Abstract
DNA damage occurs as a result of environmental insults and aging and, if unrepaired, may lead to chromosomal instability and tumorigenesis. Because GH suppresses ataxia-telangiectasia mutated kinase phosphorylation, decreases DNA repair, and increases DNA damage accumulation, we elucidated whether GH effects on DNA damage are mediated through induced IGF-1. In nontumorous human colon cells, GH, but not IGF-1, increased DNA damage. Stably disrupted IGF-1 receptor (IGF-1R) by lentivirus-expressing short hairpin RNA in vitro or treatment with the IGF-1R phosphorylation inhibitor picropodophyllotoxin (PPP) in vitro and in vivo led to markedly induced GH receptor (GHR) abundance, rendering cells more responsive to GH actions. Suppressing IGF-1R triggered DNA damage in both normal human colon cells and three-dimensional human intestinal organoids. DNA damage was further increased when cells with disrupted IGF-1R were treated with GH. Because GH induction of DNA damage accumulation appeared to be mediated not by IGF-1R but probably by more abundant GH receptor expression, we injected athymic mice with GH-secreting xenografts and then treated them with PPP. In these mice, high circulating GH levels were associated with increased colon DNA damage despite disrupted IGF-1R activity (P < 0.01), whereas GHR levels were also induced. Further confirming that GH effects on DNA damage are directly mediated by GHR signaling, GHR−/− mice injected with PPP did not show increased DNA damage, whereas wild-type mice with intact GHR exhibited increased colon DNA damage in the face of IGF-1 signaling suppression. The results indicate that GH directly induces DNA damage independent of IGF-1.
GH secreted by the pituitary mediates most growth promotion by mainly hepatic-derived IGF-1 (1–3). Although mechanisms underlying GH action have been well studied (4–8), the role of IGF-1 in mediating nongrowth effects of GH are not clear. Here we dissect the respective contribution of the two hormones and provide evidence that direct GH receptor (GHR) signaling independent of IGF-1 induces epithelial cell DNA damage.
DNA damage occurs with exposure to exogenous and endogenous genotoxic insults, giving rise to mutations and chromosomal damage, often resulting in cell transformation and tumorigenesis. To limit genomic instability, DNA damage response (DDR) pathways are activated to repair DNA (9, 10). Hormones and their cognate receptors play an important role in DNA damage control. For example, androgens promote DNA repair and tumor cell resistance to DNA damage-inducing therapy in prostate cancer (11, 12), and IGF-1 and IGF-1 receptor (IGF-1R) are necessary for nucleotide excision repair in keratinocytes (13, 14). In contrast, estradiol alters DDR and suppresses effective DNA repair in breast tissue (15). We showed recently that in colon cells, GH inhibits DDR by suppressing ataxia-telangiectasia mutated (ATM) and DNA-dependent protein kinase catalytic subunit (DNA-PKcs) kinase activity, leading to suppressed DNA repair by nonhomologous end joining (NHEJ). GH alone, and in combination with DNA-damaging etoposide, led to accumulated unrepaired DNA, thus promoting chromosomal instability (16).
GH activates IGF-1 production in the liver and in some peripheral tissues, and many GH actions are mediated by IGF-1 (3, 17–20). However, GH also exhibits direct IGF-1–independent actions in adipose, muscle, and bone tissues (21–25). Given the potential for harmful consequences of unrepaired DNA caused by GH, we examined pathways for GH-suppressed DNA repair and sought to determine whether GH effects on DDR in the colon are mediated by IGF-1/IGF-1R.
We show here that GH increased DNA damage in nontumorous human colon cells (hNCCs) and in three-dimensional (3D) intestinal organoids derived from induced human pluripotent stem cells, whereas IGF-1 had no effect on DNA damage in these cells. Suppressing GH signaling with short hairpin GHR (shGHR) RNA decreased DNA damage, whereas suppressing IGF-1 signaling with short hairpin IGF-1R (shIGF-1R) RNA or decreasing IGF-1R activity with specific inhibitor picropodophyllotoxin (PPP) (26) upregulated GHR abundance, increasing DNA damage. Indeed, in colon cells and in human 3D intestinal organoids where IGF-1R was suppressed, and in the colon of mice treated with PPP, DNA damage was increased with GHR upregulation. Furthermore, although wild-type (WT) mice showed induced DNA damage with PPP, GHR−/− mice did not exhibit increased colon DNA damage after PPP treatment. Our results suggest that GH exerts a direct IGF-1–independent effect on DNA damage mediated by the GHR.
Materials and Methods
Cells and treatments
Human colon carcinoma HCT116 cells were obtained from American Type Culture Collection (catalog no. CCL-247; Manassas, VA). HCT116 cells were cultured in McCoy 5A medium (catalog no. 16600082; Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (FBS; catalog no. 100-106; Gemini Bio-Products, West Sacramento, CA). hNCCs were purchased (catalog no. TM003; Applied Biological Materials, Richmond, BC, Canada) and cultured in PriGrow III Media (catalog no. 400-101; Applied Biological Materials) supplemented with 5% FBS. All media were supplemented with antibiotic/antimycotic solution from Gemini Bio-Products (catalog no. 400-101). Cells were infected or treated before passage four per manufacturer instructions.
Recombinant human GH1 (catalog no. 4769-500; BioVision, Milpitas, CA) was reconstituted in PBS pH 8 containing 0.1% BSA. Cells were placed in serum-free culture medium containing 0.1% BSA, GH was added at a concentration of 500 ng/mL, and cells were harvested at the indicated times. Recombinant human IGF-1 (catalog no. 4119-100; BioVision) was reconstituted in water.
IGF-1R specific inhibitor PPP (catalog no. sc-204008; Santa Cruz Biotechnology, Dallas, TX), which suppresses IGF-1R phosphorylation (activation), was reconstituted in dimethyl sulfoxide (DMSO; 50 mM stock solution), and cells were treated with 100, 300, or 600 nM PPP for 24 hours. Control cells were treated with DMSO only (1:10,000 dilution).
