Abstract
The ionotropic serotonin receptor, 5-HT3, is expressed by many developing neurons within the central nervous system. Since the olfactory epithelium continues to generate new olfactory sensory neurons (OSNs) throughout life, we investigated the possibility that 5-HT3 is expressed in the adult epithelium. Using a transgenic mouse in which the promoter for the 5-HT3a subunit drives expression of GFP, we assessed the expression of this marker in the olfactory epithelium of adult mice. Both the native 5-HT3a mRNA and GFP are expressed within globose basal cells of the olfactory and vomeronasal epithelium in adult mice. Whereas the 5-HT3a mRNA disappears relatively quickly after final cell division, the GFP label persists for about 5 days, thereby labeling immature OSNs in both the main olfactory system and vomeronasal organ. The GFP-labeled cells include both proliferative globose basal cells as well as immature OSNs exhibiting the hallmarks of ongoing differentiation including GAP43, PGP9.5, but the absence of olfactory marker protein. Some of the GFP-labeled OSNs show characteristics of more mature yet still developing OSNs including the presence of cilia extending from the apical knob and expression of Nav1.5, a component of the transduction cascade. These findings suggest that 5-HT3a is indicative of a proliferative or developmental state, regardless of age, and that the 5-HT3AGFP mice may prove useful for future studies of neurogenesis in the olfactory epithelium.
Keywords: serotonin, vomeronasal, GAP43, OMP, olfactory, neurogenesis, RRID: AB_2336819, RRID: AB_10000240, RRID: AB_796160, RRID: AB_2107282, RRID: AB_2532919, RRID: AB_2314700, RRID: AB_477413, RRID: AB_310177, RRID: AB_177503, RRID: AB_2336231
GRAPHICAL ABSTRACT
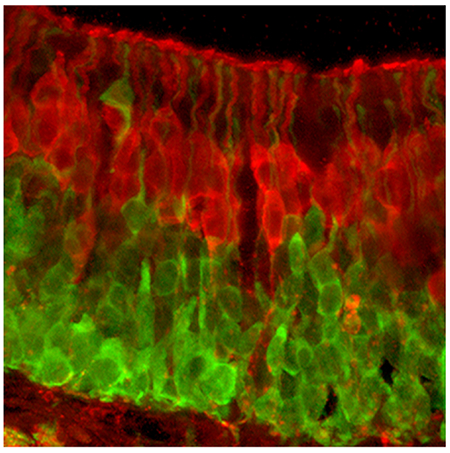
Immature olfactory receptor neurons in the olfactory epithelium of a P10 mouse, like those in adults, exhibit GFP fluorescence driven by the serotonin 5-HT3A receptor promoter whereas mature receptor neurons are colored red by staining for OMP.
The olfactory epithelium generates new receptor neurons throughout adult life in all vertebrates including mammals. In a normal, resting adult olfactory epithelium, new neurons arise by proliferation and differentiation of a population of mitotically active globose basal cells situated near the basal lamina of the epithelium. In the adult epithelium, the globose basal cells are not homogeneously distributed throughout the epithelium, but assume a patchy distribution suggesting that neuronal addition to the epithelium is nonuniform. A second population of basal cells, the horizontal basal cells, serve as a reserve stem cell population becoming activated under conditions of extreme damage (Schwob et al., 2016).
The newly generated neurons extend a dendrite to the surface of the epithelium and an axon to the olfactory bulb as they differentiate into mature olfactory sensory neurons (OSNs). During this process of maturation, the new-born OSNs undergo a stereotyped molecular and morphological maturation as their cell body migrates radially outward from the basal lamina to assume an intermediate position within the stratified epithelium (Rodriguez-Gil et al., 2015). Shortly after their terminal cell division, immature OSNs express markers of a neuronal phenotype (Packard et al., 2011; Jang et al., 2014), e.g. NCAM and PGP9.5, as well as GAP43 which is indicative of neuronal axon extension. After 4-5 days, the OSNs mature (Rodriguez-Gil et al., 2015), expressing specific odorant receptors, shortly followed by expression of elements of the olfactory transduction cascade and the characteristic olfactory marker protein (OMP). Molecular maturation is roughly coincident with the axon reaching the olfactory bulb (Rodriguez-Gil et al., 2015) and the dendrite extending long cilia along the surface of the epithelium.
In adult mammals, a limited number of neurogenic zones persist in addition to the olfactory epithelium, chiefly in the dentate gyrus of the hippocampal formation and in the subventricular zone of the telencephalon (Lepousez et al., 2015). The newly generated cells of the subventricular zone migrate tangentially along the rostral migratory stream to the olfactory bulb where they integrate as subpopulations of interneurons. In the hippocampal formation, cells generated in the subgranular zone of the dentate gyrus migrate radially into the granule cell layer where they differentiate into dentate gyrus granule cells. Immature neurons in several neuronal systems, including those in the rostral migratory stream, express the 5-HT3 receptor (Engel et al., 2013). Accordingly, in these neurogenic systems, many neuroblasts and new-born neurons can be identified by expression of green fluorescent protein (GFP) under control of the 5-HT3A promoter (Chen et al., 2012; Inta et al., 2008; Vucurovic et al., 2010). Here, we demonstrate the expression of 5-HT3A mRNA in the olfactory epithelium, and using 5HT3AGFP mice, characterize the perdurance of 5HTR3A-driven GFP after cessation of expression the 5-HT3A mRNA. We show that 5HTR3A-driven GFP is an effective marker for immature OSNs in the main olfactory epithelium, septal organ and to a lesser extent in the vomeronasal organ.
MATERIALS AND METHODS
Animals and anesthetics
All experiments were conducted on male and female mice, aged 2 to 6 months, housed on a 14 hour light cycle with ad libitum access to standard chow. The 5-HT3AGFP line, generated originally by GENSAT, was obtained from the Mutant Mouse Regional Resource Center-UNC (JAX stock # 000273-UNC; Tg(Htr3a-EGFP)DH30Gsat/Mmnc) and bred in house with C57bl/6J (JAX stock # 000664) obtained from the Jackson Laboratory. All use of experimental animals was approved by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Campus (Aurora, CO).
RT-PCR
Adult C57bl/6J mice of both sexes, 2–4.5 months old, were euthanized by CO2 asphyxiation followed by cervical dislocation. Tissue was excised and placed in RNA Later (Ambion, ThermoFisher Scientific, Grand Island NY). Samples included brainstem, olfactory bulb, olfactory epithelium, non-sensory nasal epithelium, and trigeminal ganglion. Total RNA was purified using the Qiagen Micro RNeasy Kit (Qiagen, Valencia, CA) according to manufacturer’s instructions, and incubated with DNase I to remove any contaminating genomic DNA. First strand cDNA syntheses were performed by reverse transcription of 1 μg of total RNA using Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). PCRs were carried out for mouse olfactory marker protein (OMP) and 5HT3A as well as for the housekeeping gene glyceraldehyde −3-phosphate dehydrogenase (GAPDH). The presence of a robust OMP signal in olfactory epithelium samples but not in samples of non-sensoiy epithelium confirmed the specificity of the tissue dissection although the presence of a faint band for OMP in the non-olfactory sample likely indicates the inclusion of small amounts of olfactory tissue, e.g. from Gruneberg’s ganglion. Primer sequences for each PCR are listed in Table 1.
Table 1:
Primer sequences for PCR
PCR amplifications for OMP, 5HT3A and GAPDH were performed under the following conditions: 94°C for 30 sec, 58°C for 30 sec, 72°C for 30 sec for a total of 35 cycles and elongation step at 72°C for 10 min. GAPDH annealing temperature was 56°C. A total of 100 ng/ml of each cDNA sample was used in the PCR amplification. The reverse transcriptase step was omitted in controls to confirm removal of all genomic DNA. Amplification products were analyzed on 2% agarose gels and visualized with GelRed (Biotium, Hayward, CA)
Perfusion and fixation
Animals, age P10 and 2.5 – 8 months, were deeply anesthetized by intraperitoneal injection of 50 mg/kg Fatal Plus solution (MWI, Denver CO) or sodium pentobarbital (Ovation Pharmaceuticals, Deerfield, IL). Transcardial perfusion with 25 ml of 0.9% sodium chloride was followed by 25 ml of 4% paraformaldehyde (PFA) in 0.1M phosphate buffer. The lower jaw was removed while the forebrain, including the olfactory bulbs remained in the opened skull and were post fixed in buffered PFA for 3 hours at room temperature before overnight cryoprotection with 20% sucrose in 0.1M phosphate buffer (PB, pH 7.2) at 4°C. In some cases, decalcification of the head was performed. The next day, the tissue was mounted in OCT compound (Optimal Cutting Temperature; Fisher Scientific, Pittsburgh, PA) and cut on a cryostat; 16 μm sections were collected directly onto Tanner Scientific microscope slides (Light Labs USA, Dallas TX).
Decalcification of heads
For examination of the entire nasal cavity, after fixation heads were placed in 0.45M EDTA for 1 day (<60days old) or 2 days at 4°C. They were then moved to 15% sucrose in phosphate buffer saline (PBS) for 1-2 days and finally placed in 30% sucrose for 1-2 days. Front teeth were removed prior to placing the tissue in sucrose solution. The heads were then embedded in OCT (Takara), frozen on dry ice and 16 μm coronal sections were collected from nares through to the olfactory bulb.
In Situ Hybridization
Probes.
For the in situ hybridization, RNA probes (riboprobes) were detected by the nonisotopic digoxygenin technique (DIG). An antisense 5HT3A riboprobe (660bp; F: 5’-GCCTTGACATCTACAACTTCCC-3’; R: 5’-GAGCAGTCATCAGTCTTGTTGG-3’) was produced by in vitro transcription (5HT3a clone, courtesy of Anya Voigt, Department of Molecular Genetics, German Institute of Human Nutrition Potsdam-Rehbruecke Nuthetal Germany ; characterized in Larson et al., 2015). The recombinant plasmid containing the 5HT3A cDNA insert was linearized, and the transcription was carried out utilizing in vitro transcriptions (Roche Applied Science) with T7 and T3 polymerases and DIG RNA Labeling kit (Roche Diagnostics, Indianapolis, IN).
Tissue Treatment:
Frozen sections (16mm) of mouse olfactory epithelium were cut and thawed onto Tanner Scientific positively charged slides (Light Labs USA, Dallas TX), and stored at −80°C. Before hybridization, the sections were fixed in 4% paraformaldehyde in PBS (0.1M phosphate buffer, pH7.2, 0.9% NaCl, 1mM MgCl2) and then permeabilized with 0.2M HCl for 10 minutes and 1% Triton X-100 in PBS for 2 minutes. To prevent non-specific binding, the sections were acetylated with 0.25% acetic anhydride in 0.1M triethanolamine pH 8.0. Tissue was prehybridized (prehybridization solution: 0.75M NaCl, 25mM PIPES, 25mMEDTA, 5X Denhardt’s reagent, 0.2% SDS, 250 mg/ml Escherichia coli RNA, and 500 mg/ml salmon testis DNA, pH 6.8) at room temperature for 5 hours. Riboprobes were incubated at 85°C for 10 minutes before application to sections and used at a final concentration of 500ng/ml. Hybridization was performed overnight at 56°C in a humidified chamber with 50% formamide in same prehybridization solution containing 10% dextran sulfate and 1% Normal Sheep Serum (Jackson Immunoresearch, West Grove, PA). After hybridization, slides were washed several times in 2X SSC, followed by 15 minute RNAse treatment (1mg/ml RNAse A in 0.5M NaCl, 10mM Tris-HCl, and 1.0 mM EDTA pH 7.5) to remove nonhybridized mRNA. For detection of hybrids formed, the sections were incubated with alkaline phosphatase conjugated anti digoxigenin antibody (1:750; Roche Applied Science) for 1 hour at room temperature. Colorimetric detection was accomplished with color substrate 0.175 mg/ml 5-bromo-4-chlor-indolyl-phosphate and 0.25mg/ml nitroblue- tetrazolium - chloride) applied to sections and incubated 4-5 hours in darkness. Reaction was stopped in Tris-EDTA buffer.
Controls.
Hybridizations were performed with a 5HT3A sense riboprobe that did not result in any labeling in the tissue.
Immunohistochemistry
Immunohistochemistry was performed using antibodies against green fluorescent protein (GFP), olfactory marker protein (OMP), Growth associated protein-43 (GAP43), 5-bromo-2′-deoxyuridine (BrDU), 5-ethynyl-2’-deoxyuridine (EDU), Ki67, proliferating cell nuclear antigen (PCNA), Phospho-histone H3, and the Nav1.5 sodium channel. Further antibody details including species, clonality, manufacturer, catalog and working dilution are listed in Table 2. After three 10 min washes in PBS (phosphate buffered saline, pH 7.4), slides underwent antigen retrieval. For this, the slides were placed in 10mM sodium citrate, pH 9, in 80°C water bath for 15 min. Following two 5 min PBS washes, slides were incubated in blocking solution (2% normal donkey serum, 1% bovine serum albumin, and 0.3% triton in PBS) for 1 hour. The GFP antibody was applied simultaneously with OMP or GAP43. Antibodies were diluted in blocking solution and placed on slides overnight at 4°C. The next day, slides were washed three times in PBS and then incubated for 2 hours with both Alexa-488 donkey anti chicken and Alexa-568 donkey anti rabbit secondary antibodies.
Table 2:
Primary Antisera
| Antiserum | Immunogen | Company (catalog #) | Working Dilution | Antigen Retrieval | Decalcification | AB registry # |
|---|---|---|---|---|---|---|
| Chicken polyclonal anti-GFP | purified green fluorescent protein | Aves Labs, Trigard, OR; GFP-1020 | 1:1000 | no | yes/no | AB_10000240 |
| Rabbit polyclonal anti-OMP (Olfactory Marker Protein) | synthetic peptide to rat C’ terminus (residues 119-137 conjugated to KLH) | Sigma; St. Louis, MO (07889 ; lot 017K4829) | 1:500 | yes | no | AB_796160 |
| Rabbit Polyclonal anti GAP-43; (growth associated protein-43) | recombinant rat protein (entire sequence) | Chemicon; Temecula, CA; (AB5220) | 1:250 | yes | no | AB_2107282 |
| Mouse monoclonal Biotin anti-BrDU | BrDU: Clone ZBU30 | Invitrogen, Carlsbad CA; (033940, lot 1506506A) | 1:250 | yes | yes/no | AB_2532919 |
| Rabbit monoclonal anti Ki67 | synthetic peptide to human C’ terminal | ThermoScientific Pierce;Rockford, IL; (MA1-90584; lot ML1486543) | 1:200 | yes | yes | AB_2314700 |
| Mouse monoclonal anti PCNA (proliferating cell nuclear antigen) | recognizes the acidic non-histone auxiliary protein of DNA polymerase. | Sigma, St. Louis, MO; (P-8825; lot 066H4852) | 1:1000 | yes | yes | AB_477413 |
| Rabbit polyclonal anti phospho-histone H3 (Ser10) | KLH conjugated peptide corresponding to amino acids 7-20 of human histone H3 | EMD, Millipore; Billerica, MA; (06-570; lot 32219) | 1:100 | yes | yes | AB_310177 |
| Rabbit anti sodium channel 1.5 | purified peptide corresponding to residues 493-511 of rat Nav1.5 | EMD, Millipore; Billerica, MA; (AB5493; lot LV1390273) | 1:500 | yes | no | AB_177503 |
For double-label preparations of NaV1.5 and OMP, we employed a hybrid detection method using tyramide for the first antiserum and indirect immunofluorescence for the second. Sections were incubated with anti-Nav1.5b antibody diluted 1:500, which was visualized with TSA A568 amplification (Invitrogen, Carlsbad CA). Sections were then washed, and antigen retrieval was performed consisting of 10 mM sodium citrate pH 9 for 10 min at 80°C whereupon the sections were incubated with anti-OMP (Sigma) diluted 1:1,000. Sections were then washed and reacted with secondary antiserum, Alexa-488 donkey anti rabbit IgG (1:400; Molecular Probes, Invitrogen) for 2 hours at room temperature.
All sections were viewed under an epifluorescence microscope or a Fluoview laser scanning confocal microscope (Olympus, Center Valley, PA) or scanned using a motorized stage controlled by Surveyor software by Objective Imaging (Cambridge, UK; http://www.objectiveimaging.com/Surveyor/OI_Turboscan.htm) with a monochrome Leica DFC 365FX camera on a Leica DM6000B microscope.
Proliferation markers and cell birthdating
After three 10 min washes in PBS slides were incubated in 3% H2O2 in PB for 10 min at room temperature. Three more washes in 0.1M PBS and then antigen retrieval was performed in 10mM sodium citrate pH 6 at 95°C for 15 min. Blocking solution (5% donkey serum, 1% bovine serum albumin, 0.3% triton in PBS) was applied for 2 hrs. The chicken anti GFP antibody (1:1000) was applied simultaneously with Ki67 (1:200), PCNA (1:1000) or phospho-histone H3 (1:100) diluted in blocking solution. The next day, slides were washed three times in PBS and then incubated for 2 hrs with Alexa-488 donkey anti chicken, Alexa-568 donkey anti rabbit or Alexa-568 donkey anti mouse secondary antibodies.
BrDU (bromodeoxyuridine) or EDU (ethynyl deoxyuridine) were diluted in sterile saline and injected IP (100 mg/kg). For BrDU, two injections were given separated by 5 hrs. EDU required just a single injection.
After three 10 min washes in PBS slides underwent antigen retrieval. Slides were placed in 0.1% Trypsin in 0.1M PBS for 8 min at 37°C. Following three quick rinses in PBS, slides were incubated in 2N HCl in dH2O for 18 min at 37°C. Slides were then placed in 0.1M sodium borate, pH 9.5 for 20 min at room temperature. Blocking solution (2% normal donkey serum, 1% bovine serum albumin, and 0.3% triton in PBS plus 4 drops/ml avidin solution from the avidin-biotin blocking kit (SP-2001, AB_2336231, Vector Labs, Burlingame, CA) was then applied for 1 hr. The GFP antibody was applied together with antibodies to BrDU, diluted in blocking solution containing biotin per kit instructions and placed on the slides overnight at 4°C. The next day, slides were washed three times in PBS and then incubated for 2 hrs with Alexa-488 donkey anti chicken and Streptavidin 568 secondary antibodies.
To visualize EDU labeling, we employed the Click-iT® Edu kit Alexa 594 (Cat # C10339, Invitrogen, Carlsbad CA). Initially slides were placed in 4% paraformaldehyde in 0.1M PB for 10 min at room temperature prior to washing three times in 0.1M PBS for a total of 30 min. Blocking solution (5% donkey serum, 1% bovine serum albumin, 0.3% triton in PBS) was applied for 90 min. Slides were then incubated in the provided kit components and reaction buffer for thirty minutes in the dark. Primary antibody for GFP was applied overnight 1:1000 at 4°C. The next day, slides were washed three times in PBS and then incubated for 2 hrs with Alexa-488 donkey anti chicken at 1:800 dilution along with a Cy5 Nissl.
Electron microscopy
Mice 2 months of age were anesthetized and perfused with 0.1% sodium nitrite, 0.9% sodium chloride and 100 units sodium heparin in 100 ml 0.1M PB (pH 7.3) followed by 4% PFA with 0.1% glutaraldehyde in 0.1M PB for 10 minutes with all solutions warmed to 42° C. The olfactory epithelium was removed and post-fixed in the same fixative for 4 hours before being transferred to a series of cryoprotection solutions (Eldred et al., 1983) culminating in overnight storage in 15% glycerin and 20% sucrose in 0.1 M PB (plus 0.1 ml of 2.2% calcium chloride per 100 ml). To enhance antiserum penetration, some of the pieces of epithelium were then exposed to 2 cycles of freezing on dry ice and thawing. The tissue was placed in blocking solution and primary antibody as above, but omitting the Triton-X 100 from all solutions. For control tissue, the primary antibody was omitted. The tissue pieces then were incubated with avidin-biotin complex (Elite ABC-Peroxidase Vectastain Kit; Vector Laboratories; Cat# PK-6100; RRID: AB_2336819) for 1 hr at room temperature. After rinsing, sections were transferred to 0.05M Tris buffer (pH 7.3) containing 0.05% DAB, then placed into a fresh aliquot of the DAB mixture containing H2O2 at a final concentration of 0.002%. The reaction was monitored visually but ran for only 2-5 min. Following DAB reaction, the tissue was washed in buffer then post-fixed in 1% OsO4 in 0.1M PB for 15 min. After a brief rinse in 0.05 M sodium maleate buffer (pH 5.2), the tissue was stained overnight at 4°C with 1% uranyl acetate in 0.025 M sodium maleate buffer (pH 6.0), followed by dehydration in an alcohol series, processed through propylene oxide, and embedded with Eponate 12 (Ted Pella, Inc.). Thin (70 nm) cross sections through the epithelium were then cut and mounted on slotted grids. The grids were stained with lead citrate and examined with a JEOL 1200 EXII transmission electron microscope.
Antibody Characterization
GFP (green fluorescent protein) –
In tissue from 5HT3AGFP mice, an anti-GFP antibody (Table 2) was applied to enhance detection of GFP protein. The antibody was isolated from IgY fractions of egg yolks from GFP immunized chickens. The antibody was verified by Western blot analysis and immunohistochemistry on tissue from transgenic GFP-expressing mice, where the antibody staining co-labeled with the endogenously expressed GFP (manufacturer’s technical information). This antibody was also tested in current experiments in non-GFP expressing mouse brain tissue; no label was detected (not shown).
OMP (olfactory marker protein) –
This antibody was made in rabbits against the synthetic peptide corresponding to the C terminal domain of the rat OMP sequence and was purified using antigen affinity chromatography. A single band at 19 kD was detected in immunoblot assays with rat and mouse olfactory bulb tissue (per company data sheet).
GAP 43 (Growth associated protein 43kD) –
This purified polyclonal rabbit antibody was raised against the complete sequence of the recombinant rat GAP43. According to manufacturer’s datasheet, a single band at 43kDa was detected in Western blots using mouse brain lysates.
Ki67 –
Staining for Ki67 was used to identify proliferative cells. Ki67 is a nuclear cell cycle protein, which is present in G1, S, G2 and M active phases but is absent during the resting G0 phase. Specific labeling of this antibody is therefore restricted to the nucleus. This monoclonal antibody was made in rabbits against a synthetic peptide against the human C terminus of Ki67 and according to the company data sheet a single band at 359kD is detected in Western Blots with tonsil tissue.
PCNA (proliferating cell nuclear antigen) -
This monoclonal antibody recognizes the acidic non-histone auxiliary protein of DNA polymerase that is essential for DNA replication during the S phase. PCNA begins to accumulate during the G1 phase of the cell cycle, is most abundant in the S phase and shows a decline in the G2/M phases. According to the manufacturer’s technical information, PCNA (IgG2a isotype) is derived from the PC 10 hybridoma produced by the fusion of mouse myeloma cells and splenocytes from a BALB/c mouse immunized with PCNA-Protein A fusion protein. The isotype was determined by a double diffusion immunoassay using Mouse Monoclonal Antibody Isotyping Reagents.
Phospho-Histone H3 (Ser10) –
Staining for phospho-histone H3 was used to identify proliferating cells in the M phase of the cell cycle. This rabbit polyclonal antibody detects histone H3 phosphorylated at serine 10. This highly published antibody, also known as anti-H3S10p, has been validated in immunocytochemistry, Western Blot and immunoprecipitation assays.
BrDU (5-bromo-2′-deoxyuridine) –
BrDU is a synthetic nucleoside that is an analogue of thymidine and is commonly used in the detection of proliferating cells in living tissues. BrDU is incorporated into the newly synthesized DNA of replicating cells during S phase of the cell cycle. No staining is observed in animals not injected with BrDU.
EDU (5-ethynyl-2’-deoxyuridine) –
EDU is a superior alternative to BrDU and is also a nucleoside analog of thymidine. Accurate detection methods are based on click chemistry, a protocol less harsh on the tissue as there is no denaturation step (Chehrehasa et al., 2009).
NaV1.5 (sodium channel 1.5)-
Rabbit polyclonal antibody recognizing the peptide corresponding to residues 493-511 of rat Nav1.5 and was purified using antigen affinity chromatography. The antibody has been validated for Western Blot use and was previously utilized for immunohistochemistry Frenz et al., 2014.
Secondary antibodies
Details are listed in Table 3. To test specificity, the secondary antibodies were applied to tissue without a primary antibody. Specific staining was not evident, confirming that the secondary signal is only present when bound to the primary antibody.
