Abstract
Endoplasmic reticulum (ER) stress is a major contributor to liver disease and hepatic fibrosis, but the role it plays varies depending on the cause and progression of the disease. Furthermore, ER stress plays a distinct role in hepatocytes versus hepatic stellate cells (HSCs), which adds to the complexity of understanding ER stress and its downstream signaling through the unfolded protein response (UPR) in liver disease. Here, the authors focus on the current literature of ER stress in nonalcoholic and alcoholic fatty liver diseases, how ER stress impacts hepatocyte injury, and the role of ER stress in HSC activation and hepatic fibrosis. This review provides insight into the complex signaling and regulation of the UPR, parallels and distinctions between different liver diseases, and how ER stress may be targeted as an antisteatotic or antifibrotic therapy to limit the progression of liver disease.
Keywords: unfolded protein response, nonalcoholic fatty liver disease, hepatic stellate cells, steatosis
Liver disease continues to be a leading cause of death worldwide, with metabolic liver diseases such as nonalcoholic fatty liver disease (NAFLD), its progressive component nonalcoholic steatohepatitis (NASH), and alcoholic liver disease (ALD) becoming increasingly prevalent.1,2 Experimental disease models and observational human data demonstrate that endoplasmic reticulum (ER) stress is a feature of acute and chronic liver diseases.3–8 The key signaling pathways activated by ER stress are termed the unfolded protein response (UPR) due to their characterization under conditions of accumulated misfolded or unfolded proteins in the ER lumen. Physiologically, the UPR is essential for maintaining cellular homeostasis in both hepatocytes and hepatic stellate cells (HSCs) during metabolism and protein secretion. However, UPR signaling also drives pathogenesis of liver disease through its involvement in inflammatory responses, steatosis, hepatocyte apoptosis, and fibrosis via HSC activation. We discuss the UPR signaling pathways, causes of ER stress in liver disease, and discuss UPR signaling in hepatocytes and HSCs. While ER stress plays a physiologic and pathophysiologic role in other liver cell types such as Kupffer cells, recent literature provides critical insight into the pathophysiologic mechanisms of the UPR in hepatocytes and HSCs in metabolic liver disease and fibrosis, and is the focus of this review.9–12
UPR Signaling Pathways
Unfolded protein response signaling is driven through three major pathways (Fig. 1), mediated by the ER transmembrane proteins inositol requiring enzyme 1 α (IRE1α), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 α (ATF6α).13,14 During homeostatic conditions, the luminal side of each UPR sensor interacts with ER resident chaperones, primarily immunoglobulin-binding protein/glucose regulatory protein 78 (BiP/GRP78). When the ER experiences an abundance of unfolded or misfolded proteins, the sensors activate. This mechanism involves recruitment of BiP away from the luminal domains of the sensors,15 and there is also evidence for direct binding of unfolded proteins to the luminal domains of IRE1α and PERK.16,17 Either mechanism allows for oligomerization and activation of the sensors and UPR signaling.15,18–21 At a high level, UPR signaling is initially a prosurvival mechanism; reducing the protein folding load by blocking general protein translation, increasing chaperone expression, and increasing degradative protein export from the ER. If UPR signaling is insufficient to relieve ER stress, proapoptotic signaling ensues. Chemical induction of ER stress, which mimics many aspects of canonical UPR signaling, is useful for studying the effects and mechanisms of UPR signaling. Two chemicals used in studies discussed here are tunicamycin, which blocks protein folding in the ER through inhibiting N-linked glycosylation, and brefeldin A (BFA), which disrupts protein trafficking from the ER to the Golgi leading to ER protein accumulation. We will discuss each UPR signaling pathway briefly; they are reviewed elsewhere in detail.22–25
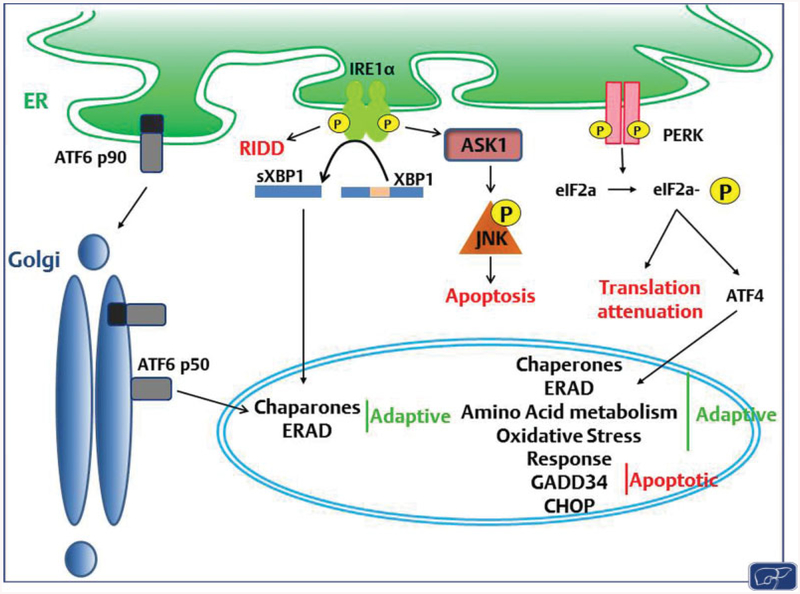
Fig. 1. UPR sensors and signaling pathways.
ER stress is sensed by three ER transmembrane proteins, ATF6α, IRE1α, and PERK, which are crucial for mediating the adaptive and apoptotic signaling of the UPR. ATF6α translocates to the Golgi upon sensing ER stress, where it is cleaved and subsequently trafficked to the nucleus where it upregulates chaperones and proteins involved in ERAD. IRE1α oligomerizes and autophosphorylates in response to ER stress, and acts through several mechanisms. The endonuclease domain is involved in RIDD, as well as transcription through activating the transcription factor XBP1. IRE1α also promotes apoptosis through activation of ASK and subsequent phosphorylation of JNK. PERK oligomerizes upon sensing ER stress, and autophosphorylates. The canonical target of PERK kinase activity is eIF2α, which acts through ATF4 to promote expression of chaperones, and proteins involved in ERAD, amino acid metabolism, the oxidative stress response, and UPR-mediated apoptosis. eIF2α also serves to attenuate nonessential mRNA translation. CHOP, a stress-induced transcription factor, is upregulated downstream of ATF4 and mediates ER stress-induced apoptosis. Gad34, another transcriptional target of this pathway, dephosphorylates eIF2α, thus resuming translation which can lead to apoptosis by increasing oxidative protein folding. ASK, apoptosis-signal-regulating kinase; CHOP, CCAAT-enhancer-binding protein homologous protein; ER, endoplasmic reticulum; ERAD, endoplasmic reticulum-associated degradation; JNK, c-Jun N-terminal kinase; PERK, protein kinase RNA-like endoplasmic reticulum kinase; RIDD, regulated IRE1-dependent decay; UPR, unfolded protein response.
