Abstract
Purpose
Relationships between tear film lipid (TFL) layer composition, structure, and function could provide insight into the etiology of dry eye. The molar ratio of cholesteryl ester (CE)/wax ester (WE) was measured in meibum from normal donors (Mn) and compared with meibum from donors with meibomian gland dysfunction (MMGD).
Methods
CE/WE was measured using nuclear magnetic resonance spectroscopy.
Results
CE/WE was distributed into two populations with 81% distributed near 0.55 and 19% near 0.3. CE/WE were higher in donors 13 to 19 years old compared with donors 1 to 12 years old and 20 to 88 years old. CE/WE for MMGD was 30% lower, 0.34 ± 0.04, compared with Mn, 0.49 ± 0.04. There were no sex differences in CE/WE. There were no significant racial differences between the CE/WE ratios for Asians and Caucasians. The CE/WE ratio was higher for blacks and lower for Hispanics compared to Caucasians. Due to the small number sampled, confirmation of the later racial results is needed. The packing of CE and WE in the TFL layer was proposed.
Conclusions
Although MMGD contains much less CE than Mn, factors other than the CE content, such as the levels of saturation and/or proteins, may be responsible for the higher order of MMGD. In addition to saturation, CE could contribute to the increase in order of Mn between 0 and 20 years of age. Observed changes in the meibum content of CE alone is not likely to influence tear film stability.
Keywords: age, dry eye, sex, lipid, meibum, meibomian gland dysfunction, NMR
A thin layer of lipid1,2 on the tear film surface could influence the stability of tears.3–8 Most of the tear film lipid (TFL) comes from meibomian gland, but other sources such as sebaceous9 and lacrimal glands10 could contribute a minor portion of lipid to the TFL layer. Relationships between TFL layer composition, structure, and function could provide insight into the etiology of dry eye. The current study focuses on human meibum cholesteryl ester (CE) and wax ester (WE) compositional changes with age, race, sex, and meibomian gland dysfunction (MGD).
CE is a very minor component, less than 2% of most tissues, and is not miscible in phospholipid membranes.11–13 However, CE is present in significant amounts in meibum and sebum.9,14 CE became the focus of research decades ago when it was discovered that the concentration of CE increases to 50% with age in human aortic intima15 and is prominent in plaques that contribute to atherosclerosis.16,17
CE and WE compose most of the TFL.4,12,18–27 The CE/WE molar ratio for adults is about 0.5:1 (Table 1); it does not change with age,28 but it decreases with MGD.23 Two populations of donors have been observed, with lower and higher ratios of CE/WE.23,29 One of the earliest studies of meibum showed that CEs contain much more anteiso- and iso-branched chains compared with WEs, and the hydrocarbon chains of CE are about three times more saturated compared with WE.18,22,26 The hydrocarbon chains of CE are among the longest measured for lipids, up to 32 carbons in length.18,30–33 CE dramatically increased the phase transition temperature and decreased the phase cooperativity of WE.28
Table 1.
WE and CE Composition of Human Meibum
|
Reference Citation, Normal Adult Unless Indicated |
Stearoyl/WE, mol/mol |
Donors Sampled,
N |
| 18 | 0.84 | 1 (76 pooled) |
| 19 | 0.69* | 4 |
| 12 | 0.54 | 72 |
| 20 | 0.53 | 22 |
| 21 | 0.85 | 4 |
| 22 | 0.72 | 4 |
| 23 | 0.57 | 27 |
| 24 | 0.53* | 14 |
| 25 | 2.7 | 10 |
| 26 | 0.46 | 45 |
| Literature average, less no. 25 | 0.64 ± 0.05 | 9 Studies |
| Literature average, n > 11 | 0.53 ± 0.01 | 5 Studies |
| Current study, adults > 13 y | 0.51 ± 0.02† | 31 |
| 25, Dry eye | 2.8 | 27 |
| 27, Dry eye | 1.14 | 4 |
| 20, Obstructive MGD seborrheic MGD | 0.50*, 0.53* | Small group |
| 23, MGD | 0.34 | 48 |
Nuclear magnetic resonance (NMR) spectroscopy has been a valuable tool for the evaluation of the lipid composition in the ocular lens34 and human meibum lipid.12,23,28,35–40 The advantages and disadvantages of using NMR spectroscopy and mass spectrometry for the compositional measurement of meibum has been discussed.40 Confirmation of NMR resonance assignments, critical to the interpretation of the changes in the NMR spectra of meibum, has been made.28,39,40 The assignments for the resonances in the CH2 and CH3 region between 2.6 and 0.6 ppm allowed for the quantification of the CE/WE molar ratio in human meibum with age, sex, race, and MGD in the current study.39,40 A major advantage of the current study compared with the previous study23 is that in the previous study the resonance used to measure CE was dependent on the esterification of the CE. In the previous study,23 it was unclear if the decrease in the CE/WE ratio with MGD was due to lysis of the CE bond or a decrease in the total content of CE. In the current study, the resonances used to measure CE were not dependent on an intact CE bond. In addition, due to the stoichiometry of the cholesterol protons, the sum of the intensities of the resonances used in the current study for CE in the CH3 region, 2.6 to 0.6 ppm, are nine times more intense than the resonance of the CE at 4.6 ppm. In the current study, the CE/WE molar ratio of meibum was quantified from 142 human donors, 94 without dry eye, one of the largest meibum compositional studies. Because of the larger number of samples, in the current study we were more accurately able to determine the distribution of the CE/WE molar ratio and if changes in the CE/WE molar ratio occurred between the ages of 1 to 12, 13 to 19, or 20 to 68 years, as the number of samples used in the previous study23 verses the current study for these age groups is 14 vs. 39, 0 vs. 17, and 26 vs. 38, respectively.
