric rhythms has been previously reported for these subjects.9 Here, we present analysis of additional ocular parameters, including IOP and mean ocular perfusion pressure (MOPP), in relation to systemic physiological rhythms and objectively measured light exposure.
Methods
Healthy subjects, ages 22 to 41 years, participated (n = 42). Subjects provided informed consent after the purpose of the study and the risks were explained. The study was approved by the Committee for Protection of Human Subjects at the University of Houston and followed the tenets of the Declaration of Helsinki.
Subjects underwent a screening to determine ocular and systemic health. All subjects had best-corrected visual acuity of 20/20 or better. Exclusion criteria included ocular disease, the use of melatonin or other pharmacologic sleep aids, and travel outside of two time zones in the month before the experiment. Noncycloplegic autorefraction (WAM-5500; Grand Seiko, AIT Industries, Bensenville, IL, USA) was performed, and subjects were classified as emmetropic (spherical equivalent refraction [SER] of +1.50 to >−0.75) or myopic (SER ≤−0.75).
Following screening and enrollment, an Actiwatch Spectrum (Philips, Respironics, Bend, OR, USA) was dispensed for 1 week of continuous wear for objective measurements of each subject's habitual light exposure, sleep, and physical activity. The light sensor in the Actiwatch measures the illuminance of broadband light in lux (range, 0.1–200,000 lux), and the irradiance of the blue (400–500 nm), green (500–600 nm), and red (600–700 nm) components (μW/cm2). Actiwatch data were analyzed by using the device software, Philips Actiware 6.0.8.43 Activity and sleep data were evaluated to ensure that subjects had normal sleep/wake patterns. Time outdoors per day was calculated as minutes spent exposed to greater than 1000 lux.43,44 Average daily white light exposure and time outdoors for the week before the lab visit were calculated.
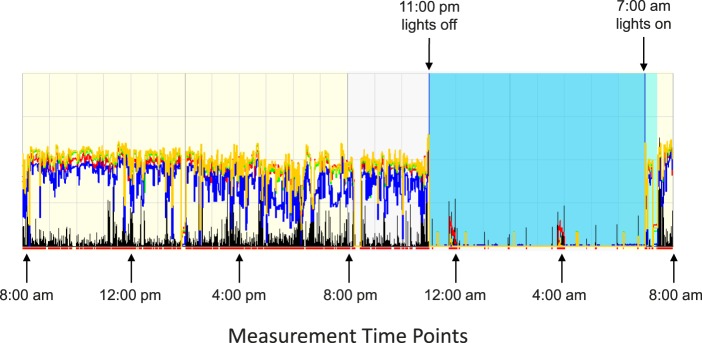
After 1 week, subjects presented to the lab at 8:00 AM for the experimental measurements, which were collected every 4 hours for 24 hours beginning at 8:00 AM, as described previously.9 Subjects were asked to refrain from caffeine, alcohol, and vigorous physical activity during the experiment, as these have been shown to affect choroidal thickness, IOP, heart rate, and body temperature.45–47 Measurements took 15 to 20 minutes at each time point. Subjects went about their activities during the day, and remained in the lab with all lights off from 11:00 PM to 7:00 AM, during which time they were encouraged to sleep.37 For the two time points during the night (12:00 AM and 4:00 AM), a dim red light was used for illumination, and the brightness of instrument monitors was decreased to minimize disruptions to circadian rhythms. A representative actigraph trace is shown for one subject during the 24-hour experimental period in which the subject slept in the lab to illustrate the protocol (Fig. 1).
Figure 1.
Actigraph trace for a representative subject for the 24-hour experimental period in lab. Measurements took place every 4 hours beginning at 8:00 AM; black trace represents physical activity, yellow trace represents broadband light exposure, blue, red, and green traces represent the spectral components of light exposure, and the blue region represents lights off (11:00 PM–7:00 AM).
At each time point except during the lights off period, subjects first rinsed their mouth with water in preparation for saliva collection, and then underwent a distance-viewing period for 10 minutes while sitting upright in a chair to minimize the influence of previous activity, relax accommodation, and standardize the conditions under which ocular imaging was performed.48 During the distance-viewing period, subjects watched a television at 4 meters, binocularly and with habitual distance correction, when necessary.
All measurements were collected in a sitting position. Subjects were asked to collect 1 mL saliva into a vial, which was immediately placed in a −20°C freezer for analysis using melatonin and cortisol ELISA kits (Salimetrics, LLC, State College, PA, USA), as previously described.22 Samples were run in duplicate within 1 month of collection (stable at −20°C for up to 6 months per Salimetrics). Body temperature was measured three times at each time point by using an under-the-tongue digital thermometer with disposable probe cover (Welch Allyn, Skaneateles Falls, NY, USA). Blood pressure and heart rate were measured three times by using an electronic cuff (Omron, Bannockburn, IL, USA).
Ocular measurements were performed on the right eye only and included IOP, retinal imaging, and biometry. IOP was measured by using a rebound tonometer (iCare, Tiolat Oy, Helsinki, Finland), and three measurements, each an average of six readings, were recorded. From the diastolic (DPB) and systolic (SBP) blood pressure and IOP, MOPP was calculated for each time point, using Equation 1:
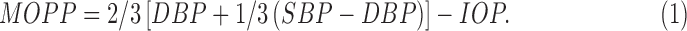 |
Ocular imaging was performed with spectral-domain optical coherence tomography (SD-OCT; Spectralis, Heidelberg, Germany) using enhanced depth-imaging mode. At each time point, two high-quality images of the back of the eye were captured. The scan protocol included a six-line 30° radial scan centered at the fovea, with 16 frame B-scan averaging. The first image at the first time point (8:00 AM) was set as the reference for each subject, and the instrument's tracking function was used for subsequent imaging. Lastly, ocular biometry (LenStar; Haag-Streit, Köniz, Switzerland) was measured. Five measurements were recorded and averaged at each time point.
