Abstract
Background:
Peritoneal free fluid (PFF) can indicate an underlying disease process; however, detection of minimal PFF in healthy children is not uncommon.
Objective:
To assess the significance of incidental PFF within healthy children by MRI and its relation to physiological changes during puberty.
Methods:
This prospective study was performed on 32 healthy volunteers (20 males) between the ages of 8 to 13 years, with consecutive follow-ups every 8–10 months for an average of 3 years. BMIz score, pubertal status, C-reactive protein and sex hormone concentrations assessed prior to MRI studies. A total of 120 pelvic MRI studies (61 males) were reviewed, and the quantity of PFF was measured. Linear mixed model accounting for within-patient correlations was used for statistical analysis.
Results:
The mean ± standard deviation volume of PFF was 4.7 ± 5.65 mL in females and 1.9 ± 3.11 mL in males with a maximum volume of 25 mL and 17 mL, respectively. The prevalence of PFF was significantly higher in females (91%) compared to males (67%) (P = 0.0035). In 15% of the females and 3% of the males the fluid was greater than 10 mL. The mean volume of PFF in 4th stage was higher and significantly different with the first stage of puberty (P = 0.01).
Conclusion:
Among healthy pubescent children, the prevalence of PFF is significantly higher in females. The volume of PFF may reach volumes greater than 10 mL during normal puberty, especially in 4th stage, and can be assumed normal in the absence of active disease.
Keywords: Magnetic resonance imaging, Peritoneal free fluid, Pubertal stage, Healthy children
Introduction:
Peritoneal free fluid (PFF), defined as a fluid collection in the pelvic cavity, can indicate an underlying disease process, such as inflammation or malignancy. As such, it has been proposed that detection of significant fluid requires further diagnostic assessment to exclude serious conditions [1]. However, detection of minimal PFF in healthy children is not uncommon, possibly due to increased peritoneal permeability [1, 2]. Previous studies using ultrasonography have indicated a higher prevalence (12 %) of PFF in asymptomatic children aged 9 to 15 years compared to other age groups, with the maximum volume ranging from 1 – 6.9 mL [1–3]. While ultrasonography remains the most readily available modality for evaluation of PFF, the sensitivity for detection of free pelvic fluid is greatly improved with MRI. To the best of our knowledge, there have been no previous studies that have investigated PFF in a healthy pubescent cohort by MRI. As a result, the prevalence and volume of fluid in pubescent populations assed with MRI could be different from the prevalence previously reported with ultrasonography [2, 4]. It may be difficult to determine whether incidentally-identified PFF is normal or abnormal; studies on prevalence and volume of PFF in healthy volunteers would assist in clarifying how much isolated fluid is physiological. In addition, the majority of studies quantifying PFF have been performed on patients with clinical indications for pelvic imaging, not truly healthy pubescent volunteers; therefore, there are few normative data regarding the volume of PFF during the pubertal transition [1–4].
In order to quantify the PFF in children, one should consider the hormonal changes during the pubertal transition that may be influential in the development of PFF. Changes in gonadal hormones, including estradiol, progesterone, 17-hydroxyprogesterone, testosterone, DHEAS, and androstenedione, during puberty not only trigger the appearance of secondary sexual characteristics and physical development but also impact homeostasis of body fluid [5, 6]. Traditionally, progression of puberty is clinically evaluated through determination of pubertal stage by a clinician employing Tanner’s methods, which divides pubescent development into five stages ranging from pre-pubescence to adulthood [5, 7], plus measurement of testicular volume via orchidometry as described by Zachmann, Prader and colleagues [8].
Another influential factor to be considered in evaluating PFF is the presence of an inflammatory condition in the body. C-reactive protein (CRP) is an acute phase reactant protein that is part of the immune system and rises during inflammatory processes. While two-thirds of people in the US have less than 3 μg/mL CRP plasma concentration, CRP concentrations of more than 10 μg/mL can be counted as clinically significant [9]. Thus, the possibility of inflammatory processes contributing to PFF can be evaluated by measuring CRP concentrations [10].