Materials and Methods
Materials
CDCl3 and d-hexane were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA).
Collection and Processing of Human Meibum
Written informed consent was obtained from all donors. Protocols and procedures were reviewed by the University of Louisville Institutional Review Board as well as the Robley Rex Veterans Affairs Institutional Review Board. All subjects were treated in accordance with the Declaration of Helsinki.
Donors were grouped into three cohorts: Cn500, donors without dry eye who donated meibum that was analyzed previously (Mn500) using a 500-mHz NMR; CMGD500, donors with MGD who donated meibum that was analyzed previously23 (MMGD500) using a 500-mHz NMR; and Cn700, donors without dry eye who donated meibum that was analyzed using a 700-mHz NMR (Mn700).
Collection and processing of human meibum for Cn500 and CMGD500 was made on the same cohorts as published previously23 and discussed below.
Cohorts Cn500 and CMGD500
Meibomian gland expression was done by compressing the eyelid between cotton-tipped applicators with strict attention to avoiding touching the eyelid margin during expression. All four eyelids were expressed, and about 1 mg of meibum was collected per individual for direct spectroscopic study. The expressate was collected with a platinum spatula and immediately spread onto the AgCl window and into 0.5 mL of tetrahydrofuran/methanol (THF/MeOH), 3:1, volume/volume, in a 9-mm microvial with a Teflon cap (Microliter Analytical Supplies, Inc., Suwanee, GA, USA). All samples were frozen under argon gas until analysis. Analyses were performed within 3 weeks of collection of the sample. Storage of the sample on AgCl windows for over 2 months under argon did not affect the sample.23 Prior to NMR analysis, the THF/MeOH in the microvial containing meibum rinsed from the spatula was evaporated with a stream of argon gas.
After infrared analysis and solvent evaporation, meibum was removed from the AgCl window using a series of solvents with different hydrophobicities to ensure that all lipid classes were extracted from the window. First, the AgCl window was placed with the meibum side down into a 15-mL glass scintillation vial containing 1 mL of hexane and then purged with argon gas. A glass vial rather than a plastic one was used in all protocols to avoid plasticizer contamination. The vial was sonicated in an ultrasonic bath (Branson 1510; Branson Ultrasonics, Danbury, CT, USA) for 10 minutes. The hexane was decanted into the microvial containing the meibum lipid rinsed from the spatula. The hexane was evaporated under a stream of nitrogen gas. Methanol (1.5 mL) was then added to the scintillation vial containing the AgCl window and purged with argon gas. The vial was sonicated in an ultrasonic bath (Branson Ultrasonics) for 10 minutes. The methanol was decanted into the microvial containing the meibum lipid rinsed from the spatula and was evaporated under a stream of nitrogen gas. THF/MeOH (1.5 mL) was added to the scintillation vial containing the AgCl window and purged with argon gas. The vial was sonicated in an ultrasonic bath (Branson Ultrasonics) for 10 minutes. The microvial containing the extracted meibum lipid was lyophilized for 12 hours to remove trace amounts of organic solvents. Finally, deuterated cyclohexane (0.5 mL) was added to the sample and sonicated in a bath sonicator (Branson Ultrasonics) for 10 minutes. The solution was transferred to glass NMR tubes (Sigma-Aldrich Corp.), and NMR spectra were collected. The samples never came in contact with any plastic so as to avoid plasticizers. Control CDCl3 spectra were run with every 11 meibum samples to ensure no impurities were present.
Cohort Mn700
Meibomian glands were gently expressed by pressing the eyelid with a fingertip with strict attention to avoiding touching the eyelid margin during expression. All four eyelids were expressed, and approximately 0.5 mg of meibum lipid was collected per individual for direct spectroscopic study. The expressate was collected with a platinum spatula under a slit lamp, and the pool of infant meibum was immediately dissolved into 0.5 mL of CDCl3 in a 9-mm microvial with a Teflon cap (Microliter Analytical Supplies, Inc.). Argon gas was blown over the samples to prevent oxidation. The sample in the vial was capped and frozen under argon gas until analysis. Analyses were performed within 3 weeks of collection of the sample. The samples never came in contact with any plastic to avoid plasticizers. Control CDCl3 spectra were run with every 11 meibum samples to ensure no impurities were present.
Clinical Diagnosis
Clinical diagnosis was made on the same cohorts as published previously23 and discussed below.
Subjects were recruited from the Kentucky Lion's Eye Center and the Robley Rex Veterans Affairs Medical Center in Louisville, Kentucky. Normal status was assigned when the subject's meibomian gland orifices showed no evidence of keratinization or plugging with turbid or thickened secretions and no dilated blood vessels were observed on the eyelid margin.
The diagnosis of MGD was made according to the criteria of Foulks and Bron.41 Plugging of the meibomian glands of at least 5 out of 10 orifices in the central portion of the upper eyelid was required for diagnosis of MGD. The secretion expressed by the meibomian gland had to be turbid, turbid with clumps, or paste-like. Inflammation of the eyelid margin, as evidenced by both swelling of the eyelid margin, and 2+ vascular engorgement of the posterior eyelid margin were necessary for diagnosis. The presence of telangiectasia of the posterior eyelid margin was confirmatory of chronic disease but not required for entry. Tear film stability was determined by instillation of sodium fluorescein into the tear film. Tear breakup time was less than 5 seconds for all subjects in CMGD500.
NMR Spectral Measurements
Cohorts Cn500 and CMGD500
Spectral data were acquired with a spectrometer (Inova-500; Varian, Lexington, MA, USA). The following parameters were used: 800 scans were acquired with a spectral width of 15 ppm, 60 degree pulse, 4K data points, 1.0-second delay time, and 2.049-second acquisition time at 25°C.
