Abstract
Background:
We have a limited understanding of the biological underpinnings of symptoms in heart failure (HF).
Objectives:
The purpose of this paper was to compare relationships between peripheral biomarkers of HF pathogenesis and physical symptoms between patients with advanced versus moderate HF.
Methods:
This was a two-stage phenotype sampling cohort study wherein we examined patients with advanced HF undergoing ventricular assist device implantation in the first stage, and then patients with moderate HF (matched adults with HF not requiring device implantation) in the second stage. Linear modeling was used to compare relationships among biomarkers and physical symptoms between cohorts.
Results:
Worse myocardial stress, systemic inflammation and endothelial dysfunction were associated with worse physical symptoms in moderate HF (n=48), but less physical symptom burden in advanced HF (n=48).
Conclusions:
Where patients are in the HF trajectory needs to be taken into consideration when exploring biological underpinnings of physical HF symptoms.
Introduction
Heart failure (HF) is a clinical diagnosis based on a history of symptoms and physical examination.1 Notwithstanding heterogeneous etiological and structural characteristics (e.g. ischemic vs. non-ischemic, reduced vs. preserved ejection fraction, dilation vs. hypertrophy), symptoms like dyspnea are what connect cases of HF together under one diagnosis.2 Among patients with chronic HF, symptoms are the primary reason why patients seek urgent care.3,4 Symptoms are also the principal drivers of quality of life in HF.5,6 Yet, we have a limited understanding of the biological underpinnings of symptoms in HF. In fact, we know more about how symptoms (i.e. subjective experiences as appraised and defined by the patient) and clinical objective parameters are unrelated than we know about how they are related in HF.
To summarize the world’s literature on the topic, there is limited-to-no association between objective markers in HF and how patients living with the condition feel, including symptoms themselves, and quality of life that in HF is primarily driven by symptoms. For example, Shah et al. found that right heart catheterization parameters are not associated significantly with dyspnea.7 Rector et al. observed that several common objective measures of HF severity, including left ventricular ejection fraction (LVEF), blood pressure, creatinine and hemoglobin were only modestly associated with quality of life.8 Meyers et al. identified that symptoms of HF correlate poorly with objective measures of peak oxygen uptake.9 Lewis et al. found that quality of life does not differ significantly by LVEF.10 Bhardwaj et al. provided evidence that values of amino-terminal pro-B type natriuretic peptide (NTproBNP), a prominent HF biomarker, were not related to quality of life.11 Finally, Guglin et al. concluded that there was no correlation between dyspnea, orthopnea, fatigue and gastrointestinal discomfort and multiple clinical factors in HF including peak oxygen uptake, LVEF, NTproBNP, right heart catheterization parameters, and echocardiographic parameters.12
There is more recent evidence of biomechanical underpinnings of HF symptoms13,14 as well as biochemical links between symptoms and HF severity including an objective metric of adrenergic dysregulation that was shown recently to be associated with a composite score of physical HF symptoms.15 Hence, one reason for limited evidence connecting symptoms and objective markers of HF may be that we have been looking at the wrong metrics to test such associations. For example, metrics of several pathogenic mechanisms in HF including systemic inflammation, endothelial dysfunction, hyper-volumetric stress, platelet activation and wasting have not been tested in relation to symptoms in HF. With such complex pathophysiological pathways in HF, it also may be that not all HF is created equal, meaning that we should not assume that links between objective markers of HF and symptoms are universally applicable. Instead, it may be that symptom biology differs by HF severity. The specific aims of this analysis were to 1) explore relationships between peripheral biomarkers of HF pathogenesis and common physical symptoms (i.e. ‘symptom biochemistry’16), and 2) compare symptom biochemistry between patients with advanced HF (i.e. patients about to receive a left ventricular assist device) and those with moderate HF (i.e. symptomatic patients without advanced HF).
Methods
Patient Cohorts
This was a two-stage, phenotype sampling cohort study17 wherein we examined the extreme sample in the first stage – advanced HF - (i.e. patients undergoing left ventricular assist device (LVAD) implantation), and then the non-extreme sample in the second stage – moderate HF - (age-, gender-, etiology- and duration of HF-matched community-dwelling adults with HF symptoms but without advanced HF and not requiring LVAD implantation). This extreme phenotype sampling approach was used to separate demographic and etiological differences in HF from variances in the clinical phenotype.
The advanced HF cohort was part of a U.S. National Institutes of Health-sponsored prospective cohort study designed to identify behavioral and biological responses to LVAD implantation.16 Adults (≥21 years of age) who were undergoing LVAD with a commercially-available and U.S. Food and Drug Administration-approved, durable, and continuous-flow device as a bridge to transplantation/decision or as destination therapy (all Interagency Registry for Mechanically Assisted Circulatory Support profile 1-418) were approached for participation and enrolled prior to LVAD implantation; all participants in the advanced HF cohort went on to receive an LVAD. Patients were not eligible if they had a heart transplantation or previous LVAD prior to enrollment, major psychiatric illness or documented major cognitive impairment such as Alzheimer’s disease, or if they had concomitant terminal illness that impeded participation in a six-month study. Plasma samples and clinical data were collected a median of 5 days prior to LVAD implantation.
To collect information on a cohort of community-dwelling adults with moderate HF, we conducted a second prospective study wherein we selectively recruited patients to match the advanced HF cohort based on age, gender, HF etiology (ischemic vs. non-ischemic), and duration of HF (≤ 1 year, 1-5 years, 5-10 years or >10 years). Adults (21 years of age or greater), with a confirmed diagnosis of HF by physical exam, symptoms and echocardiographic evidence with New York Heart Association (NYHA) class II-IV, who were able to comprehend 5th grade English were enrolled during a planned office visit at a single HF outpatient clinic. Potential participants were excluded if they had major and uncorrected hearing impairment, a diagnosis of cognitive impairment, prior heart transplantation/mechanical circulatory support, concomitant terminal illness that would impede participation, major psychiatric illness or an inability to complete the requirements of the study. Plasma samples and clinical data were collected on the same day as symptom data collection.
Both studies were reviewed and approved by our institutional review board. All participants provided written informed consent for the parent studies and also to have their data and biological samples used in future research. All data were collected between May, 2012 and July, 2015.