Mice
Animal experiments were approved by the Cedars-Sinai Institutional Animal Care and Use Committee (#5587). Athymic nude male mice and GHR−/− mice [B6N(Cg)-Ghrtm1b(KOMP)wtsi/3J] were purchased from the Jackson Laboratory (Bar Harbor, ME). Breeding of GHR−/− mice was performed with heterozygous males and females, so that WT and GHR−/− mice were obtained from the same breeding. Heterozygous mice were backcrossed with WT mice at least five times. Male and female mice 2 to 5 months old were included in the study. WT and GHR−/− pairs were matched by sex and age.
Xenograft model
Lentiviral particle expressing murine GH and control lentiviral particles were generated at the Cedars-Sinai Virus Core facility. HCT116 cells were plated 1 day before transfections. Cells were infected with 50 multiplicity of infection pLV-EF1p-hGH1-IRES-eGFP-WPRE lentiviral particles, and 8 µg/mL polybrene was added. Control cells were infected with empty pLV-EF1p-mCherry-IRES-eGFP-WPRE lentivector. HCT116 cells stably infected with lenti-murine GH (5 × 105 cells in 0.05 mL PBS) were mixed (1:1) with Matrigel (catalog no. 354248; Corning, Corning, NY) and injected subcutaneously into the right flank of athymic nude male mice (Jackson Laboratory) to establish a model of excess systemic GH. Control mice were injected with HCT116 cells infected with empty vector. All mice developed xenograft tumors. In mice bearing lentivirus-expressing murine GH (lentiGH) xenografts, increased circulating GH levels were confirmed with ELISA (catalog no. EZRMGH-45K; Millipore, Burlington, MA).
PPP model
Athymic nude male mice bearing xenografts expressing either GH or empty vector for 5 weeks, or WT and GHR−/− mice, were injected with PPP (20 mg/kg BW in 50 μL DMSO intraperitoneally) twice a day, for a total of four injections, and euthanized 16 hours after the last injection. Control mice were injected with a corresponding volume of DMSO.
Lentiviruses
Lentiviral particles expressing human, GHR, IGF-1R shRNAi, or nontargeted scramble shRNAi control (GFP Control Lentiviral Particles, catalog nos. sc40015V, sc-29358V, and sc-108080, respectively; Santa Cruz Biotechnology) were received as stock solutions (106 IU/200 µM in DMEM). hNCCs were infected with 5 multiplicity of infection lentiviral particles, 8 µg/mL polybrene (catalog no. sc-134220; Santa Cruz Biotechnology) was added, and cells were cultured overnight. Medium was then changed, cells split 48 hours later, and selected thereafter in 8 µg/mL puromycin and were used after fourth passage.
Three-dimensional intestinal organoids
Fibroblasts were obtained from healthy human volunteer donors at Cedars-Sinai (institutional review board no. 00027264). Three-dimensional intestinal organoids were generated from a control fibroblast “83i” induced pluripotent stem cell line by an episomal plasmid reprogramming system. To induce definitive endoderm formation, all induced pluripotent stem cells were cultured with a high dose of activin A (100 ng/mL; R&D Systems, Minneapolis, MN) with increasing concentrations of FBS over time [0%, 0.2%, and 2% (v/v) on days 1, 2, and 3, respectively]. Wnt3A (25 ng/mL; R&D Systems) was also added on the first day of endoderm differentiation. To induce hindgut formation, cells were cultured in advanced DMEM/F12 with 2% (v/v) FBS along with CHIR 99021 (2 μM; Tocris Bioscience, Minneapolis, MN) and FGF4 (500 ng/mL; R&D Systems). After 3 to 4 days, free-floating epithelial spheres and loosely attached epithelial tubes became visible and were harvested. Epithelial structures were subsequently suspended in Matrigel and then overlaid in intestinal medium containing CHIR99021 (2 μM; Tocris), noggin, and EGF (both 100 ng/mL; all R&D Systems) and B27 (1×; Invitrogen). Organoids were passaged every 7 to 10 days thereafter (27).
Protein analysis
Cells were homogenized and lysed in radioimmunoprecipitation assay buffer (catalog no. 98065; Cell Signaling Technology, Danvers, MA) with protease inhibitors (catalog no. p8340; Sigma-Aldrich, St. Louis, MO). Proteins were separated by SDS-PAGE, electroblotted onto Trans-Blot Turbo Transfer Pack 0.2 µm polyvinylidene difluoride membrane (BioRad), and incubated overnight with indicated primary antibodies, followed by corresponding horseradish peroxidase–conjugated secondary antibodies: anti-goat (catalog no. 805-035-180; Jackson ImmunoResearch, West Grove, PA), anti-mouse, and anti-rabbit (catalog nos. NA931V and NA934V, respectively; GE Healthcare Life Sciences, Chicago, IL).
For Western blot analysis, the following primary antibodies were used: β-actin was purchased from Sigma-Aldrich (catalog no. A1978; RRID: AB_476692) (28); GAPDH (catalog no. 2118; RRID: AB_561053) (29), IGF-1R (catalog no. 3027; RRID: AB_2122378) (30), and pIGF-1R (Y1135) (catalog no. 3918; RRID: AB_10548764) (31) were purchased from Cell Signaling; mouse GHR (catalog no. sc-137185; RRID: AB_2111405) (32) was purchased from Santa Cruz Biotechnology; and human GHR (catalog no. AF1210; RRID: AB_2294700) (33) and rat GHR (catalog no. AF-1211; RRID: AB_2111410) (34) were purchased from R&D Systems.