IRE1α:
Signaling through IRE1α is the most conserved of the three UPR pathways. There are two homologs of IRE1 in humans, IRE1α and β, though only IRE1α is expressed in the liver.26 IRE1α is a type 2 transmembrane protein and contains two cytosolic domains critical for downstream signaling: a kinase and an endoribonuclease domain. Under conditions of normal protein load, IRE1α associates with chaperones in the ER lumen, which prevents IRE1α signaling. When unfolded or misfolded proteins exceed ER capacity, IRE1α oligomerizes and autotransphosphorylates, leading to IRE1α downstream signaling.15,27 Autotransphosphorylation of IRE1α via its kinase activity activates the endoribonuclease domain, which primarily acts through unconventional splicing of the transcription factor X-boxbinding protein 1 (XBP1). IRE1α endoribonuclease activity excises a 26-base sequence from total or unspliced XBP1 which is then spliced by the conserved RNA ligase RtcB to generate a shorter messenger RNA (mRNA) with a frame shift facilitating translation of a longer, transcriptionally active XBP1 protein.28–31 Spliced XBP1 (sXBP1) translocates into the nucleus and activates transcription of several chaperones and components of the secretory pathway in an attempt to alleviate the protein load within the ER.32 sXBP1 also induces transcription of machinery involved in ER-associated degradation (ERAD),33 which facilitates the export of misfolded proteins out of the ER and targets them for degradation. Another function of the IRE1α endoribonuclease domain is to limit translation of nonessential mRNA and some micro-RNA through a process called regulated IRE1-dependent decay (RIDD).34–36 Together, the XBP1-mediated pathways are associated with the proadaptive arm of the UPR which attempts to restore cellular homeostasis; however, RIDD is associated with both prosurvival and proapoptotic effects via promiscuous mRNA decay and degradation of mRNAs that encode chaperones and microRNAs that repress caspase2, respectively.34,36,37 The IRE1α kinase domain also signals through a separate pathway via recruiting tumor necrosis factor receptor-associated factor 2 (TRAF2), an adaptor protein that activates apoptosis-signal-regulating kinase 1 (ASK1).38 ASK1 phosphorylates c-Jun N-terminal kinase (JNK), which promotes apoptosis through activating the proapoptotic protein Bim while inhibiting antiapoptotic Bcl2 proteins.39–41 In addition to ASK1 and JNK, both extracellular signal-regulated kinase (ERK) and p38 kinase are implicated downstream of IRE1α kinase activity and could play a role in prosurvival or proapoptotic UPR signaling.39,42
PERK:
PERK is a type 1 transmembrane protein that is typically activated through recruitment of chaperones away from PERK leading to oligomerization and activation of the cytosolic kinase domain. There is also evidence for an activating role for direct binding of unfolded proteins to the luminal domain of PERK.17,43 The major substrate of PERK phosphorylation is eukaryotic initiation factor 2α (eIF2α), though eIF2α is also a substrate for additional kinases (heme-regulated inhibitor, general control nonderepressible-2, and protein kinase R). eIF2α phosphorylation results in global translation attenuation while permitting selective translation of a subset of mRNAs, the best studied of which is activating transcription factor 4 (ATF4).44–46 ATF4 transcriptionally upregulates several UPR-target genes that mediate prosurvival or proapoptotic signaling. A critical protein that mediates proapoptotic signaling downstream of PERK and ATF4 is CCAAT enhancer-binding protein (C/EBP) homologous protein (CHOP), which is implicated in the progression of liver disease.47,48
ATF6α:
Upon ER stress, full length ATF6α (ATF6p90) interacts with unfolded or misfolded proteins, which exposes two Golgi localization signal sequences and initiates ATF6α translocation to the Golgi.18,49–51 At the Golgi, ATF6p90 is cleaved to the active form of ATF6α, ATF6p50: a bZIP-domain containing transcription factor. ATF6p50 translocates into the nucleus and promotes transcription of several chaperones such as BiP. ATF6p50 also heterodimerizes with sXBP1; this heterodimer upregulates components of the ERAD machinery.52
Endoplasmic Reticulum Stress in Hepatocytes
Physiologic ER functions:
Hepatocytes are structurally rich in ER, with ER membrane comprising 50% of total cell membrane, and have more abundant rough ER than smooth ER, except in the pericentral hepatocytes where hepatocytes have an equal proportion of rough and smooth ER.53,54 Hepatocytes are professional secretory cells, synthesizing and secreting the majority of plasma proteins with the exception of immunoglobulins.55,56 The majority of secreted proteins and proteins destined for the plasma membrane are cotranslationally folded in the ER followed by quality control checks before trafficking of correctly folded proteins to either the secretory pathway or the plasma membrane. The ER is also the site for the biosynthesis of several classes of lipids including cholesterol, ceramide, and phospholipids; their respective synthetic machineries enriched in the smooth ER.57 Very low density lipoprotein (VLDL) particles begin biogenesis in the rough ER where newly translated apolipoprotein B is lipidated by microsomal triglyceride transfer protein (MTTP)-mediated lipid transfer as it translocates across the ER membrane, with subsequent addition of triglycerides and other lipids in the smooth ER and Golgi.58,59 The smooth ER in hepatocytes also houses the lipophilic drugs and xenobiotic detoxification machinery that includes the family of cytochrome P450 oxidases which are involved in alcohol metabolism. Maintenance of calcium homeostasis is an integral ER function in hepatocytes, as in other cell types. Thus, the ER is central to the physiologic functions of hepatocytes.
Global disruption of ER function:
In spite of abundant ER, hepatocytes are sensitive to the disruption of normal ER function. This is highlighted by observations that liver-specific deletion of the chaperone BiP/GRP78 in mice led to disorganization and dilation of the ER compartment, an ER stress response, despite no other stimuli. Loss of BiP also led to hepatic steatosis, hepatocyte apoptosis, and spontaneous liver injury, highlighting the importance of the ER in physiologic hepatocyte processes.60 These mice were further sensitized to myriad acute and chronic hepatic insults including alcohol, high fat diet, and acetaminophen. Similarly, loss of Sec61α1 function, a component of ER translocon, led to hepatic steatosis and hepatomegaly with the activation of an ER stress response.61 Data from pharmacologic ER stress induction in genetic knockout mouse models of UPR components also supported hepatic steatosis as a common occurrence in livers with sustained, excessive, or unresolved ER stress.62 These data suggest that hepatic steatosis is a conserved response to unresolved ER stress in the liver, and perhaps UPR pathways prevent hepatic steatosis under physiologic conditions. Notably, several of these observations linking ER stress to the mechanisms leading to hepatic steatosis employed pharmacologic ER stress, mostly tunicamycin. This would be in contrast to high fat diet-induced activation of the ER stress transducers, where steatosis occurs before activation of the ER stress response, which is discussed in subsequent sections.