Clinical Parameters
Clinical and treatment characteristics, including left ventricular ejection fraction (LVEF) and left ventricular internal end-diastolic diameter (LVIDd) from echocardiographic assessments and pulmonary capillary wedge pressure (PCWP), right atrial pressure (RAP) and cardiac index (calculated by Fick) from right heart catheterization, were collected from participants’ electronic medical record. The median time from echocardiographic assessment to plasma sampling was 3 days, and the median time from right heart catheterization to plasma sampling was 2 days. We also computed projected 1-year survival based on the Seattle HF model.19
Quantification of Biomarkers
All biomarkers were quantified in a National Center for Advancing Translational Sciences-supported core lab in accordance with guidelines issued by the Clinical and Laboratory Standards Institute, using commercially-available radioimmunoassay or enzyme-linked immunosorbant assay kits in accordance with the manufacturer’s instructions. All samples were drawn, placed immediately on ice, transported directly to our core laboratory following standard transport and storage procedures, centrifuged for 15 minutes at 1000 ×g, aliquoted and stored at −80°C. Samples were thawed once for assay – there were no repeated freeze-thaw cycles.
We used a multi-marker strategy20 to capture several aspects of HF pathogenesis (Figure 1). Amino terminal pro-B-type natriuretic peptide (NTproBNP) was quantified as a measure of myocardial stress (Cusabio Technology, Houston, TX), sensitivity = 11.8 pg/mL, detection limit = 47 pg/mL, intra- and inter-assay coefficients of variation were 8% and 9% respectively). Soluble tumor necrosis factor α receptor 1 (sTNFαR1) was quantified as a metric of systemic inflammation ((R&D Systems, Minneapolis, MN), sensitivity = 11.8 pg/mL, detection limit = 7.8 pg/mL, intra- and inter-assay coefficients of variation were 5.2% and 8.8% respectively). Endothelial-leukocyte adhesion molecule 1 (E-selectin) was quantified as a peripheral blood metric of endothelial dysfunction ((R&D Systems), sensitivity = 0.03 ng/mL, detection limit = <0.03 ng/ml, and intra- and inter-assay coefficients of variation were 7% and 9%, respectively). The soluble form of ST2, an interleukin-1 receptor family, was quantified as a metric of hyper-volumetric stress ((Critical Diagnostics, San Diego, CA), sensitivity = 2.35 ng/mL, detection limit =1.31 ng/ml, and intra- and inter-assay coefficients of variation were 6.5% and 9.1%, respectively). P-Selectin (a.k.a CD62P) was quantified as a peripheral blood metric of platelet activation ((R&D Systems), sensitivity = 0.50 ng/mL, detection limit = <0.82 ng/ml, and intra- and inter-assay coefficients of variation were 6% and 9%, respectively). Finally, adiponectin was quantified as a metric of wasting ((EMD Millipore, St. Charles, MO), sensitivity = 0.89 ng/mL, detection limit = 3.9 ng/mL, intra- and inter-assay coefficients of variation were 5% and 7% respectively). Importantly, these peripheral biomarkers that have been shown by others to be associated with clinical events and/or deterioration in HF including NTproBNP,21 sTNFαR1,22 E-selectin,23 ST2,24 adiponectin25 and P-selectin.26
Figure 1:
Multi-Marker Strategy to Capture Key Elements of Heart Failure Pathogenesis. The primary source and interpretation of each biomarkers is presented.
Abbreviations: NTproBNP = amino terminal pro-B-type natriuretic peptide; sE-selectin = soluble E-selectin; sP-selectin = soluble P-selectin; sST2 = soluble interleukin-1 receptor precursor; sTNFαR1 = soluble tumor necrosis factor α receptor 1.
Symptom Measurement
Physical symptoms were measured using the 18-item Heart Failure Somatic Perception Scale (HFSPS). The HFSPS asks about how much the participant was bothered by 18 common HF symptoms during the last week and provides six response options ranging from 0 (not at all) to 5 (extremely bothersome); Cronbach’s α is 0.90 and scores range from 0-90 with higher scored indicating worse physical symptoms.27 The HFSPS has a 6-item subscale for dyspnea with a Cronbach’s α of 0.90 (range = 0-30; higher scored indicate worse dyspnea), strong concordant validity with physical limitations (r= −0.53, p<0.001), and sufficient predictive validity for 1-year event-risk controlling for the Seattle HF score (per point hazard ratio (HR) =1.031 (95%CI=1.002-1.060), p=0.031). Finally, the HFSPS also has a 7-item subscale for “early & subtle” symptoms (i.e. upset stomach, cough, feeling tired, clothes feeling tighter, waking up to urinate, needing to rest more than usual, and not feeling like eating), with a Cronbach’s α of 0.75 (range 0-35; higher scores indicate worse symptoms), moderate concordant validity with physical limitations (r = −0.390, p<0.001), and sufficient predictive validity for 1-year event risk (adjusted per point HR = 1.030 (95% CI=1.003-1.053), p=0.028).27 The HFSPS total score as well as the dyspnea and early & subtle subscales were used in this analysis.
The Brief Pain Inventory (BPI) was used as an assessment of pain severity and interference.28 The BPI consists of 4 questions about pain severity (i.e. worst, least, average, and current pain intensity), and 7 questions about pain interference (i.e. the degree to which pain interferes with 7 domains of functioning). Summary scores for pain severity and interference were then calculated (each ranging from 1-10; 0 = no pain or does not interfere, and 10 = as bad as they could imagine or interferes completely functioning).
Wake disturbances were measured using the 8-item Epworth Sleepiness Scale (ESS).29 The ESS asks respondents to rate how likely they would be to fall asleep in 8 situations (sitting and reading, watching television, sitting inactive in a public place, as a passenger in a care, lying down for a rest, sitting and talking to someone, sitting quietly after lunch, and in a car while stopped in traffic) by choosing response options that range from 0 (would never fall asleep) to 3 (high chance).
Statistical Analysis
Demographics, clinical and laboratory characteristics were tabulated by cohort. Comparisons between cohorts were made using t-tests without assuming equal variance for continuous data, and Fisher exact or χ tests for categorical data. All biomarker data were log transformed to approximate normality prior to use in linear models. Preliminary analyses for transparency included linear correlations between biomarkers and between symptoms, as well as linear correlations between biomarkers and clinical characteristics. Because of the limited associations between symptoms and biomarkers in the HF literature, and because HF symptom science is in its infancy compared with other illness contexts like cancer, ours was largely an exploratory analysis taking broad strokes at relationships between common symptoms and common elements of pathogenesis.