Analysis
Raw OCT data (*.vol files) were exported and analyzed with custom-written software in MatLab (MathWorks, Inc., Natick, MA, USA) using a semiautomated process. Data were adjusted for lateral magnification by constructing a three-surface schematic eye, as described by Bennett and Rabbetts49 and Bennett et al.,50 for each subject by using their measured axial length and corneal curvature. Individualized transverse scaling was then calculated, assuming a spherical retina as previously described.51 Bruch's membrane, the inner limiting membrane, and the retinal layers, including inner and outer photoreceptors, were automatically segmented and manually corrected when necessary. Image contrast was optimized, and the sclera/choroid border was segmented manually. Axial choroidal thickness was calculated as the distance from Bruch's membrane to the posterior choroid/sclera border for 1536 points along each of the six scan lines. Data were binned for the central 1-mm diameter and averaged for the two radial scans centered on the fovea of the right eye, which were collected at each time point. Repeatability and retinal and choroidal thicknesses in the 3-mm and 6-mm annuli by quadrant are presented in detail elsewhere.9
Statistical analyses were performed by using MedCalc Software (12.3.0; MedCalc, Mariakerke, Belgium) and Excel (Microsoft Office 2013; Microsoft, Redmond, WA, USA). On the basis of data from our previous investigations, with a between-groups difference of 59 μm, a within-subjects standard deviation of 75 μm, and a cosine change in choroidal thickness across the day of 9.6-μm amplitude, 15 subjects per group would provide power to measure a time-of-day effect at alpha = 0.05. Data are expressed as mean ± standard error unless otherwise noted. Normality was assessed with the Shapiro-Wilk test. A critical value <0.05 was considered statistically significant. For each parameter for each subject, the mean of the values from all time points was calculated, and the amplitude of diurnal variation was determined as the difference between the maximum (peak) and minimum (trough) values across 24 hours. To investigate whether significant diurnal changes occurred over a 24-hour period, a repeated measures analysis of variance (ANOVA) with one within-subject factor (time of day) and one between-subjects factor (refractive error group) was used. The ANOVA was used to detect whether average measurements changed across the day for different times and between groups, and is a sensitive test if amplitudes and phase do not show large within-group variation. However, ANOVAs do not represent the most appropriate test for assessing amplitude and phase independently. For example, groups might have different amplitudes of change across the day; however, if there is a large variation in phase within groups (i.e., variation in peak phase between subjects) then the ANOVA would not yield significant results. Likewise, there might be differences in acrophase (the time of maximum response) between groups, but because of variation between participants' amplitude, the ANOVA may not detect between-group differences in acrophase. Therefore, additional analysis was undertaken to assess whether amplitude (ignoring phase) differed between groups, and to see whether acrophase (ignoring amplitude) differed between groups. To estimate the amplitude and acrophase of diurnal rhythm for each parameter for each subject, Fourier analysis was used to determine the fundamental cosine, using Equation 2.52
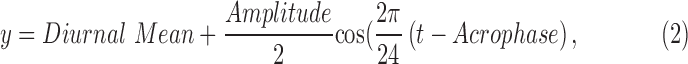 |
where t is time of measurement (on a 24-hour clock), Acrophase is the time where the fitted cosine reaches its peak, and Amplitude is the difference between maximum and minimum y values in the fitted cosine.
Mann-Whitney U tests were used to analyze refractive error group differences in the amplitude of diurnal variation. Group average peak acrophase was estimated as the vector mean of acrophases distributed around the unit circle. Rayleigh tests were used to investigate whether the acrophase distribution was significantly different from a uniform distribution across the day.53 As part of the Rayleigh test calculation, r2, an estimate of the dispersion of acrophase across the day, was estimated. An r2 = 0 indicates that acrophase is distributed evenly across the day. Higher values of r2 indicate clustering of acrophase at a given time, with r2 = 1 occurring when all acrophases occur at the same time. Watson's U2 test was used to assess whether there was a significant diurnal difference between myopes and emmetropes in terms of acrophases.53 For parameters that showed a refractive error difference, data were fit with cosine functions to compare rhythms.54 Pearson correlation was used to investigate relationships between the amplitude of diurnal variation for each parameter and mean daily light exposure.
Results
Subject demographics and ocular characteristics are shown in Table 1.9 Mean subject age was 27.2 ± 4.2 years, with 14 males and 28 females. The SER of all right eyes was −2.55 ± 3.13 diopters (D) and of left eyes was −2.52 ± 3.14 D. Right and left eyes were not significantly different (P = 0.81); only right eyes are considered further. The emmetropic group consisted of 17 subjects (>−0.75 D) and the myopic group consisted of 25 subjects (≤−0.75 D).
Table 1.
Subject Demographics, Mean ± Standard Deviation
|
Total |
Emmetropes |
Myopes |
|
| Subjects (M/F) | 42 (14/28) | 17 (10/7) | 25 (4/21) |
| Age, y | 27.18 ± 4.17 (22.37 to 41.42) | 26.35 ± 2.73 (23.02 to 32.63) | 27.79 ± 4.88 (22.37 to 41.42) |
| SER, D | −2.55 ± 3.13 (+1.32 to −11.18) | +0.18 ± 0.55 (+1.32 to −0.69) | −4.41 ± 2.75 (−1.31 to −11.18) |
| Axial length, mm | 24.66 ± 1.50 (21.69 to 29.59) | 23.77 ± 1.05 (21.69 to 25.5) | 25.26 ± 1.48 (23.17 to 29.59) |
During the week before the lab visit, all subjects demonstrated normal habitual sleep/wake patterns. Specifically, subjects had one sleep period per 24-hour period, during the night hours, and consistent wake and sleep times. Objectively measured time spent outdoors per day was similar between emmetropes (103.2 ± 12.9 minutes) and myopes (82.6 ± 8.3 minutes, P = 0.17; Table 2). Daily average white light exposure during the day was similar between emmetropes (1152.3 ± 164.7 lux) and myopes (1115.61 ± 150.5 lux, P = 0.87), and white light exposure during the night was similar for emmetropes (1.34 ± 0.41 lux) and myopes (1.28 ± 0.2 lux, P = 0.89). Light exposure during the night was calculated as average lux during the time the Actiwatch detected that the subject was sleeping. Mean sleep duration per night was similar between emmetropes (438.4 ± 13.31 minutes) and myopes (444.64 ± 9.87 minutes, P = 0.70), and sleep efficiency was similar for emmetropes (90.03% ± 0.9%) and for myopes (89.13% ± 0.69%, P = 0.42). Actigraph data also showed that physical activity was similar for emmetropes (268.67 ± 16.46 counts per minute) and myopes (262.08 ± 11.33 counts per minute, P = 0.73).
Table 2.