The goal of this prospective study was to evaluate the prevalence of incidental PFF within healthy pubescent individuals. We used MRI to delineate the relationship between PFF volume and the physiological changes during puberty and establish normative values that in theory could help reduce unnecessary work-ups and financial burden to patients [11].
Materials and Methods:
Study Population
The study sample is a cohort of children and adolescents participating in a study of normally developing children (clinicaltrials.gov NCT01434368). This Health Insurance Portability and Accountability Act (HIPAA)-compliant prospective study was approved by the Institutional Review Board of the National Institute of Mental Health. The healthy volunteer children provided written assent, and informed written consent was obtained from their parents. The study was carried out on 36 healthy volunteers (12 females, 24 males) between the ages of 8 to 13 years studied at the National Institutes of Health Hatfield Clinical Research Center. Individuals had consecutive follow-ups every 8 – 10 months for an average of 3 years from September 2012 to October 2017.
Inclusion criteria were as follow: Individuals of both sexes, race, and ethnicity with good general health were recruited at two age periods: age 8–9y and age 12–13y. The pre-pubertal sample was a group of 8-year-old children (male and female) recruited into the study after meeting criteria for being pre-pubertal due to absence of secondary sexual characteristics associated with gonadarche. Pubertal stage was assessed by a physician or a nurse practitioner trained in pubertal assessment by physical exam. Pubertal staging provides a measure of development on a scale of one to five based on emergence of secondary sex characteristics [12]. Pubertal stage in boys was determined by measuring testicular volume using the Prader orchidometer [13]. Prepubertal stage was defined as testicular volume ≤ 3mL. In girls, pubertal stage was determined by physical examination of the breasts. Prepubertal stage in girls was defined as absence of breast tissue elevation [14]. Participants were also required to be medically well, to have body mass index (BMI) between the 15th and 18th percentile for age and sex according to US Centers for Disease Control and Prevention (CDC) 2000 growth charts, and to have radiologic evidence of age-appropriate bone development. A normal tempo of growth was determined by skeletal age within ± 1.64 standard deviations of chronologic age according to the Greulich and Pyle radiograph atlas to exclude any precocious (or delayed) puberty. The study also excluded any individuals with contraindications to MRI procedures. The older cohort was a group of boys and girls recruited at ages 12 to 13 years. Their pubertal maturation was also assessed via physical examination of secondary sexual characteristics.
All menstruating females underwent MRI during the follicular phase of the menstrual cycle to decrease the effect of physiological hormonal changes and ovulation as cofounding factors for PFF [15].
Pelvic MRI
All MRI examinations were performed on the 3-T MRI scanner (Philips Achieva, Best, the Netherlands). T2-weighted turbo spin echo sequence with fat suppression (T2wFS) (TR ~ 1500–1800 ms, TE 80–100 ms) axial sequence was obtained in all 167 pelvic MRI studies. The slice interval was 2.2 mm, including 2 mm slice thickness and 0.2 mm slice space. All MRI studies were reviewed by two experienced radiologists, with 6 and 25 years of post-residency experience.
Image Analysis
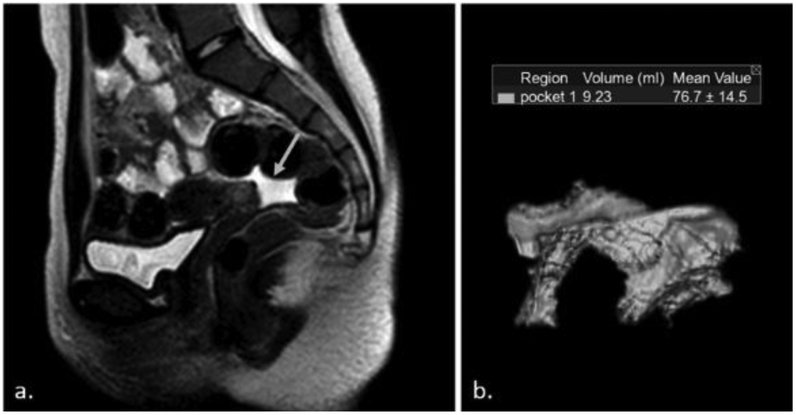
Forty seven MRIs were not evaluable due to lack of coverage of the full pelvis; therefore, a total of 32 patients (20 males) with 120 MRI scans (61 for males) were selected for analysis. Imaging software (Vitrea, v6.8.0, Vital Images Inc, Plymouth, MN), was used to measure the amount of pelvic free fluid. The area containing fluid was segmented in each slice separately, then the software automatically interpolated the segmentations and eventually calculated the volume of each pocket independently. The 3D reconstructed images were obtained for each pocket (Figure 1) [16]. The total volume of PFF in each patient was the sum of the volume in all pockets.