Unfolded protein response transducers regulate hepatic lipid homeostasis:
Several recent studies have advanced our understanding of the physiologic hepatic functions of the individual UPR transducers in regulating hepatic lipid synthesis, export and oxidation, which are distinct from their well-defined roles in the canonical UPR. XBP1 liver-specific knockout mice demonstrated profound hypocholesterolemia and hypotriglyceridemia due to reduced de novo synthesis of fatty acids.63 IRE1α hyperactivation occurred in the livers of XBP1 liver-specific knockout mice, and silencing of IRE1α partially restored plasma lipid levels, suggesting a key role for IRE1α-mediated RIDD in maintaining lipid homeostasis in the liver.64 Together, both XBP1 and RIDD regulated hepatic lipid homeostasis: genes encoding lipogenic enzymes such as Dgat2, Acacb, and Scd1 were suppressed in XBP1 liver-specific knockout mice and the Ces1 gene family and Angptl3 were identified as RIDD substrates.64 Furthermore, deletion of XBP1 in genetically obese (ob/ob) mice lowered hepatic triglycerides and plasma cholesterol. Thus, in the absence of XBP1, IRE1α-mediated RIDD predominates lipid regulation with profound reductions in plasma and hepatic lipids; however, XBP1 deletion lowers hepatic lipids in ob/ob mice.
Additional observations demonstrate that IRE1α regulates other aspects of hepatic lipid homeostasis such as suppression of lipogenic transcription factors and maintaining VLDL production. IRE1α repressed several transcriptional regulators of hepatic steatosis including C/EBPβ, C/EBPδ, and peroxisome proliferator-activated receptor γ (PPARγ).65 IRE1α-XBP1-induced protein disulfide isomerase (PDI) expression maintained VLDL assembly and secretion, as PDI is a component of the MTTP and essential for normal MTTP activity.59 Altogether these data suggest a dominant role for IRE1α in maintaining hepatic lipid homeostasis. An intact IRE1α-XBP1 signaling axis maintains hepatic VLDL secretion and prevents hepatic steatosis in acute ER stress by inhibiting lipogenic transcription factors, and XBP1 regulates a subset of hepatic lipid synthesis genes. Yet, when IRE1α is hyperactivated, RIDD-induced hypolipidemia predominates.
There is evidence that other UPR sensors are involved in mediating lipid homeostasis. Cleaved ATF6αp50 regulated lipids through binding to and inhibiting activated (cleaved) sterol-regulatory element-binding protein 2 (SREBP-2). ATF6α served this function in part through recruitment of HDAC1 and subsequent decreased transcription of SREBP-2 target genes.66 Thus, regulation of hepatic lipid homeostasis is a recently identified function of the UPR transducers that merits further study. We propose that the lipid synthesis function of the ER in hepatocytes imparts a hepatocytespecific, noncanonical role to the UPR sensors such that they regulate hepatic steatosis, as discussed above, and maintain sensitivity to lipid perturbations such as increased palmitate or phosphatidylcholine (PC) depletion (discussed in subsequent sections).
Nonalcoholic fatty liver disease:
Obesity associated-nonalcoholic NAFLD is associated with activation of all three UPR transducers in human liver samples, and genetic and dietary mouse models of NAFLD, in both isolated steatosis or nonalcoholic fatty liver (NAFL) and NASH.67–70 We have used the term NAFLD when discussing UPR signaling with respect to hepatic steatosis and insulin resistance, as this is conserved between NAFL and NASH, and the term NASH when discussing inflammation and fibrosis. In recent years, observations in genetic knockout mouse models have led to significant advances in understanding the contribution of the UPR transducers toward NAFLD. Early observations implicated UPR transducers in insulin resistance. Activation of JNK in livers of high fat-fed and ob/ob mice led to inhibitory phosphorylation of the insulin receptor substrate-1 (IRS-1) and impaired insulin signaling secondary to ER stress.68 Newer studies have implicated a regulatory role for IRE1α-XBP1 signaling in hepatic steatosis (Fig. 2), and also in liver injury and inflammation as discussed below.
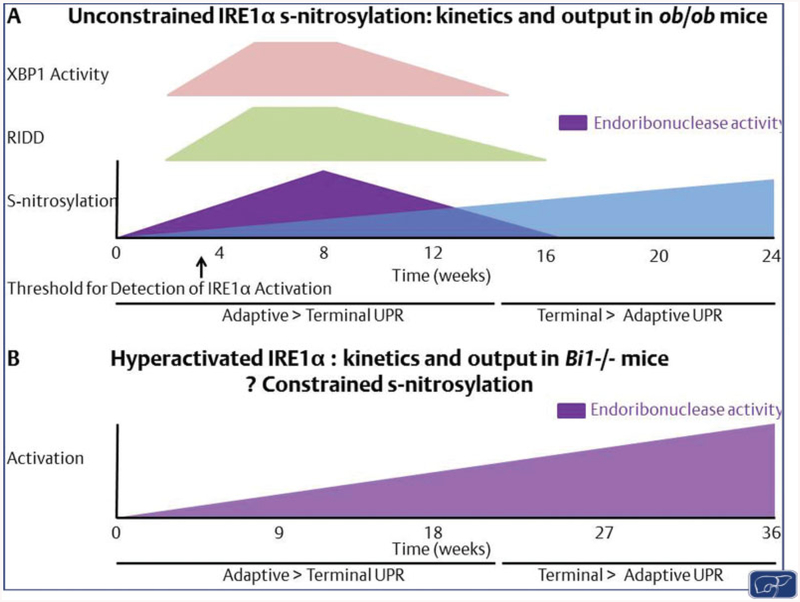
Fig. 2. IRE1α activation states in NAFLD.
In this model we propose two alternative activation states of IRE1α: (A) unconstrained IRE1α snitrosylation as observed in ob/ob mice, which may increase over time. Initial activation of XBP1 (in pink) and RIDD (in green) may favor adaptive UPR. Over time a loss of endoribonuclease activity (in purple) would occur with increased s-nitrosylation (in blue) leading to a loss of adaptive signaling. (B) In the absence of s-nitrosylation IRE1α activation increases over time and shifts from adaptive to maladaptive. However, whether this switch is a function of time; regulated by the activation of alternative signaling pathways such as PERK; a cell- and tissue-specific activation of IRE1α; or specific to accumulated toxic lipids, remain to be determined. NAFLD, nonalcoholic fatty liver disease; PERK, protein kinase RNA-like endoplasmic reticulum kinase; RIDD, regulated IRE1-dependent decay; UPR, unfolded protein response.