Objectively Measured Mean (± Standard Error) Daily Time Outdoors, Daily Light Exposure, Nightly Light Exposure, Sleep Duration, and Physical Activity for the Emmetropic and Myopic Groups
|
Total |
Emmetropes |
Myopes |
P
Value |
|
| Time outdoors, min | 92.2 ± 7.33 | 103.2 ± 12.91 | 82.59 ± 8.29 | 0.17 |
| Daily white light exposure, lux | 1130.82 ± 110.15 | 1152.28 ± 164.74 | 1115.61 ± 150.51 | 0.87 |
| Nightly white light exposure, lux | 1.31 ± 0.2 | 1.34 ± 0.41 | 1.28 ± 0.2 | 0.89 |
| Sleep duration, min | 442.1 ± 7.9 | 438.44 ± 13.31 | 444.65 ± 9.87 | 0.70 |
| Sleep efficiency, % | 89.5 ± 0.54 | 90.03 ± 0.9 | 89.13 ± 0.2 | 0.42 |
| Physical activity, counts/min | 264.81 ± 9.4 | 268.67 ± 16.46 | 262.08 ± 11.33 | 0.73 |
value for unpaired t-test.
Ocular and Systemic Diurnal Rhythms
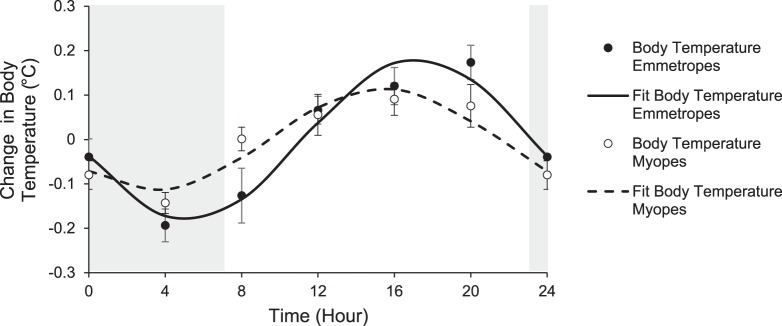
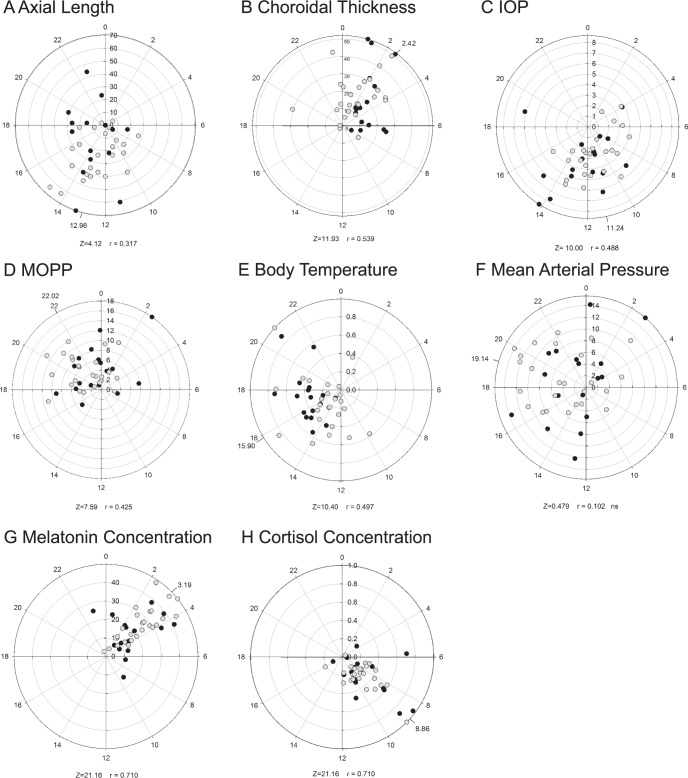
Daily mean (± standard error) for each parameter for all subjects and by refractive error group is shown in Table 3. Repeated measures ANOVA showed that all measured parameters, including axial length, choroidal thickness, IOP, body temperature, heart rate, systolic and diastolic blood pressure, mean arterial pressure, MOPP, cortisol, and melatonin, underwent significant diurnal variation (P < 0.05 for all, within-subject factor time of day; Table 4). No parameter exhibited significant refractive error effects (between-subject factor), except body temperature (P < 0.001), and no parameter exhibited significant time by refractive error effects. Cosine fits for mean 24-hour change in body temperature by refractive error group are shown in Figure 2. Further analysis using the Watson's U2 test showed a significant difference in MOPP acrophase between emmetropes and myopes. The Watson's U2 test ignores amplitude and uses nonparametric methods to assess whether the acrophase differs between the two groups. In this case, the significant result arises because the MOPP acrophases for myopes are more narrowly clustered than for emmetropes. This effect was not (and is very unlikely to have been) detected by the ANOVA. Rayleigh tests showed that subjects' acrophases were not significantly clustered at a given time of day, for heart rate, systolic and diastolic blood pressure, and mean arterial pressure, that is, acrophases for individual participants were scattered across the day. Polar plots for each parameter are shown in Figure 3.
Table 3.
Daily Mean (± Standard Error) for Each Parameter for All Subjects, for Emmetropes, and for Myopes
|
Parameter |
All |
Emmetropes |
Myopes |
P
Value |
| Axial length, mm | 24.66 ± 0.23 | 23.77 ± 0.25 | 25.26 ± 0.30 | <0.001* |
| Choroidal thickness, central 1 mm, μm | 334.62 ± 12.28 | 368.33 ± 17.72 | 305.93 ± 14.3 | 0.009* |
| Intraocular pressure, mm Hg | 14.15 ± 0.42 | 14.20 ± 0.61 | 14.12 ± 0.58 | 0.71 |
| Body temperature, °C | 36.87 ± 0.04 | 36.86 ± 0.07 | 36.88 ± 0.05 | 0.95 |
| Heart rate, bpm | 72.63 ± 1.58 | 71.52 ± 2.54 | 73.39 ± 2.03 | 0.80 |
| Mean arterial pressure, mm Hg | 83.43 ± 0.88 | 83.46 ± 1.29 | 83.42 ± 1.20 | 0.95 |
| Mean ocular perfusion pressure, mm Hg | 41.47 ± 0.73 | 41.44 ± 0.95 | 41.49 ± 0.98 | 0.95 |
| Melatonin, pg/mL | 11.72 ± 0.78 | 10.64 ± 1.22 | 12.41 ± 1.0 | 0.25 |
| Cortisol, dg/mL | 0.23 ± 0.01 | 0.25 ± 0.02 | 0.21 ± 0.01 | 0.18 |
values from Mann-Whitney U test.
Significance at P < 0.05.
Table 4.
