Abstract
This study focused on an estuarine wildlife species exhibiting high site fidelity and ubiquitous distribution in coastal environments along the Atlantic and Gulf coasts of the United States to monitor per- and polyfluoroalkyl substances (PFAS). A total of 75 diamondback terrapin (Malaclemys terrapin) plasma samples were collected from five creeks associated with Kiawah (Oyster Creek, Fiddler Creek, Sandy Creek, Gnat Creek) and Edisto (Townsend Creek) islands in Charleston County, South Carolina and investigated for 15 legacy PFAS. Of those, PFHxS was the only PFAS found in all terrapin plasma samples. Four additional PFAS were routinely detected (greater than 90 % of the samples) and were included in statistical analyses: PFOS, PFNA, PFDA, and PFUnA. Sex-differences were observed for two creeks with male plasma containing higher PFAS than female plasma (PFHxS at Townsend Creek, PFOS at Oyster Creek). Sex-specific site differences in PFAS concentrations were observed primarily for males, suggesting male terrapins may be more sensitive indicators of localized contaminant profiles than females. Three PFAS were observed to have negative correlations with body mass: PFOS in males (p=0.045, tau=−0.220), PFNA in males (p=0.016, tau=−0.269), and PFHxS in both males (p=0.007, tau=−0.302) and females (p=0.001, tau=−0.379). No relationships for body mass and PFDA and PFUnA were observed.
1. INTRODUCTION
Sentinel species are defined as organisms whose health, presence, and prevalence in the environment can act as an early indicator of potential hazards and/or risks to environmental and human health [1]. In the past, a variety of sentinel species have been used to monitor environmental health including numerous amphibians [2], birds [3], fish [4], mammals [5], and reptiles [6]. These species help researchers identify and understand potential dangers from a wide variety of environmental hazards including pollution by inorganic (e.g. arsenic [7] and mercury [5]) and organic contaminants (e.g., organochlorine pesticides [4], polychlorinated biphenyls (PCBs) [3], polybrominated diphenyl ethers (PBDEs) [8]).
A group of contaminants emerging as chemicals of concern are per- and polyfluoroalkyl substances (PFAS) [9], a class of man-made chemicals commonly utilized for their non-stick, surfactant like qualities. Since the 1950s, the use of PFAS in industrial and commercial products has become widespread, and currently these chemicals can be found in nonstick pans, food packaging, lubricants, paints, and aqueous film-forming foams (AFFF), to name a few [10, 11]. From the global use of these and other PFAS-containing products around the globe, PFAS steadily partition into the surrounding environment and have been measured in a variety of wildlife species [12]. The chemical stability that makes PFAS desirable for commercial and industrial uses also prevents their degradation in the environment.
Like many other turtles, the diamondback terrapin (Malaclemys terrapin) has been shown to be a useful sentinel species for the biomonitoring of contaminants including mercury, methyl mercury, and historic persistent organic pollutants [13, 14]. Terrapins exhibit multiple life history traits characteristic of model sentinel species such as a long-life span, an extensive geographic range along the United States (US) southeastern coast, and a relatively high trophic position. In addition, terrapins maintain high site fidelity in tidal creeks [15] and estuaries where anthropogenic run-off accumulates. As a result, contaminant profiles in terrapins may closely reflect those of their immediate surrounding environment.
American alligator (Alligator mississippiensis) plasma from Kiawah Island, South Carolina (SC) has shown greater than average perfluorooctane sulfonate (PFOS) burden when compared to alligators at 11 other sites along the southeastern US, as well as an unusual trend of higher levels of perfluorodecanoic acid (PFDA) when compared to the other sites [16]. The objective of this study was to examine PFAS concentrations in plasma of diamondback terrapins at Kiawah Island to determine if the PFAS trends observed in alligators at the site also occur in another reptile of lower trophic stature. In addition, plasma PFAS concentrations were also measured in terrapins from nearby Edisto Island to determine if contaminant profiles observed in Kiawah turtles are similar at adjacent sites.
2. MATERIALS AND METHODS
2.1. Sample Collection
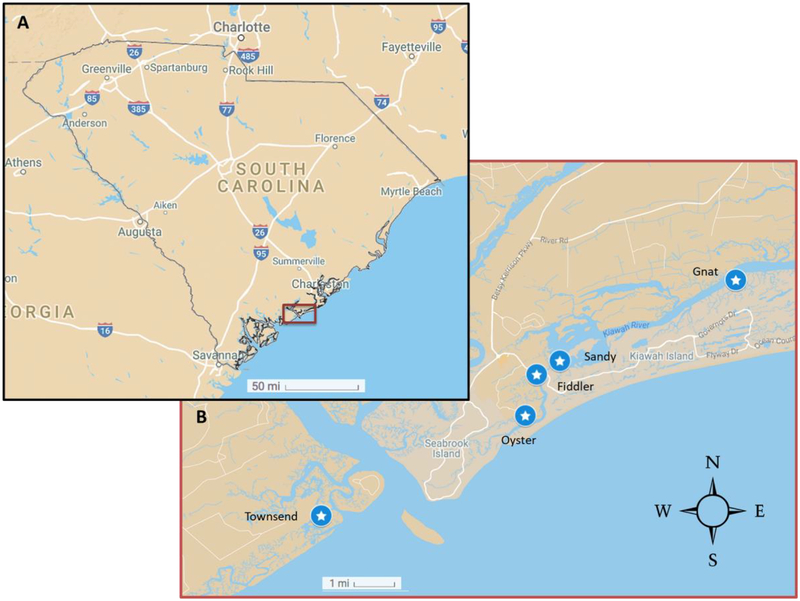
This study was conducted in the tidal creek tributaries of the Kiawah River and North Edisto River, Charleston County, SC as part of ongoing, long-term ecological research on diamondback terrapins in the area [15, 17–27]. Terrapins were captured from four creeks associated with the Kiawah River (Oyster Creek, Fiddler Creek, Sandy Creek, Gnat Creek) and one creek associated with the North Edisto River (Townsend Creek) (Figure 1). Terrapins were captured at Kiawah in October 2011 and May 2012, while animals from Edisto were captured only during May 2012. All turtles were captured at low tide by trammel nets, seines, and by hand and brought to a central location for processing. Straight-line carapace and plastron length were measured with calipers (± 1 mm), and individual turtles were uniquely marked by notching marginal scutes [25, 28]. Sex was determined by tail length and age was estimated at initial capture based on growth rings on the carapace and plastron when possible [25]. Blood (≤ 3 mL per turtle) was collected from the subcarapacial vein using a 2.5 cm, 22-gauge needle attached to a plastic syringe and transferred to a sterile 3 mL plastic Vacutainer tube containing lithium heparin [29–35]. Blood was kept on ice (< 30 minutes) until centrifuged at an RCF of 2000 g for 10 min. Plasma was then transferred to sterile cryovials and stored at −80 °C until analysis. Following sampling, all turtles were released at their site of capture.