Comet assay
The extent of nuclear DNA damage in an individual cell was detected by analyzing accumulation of DNA breaks by using an OxiSelect Comet Assay kit (catalog no. STA-350; Cell Biolabs, San Diego, CA) per manufacturer instructions. Single-cell alkaline electrophoresis was used for 30 minutes at 1 V/cm. The level of DNA damage (intensity of the staining) was measured by ImageJ as a percentage of damaged DNA in the tail of the entire cell DNA and multiplied by the length of the tail (olive tail moment, tail DNA% × tail moment length). Data were collected from at least three independent experiments. For in vivo experiments, colon tissue was resected from mice, and epithelial mucosal cells were gently scraped out, washed in ice-cold PBS, and incubated in 20 mM EDTA in PBS without magnesium and calcium for 10 minutes to dissociate the tissue into a single-cell suspension.
At least 1000 nuclei per group were analyzed for DNA damage in colon mucosal cells, and at least 200 nuclei per group were analyzed in cultured cells.
Statistics
Differences between groups were tested with mixed model regression to allow random effects of intra-assay variation. Post hoc testing was performed by Tukey test to control for multiple comparisons. Concentrations of circulating GH were assessed by two-tailed Student t test. The in vivo effects of GH and GHR−/− genotype were analyzed via two-way ANOVA. Residuals were inspected to confirm that data met assumptions necessary for parametric testing. Differences were considered significant where P < 0.05. Data are graphed as fold change or percentage of control, and statistical testing was performed on individual values.
Results
GH but not IGF-1 induces DNA damage in colon cells
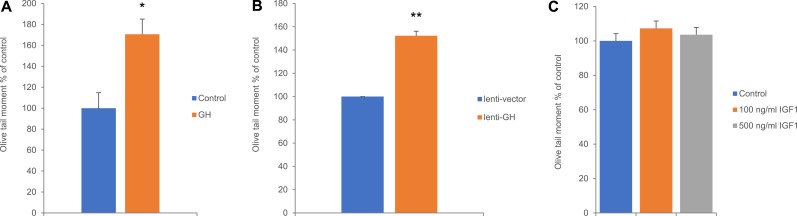
To investigate the effects of GH on DNA damage, we used high dosages of GH to recapitulate levels observed in patients with acromegaly (35). Treatment of hNCCs with 500 ng/mL GH for 24 hours increased unrepaired DNA damage (70 ± 14.9%) as assessed by Comet assay (Fig. 1A). Similar results were observed in lentiviral human GH hNCC transfectants, which showed nearly 50% higher levels of DNA damage compared with empty vector transfectants (Fig. 1B). To examine the role of IGF-1 in this process, cells were treated with human IGF-1 (100 ng/mL or 500 ng/mL), and when levels of DNA damage assessed by Comet assay 24 hours after treatment, differences in DNA damage were not observed between control and IGF-1–treated cells (Fig. 1C).
Figure 1.
GH induces DNA damage in hNCCs. Olive tail moment in hNCCs (A) treated with 500 ng/mL GH and harvested 24 h later, (B) infected with lentiGH or lentiV and harvested 7 d after infection, and (C) treated with 100 and 500 ng/mL human IGF-1, respectively, and harvested 24 h later. Results shown are mean ± SEM of three independent experiments. Differences were assessed with Tukey-adjusted mixed model regression. *P < 0.05, **P < 0.01 vs control. Data are graphed as percentage of control, and statistical testing was performed on individual values.
Suppression of IGF-1 signaling increases DNA damage mediated by GHR
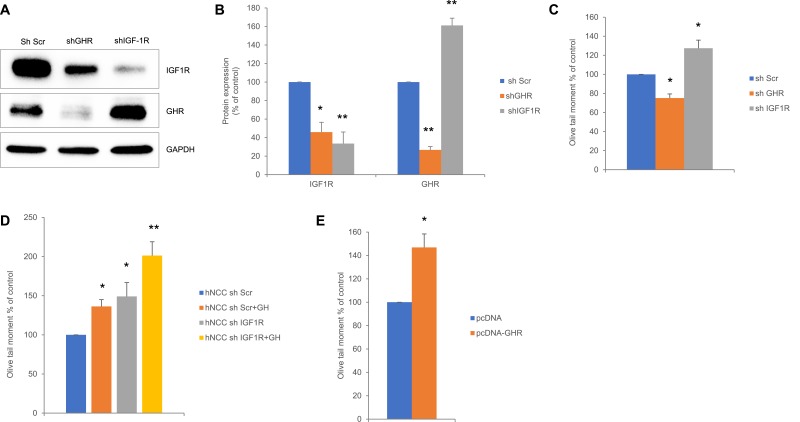
hNCCs were stably infected with lentivirus expressing either shGHR or shIGF-1R, or with lentivirus expressing scramble shRNA (shSCR). Western blot analysis showed that IGF-1R expression was lower in cells with suppressed GHR, whereas GHR expression was markedly induced in cells with suppressed IGF-1R (Fig. 2A and 2B). Comet assay showed decreased DNA damage in cells with suppressed GHR, but DNA damage was increased with suppressed IGF-1R (Fig. 2C). To determine whether this increase was due to GHR upregulation, we treated cells infected with lentiviruses with exogenous GH (500 ng/mL) for 24 hours. DNA damage was increased in control cells treated with GH and similarly induced in cells with suppressed IGF-1R. However, when cells with suppressed IGF-1R and exhibiting high GHR expression were treated with GH, levels of DNA damage were further increased compared with control untreated and GH-treated cells (Fig. 2D). These results suggest that induced DNA damage occurring in shIGF-1R cells in response to GH treatment is associated with high GHR expression.
Figure 2.