Figure 1.
(a) Sagittal T2-weighted MRI shows free fluid in a 15-year-old female (arrow). (b) Corresponding 3 dimensional image shows a 9.23 mL pocket of fluid in the same individual.
Anthropometrics, Pubertal Status, Inflammation, and Sex Hormones
Height and weight were used to calculate BMI (kg/m2), which was converted to BMIz scores for age and sex. Reproductive endocrine, metabolic, and physical status was measured by trained observers to identify the stage of puberty. In all 167 follow-ups, the pubertal status and sex hormone concentrations, including estradiol, progesterone, 17-hydroxyprogesterone, testosterone, DHEAS, and androstenedione, were assessed prior to MRI studies. Levels of 11-Desoxycortisol, Testosterone, Androstenedione, and 17-hydroxyprogesterone were analyzed using Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). [ng/dL]. Progesterone serum levels were measured with mass spectrometry. [ng/mL]; estradiol, was measured by Electrochemiluminescence Immunoassay on Roche Cobas e601 analyzer. [pg/mL]; Dehydroepiandrosterone Sulfate was measured via Chemiluminescence immunoassay on Siemens Immulite 2000 XPi analyzer. [mcg/mL]. In addition, C-reactive protein (CRP) was evaluated before each imaging study. C-reactive protein high sensitivity was run on Roche Cobas 6000 Analyzer.
Statistical Analysis
R Statistical Software (R Studio: Integrated Development Environment for R, v1.0.44, Boston, MA) was used for statistical analysis. Linear mixed modeling was used to determine whether levels of pelvic free fluid varied across different pubertal stage groups in boys or girls. Pubertal stages 1–5 were selected as fixed effects of the test, and patient IDs were assigned as the random effect to account for repeated measures within each patient. The relationship between level of sex hormones, CRP, BMIz score, and age with volume of free pelvic fluid was measured by the bootstrapping procedure. Models were calculated using the R”lme4” [17] and “rmcorr” [18] packages. A statistically significant difference was defined as a P value <0.05.