To address aim 1, pairwise linear correlations between biomarkers and between symptoms were calculated by cohort. By convention, relationships with a statistical significance less than 0.20 were considered for subsequent formal testing of aim 2. To address aim 2, linear regression modeling was used to examine the differential influence of biomarkers on symptoms comparing the moderate and advanced HF cohorts; results were presented as slope coefficients for each cohort and their respective standard errors (in tables) and 95% confidence intervals (in figures), as well as the statistical significance of the interaction term (i.e. biomarker x cohort) comparing cohorts (reported as p-value). Based on linear modeling, marginal means were estimated across observed ranges of symptoms and log-transformed biomarkers and then graphed to represent differences in the relationship between biomarkers and symptoms between patients with moderate vs. patients with advanced HF. All analyses were performed using StataMP v15 (College Station, TX). Assuming alpha of 0.05, and an average difference in correlation of ±0.5 using experimental-group correlation for a two-sample correlations tests, a sample size of 96 was necessary to preserve 80% power.
Results
There were 48 participants in the advanced HF cohort and 48 participants in the matched moderate HF cohort (Table 1). As a function of the study design, the two groups were similar with respect to age, gender, etiology and duration of HF. As expected, patients in the advanced HF cohort had more severe HF with respect to functional classification, contractility, ventricular dilatation, filling pressures, clinical biochemistries and projected one-year survival compared with the moderate HF cohort. Based on biomarker results, the advanced HF cohort also had significantly worse myocardial stress, systemic inflammation, hyper-volumetric stress, platelet activation and wasting compared with the moderate HF cohort (Table 2). The only symptom that was significantly worse in the advanced HF cohort compared with the moderate HF cohort was dyspnea; all other symptom were statistically similar between cohorts.
Table 1:
Demographic and Clinical Characteristics of the Cohorts
| Moderate HF (n=48) |
Advanced HF (n=48) |
p-value | |
|---|---|---|---|
| Age in years | 54.6±12.5 | 54.0±14.5 | 0.851 |
| Female | 11 (22.9%) | 11 (22.9%) | 1.000 |
| Married | 31 (64.6%) | 27 (56.3%) | 0.567 |
| Non-Hispanic Caucasian | 42 (87.5%) | 42 (87.5%) | 1.000 |
| Ischemic etiology | 20 (41.7%) | 17 (35.4%) | 0.529 |
| Duration of heart failure: | |||
| ≤ 1 year | 12 (25.0%) | 14 (29.2%) | |
| 1-5 years | 10 (20.8%) | 7 (14.6%) | |
| 5-10 years | 11 (22.9%) | 10 (20.8%) | 0.836 |
| >10 years | 15 (31.3%) | 17 (35.4%) | |
| NYHA | |||
| Class II | 13 (27.1%) | 3 (6.3%) | |
| Class III | 32 (66.7%) | 23 (47.9%) | <0.001 |
| Class IV | 3 (6.2%) | 22 (45.8%) | |
| Body mass index | 32.4±7.7 | 27.5±4.6 | <0.001 |
| LVEF (%) | 26.5±12.7 | 19.8±2.3 | <0.001 |
| LVIDd (cm) | 6.4±1.2 | 7.4±1.3 | <0.001 |
| PCWP (mm/Hg) | 16.8±9.6 | 23.6±8.6 | <0.001 |
| RAP (mm/Hg) | 7.2±4.2 | 9.3±4.3 | 0.030 |
| Cardiac Index (L/min/m2) | 2.1±0.4 | 1.9±0.5 | 0.148 |
| V02Max (L/min) | 17.6±6.7 | 15.3±5.4 | 0.279 |
| Sodium (mEq/L) | 138.5±2.7 | 134.2±4.5 | <0.001 |
| Hemoglobin (%) | 13.6±1.6 | 12.1±2.0 | <0.001 |
| BUN/Cr ratio | 20.3±13.8 | 23.7±8.1 | 0.146 |
| Projected 1-year survival | 92.0%±10.1% | 48.8%±32.4% | <0.001 |
Abbreviations: BUN/Cr = blood urea nitrogen/creatinine ratio; LVEF = left ventricular ejection fraction; LVIDd = left ventricular internal diastolic diameter; NYHA = New York Heart Association; PCWP = pulmonary capillary wedge pressure; RAP = right atrial pressure; V02max = maximal oxygen consumption.
Table 2:
Biomarker and Symptom Characteristics of the Cohorts
| Moderate HF (n=48) |
Advanced HF (n=48) |
p-value | |
|---|---|---|---|
| NTproBNP | 1,999.6±1,386.3 | 4,021.7±2,772.5 | <0.001 |
| logNTproBNP | 7.4±0.7 | 8.0±0.9 | <0.001 |
| sTNFαR1 | 1,634.4±1,367.3 | 2,390.0±1,846.7 | 0.025 |
| logsTNFαR1 | 7.2±0.5 | 7.6±0.6 | 0.008 |
| E-Selectin | 41.7±21.0 | 46.4±22.9 | 0.300 |
| logsE-Selectin | 3.6±0.5 | 3.7±0.5 | 0.254 |
| sST2 | 45.1±31.0 | 76.2±51.3 | 0.005 |
| logsST2 | 3.7±0.5 | 4.1±0.6 | <0.001 |
| P-Selectin | 44.6±12.0 | 53.7±22.1 | 0.014 |
| logsP-Selectin | 3.8±0.3 | 3.9±0.4 | 0.030 |
| Adiponectin | 9.3±7.3 | 19.0±11.7 | <0.001 |
| logAdiponectin | 2.0±0.7 | 2.7±0.7 | <0.001 |
| Physical Symptoms (HFSPS) | 32.8.1±21.6 | 38.1±14.8 | 0.168 |
| Dyspnea (HFSPS) | 10.7±9.3 | 14.2±7.6 | 0.049 |
| Early & Subtle (HFSPS) | 14.3±8.2 | 16.2±6.1 | 0.198 |
| Pain Severity (BPI) | 2.8±2.2 | 2.8±2.5 | 0.861 |
| Pain Interference (BPI) | 3.8±3.1 | 3.2±3.0 | 0.364 |
| Wake Disturbances (ESS) | 9.2±5.6 | 9.6±5.4 | 0.754 |
Abbreviations: BPI = brief pain inventory; ESS = Epworth Sleepiness Scale; HFSPS = heart failure somatic perception scale, log = natural logarithm; NTproBNP = amino terminal pro-B-type natriuretic peptide; sE-selectin = soluble E-selectin; sP-selectin = soluble P-selectin; sST2 = soluble interleukin-1 receptor precursor; sTNFαR1 = soluble tumor necrosis factor α receptor 1.