Suppression of IGF-1R induces GHR expression and increases DNA damage in hNCCs. (A) Representative Western blot of hNCCs infected with lentivirus expressing either scramble shRNA (shScr) as control or shGHR or shIGF-1R RNAi and analyzed 24 h after infection and then assessed for GHR and IGF-1R expression. (B) ImageJ quantification of protein expression in (A) normalized to loading controls. Results shown are mean ± SEM of three independent experiments. Differences were assessed with Tukey-adjusted mixed model regression. (C) Olive tail moment of hNCCs stably infected with shScr (control), shGHR, or shIGF-1 RNAi. (D) Olive tail moment of hNCCs stably infected with shScr (control) or shIGF-1R, or infected with shIGF-1R RNAi and treated with 500 ng/mL hGH (shScr + GH and shIGF-1R + GH, respectively) and harvested 24 h later. (E) Olive tail moment of hNCCs nucleofected with either pcDNA (control) or pcDNA-GHR and harvested 48 h after nucleofection. Results shown are mean ± SEM of three independent experiments. Differences were assessed with Tukey-adjusted mixed model regression. *P < 0.05, **P < 0.01 vs control. Data are graphed as percentage of control, and statistical testing was performed on individual values.
To test this hypothesis, we nucleofected hNCCs with plasmids expressing GHR and at 48 hours observed a 46% ± 5% increase in DNA damage (Fig. 2E).
Suppressing IGF-1R activity dose-dependently increases GHR expression and DNA damage in human colon cells and in 3D intestinal organoids
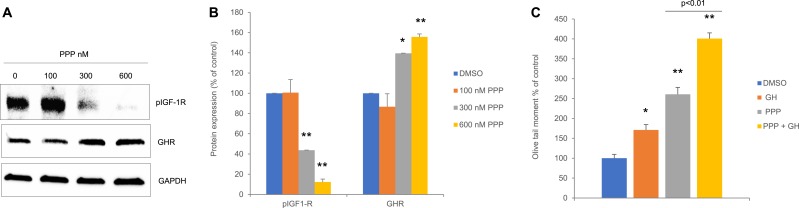
hNCCs were treated with PPP, a nontoxic, noncompetitive, specific inhibitor of IGF-1R tyrosine autophosphorylation (26). At 24 hours, IGF-R phosphorylation was decreased, concomitantly with increased GHR expression (Fig. 3A and 3B) and induced DNA damage levels (Fig. 3C). These results are concordant with the observed increased GHR expression and induced DNA damage in hNCCs transfectant with shIGF-1R (Fig. 2A–2D).
Figure 3.
Suppression of IGF-1R activity induces GHR expression and increases DNA damage in hNCCs. (A) Representative Western blot of hNCCs treated with 300 and 600 nM PPP and harvested 24 h later. (B) ImageJ quantification of protein expression in (A) normalized to loading control. Results shown are mean ± SEM of three independent experiments. (C) Olive tail moment in hNCCs treated with DMSO control or with 300 and 600 nM PPP and harvested 24 h later. Results shown are mean ± SEM of three independent experiments. Differences were assessed with Tukey-adjusted mixed model regression. *P < 0.05, **P < 0.01 vs control. Data are graphed as percentage of control, and statistical testing was performed on individual values. C, control (DMSO).
We next tested effects of IGF-1R suppression in human 3D intestinal organoids derived from induced pluripotent stem cells. As in hNCCs, suppression of IGF-1R activity with PPP led to increased GHR expression (Fig. 4A and 4B). Comet assay of these cells showed increased DNA damage after treatment of organoids with 300 nM PPP, and signs of DNA damage were further increased in organoids treated with both PPP and GH (Fig. 4C), suggesting that GH signaling is directly associated with increased DNA damage.
Figure 4.
Suppression of IGF-1R activity induces GHR expression and increases DNA damage in human 3D intestinal organoids. (A) Representative Western blot of organoids treated with 100, 300, and 600 nM PPP and harvested 24 h later. (B) ImageJ quantification of protein expression in (A) normalized to loading control. Results shown are mean ± SEM of three independent experiments. (C) Olive tail moment in organoids treated with 500 ng/mL GH (GH), 300 nM PPP (PPP), or 300 nM PPP and 500 ng/mL GH (PPP + GH) and harvested 24 h later. Results shown are mean ± SEM of three independent experiments. Differences were assessed with Tukey-adjusted mixed model regression. *P < 0.05, **P < 0.01 vs control. Data are graphed as percentage of control, and statistical testing was performed on individual values.
High circulating GH increases colon DNA damage in mice independent of IGF-1R activity
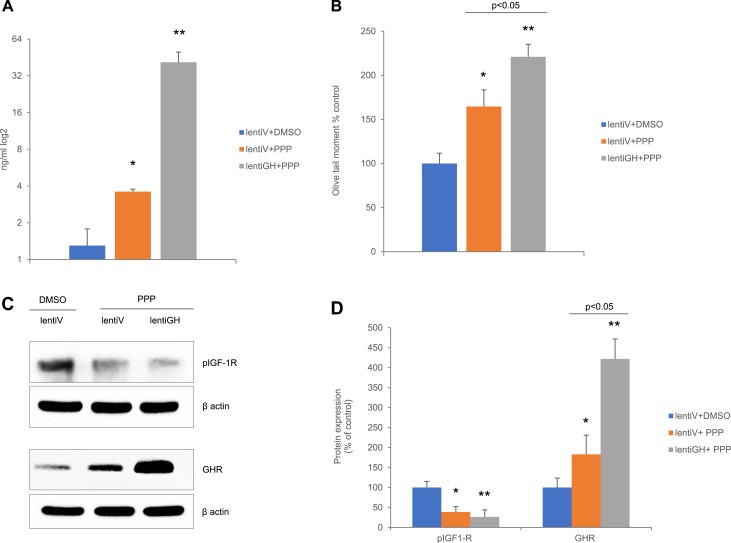
To confirm our findings in vivo, athymic nude mice were injected with HCT116 cells stably infected with lentiGH or empty vector (lentiV). Five weeks after xenograft inoculation and 2 days before euthanasia, lentiV and lentiGH mice were injected with PPP. Suppression of IGF-1R with PPP in lentiV mice led to a twofold increase in circulating GH levels, probably because of release of pituitary GH from IGF-1 negative feedback. By contrast, mice bearing GH xenografts exhibited ∼20-fold increased circulating GH levels (Fig. 5A). Similarly, suppression of IGF-1R with PPP caused ∼60% more colon DNA damage compared with control animals, whereas lentiGH mice treated with PPP demonstrated 220% increase in colon DNA damage (Fig. 5B). Western blot analysis showed that suppressed IGF-1R in control lentiV mice resulted in increased colon GHR expression, which was further induced in lentiGH mice (Fig. 5C and 5D). Induced GHR was probably responsible for the observed increased colon DNA damage, which was further increased after PPP treatment (Fig. 5B), suggesting that GHR abundance renders mice susceptible to DNA-damaging effects of GH.