Figure 1.
Creek sampling locations (stars) for Diamondback terrapin (Malaclemys terrapin) at Edisto Island (Creek: Townsend, n = 33) and Kiawah Island (Creeks: Oyster, n = 9; Fiddler, n = 17; Sandy, n = 11; and Gnat, n = 5), South Carolina.
2.2. Chemicals
A calibration solution was prepared using the National Institute of Standards and Technology (NIST) Reference Materials (RMs) 8446 Perfluorinated Carboxylic Acids and Perfluorooctane Sulfonamide in Methanol and RM 8447 Perfluorinated Sulfonic Acids in Methanol. The calibration solution contained 15 PFAS as follows: perfluorobutyric acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), PFDA, perfluoroundecanoic acid (PFUnA), perfluorododecanoic acid (PFDoA), perfluorotridecanoic acid (PFTriA), perfluorotetradecanoic acid (PFTA), perfluorobutanesulfonic acid (PFBS), perfluorohexanesulfonic acid (PFHxS), PFOS, and perfluorooctanesulfonamide (PFOSA).
The internal standard (IS) mixture was comprised of a total of eleven isotopically labeled PFAS, and they were as follows: [13C4]PFBA, [13C2]PFHxA, [13C8]PFOA, [13C9]PFNA, [13C9]PFDA, [13C2]PFUnA, [13C2]PFDoA, [18O2]PFBS, [18O2]PFHxS, [13C4]PFOS, and [18O2]PFOSA. The internal standards (IS) were purchased from Cambridge Isotope Laboratories (Andover, MA), RTI International (Research Triangle Park, NC), and Wellington Laboratories (Guelph, Ontario).
2.3. Sample Preparation
Terrapin plasma samples were extracted using previously described methods [36]. Briefly, approximately 1 mL of each terrapin plasma sample, Standard Reference Material (SRM) 1950 Metabolites in Frozen Human Plasma (used as control material), the calibrants, and blank samples were spiked with the internal standard mixture (comprised of RM 8446 and 8447) and allowed to come to equilibrium. The samples were extracted using basic methanol and cleaned using graphitized carbon solid phase extraction. After evaporation to approximately 1 mL, the extracts were analyzed by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS).
Samples (5 μL) were analyzed using an Agilent 1100 HPLC system (Santa Clara, CA) coupled to an Applied Biosystems API 4000 triple quadrupole mass spectrometer (Foster City, CA) with electrospray ionization in negative mode. A Phenomenex Kinetex PFP analytical column (2.1 mm x 150 mm x 2.6 μm) was used for separation of the analytes. Each run involved a ramping LC solvent gradient with methanol and de-ionized water both containing 20 mmol/L ammonium acetate (Table S1). Two multiple reaction monitoring (MRM) transitions for each PFAS were monitored to ensure no interferences, one MRM was employed for quantitation and the other one was used for confirmation.
2.4. Quality Control
All samples were processed alongside SRM 1950 and process blanks to determine the quality of the method. The PFAS concentrations measured in SRM 1950 agreed with previously established values reported on the Certificate of Analysis. Measured compounds were considered above the reporting limit (RL) if the mass of an analyte in the sample was greater than the mean plus three standard deviations of all blanks or lowest calibrant detected.
2.5. Statistical Methods
Statistical analyses and visualizations used the open-source program R [37] (primarily packages “tidyverse” and “NADA”) [38, 39]. Concentration data were grouped by analyte, sex, and capture location. Groups with 100 % detection used standard distribution-based estimates of central tendency and spread. Hypothesis testing between such groups with normal or log-normal distributions (values were log10 transformed) used either t-tests (2 groups) or ANOVA (3+ groups) followed by pairwise Tukey’s honest significant difference test. Comparisons including nonparametric distributions used either Wilcoxon (2 groups) or Kruskal-Wallis (3+ groups) tests followed by pairwise Wilcoxon tests. Helsel’s approaches for central tendency estimates and significance testing as implemented in NADA were used for data sets with < 100 % detection frequency [40]. Briefly, percent of samples above the maximum reporting limit was reported in groups where detection frequency was less than 20 %, groups where detection frequencies were 20 % to 50 % used robust regression on order statistics, and groups with detection frequencies 50 % to 99.9 % used the Kaplan-Meier method to estimate empirical cumulative distribution functions (ECDFs). Hypothesis testing between groups with < 100 % detection in all groups used NADA’s “cendiff()” comparison between ECDFs; pairwise ECDF comparisons followed significant results with 3+ groups. Significance levels (α=0.05) for rejection of H0 were consistent throughout; all tests were two-sided.