IRE1α-XBP1:
In ob/ob mice and high fat diet-fed mice, s-nitrosylation of IRE1α was reported.69 This resulted in a progressive decline in IRE1α endoribonuclease activity with reduced XBP1 splicing; however, kinase function was not compromised. Furthermore, reconstitution of hepatic IRE1α expression with s-nitrosylation-resistant IRE1α restored XBP1 levels, which is associated with improved glucose homeostasis in ob/ob mice. Interestingly, XBP1 splicing and other UPR target genes were significantly upregulated in 7- and 12-week-old ob/ob mouse livers, before a reduction was noted in 16-week-old mouse livers; however, no other analyses of hepatic steatosis, inflammation, and injury were reported. Separately, Wang et al reported an increase in hepatic triglyceride content due to lack of IRE1α-mediated degradation of precursor forms of microRNAs, miR-200 and miR-34 families, resulting from impaired endoribonuclease activity of s-nitrosylated IRE1α,70 suggesting that functional IRE1α mitigates hepatic steatosis by RIDD-mediated decay of target microRNA. They also observed increased hepatic inflammation and fibrosis in 20 week high fat-fed hepatocyte-specific IRE1α knockout mice, suggesting that IRE1α protects from NASH.
Liver-specific XBP1 overexpression in dietary or genetic obesity models had an antisteatotic effect due to reduced fatty acid synthesis rates resultant of Srebp1c inhibition, reduced expression of fatty acid synthase (FasN) and stearoyl-CoA desaturase 1 (Scd1), and increased macrolipophagy.71 These observations were contrary to earlier reports that XBP1 liver-specific knockout mice manifest hypocholesterolemia and hypotriglyceridemia and low hepatic lipid synthesis rates.63 Later work clarified that the hypolipidemia in XBP1 liver-specific knockout mice is mostly due to IRE1α hyperactivation and ensuant RIDD.64 On the other hand, when challenged with a diet known to recapitulate human NASH in mice,72 hepatocyte-specific XBP1 knockout mice exhibited greater liver injury, apoptotic and inflammatory signaling, hepatocyte apoptosis, and fibrosis; however, reduced hepatic steatosis was observed which may be attributable to RIDD.73 IRE1α was hyperactivated in the hepatocyte-specific XBP1 knockout mice, linking a deleterious outcome with IRE1α hyperactivation. A similar pheno-type was observed in the absence of Bax inhibitor 1 (BI-1), an ER membrane protein which negatively regulates IRE1α activity. Nine month high fat diet-fed BI-1 knockout mice (BI-1¯/¯) developed exaggerated hepatocyte cell death, liver injury, inflammation, and liver fibrosis.74 This was associated with overactivation of IRE1α endonuclease activity as measured by sXBP1 protein levels and the nod-like receptor protein 3 (NLRP3) inflammasome. Inhibition of IRE1α endo-nuclease activity with the pharmacologic inhibitor STF-083010 was associated with a reduction in NLRP3 inflammasome and proinflammatory markers in BI-1¯/¯ mice. Thus, BI-1 constrained IRE1α endonuclease activity, and in its absence, hyperactivation of IRE1α endonuclease activity with resultant increased sXBP1 expression was associated with increased hepatocyte apoptosis, worse liver injury, and inflammation.
How do we reconcile these observations to explain the important yet variable role of the IRE1α-XBP1 axis in NAFLD pathogenesis? We propose that IRE1α s-nitrosylation may facilitate the switch between NAFL and NASH, and that BI-1 facilitates IRE1α s-nitrosylation (Fig. 2). However, we propose this cautiously as these models are based mostly on knockout mouse studies and require further validation. Given the well-established paradigm that inflammation and fibrosis in NASH occur secondary to hepatocyte apoptosis and injury, the question whether the increase in hepatocyte apoptosis occurs in high fat-fed hepatocyte-specific IRE1α knockout mice needs to be addressed. Kinetic analysis will be needed to determine if the UPR shifts from adaptive to maladaptive due to sustained ER stress, leading to hepatocyte apoptosis and proinflammatory signaling (Fig. 2).
Hepatic steatosis is promoted by free fatty acids derived from enhanced adipose tissue lipolysis in an insulin-resistant state.75 This is driven by macrophage-mediated adipose tissue inflammation. In keeping with this, IRE1α signaling in immune cells or adipose tissue may playan understudied role in NAFLD. Macrophage IRE1α promoted proinflammatory polarization of adipose tissue macrophages, such that when IRE1α was deleted from myeloid cells, mice were resistant to diet-induced weight gain, insulin, hyperlipidemia, and hepatic steatosis.76 Thus, the tissue-specific role of IRE1α in hepatic macrophages merits examination. IRE1α s-nitrosylation and BI-1 function in adipose tissue may also influence the development of NAFLD, providing another scenario for experimental testing. In summary, negative regulation of steatosis by XBP1 and RIDD appears to be beneficial in reducing hepatic steatosis, whereas inactivation of IRE1α endoribonuclease activity promotes hepatic steatosis and hyperactivation of IRE1α promotes NASH. There remain multiple unanswered mechanistic questions, as discussed above, on cell autonomous functions and the tissue-specific role of IRE1α in NAFLD pathogenesis.
PERK:
PERK signaling is also implicated in NAFLD. One of the earliest observations in this regard demonstrated that enforced expression of GADD34, a downstream effector of PERK, improved hepatic steatosis and insulin tolerance in high fat-fed mice due to attenuated expression of the lipogenic transcriptional regulators PPARγ and C/EBPα and β.77 ATF4 deficiency protected against high carbohydrate diet (HCD)-induced hepatic steatosis by downregulating HCD-induced SCD1 expression.78 When challenged with a high fructose diet, ATF4 knockout mice were protected from hepatic steatosis and hypertriglyceridemia due to reduced induction of PPARγ and other lipogenic genes.79 Mechanistically, CHOP-dependent hepatocyte lipotoxicity is an important mediator of NASH pathogenesis and links ER stress to NASH (Fig. 3). In isolated hepatocytes, saturated free fatty acid-induced apoptosis was CHOP-dependent by increasing the expression of the death receptor 5 (DR5) and proapoptotic Bcl-2 family protein p53 upregulated modulator of apoptosis (PUMA).80,81 The palmitate-repressed microRNA miR-615–3p repressed CHOP expression in hepatocytes such that augmentation of miR615–3p led to reduced CHOP expression and protected hepatocytes from palmitate-induced apoptosis.82 CHOP knockout mice (CHOP¯/¯), however, developed greater liver injury, inflammation, and hepatocyte apoptosis when fed a high-fat diet.83,84 This was reportedly due to impaired macrophage apoptosis in CHOP¯/¯ mice which increased proinflammatory signaling. In contrast, it was reported that CHOP¯/¯ mice are either protected from a methionineand choline-deficient diet-induced steatohepatitis,85 or demonstrated a similar degree of liver injury.86 Further, hepatocyte-specific CHOP deletion did not protect or worsen liver injury and ER stress in high fat-fed MUP-uPA mice. These mice express the urokinasetype plasminogen activator under the hepatocyte-specific major urinary protein promoter (MUP-uPA); uPA accumulation in the ER activates the ER stress response.87,88 Thus, the role of CHOP remains incompletely understood in NASH pathogenesis. Complicating these studies is the varied mechanism of dietary injury, which may account for some of the observed differences in the role of CHOP in steatohepatitis. Additionally, tissue-specific roles of CHOP, especially in myeloid cells, may explain some of the disparate observations.