Figure 5.
High circulating GH increases colon DNA damage in mice independent of IGF-1R activity. Nude male mice were injected with xenograft bearing HCT116 lentiGH or lentiV and treated with PPP or DMSO (control). (A) Concentration of circulating GH. Results shown are mean ± SEM of 8 to 10 mice per group. Differences are assessed by two-tail Student t test. (B) Olive tail moment. Results shown are mean ± SEM. Four mice per group were analyzed. (C) Representative Western blot of colon tissue. (D) ImageJ quantification of protein expression in (C) normalized to loading controls. Results shown are mean ± SEM of 8 to 10 mice per group. In (B) and (D), differences were assessed with Tukey-adjusted mixed model regression. *P < 0.05, **P < 0.01 vs control. Data are graphed as percentage of control, and statistical testing was performed on individual values.
GH signaling promotes DNA damage independent of IGF-1R
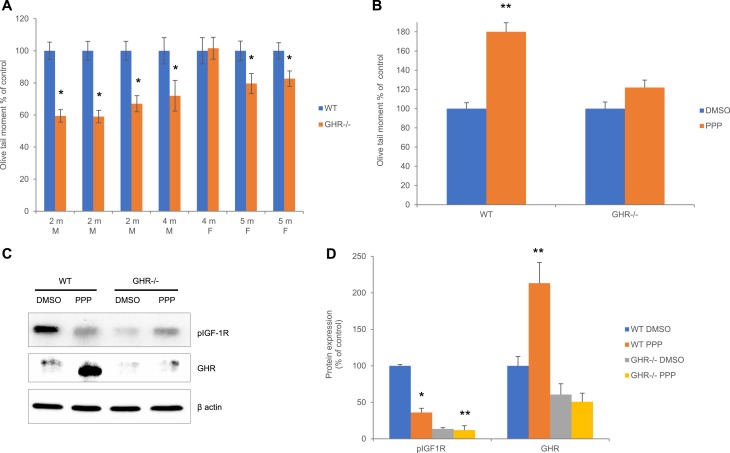
We next sought to confirm a role for GHR in promoting DNA damage in GHR−/− mice by testing endogenous colon DNA damage. We tested colon epithelial cell in males at 2 to 4 months and females at 4 to 5 months, comparing WT and GHR−/− paired by age and sex at each time point. Global results of this experiment were reported earlier (16); here, we present individual levels of colon DNA damage in each pair. Similar to what we observed in hNCCs treated with shGHR RNA (Fig. 2C), GHR−/− mice exhibited decreased levels of colon DNA damage in nearly all pairs (Fig. 6A). We next treated WT and GHR−/− mice with PPP or DMSO as control, and two-way ANOVA showed significant interaction of genotype (P = 0.032) and PPP treatments (P = 0.0023) with colon epithelial DNA damage. Unlike GHR−/−, WT mice exhibited greater DNA damage in response to PPP (Fig. 6B). Furthermore, Western blot analysis of colon tissue showed that suppressed IGF-1R resulted in GHR upregulation in WT but not in GHR−/− mice (Fig. 6C and 6D). Thus, as we observed in lentiV mice, suppressing IGF-1R in WT mice induced high GHR expression and correspondingly increased DNA damage. Accordingly, GHR−/− mice, which weakly express nonfunctional GHR, did not respond to PPP with either increased GHR or increased DNA damage. These results support the hypothesis that GHR induced in response to IGF-1R suppression mediates DDR and suggest that GHR signals independent of IGF and IGF-1R.
Figure 6.
GH signaling promotes DNA damage independent of IGF-1R. (A, B) Olive tail moment in the colon of GHR−/− and WT (A) paired according to age and sex and (B) after treatment with 300 nM PPP or DMSO (control), n = 4 (two males + two females) per group. Data were tested by two-way ANOVA, followed by Tukey test to adjust for multiple group comparisons showing significant interaction of genotype (P = 0.032) and PPP treatments (P = 0.0023) with DNA damage. Pairwise differences were assessed with Tukey-adjusted mixed model regression. (C) Representative Western blot of the colon tissue of WT and GHR−/− male mice treated with 300 nM PPP. (D) ImageJ quantification of protein expression in (C) normalized to loading controls. Results shown are mean ± SEM of five to six mice per group. Differences were assessed with Tukey-adjusted mixed model regression. *P < 0.05, **P < 0.01 vs control. Data are graphed as percentage of control, and statistical testing was performed on individual values. F, female; M, male.
Discussion
Numerous GH functions are mediated by IGF-1, and GHR also interacts with IGF-1R to activate JAK/STAT pathways in response to GH (36, 37). DNA damage and repair mechanisms play a crucial role in tumorigenesis, because most oncogenic alterations are associated with inefficient DNA damage repair (38). The observations shown here support the notion that GH elicits a direct, IGF-1–independent effect on DNA damage repair and provide a mechanism for nongrowth GH action.
GH action may be cell specific; for example, in human LNCaP prostate cancer cells, suppressed IGF-1R was shown to diminish GH signaling (36). High circulating murine GH also decreased p53 in murine colon but not in liver (39), whereas transgenic mice overexpressing bovine GH showed induced white adipose tissue p53 (40).