3. RESULTS AND DISCUSSION
In this study, we collected a total of 75 terrapin plasma samples from four creeks on Kiawah Island (Oyster, Fiddler, Sandy, and Gnat) and one creek on Edisto Island (Townsend) (Figure 1) to examine PFAS concentrations. Of the 15 PFAS investigated, PFHxS was the only PFAS found in all terrapin plasma samples (range 0.159 ng/g to 4.38 ng/g). Four additional PFAS were routinely detected (greater than 90 % of the samples) and were included in statistical analyses: PFOS (range < 0.064 ng/g to 10.5 ng/g), PFNA (range < 0.005 ng/g to 3.07 ng/g), PFDA (range < 0.043 ng/g to 1.60 ng/g), and PFUnA (range < 0.009 ng/g to 0.838 ng/g) (Supplemental Information, Figure S1, Table 1). Of all PFAS examined, PFOS concentrations were the highest measured in terrapin plasma, similar to that observed for other wildlife in previous studies (Tables 2 & 3) [12]. Results were examined among tidal creeks of capture, sex, and terrapin morphometrics.
Table 1.
Summary of PFAS (ng/g) in diamondback terrapin (Malaclemys terrapin) plasma collected from South Carolina examined by creek of capture on (A) all samples, (B) Edisto Island creeks and (C) Kiawah Island creeks.
| (A) | |||
|---|---|---|---|
| All terrapin (n = 75) | |||
| Range | Median | n > RL | |
| PFOS | < 0.064 – 10.5 | 1.56 | 72 |
| PFHxS | 0.249 – 4.38 | 0.695 | 75 |
| PFNA | < 0.005 −3.07 | 0.426 | 72 |
| PFDA | < 0.043 – 1.60 | 0.326 | 72 |
| PFUnA | < 0.009 – 0.838 | 0.249 | 71 |
| (B) | |||
|---|---|---|---|
| Townsend (n = 33) | |||
| Range | Median | n > RL | |
| PFOS | < 0.064 – 10.5 | 1.46 | 30 |
| PFHxS | 0.159 – 4.35 | 0.511 | 33 |
| PFNA | < 0.005 −3.07 | 0.405 | 30 |
| PFDA | < 0.043 – 0.942 | 0.324 | 30 |
| PFUnA | < 0.009 – 0.436 | 0.193 | 30 |
| (C) | ||||||
|---|---|---|---|---|---|---|
| Oyster (n = 9) | Fiddler (n = 17) | |||||
| Range | Median | n > RL | Range | Median | n > RL | |
| PFOS | 0.788 – 4.06 | 1.71 | 9 | 0.231 – 8.18 | 1.44 | 17 |
| PFHxS | 0.325 – 1.09 | 0.428 | 9 | 0.344 – 2.32 | 0.729 | 17 |
| PFNA | 0.084 – 0.644 | 0.229 | 9 | 0.078 – 1.46 | 0.341 | 17 |
| PFDA | 0.171 – 0.62 | 0.326 | 9 | 0.091 – 1.60 | 0.281 | 17 |
| PFUnA | < 0.011 – 0.393 | 0.252 | 8 | 0.054 – 0.838 | 0.21 | 17 |
| Sandy (n = 11) | Gnat (n = 5) | |||||
| Range | Median | n > RL | Range | Median | n > RL | |
| PFOS | 1.16 – 4.47 | 3.3 | 11 | 0.875 – 3.86 | 2.66 | 5 |
| PFHxS | 0.382 – 4.38 | 0.969 | 11 | 0.695 – 2.97 | 1.38 | 5 |
| PFNA | 0.31 – 1.85 | 0.954 | 11 | 0.288 – 1.24 | 0.726 | 5 |
| PFDA | 0.162 – 0.721 | 0.567 | 11 | 0.262 – 0.827 | 0.676 | 5 |
| PFUnA | 0.069 – 0.514 | 0.288 | 11 | 0.229 – 0.590 | 0.513 | 5 |
Table 2.
Summary of studies investigating several PFAS in various turtle species.
| Reference | Species | Matrix | Location | Sub-location | Sampling Dates | Reported values & units | n | PFOS | PFHxS | PFDA | PFNA | PFUnA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bangma et al. this study | Diamondback terrapin (Malaclemys terrapin) | Plasma | East coast USA |
Edisto Island Kiawah Island | 2011–2012 | median (range) ng/g | 33 42 |
1.46 (<0.064 – 10.5) 1.67 (0.231–8.18) | 0.511 (0.159–4.35) 0.739 (0.325–4.38) | 0.324 (< 0.043–0.942) 0.341 (0.091–1.60) | 0.405 (< 0.005–3.07) 0.437 (0.078–1.85) | 0.193 (<0.009–0.436) 0.269 (< 0.011–0.838) |
| de Solla et al. 2012 | Snapping turtle (Chelydra serpentina) | Plasma | Ontario, Canada |
Lake Niapenco (E) Lake Niapenco (W) Upper Welland River | 2008 & 2010 | Arithmetic mean (SD) ng/g w. w. | 9 9 1 |
2376.7 (1460.3) 2065.2 (649.6) 2269.4 | 2.3 (2) 3.2 (1.7) 8.2 | 4.3 (2.1) 2 (1) 3.4 | 0.6 (0.2) 0.1 (0.1) 0.3 | (2.5) (0.4) 1.8 |
| Credit River | 10 | 171.4 (120) | 0.2 (0.2) | 2.7 (1.4) | 0.3 (0.2) | 2.6 (1.2) | ||||||
| Cootes Paradise | 7 | 53 (17.1) | 0.1 (0.1) | 4.5 (3.4) | < 0.1 | 0.8 (0.3) | ||||||
| Humber River | 7 | 121.4 (90.1) | 0.2 (0.2) | 2.3 (0.9) | 0.2 (0.2) | 2 (0.7) | ||||||
| Island Lake | 4 | 15.1 (9) | < 0.1 | 1.2 (0.7) | < 0.1 | 1.3 (0.7) | ||||||
| Keller at al. 2005 | Loggerhead turtle (Caretta caretta) | Serum | East coast USA |
2003 | mean (SD) ng/mL | 73 | 11.0 (17.2) | 0.155 (0.201) | 0.515 (0.858) | 0.298 (0.588) | 0.355 (0.515) | |
| Kemp's ridley sea turtles (Lepidochelys kempii) | Serum | East coast USA |
6 | 39.4 (17.1) | 0.253 (0.0895) | 2.37 (1.25) | 1.654 (2.40) | 1.42 (0.789) | ||||