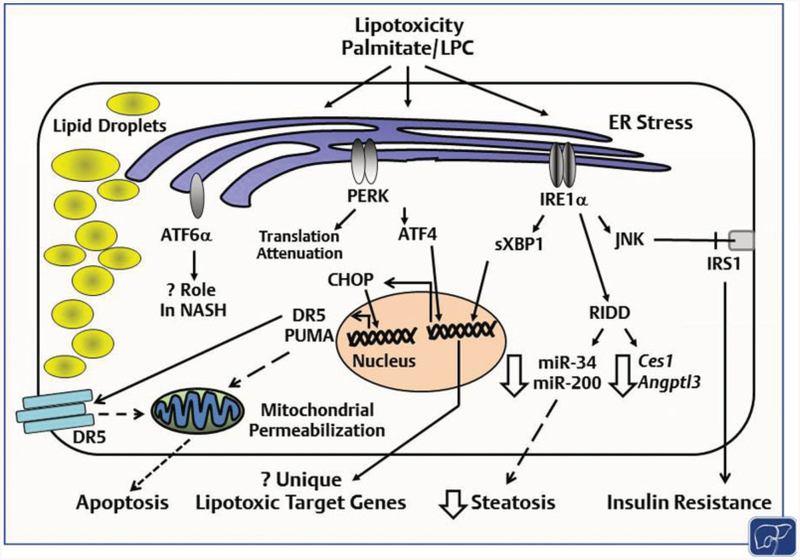
Fig. 3. Lipotoxicity and ER stress in NASH.
The saturated free fatty acid palmitate, and other toxic lipids such as lysophosphatidylcholine (LPC) are known to activate the three UPR sensors. IRE1α, via phosphorylation and activation of the stress kinase c-Jun N-terminal kinase (JNK) targets the insulin receptor substrate 1 (IRS1) for inhibitory phosphorylation leading to insulin resistance. IRE1α-dependent RNA degradation (RIDD) negatively regulates hepatic steatosis via degradation of lipogenic mRNAs and regulatory microRNA. IRE1α s-nitrosylation inhibits its endoribonuclease activity with a resultant decrease in XBP1 splicing and an increase in microRNAs that are degraded via RIDD. The PERK pathway is implicated in the regulation of hepatic steatosis; CHOP mediates palmitate-induced hepatocyte apoptosis, though its in vivo role in NASH is less clear. ATF6α is activated in NASH; however, its contribution is not well defined. Lipid perturbation-induced UPR activation activates a subset of genes distinct from misfolded protein-induced UPR; however, the specific ATF4 and XBP1 induced lipotoxic target genes have not been defined. CHOP, CCAAT-enhancer-binding protein homologous protein; ER, endoplasmic reticulum; NASH, nonalcoholic steatohepatitis; PERK, protein kinase RNA-like endoplasmic reticulum kinase; UPR, unfolded protein response.
ATF6α and sarco/endoplasmic reticulum calcium ATPase (SERCA):
ATF6α signaling is likely important in NASH pathophysiology; however, not much is known. High fat-fed ATF6α¯/¯ mice demonstrated greater hepatic steatosis with associated increased XBP1 splicing,89 suggesting a role for ATF6α in promoting adaptation to dietary obesityinduced fatty liver. Additionally, disrupted phospholipid composition, leading to inhibition of the SERCA activity, was reported in livers of obese mice.90 A limiting factor in these studies is the lack of validated reagents to test the ATF6α pathway, highlighting a major limitation in fully understanding ATF6α in NASH and other liver diseases.
Endoplasmic reticulum stress and inflammasome activation:
NLRP3, an inflammasome component, is associated with NASH progression.91 ER stress is also associated with activation of the NLRP3 inflammasome by lipopolysaccharide and hepatocyte cell death,92 with a correlation in liver biopsies from NASH subjects between NLRP3 expression, ER stress markers, and liver injury. ER stress-induced inflammasome activation likely occurred independently of classical UPR signaling pathways.93 Thus the UPR transducers and ER stress play myriad roles in NAFLD pathogenesis. It is possible that IRE1α hyperactivation, CHOP-dependent macrophage apoptosis, and ER stress-induced inflammasome activation are determinants of NASH; these concepts require further experimental validation.
Mechanism of lipid-induced ER stress:
Lastly, though we have focused mostly on in vivo observations, there have been considerable advancements in the mechanisms by which ER stress pathways are activated in hepatocytes (Fig. 3). NASH is a lipotoxic disease, where the following lipids have been identified as being elevated and injurious to hepatocytes: the saturated free fatty acid palmitate, the phospholipid lysophosphatidylcholine (LPC), sphingolipids including C16:0 ceramide, and free cholesterol.94 Palmitate-induced increases in membrane saturation activated IRE1α and PERK in a mechanism that relied on the transmembrane domain of each protein.95 This and additional studies demonstrated activation of all three UPR sensors by palmitate via induction of downstream signaling molecules including sXBP1, eIF2α phosphorylation, increased abundance of ATF4 and CHOP, and transcriptional targets of ATF6α.80–82 CHOP was of particular interest, as it was implicated in hepatocyte apoptosis via upregulation of DR5, which induces apoptosis upon engagement of its ligand tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and the proapoptotic protein PUMA.80,81 CHOP also suppress the antiapoptotic protein Bcl-2,96 though this has limited relevance to hepatocytes, which do not express Bcl-2.97
In contrast to palmitate, monounsaturated fatty acids such as oleate and palmitoleate are known not to be toxic, but rather mitigate the palmitate toxicity by promoting more efficient esterification into neutral triglyceride.98,99 In keeping with this, palmitoleate attenuated palmitate-induced XBP1 splicing.100 However, prolonged (16 hours) or high concentration (800 and 1600 μM) exposure to oleate activated the UPR sensors in a rat hepatocyte cell line.101 This may be due to conversion of exogenously loaded oleate to palmitate, as has been previously reported;102 though, this is unclear as this study did not measure palmitate levels in oleate loaded cells. At equimolar concentrations, the magnitude of BiP induction and eIF2α phosphorylation was greater in palmitate-treated cells in comparison to oleate-treated cells,103 perhaps due to only partial conversion of exogenously loaded oleate into palmitate.