The role of IGF-1R in DDR includes promotion of DNA repair. In the skin, IGF-1R signaling regulates DNA repair through downstream targets PI3K/AKT (41, 42) or through the ATR-Chk1 kinase pathway (43), and IGF1-R promotes DNA repair in primary human lung fibroblasts, in several human cancer cells (13), and in irradiated salivary glands (44). Disrupted GH signaling leads to partial suppression of IGF-1R expression, possibly because of decreased colon cell IGF-1 expression. Suppression of IGF-1R caused decreased ATM phosphorylation, which plays a critical role in homologous recombination and DNA-PKc phosphorylation, the key kinase involved in NHEJ (45, 46). These signaling defects may result in decreased DNA damage repair and accumulated endogenous DNA damage observed in colon cells with suppressed IGF-1R. Furthermore, we previously showed that p53 directly stimulates the GH promoter (47). Therefore, we propose that when IGF-1R activity is blocked by shRNA or PPP, endogenous DNA damage accumulates, with subsequently increased p53 and induced GH, leading to markedly upregulated human colon cell GHR, also observed in human intestinal organoids and murine colon tissue, and rendering them all more susceptible to GH action. Accumulation of unrepaired DNA damage enabled GH/GHR signaling, which further amplified the DNA damage observed in cells treated with GH.
Our results showing induced DNA damage in the face of IGF-1R suppression in hNCC, human intestinal organoids, and murine colon support these observations. We showed recently that GH signaling suppresses DNA damage responses and repair by inhibiting phosphorylation of ATM, a key sensor of DNA damage and a major regulator of DDR signaling pathway (16). As we show here that inhibition of IGF-1R results in GHR induction, our results suggest that in normal colon cells and tissue, increased GHR signaling may directly contribute to increased DNA damage.
GH effects on DDR are cell type dependent, and in many cell types GH decreases DNA damage. Thus, GH decreased apoptosis, increased proliferation, and increased DNA damage repair in Chinese hamster ovary-4 cells treated with bleomycin (48) and also protected human breast cancer cells from the DNA-damaging effects of cytotoxic drugs (49). Autocrine human GH (hGH) also reduced sensitivity to radiation treatment in human mammary and endometrial carcinoma cell lines by attenuating DNA damage (50).
In contrast, injections of supraphysiological doses of hGH to human subjects for 6 weeks resulted in increased lymphocyte DNA damage, and unrepaired DNA damage remained elevated for 30 days after hGH withdrawal in some subjects (51). Patients with acromegaly with elevated GH levels from a GH-secreting pituitary tumor show chromosomal and DNA damage in peripheral blood lymphocytes (52). A zebrafish model of acromegaly also exhibited elevated DNA damage and impaired DNA damage pathways (53). Although little is known about tissue GHR expression in acromegaly, transgenic mice expressing bovine GH show induced liver GHR levels (54).
p53 regulates DNA damage repair proteins (9, 10), and p53 accumulates in response to DNA damage, causing either cell cycle arrest, apoptosis, or senescence depending on cell type, and intensity of DNA damage. IGF-1 and GH differ in their regulation of p53. Acute IGF-1 treatment of human fibroblasts increases cell proliferation, whereas prolonged IGF-1 exposure leads to p53 accumulation, cell cycle arrest, and senescence in human and mouse fibroblasts (55) and in murine hepatic stellate cells (56). High p53, in turn, suppresses IGF-1R transcription (57). In contrast, both acute and prolonged GH treatment suppress p53 in hNCC, human 3D intestinal organoids, and murine colon tissue (39). In turn, induced p53 triggers GH promoter activity (47). Thus, GH and IGF-1 have apparent opposing effects on the DNA damage signaling pathway.
We demonstrate that suppression or inactivation of IGF-1R induces GHR associated with increased DNA damage. Our findings showing that GHR−/− mice devoid of functional GHR do not respond to suppression of IGF-1R with increased DNA damage buttress the hypothesis suggesting an IGF-1–independent role for GHR in mediating DNA damage. Together with our recent reports showing that GH suppresses DNA damage response and repair (16), we propose that in colon cells, GH at the dosages used may elicit a direct effect on the DNA damage signaling pathway, independent of IGF-1 and IGF-1R activity. Demonstrating direct GH action elucidates a mechanism for non–growth-associated GH action.
Acknowledgments
We are grateful to Catherine Bresee for statistical analysis and to Shira Berman for assistance with manuscript preparation.