Amplitude of Diurnal Variation (Mean ± Standard Error) and Acrophase for Each Measured Parameter for All Subjects (n = 42)
|
Parameter |
Amplitude |
Acrophase, Peak, h |
Time of Day |
Refractive Error |
Time by Refractive Error |
| Axial length, μm | 35.71 ± 2.99 | 12.96 | <0.001* | 1.00 | 0.501 |
| Choroidal thickness, central 1 mm, μm | 25.80 ± 2.08 | 2.42 | <0.001* | 0.32 | 0.37 |
| Intraocular pressure, mm Hg | 4.92 ± 0.25 | 11.42 | <0.001* | 0.96 | 0.42 |
| Body temperature, °C | 0.52 ± 0.04 | 15.9 | <0.001* | <0.001* | 0.16 |
| Heart rate, bpm | 14.71 ± 0.77 | 21.66 | 0.034* | 0.11 | 0.73 |
| Systolic blood pressure, mm Hg | 16.38 ± 0.88 | 17.97 | <0.001* | 0.14 | 0.71 |
| Diastolic blood pressure, mm Hg | 11.43 ± 0.7 | 20.85 | 0.015* | 0.94 | 0.70 |
| Mean arterial pressure, mm Hg | 11.89 ± 0.67 | 19.14 | <0.001* | 0.36 | 0.88 |
| Mean ocular perfusion pressure, mm Hg | 9.70 ± 0.49 | 22.02 | <0.001* | 0.88 | 0.55 |
| Melatonin, pg/mL | 26.95 ± 1.88 | 3.19 | <0.001* | 0.69 | 0.19 |
| Cortisol, dg/mL | 0.50 ± 0.05 | 8.86 | <0.001* | 0.16 | 0.87 |
values are from two-factor repeated measures ANOVA for time of day, refractive error, and time of day by refractive error.
Significance at P < 0.05.
Figure 2.
Mean (± standard error) 24-hour change in body temperature (°C) for emmetropes (filled symbols) and myopes (open symbols), lines are cosine fits to the data, gray area represents the dark period.
Figure 3.
Diurnal amplitudes and acrophase (hours) calculated by Fourier analysis (Equation 2). (A) Axial length (μm), (B) choroidal thickness (μm), (C) IOP (mm Hg), (D) MOPP (mm Hg), (E) body temperature (°C), (F) mean arterial pressure (mm Hg), (G) melatonin concentration (pg/mL), (H) cortisol concentration (dg/mL). Median acrophases are indicated, calculated by averaging unit vectors. Emmetropes, filled circles; myopes, unfilled circles.
Mean axial length was significantly longer for myopes (25.26 ± 0.30 mm) than for emmetropes (23.77 ± 0.25 mm, P < 0.001), and the choroid was significantly thinner for myopes (305.93 ± 14.3 μm) than for emmetropes (368.33 ± 17.72 μm, P = 0.009). Axial length acrophase was at 12.96 hours, with a mean diurnal variation of 35.71 ± 2.99 μm. Choroidal thickness acrophase was at 2.42 hours, with a mean diurnal variation of 25.80 ± 13.44 μm. Mean IOP was similar for myopes (14.12 ± 0.58 mm Hg) and emmetropes (14.20 ± 0.61 mm Hg, P = 0.71). IOP acrophase was at 11.24 hours, with a mean diurnal variation of 4.92 ± 1.57 mm Hg. Choroidal thickness variations were in approximate antiphase with axial length and IOP variations. Regression analysis showed that diurnal changes in IOP were significantly associated with diurnal changes in axial length (F1,244 = 30.45, R2 = 0.11, P < 0.0001; Fig. 4).
Figure 4.
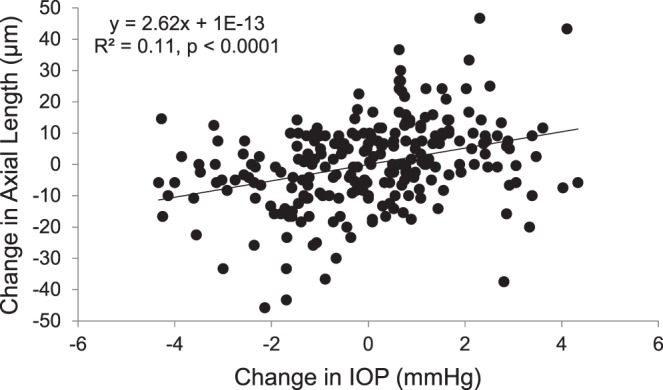
Change in axial length (μm) with change in IOP for all subjects at each time point over a 24-hour period; solid line represents the linear regression, P < 0.0001.
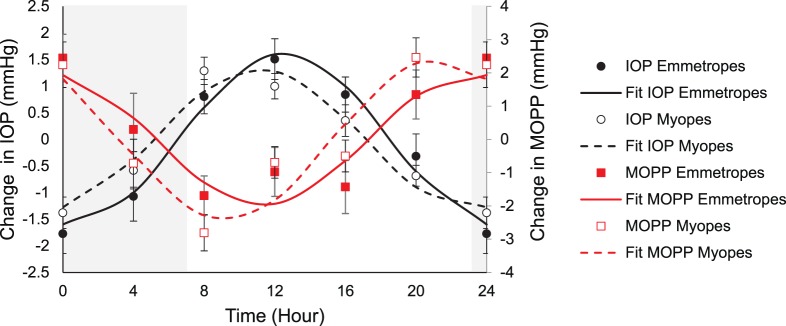
MOPP was calculated from systolic and diastolic blood pressure and IOP. Mean MOPP for all subjects was 41.47 ± 0.69 mm Hg, which underwent a diurnal amplitude change of 9.7 ± 0.49 mm Hg (P < 0.001) with a mean acrophase at 22.02 hours. Standard ANOVA analysis showed that there were no significant differences in mean daily MOPP between emmetropes and myopes (P = 0.95). However, further analysis showed that the acrophase for MOPP was slightly but significantly different for emmetropes (22.6 hours) versus myopes (21.44 hours, Watson's U2 25,17 = 0.216, P < 0.05). MOPP and IOP were in approximate antiphase with each other. Cosine fits for IOP and MOPP by refractive error group are shown in Figure 5. This antiphase relationship is expected, as based on Equation 1. MOPP is not an independent variable, but dependent on both IOP and blood pressure. This finding shows that IOP variations are likely to be the major determinant of diurnal changes in MOPP.
Figure 5.
Mean (± standard error) change in IOP (mm Hg; emmetropes, filled circles; myopes, open circles) and MOPP (mm Hg; emmetropes, filled squares; myopes, open squares) across 24 hours; lines are cosine fits to the data, gray area represents the dark period.
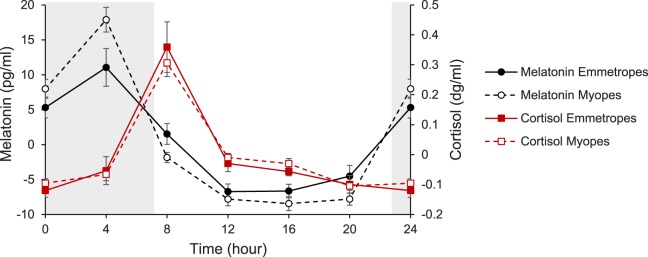
Melatonin concentration exhibited a sharp rise between the 8:00 PM and midnight measurements, following lights off at 11:00 PM, with an acrophase at 3.19 hours (Fig. 6). On the other hand, cortisol concentration exhibited a sharp rise between the 4:00 AM and the 8:00 AM measurements, following lights on at 7:00 AM, with an acrophase at 8.86 hours. There were no differences between emmetropes and myopes for mean daily melatonin (P = 0.25) or cortisol (P = 0.18) concentrations, or in diurnal variation for melatonin (P = 0.69) or cortisol (P = 0.16).
Figure 6.
Mean (± standard error) change in melatonin concentration (emmetropes, filled circles; myopes, open circles) and cortisol concentration (emmetropes, filled squares; myopes, open squares) across 24 hours; gray area represents the dark period.
Pearson correlation analysis showed that mean daily light exposure was not significantly correlated to the amplitude of diurnal change for axial length (R2 = 0.02, P = 0.92), choroidal thickness (R2 = 0.06, P = 0.69), IOP (R2 = −0.04, P = 0.82), MOPP (R2 = −0.19, P = 0.24), body temperature (R2 = 0.25, P = 0.12), melatonin concentration (R2 = −0.11, P = 0.48), or cortisol concentration (R2 = −0.14, P = 0.38).
Discussion
Ocular factors, including the choroid, IOP, and perfusion pressure, and systemic factors, including sleep and stress hormones and body temperature, have potential roles in refractive development, and valuable information can be gained by investigating how these parameters fluctuate across the day in refractive groups. This study examined diurnal rhythms of ocular and systemic processes, and extended previous work to investigate potential association with light exposure and refractive status in young adults. Our results showed that there were significant diurnal variations in ocular parameters, including axial length, choroidal thickness, IOP, and MOPP, as well as systemic parameters, including blood pressure and heart rate, body temperature, and melatonin and cortisol levels, consistent with previous reports.4,55 Furthermore, we showed small but significant differences in diurnal rhythms of body temperature and MOPP between emmetropic and myopic groups.
For all parameters investigated, the amplitudes of diurnal variations were not associated with light exposure during the previous week. We made use of an ambulatory, noninvasive device, the Actiwatch, for measures of habitual light exposure and sleep patterns before the experimental visit. We found that time per day exposed to outdoor light (>1000 lux) in our young adult emmetropic group was not significantly different from that of the myopic group (103 and 82 minutes, respectively). This is similar to findings in previous reports using objective methods to measure light exposure in young adult populations, which range from 74 to 112 minutes per day of exposure to outdoor light levels.43,56 These latter young adult populations were also drawn from university settings.
In accordance with previous reports,4 diurnal variations in IOP and axial length were in phase with each other and demonstrated a weak but significant correlation (R2 = 0.11); as IOP increased, axial length increased. It has been proposed that increased IOP may induce axial length increases in myopia. Evidence from studies in chicks suggests that an increase in IOP can lead to biomechanical changes in the sclera that result in an increase in axial length.57 Similarly, studies in humans who have undergone glaucoma filtration surgery to lower IOP have found decreases in axial length.58,59 However, increasing IOP does not result in an increase in axial length of the tree shrew,57 whose sclera is structurally more similar to that of humans than of the chick. Additionally, it is possible to dissociate IOP and axial length rhythms in chicks.60 These latter findings suggest that increases in axial length are not a result of biomechanical changes due to increases in IOP.
Previous studies assessing diurnal variations in IOP with refractive error show conflicting results, with some demonstrating that patterns in IOP rhythms are different between emmetropic and myopic subjects,36,37 and others showing no difference.4 Liu et al.37 have collected 24-hour data of IOP, axial length, blood pressure, and heart rate in subjects with either emmetropia or moderate to severe myopia. The authors have found significant differences in the magnitude of nighttime increases in IOP with refractive error, as well as timing for supine IOP measurements. The mechanism behind the refractive error group differences is unclear, although the authors speculate that choroidal vascular volume and episcleral pressure may play a role. Additionally, studies have shown that myopic scleras are biomechanically weaker than emmetropic scleras, and more susceptible to axial elongation with increased short-term IOP elevation.61 Here, we found that diurnal patterns in IOP were not significantly different between emmetropes and myopes, similar to a report by Chakraborty et al.4 While qualitative differences can be observed in the cosine fits for each group (Fig. 5), with the myopic group tending to have a smaller amplitude in diurnal variation, similar to results reported by Liu et al.,37 differences did not reach statistical significance. Both of the previous studies were carried out in a young adult population of emmetropes and myopes, similar to the current study. Differing results between studies could be due to differences in how IOP was measured; our measurements were recorded in a seated position, while Liu et al.37 have found differences between groups when IOP is recorded in a supine position.
Myopia has been shown to carry an increased risk of primary open angle glaucoma.42 IOP and MOPP are well known to be associated with glaucoma. Ocular perfusion pressure is the pressure available to drive blood through the intraocular vasculature.62 Larger 24-hour fluctuations in MOPP have been shown to be a risk factor for glaucoma severity in normal tension glaucoma.63 Here, Rayleigh test for acrophase detected a small but significant difference in the acrophase of MOPP between emmetropes and myopes, while amplitude was not significantly different between groups. It is uncertain if this observed phase shift is a clinically meaningful result, and whether it has implications in ocular perfusion.
With known relationships between light exposure and melatonin release, and increasing evidence that light exposure is protective for myopia, speculation exists whether melatonin may play a role in refractive error development. Melatonin is a hormone that is released in darkness from the pineal gland.12 In the retina, melatonin has a reciprocal relationship with dopamine,64 a neurotransmitter linked to regulate eye growth, as demonstrated in animal models.65 Systemic melatonin production is regulated by the suprachiasmatic nucleus and plays a significant role in regulating sleep/wake patterns. Recent studies66,67 have suggested that sleep patterns may vary between nonmyopic and myopic individuals, with myopes reporting poor sleep quality compared to nonmyopes. Ayaki et al.68 have found decreased sleep quality in myopic children, with no differences in sleep between adult myopes and nonmyopes. Here, we found that diurnal variations in salivary melatonin concentration were similar between emmetropic and myopic subjects. Our subject population was from a group of young adults, drawn from students, faculty, and staff at the university. Subjects demonstrated similar sleep/wake cycles and light exposure patterns, as measured objectively with a wrist-worn sensor during the week previous to diurnal measurements. We speculate that evaluating subjects with a wider range of light exposure patterns may reveal associations between light exposure and diurnal rhythms.
Cortisol is a hormone that is associated with waking, alertness, and stress,69 and is known to exhibit a diurnal rhythm that peaks in the early morning.70 While melatonin synthesis is known to be light dependent, cortisol has been shown to be regulated internally and remain phase locked to melatonin rhythms.71 Therefore, cortisol is considered to be a reliable marker of circadian rhythm. Similar to findings for melatonin concentration, the diurnal pattern in cortisol concentration observed here was not significantly different between refractive error groups, with a peak cortisol concentration at 8.86 hours, shortly after the dark period ended.
Body temperature is a well-described, simple, and noninvasive marker of a systemic diurnal rhythm.72 Physical activity is known to elevate body temperature and modify an individual's capacity for thermoregulation, an effect that can persist for hours after activity has ceased.73 For these reasons, it was of interest to assess body temperature in this study. We found that diurnal variations in body temperature were significantly different for myopic and emmetropic subjects (two-factor repeated measures ANOVA), with myopic subjects exhibiting less diurnal variation than emmetropes. Body temperature is known to undergo diurnal variations with the lowest temperature in the early morning and the highest temperature in the late evening. Many endogenous and exogenous factors contribute to body temperature, including hormones, fitness level, age, diet, and lifestyle.74 Emmetropic and myopic groups in this study did not show a difference in objectively measured physical activity levels. It is unclear why a difference was observed between refractive error groups. Future investigations may be able to provide insight into this unexpected finding.
Studies1,8,75 have shown that when changes in ocular growth patterns are induced in animal models, disruptions in diurnal patterns are observed. For example, in chick eyes undergoing decreased growth through induced myopic defocus, the rhythm in axial length phase-delays, while the rhythm in choroidal thickness phase-advances, bringing the two into phase with each other (Nickla DL, et al. IOVS 1996;37:ARVO Abstract 3141). In eyes that are developing myopia through the application of negative powered lenses, axial length phase-advances, bringing choroidal thickness and axial length patterns in exact antiphase (12 hours apart). These findings suggest that the timing of diurnal rhythms with respect to each other is important in the visual regulation of eye growth. However, our results showed that the amplitude and timing of axial length and choroidal thickness variations were not significantly different between emmetropic and myopic adults. It is likely that we failed to detect a difference between refractive error groups because the subjects in our myopic group were stable myopes, whereas in chick studies, the animals were undergoing active eye growth and myopia development when altered rhythms were observed. To address this difference, studies similar to this one, carried out in children or young adults who are undergoing myopia development and progression, will be important in clarifying the relationship of axial length and choroidal thickness rhythms in eye growth.
The study design used here presented some limitations. While all subjects demonstrated regular sleep/wake patterns and similar light exposure as recorded by the Actiwatch, it is possible that behaviors were influenced by virtue of being observed; a longer observation period or administration of behavioral questionnaires may have revealed variations in behaviors that might influence diurnal rhythms. During the experimental measures in lab, it was necessary to wake subjects twice during the night and use dim illumination for measurements. A previous study76 has shown that brief periods of moderate illumination during the night do not interrupt diurnal variations in IOP; it is unlikely that the dim red illumination used here altered diurnal rhythms. Additionally, all measurements were recorded with the subjects in a seated position, as opposed to a more natural prone or supine position during the night. However, a study77 has shown that IOP diurnal variations are observable for both sitting and supine positions, with no significant differences in the amplitude based on position. Another study78 has shown that choroid volume does not differ in subjects between recumbent and sitting positions. It is unlikely that a change in position would have altered observed diurnal rhythms. Finally, subjects' refraction was only measured on one occasion, so we were unable to assess whether myopic subjects were progressing or stable myopes. However, subjects were likely to be stable myopes, given their age range of 22 to 41 years, which is outside the range over which juvenile-onset myopia typically progresses.79
In summary, we described diurnal variations in ocular and systemic parameters over a 24-hour period. Emmetropic and myopic refractive error groups showed small but significant differences in body temperature and MOPP rhythms, while other ocular and systemic patterns were similar. Previous light exposure was not associated with the amplitude of diurnal variation in axial length, choroidal thickness, MOPP, body temperature, melatonin concentration, or cortisol concentration in this group of young adults.
Acknowledgments
The authors thank Krista Beach for help with analysis and Nimesh Patel for OCT image analysis software.
Supported by National Institutes of Health T35EY007088 (Bethesda, MD, USA).
Disclosure: H.J. Burfield, None; A. Carkeet, None; L.A. Ostrin, None
References
- 1.Papastergiou GI, Schmid GF, Riva CE, Mendel MJ, Stone RA, Laties AM. Ocular axial length and choroidal thickness in newly hatched chicks and one-year-old chickens fluctuate in a diurnal pattern that is influenced by visual experience and intraocular pressure changes. Exp Eye Res. 1998;66:195–205. doi: 10.1006/exer.1997.0421. [DOI] [PubMed] [Google Scholar]
- 2.Lozano DC, Hartwick AT, Twa MD. Circadian rhythm of intraocular pressure in the adult rat. Chronobiol Int. 2015;32:513–523. doi: 10.3109/07420528.2015.1008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickla DL, Wildsoet CF, Troilo D. Diurnal rhythms in intraocular pressure, axial length, and choroidal thickness in a primate model of eye growth, the common marmoset. Invest Ophthalmol Vis Sci. 2002;43:2519–2528. [PubMed] [Google Scholar]
- 4.Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52:5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- 5.Stone RA, Quinn GE, Francis EL, et al. Diurnal axial length fluctuations in human eyes. Invest Ophthalmol Vis Sci. 2004;45:63–70. doi: 10.1167/iovs.03-0294. [DOI] [PubMed] [Google Scholar]
- 6.Liu JH, Kripke DF, Hoffman RE, et al. Nocturnal elevation of intraocular pressure in young adults. Invest Ophthalmol Vis Sci. 1998;39:2707–2712. [PubMed] [Google Scholar]
- 7.Nickla DL. Ocular diurnal rhythms and eye growth regulation: where we are 50 years after Lauber. Exp Eye Res. 2013;114:25–34. doi: 10.1016/j.exer.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 9.Burfield HJ, Patel NB, Ostrin LA. Ocular biometric diurnal rhythms in emmetropic and myopic adults. Invest Ophthalmol Vis Sci. 2018;59:5176–5187. doi: 10.1167/iovs.18-25389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 11.Cajochen C, Krauchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 12.Ostrin LA. Ocular and systemic melatonin and the influence of light exposure. Clin Exp Optom. 2018;102:99–108. doi: 10.1111/cxo.12824. [DOI] [PubMed] [Google Scholar]
- 13.te Kulve M, Schellen L, Schlangen LJ, van Marken Lichtenbelt WD. The influence of light on thermal responses. Acta Physiol (Oxf) 2016;216:163–185. doi: 10.1111/apha.12552. [DOI] [PubMed] [Google Scholar]
- 14.Cajochen C, Munch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 15.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 17.Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 18.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 21.Abbott KA, Queener H, Ostrin LA. Relationship between intrinsically photosensitive retinal ganglion cells and refractive error, light exposure, and melatonin. Optom Vis Sci. 2018;95:323–331. doi: 10.1097/OPX.0000000000001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostrin LA, Abbott KS, Queener HM. Attenuation of short wavelengths alters sleep and the ipRGC pupil response. Ophthalmic Physiol Opt. 2017;37:440–450. doi: 10.1111/opo.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kearney S, O'Donoghue L, Pourshahidi LK, Cobice D, Saunders KJ. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthalmic Physiol Opt. 2017;37:557–567. doi: 10.1111/opo.12396. [DOI] [PubMed] [Google Scholar]
- 24.Li T, Troilo D, Glasser A, Howland HC. Constant light produces severe corneal flattening and hyperopia in chickens. Vision Res. 1995;35:1203–1209. doi: 10.1016/0042-6989(94)00231-a. [DOI] [PubMed] [Google Scholar]
- 25.Li T, Howland HC, Troilo D. Diurnal illumination patterns affect the development of the chick eye. Vision Res. 2000;40:2387–2393. doi: 10.1016/s0042-6989(00)00098-5. [DOI] [PubMed] [Google Scholar]
- 26.Lauber JK, Boyd JE, Boyd TA. Intraocular pressure and aqueous outflow facility in light-induced avian buphthalmos. Exp Eye Res. 1970;9:181–187. doi: 10.1016/s0014-4835(70)80074-4. [DOI] [PubMed] [Google Scholar]
- 27.Jensen LS, Matson WE. Enlargement of avian eye by subjecting chicks to continuous incandescent illumination. Science. 1957;125:741. doi: 10.1126/science.125.3251.741. [DOI] [PubMed] [Google Scholar]
- 28.Loman J, Quinn GE, Kamoun L, et al. Darkness and near work: myopia and its progression in third-year law students. Ophthalmology. 2002;109:1032–1038. doi: 10.1016/s0161-6420(02)01012-6. [DOI] [PubMed] [Google Scholar]
- 29.Xiong S, Sankaridurg P, Naduvilath T, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017;95:551–566. doi: 10.1111/aos.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He M, Xiang F, Zeng Y, et al. Effect of time spent outdoors at school on the development of myopia among children in China: a randomized clinical trial. JAMA. 2015;314:1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 31.Jin JX, Hua WJ, Jiang X, et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun Eye Care Study. BMC Ophthalmol. 2015;15:73. doi: 10.1186/s12886-015-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125:1239–1250. doi: 10.1016/j.ophtha.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 33.French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68. doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010;51:5247–5253. doi: 10.1167/iovs.09-4689. [DOI] [PubMed] [Google Scholar]
- 35.Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989;2:465–471. doi: 10.1017/s0952523800012360. [DOI] [PubMed] [Google Scholar]
- 36.Loewen NA, Liu JH, Weinreb RN. Increased 24-hour variation of human intraocular pressure with short axial length. Invest Ophthalmol Vis Sci. 2010;51:933–937. doi: 10.1167/iovs.09-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in young adults with moderate to severe myopia. Invest Ophthalmol Vis Sci. 2002;43:2351–2355. [PubMed] [Google Scholar]
- 38.Quinn GE, Berlin JA, Young TL, Ziylan S, Stone RA. Association of intraocular pressure and myopia in children. Ophthalmology. 1995;102:180–185. doi: 10.1016/s0161-6420(95)31038-x. [DOI] [PubMed] [Google Scholar]
- 39.Parssinen O. Intraocular pressure in school myopia. Acta Ophthalmol (Copenh) 1990;68:559–563. doi: 10.1111/j.1755-3768.1990.tb04787.x. [DOI] [PubMed] [Google Scholar]
- 40.Goss DA, Caffey TW. Clinical findings before the onset of myopia in youth: 5, intraocular pressure. Optom Vis Sci. 1999;76:286–291. doi: 10.1097/00006324-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Tornquist R, Stenkula S, Tornquist P. Retinal detachment: a study of a population-based patient material in Sweden 1971-1981—I, epidemiology. Acta Ophthalmol (Copenh) 1987;65:213–222. doi: 10.1111/j.1755-3768.1987.tb07003.x. [DOI] [PubMed] [Google Scholar]
- 42.Marcus MW, de Vries MM, Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–1994. doi: 10.1016/j.ophtha.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Ostrin LA. Objectively measured light exposure in emmetropic and myopic adults. Optom Vis Sci. 2017;94:229–238. doi: 10.1097/OPX.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 44.Read SA, Collins MJ, Vincent SJ. Light exposure and eye growth in childhood. Invest Ophthalmol Vis Sci. 2015;56:6779–6787. doi: 10.1167/iovs.14-15978. [DOI] [PubMed] [Google Scholar]
- 45.Houle RE, Grant WM. Alcohol, vasopressin, and intraocular pressure. Invest Ophthalmol. 1967;6:145–154. [PubMed] [Google Scholar]
- 46.Dervisogullari MS, Totan Y, Yuce A, Kulak AE. Acute effects of caffeine on choroidal thickness and ocular pulse amplitude. Cutan Ocul Toxicol. 2016;35:281–286. doi: 10.3109/15569527.2015.1104330. [DOI] [PubMed] [Google Scholar]
- 47.Kinoshita T, Mori J, Okuda N, et al. Effects of exercise on the structure and circulation of choroid in normal eyes. PLoS One. 2016;11:e0168336. doi: 10.1371/journal.pone.0168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chakraborty R, Read SA, Collins MJ. Hyperopic defocus and diurnal changes in human choroid and axial length. Optom Vis Sci. 2013;90:1187–1198. doi: 10.1097/OPX.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 49.Bennett AG, Rabbetts RB. The schematic eye. In: AG Bennett, Rabbetts RB., editors. Clinical Visual Optics. London: Butterworths;; 1989. pp. 249–274. [Google Scholar]
- 50.Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann's method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232:361–367. doi: 10.1007/BF00175988. [DOI] [PubMed] [Google Scholar]
- 51.Patel NB, Wheat JL, Rodriguez A, Tran V, Harwerth RS. Agreement between retinal nerve fiber layer measures from Spectralis and Cirrus spectral domain OCT. Optom Vis Sci. 2012;89:E652–E666. doi: 10.1097/OPX.0b013e318238c34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.James JF. A Student's Guide to Fourier Transforms With Applications in Physics and Engineering. Cambridge, UK: Cambridge University Press;; 1995. [Google Scholar]
- 53.Batschelet E. Circular Statistics in Biology. London, UK: Academic Press;; 1981. [Google Scholar]
- 54.Refinetti R, Lissen GC, Halberg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38:275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hastings MH. Central clocking. Trends Neurosci. 1997;20:459–464. doi: 10.1016/s0166-2236(97)01087-4. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez AA, Wildsoet CF. Quantifying light exposure patterns in young adult students. J Mod Opt. 2013;60:1200–1208. doi: 10.1080/09500340.2013.845700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips JR, McBrien NA. Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera. Invest Ophthalmol Vis Sci. 2004;45:758–763. doi: 10.1167/iovs.03-0732. [DOI] [PubMed] [Google Scholar]
- 58.Cashwell LF, Martin CA. Axial length decrease accompanying successful glaucoma filtration surgery. Ophthalmology. 1999;106:2307–2311. doi: 10.1016/S0161-6420(99)90531-6. [DOI] [PubMed] [Google Scholar]
- 59.Francis BA, Wang M, Lei H, et al. Changes in axial length following trabeculectomy and glaucoma drainage device surgery. Br J Ophthalmol. 2005;89:17–20. doi: 10.1136/bjo.2004.043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmid GF, Papastergiou GI, Lin T, Riva CE, Laties AM, Stone RA. Autonomic denervations influence ocular dimensions and intraocular pressure in chicks. Exp Eye Res. 1999;68:573–581. doi: 10.1006/exer.1998.0649. [DOI] [PubMed] [Google Scholar]
- 61.Sergienko NM, Shargorogska I. The scleral rigidity of eyes with different refractions. Graefes Arch Clin Exp Ophthalmol. 2012;250:1009–1012. doi: 10.1007/s00417-012-1973-0. [DOI] [PubMed] [Google Scholar]
- 62.Schmidl D, Garhofer G, Schmetterer L. The complex interaction between ocular perfusion pressure and ocular blood flow: relevance for glaucoma. Exp Eye Res. 2011;93:141–155. doi: 10.1016/j.exer.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Choi J, Kim KH, Jeong J, Cho HS, Lee CH, Kook MS. Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2007;48:104–111. doi: 10.1167/iovs.06-0615. [DOI] [PubMed] [Google Scholar]
- 64.Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM. Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis Neurosci. 2000;17:63–70. doi: 10.1017/s0952523800171068. [DOI] [PubMed] [Google Scholar]
- 65.Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- 66.Abbott KS, Queener HM, Ostrin LA. The ipRGC-driven pupil response with light exposure, refractive error, and sleep. Optom Vis Sci. 2018;95:323–331. doi: 10.1097/OPX.0000000000001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Z, Morgan IG, Chen Q, Jin L, He M, Congdon N. Disordered sleep and myopia risk among Chinese children. PLoS One. 2015;10:e0121796. doi: 10.1371/journal.pone.0121796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ayaki M, Torii H, Tsubota K, Negishi K. Decreased sleep quality in high myopia children. Sci Rep. 2016;6:33902. doi: 10.1038/srep33902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chapotot F, Gronfier C, Jouny C, Muzet A, Brandenberger G. Cortisol secretion is related to electroencephalographic alertness in human subjects during daytime wakefulness. J Clin Endocrinol Metab. 1998;83:4263–4268. doi: 10.1210/jcem.83.12.5326. [DOI] [PubMed] [Google Scholar]
- 70.Sherman B, Wysham C, Pfohl B. Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab. 1985;61:439–443. doi: 10.1210/jcem-61-3-439. [DOI] [PubMed] [Google Scholar]
- 71.Weibel L, Brandenberger G. The start of the quiescent period of cortisol remains phase locked to the melatonin onset despite circadian phase alterations in humans working the night schedule. Neurosci Lett. 2002;318:89–92. doi: 10.1016/s0304-3940(01)02496-x. [DOI] [PubMed] [Google Scholar]
- 72.Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L'Hermite-Baleriaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–188. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 73.Kenny GP, McGinn R. Restoration of thermoregulation after exercise. J Appl Physiol. 2017;122:933–944. doi: 10.1152/japplphysiol.00517.2016. [DOI] [PubMed] [Google Scholar]
- 74.Kelly GS. Body temperature variability (part 2): masking influences of body temperature variability and a review of body temperature variability in disease. Altern Med Rev. 2007;12:49–62. [PubMed] [Google Scholar]
- 75.Nickla DL. The phase relationships between the diurnal rhythms in axial length and choroidal thickness and the association with ocular growth rate in chicks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:399–407. doi: 10.1007/s00359-005-0077-2. [DOI] [PubMed] [Google Scholar]
- 76.Liu JH, Kripke DF, Hoffman RE, et al. Elevation of human intraocular pressure at night under moderate illumination. Invest Ophthalmol Vis Sci. 1999;40:2439–2442. [PubMed] [Google Scholar]
- 77.Liu JH, Bouligny RP, Kripke DF, Weinreb RN. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci. 2003;44:4439–4442. doi: 10.1167/iovs.03-0349. [DOI] [PubMed] [Google Scholar]
- 78.Seidel G, Hausberger S, Herzog SA, et al. Circadian macular volume changes in the healthy human choroid. Am J Ophthalmol. 2015;159:365–371. doi: 10.1016/j.ajo.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Goss DA, Winkler RL. Progression of myopia in youth: age of cessation. Am J Optom Physiol Opt. 1983;60:651–658. doi: 10.1097/00006324-198308000-00002. [DOI] [PubMed] [Google Scholar]