| O'Connell et al. 2010 | Juvenile loggerhead turtle (Caretta caretta) | Serum | East coast USA |
Florida Bay Cape Canaveral, FL | 2000–2008 | means (SD) ng/g | 11 10 |
3.67 (1.03) 1.44 (0.94) | 0.138 (0.051) <0.053 | 1.04 (0.233) 0.157 (0.123) | 2.21 (1.07) 0.141 (0.164) | 1.42 (0.546) 0.227 (0.111) |
| Charleston, SC | 9 | 3.86 (2.92) | 0.039 (0.038) | 0.316 (0.398) | 0.059 (0.050) | <0.194 | ||||||
| Core Sound, NC | 15 | 6.47 (7.53) | 0.135 (0.107) | 0.487 (0.601) | 1.30 (3.63) | 0.668 (0.751) | ||||||
| Chesapeake Bay, MD | 14 | 9.34 (11.4) | 0.065 (0.034) | 0.720 (0.817) | 0.974 (1.37) | 2.27 (4.20) | ||||||
| Keller et al. 2012 | Loggerhead turtle (Caretta caretta) | Plasma | East coast USA |
Core Sound, NC | 2006–2007 | median (SD) ng/g wet mass | 15 | 3.13 (7.52) | 0.110 (0.106) | 0.220 (0.602) | 0.240 (3.680) | 0.340 (0.754) |
| Kemp's ridley sea turtles (Lepidochelys kempii) | Plasma | Core Sound, NC | 10 | 10.8 (9.86) | 0.505 (0.378) | 1.910 (0.965) | 2.18 (1.60) | 1.74 (1.26) | ||||
| Herbivorous green turtles (Chelonia mydas) | Plasma | Core Sound, NC | 10 | 2.37 (1.12) | 0.030 (0.044) | 0.160 (0.117) | <RL | 0.293 (0.203) | ||||
| Hawksbill sea turtles (Eretmochelys imbreicata) | Plasma | Juno Beach, FL | 5 | 11.9 (6.27) | 0.550 (0.486) | 4.130 (0.789) | 17.0 (1.21) | 3.81 (2.18) | ||||
| Leatherbacks (Dermochelys coriacea) | Plasma | Juno Beach, FL | 7 | 4.42 (2.51) | 0.020 (0.005) | 0.190 (0.206) | 0.184 (0.204) | 0.470 (0.294) |
Table 3.
Summary of additional studies investigating PFOS in various turtle species.
| Reference | Species | Matrix | Location | Sub-Location | Sampling Dates |
Reported values & units | n | PFOS |
|---|---|---|---|---|---|---|---|---|
| Guerranti et al. 2013 | Loggerhead turtle (Caretta caretta) | Whole blood | Italy | Lesina | 2002–2008 | mean (SD) ng/g w.w. | 26 | 1.37 (1.52) |
| Linosa | 13 | 2.29 (7.89) | ||||||
| Livorno | 5 | <LOD | ||||||
| Talamone | 5 | 0.23 | ||||||
| Kannan et al. 2005 | Sanpping turtle (Chelydra serpentina) | Plasma | Michigan, USA | Macomb County | 1999 | mean (range) ng/mL | 2 male | 137(105–169) |
| 3 female | 6.13 (<1–8.8) | |||||||
| Morikawa et al. 2006 | (Trachemys scripta elegans) and (Chinemys reevesii) | Serum | Japan | Ai River A | 2003–2004 | Geometric mean (geometric SD) ng/mL | 3 | 108.2(2.8) |
| Ai River B | 10 | 283.3(1.6) | ||||||
| Ai River C | 16 | 89.5(3.8) | ||||||
| Ai River D | 10 | 127.2(1.9) | ||||||
| Ai River E | 14 | 187.2(1.7) | ||||||
| Taisyo River F | 2003–2004 | 1 | 252.4 | |||||
| Taisyo River G | 15 | 398.3(2.0) | ||||||
| Taisyo River H | 2 | 100.8(1.3) | ||||||
| Daijagaike Pond I | 3 | 50.1(2.5) | ||||||
| Uji River Waterway J | 20 | 25.1(2.0) |
3.1. Sex and site differences
Sex and site differences across the five PFAS routinely detected in terrapin plasma were assessed and resulted in five significant findings (Table 4A) on which post-hoc analyses were performed (Table 4B). Two differences were found between male and female terrapins: PFHxS at Townsend Creek, and PFOS at Oyster Creek. In both cases, males exhibited higher plasma concentrations of each respective PFAS than females. Regarding site differences within each sex, females showed few differences in PFAS among sites, with one exception. The single female captured at Gnat Creek had higher concentrations of PFUnA compared to Townsend Creek females; however, this difference may have been influenced by the small sample size at Gnat Creek. No other site differences among sites were found for female terrapins. For males, several site differences were found for several PFAS. Male terrapins from Oyster Creek had significantly lower plasma PFNA compared to all other sites except Townsend Creek. However, it should be noted that the comparison between Townsend Creek and Oyster Creek was also near statistical significance (Townsend Creek > Oyster Creek) with a p-value of 0.057. In addition, male terrapins at Oyster Creek had significantly lower PFHxS than at all other locations, while Sandy Creek male terrapins PFHxS levels were significantly higher than Townsend Creek PFHxS. These findings suggest male terrapin likely represent more sensitive indicators of site-specific differences in contamination because males maintain higher PFAS concentrations than females, and differences can be observed with as few as seven male plasma samples.
Table 4.