Financial Support: Support was provided by National Institutes of Health Grant DK113998 (S.M.), Pfizer ASPIRE Award WI215910 (S.M.), and the Doris Factor Molecular Endocrinology Laboratory at Cedars-Sinai. Funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 3D
three-dimensional
- ATM
ataxia-telangiectasia mutated
- DDR
DNA damage response
- DMSO
dimethyl sulfoxide
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- FBS
fetal bovine serum
- GHR
GH receptor
- hGH
human GH
- hNCC
nontumorous human colon cell
- IGF-1R
IGF-1 receptor
- lentiGH
lentivirus-expressing murine GH
- lentiV
empty vector
- NHEJ
nonhomologous end joining
- PPP
picropodophyllotoxin
- shGHR
short hairpin GH receptor
- shIGF-1R
short hairpin IGF-1 receptor
- shRNA
short hairpin RNA
- shRNAi
short hairpin RNA interference
- shSCR
scramble short hairpin RNA
- WT
wild-type
References and Notes
- 1. Laron Z. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol. 2001;54(5):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clayton PE, Gill MS, Tillmann V, Westwood M. Translational neuroendocrinology: control of human growth. J Neuroendocrinol. 2014;26(6):349–355. [DOI] [PubMed] [Google Scholar]
- 3. Chia DJ. Minireview: mechanisms of growth hormone–mediated gene regulation. Mol Endocrinol. 2014;28(7):1012–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartke A, Quainoo N. Impact of growth hormone–related mutations on mammalian aging. Front Genet. 2018;9:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartke A. Healthspan and longevity can be extended by suppression of growth hormone signaling. Mamm Genome. 2016;27(7–8):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perry JK, Liu DX, Wu ZS, Zhu T, Lobie PE. Growth hormone and cancer: an update on progress. Curr Opin Endocrinol Diabetes Obes. 2013;20(4):307–313. [DOI] [PubMed] [Google Scholar]
- 7. Waters MJ, Brooks AJ. Growth hormone and cell growth. Endocr Dev. 2012;23:86–95. [DOI] [PubMed] [Google Scholar]
- 8. Buchman M, Bell S, Kopchick JJ. Growth hormone discovery and structure. Pediatr Endocrinol Rev. 2018;16(suppl 1):2–10. [DOI] [PubMed] [Google Scholar]
- 9. Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16(1):20–33. [DOI] [PubMed] [Google Scholar]
- 10. Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40(2):179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, Ma T, Den RB, Dicker AP, Feng FY, Knudsen KE. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3(11):1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, Arora VK, Yen WF, Cai L, Zheng D, Carver BS, Chen Y, Watson PA, Shah NP, Fujisawa S, Goglia AG, Gopalan A, Hieronymus H, Wongvipat J, Scardino PT, Zelefsky MJ, Jasin M, Chaudhuri J, Powell SN, Sawyers CL. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3(11):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Auclair Y, Rouget R, Affar B, Drobetsky EA. ATR kinase is required for global genomic nucleotide excision repair exclusively during S phase in human cells. Proc Natl Acad Sci USA. 2008;105(46):17896–17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loesch MM, Collier AE, Southern DH, Ward RE, Tholpady SS, Lewis DA, Travers JB, Spandau DF. Insulin-like growth factor-1 receptor regulates repair of ultraviolet B-induced DNA damage in human keratinocytes in vivo. Mol Oncol. 2016;10(8):1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caldon CE. Estrogen signaling and the DNA damage response in hormone dependent breast cancers. Front Oncol. 2014;4:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chesnokova V, Zonis S, Barrett R, Kameda H, Wawrowsky K, Ben-Shlomo A, Yamamoto M, Gleeson J, Bresee C, Gorbunova V, Melmed S. Excess growth hormone suppresses DNA damage repair in epithelial cells. JCI Insight. 2019;4(3):e125762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7(1):11–24. [DOI] [PubMed] [Google Scholar]
- 18. Lanning NJ, Carter-Su C. Recent advances in growth hormone signaling. Rev Endocr Metab Disord. 2006;7(4):225–235. [DOI] [PubMed] [Google Scholar]
- 19. Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol. 2013;9(6):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clemmons DR. The relative roles of growth hormone and IGF-1 in controlling insulin sensitivity. J Clin Invest. 2004;113(1):25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yakar S, Isaksson O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: lessons from mouse models. Growth Horm IGF Res. 2016;28:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29(5):535–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miquet JG, Freund T, Martinez CS, González L, Díaz ME, Micucci GP, Zotta E, Boparai RK, Bartke A, Turyn D, Sotelo AI. Hepatocellular alterations and dysregulation of oncogenic pathways in the liver of transgenic mice overexpressing growth hormone [published correction appears in Cell Cycle. 2015;14(15):2537]. Cell Cycle. 2013;12(7):1042–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chikani V, Ho KK. Action of GH on skeletal muscle function: molecular and metabolic mechanisms. J Mol Endocrinol. 2013;52(1):R107–R123. [DOI] [PubMed] [Google Scholar]
- 25. Sjögren K, Leung KC, Kaplan W, Gardiner-Garden M, Gibney J, Ho KK. Growth hormone regulation of metabolic gene expression in muscle: a microarray study in hypopituitary men. Am J Physiol Endocrinol Metab. 2007;293(1):E364–E371. [DOI] [PubMed] [Google Scholar]
- 26. Girnita A, Girnita L, del Prete F, Bartolazzi A, Larsson O, Axelson M. Cyclolignans as inhibitors of the insulin-like growth factor-1 receptor and malignant cell growth. Cancer Res. 2004;64(1):236–242. [DOI] [PubMed] [Google Scholar]
- 27. Barrett R, Ornelas L, Yeager N, Mandefro B, Sahabian A, Lenaeus L, Targan SR, Svendsen CN, Sareen D. Reliable generation of induced pluripotent stem cells from human lymphoblastoid cell lines. Stem Cells Transl Med. 2014;3(12):1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.RRID:AB_476692, https://scicrunch.org/resolver/AB_476692.
- 29.RRID:AB_561053, https://scicrunch.org/resolver/AB_561053.
- 30.RRID:AB_2122378, https://scicrunch.org/resolver/AB_2122378.
- 31.RRID:AB_10548764, https://scicrunch.org/resolver/AB_10548764.
- 32.RRID:AB_2111405, https://scicrunch.org/resolver/AB_2111405.
- 33.RRID:AB_2294700, https://scicrunch.org/resolver/AB_2294700.
- 34.RRID:AB_2111410, https://scicrunch.org/resolver/AB_2111410.
- 35. D’Arcy R, Courtney CH, Graham U, Hunter S, McCance DR, Mullan K. Twenty-four-hour growth hormone profiling in the assessment of acromegaly. Endocrinol Diabetes Metab. 2017;1(1):e00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gan Y, Buckels A, Liu Y, Zhang Y, Paterson AJ, Jiang J, Zinn KR, Frank SJ. Human GH receptor-IGF-1 receptor interaction: implications for GH signaling. Mol Endocrinol. 2014;28(11):1841–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gan Y, Zhang Y, Digirolamo DJ, Jiang J, Wang X, Cao X, Zinn KR, Carbone DP, Clemens TL, Frank SJ. Deletion of IGF-I receptor (IGF-IR) in primary osteoblasts reduces GH-induced STAT5 signaling. Mol Endocrinol. 2010;24(3):644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khanna A. DNA damage in cancer therapeutics: a boon or a curse? Cancer Res. 2015;75(11):2133–2138. [DOI] [PubMed] [Google Scholar]
- 39. Chesnokova V, Zonis S, Zhou C, Recouvreux MV, Ben-Shlomo A, Araki T, Barrett R, Workman M, Wawrowsky K, Ljubimov VA, Uhart M, Melmed S. Growth hormone is permissive for neoplastic colon growth [published correction appears in Proc Natl Acad Sci USA. 2016;113(35):E5251]. Proc Natl Acad Sci USA. 2016;113(23):E3250–E3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bogazzi F, Raggi F, Russo D, Bohlooly-Y M, Sardella C, Urbani C, Lombardi M, Manetti L, Lupi I, Tornell J, Martino E. Growth hormone is necessary for the p53-mediated, obesity-induced insulin resistance in male C57BL/6J x CBA mice. Endocrinology. 2013;154(11):4226–4236. [DOI] [PubMed] [Google Scholar]
- 41. Kemp MG, Spandau DF, Simman R, Travers JB. Insulin-like growth factor 1 receptor signaling Is required for optimal ATR-CHK1 kinase signaling in ultraviolet B (UVB)-irradiated human keratinocytes. J Biol Chem. 2017;292(4):1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kemp MG, Spandau DF, Travers JB. Impact of age and insulin-like growth factor-1 on DNA damage responses in UV-irradiated human skin. Molecules. 2017;22(3):E356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strozyk E, Kulms D. The role of AKT/mTOR pathway in stress response to UV-irradiation: implication in skin carcinogenesis by regulation of apoptosis, autophagy and senescence. Int J Mol Sci. 2013;14(8):15260–15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meyer S, Chibly AM, Burd R, Limesand KH. Insulin-like growth factor-1-mediated DNA repair in irradiated salivary glands is sirtuin-1 dependent. J Dent Res. 2017;96(2):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chitnis MM, Lodhia KA, Aleksic T, Gao S, Protheroe AS, Macaulay VM. IGF-1R inhibition enhances radiosensitivity and delays double-strand break repair by both non-homologous end-joining and homologous recombination. Oncogene. 2014;33(45):5262–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Turney BW, Kerr M, Chitnis MM, Lodhia K, Wang Y, Riedemann J, Rochester M, Protheroe AS, Brewster SF, Macaulay VM. Depletion of the type 1 IGF receptor delays repair of radiation-induced DNA double strand breaks. Radiother Oncol. 2012;103(3):402–409. [DOI] [PubMed] [Google Scholar]
- 47. Chesnokova V, Zhou C, Ben-Shlomo A, Zonis S, Tani Y, Ren SG, Melmed S. Growth hormone is a cellular senescence target in pituitary and nonpituitary cells. Proc Natl Acad Sci USA. 2013;110(35):E3331–E3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Madrid O, Varea S, Sanchez-Perez I, Gomez-Garcia L, De Miguel E, Gomez De Segura IA, Perona R. Growth hormone protects against radiotherapy-induced cell death. Eur J Endocrinol. 2002;147(4):535–541. [DOI] [PubMed] [Google Scholar]
- 49. Zatelli MC, Minoia M, Molè D, Cason V, Tagliati F, Margutti A, Bondanelli M, Ambrosio MR, degli Uberti E. Growth hormone excess promotes breast cancer chemoresistance. J Clin Endocrinol Metab. 2009;94(10):3931–3938. [DOI] [PubMed] [Google Scholar]
- 50. Bougen NM, Steiner M, Pertziger M, Banerjee A, Brunet-Dunand SE, Zhu T, Lobie PE, Perry JK. Autocrine human GH promotes radioresistance in mammary and endometrial carcinoma cells. Endocr Relat Cancer. 2012;19(5):625–644. [DOI] [PubMed] [Google Scholar]
- 51. Fantini C, Sgrò P, Pittaluga M, de Perini A, Dimauro I, Sartorio A, Caporossi D, Di Luigi L. Short-term, supra-physiological rhGH administration induces transient DNA damage in peripheral lymphocytes of healthy women. J Endocrinol Invest. 2017;40(6):645–652. [DOI] [PubMed] [Google Scholar]
- 52. Bayram F, Bitgen N, Donmez-Altuntas H, Cakir I, Hamurcu Z, Sahin F, Simsek Y, Baskol G. Increased genome instability and oxidative DNA damage and their association with IGF-1 levels in patients with active acromegaly. Growth Horm IGF Res. 2014;24(1):29–34. [DOI] [PubMed] [Google Scholar]
- 53. Elbialy A, Asakawa S, Watabe S, Kinoshita S. A zebrafish acromegaly model elevates DNA damage and impairs DNA repair pathways. Biology (Basel). 2018;7(4):E47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iida K, Del Rincon JP, Kim DS, Itoh E, Nass R, Coschigano KT, Kopchick JJ, Thorner MO. Tissue-specific regulation of growth hormone (GH) receptor and insulin-like growth factor-I gene expression in the pituitary and liver of GH-deficient (lit/lit) mice and transgenic mice that overexpress bovine GH (bGH) or a bGH antagonist. Endocrinology. 2004;145(4):1564–1570. [DOI] [PubMed] [Google Scholar]
- 55. Tran D, Bergholz J, Zhang H, He H, Wang Y, Zhang Y, Li Q, Kirkland JL, Xiao ZX. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell. 2014;13(4):669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nishizawa H, Iguchi G, Fukuoka H, Takahashi M, Suda K, Bando H, Matsumoto R, Yoshida K, Odake Y, Ogawa W, Takahashi Y. IGF-I induces senescence of hepatic stellate cells and limits fibrosis in a p53-dependent manner. Sci Rep. 2016;6(1):34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Werner H, Maor S. The insulin-like growth factor-I receptor gene: a downstream target for oncogene and tumor suppressor action. Trends Endocrinol Metab. 2006;17(6):236–242. [DOI] [PubMed] [Google Scholar]