Preliminary Analyses
Linear associations among biomarkers are presented in Table 3 and ranged in effect size between small and medium. Linear associations among symptoms also are presented in Table 3. Because the HFSPS subscales were nearly completely redundant to the HFSPS total score (both r ≥ 0.88), subsequent analyses focused on the dyspnea and early & subtle subscales for better symptom granularity. Linear associations between biomarkers and clinical characteristics are presented in Table 4.
Table 3:
Linear Relationships Among Biomarkers and Among Symptoms
| logNTproBNP | logsTNFαR1 | logsE-Selectin | logsST2 | logsP-Selectin | |
|---|---|---|---|---|---|
| logsTNFαR1 | 0.203 | - | |||
| logsE-Selectin | 0.379‡ | 0.190 | - | ||
| logsST2 | 0.374‡ | 0.398‡ | 0.313‡ | - | |
| logsP-Selectin | 0.410† | 0.195† | 0.553‡ | 0.228† | - |
| logAdiponectin | 0.368‡ | 0.167 | −0.019 | 0.398‡ | 0.194 |
| Physical Symptoms |
Dyspnea | Early & Subtle | Pain Severity | Pain Interference |
|
| Dyspnea | 0.906‡ | - | |||
| Early & Subtle | 0.879‡ | 0.681‡ | - | ||
| Pain Severity | 0.310‡ | 0.220† | 0.310‡ | - | |
| Pain Interference | 0.411‡ | 0.290‡ | 0.412‡ | 0.691‡ | - |
| Wake Disturbances | 0.378‡ | 0.358‡ | 0.387‡ | 0.178 | 0.297‡ |
Abbreviations: log = natural logarithm; NTproBNP = amino terminal pro-B-type natriuretic peptide; sE-selectin = soluble E-selectin; sP-selectin = soluble P-selectin; sST2 = soluble interleukin-1 receptor precursor; sTNFαR1 = soluble tumor necrosis factor α receptor 1.
= p<0.05,
= p<0.01
Table 4:
Linear Relationships Between Biomarkers and Clinical Characteristics
| log NTproBNP |
log sTNFαR1 |
log sE-Selectin |
log sST2 |
log sP-Selectin |
log Adiponectin |
|
|---|---|---|---|---|---|---|
| LVEF (%) | −0.183 | 0.041 | −0.080 | −0.172 | −0.186 | −0.128 |
| LVIDd (cm) | 0.304‡ | 0.020 | 0.103 | 0.214† | 0.232† | 0.295‡ |
| CI (L/min/m2) | −0.089 | 0.213 | −0.071 | −0.110 | −0.010 | −0.244† |
| RAP (mm/Hg) | 0.327‡ | 0.338‡ | 0.185 | 0.374‡ | 0.076 | 0.267† |
| PCWP (mm/Hg) | 0.394‡ | 0.280‡ | 0.295‡ | 0.488‡ | 0.168 | 0.328‡ |
| Sodium (mEq/L) | −0.330‡ | −0.322‡ | −0.302‡ | −0.443‡ | −0.304‡ | −0.364‡ |
| Hemoglobin (g/dL) | −0.120 | −0.592‡ | 0.030 | −0.314‡ | −0.085 | −0.281‡ |
| Creatinine (mg/dL) | 0.094 | 0.680‡ | 0.197 | 0.325‡ | 0.193 | 0.058 |
| BMI (kg/m2) | −0.102 | 0.037 | 0.290‡ | −0.150 | 0.049 | −0.533‡ |
Abbreviations: CI = cardiac index; BMI = body mass index; LVEF = left ventricular ejection fraction; LVIDd = left ventricular internal diastolic diameter; NTproBNP = amino terminal pro-B-type natriuretic peptide; PCWP = pulmonary capillary wedge pressure; RAP = right atrial pressure; sE-selectin = soluble E-selectin; sP-selectin = soluble P-selectin; sST2 = soluble interleukin-1 receptor precursor; sTNFαR1 = soluble tumor necrosis factor α receptor 1.
= p<0.05,
= p<0.01
Results of Aim 1
Linear associations among biomarkers and symptoms are presented by cohort in Table 5. There were several examples where the linear correlations between biomarkers and symptoms were different in direction, magnitude and/or significance when comparing the two cohorts. For example, NTproBNP and early & subtle symptoms were significantly and positively correlated in the moderate HF cohort (r = 0.399); but, in the advanced HF cohort the correlation was negative and non-significant (r = −0.034). Similarly, sTNFαR1 was significantly and positively associated with pain severity in the moderate HF cohort (r = 0.293), but the correlation was negative and non-significant in the advanced HF cohort (r = −0.076). Based on differences in linear correlations between cohorts, we tested 11 interactions between biomarkers and symptoms by cohort.
Table 5:
Linear Relationships Between Biomarkers and Symptoms by Cohort
| Dyspnea (HFSPS) |
Early & Subtle (HFSPS) |
Pain Severity (BPI) |
Pain Interference (BPI) |
Wake Disturbances (ESS) |
|
|---|---|---|---|---|---|
| Moderate Heart Failure (n=48) | |||||
| logNTproBNP | 0.239* | 0.399‡ | 0.189* | 0.303† | 0.266* |
| logsTNFαR1 | −0.073 | −0.035 | 0.293† | 0.261* | −0.095 |
| logsE-Selectin | 0.236* | 0.406‡ | 0.174 | 0.323† | 0.304† |
| logsST2 | 0.103 | 0.079 | 0.067 | 0.007 | 0.085 |
| logsP-Selectin | 0.185 | 0.288* | 0.170 | 0.193* | 0.130 |
| logAdiponectin | −0.104 | 0.021 | −0.009 | −0.117 | −0.228* |
| Advanced Heart Failure (n=48) | |||||
| logNTproBNP | 0.136 | −0.034 | −0.217* | −0.116 | −0.287† |
| logsTNFαR1 | −0.347† | −0.327† | −0.076 | −0.044 | −0.108 |
| logsE-Selectin | −0.114 | −0.034 | 0.026 | 0.051 | 0.006 |
| logsST2 | −0.151 | −0.124 | −0.175 | −0.090 | 0.023 |
| logsP-Selectin | −0.169 | 0.027 | 0.178 | 0.178 | 0.023 |
| logAdiponectin | 0.080 | 0.140 | −0.093 | −0.112 | −0.078 |
Abbreviations: BPI = brief pain inventory; ESS = Epworth Sleepiness Scale; HFSPS = heart failure somatic perception scale, log = natural logarithm; NTproBNP = amino terminal pro-B-type natriuretic peptide; sE-selectin = soluble E-selectin; sP-selectin = soluble P-selectin; sST2 = soluble interleukin-1 receptor precursor; sTNFαR1 = soluble tumor necrosis factor α receptor 1.