Results:
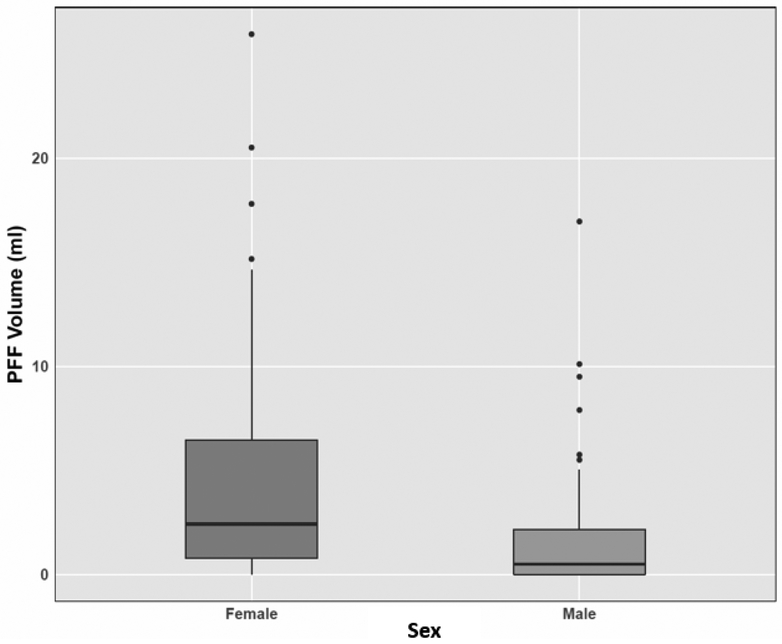
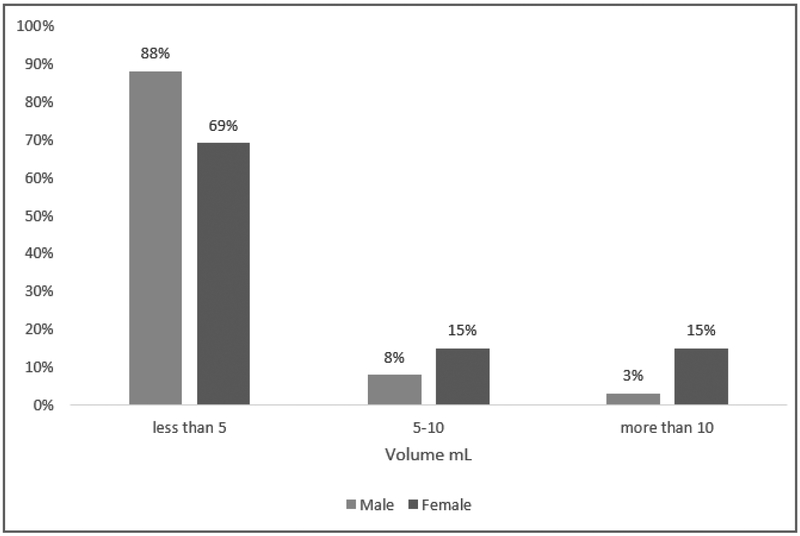
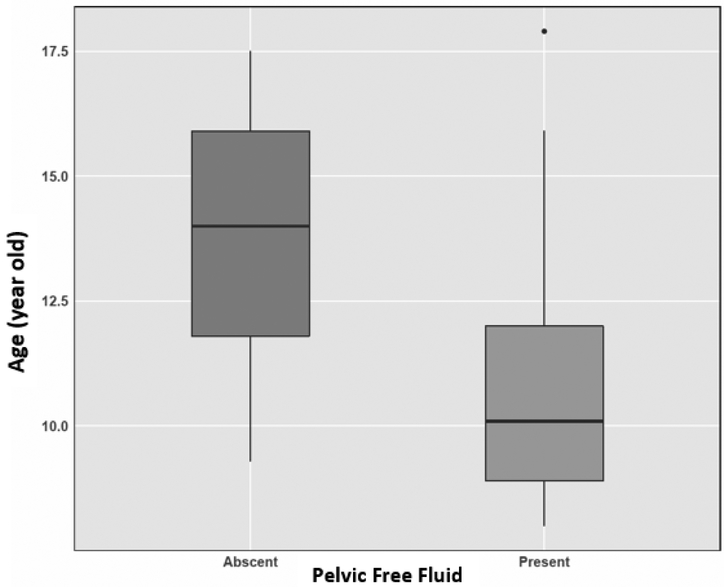
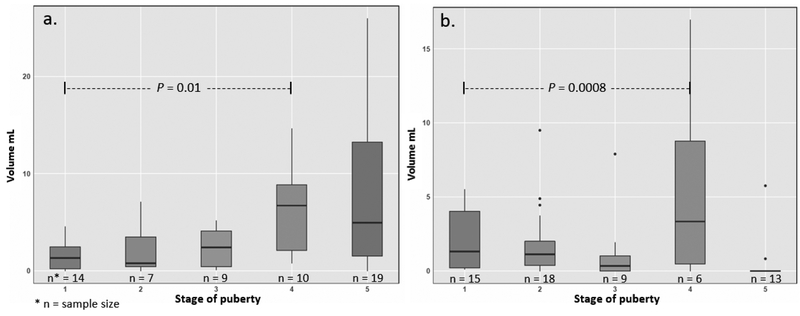
The prevalence of PFF was significantly higher in females (91%) compared to males (67%) (P = 0.0035) (Table 1). The mean ± standard deviation (SD) volume of PFF was 4.7 ± 5.65 mL in females and 1.9 ± 3.11 mL in males with a maximum volume of 25 mL and 17 mL, respectively (Figure 2). While 69% of females and 88% of males had less than 5 mL PFF, the volume of PFF was greater than 10 mL in 15% of the females and 3% of the males (Figure 3). There was no relationship between chronological age or BMIz score with volume of PFF in either males or females (all P > 0.05) in the linear regression model. There was a significant difference (P = 0.006) in mean age of volunteers with (10.7 -years -olds) and without (13.7 -years -olds) pelvic fluid (Figure 4). The sample sizes for first through fifth stages of puberty were 14, 7, 9, 10, 19 in female and 15, 18, 9, 6, 13 in male population, respectively. In the stages of puberty, mean volume of PFF in the 4th stage was higher than other stages in both sexes. Furthermore, the mean volume in the 4th stage was significantly different from the first pubertal stage in both sexes (P = 0.01) (Figure 5) [5]. Among sex hormones, the gonadal hormones (estradiol and progesterone) had positive linear correlations with PFF in females (r = +0.30, P = 0.03 and r = +0.42, P= 0.002). In the male population there were no significant relationships between sex hormones and PFF volume (all P > 0.05) (Table 2). The mean (± SD) BMIz score was 0.22 ± 0.61 and −0.09 ± 0.56 in these healthy female and male volunteers, respectively. The CRP level was under 3 μg/ml in 99% (119/120) of this population, regardless of sex. In addition, there was no correlation between CRP and fluid volume (P > 0.05).