(A) Significant statistical comparisons for diamondback terrapins (Malaclemys terrapin) in South Carolina by site, sex and PFAS, and (B) post-hoc analyses.
| (A) | |||
|---|---|---|---|
| PFAS | Comparison | n | p - value |
| PFUnA | Females across sites | 36 | 0.0199 |
| PFNA | Males across sites | 39 | 0.0460 |
| PFHxS | Males across sites | 39 | 0.002 |
| PFHxS | Townsend male/female | 33 | 0.0026 |
| PFOS | Oyster male/female | 9 | 0.0275 |
| (B) | |||
|---|---|---|---|
| PFAS | Comparison | Post-Hoc (n) | p - value |
| PFUnA | Females across sites | Townsend (19) vs Gnat (1) | 2.21E-05 |
| PFNA | Males across sites | Oyster (6) vs Fiddler (7) | 0.004 |
| Males across sites | Oyster (6) vs Sandy (7) | 0.002 | |
| Males across sites | Oyster (6) vs Gnat (4) | 0.016 | |
| PFHxS | Males across sites | Townsend (15) vs Oyster (6) | 4.42E-04 |
| Males across sites | Townsend (15) vs Sandy (7) | 0.047 | |
| Males across sites | Oyster (6) vs Fiddler (7) | 0.008 | |
| Males across sites | Oyster (6) vs Sandy (7) | 0.001 | |
| Males across sites | Oyster (6) vs Gnat (4) | 0.010 | |
Previous studies have observed similar sex-specific differences in PFAS concentrations in both mammals [41] and other reptiles [16], with adult males exhibiting higher concentrations of PFAS in plasma compared to females. One potential hypothesis for sex-based differences is maternal depuration into eggs during nesting, and while no studies have assessed PFAS levels in terrapin eggs to date, studies on other oviparous species such as herring gulls (Larus argentatus) [42] have observed measurable levels of PFOS in eggs. In addition to maternal depuration, studies have also shown sex-based differences in the half-lives of PFASs are greatly influenced by sex hormone expression differences between the sexes. For example, biological half lives in male rats and male fathead minnow (Pimephales promelas) for PFOA are longer than in the respective female species, and castration of male rats shortens the half-life, and pretreatment of female fathead minnows with synthetic androgens lengthens the half-life of PFOA [43, 44]. Changes in testosterone levels likely effect organic anion transporters (OATs) in the kidney, which then influence PFAS excretion rates [44]. This would suggest the elimination half-life of PFAS is partially modulated by testosterone [45].
Male and female terrapin in this study were captured in the months May and October. Sample sizes were not large enough to account for season of capture as a covariate in the original model due to having five sampling sites of interest. However, seasonality was investigated separately to determine potential effects on PFAS levels. For males, only PFUnA showed a slight elevation during October compared with May (Table S2). However, females showed higher levels of all measurable PFAS with the exception of PFUnA during October compared with May (Table S2). Previous studies with similar findings hypothesize this may be due to animals increased dietary intake in fall months (such as October) in preparation for winter months [14]. Females may show more significance in PFAS seasonality compared to males because females may need to consume larger quantity of their diet to account for energy lost during ovoposition in the summer months. It is possible this observed PFAS seasonality in females reduced the number of significant findings for site to site differences. Future studies investigating site differences in terrapin, should account for seasonality in their study designs in order to reduce variation due to month of capture.
3.2. Morphometrics
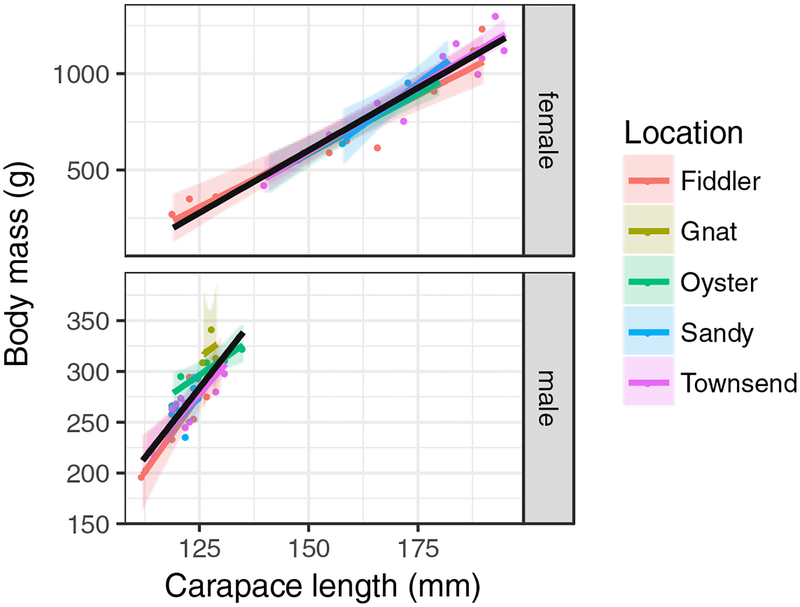
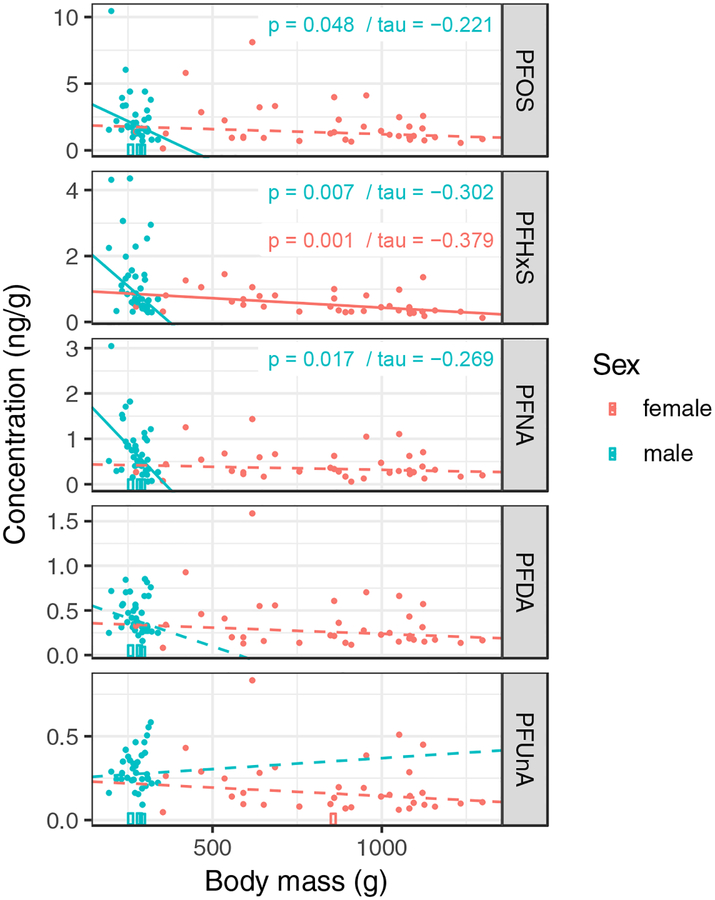
Terrapin body mass and carapace length were highly positively correlated, with males (Pearson rho = 0.856) much smaller than females (Pearson rho = 0.966) (Figure S2). No differences were observed for carapace length correlations between sites (p=0.485) when controlling for the significant difference between sex (p=0.007) (Figure 2). With no observable differences in carapace length by location and high correlation coefficients between body mass and carapace length, we examined the relationship between PFAS and body mass for each sex. Using Kendall’s tau correlation, three PFAS were observed to have negative correlations with body mass: PFOS in males (p=0.045, tau=−0.22), PFNA in males (p=0.016, tau=−0.269), and PFHxS in both males (p=0.007, tau=−0.302) and females (p=0.001, tau=−0.379) (Figure 3). No relationships for body mass and PFDA and PFUnA were observed. Overall, all observed significant correlations were negative. Males exhibited more correlations between body mass and various PFAS, while the strongest correlation observed was for PFHxS and body mass in females. Prior to this study, negative correlations between PFAS and body size had not been reported in turtles, although such correlations have been observed in bottlenose dolphins (Tursiops truncatus) [46]. Previous studies on sea turtles have found positive correlations between several PFAS and carapace length [31], while other investigations have found no correlation between PFAS and sea turtle morphometrics [35]. Positive correlations between several PFAS and body length have also been observed in adult alligators [16].