= p<0.20,
= p<0.05,
= p<0.01
Results of Aim 2
The relationship between NTproBNP and both early & subtle symptoms and wake disturbances were different between patients with moderate and those with advanced HF (Table 6; Figure 2). That is, worse myocardial stress was associated with worse early & subtle symptoms in moderate HF, but less early & subtle symptoms in advanced HF. Worse myocardial stress also was associated with worse wake disturbances in moderate HF, but less wake disturbances in advanced HF. Similar results were observed between NTproBNP and pain interference (Table 6).
Table 6:
Regression Slopes and Interactions Comparing Moderate and Advanced Heart Failure
| Moderate Heart Failure β±SE |
Advanced Heart Failure β±SE |
Interaction Term p-value |
|
|---|---|---|---|
| logNTproBNP | |||
| Early & Subtle | 4.50±1.45 | −0.22±0.07 | 0.006 |
| Wake Disturbances | 2.06±0.99 | −1.68±0.59 | 0.005 |
| Pain Interference | 1.31±0.55 | −0.40±0.18 | 0.025 |
| logsTNFαR1 | |||
| Pain Severity | 1.56±0.61 | −0.26±0.12 | 0.027 |
| Pain Interference | 1.71±0.59 | −0.19±0.08 | 0.026 |
| Dyspnea | −1.41±1.73 | −3.98±2.39 | 0.267 |
| Early & Subtle | −0.60±1.85 | −3.04±2.11 | 0.248 |
| logsE-Selectin | |||
| Dyspnea | 4.23±1.89 | −0.31±0.15 | 0.034 |
| Early & Subtle | 6.39±2.17 | −0.41±0.15 | 0.007 |
| Pain Interference | 1.94±.061 | 0.31±0.23 | 0.170 |
| Wake Disturbances | 3.30±1.60 | 0.07±0.05 | 0.171 |
Note: The interaction term is the formal statistical test of difference in the relationship between biomarkers and symptoms by group.
Abbreviations: β = regression slope; NTproBNP = amino terminal pro-B-type natriuretic peptide; SE = standard error; sE-selectin = soluble E-selectin; sP-selectin = soluble P-selectin; sTNFαR1 = soluble tumor necrosis factor α receptor 1.
Figure 2: Relationships between NTproBNP and Symptoms Comparing Moderate to Advanced Heart Failure.
In each image, the symptoms are presented on the y-axis, the natural log of NTproBNP (a metric of myocardial stress) is presented on the x-axis (within the range observed in this study), results from the moderate heart failure cohort are represented by dashed lines with circles at the mean, and results from the advanced heart failure cohort are represented by solid gray lines with squares at the mean. Higher levels of NTproBNP were associated with worse early & subtle symptoms in moderate heart failure, but were associated with fewer early & subtle symptoms in advanced heart failure (interaction p=0.006). Similarly, higher levels of NTproBNP were associated with worse wake disturbances in moderate heart failure but fewer wake disturbances in advanced heart failure (interaction p=0.005). Higher levels of NTproBNP also were associated with worse pain interference in moderate heart failure but less pain interference in advanced heart failure (interaction p=0.025) – data not shown in graph for economy of presentation. CI = confidence interval; NTproBNP = amino terminal pro-B-type natriuretic peptide.
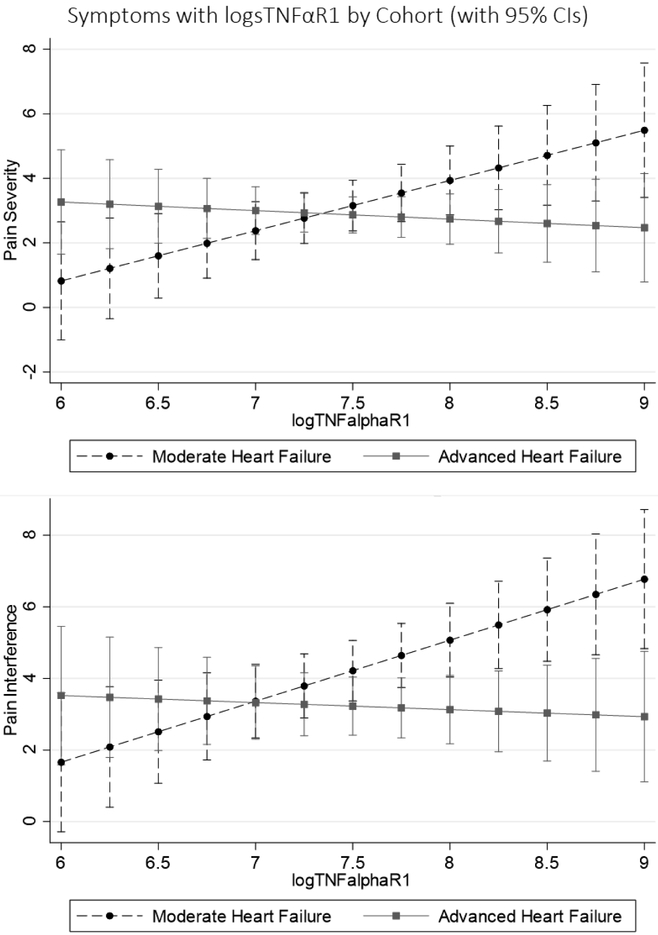
The relationship between sTNFαR1 and both pain severity and interference were different between patients with advanced and moderate HF (Table 6; Figure 3). Worse systemic inflammation was associated with worse pain severity in moderate HF, but less pain severity in advanced HF. Worse systemic inflammation also was associated with worse pain interference in moderate HF, but less pain interference in advanced HF. There were no differences in the relationships between sTNFαR1 and dyspnea or early & subtle symptoms by cohort (Table 6).
Figure 3: Relationships between sTNFαR1 and Symptoms Comparing Moderate to Advanced Heart Failure.
In each image, the symptoms are presented on the y-axis, the natural log of sTNFαR1 (a metric of systemic inflammation) is presented on the x-axis (within the range observed in this study), results from the moderate heart failure cohort are represented by dashed lines with circles at the mean, and results from the advanced heart failure cohort are represented by solid gray lines with squares at the mean. Higher levels of sTNFαR1 were associated with greater pain severity and interference in moderate heart failure, but were associated with less pain severity and interference in advanced heart failure (both interaction p<0.05). CI = confidence interval; sTNFαR1 = soluble tumor necrosis factor α receptor 1.
The relationship between sE-Selectin and both dyspnea and early & subtle symptoms were different between patients with advanced and moderate HF (Table 6; Figure 4). Worse endothelial dysfunction was associated with worse dyspnea in moderate HF, but less dyspnea in advanced HF. Worse endothelial dysfunction also was associated with worse early & subtle symptoms in moderate HF, but less early & subtle symptoms in advanced. There were no differences in the relationships between sE-Selectin and pain interference or wake disturbances by cohort (Table 6).
Figure 4: Relationships between sE-Selectin and Symptoms Comparing Moderate to Advanced Heart Failure.
In each image, the symptoms are presented on the y-axis, the natural log of sE-Selectin (a metric of endothelial dysfunction) is presented on the x-axis (within the range observed in this study), results from the moderate heart failure cohort are represented by dashed lines with circles at the mean, and results from the advanced heart failure cohort are represented by solid gray lines with squares at the mean. Higher levels of sE-Selectin were associated with worse dyspnea in moderate heart failure, but were associated with less dyspnea in advanced heart failure (interaction p=0.034). Similarly, higher levels of sE-Selectin were associated with worse early & subtle symptoms in moderate heart failure but less early & subtle symptoms in advanced heart failure (interaction p=0.007). CI = confidence interval; sE-Selectin = soluble E-Selectin.
Discussion
In this two-stage phenotype sampling cohort study of 96 adults with HF, we observed that relationships between peripheral biomarkers of HF pathogenesis and physical symptoms diverged considerably comparing adults with advanced HF (i.e. pre-LVAD implantation) to those with moderate HF (i.e. age-, gender-, etiology- and duration of HF-matched adults with moderate HF). Specifically, worse myocardial stress (higher NTproBNP) was associated with worse early & subtle symptoms, wake disturbances and pain interference in moderate HF, but less early & subtle symptoms, wake disturbances and pain interference in advanced HF. Worse systemic inflammation (higher sTNFαR1) was associated with worse pain severity and interference in moderate HF, but less pain severity and interference in advanced HF. Finally, worse endothelial dysfunction (higher sE-Selectin) was associated with worse dyspnea and early & subtle symptoms in moderate HF, but less dyspnea and early & subtle symptoms in advanced HF. The purpose of this line of inquiry is to better understand symptoms of chronic HF. According to these results, where patients are in the trajectory of HF needs to be taken into consideration when exploring the biological underpinnings of physical HF symptoms.
The dominant mechanism of NTproBNP secretion in HF is stretch-secretion coupling from myocardial wall stress.30,31 Hence, it make sense that higher levels of NTproBNP were associated with worse early & subtle physical symptoms linked to left-sided (e.g. cough) and right-sided failure (e.g. clothes feeling tighter), as well as symptoms related to low output (e.g. fatigue, satiety, wake disturbances) among adults with moderate HF. Exactly why higher levels of NTproBNP were associated with less physical symptom burden in advanced HF is unclear. One reason could be that patients with advanced HF have reduced their activity to nearly mitigate symptoms despite more advanced illness and higher levels of NTproBNP (1,999±1,386 pg/mL in moderate HF vs. 4,022±2,772 pg/mL in advanced HF). Another reason may be that a single measure of NTproBNP can be misleading, particularly in advanced HF, whereas knowing change in NTproBNP may be more important.32
Elevated levels of sTNFαR1 reflect high levels of TNFα as triggered in HF by direct antigenic stimulation,33 endothelial disruption, or direct hemodynamic stress.34 To the best of our knowledge, ours is the first evidence of a link between higher levels of sTNFαR1 and worse pain in HF (and only in moderate HF), although based on animal models TNFα has been proposed as having an important role in neuropathic pain.35 Although there were no differences between groups in pain severity or interference, there was a difference in sTNFαR1 comparing patients with moderate HF (1,634±1,367 pg/mL) to those with advanced HF (2,390±1,847 pg/mL). It may be that in moderate HF nociceptive responses persist beyond the resolution of pain stimuli, or more simply that the direct hemodynamic stress that causes elevated sTNFαR1 also causes pain. In advanced HF, there was an approximate difference of 1 point on the pain severity and interference scores (out of 10) across the full range of sTNFαR1. Hence, the relationship between sTNFαR1 and pain in advanced HF could be interpreted as clinically irrelevant, or simply that pain is a function of other mechanisms in advanced HF. It also may be that the location and etiology of pain are different in patients with moderate compared with advanced HF.
Elevated levels of cell adhesion molecules like sE-Selectin reflect enhanced expression and shedding36 caused by endothelial disturbances.37 Endothelial dysfunction is systemic in HF involving major vessels through capillary beds including those in the lung and periphery.38 Hence, although ours is a novel finding, it is not surprising to observe that higher levels of sE-Selectin were associated with worse dyspnea and early & subtle symptoms in moderate HF. Patients with advanced HF may require greater changes in underlying pathogenic mechanisms to be perceived as symptoms, and/or there is a relative ceiling effect of the biological underpinnings of symptoms in advanced HF. Moreover, patients with advanced HF also may reduce activity (intentionally or otherwise) to minimize symptoms. Hence, soliciting information about symptoms without provocation (e.g. standing, walking etc.) may be insufficient to understand biological underpinning of symptoms in advanced HF.
A surprising finding was that sST2 was not associated significantly with any symptom in either group even though it was correlated significantly with several clinical HF characteristics (Table 4). It may be that sST2 is more useful in other contexts like when detecting residual congestion after diuresis.39,40 It also was interesting that adiponectin was not associated significantly with any symptom in either group. Adiponectin in HF is complicated because it is higher in cachexic patients,41 and because HF is associated with functional adiponectin resistance.42 Hence, higher levels of adiponectin that in other conditions would be considered anti-inflammatory and insulin-sensitizing are mainly reflective of wasting in HF,43 and adiponectin levels alone may tell an incomplete picture of the adipokine function.42 sP-selectin having no significant association with any symptom in either group also was an interesting finding. Although HF is a pro-thrombotic condition, others have shown that sP-Selectin may reflect comorbidities in HF and not the HF itself.44 In this sample, sP-Selectin was only correlated significantly with LVIDd and sodium. It also may be that the symptoms chosen to be measured in this study do not include those sensitive enough to be associated with platelet activation in HF.
Finally, attenuation of relationships between biomarkers and physical symptoms in advanced compared with moderate HF also may reflect differences in patients’ ability to detect and/or interpret bodily changes, and/or changes in the anchor or normalization of symptoms over time. That is, here are established antecedents to detecting and interpreting bodily changes as symptoms including knowledge (awareness and familiarity with bodily sensations), attention (focusing on relevant bodily sensations as opposed to competing cues) and expectation (experiential and contextual biases)45 that may help us understand the relatively flat and in some cased inverse relationships between pathogenic biomarkers and symptoms in advanced HF. Moreover, more work is needed to understand how medical treatment of advanced vs. moderate HF may be associated with a blunting between pathogenesis and symptomatology.
Strengths and Limitations
A strength of this study was that by design it provides insight into biochemical underpinnings of physical symptoms in two different phases of the HF trajectory. Another strength is that we included a range of common peripheral biomarkers reflective of several major pathophysiological mechanisms, used commercially available kits such that these data could easily be replicated, and also included a range of common physical symptoms that are known to be bothersome in HF. A possible weakness is that we chose to measure common proteins as opposed to other omics, although our findings set a foundation for future omic work related to specific pathways that had relevance in symptom science. Another limitation is that our data was cross-sectional at this phase in the evolution of our science and in this paper was focused on physical symptoms; future work must focus on how symptom biochemistry changes over time in HF and on the interdependence between physical and affective symptoms in HF. Additionally, our goal was to provide evidence of relationships between peripheral biomarkers and symptoms but certainly not to imply causality using a cross-sectional design. Finally, since we were limited by a small sample size at this proof of concept phase, future work by ours and other groups must account for other factors that influence biomarkers, symptoms or both.
Conclusion
Relationships between peripheral biomarkers of HF pathogenesis and physical symptoms differed in magnitude and direction comparing adults with advanced HF to those with moderate HF. Where patients are in the trajectory of HF needs to be taken into consideration when exploring the biological underpinnings of physical HF symptoms.
Acknowledgments
Sources of Funding:
This project was supported by the National Institutes of Health/ National Institute of Nursing Research (1R01NR013492). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health. Research reported in this paper also was supported by National Center for Advancing Translational Sciences of the National Institutes of Health (UL1TR000128), from the Oregon Health & Science University Hartford Center, and from the Oregon Health & Science University School of Nursing Innovation Grants program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher S. Lee, Boston College William F. Connell School of Nursing, Chestnut Hill, MA.
Quin E. Denfeld, Oregon Health & Science School of Nursing, Portland, OR.
Bradley E. Aouizerat, New York University Department of Oral and Maxillofacial Surgery, New York, NY.
Corrine Y. Jurgens, Stony Brook University School of Nursing, Stony Brook, NY.
Christopher V. Chien, University of North Carolina REX Healthcare, Raleigh, NC.
Emily Aarons, Boston College William F. Connell School of Nursing, Chestnut Hill, MA.
Jill M. Gelow, Providence Health, Portland, OR.
Shirin O. Hiatt, Oregon Health & Science University School of Nursing, Portland, OR.
James O. Mudd, Oregon Health & Science University Knight Cardiovascular Institute, Portland, OR.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128(16):1810. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):1. doi: 10.1016/j.cardfail.2010.04.004 [doi]. [DOI] [PubMed] [Google Scholar]
- 3.Adams KF, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the united states: Rationale, design, and preliminary observations from the first 100,000 cases in the acute decompensated heart failure national registry (ADHERE). Am Heart J. 2005;149(2):209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg RJ, Spencer FA, Szklo-Coxe Mariana, et al. Symptom presentation in patients hospitalized with acute heart failure. Clin Cardiol. 2010;33(6):E80. doi: 10.1002/clc.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekelman DB, Havranek EP, Becker DM, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail. 2007;13(8):643–648. doi: S1071-9164(07)00167-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 6.Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4(3):198–206. doi: S1474-5151(05)00034-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 7.Shah MR, Hasselblad V, Stinnett SS, et al. Dissociation between hemodynamic changes and symptom improvement in patients with advanced congestive heart failure. Eur J Heart Fail. 2002;4(3):297–304. doi: S1388984201002021 [pii]. [DOI] [PubMed] [Google Scholar]
- 8.Rector TS, Anand IS, Cohn JN. Relationships between clinical assessments and patients' perceptions of the effects of heart failure on their quality of life. J Card Fail. 2006;12(2):87–92. doi: S1071-9164(05)01322-9 [pii]. [DOI] [PubMed] [Google Scholar]
- 9.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of functional and health status measures in heart failure. J Card Fail. 2006;12(6):439–445. doi: S1071-9164(06)00213-2 [pii]. [DOI] [PubMed] [Google Scholar]
- 10.Lewis EF, Lamas GA, O'Meara E, et al. Characterization of health-related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail. 2007;9(1):83–91. doi: S1388-9842(06)00339-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 11.Bhardwaj A, Rehman SU, Mohammed AA, et al. Quality of life and chronic heart failure therapy guided by natriuretic peptides: Results from the ProBNP outpatient tailored chronic heart failure therapy (PROTECT) study. Am Heart J. 2012;164(5):799.e1. doi: 10.1016/j.ahj.2012.08.015 [doi]. [DOI] [PubMed] [Google Scholar]
- 12.Guglin M, Patel T, Darbinyan N. Symptoms in heart failure correlate poorly with objective haemodynamic parameters. Int J Clin Pract. 2012;66(12):1224–1229. doi: 10.1111/j.1742-1241.2012.03003.x [doi]. [DOI] [PubMed] [Google Scholar]
- 13.Lee CS, Hiatt SO, Denfeld QE, Chien CV, Mudd JO, Gelow JM. Gender-specific physical symptom biology in heart failure. J Cardiovasc Nurs. 2015;30(6):517–521. doi: 10.1097/JCN.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denfeld QE, Mudd JO, Gelow JM, Chien C, Hiatt SO, Lee CS. Physical and psychological symptom biomechanics in moderate to advanced heart failure. J Cardiovasc Nurs. 2015;30(4):346–350. doi: 10.1097/JCN.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denfeld QE, Mudd JO, Hasan W, et al. Exploring the relationship between beta-adrenergic receptor kinase-1 and physical symptoms in heart failure. Heart Lung. 2018. doi: S0147-9563(18)30107-9 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CS, Mudd JO, Gelow JM, et al. Background and design of the profiling biobehavioral responses to mechanical support in advanced heart failure study.(report). J Cardiovasc Nurs. 2014;29(5):405. doi: 10.1097/JCN.0b013e318299fa09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Lewinger JP, Gauderman WJ, Murcray CE, Conti D. Using extreme phenotype sampling to identify the rare causal variants of quantitative traits in association studies. Genet Epidemiol. 2011;35(8):790–799. doi: 10.1002/gepi.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–1504. doi: 10.1016/j.healun.2015.10.003 [doi]. [DOI] [PubMed] [Google Scholar]
- 19.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle heart failure model: Prediction of survival in heart failure. Circulation. 2006;113(11):1424. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 20.Allen LA, Felker GM. Multi-marker StrategieS in heart failure: Clinical and StatiStical approaches. Heart Fail Rev. 2010;15(4):343–349. doi: 10.1007/s10741-009-9144-z [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bettencourt P, Azevedo A, Pimenta J, Fries F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110(15):2168. doi: 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 22.Rauchhaus M, Doehner W, Francis DP, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102(25):3060. doi: 10.1161/01.CIR.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 23.Potapov EV, Hennig F, Wagner FD, et al. Natriuretic peptides and E-selectin as predictors of acute deterioration in patients with inotrope-dependent heart failure. Eur J Cardiothorac Surg. 2005;27(5):899–905. doi: S1010-7940(05)00126-0 [pii]. [DOI] [PubMed] [Google Scholar]
- 24.Felker GM, Fiuzat M, Thompson V, et al. Soluble ST2 in ambulatory patients with heart failure: Association with functional capacity and long-term outcomes. Circ Heart Fail. 2013;6(6):1172–1179. doi: 10.1161/CIRCHEARTFAILURE.113.000207 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamura T, Furukawa Y, Taniguchi R, et al. Serum adiponectin level as an independent predictor of mortality in patients with congestive heart failure. Circ J. 2007;71(5):623–630. doi: JST.JSTAGE/circj/71.623 [pii]. [DOI] [PubMed] [Google Scholar]
- 26.Chung I, Choudhury A, Lip GY. Platelet activation in acute, decompensated congestive heart failure. Thromb Res. 2007;120(5):709–713. doi: S0049-3848(07)00012-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 27.Jurgens CY, Lee CS, Riegel B. Psychometric analysis of the heart failure somatic perception scale as a measure of patient symptom perception. J Cardiovasc Nurs. 2017;32(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cleeland CS, Ryan KM. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep. 1991;14(6):540–545. 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.de Bold AJ, Bruneau BG, Kuroski de Bold ML. Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc Res. 1996;31(1):7–18. doi: 0008-6363(95)00121-2 [pii]. [PubMed] [Google Scholar]
- 31.Ogawa T, Linz W, Stevenson M, et al. Evidence for load-dependent and load-independent determinants of cardiac natriuretic peptide production. Circulation. 1996;93(11):2059–2067. [DOI] [PubMed] [Google Scholar]
- 32.Eurlings LW, Sanders-van Wijk S, van Kraaij DJ, et al. Risk stratification with the use of serial N-terminal pro-B-type natriuretic peptide measurements during admission and early after discharge in heart failure patients: Post hoc analysis of the PRIMA study. J Card Fail. 2014;20(12):881–890. doi: 10.1016/j.cardfail.2014.08.014 [doi]. [DOI] [PubMed] [Google Scholar]
- 33.Torre-Amione G. Immune activation in chronic heart failure. Am J Cardiol. 2005;95(11A):40C. doi: S0002-9149(05)00385-1 [pii]. [DOI] [PubMed] [Google Scholar]
- 34.Aukrust P, Gullestad L, Ueland T, Damas JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: Potential therapeutic implications. Ann Med. 2005;37(2):74–85. [DOI] [PubMed] [Google Scholar]
- 35.Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. J Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin WH, Chen JW, Jen HL, et al. The prognostic value of circulating soluble cell adhesion molecules in patients with chronic congestive heart failure. Eur J Heart Fail. 2003;5(4):507–516. doi: S1388984203000096 [pii]. [DOI] [PubMed] [Google Scholar]
- 37.Chong AY, Lip GY, Freestone B, Blann AD. Increased circulating endothelial cells in acute heart failure: Comparison with von willebrand factor and soluble E-selectin. Eur J Heart Fail. 2006;8(2):167–172. doi: S1388-9842(05)00164-9 [pii]. [DOI] [PubMed] [Google Scholar]
- 38.Shantsila E, Wrigley BJ, Blann AD, Gill PS, Lip GY. A contemporary view on endothelial function in heart failure. Eur J Heart Fail. 2012;14(8):873–881. doi: 10.1093/eurjhf/hfs066 [doi]. [DOI] [PubMed] [Google Scholar]
- 39.Ky B, French B, McCloskey K, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4(2):180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Serum levels of the interleukin-1 receptor family member ST2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ Heart Fail. 2009;2(4):311–319. doi: 10.1161/CIRCHEARTFAILURE.108.833707 [doi]. [DOI] [PubMed] [Google Scholar]
- 41.Szabo T, Scherbakov N, Sandek A, et al. Plasma adiponectin in heart failure with and without cachexia: Catabolic signal linking catabolism, symptomatic status, and prognosis. Nutr Metab Cardiovasc Dis. 2014;24(1):50–56. doi: 10.1016/j.numecd.2013.04.015 [doi]. [DOI] [PubMed] [Google Scholar]
- 42.Van Berendoncks AM, Garnier A, Beckers P, et al. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail. 2010;3(2):185–194. doi: 10.1161/CIRCHEARTFAILURE.109.885525 [doi]. [DOI] [PubMed] [Google Scholar]
- 43.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112(12):1756–1762. doi: CIRCULATIONAHA.104.530972 [pii]. [DOI] [PubMed] [Google Scholar]
- 44.Chung I, Choudhury A, Patel J, Lip GY. Soluble, platelet-bound, and total P-selectin as indices of platelet activation in congestive heart failure. Ann Med. 2009;41(1):45–51. doi: 10.1080/07853890802227089 [doi]. [DOI] [PubMed] [Google Scholar]
- 45.Whitaker KL, Scott SE, Wardle J. Applying symptom appraisal models to understand sociodemographic differences in responses to possible cancer symptoms: A research agenda. Br J Cancer. 2015;112 Suppl 1:27. doi: 10.1038/bjc.2015.39 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]