Ceramide is also implicated in NASH. Ceramide accumulation from de novo synthesis occurs in palmitate-treated cells, and in rat hepatoma cells this was variably reported as decreasing or not impacting palmitate-induced ER stress.103,104 However, release of extracellular vesicles from palmitate-treated cells depended on the de novo synthesis of ceramide in an IRE1α-XBP1 dependent fashion.105 Another possible mechanism for palmitate-induced UPR signaling may be post-translational s-palmitoylation, which has been described for the ER chaperone calnexin but not for any of the three UPR sensors.106,107
Another lipotoxic lipid implicated in NASH is LPC, which recapitulates many of the features of palmitate-induced ER stress and lipotoxicity when applied exogenously.108,109 Endogenous LPC is synthesized from several pathways: (1) hydrolysis of the fatty acid at sn-2 position by the action of phospholipase A2 (PLA2) on PC, (2) lecithin cholesterol acetyl transferase (LCAT) activity in high-density lipoprotein and low-density lipoprotein, and (3) endothelial lipase and hepatic lipase.110 Circulating LPC levels in plasma are high under normal conditions and its hepatocyte lipotoxicity was dependent on hepatocyte-PLA2, implying intracellular generation of LPC from PC.108,111 Unlike palmitate, LPC generation from PC may lead to plasma membrane PC depletion with subsequent activation of an ER stress response. This mechanism has been demonstrated in budding yeast and Caenorhabditis elegans but not yet in mammalian hepatocytes.112,113 Interestingly, PC depletion activated a subset of genes unique from those activated following tunicamycin treatment, suggesting that lipid perturbation-induced ER stress differs from chemically induced ER stress.
In summary, lipid-induced perturbations promote ER stress, the best described of which are palmitate-induced increased membrane saturation and PC depletion. It remains to be seen whether mammalian lipid perturbation-induced ER stress differs from unfolded protein-induced ER stress, but this would provide an interesting avenue of study and increase our understanding of pathological mechanisms of NAFLD and NASH.
Alchoholic liver disease:
Alcohol metabolism is a major driver of ER stress in hepatocytes in ALD. Alcohol is primarily metabolized into acetaldehyde in the cytosol by alcohol dehydrogenase, followed by conversion to acetate in the mitochondria. During excessive ethanol consumption, the smooth ER localized p450 cytochrome CYP2E1 participates in the ethanol-to-acetaldehyde metabolism; however, this process generates reactive oxygen species (ROS) and activates ROS-induced signaling pathways. Recent studies showed that ethanol-induced hyperhomocysteinemia due to reduced methionine synthase activity led to ER stress due to interference with protein folding.114,115 The administration of betaine, a methyl donor used for the conversion of homocysteine to methionine, to mice receiving intragastric ethanol to induce alcoholic steatohepatitis (AH) prevented an increase in homocysteine, while reducing ER stress.116 ER chaperones including GRP78 and glucose regulated protein 94 (GRP94) were upregulated in an early and sustained manner in mice fed a chronic ethanol diet.116 This was associated with increases in SREBP-1 and HMG CoA reductase expression, possibly contributing to ethanol-induced steatosis. CHOP was also induced by ethanol feeding; furthermore, ethanol-fed CHOP ¯/¯ mice were protected from hepatocyte apoptosis, though they were equally susceptible as wild-type mice to ethanol-induced hyperhomocysteinemia, liver steatosis, and injury.115 Acid sphingomyelinase (ASMase) was upregulated in human liver biopsies in AH, and ASMase knockout mice were resistant to alcohol-induced activation of ER stress response in spite of comparable increases in homocysteine levels. This suggested that homocysteine-independent effects of alcohol also contributed to the activation of the ER stress response in AH.117 Mallory–Denk bodies (MDBs), protein aggregates of ubiquitinated keratin 8 and keratin 18, were a hallmark feature of AH. Failure of protein quality control along with activation of an ER stress response was associated with the formation of MDBs in human liver biopsy samples.118 Overall, though ER stress is associated with many features of ALD, the mechanistic pathways and contribution of each UPR sensor and their downstream effectors in pathogenesis of AH are less defined than NAFLD.
Unfolded Protein Response Signaling in HSC Activation and Fibrogenesis
Hepatic stellate cells are the key fibrogenic cells in the liver. Activation of HSCs in response to liver injury leads to production and secretion of extracellular matrix (ECM) proteins.119 Enhanced protein secretion is associated with ER stress and UPR signaling in numerous secretory cells, indicating that UPR signaling may be crucial for processing and secretion of ECM proteins.120 Indeed, UPR signaling was observed in HSCs in response to liver injury in vivo (i.e., alcohol and carbon tetrachloride (CCl4)), and in response to fibrogenic stimuli in vitro (i.e., transforming growth factor β [TGFβ], oxidative stress, and stiffness).8,121–124 This suggests that UPR induction results from increased ECM production; however, UPR-mediated ER expansion prior to increased protein production was observed in other cell types.125 Conversely, chemical induction of UPR signaling through tunicamycin and BFA promoted expression of fibrotic genes such as procollagen I, and these effects were reversed by inhibiting one or all three UPR sensors.8,121,122,126 Similar to the paradox in hepatocytes that ER stress can induce cellular injury but cellular injury can cause ER stress, ER stress may be a driver and a result of HSC activation. Adding another layer of complexity to UPR signaling in HSCs, inducing the UPR in HSCs could serve as an antifibrotic strategy through activating proapoptotic signaling pathways. Here we will discuss recent studies looking at each of the three UPR sensors and their role in HSC activation, and how they mediate adaptive or apoptotic responses (Fig. 4).
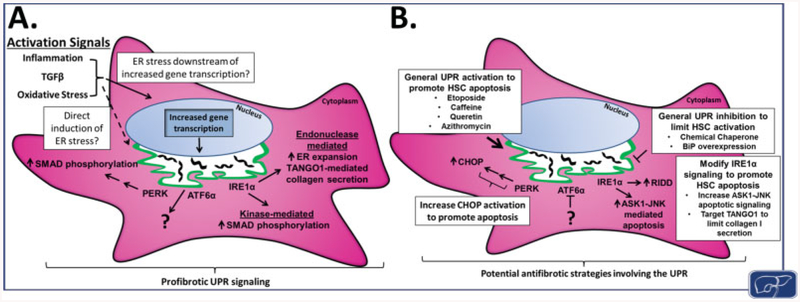
Fig. 4. UPR signaling pathways during HSC activation and potential strategies for antifibrotic targeting.
(A) HSC activation through signals such as inflammation, TGFβ, or oxidative stress leads to ER stress and induction of the UPR. It is yet unclear how activation signals lead to UPR induction, whether through upregulation of ECM proteins or a separate mechanism independent of increased gene transcription. UPR signaling downstream of PERK, ATF6α, and IRE1α further promotes HSC activation and fi(A) HSC ac through increased SMAD2/3 phosphorylation, ER expansion, and enhanced protein secretion, as well as additional, unexplored mechanisms. (B) Targeting the UPR provides potential strategies for either limiting HSC activation or promoting apoptosis of activated HSCs, both favorable for fibrosis resolution. General UPR inhibition through chemical chaperones or BiP overexpression has already shown to be antifibrotic in animal models, and could be pursued further. In addition, preferential targeting of activated HSCs for apoptosis, whether through general activation of UPR signaling (etoposide, caffeine, quercetin, or azithromycin), increased CHOP expression, ASK1-JNK signaling, or disrupted protein export from the ER (inhibition of TANGO1), could prove to be a useful strategy for antifibrotic therapies. ASK1, ASK, apoptosis-signal-regulating kinase 1; BiP, immunoglobulin-binding protein; CHOP, CCAAT-enhancer-binding protein homologous protein; ECM, extracellular matrix; ER, endoplasmic reticulum; HSC, hepatic stellate cell; JNK, c-Jun N-terminal kinase; PERK, protein kinase RNA-like endoplasmic reticulum kinase; UPR, unfolded protein response.
IRE1α:
IRE1α signaling plays an important role in HSC activation. Inhibition of IRE1α through 4μ8C, a noncompetitive inhibitor that blocks IRE1α kinase and endoribonuclease signaling, effectively blocked both TGFβ- and BFA-induced activation of HSCs in vitro, and in an alcohol/CCl4 liver fibrosis model in vivo.122,127,128 Despite this clear relationship, the mechanisms by which IRE1α mediates HSC activation and the regulation of IRE1α signaling during HSC activation are less understood. Both the endonuclease and the kinase domains of IRE1α are implicated in HSC activation and will be reviewed in detail.
IRE1α endonuclease activity and HSC activation:
A critical role for IRE1α signaling in HSC activation was initially reported upon observations of increased sXBP1 in response to ethanol or oxidative stress.121 Furthermore, TGFβ-promoted XBP1 splicing in HSCs, while 4μ8C-mediated IRE1α inhibition reduced TGFβ-induced sXBP1 and expression of BiP, α smooth muscle actin (αSMA), collagen Iα1, and connective tissue growth factor (CTGF).121,122,124 Several pieces of evidence suggested that XBP1 acts to facilitate HSC activation through increasing protein secretion. First, sXBP1 correlated with TGFβ-induced ER dilation during HSC activation, a phenotype associated with increased ER secretory capacity.122 Second, XBP1 was critical for expression of transport and Golgi organization protein 1 (TANGO1), a critical component of the collagen I ER export machinery.124 Furthermore, XBP1 overexpression was sufficient to drive HSC activation and increased expression of several genes involved in protein secretion.123 Interestingly, signaling pathways activated by XBP1 overexpression did not include TGFβ- responsive signaling, as evidenced by unchanged expression of SMAD2, a predominant mediator of TGFβ signaling, or other downstream components.123 This suggested that XBP1 contributes to HSC activation through facilitating cargo secretion by expanding ER capacity and upregulating protein secretion machinery as opposed to enhanced TGFβ signaling. Another less explored role for the IRE1α endonuclease domain in HSC activation may be through cleavage of miRNAs. IRE1α-mediated cleavage, and subsequent degradation of miR-150, led to increased αSMA expression, a known miR150 target.122 Additional miRNA targets of RIDD may mediate HSC activation and fibrogenesis, and remain to be explored.
IRE1α kinase activity and HSC activation:
IRE1α kinase activity also influences HSC activation through one of its downstream targets, p38. p38 is a mitogen-activated protein kinase (MAPK) involved in regulating cell proliferation and autophagy, but also promotes apoptosis downstream of IRE1α.42,129 In HSCs, UPR induction of p38 activity regulated TGFβ signaling. Tunicamycin induced p38 phosphorylation downstream of IRE1α, and p38 inhibition decreased expression of collagen Iα1 and αSMA.121 Later studies showed that IRE1α activation in response to BFA led to SMAD2/3 phosphorylation and procollagen Iα1 expression in a p38-dependent manner, suggesting crosstalk between TGFβ and IRE1α kinase activity.126 Finally, little is understood in regards to UPR regulation of the other downstream effectors of the IRE1α kinase domain (ASK1 and JNK) during HSC activation, but the importance of JNK in HSC activation suggests that UPR signaling could further regulate HSC activation and fiuggests t, through modulation of these kinases.130
PERK:
Mouse models of liver disease, such as CCl4 injection or administration of a high-fat diet, identified a positive correlation between increased PERK phosphorylation and elevated expression of HSC activation markers. The mechanisms by which PERK signaling promotes HSC activation have not received much attention, but recent evidence suggested that PERK augments TGFβ signaling to maintain HSC activation in the presence of chronic liver injury. A noncanonical PERK substrate found to play a role in liver fibrosis is heterogeneous nuclear riboprotein A1 (HNRNPA1). Phosphorylation of HNRNPA1 by PERK was necessary for tunicamycin to upregulate proteins involved in TGFβ signaling, including SMAD2.8 HNRNPA1 processed miR-18, which in turn induced the degradation of SMAD2 mRNA. Upon phosphorylation by PERK, HNRNPA1 was targeted for degradation, leading to reduced miR-18 processing and increased SMAD2 expression. Thus, PERK promoted SMAD2/3-mediated expression of profibrotic genes. This study also identified HNRNPA1 as a potential drug target as lentiviral delivery of HNRNPA1 limited CCl4-induced fibrosis.8 While a role for PERK in HSC activation is clear, no direct role for the primary target of PERK, eIF2α, has been identified, and merits further investigation.
ATF6α:
Little is known regarding ATF6α signaling in HSC activation. ATF6α inhibition reduced HSC activation in response to BFA; however, no mechanistic studies are reported thus far.126 ATF6α likely plays a role in HSC activation, as ATF6α was implicated in cardiac fibrosis, where ATF6α activation promoted production and folding of ECM proteins.131,132 Furthermore, UPR signaling in cardiomyocytes, the primary fiytes, th cells in the heart, was implicated in prolonged TGFβ signaling, and may play a wider role in cardiac fibrogenesis. More studies are required to understand the role of ATF6α signaling during HSC activation.
Potential therapies:
Therapeutic targeting of ER stress in HSCs is attractive as an antifibrotic therapy, through either inhibition of ER stress and HSC activation, or induction of UPR-mediated apoptosis. Fibrosis was limited in murine models through inhibition of ER stress by 4μ8C or 4-phenylbuterate (4-PBA), a small molecule that functionally reduces ER stress via a mechanism that remains poorly understood. Another study utilized a viral delivery system where BiP expression was driven by an αSMA promoter, intended to specifically overexpress BiP in activated HSCs.8 This treatment effectively reduced CCl4- or tunicamycindriven fibrogenesis. While not yet feasible for human application, this work shows that targeting UPR signaling in HSCs may be just as important as targeting UPR signaling in hepatocytes.