Figure 2.
Carapace length by creek of capture for female and male diamondback terrapins (Malaclemys terrapin). For each sex, across-location correlation is indicated by a black line.
Figure 3.
Kendall’s tau correlations for PFOS, PFHxS, PFNA, PFDA, and PFUnA (ng/g) and body mass (g) in male and female diamondback terrapins (Malaclemys terrapin). Lines represent significant (solid) and non-significant (dashed) correlations.
The causes of the negative correlations between PFAS concentrations and terrapin body mass are currently unknown. However, this relationship may possibly be a reflection of ontogenetic shifts in diet among terrapins at our study site. A previous study on the diet of terrapins at Kiawah Island found that larger terrapins generally consume larger prey and a wider diversity of prey items than smaller terrapins [47]. If the additional prey items consumed by larger terrapins contain lower concentrations of PFAS than prey items consumed by smaller terrapins, this may contribute to the lower overall body burdens of PFAS in the former. Data on PFAS concentrations in terrapin prey items are needed to adequately examine this possibility. Likewise, negative correlations between PFAS concentrations and terrapin body mass may also be a function of size-specific differences in the toxicokinetics and toxicodynamics of PFAS in terrapins following exposure, but no such data are currently available.
3.3. Comparisons with other studies
Of the different PFAS detected in both terrapins and alligators [16] at Kiawah Island, concentrations were much lower in the former compared to the latter. Median concentrations of PFOS, PFDA, PFUnA, and PFNA were more than 35-, 19-, 15-, and 2-fold lower in terrapins than alligators, respectively, while PFHxS concentrations were similar [16]. As both species are long-lived [15, 48] and therefore exhibit potentially similar chemical exposure durations, differences in PFAS burdens between terrapins and alligators at Kiawah are likely related to species-specific differences in habitat use and diet, additional PFAS contamination at Kiawah following terrapin sampling (2011–2012) and prior to alligator sampling (2015), species-specific differences in PFAS toxicokinetics and toxicodynamics, or a combination of these.
To our knowledge, this is the first study to examine PFAS in diamondback terrapins, an estuarine species. However, multiple other studies have investigated PFAS in both marine and freshwater turtles (Table 2). Along the east coast of the US, similar median levels of PFOS were observed in green turtles (Chelonia mydas) and leatherback sea turtles (Dermochelys coriacea), while similar levels of PFHxS were observed in Kemp’s ridley sea turtles (Lepidochelys kempii) and hawksbill turtles (Eretmochelys imbricata). Significantly higher levels of PFAS were found in plasma from freshwater species (i.e., snapping turtle (Chelydra serpentina) downstream from an airport due to point source contamination of AFFF [34]. Overall, terrapin plasma collected at Kiawah and Edisto islands were similar to those previously reported for other turtle species, suggesting terrapins could represent a model sentinel for some larger and potentially more difficult to sample chelonians.
4. CONCLUSIONS
Detectability for PFAS was high in diamondback terrapins, an estuarine species exhibiting high site fidelity and ubiquitous distribution in coastal environments along the Atlantic and Gulf coasts of the US. In addition, for the first time a negative correlation between body size and PFAS was observed for a turtle species. Overall, male terrapins appear to be more sensitive indicators of site differences than females.
Supplementary Material
Acknowledgments-
We thank researchers who contributed with collection of diamondback terrapins, particularly Michael Dorcas, Cris Hagen, and Meg Hoyle. Field research was supported by the Department of Energy under Award Numbers DE-FC09–07SR22506 and DE-EM0004391 to the Savannah River Ecology Laboratory and University of Georgia Research Foundation. This paper represents Technical Contribution Number 6597 of the Clemson University Experiment Station.
Footnotes
Publisher's Disclaimer: Disclaimer - Certain commercial equipment or instruments are identified in the paper to specify adequately the experimental procedures. Such identification does not imply recommendations or endorsement by the NIST nor does it imply that the equipment or instruments are the best available for the purpose.