Cohort Cn700
Spectral data were acquired using a 700-MHz NMR spectrometer (VNMRS; Varian) equipped with a 5-mm 1H{13C/15N} 13C-enhanced cold probe (Varian Inc., Palo Alto, CA, USA). Spectra were acquired with a minimum of 250 scans, 45-degree pulse width, and a relaxation delay of 1.000 second. All spectra were obtained at 25°C. The tetra methyl silane resonance was set to 0 ppm.
Commercial software (GRAMS 386; Galactic Industries Corp., Salem, NH, USA) was used for phasing, curve fitting, and integrating.
Results
The demographics of human meibum donors are presented in Table 2.
Table 2.
Cohort Demographics of Donors Without Dry Eye
|
Cohort Without Dry Eye |
Number |
Average Age, y |
CE/WE, mol/mol |
P |
| Female | 27 | 20 ± 3 | 0.51 ± 0.04 | Male vs. female |
| Male | 64 | 23 ± 3 | 0.50 ± 0.03 | 0.85 |
| Caucasian | 72 | 22 ± 1 | 0.51 ± 0.01 | Race vs. Caucasian |
| Asian | 7 | 23 ± 1 | 0.45 ± 0.02 | 0.99 |
| Black | 6 | 24 ± 2 | 0.60 ± 0.02 | <0.001 |
| Hispanic | 3 | 11 ± 3 | 0.40 ± 0.04 | <0.001 |
Values ± standard error of the mean.
Resonance 4.6 ppm From CE
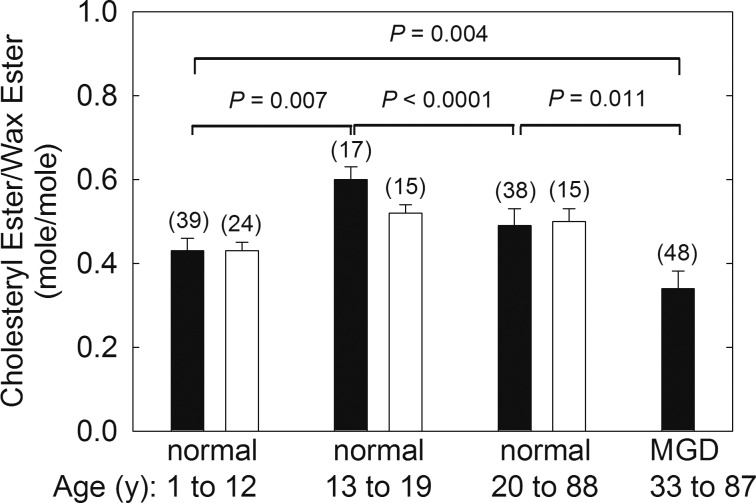
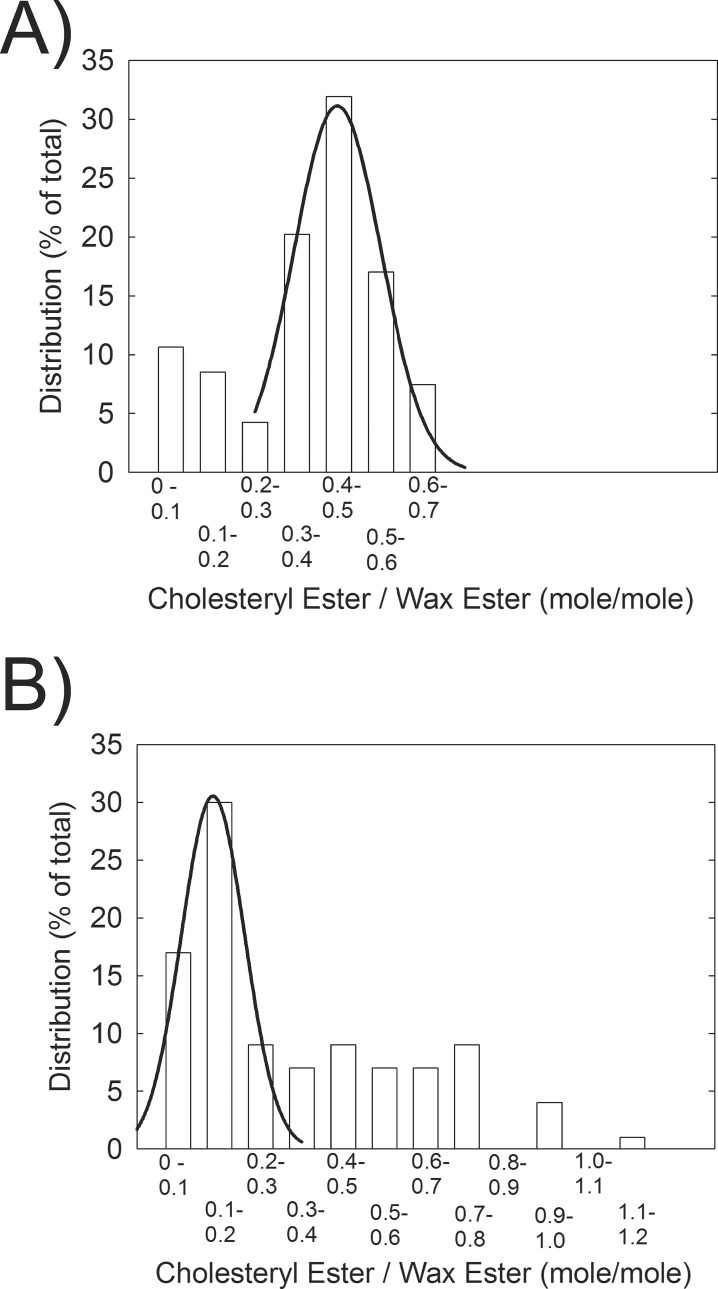
The 1H-NMR spectra of all CEs, regardless of the ester hydrocarbon type, have a well-resolved broad resonance at 4.6 ppm (Fig. 1b). The 1H-NMR spectra of all WEs, regardless of the type of ester hydrocarbons, have a well-resolved triplet resonance near 4 ppm (Fig. 1b). From the relative intensity of these resonances, we can calculate the molar ratio of CE to WE in meibum (Fig. 2, solid bar). CE/WE was distributed into two populations, with 81% normally distributed, with a peak between 0.5 and 0.59 mol/mol, and 19% between 0.1 and 0.29 mol/mol (Fig. 3). The CE/WE molar ratios were mostly distributed in the higher ratio group for Mn, whereas the ratios were mostly distributed in the lower ratio group for MMGD (Fig. 3). The distribution was not related to age as the average age of the low and high CE/WE populations was 20.5 and 19.9 years old, respectively. The molar ratio of CE/WE were slightly but significantly higher in donors 13 to 88 years old compared with donors 1 to 12 years old and 20 to 88 years old (Fig. 2). The molar ratio of CE/WE for MMGD500 was significantly 30% lower, 0.34 ± 0.04, compared with Mn, 0.49 ± 0.04 (Fig. 2). Data measured on the 500-mHz instrument was not significantly different from the data collected on the 700-mHz instrument, P = 0.888.
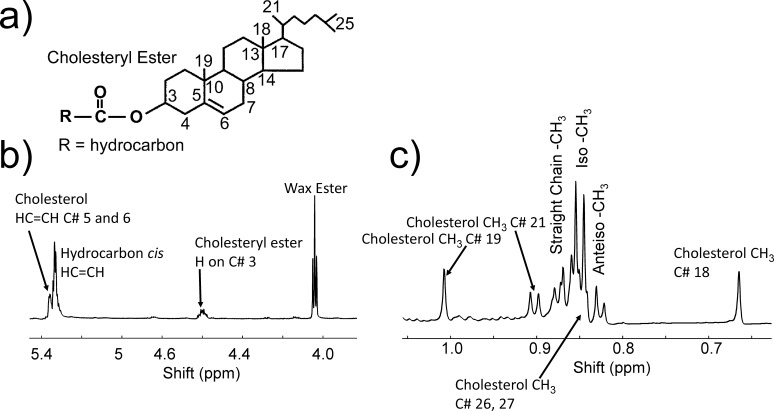
Figure 1.
(a) Numbering used in (b) and (c) associated with CEs. (b, c) A typical NMR spectrum of meibum from a 31-year-old male Caucasian donor. The resonance due to WEs near 4.0 ppm is a triplet. The resonance due to cholesterol number C21 is a doublet. Resonances for straight chain CH3 and anteiso-CH3 moieties are composed of two major resonances, and the iso-CH3 moieties are composed of two major and one minor (left shoulder) resonance. The resonances due to cholesterol numbers C26 and 27 are not resolved but rather are buried under the straight chain resonances.
Figure 2.
CE/WE molar ratios calculated from the NMR spectra of meibum. (Solid bar) Molar ratios calculated from the intensity of the CE resonance at 4.6 ppm and the WE resonance at 4.0 ppm. Correcting for (O)-acylated ω-hydroxy fatty acids, the solid bars would be lower. For instance, the value for MGD corrected would be 31, lower than the reported value of 34. (Open bar) Molar ratios calculated from the intensity of the CE resonances from cholesteryl numbers C18 and 19 and the WE resonance at 4.0 ppm. See Figure 1 for details.
Figure 3.
Distribution of CE/WE molar ratios calculated from the intensity of the CE resonance at 4.6 ppm and the WE resonance at 4.0 ppm. (A) Meibum from donors without dry eye. (B) Meibum for donors with dry eye.
Cholesterol CH3 Resonances From Carbons 18 and 19 of Cholesterol Near 0.66 and 1 ppm
The CE/WE molar ratios were also measured using the areas of the cholesterol CH3 resonances from carbons 18 and 19 of cholesterol near 0.66 and 1 ppm (Fig. 1a and c) and the WE resonance near 4 ppm (Fig. 1b). The molar ratios of CE/WE calculated using the 4.6-ppm resonance (Fig. 2, solid bars) and the 0.66- and 1-ppm resonances (Fig. 2, open bars) were almost identical. The intensity of the resonances at 0.66 and 1 ppm measured for Mn500 and MMGD500 were low compared with those measured for Mn700, which resulted in the intensity ratio (0.66 ppm + 1 ppm)/4.6 ppm of 1.3 for Mn500 and MMGD500, not near the expected stoichiometry of 6. Thus, the data for Mn500 and MMGD500 were not included in Figure 2 (open bars).
There were no differences in the CE/WE molar ratio of meibum from female donors compared with male donors (Table 2). There were no significant racial differences between the CE/WE ratios for Asians and Caucasians (Table 2). The CE/WE ratio was higher for blacks and lower for Hispanics compared to Caucasians (Table 2).
Discussion
Relationships between TFL layer composition, structure, and function could provide insight into the etiology of dry eye.
Meibum CE/WE Composition and Distribution
In the current meibum compositional study of 142 donors, one of the largest to date, the CE/WE molar ratio for donors without dry eye was found to be distributed into two populations, one with a peak near 0.55 and the other between 0 and 0.2. The distribution is much greater than the experimental error of approximately 5%. The distribution pattern confirms earlier studies with much smaller cohorts.23,29 The wide distribution could explain the variation in the literature values for the CE/WE molar ratio, especially for studies with fewer than 10 donors (Table 1). The CE/WE molar ratio calculated herein, 0.51 ± 0.02, was almost identical to the average literature value of studies with n > 11 (Table 1) and was very similar to 0.54, a NMR study from another group.14 The agreement between the ratios measured in the current study using different resonances for CE and the agreement with other studies confirms the reliability of the results of the current study.
There is another advantage of using the 1- and 0.66-ppm resonances to calculate CE as was done in this study and not in our previous study.23 The NMR data ratio from the 4.6- and 4-ppm resonances in the previous study23 does not account for the esters from (O)-acylated ω-hydroxy fatty acids (OAHFA). CE of OAHFA and free OAHFA account for about 3% each of the total meibum esters.22,24 OAHFA esters would add to the WE resonance near 4 ppm. CE of OAHFA would contribute one ester bond, and free OAHFA would contribute two esters to the NMR WE value. Correcting the NMR value of WE for the OAHFA esters, the corrected CE/WE ratio for MMGD would be 0.31, lower than the value of 0.34 reported.
The CE/WE ratio for the age group 1 to 12 years old was statistically 28% lower than the groups older than 12 years. There were no sex differences in the CE/WE ratio. It is interesting that the CE/WE ratio was higher for blacks and lower for Hispanics compared to Caucasians. Due to the small sample sizes, even though the results were statistically significant, the results should be viewed cautiously until cohorts of at least 15 donors are analyzed. No difference was found between the CE/WE of meibum from Asians and Caucasians, but the sample size was small. One compositional study of Asians25 gave a CE/WE molar ratio four times higher than the average value for Caucasians (Table 1), but the accuracy of the ratio has been challenged.24 Therefore, further studies are needed to determine the contribution of race to the CE/WE molar ratio.
The molar ratio of CE/WE for MMGD500 was, significantly, 30% lower compared with Mn. In a comprehensive study of Asians with dry eye, no difference between Mn and meibum from donors with dry eye was observed.25 However, the molar ratios in the study25 were very high as discussed above.25 In a later, smaller study by the same group,27 the CE/WE ratio was 59% lower with dry eye compared with Mn,25 a percentage change that is in agreement with the current study. Another study using thin-layer chromatography noted a 6% decrease in the ratio of CE/WE for meibum from donors with obstructive MGD compared with Mn.20
Contribution of CE/WE to Structure
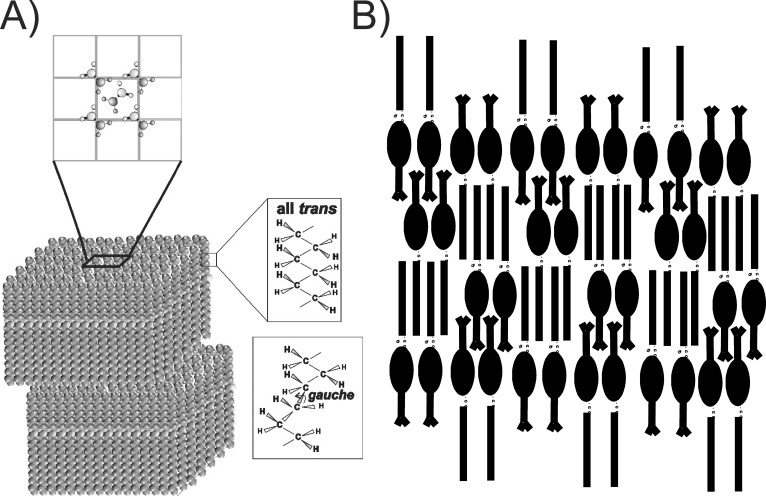
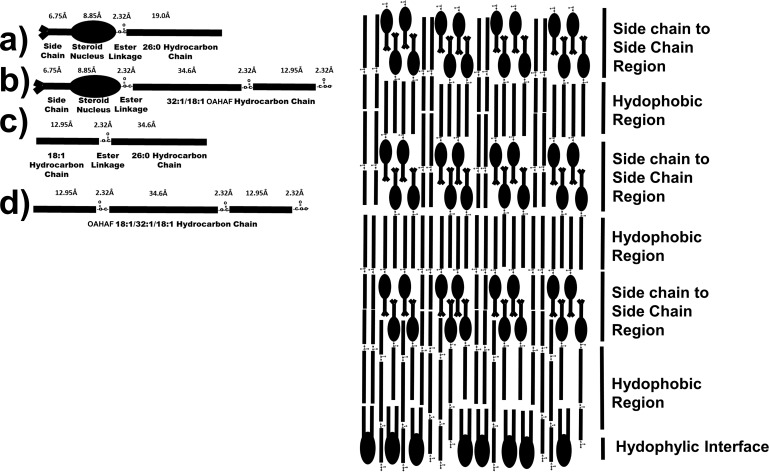
Infrared9,42–54 and fluorescence anisotropy42 studies show that meibum lipid hydrocarbons align to maximize Van der Waal's interactions between chains. Therefore, the WE and CE hydrocarbon chains are not randomly orientated as they are in an oil phase, as many schematic pictures in literature show them to be. At lower temperatures, meibum is in a liquid crystalline phase. The term “liquid crystalline phase” is used because meibum is not a solid crystal (100% trans) as the meibum hydrocarbon chains contain 72% trans rotamers, allowing them to pack tightly together (Fig. 4A).54 Thus, the term “liquid crystalline phase” is used rather than crystalline phase. At higher temperatures, meibum is in the gel phase, and the conformation of the meibum lipid hydrocarbon chains are 18% trans rotamers (Fig. 4A).54 Thus, meibum is not a liquid (0% trans) but rather in the gel phase. X-ray crystallography of WE (Fig. 4A) shows that WE packs together in lamellar layers with their hydrocarbons, with terminal methyl and ester carbonyl moieties adjacent to one another (Fig. 4A).55–57 X-ray crystallography and biophysical studies of CE (Fig. 4B) show that at lower temperatures, in the smectic phase, they also pack together in lamellar layers with their hydrocarbons, steroid nuclei, with terminal methyl and ester carbonyl moieties adjacent to one another and their side chains interdigitated.17,58 Adjacent terminal methyl moieties would be expected, especially with CE that contains branched terminal methyl groups. It is unknown how a mixture of WE and CE pack together, but it is reasonable that the minimal energy structures of CE and WE crystals alone are maintained in a mixture of the two. In the mixture, we speculate that the lipids would align to maximize hydrocarbon chain interactions just as they do for CE and WE alone (Fig. 5). The arrangement of molecules in Figure 5 allows for the interdigitation of CE side chains and maximizes the adjacent packing of the steroid nuclei and CE carbonyl moieties, just as they are in the crystalline state alone. The speculative model for the mixture of CE and WE (Fig. 5) has phospholipids with their hydrophilic head group facing the tear aqueous layer. Phospholipids do not interact with CE.11–13 It is speculative if the phospholipids in tears arise from debris59 or form a monolayer alone with other amphipathic molecules, such as OAHFA. Note that the carboxylate groups of the diester of OAHFA and the CE of OAHFA extend to the interface region where they may interact with water. For a 100-nm thick TFL layer, the motif structure of hydrophobic region and side chain-to-side chain region above the phospholipid monolayer in Figure 5 would stack about 26 times.
Figure 4.
(A) Potential lamellar packing of WE. (Top) Shows rhombic packing of the hydrocarbon chains. (Right) Trans orientation for ordered hydrocarbons, gauche rotamer orientations for disordered hydrocarbon chains.47 (B) Smectic phase packing of CE.17,61
Figure 5.
(Right) Speculative schematic of WE, CE, phospholipid, OAHFA, cholesteryl OAHFA, and OAHFA diester packing on an aqueous surface from X-ray crystallography of pure WE and CE in Figure 4 (a–d). The molecular size of the moieties were calculated from the data in Janiak et al.12
There are numerous limitations to the model in Figure 5. Proteins, especially mucin, are likely to associate with the tear lipid layer but are not shown in the model.51 Also, hydrocarbon chain kinks due to unsaturation as in Figure 4A are not shown in the model.
Cholesteryl palmitate had little effect on the phase-transition parameters of a mixture of stearyl palmitate and oleyloleate.50 However, cholesteryl behenate dramatically ordered and lowered the cooperativity of stearyl palmitate.28 From the model studies, it is difficult to predict the effect on structure of a major loss of CE with MMGD. As MMGD is more ordered than Mn,50 other factors such as saturation44,54 and/or proteins49,51 may be responsible for the higher order of MMGD. Mn order increases with age between 0 and 20 years of age. The CE/WE ratio increased with age between 0 and 20 years of age; thus, in addition to saturation,44,54 CE could contribute to the increase in order, with age between 0 and 20 years,43 as a cholesteryl behenate increased the order of WE.28 Experiments are underway to determine the contribution of CE to the order of meibum by separating meibum CE from WE and adding the moieties back together, as was done for saturated meibum.44,54
Contribution of CE/WE to Function
The meibum content of CE alone is not likely to influence tear film (TF) stability given that a significant number of donors with a stable TF have lower meibum levels of CE, and a significant number of donors with MGD and an unstable TF have higher meibum levels of CE. Similarities in the rheology of feline, canine, and human meibum suggest that “comparable tear film dynamics have developed quite a robust mixture of meibomian lipids that is tolerable to significant variances in the ratios of some of its compounds and still establishes similar functionality.”60 In terms of function, such as for the cat and dog, because CE and WE are preferentially distributed within upper layers of the duplex tear film lipid (TFL) layer, they do not significantly contribute to the properties and lipid composition of the tear interface. Thus, large changes in the amount of CE in the TFL layer of humans can also be tolerated, resulting in comparable tear film dynamics. One should note that lipid hydrocarbon chain order driven by saturation does influence the surface properties of human meibum.44,45 There is no sex difference in TF stability (breakup times),61–63 which correlates with no sex difference in the CE of Mn in the current study. Correlations between TFL layer composition, structure and function may help elucidate the contribution of the TFLL to the signs and symptoms of dry eye and decreased tear film stability with age.
Conclusions
Although MMGD contains much less CE than Mn, factors other than the CE content of meibum, such as the levels of saturation and/or proteins, may be responsible for the higher order of MMGD. In addition to saturation, CE could contribute to the increase in order of Mn between the ages of 0 and 20 years. Observed changes in the meibum content of CE alone is not likely to influence tear film stability.
Acknowledgments
Supported by the National Institute of Health Grant R01EY026180 and an unrestricted grant (GN151619B) from Research to Prevent Blindness, Inc., New York, New York, United States. The authors alone are responsible for the content and writing of the paper.
Disclosure: D. Borchman, None; A. Ramasubramanian, None; G.N. Foulks, None
References
- 1.King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci. 2010;51:2418–2423. doi: 10.1167/iovs.09-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King-Smith PE, Fink BA, Fogt N, Nichols KK, Hill RM, Wilson GS. The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci. 2000;41:3348–3359. [PubMed] [Google Scholar]
- 3.Green-Church KB, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–1993. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pucker AD, Nichols JJ. Analysis of meibum and tear lipids. Ocul Surf. 2012;10:230–250. doi: 10.1016/j.jtos.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids–a review. Curr Eye Res. 2008;33:405–420. doi: 10.1080/02713680802018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on Meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murube J. The origin of tears. III. The lipid component in the XIX and XX centuries. Ocul Surf. 2013;10:200–209. doi: 10.1016/j.jtos.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Georgiev GA, Eftimov P, Yokoi N. Structure-function relationship of tear film lipid layer: a contemporary perspective. Exp Eye Res. 2017;163:17–28. doi: 10.1016/j.exer.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Mudgil P, Borchman D, Gerlach D, Yappert MC. Sebum/meibum surface film interactions and phase transitional differences. Invest Ophthalmol Vis Sci. 2016;57:2401–2411. doi: 10.1167/iovs.16-19117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glasgow BJ, Abduragimov AR. Interaction of ceramides and tear lipocalin. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:399–408. doi: 10.1016/j.bbalip.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salmon A, Hamilton JA. Magic-angle spinning and solution C-13 nuclear magnetic resonance studies of medium- and long-chain cholesteryl esters in model bilayers. Biochemistry. 1995;34:16065–16073. doi: 10.1021/bi00049a021. [DOI] [PubMed] [Google Scholar]
- 12.Janiak MJ, Small DM, Shipley GG. Interactions of cholesterol esters with phospholipids–cholesteryl myristate and dimyristoyl lecithin. J Lipid Res. 1979;20:183–199. [PubMed] [Google Scholar]
- 13.Souza SL, Hallock KJ, Funari SS, Vaz WLC, Hamilton JA, Melo E. Study of the miscibility of cholesteryl oleate in a matrix of ceramide, cholesterol and fatty acid. Chem Phys Lipids. 2011;164:664–671. doi: 10.1016/j.chemphyslip.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Robosky LC, Wade K, Woolson D, et al. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. J Lipid Res. 2008;49:686–692. doi: 10.1194/jlr.D700035-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Katz SS. The lipids of grossly normal human aortic intima from birth to old age. J Biol Chem. 1981;256:12275–12280. [PubMed] [Google Scholar]
- 16.Small DM, Shipley GG. Physical-chemical basis of lipid deposition in atherosclerosis. Science. 1974;185:222–229. doi: 10.1126/science.185.4147.222. [DOI] [PubMed] [Google Scholar]
- 17.Small DM. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988;8:103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536. [PubMed] [Google Scholar]
- 19.Tiffany JM. Individual variations in human meibomian lipid composition. Exp Eye Res. 1978;27:289–300. doi: 10.1016/0014-4835(78)90164-1. [DOI] [PubMed] [Google Scholar]
- 20.Mathers WD, Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360. doi: 10.1007/978-1-4615-5359-5_50. [DOI] [PubMed] [Google Scholar]
- 21.Brown SH, Kunnen CM, Duchoslav E, et al. A comparison of patient matched meibum and tear lipidomes. Invest Ophthalmol Vis Sci. 2013;54:7417–7424. doi: 10.1167/iovs.13-12916. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Green KB, Nichols KK. Quantitative profiling of major neutral lipid classes in human meibum by direct infusion electrospray ionization mass spectrometry. Invest Ophthalmol Vis Sci. 2013;54:5730–5753. doi: 10.1167/iovs.12-10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha RK, Borchman D, Foulks GN, Yappert MC. Analysis of the composition of lipid in human meibum from normal infants, children, adolescents, adults and adults with meibomian gland dysfunction using 1H-NMR spectroscopy. Invest Ophthalmol Vis Sci. 2011;52:7350–7358. doi: 10.1167/iovs.11-7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butovich IA. Tear film lipids. Exp Eye Res. 2013;117:4–27. doi: 10.1016/j.exer.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam SM, Tong L, Yong SS, et al. Meibum lipid composition in Asians with dry eye disease. PLoS One. 2011;6:e24339. doi: 10.1371/journal.pone.0024339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest Ophthalmol Vis Sci. 2010;51:6220–6231. doi: 10.1167/iovs.10-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. 2014;55:289–298. doi: 10.1194/jlr.M044826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borchman D, Yappert MC, Milliner S, et al. 13C and 1H NMR ester region resonance assignments and the composition of human infant and child meibum. Exp Eye Res. 2013;112:151–159. doi: 10.1016/j.exer.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–2280. [PubMed] [Google Scholar]
- 30.Butovich IA. Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids. 2010;75:726–733. doi: 10.1016/j.steroids.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res. 2009;50:2471–2485. doi: 10.1194/jlr.M900252-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Nichols KK. Comprehensive shotgun lipidomics of human meibomian gland secretions using MS/MSall with successive switching between acquisition polarity modes. J Lipid Res. 2018;59:2223–2236. doi: 10.1194/jlr.D088138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009;50:501–513. doi: 10.1194/jlr.M800426-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borchman D, Yappert MC. Lipids and the ocular lens. J Lipid Res. 2010;51:2473–2488. doi: 10.1194/jlr.R004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borchman D, Foulks GN, Yappert MC, Milliner SE. Differences in human meibum lipid composition with meibomian gland dysfunction using NMR and principal component analysis. Invest Ophthalmol Vis Sci. 2012;53:337–347. doi: 10.1167/iovs.11-8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borchman D, Foulks GN, Yappert MC, Milliner SE. Changes in human meibum lipid composition with age using NMR spectroscopy. Invest Ophthalmol Vis Sci. 2012;53:475–482. doi: 10.1167/iovs.11-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foulks GN, Borchman D, Yappert MC, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2013;32:44–53. doi: 10.1097/ICO.0b013e318254205f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanova S, Tonchev V, Yokoi N, Yappert MC, Borchman D, Georgiev GA. Surface properties of squalene/meibum films and NMR confirmation of squalene in tears. Colloids and Interface Surfaces B. 2015;16:21813–21831. doi: 10.3390/ijms160921813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butovich IA, McMahon A, Wojtowicz JC, Lin F, Mancini R, Itani K. Dissecting lipid metabolism in meibomian glands of humans and mice: an integrative study reveals a network of metabolic reactions not duplicated in other tissues. Biochim Biophys Acta. 2016;1861:538–553. doi: 10.1016/j.bbalip.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borchman D, Ramasubramanian A. Human meibum chain branching variability with age, gender and meibomian gland dysfunction. Ocul Surf. 2018 doi: 10.1016/j.jtos.2018.12.005. published online ahead of print December 12. [DOI] [PMC free article] [PubMed]
- 41.Foulks GN, Bron AJ. Meibomian-gland dysfunction: a clinical scheme for description, diagnosis, classification and grading. Ocul Surf. 2003;1:17–36. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- 42.Borchman D, Foulks GN, Yappert MC, Tang D, Ho DV. Spectroscopic evaluation of human tear lipids. Chem Phys Lipids. 2007;147:87–102. doi: 10.1016/j.chemphyslip.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Mudgil P, Borchman D, Ramasubramanian A. Insights into tear film stability from babies and young adults; a study of human meibum lipid conformation and rheology. Int J Mol Sci. 2018;19:E3502. doi: 10.3390/ijms19113502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mudgil P, Borchman D, Yappert MC, et al. Lipid order, saturation and surface property relationships: a study of human meibum saturation. Exp Eye Res. 2013;116:79–85. doi: 10.1016/j.exer.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Nencheva Y, Ramasubramanian A, Eftimov P, et al. Effects of lipid saturation on the surface properties of human meibum films. Int J Mol Sci. 2018;19:E2209. doi: 10.3390/ijms19082209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borchman D, Foulks GN, Yappert MC, Ho DV. Temperature-induced conformational changes in human tear lipids hydrocarbon chains. Biopolymers. 2007;87:124–133. doi: 10.1002/bip.20798. [DOI] [PubMed] [Google Scholar]
- 47.Borchman D, Foulks GN, Yappert MC, et al. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 2010;44:34–42. doi: 10.1159/000283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borchman D, Foulks GN, Yappert MC. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr Eye Res. 2010;35:778–86. doi: 10.3109/02713683.2010.490895. [DOI] [PubMed] [Google Scholar]
- 49.Borchman D, Foulks GN, Yappert MC. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp Eye Res. 2010;91:24656. doi: 10.1016/j.exer.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:3805–3817. doi: 10.1167/iovs.10-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faheem S, Kim S, Nguyen J, et al. Wax-tear and meibum protein, wax-β-carotene interactions in vitro using infrared spectroscopy. Exp Eye Res. 2012;100:32–39. doi: 10.1016/j.exer.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter M, Bhola R, Yappert MC, Borchman D, Gerlach D. Pilot study of the influence of eyeliner cosmetics on the molecular structure of human meibum. Ophthalmic Res. 2015;53:131–135. doi: 10.1159/000371852. [DOI] [PubMed] [Google Scholar]
- 53.Mudgil P, Borchman D, Gerlach D, Yappert MC. Sebum/meibum surface film interactions and phase transitional differences. Invest Ophthalmol Vis Sci. 2016;57:2401–2411. doi: 10.1167/iovs.16-19117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sledge S, Henry C, Borchman D, et al. Human meibum age, lipid-lipid interactions and lipid saturation in meibum from infants. Int J Mol Sci. 2017;18:E1862. doi: 10.3390/ijms18091862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simpson TD, Miwa TK. X-ray study of hydrogenated jojoba wax. J Am Oil Chem Soc. 1977;54:54–58. [Google Scholar]
- 56.Kreger DR, Schamhart C. On the long crystal-spacings in wax esters and their value in micro-analysis of plant cuticle waxes. Biochim Biophys Acta. 1956;19:22–44. doi: 10.1016/0006-3002(56)90382-1. [DOI] [PubMed] [Google Scholar]
- 57.Weinbach SP, Weissbuch I, Kjaer K, et al. Self-assembled crystalline monolayers and multilayers of N-alkanes on the water surface. Adv Mater. 1995;7:857–862. [Google Scholar]
- 58.Ginsburg GS, Atkinson D, Small DM. Physical properties of cholesteryl esters. Prog Lipid Res. 1984;23:135–167. doi: 10.1016/0163-7827(84)90002-x. [DOI] [PubMed] [Google Scholar]
- 59.Brown SH, Kunnen CM, Papas EB, et al. Intersubject and interday variability in human tear and meibum lipidomes: a pilot study. Ocul Surf. 2016;14:43–48. doi: 10.1016/j.jtos.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Eftimov P, Yokoi N, Tonchev V, Nencheva Y, Georgiev GA. Surface properties and exponential stress relaxations of mammalian meibum films. Eur Biophys J. 2017;46:129–140. doi: 10.1007/s00249-016-1146-x. [DOI] [PubMed] [Google Scholar]
- 61.Mohidin N, Bay TC, Yap M. Non-invasive tear break-up time in normal Malays. Clin Exp Optom. 2002;85:37–41. doi: 10.1111/j.1444-0938.2002.tb03070.x. [DOI] [PubMed] [Google Scholar]
- 62.Ozdemir M, Temizdemir H. Age- and sex- related tear function changes in normal population. Eye. 2010;24:79–83. doi: 10.1038/eye.2009.21. [DOI] [PubMed] [Google Scholar]
- 63.Cho P, Yap M. Age, sex, and tear break-up time. Optom Vis Sci. 1993;70:828–831. doi: 10.1097/00006324-199310000-00009. [DOI] [PubMed] [Google Scholar]