TABLE 1:
Significant P value shows greater prevalence of PFF in girls.
| Peritoneal free fluid | Male | Female |
|---|---|---|
| Presence* | 41 (67%) | 54 (91%) |
| Absence | 20 | 5 |
P = 0.0035
Figure 2.
Box plot shows peritoneal free fluid volume in female and male. The mean volume is significantly larger in females compared to males (P = 0.001).
Figure 3.
The prevalence of PFF in the different categories of volume in both sex.
Figure 4.
Box plot shows the difference of mean age in presence and absence of fluid in males. The mean age of participants with free fluid (10.7 years old) was significantly lower than participants without free fluid (13.7 years old) in the pelvis (P = 0.006).
Figure 5.
Graph showing the relation of PFF volume and pubertal stage in females (a) and males (b). The mean volume of PFF in the 4th stage of puberty was higher than other stages and it was significantly different from the first pubertal stage in both sexes (P < 0.05).
TABLE 2:
Comparison of sex hormones with PFF in both sex.
| Hormones | r | P Value* |
|---|---|---|
| Estradiol | ||
| Male | −0.13 | 0.390 |
| Female | 0.30 | 0.032 |
| Progesterone | ||
| Male | 0.03 | 0.823 |
| Female | 0.42 | 0.002 |
| 17OH-progesterone | ||
| Male | −0.10 | 0.489 |
| Female | 0.27 | 0.052 |
| Testosterone | ||
| Male | 0.04 | 0.773 |
| Female | −0.11 | 0.423 |
| Androstenedione | ||
| Male | −0.01 | 0.934 |
| Female | 0.01 | 0.908 |
| DHEAS | ||
| Male | −0.14 | 0.359 |
| Female | −0.12 | 0.390 |
P-value is significant at < 0.05
Discussion:
Although free fluid in the pelvis can be a sign of underlying disease, minimal PFF in children has been considered normal. To the best of our knowledge, there is no specific definition for minimal PFF. In this study, we focused on healthy pubescent volunteers to evaluate the prevalence and volume of PFF, and to investigate the relationship between PFF volume and its prevalence with adolescent developmental changes using MRI.
In previous studies, prevalence of fluid in asymptomatic children with a few comorbidities during their visits was approximately 12% [1–3]. In this study, the prevalence of fluid within both female (91%) and male (67%) groups was much higher. This result may be due to higher sensitivity for detection of fluid as measured by MRI compared to ultrasonography and CT scan. We chose our study population to be of ages that would encompass the full range of pubertal stages since this age group is associated with hormonal changes [2].
Our results showed a significant difference in mean PFF volume between females (4.7 mL) and males (1.7 mL). This difference may be due to the effect of estradiol on capillary permeability and the existence of residual ovarian exudate in the pelvis in females. It has been shown that PFF volume increases at the end of the follicular phase during each menstrual cycle which might be related to the estradiol surge [19]. During puberty, testosterone and estradiol have a major role in secondary sexual development in boys and girls, respectively. Testosterone can be transformed to estradiol through aromatization (frequently in adipose tissue), so with increasing testosterone levels in males, estradiol also rises, albeit to a lesser degree compared to females [20]. According to previous studies, estradiol in girls and testosterone in boys are at the highest level in the 4th stage of puberty compared to the 1st stage that has the lowest level of sexual hormones [5]. The latter may be related to higher volumes of PFF in the 4th stage of puberty in both sexes in our study compared to the 1st stage.
In rat studies, progesterone increases the capillary permeability through vascular endothelial growth factors, but this has not been established in humans [21]. In this study, there was a positive correlation between the volume of PFF and both estradiol and progesterone in the female population. These data are consistent with the hypothesis that progesterone and estradiol may have similar effects on vessel permeability in children.
CRP as a phase reactant protein can be a sign of inflammatory processes in the body. In this study, almost all patients had a negative CRP result before undergoing MRI studies, correlating with the healthy status of our participants.
In all previous studies, more than 10 mL pelvic free fluid was rarely reported in asymptomatic individuals. Less than 10 mL fluid has been assumed normal in all age groups without evidence of underlying disease [2, 4, 22]. However in this study, 15% of females and 3% of males had more than 10 mL fluid during puberty transition. In addition, the maximum volume of detected fluid was 25 mL and 17 mL in females and males, respectively. It seems the PFF prevalence and volume are much greater during puberty than prior studies have detected. Therefore, if there is no sign of underlying disease in the pubescent population, the range of normal values for PFF volume appears to exceed 10 mL. More than 10 mL physiological PFF, found incidentally in an MRI study in a mid-pubertal child may mislead radiologists into reporting this as an abnormal finding.
There are limitations to this study. First, we had to exclude MRI studies of 45 males and 2 females that also detected PFF because MRIs of the full pelvis were not included in the imaging studies. This could have impacted the reported averages, especially in studies of male individuals where free fluid was detected. Second, we could not evaluate peritoneal free fluid in the upper part of the abdomen due to the pelvic MRI protocol. However, according to previous studies, peritoneal fluid usually accumulates in the pelvis rather than abdomen due to gravity [23].
Conclusion:
Our findings showed a high prevalence of PFF in both sexes (91% female, 67% male), and a high incidence of more than 10 mL PFF (15% female, 3% male) during puberty. We suggest that volume of PFF in the pubescent population needs further investigation to define baseline values as a guideline for clinicians, especially radiologists, to differentiate physiological from pathological fluid when encountering incidental PFF. An appreciation of the prevalence of measurable PFF in healthy children could help physicians by preventing unnecessary work-ups and associated costs.
References
- 1.Simanovsky N, Hiller N, Lubashevsky N, Rozovsky K. Ultrasonographic evaluation of the free intraperitoneal fluid in asymptomatic children. Pediatric Radiology. 2011;41(6):732–5. [DOI] [PubMed] [Google Scholar]
- 2.Fox MG, Balin JI, Stephens T, Patrie JT, Brant WE, de Lange EE. Isolated Pelvic Fluid in Males on Outpatient Magnetic Resonance Imaging Examinations: Differences in Incidence Based on Age. Journal of Computer Assisted Tomography. 2014;38(6):869–73. [DOI] [PubMed] [Google Scholar]
- 3.Rathaus V, Grunebaum M, Konen O, Odsatchy A, Zissin R, Shapiro M, et al. Minimal Pelvic Fluid in Asymptomatic Children. Journal of ultrasound in medicine. 2003;22(1):13–7. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa T, Hayashi N, Maeda E, Matsuda I, Sasaki H, Ohtsu H, et al. Peritoneal Fluid Accumulation in Healthy Men and Postmenopausal Women: Evaluation on Pelvic MRI. American Journal of Roentgenology. 2013;200(6):1181–5. [DOI] [PubMed] [Google Scholar]
- 5.Shirtcliff EA, Dahl RE, Pollak SD. Pubertal Development: Correspondence between hormonal and physical development. Child development. 2009;80(2):327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stachenfeld NS. Sex Hormone Effects on Body Fluid Regulation. Exercise and sport sciences reviews. 2008;36(3):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauren BA, Jessica LH, Elissa JH, Lyn YA. Pubertal Development, Emotion Regulatory Styles, and the Emergence of Sex Differences in Internalizing Disorders and Symptoms in Adolescence. Clinical Psychological Science. 2016;4(5):867–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ZACHMANN M Testicular volume during adolescence: cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974;29:61–72. [PubMed] [Google Scholar]
- 9.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. Journal of Clinical Investigation. 2003;111(12):1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landry A, Docherty P, Ouellette S, Cartier LJ. Causes and outcomes of markedly elevated C-reactive protein levels. Canadian Family Physician. 2017;63(6):e316–e23. [PMC free article] [PubMed] [Google Scholar]
- 11.Ding A, Eisenberg JD, Pandharipande PV. The Economic Burden of Incidentally Detected Findings. Radiologic clinics of North America. 2011;49(2):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of disease in childhood. 1970;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prader A Testicular size: assessment and clinical importance. Triangle; the Sandoz journal of medical science. 1966;7(6):240. [PubMed] [Google Scholar]
- 14.Bonat S, Pathomvanich A, Keil MF, Field AE, Yanovski JA. Self-assessment of pubertal stage in overweight children. Pediatrics. 2002;110(4):743–7. [DOI] [PubMed] [Google Scholar]
- 15.Davis JA, Gosink BB. Fluid in the female pelvis: cyclic patterns. Journal of Ultrasound in Medicine. 1986;5(2):75–9. [DOI] [PubMed] [Google Scholar]
- 16.Nasis A, Moir S, Seneviratne SK, Cameron JD, Mottram PM. Assessment of left ventricular volumes, ejection fraction and regional wall motion with retrospective electrocardiogram triggered 320-detector computed tomography: a comparison with 2D-echocardiography. The International Journal of Cardiovascular Imaging. 2012;28(4):955–63. [DOI] [PubMed] [Google Scholar]
- 17.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. 2015. 2015;67(1):48. [Google Scholar]
- 18.Bakdash JZ, Marusich LR. Repeated Measures Correlation. Frontiers in Psychology. 2017;8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarali H, Fleige-Zahradka BG, Ho Yuen B, McComb PF. The ascites in the ovarian hyperstimulation syndrome does not originate from the ovary*. Fertility and Sterility. 1993;59(3):657–61. [DOI] [PubMed] [Google Scholar]
- 20.Santen RJ. Is aromatization of testosterone to estradiol required for inhibition of luteinizing hormone secretion in men? The Journal of Clinical Investigation. 1975;56(6):1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ujioka T, Matsuura K, Kawano T, Okamura H. Role of progesterone in capillary permeability in hyperstimulated rats. Human reproduction (Oxford, England). 1997;12(8):1629–34. [DOI] [PubMed] [Google Scholar]
- 22.Brown SE, Dubbins PA. Detection of Free Intraperitoneal Fluid in Healthy Young Men. Journal of Ultrasound in Medicine. 2012;31(10):1527–30. [DOI] [PubMed] [Google Scholar]
- 23.Healy JC, Reznek RH. The peritoneum, mesenteries and omenta: normal anatomy and pathological processes. European Radiology. 1998;8(6):886–900. [DOI] [PubMed] [Google Scholar]