Table 3:
Secondary Antibodies
| Antibodies | Company (Catalog #) | Dilution | Antibodies |
|---|---|---|---|
| dk anti-ck, Alexa 488 | Jackson Immuno (703-545-155) | 1:400 | dk anti-ck, Alexa 488 |
| dk anti-rb, Alexa 568 | Invitrogen (A10042) | 1:400 | dk anti-rb, Alexa 568 |
| dk anti-ms Alexa 568 | Invitrogen (A10037) | 1:400 | dk anti-ms Alexa 568 |
| Streptavidin 568 | Invitrogen (S11226) | 1:1000 | Streptavidin 568 |
| Neurotrace 640/660 | Invitrogen (N21483) | 1:500 | Neurotrace 640/660 |
RESULTS:
The olfactory epithelium of adult (>8 weeks) 5-HT3AGFP mice shows numerous GFP-labeled cells lying within the basal half of the epithelium (Fig. 1). Many of these GFP-labeled cells appear to be round, like globose basal cells (Fig. 1A, arrows). Other basally-situated cells, have an apical extension, characteristic of the OSN dendrite, reaching to the surface of the epithelium where it apparently terminates in an enlargement or knob. GFP-labeled fibers are also apparent in the nerve fascicles beneath the basal lamina of the epithelium. Based on these morphological characteristics, the GFP-labeled cells in the olfactory epithelium evidently include both globose basal cells and immature OSNs (Fig 1 OSNi).
Fig. 1.
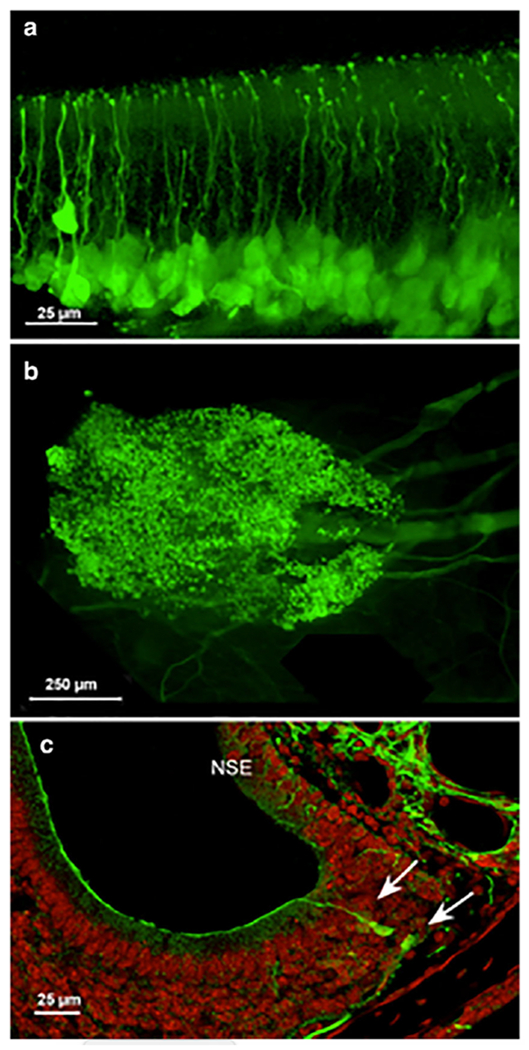
A. Whole mount of olfactory epithelium from 5-HT3AGFP mouse. The approximate position of mature OSNs (OSNm), immature OSNs (OSNi) and GBC is indicated. Arrows indicate likely globose basal cells. B. Surface view of a whole mount of the septal organ of Masera from an adult mouse. Numerous GFP-labeled OSNs are evident as well as fascicles of GFP-labeled axons coursing posteriorly below the epithelium. Anterior is to the left. C. Transverse section through the vomeronasal organ showing GFP-labeled OSNs at the lateral margin of the organ. The non-sensoiy epithelium (NSE) also shows numerous GFP-labeled axons, presumably of trigeminal origin. Counterstain is Fluoronissl.
GFP-labeled cells are present, not only within the main olfactory epithelium, but also in the septal organ or organ of Masera (Fig. 1B), and to a lesser extent in the vomeronasal organ (Fig. 1C). In the vomeronasal organ, GFP-labeled cells are restricted to the margins of the sensory epithelium, i.e. in the areas of cell proliferation and addition in young adults (Giacobini et al., 2000; De La Rosa-Prieto et al., 2009; Brann and Firestein, 2010; Brann et al., 2015).
The distribution of GFP-labeled cells within the main olfactory epithelium of animals 2 months of age and older is heterogeneous. The dorsomedial domain (Zone 1) of the epithelium contains few GFP-labeled cells (Fig. 2B, C arrows), whereas the lateral and ventral portions of the epithelium covering the endo- and ectoturbinates have many GFP-labeled cells (Fig. 2). GFP-labeled cells are especially numerous at the edges of olfactory epithelium, at the junction with non-sensory respiratory epithelium. The sparse GFP label in the dorsomedial zone (Zone 1) in the 2 month old animal is in keeping with the report of reduced neurogenesis in this area (Vedin et al., 2009).(Fig. 2B).
Fig. 2:
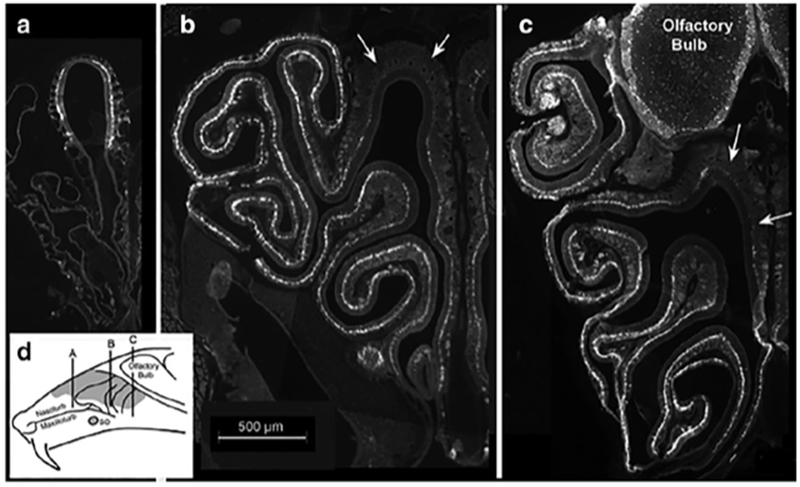
A-C. Micrographs of a series of transverse sections from anterior (A) to posterior (C) showing the distribution of GFP-labeled cells within the olfactory epithelium of an adult mouse. Note that the dorsomedial portion of the epithelium, indicated by the arrows in panels B & C, contains few labeled cells at mid- and posterior sections. Medial is to the left. (D) Schematic diagram of a sagittal section through the head of a mouse showing the approximate location of each of the images shown in A-C.
5-HT3A mRNA
The presence of GFP label in cells of BAC-transgenic mice does not necessarily reflect expression of the corresponding mRNA. First, there may be mis-expression of the marker compared to the native mRNA. Second, the GFP protein may persist longer than the encoding mRNA. To assess the expression of 5-HT3A mRNA, we undertook PCR and in situ hybridization (ISH) analysis of the olfactory epithelium.
PCR showed the presence of 5-HT3A mRNA in samples of olfactory epithelium (Fig. 3A, B). In keeping with the PCR result, 5-HT3A ISH signal was evident in basal cells of the olfactory epithelium, but there were substantially fewer cells than seen with the expression of the GFP protein. The ISH signal was limited to cells situated near the basement membrane whereas GFP signal includes cell somata that have migrated a short distance radially outward from the basal lamina. These results suggest that 5-HT3A mRNA is expressed predominantly in globose basal cells whereas the GFP protein perdures in immature OSNs even after loss of the 5-HT3A mRNA. The absence of GFP signal in mature OSNs suggests that the GFP protein persists for only a limited time and therefore can serve as a molecular clock of the age or state of maturation of immature OSNs.
Fig. 3.
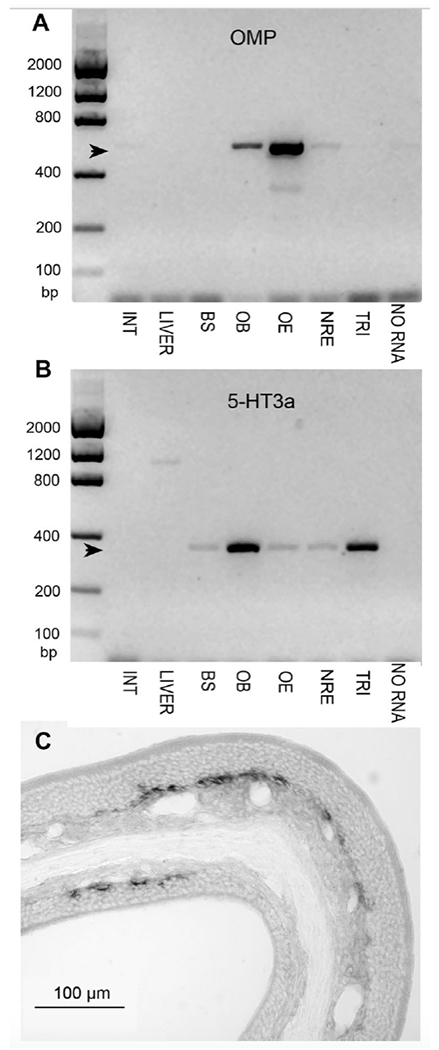
A & B. PCR of tissue samples from a WT mouse testing for the presence of OMP (A) or 5-HT3A (B) mRNA. INT, duodenum; LIVER, liver; BS, brainstem; OB, olfactory bulb; OE, olfctory epithelium; NOE, nasal respiratory epithelium; TRI, trigeminal ganglion; NO RNA, negative control. Photoshop® Dust & Scratches Filter was applied uniformly to reduce background noise in these images. A. Strong OMP signal is detected in olfactory bulb and epithelium, with only a faint band in the sample of nasal respiratory epithelium, verifying that this sample contained little olfactory tissue. B. 5-HT3A signal is strong in the olfactory bulb and trigeminal ganglion, but still detected in the brainstem, olfactory epithelium and nasal respiratory epithelium. C. In situ hybridization for 5-HT3A in turbinates from an adult mouse shows the presence of this mRNA in basal layers of the olfactory epithelium.
Birth-dating relative to GFP expression
To quantitatively estimate the perdurance of GFP, we used thymidine analog birthdating, assuming that GFP production ceased at the time of disappearance of the mRNA in situ signal, i.e. shortly after the final cell division of the globose basal cell. In keeping with our observation of GFP expression in round cells lying at the base of the epithelium, short-term (24 hr) labeling with BrDU or EDU marks numerous GFP-labeled cells immediately adjacent to the basal lamina consistent with their identity as globose basal cells (Fig. 4A). Longer term labeling, 2-4 days post injection with a thymidine analogue also shows numerous double-label cells (Fig. 4B), although this double-label population is now superficial to the basal lamina (Fig. 4B violet dotted line) consistent with reports from other laboratories studying cell dynamics in the olfactory epithelium e.g. Rodriguez-Gil et al., 2015, Jang et al., 2014. At 5 days post-injection, double-label cells are rare, with most BrDU-labeled cells lying above the GFP-labeled population in the epithelium (Fig. 4C; yellow arrows). Similarly, at 5 days post-thymidine analog labeling, double-labeled cells occur at the lateral margin of the vomeronasal organ (Fig. 4D). These findings indicate that immunohistochemical detectability of the GFP signal lasts for about 4-5 days after disappearance of the mRNA signal as assessed by in situ hybridization.
Fig. 4.
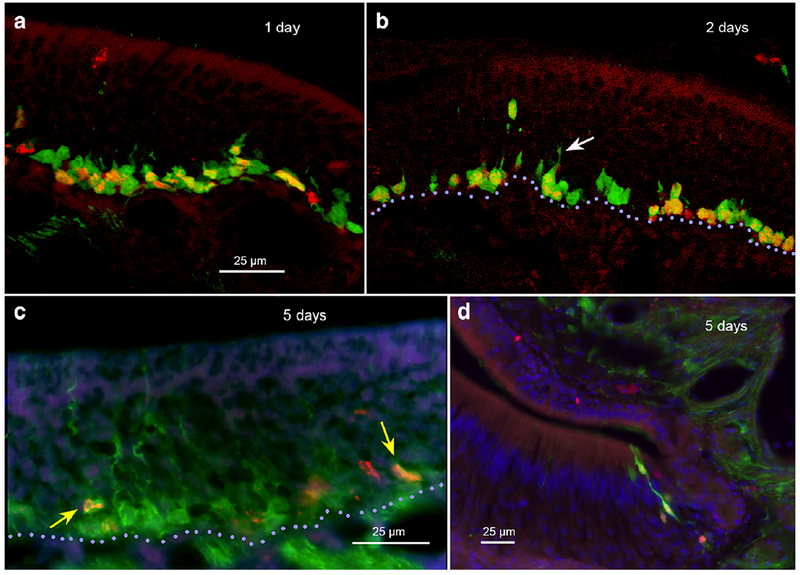
Labeling of olfactory epithelium with thymidine analogs. A. EdU labeling for 1 day shows numerous double-label (EdU + 5-HT3AGFP) cells along the basal lamina with a few non-EdU-labeled GFP positive cells sitting just outward from the double-label cells. B. 2-day post EdU, numerous double-label cells lie both near the basal lamina (violet dotted line) and just above it. The more distal cells exhibit a more elongate morphology, some with an apically directed dendrite. (arrow). C. At 5 days post-BrDU, only scattered doublelabel cells occur (yellow arrows). These are mostly elongate and well above the basal lamina (violet dotted line). D. At 5 days post-BrDU, double-labeled elongate cells occur along the lateral margin of the vomeronasal organ.
5HTR3A-driven GFP in Globose Basal Cells and Immature OSNs
Based on the experiments above, the 5HTR3A-driven GFP appears within globose basal cells and in the deep epithelium containing immature OSNs. To test directly whether the GFP was present in these two populations, we undertook a series of double-label experiments using antisera to characteristic markers for each of these developmental stages.
Globose basal cells are identified by their position in the epithelium, by a high mitotic rate and by markers of cell division including Ki67, PCNA and phospho-histone H3 (pH3). Co-localization experiments showed that each of the phenotypic markers colocalizes with the GFP (Fig 5A,B,C). The proliferative markers all co-localize in GFP labeled cells situated in the lowest level in the epithelium, proximal to the basal lamina, consistent with their identification as basal cells. GFP+/Ki67-negative cells exhibit the morphology of immature OSNs including an apical dendrite (white arrow Fig. 5A). Conversely, some Ki67 positive cells are not labeled with GFP (red arrow, Fig. 5A). The nuclei of these Ki67+/GFP− cells lie along the basal lamina and tend to be oval being flattened in the apical-to-basal dimension. Labeling with Ph3 yields many fewer labeled cells than the other markers of cell division since Ph3 labels only those cells in M-phase. Despite this more limited label, both single label Ph3-only (Fig. 5C insert a) and double-label (Fig. 5C inset b) basal cells occur, confirming that GFP label is expressed while some cells are still actively dividing.
Fig. 5.
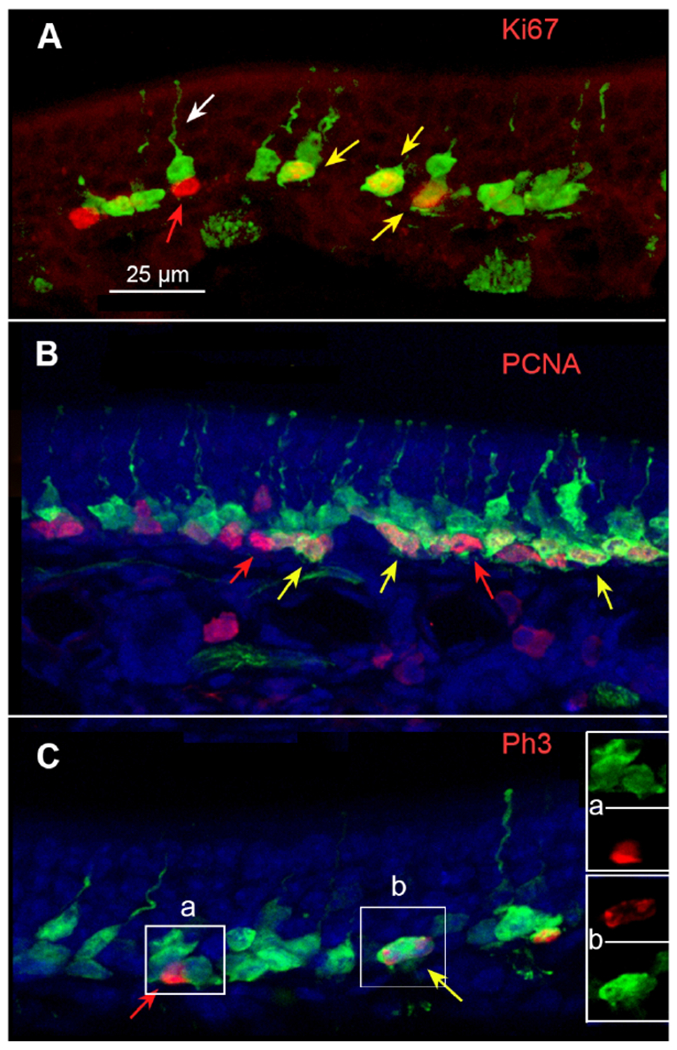
Double immunofluorescence for GFP and markers of proliferation. A. Ki67 (red) co-localizes with GFP label in many cells (yellow arrows) although many GFP labeled cells do not exhibit Ki67 label (e.g. white arrow) and some Ki67-positive cells show no apparent GFP (red arrow). Z-stack = 3.9μm. B. PCNA gives a similar pattern of staining to Ki67 showing single labeled cells of each type as well as numerous double-labeled cells. Z-stack = 8.25 μm. C. Double label for 5-HT3AGFP and Ph3. Red and yellow arrows indicate cells single labeled (Ph3 only; red) and double-labeled (Ph3 and GFP; yellow arrow). Inserts A & B show color separation images of areas indicated on the main figure. Z-stack = 6 μm.
Shortly after their final cell division, newly generated OSNs express several characteristic markers including NCAM, PGP9.5 and GAP43. These markers of immature OSNs co-localize with GFP in somata situated slightly more superficial than the markers of cell division, but still in the lower half of the epithelium (Fig. 6A). In contrast, OMP, the canonical marker of OSN maturation, rarely co-localized with the GFP signal (Fig 6B). Even under conditions of rapid growth typified by the epithelium in a P10 animal (Fig. 6C), GFP does not co-localize with OMP although many more cells exhibit GFP label than in adult epithelium, consistent with a higher rate of cell addition during this growth phase compared to the relatively static conditions of the adult epithelium. PGP9.5 immunoreactive cells appear nearly the full height of the epithelium excluding the basal cell layer (Fig. 6D). The most basal of the PGP9.5 positive population show co-localization with the GFP label (Fig. 6D). Occasionally, vertically adjacent GFP-positive cells occur (Fig. 6E, F) with the upper cell being PGP9.5 positive and the lower being negative. This arrangement is consistent with these two adjacent cells arising from an asymmetric division of a single progenitor in which the lower, GFP-only cell stays attached to the basal lamina and remains in the proliferative cycle whereas the cell above (green arrow) detaches from the basal lamina and begins terminal differentiation including expression of PGP. Like OMP, which appears late in the process of OSN maturation, the sodium channel Nav1.5, a key component regulating spike firing in the mature OSNs, rarely co-localized with 5HTR3A-driven GFP (Fig. 6G, H).
Fig. 6.
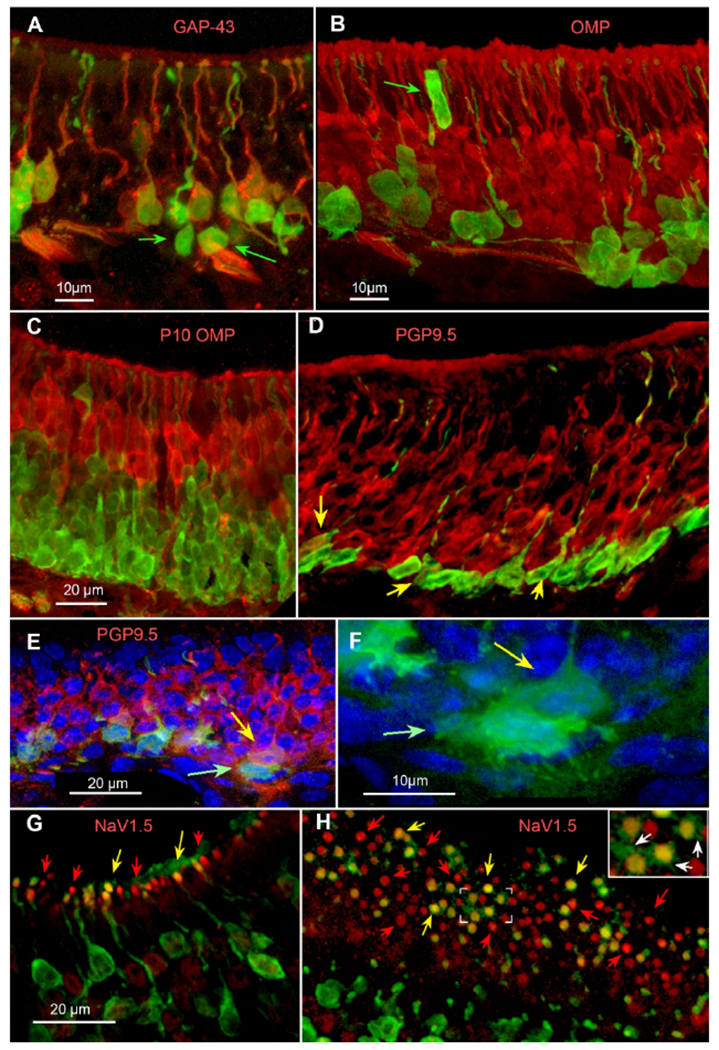
Double immunofluorescence for GFP and markers of maturation of sensory neurons. A. GAP43 (red) largely co-localizes with 5-HT3AGFP although some deeper GFP+ cells lack GAP43 (green arrows). B. OMP (red) immunoreactivity in adult epithelium rarely co-localizes with GFP. Noteworthy is that rare GFP+ cells are supporting cells of the epithelium such as an apparent microvillous sustentacular cell labeled by GFP (green arrow). C. OMP label in epithelium from a P10 pup; GFP does not co-localize with OMP. D. PGP9.5, another marker of cellular maturation, does co-localize with the GFP signal in lower levels of the adult epithelium but persists into more mature cells higher in the epithelium. Yellow arrows indicate double-labeled cells although many of the basal GFP+ cells exhibit low levels of PGP reactivity. E. & F. PGP and GFP label in turbinate epithelium. Many of the GFP+ cells along the basal membrane show only GFP fluorescence (green arrow), whereas those just above are co-labeled with PGP (yellow arrow). F. Higher magnification of only the GFP immunofluorescence and counterstain of the labeled cell pair indicated by the arrows in panel E. Note the multiple fine extensions and irregular shape of the lower, GFP− only cell (green arrow; brighter green) compared to the PGP positive cell (see panel E for PGP immunoreactivity) just above (yellow arrow; duller green). G & H. Label for NaV1.5 (red) shows localization within the dendritic knob of OSNs. As reported in Frenz et al (Frenz et al., 2014), NaV1.5 largely c0-localizes with OMP and hence should not be expected in immature OSNs. Many apical knobs show the presence of NaV1.5 but lack GFP label (red arrows). Yet we find many OSN apical knobs showing co-localization of NaV1.5 with GFP (yellow arrows) suggesting that NaV1.5 may be an early marker of OSN maturation. G. Cross-section of olfactory epithelium. H. Glancing tangential section through the surface of the epithelium showing the layer of dendritic knobs. The boxed area at the center of this panel is shown at 2× enlargement in the inset at upper right. Green (GFP immunofluorescent) processes (arrows) extend radially outward from double-labeled knobs.
GFP labeled cilia
Although immature OSNs do not express ORs or elements of the olfactory transduction cascade, the apical extension of the immature OSNs terminates in an apparent dendritic knob at the epithelial surface, suggesting that cilia may be present. At light microscopic levels, we observed short, GFP-labeled processes extending from apical knobs double-labeled for GFP and NaV1.5 (Fig. 6H, insert). To determine whether these were indeed ciliary extensions, we employed DAB-based immuno-electron microscopy to determine if labeled cilia were present. In control preparations, dendritic knobs have a relatively electron lucent character (7D, E) whereas sections immunoreacted for GFP show both electron dense (dark) and electron lucent (light) knobs. Tangential sections through the surface of the olfactory epithelium revealed that both labeled and unlabeled dendritic knobs appear morphologically similar: round profiles ~1-2 μm in diameter that contain multiple basal bodies from which the cilia extend (Fig. 7A–C). No clear morphological features distinguish the labeled from the unlabeled dendritic knobs and cilia, but it seems parsimonious to suggest that the latter are mature OSNs that have down-regulated GFP expression.
Fig. 7.
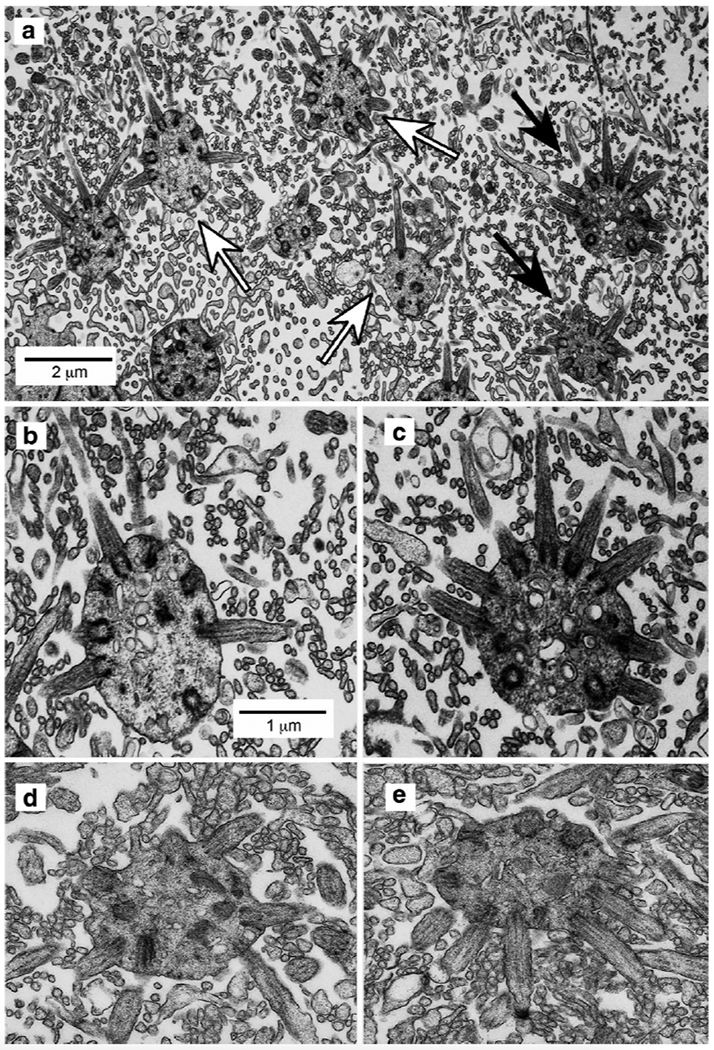
Immunoelectron microscopy showing the localization of GFP-labeled dendritic knobs and cilia of olfactory sensory neurons in tangential sections at the surface of the epithelium. A. Dendritic knobs appear as spherical profiles that contain multiple basal bodies from which the cilia extend. Both unlabeled knobs (e.g., white arrows) and GFP− labeled knobs (black arrows) extend cilia characterized by the presence of microtubules and basal bodies. Also present in the image are microvilli of sustantacular cells, appearing as smaller circular profiles lacking defined cytoplasmic structure or details. B. & C. Higher magnifications of corresponding unlabeled (leftmost white arrow) and GFP-labeled (upper black arrow) dendritic knobs in panel A. Scale bar in panel B also applies to panel C. D & E. Examples of dendritic knobs from control tissues not exposed to primary antiserum. No dark knobs are evident.
GFP labeled axons in the Olfactory Nerve
Sections through the olfactory bulb and posterior portions of the olfactory epithelium of 5-HT3AGFP animals showed numerous GFP-labeled axons within olfactory nerve fascicles beneath the epithelium and continuing into the nerve layer of the olfactory bulb (Fig. 8A). In many, but not all specimens and sections, the outer olfactory nerve layer exhibited more GFP label than inner nerve layer, which is proximal to the glomeruli (Fig. 8B, C). This observation is consistent with the notion that the outer nerve layer contains the most immature axons while the inner nerve layer has a higher percentage of mature axons (Rodriguez-Gil et al., 2015).
Fig. 8.
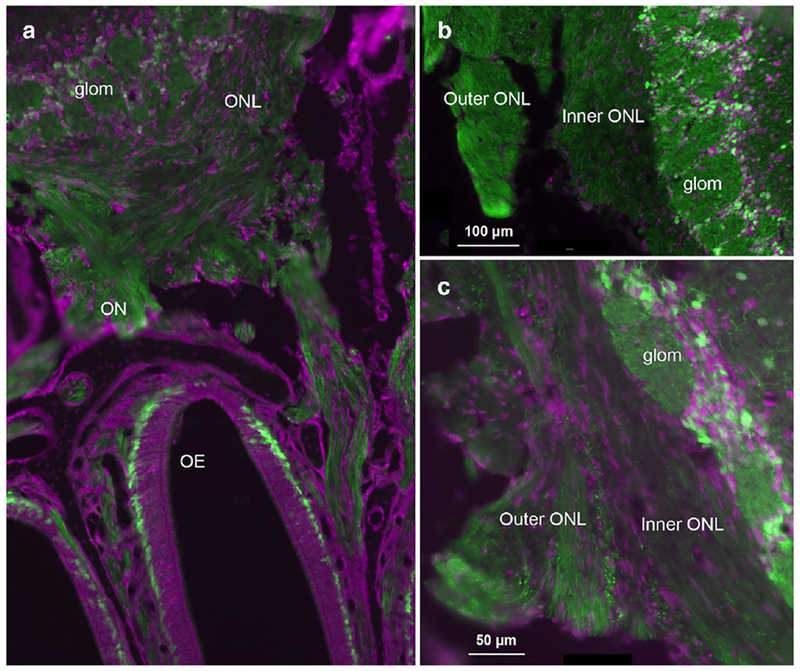
Transverse sections through the olfactory bulb and nerve layer of adult 5-HT3AGFP mice. Fluorescent Nissl stain shown in magenta. Numerous GFP-labeled periglomerular cells surrounding the glomeruli provide a clear boundary for the inner edge of the olfactory nerve layer (ONL). A. Section showing the olfactory epithelium (OE) and olfactory nerve (ON) coursing through the cribriform plate, as well as the olfactory nerve layer (ONL) and glomerular layer of the olfactory bulb. GFP-labeled fibers are evident along the entire course of the olfactory nerve although in many sections, GFP staining appears brighter in the outer ONL compared to the inner ONL (e.g. see B and C).
GFP labeled non-neuronal cells
While the vast majority of GFP-labeled cells in the epithelium are globose basal cells or immature OSNs, the epithelium also contains rare GFP-labeled non-neuronal cells. As shown in Fig. 6B, rare microvillar cells and elongate cells (likely sustentacular [SUS] cells) high in the epithelium exhibit GFP-label. This possibly reflects the findings of others that SUS and OSNs can arise from a common epithelial progenitor (Schwob et al., 2016).
DISCUSSION
Throughout adult life, as well as during postnatal development, the olfactory epithelium retains the capacity for generating new sensory neurons, which arise from one or more populations of proliferative basal cells. Some globose basal cells undergo a terminal cell division giving rise to daughter cells that differentiate into mature OSNs Schwob et al., 2016. The progression from globose basal cell to mature OSN follows a stereotypical process of molecular and morphological maturation (Kam et al., 2016;Schwob et al., 2016). Within 24 hours of terminal division, the immature OSNs express highly glycoslylated forms of NCAM (Key and Akeson, 1990), GAP43 (Jang et al., 2014) and PGP9.5 (Packard et al., 2011) as the cells extend an apical dendrite toward the epithelial surface and an axon toward the olfactory bulb (Rodriguez-Gil et al., 2015). After approximately 3-4 days, odorant receptor (OR) protein begins to accumulate in the dendritic knob at the surface of the epithelium (Schwarzenbacher et al., 2005) as the axon nears the surface of the olfactory bulb (Rodriguez-Gil et al., 2015). Over the next 24-48 hours, the OSNs mature, down-regulating GAP43 in favor of elements of the transduction and transmission cascades including OMP, adenylate cyclase 3, and NaV1.5 (current study and Rodriguez-Gil et al., 2015). We found 5HTR3A-driven GFP in both the proliferative basal cells and in immature OSNs although 5-HT3A mRNA itself is detectable only in the basal cell population. The most reasonable explanation is the longer-lasting GFP is due to perdurance of the protein generated by the basal cells, which is retained for only a few days and is lost as the OSNs mature. This is similar to neurogenin-driven GFP and Sox2-driven GFP in the olfactory epithelium, both of which down-regulate leading to the loss of GFP expression (Krolewski et al., 2013). In the 5-HT3AGFP line as well as these other GFP-expressing transgenic lines, the location and number of GFP-labeled cells nevertheless serve as a useful index of the degree of proliferation and OSN generation across the epithelium.
In the 5-HT3AGFP mice, GFP-labeled immature OSNs are not distributed uniformly throughout the epithelium. Rather, pockets of neurogenesis are interspersed within swatches of relatively quiescent epithelium, as originally described by Graziadei and co-workers (Graziadei and Graziadei, 1979). In addition, areas of high neurogenesis lie within the ventrolateral areas of the epithelium and especially at the junctures between olfactory and respiratory epithelium. This pattern of neurogenesis likely reflects continuing growth and expansion of the olfactory epithelium within the nasal cavity as the mice mature, similar to the situation described in rats (Weiler and Farbman, 1997) where addition of OSNs at the edges of the epithelium allows for continuing expansion of the receptor sheet to accommodate ongoing growth of the head and nasal cavity well into adulthood. While further insight is not possible at this time, the patchy distribution of the 5-HT3AGFP labeled immature cells may also suggest that subpopulations of OSNs that are regionally distributed in the olfactory epithelium may undergo heterogeneous patterns of replacement (Khan et al., 2013; Rodriguez-Gil et al., 2015).
The capacity for adult neurogenesis in the mammalian nervous system resides in the olfactory epithelium, as shown here, as well as in a limited number of other areas in the brain. The best studied are the dentate gyrus of the hippocampal formation and the subventricular zone of the telencephalon. In both of these regions, the dividing neuronal precursors as well as immature neurons express GFP in the 5-HT3AGFP line used in the current experiments (Ming and Song, 2005; Chen et al., 2012). Similarly, the globose basal cells and immature OSNs express 5HTR3A-driven GFP. In the telencephalon, many of the immature 5-HT3AGFP neurons differentiate into GABAergic cortical interneurons that continue to express 5-HT3a mRNA and the functional 5-HT3 receptor as well as the 5HTR3A-driven GFP (Inta et al., 2008; Vucurovic et al., 2010). Unlike these cortical interneurons, the immature neurons of the olfactory epithelium apparently cease expression of the 5-HT3A mRNA although the GFP signal persists for some days in the absence of detectable 5-HT3A mRNA. We propose that the expression of 5-HT3A mRNA is a reliable reporter of 5-HT3 protein and functional 5-HT3 receptors as in other systems (Inta et al., 2008; Vucurovic et al., 2010; Larson et al., 2015). However, then what is the source of 5-HT3 ligand for the globose basal cells? Immunohistochemistry for 5-HT itself shows no accumulation in any epithelial cell type even after pre-loading with 5-HTP (data not shown). Scattered mast cells exhibit 5-HT immunoreactivity but these are not obviously associated with or even in the vicinity of GFP-labeled basal cells. While the question of whether the 5-HT3 receptors we identified in the olfactory epithelium are functional remains to be determined, as does the native ligand. Regardless, the data reported here provide new insights into a novel molecular marker that can now be employed to isolate and study further the earliest stages of OSN maturation following basal cell division as well as regional changes in basal cell division throughout the epithelium.
Acknowledgments
Grant Support:
National Institute on Deafness and Other Communication Disorders, National Institutes of Health . Grant Numbers: R01 DC006070 (to D.R. and T.E.F.) , P30 DC04657 (to D.R.), DC00210, DC012441 and DC015438 (to C.A.G.) and U. Colo. Program in Neuroscience training grant T32 HD 041697.
REFERENCES
- Brann JH, Ellis DP, Ku BS, Spinazzi EF, Firestein S. 2015. Injury in aged animals robustly activates quiescent olfactory neural stem cells. Front Neurosci 9:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann JH, Firestein S. 2010. Regeneration of new neurons is preserved in aged vomeronasal epithelia. J Neurosci 30:15686–15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehrehasa F, Meedeniya ACB, Dwyer P, Abrahamsen G, Mackay-Sim A. 2009. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. Journal of neuroscience methods 177:122–130. [DOI] [PubMed] [Google Scholar]
- Chen R, Lin C, You Y, Liu F. 2012. Characterization of immature and mature 5- hydroxytryptamine 3A receptor-expressing cells within the adult SVZ-RMS-OB system. Neuroscience 227:180–190. [DOI] [PubMed] [Google Scholar]
- De La Rosa-Prieto C, Saiz-Sanchez D, Ubeda-Bañon I, Argandoña-Palacios L, Garcia-Muñozguren S, Martinez-Marcos A. 2009. Fate of marginal neuroblasts in the vomeronasal epithelium of adult mice. The Journal of Comparative Neurology 517:723–736. [DOI] [PubMed] [Google Scholar]
- Eldred WD, Zucker C, Karten HJ, Yazulla S. 1983. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J Histochem Cytochem 31:285–292. [DOI] [PubMed] [Google Scholar]
- Engel M, Smidt MP, van Hooft JA. 2013. The serotonin 5-HT3 receptor: a novel neurodevelopmental target. Front Cell Neurosci 7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenz CT, Hansen A, Dupuis ND, Shultz N, Levinson SR, Finger TE, Dionne VE. 2014. NaV1.5 sodium channel window currents contribute to spontaneous firing in olfactory sensory neurons. J Neurophysiol 112:1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini P, Benedetto A, Tirindelli R, Fasolo A. 2000. Proliferation and migration of receptor neurons in the vomeronasal organ of the adult mouse. Brain Res Dev Brain Res 123:33–40. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Graziadei GAM. 1979. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. Journal of neurocytology 8:1–18. [DOI] [PubMed] [Google Scholar]
- Inta D, Alfonso J, von Engelhardt J, Kreuzberg MM, Meyer AH, van Hooft JA, Monyer H. 2008. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci U S A 105:20994–20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Chen X, Flis D, Harris M, Schwob JE. 2014. Label-retaining, quiescent globose basal cells are found in the olfactory epithelium. Journal of comparative neurology 522:731–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JW, Dumontier E, Baim C, Brignall AC, Mendes da Silva D, Cowan M, Kennedy TE, Cloutier JF. 2016. RGMB and neogenin control cell differentiation in the developing olfactory epithelium. Development 143:1534–1546. [DOI] [PubMed] [Google Scholar]
- Key B, Akeson RA. 1990. Olfactory neurons express a unique glycosylated form of the neural cell adhesion molecule (N-CAM). The Journal of Cell Biology 110:1729–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Vaes E, Mombaerts P. 2013. Temporal patterns of odorant receptor gene expression in adult and aged mice. Mol Cell Neurosci 57:120–129. [DOI] [PubMed] [Google Scholar]
- Krolewski RC, Packard A, Schwob JE. 2013. Global expression profiling of globose basal cells and neurogenic progression within the olfactory epithelium. Journal of comparative neurology 521:833–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ED, Vandenbeuch A, Voigt A, Meyerhof W, Kinnamon SC, Finger TE. 2015. The Role of 5-HT3 Receptors in Signaling from Taste Buds to Nerves. J Neurosci 35:15984–15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepousez G, Nissant A, Lledo PM. 2015. Adult neurogenesis and the future of the rejuvenating brain circuits. Neuron 86:387–401. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. 2005. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 28:223–250. [DOI] [PubMed] [Google Scholar]
- Packard A, Schnittke N, Romano RA, Sinha S, Schwob JE. 2011. DeltaNp63 regulates stem cell dynamics in the mammalian olfactory epithelium. J Neurosci 31:8748–8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gil DJ, Bartel DL, Jaspers AW, Mobley AS, Imamura F, Greer CA. 2015. Odorant receptors regulate the final glomerular coalescence of olfactory sensory neuron axons. Proceedings of the National Academy of Sciences 112:5821–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenbacher K, Fleischer J, Breer H. 2005. Formation and maturation of olfactory cilia monitored by odorant receptor-specific antibodies. Histochemistry and Cell Biology 123:419–428. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Jang W, Holbrook EH, Lin B, Herrick DB, Peterson JN, Hewitt Coleman J. 2016. Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license. J Comp Neurol. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedin V, Molander M, Bohm S, Berghard A. 2009. Regional differences in olfactory epithelial homeostasis in the adult mouse. J Comp Neurol 513:375–384. [DOI] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, Monyer H, Rossier J, Vitalis T. 2010. Serotonin 3A Receptor Subtype as an Early and Protracted Marker of Cortical Interneuron Subpopulations. Cerebral Cortex 20:2333–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. 1997. Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci 17:3610–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]