REFERENCES
- 1.Tabor GM and Aguirre AA, Ecosystem health and sentinel species: adding an ecological element to the proverbial “canary in the mineshaft”. EcoHealth, 2004. 1(3): p. 226–228. [Google Scholar]
- 2.Sparling DW, Fellers GM, and McConnell LL, Pesticides and amphibian population declines in California, USA. Environmental Toxicology and Chemistry, 2001. 20(7): p. 1591–1595. [DOI] [PubMed] [Google Scholar]
- 3.Jackson AK, et al. , Songbirds as sentinels of mercury in terrestrial habitats of eastern North America. Ecotoxicology, 2015. 24(2): p. 453–467. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira M, et al. , Organochlorine contaminants in flounder (Platichthys flesus) and mullet (Mugil cephalus) from Douro estuary, and their use as sentinel species for environmental monitoring. Aquatic Toxicology, 2004. 69(4): p. 347–357. [DOI] [PubMed] [Google Scholar]
- 5.Basu N, et al. , Mink as a sentinel species in environmental health. Environmental Research, 2007. 103(1): p. 130–144. [DOI] [PubMed] [Google Scholar]
- 6.Milnes MR and Guillette LJ, Alligator tales: new lessons about environmental contaminants from a sentinel species. Bioscience, 2008. 58(11): p. 1027–1036. [Google Scholar]
- 7.Baos R, et al. , Evaluation of genotoxic effects of heavy metals and arsenic in wild nestling white storks (Ciconia ciconia) and black kites (Milvus migrans) from southwestern Spain after a mining accident. Environmental Toxicology and Chemistry, 2006. 25(10): p. 2794–2803. [DOI] [PubMed] [Google Scholar]
- 8.Boon JP, et al. , Levels of polybrominated diphenyl ether (PBDE) flame retardants in animals representing different trophic levels of the North Sea food web. Environmental Science & Technology, 2002. 36(19): p. 4025–4032. [DOI] [PubMed] [Google Scholar]
- 9.Bangma JT, et al. , Perfluorinated alkyl acids and fecundity assessment in striped mullet (Mugil cephalus) at Merritt Island national wildlife refuge. Science of The Total Environment, 2018. 619: p. 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renner R, The long and the short of perfluorinated replacements. Environmental Science and Technology, 2006. 40(1): p. 12–13. [DOI] [PubMed] [Google Scholar]
- 11.Betts KS, Perfluoroalkyl acids-What is the evidence telling us? 2007, US Dept Health Human Sciences Public Health Science National Institute of Helath, Environmental Helath Sciences, PO BOX 12233, Research Triangle Park, NC 27709–2233 USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houde M, et al. , Monitoring of Perfluorinated Compounds in Aquatic Biota: An Updated Review. Environmental Science and Technology, 2011. 45(19): p. 7962–7973. [DOI] [PubMed] [Google Scholar]
- 13.Blanvillain G, et al. , Diamondback terrapins, Malaclemys terrapin, as a sentinel species for monitoring mercury pollution of estuarine systems in South Carolina and Georgia, USA. Environmental Toxicology and Chemistry, 2007. 26(7): p. 1441–1450. [DOI] [PubMed] [Google Scholar]
- 14.Basile ER, et al. , Diamondback terrapins as indicator species of persistent organic pollutants: using Barnegat Bay, New Jersey as a case study. Chemosphere, 2011. 82(1): p. 137–144. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons J, et al. , Demographic and ecological factors affecting conservation and management of the diamondback terrapin (Malaclemys terrapin) in South Carolina . Chelonian Conservation and Biology, 2001. 4(1): p. 66–74. [Google Scholar]
- 16.Bangma JT, et al. , Perfluorinated Alkyl Acids in plasma of American alligators (Alligator mississippiensis) from Florida and South Carolina. Environ Toxicol Chem, 2017. 36(4): p. 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbons J and Harrison J, Reptiles and amphibians of Kiawah and Capers islands, South Carolina. Brimleyana, 1981. 5: p. 145–162. [Google Scholar]
- 18.Lovich JE and Gibbons JW, Age at maturity influences adult sex ratio in the turtle Malaclemys terrapin. Oikos, 1990: p. 126–134. [Google Scholar]
- 19.Lovich J, et al. , Behavior of hatchling diamondback terrapins (Malaclemys terrapin) released in a South Carolina salt marsh. Herpetological Review, 1991. 22(3): p. 81–83. [Google Scholar]
- 20.Tucker AD, FitzSimmons NN, and Gibbons JW, Resource partitioning by the estuarine turtle Malaclemys terrapin: trophic, spatial, and temporal foraging constraints. Herpetologica, 1995: p. 167–181. [Google Scholar]
- 21.Tucker AD, Gibbons JW, and Greene JL, Estimates of adult survival and migration for diamondback terrapins: conservation insight from local extirpation within a metapopulation. Canadian Journal of Zoology, 2001. 79(12): p. 2199–2209. [Google Scholar]
- 22.Tucker AD, Yeomans SR, and Gibbons JW, Shell strength of mud snails (Ilyanassa obsoleta) may deter foraging by diamondback terrapins (Malaclemys terrapin). American Midland Naturalist, 1997: p. 224–229. [Google Scholar]
- 23.Hoyle ME and Gibbons J, Use of a Marked Population of Diamondback Terrapins (Malaclemys terrapin) to Determine Impacts of Recreational Crab Pots. Chelonian Conservation and Biology, 2000. 3(4): p. 735–737. [Google Scholar]
- 24.McKee RK, Cecala KK, and Dorcas ME, Behavioural interactions of diamondback terrapins with crab pots demonstrate that bycatch reduction devices reduce entrapment. Aquatic Conservation: Marine and Freshwater Ecosystems, 2016. 26(6): p. 1081–1089. [Google Scholar]