Another approach to targeting ER stress as an antifibrotic therapy is driving UPR-mediated HSC apoptosis. This approach is more difficult, since any drug would need to specifically target activated HSCs for apoptosis, with little effect on hepatocytes. Studies using the anticancer drug etoposide showed increased apoptosis of LX-2 cells through a UPR signaling mechanism, with reduced effects on the hepatocyte cell lines LO-2 and QSG-7701.133 Compounds such as caffeine and quercetin also promoted HSC apoptosis through UPR signaling.134,135 Additionally, a promising treatment for idiopathic pulmonary fibrosis (IPF) may translate to liver fibrosis. The drug azithromycin significantly increased survival of patients with acute exacerbation of IPF (AE-IPR), and acted in vitro to limit differentiation of lung fibroblasts in part through increased UPR signaling.136,137 HSC apoptosis was also induced through forced accumulation of secretory proteins in the ER. RNA interference (RNAi)-mediated knockdown of TANGO1 led to procollagen I retention within the ER and increased cell death in vitro, whereas HepG2 cells were unaffected by TANGO1 knockdown.138 The drawback of targeting HSCs directly lies in the lack of effective and selective delivery mechanisms. HSC-specific targeting by adenoviral delivery or targeted liposomes have been explored, but with only moderate success. This is a major hurdle which needs to be addressed if antifibrotic therapies are to specifically target activated HSCs for apoptosis.
Conclusion
This review focuses on the known contribution of the UPR sensors in the pathogenesis of NAFLD, ALD, and hepatic fibrosis. Following recently recognized links between the UPR sensors, hepatic lipid metabolism, insulin resistance, and obesity-associated activation of an ER stress response, a vast literature has emerged highlighting the complexities of each UPR sensor and their downstream signaling pathways in the pathogenesis of NAFLD. Accumulated observations highlight an important role for each UPR sensor in NAFLD pathogenesis with several unanswered questions; importantly, whether failure of IRE1α s-nitrosylation leading to hyperactivation over time marks a transition from NAFL to NASH. Alternatively, loss of IRE1α endoribonuclease activity over time in conjunction with sustained PERK activation may mark this transition.139 Mechanistically, lipid perturbations activate ER stress in a manner distinct from unfolded proteins. Palmitate is elevated in human and murine models of NAFLD; however, dissecting the mechanism of activation of ER stress pathways by other lipids known to be perturbed in NAFLD may help understand the differences between ER stresses in NAFL and ER stress in NASH. Interfering with lipid perturbations such that the adaptive UPR can be protractedly activated may be beneficial in NAFLD. Additionally, studies with IRE1α suggest that inhibition of IRE1α s-nitrosylation may be of therapeutic benefit in NAFLD.
We also focus on the activated HSC, as upon activation, these cells undergo a remarkable shift from quiescence to professional matrix secretory cells, leading eventually to hepatic fibrosis. The pathophysiology of UPR signaling in HSCs is an especially crucial area for study due to the potential therapeutic implications. It is not well understood how the UPR is induced during HSC activation, whether as a preceding event in response to activating stimuli or a result of increased production of secretory proteins. In this way, the diverse stimuli that promote HSC activation may be critical for differential induction and timing of the UPR and how it influences HSCs. Another interesting area of study is how the proadaptive and proapoptotic arms of the UPR are regulated during prolonged HSC activation. A better understanding of the machinery suppressing UPR-mediated apoptosis in this context could provide a potential therapeutic modality. Lastly, we highlighted the role of the UPR sensors in both hepatocytes and HSCs in disease states, to highlight the complexity inherent to targeting the UPR for therapy of chronic liver diseases.
Main Concepts and Learning Points.
ER stress plays a critical but varied role in metabolic liver diseases depending of the cellular and disease context.
UPR sensors regulate hepatic steatosis and lipid metabolism; conversely, the UPR sensors are dysregulated in fatty liver disease.
UPR signaling pathways play distinct roles during HSC activation and fibrogenesis.
Targeting ER stress in HSCs to block the UPR or promote UPR-mediated apoptosis may be a favorable antifibrotic therapy.
Funding
This work was supported in part by NIH grants DK111378 (H. M.), DK112915 (J. L. M), and by Gilead Sciences Research Scholars Program in Liver Disease (H. M.).
Footnotes
Conflict of Interest
None.
References
- 1.Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018; 4(01):16. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Loomba R, Rinella ME, et al. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018; 68(01):361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshiuchi K, Kaneto H, Matsuoka TA, et al. Pioglitazone reduces ER stress in the liver: direct monitoring of in vivo ER stress using ER stress-activated indicator transgenic mice. Endocr J 2009; 56 (09):1103–1111 [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology 2006; 147(02):943–951 [DOI] [PubMed] [Google Scholar]
- 5.Tardif KD, Mori K, Siddiqui A. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J Virol 2002; 76(15):7453–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puri P, Mirshahi F, Cheung O, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 2008; 134(02):568–576 [DOI] [PubMed] [Google Scholar]
- 7.Sakon M, Ariyoshi H, Umeshita K, Monden M. Ischemia-reperfusion injury of the liver with special reference to calcium-dependent mechanisms. Surg Today 2002; 32(01):1–12 [DOI] [PubMed] [Google Scholar]
- 8.Koo JH, Lee HJ, Kim W, Kim SG. Endoplasmic reticulum stress in hepatic stellate cells promotes liver fibrosis via PERK-mediated degradation of HNRNPA1 and up-regulation of SMAD2. Gastroenterology 2016;150(01):181.e8–193.e8 [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Zhang K, Li Z, Guo B. ER stress-induced inflammasome activation contributes to hepatic inflammation and steatosis. J Clin Cell Immunol 2016; 7(05):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang F, Wang S, Liu Y, et al. IRE1α aggravates ischemia reperfusion injury of fatty liver by regulating phenotypic transformation of kupffer cells. Free Radic Biol Med 2018; 124:395–407 [DOI] [PubMed] [Google Scholar]
- 11.Wenfeng Z, Yakun W, Di M, Jianping G, Chuanxin W, Chun H. Kupffer cells: increasingly significant role in nonalcoholic fatty liver disease. Ann Hepatol 2014; 13(05):489–495 [PubMed] [Google Scholar]
- 12.Gao J, Jiang Z, Wang S, Zhou Y, Shi X, Feng M. Endoplasmic reticulum stress of Kupffer cells involved in the conversion of natural regulatory T cells to Th17 cells in liver ischemia-reperfusion injury. J Gastroenterol Hepatol 2016; 31(04):883–889 [DOI] [PubMed] [Google Scholar]
- 13.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev 2008; 29(03):317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011; 334 (6059):1081–1086 [DOI] [PubMed] [Google Scholar]
- 15.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2000; 2(06):326–332 [DOI] [PubMed] [Google Scholar]
- 16.Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 2011; 333(6051):1891–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang P, Li J, Tao J, Sha B. The luminal domain of the ER stress sensor protein PERK binds misfolded proteins and thereby triggers PERK oligomerization. J Biol Chem 2018; 293(11): 4110–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 2002; 3(01): 99–111 [DOI] [PubMed] [Google Scholar]
- 19.Ali MM, Bagratuni T, Davenport EL, et al. Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. EMBO J 2011; 30(05):894–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karagöz GE, Acosta-Alvear D, Nguyen HT, Lee CP, Chu F, Walter P. An unfolded protein-induced conformational switch activates mammalian IRE1. eLife 2017; 6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp MC, