Abstract
Background:
Consensus criteria for classifying tremor disorders were published by the International Parkinson and Movement Disorder Society in 1998. Subsequent advances with regard to essential tremor, tremor associated with dystonia, and other monosymptomatic and indeterminate tremors make a significant revision necessary.
Objectives:
Convene an international panel of experienced investigators to review the definition and classification of tremor.
Methods:
Computerized MEDLINE searches in January 2013 and 2015 were conducted using a combination of text words and MeSH terms: “tremor”, “tremor disorders”, “essential tremor”, “dystonic tremor”, and “classification” limited to human studies. Agreement was obtained using consensus development methodology during four in-person meetings, two teleconferences, and numerous manuscript reviews.
Results:
Tremor is defined as an involuntary, rhythmic, oscillatory movement of a body part and is classified along two axes: Axis 1—clinical characteristics, including historical features (age at onset, family history, and temporal evolution), tremor characteristics (body distribution, activation condition), associated signs (systemic, neurological), and laboratory tests (electrophysiology, imaging); and Axis 2—etiology (acquired, genetic, or idiopathic). Tremor syndromes, consisting of either isolated tremor or tremor combined with other clinical features, are defined within Axis 1. This classification scheme retains the currently accepted tremor syndromes, including essential tremor, and provides a framework for defining new syndromes.
Conclusions:
This approach should be particularly useful in elucidating isolated tremor syndromes and syndromes consisting of tremor and other signs of uncertain significance. Consistently defined Axis 1 syndromes are needed to facilitate the elucidation of specific etiologies in Axis 2.
Keywords: tremor, classification, diagnostic axes, etiology, tremor syndromes
Consensus criteria for classifying tremor disorders were published by the Movement Disorders Society (now called the International Parkinson and Movement Disorder Society [IPMDS]) in 1998.1 Subsequent advances have highlighted limitations of these criteria, particularly with regard to essential tremor (ET), tremor associated with dystonia or other additional neurological abnormalities, and focal tremors. The previous consensus did not use a consistent approach to tremor classification. In some instances, a tremor was defined according to its presumed anatomical origin, such as cerebellar tremor pertaining to intention tremor. In other instances, tremor was defined according to a presumed etiology, for example, parkinsonian tremor and tremors associated with neuropathies. Classification of other tremors was based purely on clinical phenomenology, as in primary writing tremor, orthostatic tremor, and isolated voice tremor. None of these approaches was adequate when a particular type of tremor had multiple etiologies.
ET may serve here to illustrate many of the existing problems with tremor classification. First, clinicians vary greatly in their concept of ET,8 particularly in regard to whether ET is an isolated tremor disorder.9 A variety of other signs and symptoms and considerable phenotypic variability have been observed in patients diagnosed with ET.4,9 Therefore, a new classification scheme is needed to facilitate deeper and consensual phenotyping of patients with ET and other forms of tremor that are traditionally viewed as occurring in the absence of other neurological abnormalities. Second, a tremor syndrome like ET may have more than one etiology, and vice versa an etiology of ET could conceivably produce more than one clinical syndrome. A classification scheme that promotes detailed phenotyping is needed to uncover potential differences among patients with various etiologies of ET, such as the rare mutation in the fused in sarcoma gene13 and fragile X premutations.21
Given these limitations and uncertainties in tremor classification, a task force on tremor was convened by the IPMDS to review the 1998 consensus criteria and to develop a revised classification scheme that will allow a deeper phenotyping of ET and other idiopathic tremor syndromes, thereby facilitating the discovery of specific etiologies.
Materials and Methods
An international task force, consisting of investigators with clinical and research experience in tremor, was convened to review the 1998 consensus criteria in the context of subsequent published work. Computerized MEDLINE searches in January 2013 and 2015 were conducted using a combination of text words and MeSH terms: “tremor”, “tremor disorders”, “essential tremor”, “dystonic tremor”, and “classification” limited to human studies. No language restrictions were applied. The first draft of the manuscript for this article was based on the results of the literature review, data analysis, and comments from task force members. To reach consensus, the draft and the preliminary conclusions were critically discussed during a conference held in May 2013, in Lisbon, Portugal, and during the 2013–2016 International Congresses of the IPMDS. There were also numerous e-mail exchanges and teleconferences. Consensus development conference methodology was used to develop the following tremor classification scheme.30
Results
Definition of Tremor
The task force defines tremor as follows: Tremor is an involuntary, rhythmic, oscillatory movement of a body part. It is important to note that the limbs and head, when unsupported, exhibit slight tremor, referred to as physiological tremor.31 Physiological tremor is generally not visible or symptomatic unless it is enhanced by fatigue or anxiety, whereas pathological tremor is usually visible and persistent. Several movement disorders need to be differentiated from tremor, as discussed previously.1
Classification of Tremor
The proposed classification has two main axes: clinical features (Axis 1) and etiology (Axis 2) (Fig. 1). This approach to disease classification is common in epidemiological studies in which clinical features of a disease are used to define a clinical syndrome, and studies of this syndrome ultimately lead to the discovery of one or more etiologies.33,34 Here, it should be emphasized that a syndrome may have multiple etiologies, and a particular etiology may produce multiple clinical syndromes. This two-axis approach is similar to the new classification scheme for dystonia,35 and it was designed to facilitate the collection of clinically important information from tremor patients and to serve as a tool for clinical diagnosis and research.
FIG. 1.

(A) Axis 1 classification of tremor is based on clinical features from the patient’s medical history and physical examination. Additional tests are sometimes useful. (B) Axis 2 classification is etiology. A syndrome in Axis 1 may have multiple etiologies, and a particular etiology may produce multiple syndromes.
Axis 1: Clinical Features
In this axis, the clinical features of tremor in a given patient are specified in terms of medical history (age of onset, family history, temporal evolution, and exposure to drugs and toxins), tremor characteristics (body distribution, activation condition, and frequency), and associated signs (Fig. 1A). For some tremors, laboratory tests that provide additional characterization may be included (e.g., frequency of polymyographic recordings in orthostatic tremor, structural imaging for lesion locations).32 Receptor imaging and serum and tissue biomarkers can be included and may lead to the discovery of Axis 2 etiologies.
Age of onset is important in guiding diagnostic thinking in patients with tremor. We suggest a rough categorization of patients into the following age groups: infancy (birth to 2 years); childhood (3–12 years); adolescence (13–20 years); early adulthood (21–45 years); middle adulthood (46–60 years); and late adulthood (>60 years). However, age of onset should always be documented as accurately as possible.
The anatomical distribution of tremor is very important and should be carefully documented. Tremor can be focal (only one body region is affected, such as voice, head, jaw, one limb, etc.), segmental (when two or more contiguous body parts in the upper or lower body are affected (e.g., head and arm, or when tremor is bibrachial or bicrural), hemitremor (when one side of the body is affected), and generalized (when tremor affects the upper and lower body). Tremor in the lower limbs or trunk during standing is called orthostatic tremor.
The identification of activation conditions is also critical (Fig. 2). In the 1998 consensus criteria, the two main activation classifications are rest-tremor and action-tremor. The latter includes postural, kinetic (simple and intention), task-specific, and isometric tremors. We propose no change in this nomenclature.
FIG. 2.
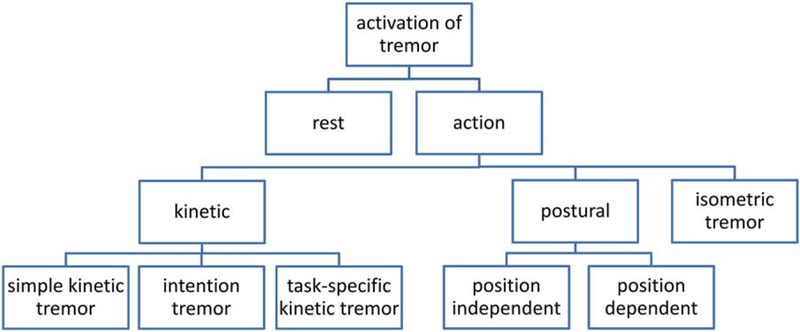
Activation conditions and related nomenclature. The terms action tremor and kinetic tremor are frequently used interchangeably, but they have different meanings.
Rest tremor is tremor in a body part that is not voluntarily activated. It should be assessed when the patient is attempting to relax and is given adequate opportunity to relax the affected body part. This may require complete support of the body part (e.g., the head) against gravity. In Parkinson’s disease (PD), the amplitude of rest tremor almost always diminishes or is abolished, at least transiently, during goal-directed voluntary movements, and tremor amplitude typically increases during mental stress (counting backward, Stroop test, and so forth).36 In addition, rest tremor may appear or increase while walking or when performing movements of another body part. Rest tremor may also occur in advanced ET, but rest tremor does not subside during voluntary movements in ET and possibly also not in dystonic tremor.37 There are ambiguities in the definition of rest tremor. When patients with rest tremor also have tremor during posture or movement, the latter is logically no longer rest tremor; it is an action tremor. Hence, the re-emergent tremor of PD38 should be regarded as an action tremor irrespective of the waveform similarities with rest tremor.
Action tremor occurs while voluntarily maintaining a position against gravity (postural tremor and orthostatic tremor) or during any voluntary movement (kinetic tremor). Kinetic tremor is subdivided into simple kinetic tremor, in which tremor is roughly the same throughout a movement (e.g., waiving with the hands at a slow speed), and intention tremor in which a crescendo increase in tremor occurs as the affected body part approaches its visual target. Other forms of action tremor are position-specific postural tremor, when maintaining a specific position or posture, and task-specific kinetic tremor, which occurs during a specific task such as writing. Isometric tremor occurs during a muscle contraction against a rigid stationary object, as when making a fist or squeezing an examiner’s fingers.
Tremor frequency is commonly used in characterizing tremor, but with a few exceptions, frequency is not very helpful in diagnosis because the frequency of most pathological tremors is 4 to 8 Hz. However, myorhythmia and some palatal tremors have a slow frequency below 4 Hz, and primary orthostatic tremor typically has a frequency of 13 to 18 Hz. The central neurogenic component of physiological tremor is 8 to 12 Hz, and rhythmic cortical myoclonus typically has a frequency greater than 8 Hz. Therefore, tremor frequency is often categorized as <4, 4 to 8, 8 to 12, and >12 Hz. Tremor frequency is most accurately measured with a motion transducer or electromyography.
In addition to characterizing tremor, the physical exam is devoted to the identification of associated or concomitant signs that may aid in clinical diagnosis. We propose two broad categories of tremor in Axis 1: isolated tremor in which tremor is the only abnormal sign and combined tremor in which other abnormal signs are present. Combined tremor may occur with other neurological signs (e.g., dystonic postures, rigidity, bradykinesia, or myoclonus) or with relevant systemic signs (e.g., Kayser-Fleischer ring, hepatosplenomegaly, or exophthalmos).
Axis 2: Etiological Classification
The second axis is etiology (Fig. 1B). Etiologies may be genetic, acquired, or idiopathic. Specific examples are listed in Table 2.
TABLE 2.
Etiological causes of tremor (selection)
| Neurodegenerative disease |
| ○ PD |
| ○ Multiple system atrophy |
| ○ Corticobasal degeneration |
| ○ PSP |
| ○ Genetic disorders: genes causing predominantly parkinsonism |
| ○ Genes causing frontotemporal dementia with parkinsonism |
| ○ Genes causing predominantly dystonia |
| ○ Neuroferritinopathy |
| ○ Spinocerebellar ataxias |
| ○ Genes causing Fahr’s disease |
| ○ Genes causing peripheral neuropathies that produce tremor |
| ○ Wilson’s disease |
| ○ X-linked dystonia parkinsonism/Lubag |
| ○ Lesch-Nyhan’s syndrome |
| ○ Fragile X–associated tremor/ataxia syndrome |
| ○ Spinal muscular atrophy |
| Chromosomal aneuploidy |
| ○ XYY, XXY (Klinefelter’s syndrome), and XXYY syndromes |
| Mitochondrial genetic disorders |
| ○ Leigh’s syndrome |
| ○ Mitochondrial polymerase gamma mutations |
| Infectious and other inflammatory diseases |
| ○ Demyelinating diseases such as multiple sclerosis |
| ○ Encephalitis lethargica, subacute sclerosing panencephalitis, |
| ○ HIV |
| ○ Tuberculosis, syphilis, measles, typhus, neuroborreliosis |
| ○ Bacterial or viral encephalitis |
| ○ Antineuronal antibody disease |
| Endocrine and metabolic disorders |
| ○ Nephrotic or liver failure |
| ○ Hyperthyroidism |
| Neuropathies and spinal muscular atrophies |
| ○ Kennedy’s syndrome |
| ○ Guillain-Barre’s syndrome |
| ○ Gammopathy-induced neuropathies |
| Toxins |
| ○ Mercury |
| ○ Lead |
| ○ Manganese |
| ○ Arsenic |
| ○ Cyanide, DDT, CO |
| ○ Naphthalene |
| ○ Toluene |
| ○ Lindane |
| Drugs |
| ○ Anticonvulsants: valproate, carbamazepine, phenytoin |
| ○ Tetrabenazine, antidepressants, sympathomimetics, bronchodilators, beta-2 agonists |
| ○ Lithium |
| ○ Neuroleptics, metoclopramide |
| ○ Amiodarone |
| ○ Thyroid hormone replacement |
| ○ Anticancer drugs: vincristine, cisplatin, paclitaxel, doxorubicin, cytosine arabinoside, ifosfamide, tacrolimus, 5-fluorouracil, methotrexate |
| ○ Drug and alcohol withdrawal |
| Others |
| ○ Brain neoplasms |
| ○ Brain injury: head trauma, brain surgery, and electrical injury |
| ○ Vascular: ischemia, hemorrhage, and arteriovenous malformations |
| ○ Anxiety and stress |
| ○ Fatigue |
| ○ Cooling |
| ○ Trauma of peripheral tissues |
| ○ HIV, human immunodeficiency virus. |
Axis 1 Tremor Syndromes
Some Axis 1 signs and symptoms will coexist so commonly that a specific clinical syndrome is suggested. A syndrome consisting only of tremor is called an isolated tremor syndrome. A syndrome of consisting of tremor and other systemic or neurological signs is called a combined tremor syndrome. Syndromes are defined for the purpose of facilitating a search for specific etiologies in Axis 2. Syndromes are tools of pattern recognition. The utility of any syndrome will depend on how carefully it is defined and on how consistently it is used.
A tremor syndrome may have multiple etiologies. Theoretically, any combination of features in Axis 1 is a possible syndrome. This approach to tremor classification provides ample flexibility for elucidating new syndromes or phenotypes without etiological assumptions. This is quite important, in particular, for isolated tremors of unknown etiology.
It is self-evident that Axis 1 features may change over time, making an initial syndromic classification invalid. In such cases, the initial syndrome should be documented and retained in the medical history.
Currently accepted Axis 1 syndromes are summarized in the following paragraphs and in Figure 3. For clinical and research purposes, each patient usually can be classified into one of the syndromes listed here. For indeterminate tremors, the question arises of whether this is a new tremor syndrome or whether time is needed for an existing syndrome to become sufficiently established for Axis 1 diagnosis.
FIG. 3.
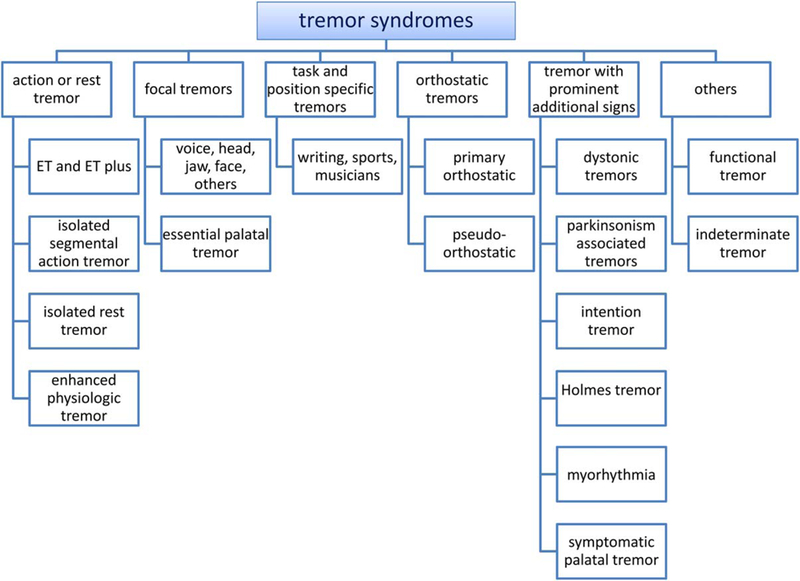
Axis 1 tremor syndromes. Tremor syndromes are listed in this figure according to the predominant presenting symptoms.
Essential Tremor
ET in the 19th century was viewed as an inherent tendency to develop tremor, often hereditary, in the absence of other neurological signs.39 Clinicians ultimately recognized a common isolated tremor syndrome of bilateral upper limb postural or kinetic tremor, with or without head tremor or tremor in other locations, and called this ET. However, the diagnostic criteria for ET have varied substantially among clinicians,1,7,8,40,41 reducing the value of this syndrome in clinical diagnosis and research. An Axis 1 syndrome must be defined and used consistently to have clinical value.
It is important to emphasize that the definition of ET in Axis 1 allows for the existence of multiple etiologies for this common syndrome. Patients frequently have a family history, and small doses of alcohol may improve the tremor. However, these clinical features are not consistent enough to be included in the definition of ET. It was discussed to include onset of tremor in the upper limbs as a further criterion, but there are no convincing data that support this criterion. Some studies have included patients with neurological signs of uncertain relationship to tremor (i.e., “soft neurological signs”), such as mild memory impairment, impaired tandem gait, and subtle body posturing that could be dystonic. There is no consensus on which of these additional signs are acceptable within the definition of ET. In clinical practice, the interpretation of soft signs is subjective and left to the investigator. We propose to clearly label these cases with soft signs as ET plus.
The ET plus syndrome does not include other clearly defined syndromes like dystonic tremor and task-specific tremor.
These definitions of ET and ET plus are refinements of previous definitions of ET, in which soft signs were accepted as ET as long as the clinician deemed these additional signs as related to ET or as insignificant. Such ambiguities and assumptions are a source of diagnostic confusion and etiological heterogeneity within the ET syndrome. An optional refinement of the definition of ET and ET plus is that electrophysiological analysis confirms the presence of central neurogenic oscillation, in contrast to the mechanical-reflex oscillation of physiological tremor.32 This requires special clinical neurophysiologic laboratory testing,42,43 but is valuable in certain research and clinical settings.
The proposed definition of ET requires at least a 3-year history of tremor and excludes isolated head and isolated voice tremors. A 3-year history is included to reduce the odds of subsequent development of other neurological signs (e.g., dystonia, parkinsonism, or ataxia). Even with this safeguard, patients with ET may ultimately develop other signs, at which time they would have a combined tremor syndrome, not ET. In other words, ET is a syndrome that may evolve into another tremor syndrome. Tremor of less than 3 years’ duration that otherwise fulfills criteria for ET should be labeled during the observation period as indeterminate tremor.
In addition, we provide the following important exclusion criteria for the diagnosis of an ET syndrome. If an etiology for patients or a patient group with the Axis 1 syndrome ET is found (e.g., a new gene), the diagnosis will be the specific Axis 2 disease or etiology, replacing the less-specific syndromic diagnosis or classification, but the Axis 1 clinical syndrome will remain an ET syndrome. For example, a patient with ET could be discovered to have an inherited dystonia, ataxia, or parkinsonism, and this patient would there-after be labeled with the specific Axis 2 diagnosis, but would retain the Axis 1 classification until other signs developed. The medical record would document ET syndrome as the initial presentation. This approach is consistent with the general concept of dual classification into syndromic and etiological axes.
Other Axis 1 Isolated Tremor Syndromes
Many patients with this syndrome ultimately fulfill the criteria for ET. Some patients later develop focal or segmental dystonia that is idiopathic or attributed to genetic abnormalities such as anoctamin 3 (ANO3).44 Age of onset and family history may help to identify these cases. Other patients suffer from enhanced physiological tremor.
Enhanced physiological tremor is a very common bilateral upper limb action tremor. It is caused by enhancement of the normal mechanical reflex and 8- to 12-Hz central neurogenic oscillations of physiological tremor by a variety of reversible conditions, such as anxiety, fatigue, hyperthyroidism, and different drugs. We define enhanced physiological tremor as a symptomatic upper extremity action tremor that is potentially reversible if the cause of the tremor is eliminated. The diagnosis of enhanced physiological tremor is confirmed when a specific Axis 2 etiology is found (e.g., hyperthyroidism, sympathomimetic drugs) and when the tremor is normalized with successful management. The most common differential diagnosis is ET, but the duration of enhanced physiological tremor is usually far less than 3 years. Electrophysiological studies are helpful in distinguishing mild ET from enhanced physiological tremor, but the specificity and sensitivity of this approach are not perfect.32 Enhanced physiological tremor also may be mistaken for a whole-body tremulousness that is usually rhythmic cortical myoclonus.45 The suppression of tremor amplitude during movement onset is in favor of a dopaminergic deficiency syndrome like PD.37 It is important to look for associated signs and symptoms of PD (e.g., bradykinesia, rigidity, rapid eye movement sleep behavior disorder). Even if the tremor is isolated, dopamine transporter imaging (e.g., ioflupane I-123 single-photon emission computed tomography; DaTscan]) may be needed to exclude a parkinsonian condition, given that some early tremor-dominant PD patients (monosymptomatic tremor at rest) may exhibit no appreciable bradykinesia or rigidity. The etiology is usually unknown when the rest tremor is isolated and the DaTscan is normal, but some of these cases are considered to have dystonia46 or striatal dopaminergic deficiency without nigral degeneration.47
Isolated Focal Tremors
Several syndromes of focal tremors other than hand tremor are well described. There is ongoing debate whether these tremors are similar in pathophysiology to ET2 or dystonia.5,48
Patients with no signs of dystonia in the vocal apparatus and no tremor, dystonia, or other neurological signs elsewhere are considered to have isolated voice tremor. Cases with hyperabduction or -adduction of the vocal cords, as observed in laryngeal dystonia, or with dystonia in other body parts are classified as dystonic voice tremor of known or idiopathic etiology (see dystonic tremor syndromes). Voice tremor may be observed in dystonia 6 and ANO3 mutation carriers without clinical dystonia.44,49 Voice tremor in ET occurs, by definition, in combination with hand tremor.
Head tremor is common in the context of ET. It is also a common manifestation of tremulous dystonia.48 The relationship between isolated head tremor and focal tremulous cervical dystonia is a topic of ongoing controversy.
There are other rare focal tremors that may occur in the absence of other neurological signs, such as hereditary geniospasm,50 isolated jaw tremor, isolated tongue tremor, rabbit syndrome, and tremor during smiling.51,52
Several syndromes with palatal tremor exist.53,54 Essential palatal tremor presents with the symptom of an ear click, mostly attributed to rhythmic contraction of the tensor veli palatini. Other throat muscles may be involved, but there are no other neurological abnormalities. Essential palatal tremor is therefore an isolated focal tremor syndrome. MRI of the inferior olives is normal, and extremity or eye muscles are not involved. Mostly, the etiology is unknown, but a functional origin has been described.55
Symptomatic palatal tremor presents usually without an ear click and involves the levator veli palatini and other muscles innervated by brainstem nuclei (eye movements, face) or spinal motoneurons (trunk and extremity tremor). This tremor is typically combined with ataxia and is usually attributed to a lesion in the dentato-olivary pathway. Thus, symptomatic palatal tremor is typically a combined tremor syndrome, and olivary pseudohypertrophy is usually observed on MRI.56 This syndrome has been described with focal Guillain-Mollaret triangle lesions, glial fibrillary acidic protein,57,58 and polymerase-g mutations,59 neuroferritinopathy,60 and the syndrome of progressive ataxia and palatal tremor.61 In addition to these palatal tremor syndromes, there are other partly rhythmic lower cranial nerve dyskinesias that have been recently reviewed.62
Task-specific tremors are not uncommon in persons who perform the affected motor task repetitively and frequently. They are usually focal. As with focal tremor in general, there is an ongoing discussion as to whether they are a variant of dystonia, an overuse syndrome, or simply a unique isolated tremor syndrome. Isolated task- and position-specific tremors should be distinguished from similar syndromes that occur in combination with other neurological signs, such as dystonia (e.g., writer’s cramp) and parkinsonism (e.g., young-onset PD with dystonia).
Primary writing tremor is probably the most common form of task-specific tremor.63 It occurs only when writing or attempting to write.64 This condition has been studied extensively.65
Task-specific tremors of the hand or mouth occur in musicians66 and sportsmen. Yips in golfers is mostly considered a task-related dystonia, sometimes manifesting with tremor as the main symptom.67
Orthostatic Tremors
Different forms of orthostatic tremor share the core symptom of tremor during standing. Primary orthostatic tremor is defined as an isolated tremor syndrome, and its accurate diagnosis requires electrophysiological testing.68
Orthostatic tremor with the electrophysiological properties of primary orthostatic tremor has occurred in combination with other neurological conditions (e.g., dementia, PD, and spinocerebellar ataxia), and it should then be labeled as primary orthostatic tremor plus.69 The pathophysiological relation of primary orthostatic tremor and the other conditions is unclear and may be coincidental. Primary orthostatic tremor may be palpable but not visible, and the diagnosis needs confirmation with EMG recordings that reveal a 13- to 18-Hz tremor that is uniquely highly coherent among affected body parts.3,68,69 An audible rhythm called the “helicopter sign” may be detected by auscultating the lower limb muscles or by listening to EMG.70
There are additional clinical syndromes with tremor during standing that have a lower frequency than 13 Hz and have been labeled as slow orthostatic tremor, tremor in orthostatism, and pseudo-orthostatic tremor. We propose the term pseudo-orthostatic tremor.71 These slower orthostatic tremors are frequently associated with other neurological signs and have a slower tremor frequency than 13 Hz. Cases with various forms of parkinsonism, ataxia (spinocerebellar ataxia type 3), and dystonia72,73 have been described. A rare differential diagnosis (which is not tremor) with a similar clinical picture is orthostatic myoclonus syndrome.71
Axis 1 Tremor Syndromes With Prominent Additional Signs
In the preceding review of isolated tremor syndromes, we described two forms of tremor—symptomatic palatal tremor and pseudo-orthostatic tremor—that are usually associated with other neurological abnormalities or signs. Such syndromes are called combined tremor syndromes to emphasize the distinction from those forms of tremor that occur in isolation of other neurological and systemic signs. Here, we describe other forms of tremor that rarely, if ever, occur in isolation.
It is now generally agreed that tremor can be a basic element or feature of a dystonic contraction. Tremor in a body part affected by dystonia is labeled as dystonic tremor. Common examples include tremulous cervical dystonia (dystonic head tremor) and segmental tremulous dystonia affecting the head and upper limbs. The geste antagoniste (sensory trick) may be helpful in separating dystonic head tremor from essential head tremor.74 Sensory tricks have also been convincingly demonstrated for other cranial dystonic tremors.75 Cranial and other dystonic tremors may be relieved by allowing the abnormal dystonic posture to develop without resistance (“null point”).35 Dystonic tremor can be exacerbated by an attempt to maintain certain postures. Overflow dystonia and action dystonia when initiating a movement have been proposed as further diagnostic features of dystonia.35 The sensitivity and specificity of these examination methods are unknown, so these methods are not included in the definition of dystonic tremor syndromes.
If dystonia and tremor are found in different body parts, this is called tremor associated with dystonia. The dystonia can sometimes manifest only during challenging motor or cognitive tasks. Mirror dystonia has also been proposed to be a feature of dystonic movements.35
A specific cranial tremor syndrome has been labeled jaw tremor and dystonia in which jaw tremor is combined with dystonia in the jaw or elsewhere.76
The etiology of dystonic tremor syndromes may be known or idiopathic, and may be sporadic or familial.35,44
Rest tremor combined with parkinsonism is usually asymmetric and commonly unilateral in onset (classical Parkinsonian rest tremor). A characteristic feature of rest tremor in PD is that it ceases or greatly subsides, at least transiently, when the muscles are activated voluntarily to execute a posture or movement. The subsidence of rest tremor may be followed by delayed re-emergence of tremor when a new limb posture is sustained (re-emergent tremor). These features separate parkinsonian rest tremor from the rest tremor in ET plus.37 Rest tremor is uncommon in other parkinsonian conditions such as MSA, corticobasal degeneration, and PSP.77–79
Intention tremor is usually caused by a lesion in the cerebellothalamic pathway. Focal or unilateral intention tremor is rarely an isolated tremor syndrome. Cerebellothalamic dysfunction is caused by a wide range of disorders, and syndromic associations help in the differential diagnosis for each patient.
The etiology is frequently an acquired lesion in the brainstem in the vicinity of the red nucleus (hence the old terms rubral tremor and midbrain tremor).81 Therefore, it is important to recognize this syndrome and look for pathology in this anatomical region.82,83 Holmes tremor usually occurs with other localizing signs, but is rarely isolated. Dystonia and abnormal proprioception are often present when the underlying pathology is in the thalamus.
Myorhythmia has been recently reviewed84 and is distinguished from palatal tremor, rhythmic skeletal myoclonus, and epilepsia partialis continua. It is usually caused by pathology in the brainstem, diencephalon, or cerebellum, and it is usually associated with other brainstem or cerebellar signs. An underlying etiology is often found and may be treatable.
Functional Tremor Syndrome
The diagnostic features of functional tremors are all part of the Axis 1 description of this syndrome and include medical history, inconsistency of symptoms, sudden onset of symptoms, and widely fluctuating features (e.g., topographic distribution, frequency, and activation characteristics). Other functional neurological symptoms or signs are frequently present. Although there are often psychiatric features like depression in patients with functional disorders, their pathophysiological relationship with the functional tremor is often unclear. Test batteries have been proposed.88 There are probably multiple etiologies. A search for underlying psychiatric disorders is required in all cases. Cases are usually sporadic, but familial cases have also been described.89
Indeterminate Tremor Syndrome
Discussion
We have described a new classification scheme for tremor that is based on two axes. Axis 1 is a detailed clinical characterization of the patient with tremor that is based on the medical history, exam, and relevant ancillary tests. Axis 1 characterization will often reveal a syndrome or phenotype that leads to the identification of one or more underlying etiologies in Axis 2. This classification scheme was motivated by the common diagnostic process of syndrome identification and subsequent search for an underlying etiology. Another motivation was the need for classifying the many tremors with no known etiology, particularly tremors that occur in the absence of other clinical signs. Figure 3 contains many important clinical tremor syndromes according to their main presenting symptoms. The proposed classification scheme should aid in identifying homogenous populations of patients with specific tremor syndromes. New tremor syndromes are easily incorporated into this classification scheme.
An Axis 1 syndrome should not be viewed as a final diagnosis or a single disease; rather, these syndromes are merely an initial step in elucidating etiology and pathophysiology.33,34 Patients with an isolated tremor may develop additional diagnostic signs, so Axis 1 classification for a patient may change. For example, a patient with isolated bilateral upper limb action tremor may develop dystonia or bradykinesia, leading to a different and more appropriate Axis 1 classification. One cannot overstate the importance of prospective follow-up and re-examination in the evaluation of Axis 1 syndromes. Indeed, prospective longitudinal studies of ET and other isolated tremor syndromes are sorely needed.
It is also important to recognize that an Axis 1 syndrome may have multiple etiologies, and any of these etiologies might produce multiple Axis 1 syndromes (Fig. 1). For example, isolated bibrachial action tremor can be caused by the ANO3 mutation, but this genetic variant is more commonly a cause of dystonic tremor.44 Recognizing this complexity is particularly important in studies of ET. Occasionally, Axis 1 syndromes will be combined to summarize the patient’s condition, particularly when the combination is not covered by a single established syndrome. For example, a patient with long-standing tremor in the head, voice, and upper limbs (ET syndrome) may ultimately develop PD.
An important question is what to do with a long-standing tremor syndrome when a patient develops additional diagnostic signs. For example, a patient may have ET for decades before developing dystonia. We recommend that such patients be reclassified as having dystonic tremor with antecedent ET. Assumptions regarding etiological relationships are thereby avoided. The etiology of ET may not be the same as the etiology of the subsequent additional neurological signs.
Although the term ET seems entrenched in clinical neurology, we found it extraordinarily difficult to achieve a consensus definition of ET. Historically, ET has not been defined consistently by clinicians and researchers, and the definition of ET is admittedly some-what arbitrary.9,41 Some task force members thought that the term ET should be abandoned entirely, but it was retained as a specific tremor syndrome attributed to the widespread use of this term by clinicians, its implementation in the International Classification of Diseases system, and requests from lay people. However, there are now two forms of ET: ET and ET plus, and focal isolated tremors (e.g., head and voice) are not included. Thus, the clinician is obliged to identify any associated signs or symptoms, even those of uncertain diagnostic significance or etiological relevance (e.g., memory loss or impaired balance). Indeterminate tremor syndrome may be used when the clinician cannot confidently classify a patient with ET or ET plus.
Tremor in a dystonic body part is considered to be part and parcel of the dystonia35 and was called dystonic tremor in the 1998 consensus criteria.1 We agree fully with this terminology. In addition, dystonia patients may exhibit tremor in body parts that are not dystonic, and such tremor is currently called tremor associated with dystonia. We chose to retain this terminology even though the distinction between dystonic tremor and tremor associated with dystonia is increasingly questioned. There is no reason to suspect a different etiology for the tremor in nondystonic body parts, even though such tremor would be regarded as a form of ET9 if dystonia were not present. However, some electrophysiological tests and clinical arguments are in favor of maintaining the distinction between tremors that are and are not topographically located in the dystonic contractions,90 because there may be pathophysiological differences. Temporal discrimination threshold studies may be useful in resolving this controversy,91–94 but more specific, definitive biomarkers are needed. Meanwhile, we need to describe the phenomenology as precisely as possible, including the locations of tremor and dystonia.
The proposed classification scheme was designed primarily to facilitate the clarification of idiopathic tremor syndromes and the discovery of their etiologies. We encourage clinicians and researchers to use and test this new classification scheme, and we also encourage the incorporation of the new terminology into clinical rating scales, clinical trials, and other research. We acknowledge that the classification of tremors will change with the discovery of new and more accurate tremor syndromes, leading to and stemming from a better understanding of their pathophysiology and etiology.
TABLE 1.
Tests that are useful for delineating Axis 1 syndromes (1, 2, and 3) and for elucidating Axis 2 etiologies (2, 3, and 4)
| 1. Electrophysiological tests | Surface EMG to document the presence of tremor, measure tremor frequency, and evaluate EMG burst morphology and rhythmicity (e.g., to identify myoclonus and asterixis) |
| Fourier analysis of accelerometric and EMG recordings with and without loading the hand with a weight to identify mechanical-reflex and central neurogenic tremors | |
| Fourier and coherence analysis of EMG recordings from multiple limbs to diagnose primary orthostatic tremor | |
| 2. Structural imaging | MRI, CT for detection of lesions, metabolic disorders, etc. |
| 3. Receptor imaging | Dopamine and serotonin transporter imaging for disturbances or deficiency syndromes |
| 4. Serum and tissue markers | Metabolic blood tests, tests for infections, genetic tests, etc. |
Essential tremor.
isolated tremor syndrome of bilateral upper limb action tremor
at least 3 years’ duration
with or without tremor in other locations (e.g., head, voice, or lower limbs)
absence of other neurological signs, such as dystonia, ataxia, or parkinsonism.
Essential tremor plus:
Tremor with the characteristics of ET and additional neurological signs of uncertain significance such as impaired tandem gait, questionable dystonic posturing, memory impairment, or other mild neurologic signs of unknown significance that do not suffice to make an additional syndrome classification or diagnosis. ET with tremor at rest should be classified here.
Exclusion criteria for ET and ET plus.
Isolated focal tremors (voice, head)
Orthostatic tremor with a frequency >12 Hz
Task- and position-specific tremors
Sudden onset and step-wise deterioration
Isolated segmental postural or kinetic tremor syndromes commonly involve the upper limbs, but also may involve the head, voice, tongue, and face.
Isolated rest tremor syndromes most commonly occur in an upper or lower limb or as a hemitremor, but may occur elsewhere (e.g., lips, jaw, or tongue). It is crucial to determine, using Axis 1 characteristics, whether the rest tremor is isolated or combined with other clinical features.
Isolated voice tremor is a visible and/or audible tremor of the vocal apparatus.
Isolated head tremor is a shaking of the head in yes-yes, no-no, or variable directions.
Palatal tremor is characterized by rhythmic movement of the soft palate at 0.5 to 5 Hz.
Isolated task- and position-specific tremors occur during a specific task or posture.
Primary orthostatic tremor is a generalized high-frequency (13–18 Hz) isolated tremor syndrome that occurs when standing. Confirmation of the tremor frequency is needed, typically with an electromyography (EMG).
Dystonic tremor syndromes are tremor syndromes combining tremor and dystonia as the leading neurological signs. Different syndromes are separated on clinical grounds.
Tremor combined with parkinsonism (bradykinesia and rigidity) is typically a 4- to 7-Hz rest tremor of the hand (“pill-rolling” tremor), lower limb, jaw, tongue, or foot. This is called classic parkinsonian tremor. Other types of tremor may coexist in patients with parkinsonism, such as postural or kinetic tremor with the same or different frequency as the rest tremor. Although 4 to 7 Hz is characteristic, higher frequencies have been described.
Intention tremor syndromes consist of intention tremor at <5 Hz, with or without other localizing signs.
Holmes tremor is a syndrome of rest, postural, and intention tremor that usually emerges from proximal and distal rhythmic muscle contraction at low frequency (<5 Hz).
Myorhythmia is a very rare rhythmic movement disorder of cranial or limb muscles at rest or during action and is classified here as a tremor. The frequency is 1 to 4 Hz. It is usually associated with localizing brainstem signs and usually with a diagnosable etiology.
Functional (a.k.a., psychogenic) tremor is characterized by distractibility, frequency entrainment, or antagonistic muscle coactivation.85–87
Indeterminate tremor syndrome is reserved for a patient who does not fit into an established syndrome or who needs further observation to clarify the tremor syndrome.
Footnotes
Further members of the IPMDS Task Force: Dietrich Haubenberger, MHSc, MD; Giovanni Abbruzzese, MD; Julian Benito-Leon, MD, PhD; Maria Joao Forjaz, PhD; Kelly E. Lyons, PhD; Tiago A. Mestre, MSc, MD; Eng-King Tan, MD; Joachim Ferreira, MD, PhD
Relevant conflicts of interest/financial disclosures: Nothing to report.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 1998;13(Suppl 3):2–23. [DOI] [PubMed] [Google Scholar]
- 2.Marsden CD, Obeso JA, Rothwell J. Benign essential tremor is not a single entity. In: Yahr MD, ed. Current Concepts of Parkinson Disease and Related Disorders Amsterdam: Excerpta Medica; 1983:31–46. [Google Scholar]
- 3.Elble R, Deuschl G. Milestones in tremor research. Mov Disord 2011;26:1096–1105. [DOI] [PubMed] [Google Scholar]
- 4.Louis ED. Essential tremors: a family of neurodegenerative disorders? Arch Neurol 2009;66:1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinn NP, Schneider SA, Schwingenschuh P, Bhatia KP. Tremor—some controversial aspects. Mov Disord 2011;26:18–23. [DOI] [PubMed] [Google Scholar]
- 6.Deuschl G, Petersen I, Lorenz D, Christensen K. Tremor in the elderly: essential and aging-related tremor. Mov Disord 2015;30: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology 1991;41(2 (Pt1)):234–238. [DOI] [PubMed] [Google Scholar]
- 8.Chouinard S, Louis ED, Fahn S. Agreement among movement disorder specialists on the clinical diagnosis of essential tremor. Mov Disord 1997;12:973–976. [DOI] [PubMed] [Google Scholar]
- 9.Jankovic J Essential tremor: a heterogenous disorder. Mov Disord 2002;17:638–644. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Ferreira JJ. How common is the most common movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 2010;25:534–541. [DOI] [PubMed] [Google Scholar]
- 11.LaRoia H, Louis ED . Association between essential tremor and other neurodegenerative diseases: what is the epidemiological evidence? Neuroepidemiology 2011;37:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhlenbaumer G, Hopfner F, Deuschl G. Genetics of essential tremor: meta-analysis and review. Neurology 2014;82:1000–1007. [DOI] [PubMed] [Google Scholar]
- 13.Merner ND, Girard SL, Catoire H, et al. Exome sequencing identifies FUS mutations as a cause of essential tremor. Am J Hum Genet 2012;91:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parmalee N, Mirzozoda K, Kisselev S, et al. Genetic analysis of the FUS/TLS gene in essential tremor. Eur J Neurol 2013;20:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortega-Cubero S, Lorenzo-Betancor O, Lorenzo E, et al. Fused in Sarcoma (FUS) gene mutations are not a frequent cause of essential tremor in Europeans. Neurobiol Aging 2013;34:2441 e2449-e2441.e11. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED. Treatment of essential tremor: are there issues we are overlooking? Front Neurol 2011;2:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain 2001;124(Pt 11): 2278–2286. [DOI] [PubMed] [Google Scholar]
- 18.Simões RM, Constantino A, Gibadullina E, Houghton D, Louis ED, Litvan I. Examining the motor phenotype of patients with both essential tremor and Parkinson’s disease. Tremor Other Hyperkinet Mov (N Y) 2012;2 pii: tre-02–47-149–3. doi: 10.7916/D8CN72N0. Epub 2012 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao AK, Gillman A, Louis ED. Quantitative gait analysis in essential tremor reveals impairments that are maintained into advanced age. Gait Posture 2011;34:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord 2010;25:1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apartis E, Blancher A, Meissner WG, et al. FXTAS: new insights and the need for revised diagnostic criteria. Neurology 2012;79: 1898–1907. [DOI] [PubMed] [Google Scholar]
- 22.Cohen LG, Hallett M, Sudarsky L. A single family with writer’s cramp, essential tremor, and primary writing tremor. Mov Disord 1987;2:109–116. [DOI] [PubMed] [Google Scholar]
- 23.Hedera P, Phibbs FT, Fang JY, Cooper MK, Charles PD, Davis TL. Clustering of dystonia in some pedigrees with autosomal dominant essential tremor suggests the existence of a distinct subtype of essential tremor. BMC Neurol 2010;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiebler S, Schmidt A, Zittel S, et al. Arm tremor in cervical dystonia—is it a manifestation of dystonia or essential tremor? Mov Disord 2011;26:1789–1792. [DOI] [PubMed] [Google Scholar]
- 25.Lalli S, Albanese A. The diagnostic challenge of primary dystonia: evidence from misdiagnosis. Mov Disord 2010;25:1619–1626. [DOI] [PubMed] [Google Scholar]
- 26.Defazio G The epidemiology of primary dystonia: current evidence and perspectives. Eur J Neurol 2010;17(Suppl 1):9–14. [DOI] [PubMed] [Google Scholar]
- 27.Elble RJ. What is essential tremor? Curr Neurol Neurosci Rep 2013;13:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwingenschuh P, Ruge D, Edwards MJ, et al. Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson’s disease: a clinical and electrophysiological study. Mov Disord 2010;25:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erro R, Schneider SA, Stamelou M, Quinn NP, Bhatia KP. What do patients with scans without evidence of dopaminergic deficit (SWEDD) have? New evidence and continuing controversies. J Neurol Neurosurg Psychiatry 2016;87:319–323. [DOI] [PubMed] [Google Scholar]
- 30.Halcomb E, Davidson P, Hardaker L. Using the consensus development conference method in healthcare research. Nurse Res 2008;16:56–71. [DOI] [PubMed] [Google Scholar]
- 31.Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 2005;6:285–296. [DOI] [PubMed] [Google Scholar]
- 32.Elble RJ, Deuschl G. Tremor. In: Brown WF, Bolton CF, Aminoff MJ, eds. Neuromuscular Function and Disease: Basic, Clinical and Electrodiagnostic Aspects Philadelphia, PA: W.B. Saunders; 2002: 1759–1779. [Google Scholar]
- 33.Schoenbach VJ, Rosamond WD. The phonomenon of disease. Understanding the fundamentals of epidemiology—an evolving text http://www.epidemiolog.net/evolving/PhenomenonofDisease.pdf.
- 34.MacMahon B, Pugh TF. Causes and entities of disease. In: Clark DW, MacMahon B, eds. Preventive Medicine Boston, MA: Little, Brown; 1967:26–33. [Google Scholar]
- 35.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013; 28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raethjen J, Austermann K, Witt K, Zeuner KE, Papengut F, Deuschl G. Provocation of Parkinsonian tremor. Mov Disord 2008;23:1019–1023. [DOI] [PubMed] [Google Scholar]
- 37.Papengut F, Raethjen J, Binder A, Deuschl G. Rest tremor suppression may separate essential from parkinsonian rest tremor. Parkinsonism Relat Disord 2013;19:693–697. [DOI] [PubMed] [Google Scholar]
- 38.Jankovic J, Schwartz KS, Ondo W. Re-emergent tremor of Parkinson’s disease. J Neurol Neurosurg Psychiatry 1999;67:646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louis ED, Broussolle E, Goetz CG, Krack P, Kaufmann P, Mazzoni P. Historical underpinnings of the term essential tremor in the late 19th century. Neurology 2008;71:856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louis ED. Twelve clinical pearls to help distinguish essential tremor from other tremors. Expert Rev Neurother 2014;14:1057–1065. [DOI] [PubMed] [Google Scholar]
- 41.Louis ED, Ford B, Lee H, Andrews H, Cameron G. Diagnostic criteria for essential tremor: a population perspective. Arch Neurol 1998;55:823–828. [DOI] [PubMed] [Google Scholar]
- 42.van der Stouwe AM, Elting JW, van der Hoeven JH, et al. How typical are ‘typical’ tremor characteristics? Sensitivity and specificity of five tremor phenomena. Parkinsonism Relat Disord 2016;30: 23–28. [DOI] [PubMed] [Google Scholar]
- 43.Gironell A, Kulisevsky J, Pascual-Sedano B, Barbanoj M. Routine neurophysiologic tremor analysis as a diagnostic tool for essential tremor: a prospective study. J Clin Neurophysiol 2004;21:446–450. [DOI] [PubMed] [Google Scholar]
- 44.Stamelou M, Charlesworth G, Cordivari C, et al. The phenotypic spectrum of DYT24 due to ANO3 mutations. Mov Disord 2014; 29:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKeon A, Pittock SJ, Glass GA, et al. Whole-body tremulousness: isolated generalized polymyoclonus. Arch Neurol 2007;64: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 46.Schwingenschuh P, Ruge D, Edwards MJ, et al. Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson’s disease: a clinical and electrophysiological study. Mov Disord 2011;25:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ling H, Kearney S, Yip HL, et al. Parkinson’s disease without nigral degeneration: a pathological correlate of scans without evidence of dopaminergic deficit (SWEDD)? J Neurol Neurosurg Psychiatry 2016;87:633–641. [DOI] [PubMed] [Google Scholar]
- 48.Albanese A, Sorbo FD. Dystonia and tremor: the clinical syndromes with isolated tremor. Tremor Other Hyperkinet Mov (N Y) 2016;6:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiromerisiou G, Houlden H, Scarmeas N, et al. THAP1 mutations and dystonia phenotypes: genotype phenotype correlations. Mov Disord 2012;27:1290–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soland VL, Bhatia KP, Volonte MA, Marsden CD. Focal task-specific tremors. Mov Disord 1996;11:665–670. [DOI] [PubMed] [Google Scholar]
- 51.Schwingenschuh P, Cordivari C, Czerny J, Esposito M, Bhatia KP. Tremor on smiling. Mov Disord 2009;24:1542–1545. [DOI] [PubMed] [Google Scholar]
- 52.Jacome DE, Yanez GF. Tremors of the smile. J Neurol Neurosurg Psychiatry 1987;50:489–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zadikoff C, Lang AE, Klein C. The ‘essentials’ of essential palatal tremor: a reappraisal of the nosology. Brain 2006;129(Pt 4):832–840. [DOI] [PubMed] [Google Scholar]
- 54.Deuschl G, Mischke G, Schenck E, Schulte-Monting J, Lucking CH. Symptomatic and essential rhythmic palatal myoclonus. Brain 1990;113(Pt 6):1645–1672. [DOI] [PubMed] [Google Scholar]
- 55.Stamelou M, Saifee TA, Edwards MJ, Bhatia KP. Psychogenic palatal tremor may be underrecognized: reappraisal of a large series of cases. Mov Disord 2012;27:1164–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deuschl G, Toro C, Valls-Sole J, Zeffiro T, Zee DS, Hallett M. Symptomatic and essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain 1994;117(Pt 4):775–788. [DOI] [PubMed] [Google Scholar]
- 57.Thyagarajan D, Chataway T, Li R, Gai WP, Brenner M. Dominantly-inherited adult-onset leukodystrophy with palatal tremor caused by a mutation in the glial fibrillary acidic protein gene. Mov Disord 2004;19:1244–1248. [DOI] [PubMed] [Google Scholar]
- 58.Howard KL, Hall DA, Moon M, Agarwal P, Newman E, Brenner M. Adult-onset Alexander disease with progressive ataxia and palatal tremor. Mov Disord 2008;23:118–122. [DOI] [PubMed] [Google Scholar]
- 59.Johansen KK, Bindoff LA, Rydland J, Aasly JO. Palatal tremor and facial dyskinesia in a patient with POLG1 mutation. Mov Disord 2008;23:1624–1626. [DOI] [PubMed] [Google Scholar]
- 60.Wills AJ, Sawle GV, Guilbert PR, Curtis AR. Palatal tremor and cognitive decline in neuroferritinopathy. J Neurol Neurosurg Psychiatry 2002;73:91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuel M, Torun N, Tuite PJ, Sharpe JA, Lang AE . Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain 2004;127(Pt 6):1252–1268. [DOI] [PubMed] [Google Scholar]
- 62.Ellenstein A, Yusuf N, Hallett M. Middle ear myoclonus: two informative cases and a systematic discussion of myogenic tinnitus. Tremor Other Hyperkinet Mov (N Y) 2013;3 pii: tre-03–103-3713–1. doi: 10.7916/D8RX9BS1. Print 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rothwell JC, Traub MM, Marsden CD. Primary writing tremor. J Neurol Neurosurg Psychiatry 1979;42:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bain PG, Findley LJ, Britton TC, et al. Primary writing tremor. Brain 1995;116:203–209. [DOI] [PubMed] [Google Scholar]
- 65.Ondo WG, Satija P. Task-specific writing tremor: clinical phenotypes, progression, treatment outcomes, and proposed nomenclature. Int J Neurosci 2012;122:88–91. [DOI] [PubMed] [Google Scholar]
- 66.Lee A, Furuya S, Altenmu€ller E. Epidemiology and treatment of 23 musicians with task specific tremor. J Clin Mov Disord 2014;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhungana S, Jankovic J. Yips and other movement disorders in golfers. Mov Disord 2013;28:576–581. [DOI] [PubMed] [Google Scholar]
- 68.Koster B, Lauk M, Timmer J, et al. Involvement of cranial muscles and high intermuscular coherence in orthostatic tremor. Ann Neurol 1999;45:384–388. [DOI] [PubMed] [Google Scholar]
- 69.Hassan A, Ahlskog JE, Matsumoto JY, Milber JM, Bower JH, Wilkinson JR. Orthostatic tremor: clinical, electrophysiologic, and treatment findings in 184 patients. Neurology 2016;86:458–464. [DOI] [PubMed] [Google Scholar]
- 70.DeOrchis VS, Geyer HL, Herskovitz S. Teaching video neuroimages: orthostatic tremor: the helicopter sign. Neurology 2013;80:e161. [DOI] [PubMed] [Google Scholar]
- 71.Erro R, Bhatia K, Cordivari C. Shaking on standing: a critical review. Mov Disord Clin Pract 2014;1:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobylecki C, Silverdale MA, Dick JP, Kellett MW, Marshall AG. Dystonia associated with idiopathic slow orthostatic tremor. Tremor Other Hyperkinet Mov (N Y) 2015;5:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rigby HB, Rigby MH, Caviness JN. Orthostatic tremor: a spectrum of fast and slow frequencies or distinct entities? Tremor Other Hyperkinet Mov (N Y) 2015;5:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Masuhr F, Wissel J, Muller J, Scholz U, Poewe W. Quantification of sensory trick impact on tremor amplitude and frequency in 60 patients with head tremor. Mov Disord 2000;15:960–964. [DOI] [PubMed] [Google Scholar]
- 75.Albanese A, Lalli S. Is this dystonia? Mov Disord 2009;24:1725–1731. [DOI] [PubMed] [Google Scholar]
- 76.Schneider SA, Bhatia KP. The entity of jaw tremor and dystonia. Mov Disord 2007;22:1491–1495. [DOI] [PubMed] [Google Scholar]
- 77.Litvan I, Mangone CA, McKee A, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 1996;60:615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wenning GK, Geser F, Krismer F, et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 2013;12:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Armstrong MJ, Litvan I, Lang AE, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qureshi F, Morales A, Elble RJ. Tremor due to infarction in the ventrolateral thalamus. Mov Disord 1996;11:440–444. [DOI] [PubMed] [Google Scholar]
- 81.Lehericy S, Grand S, Pollak P, et al. Clinical characteristics and topography of lesions in movement disorders due to thalamic lesions. Neurology 2001;57:1055–1066. [DOI] [PubMed] [Google Scholar]
- 82.Kim JS. Delayed onset mixed involuntary movements after thalamic stroke: clinical, radiological and pathophysiological findings. Brain 2001;124(Pt 2):299–309. [DOI] [PubMed] [Google Scholar]
- 83.Raina GB, Cersosimo MG, Folgar SS, et al. Holmes tremor: clinical description, lesion localization, and treatment in a series of 29 cases. Neurology 2016;86:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baizabal-Carvallo JF, Cardoso F, Jankovic J. Myorhythmia: phenomenology, etiology, and treatment. Mov Disord 2015;30: 171–179. [DOI] [PubMed] [Google Scholar]
- 85.Fahn S, Williams DT. Psychogenic dystonia. Adv Neurol 1988;50: 431–455. [PubMed] [Google Scholar]
- 86.Schwingenschuh P, Katschnig P, Seiler S, et al. Moving toward “laboratory-supported” criteria for psychogenic tremor. Mov Disord 2011;26:2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raethjen J, Kopper F, Govindan RB, Volkmann J, Deuschl G. Two different pathogenetic mechanisms in psychogenic tremor. Neurology 2004;63:812–815. [DOI] [PubMed] [Google Scholar]
- 88.Schwingenschuh P, Saifee TA, Katschnig-Winter P, et al. Validation of “laboratory-supported” criteria for functional (psychogenic) tremor. Mov Disord 2016;31:555–562. [DOI] [PubMed] [Google Scholar]
- 89.Stamelou M, Cossu G, Edwards MJ, et al. Familial psychogenic movement disorders. Mov Disord 2013;28:1295–1298. [DOI] [PubMed] [Google Scholar]
- 90.Schiebler S, Schmidt A, Zittel S, et al. Arm tremor in cervical dystonia—is it a manifestation of dystonia or essential tremor? Mov Disord 2011;26:1789–1792. [DOI] [PubMed] [Google Scholar]
- 91.Bradley D, Whelan R, Walsh R, et al. Temporal discrimination threshold: VBM evidence for an endophenotype in adult onset primary torsion dystonia. Brain 2009;132(Pt 9):2327–2335. [DOI] [PubMed] [Google Scholar]
- 92.Tinazzi M, Fasano A, Di Matteo A, et al. Temporal discrimination in patients with dystonia and tremor and patients with essential tremor. Neurology 2013;80:76–84. [DOI] [PubMed] [Google Scholar]
- 93.Hutchinson M, Kimmich O, Molloy A, et al. The endophenotype and the phenotype: temporal discrimination and adult-onset dystonia. Mov Disord 2013;28:1766–1774. [DOI] [PubMed] [Google Scholar]
- 94.Kimmich O, Molloy A, Whelan R, et al. Temporal discrimination, a cervical dystonia endophenotype: penetrance and functional correlates. Mov Disord 2014;29:804–811. [DOI] [PubMed] [Google Scholar]