- 25.Dorcas ME, Willson JD, and Gibbons JW, Crab trapping causes population decline and demographic changes in diamondback terrapins over two decades. Biological Conservation, 2007. 137(3): p. 334–340. [Google Scholar]
- 26.Harden L, et al. , Spatial and thermal ecology of diamondback terrapins (Malaclemys terrapin) in a South Carolina salt marsh. Journal of the North Carolina Academy of Science, 2007: p. 154–162. [Google Scholar]
- 27.Cecala K, Gibbons J, and Dorcas M, Ecological effects of major injuries in diamondback terrapins: implications for conservation and management. Aquatic Conservation: Marine and Freshwater Ecosystems, 2009. 19(4): p. 421–427. [Google Scholar]
- 28.Sexton OJ, Spatial and temporal movements of a population of the painted turtle, Chrysemys picta marginata (Agassiz). Ecological Monographs, 1959. 29(2): p. 113–140. [Google Scholar]
- 29.Kannan K, et al. , Perfluorinated Compounds in Aquatic Organisms at Various Trophic Levels in a Great Lakes Food Chain . Archives of Environmental Contamination and Toxicology, 2005. 48(4): p. 559–566. [DOI] [PubMed] [Google Scholar]
- 30.Keller JM, et al. , Perfluoroalkyl contaminants in plasma of five sea turtle species: Comparisons in concentration and potential health risks. Environmental Toxicology and Chemistry, 2012. 31(6): p. 1223–1230. [DOI] [PubMed] [Google Scholar]
- 31.Keller JM, et al. , Perfluorinated Compounds in the Plasma of Loggerhead and Kemp’s Ridley Sea Turtles from the Southeastern Coast of the United States. Environmental Science and Technology, 2005. 39(23): p. 9101–9108. [DOI] [PubMed] [Google Scholar]
- 32.Morikawa A, et al. , The bioconcentration factor of perfluorooctane sulfonate is significantly larger than that of perfluorooctanoate in wild turtles (Trachemys scripta elegans and Chinemys reevesii): An Ai river ecological study in Japan . Ecotoxicology and Environmental Safety, 2006. 65(1): p. 14–21. [DOI] [PubMed] [Google Scholar]
- 33.O’Connell SG, et al. , Temporal and Spatial Trends of Perfluorinated Compounds in Juvenile Loggerhead Sea Turtles (Caretta caretta) along the East Coast of the United States. Environmental Science and Technology, 2010. 44(13): p. 5202–5209. [DOI] [PubMed] [Google Scholar]
- 34.de Solla SR, De Silva AO, and Letcher RJ, Highly elevated levels of perfluorooctane sulfonate and other perfluorinated acids found in biota and surface water downstream of an international airport, Hamilton, Ontario, Canada . Environmental International, 2012. 39(1): p. 19–26. [DOI] [PubMed] [Google Scholar]
- 35.Guerranti C, et al. , Perfluorinated compounds in blood of Caretta caretta from the Mediterranean Sea. Marine Pollution Bulletin, 2013. 73(1): p. 98–101. [DOI] [PubMed] [Google Scholar]
- 36.Reiner JL, Phinney KW, and Keller JM, Determination of perfluorinated compounds in human plasma and serum Standard Reference Materials using independent analytical methods. Analytical and Bioanalytical Chemistry, 2011. 401(9): p. 2899–907. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2013. http://www.R-project.org/ [Google Scholar]
- 38.Lee L and Lee ML, NADA: Nondetects and Data Analysis for Environmental Data. R package version 1.6-1, 2017. https://CRAN.R-project.org/package=NADA.
- 39.Wickham H, tidyverse: Easily install and load “Tidyverse” packages (Version 1.1. 1). R Core Team: Vienna, Austria, 2017. https://CRAN.R-project.org/package=tidyverse. [Google Scholar]
- 40.Helsel DR, Statistics for censored environmental data using Minitab and R. 2nd ed. Vol. 77 2012, New York: John Wiley & Sons. [Google Scholar]
- 41.Houde M, et al. , Biological Monitoring of Polyfluoroalkyl Substances: A Review. Environmental Science and Technology, 2006. 40(11): p. 3463–3473. [DOI] [PubMed] [Google Scholar]
- 42.Letcher RJ et al. Perfluorinated sulfonate and carboxylate compounds and precursors in herring gull eggs from across the Laurentian Great Lakes of North America: Temporal and recent spatial comparisons and exposure implications. Science of the Total Environment, 2015. 538: p. 468–477. [DOI] [PubMed] [Google Scholar]
- 43.Lee JJ, & Schultz IR, Sex differences in the uptake and disposition of perfluorooctanoic acid in fathead minnows after oral dosing. Environmental Science and Technology, 2009. 44: p. 491–496. [DOI] [PubMed] [Google Scholar]
- 44.Kudo N, Katakura M, Sato Y, and Kawashima Y, Sex hormone-regulated renal transport of perfluorooctanoic acid. Chemico-Biological Interactions, 2002. 139: p. 301–316. [DOI] [PubMed] [Google Scholar]
- 45.Kudo N, Suzuki E, Katakura M, Ohmori K, Noshiro R, Kawashima Y, Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chemico-Biological Interactions, 2001. 134: p. 203–216. [DOI] [PubMed] [Google Scholar]
- 46.Fair PA, et al. , Assessment of perfluorinated compounds (PFCs) in plasma of bottlenose dolphins from two southeast US estuarine areas: relationship with age, sex and geographic locations. Marine Pollution Bulletin, 2012. 64(1): p. 66–74. [DOI] [PubMed] [Google Scholar]
- 47.Tucker Anton D., FitzSimmons Nancy N., and Gibbons J. Whitfield. Resource partitioning by the estuarine turtle Malaclemys terrapin: trophic, spatial, and temporal foraging constraints. Herpetologica. 1995: p. 167–181. [Google Scholar]
- 48.Wilkinson PM, et al. , Determinate growth and reproductive lifespan in the American alligator (Alligator mississippiensis): evidence from long-term recaptures. Copeia, 2016. 104(4): p. 843–852. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.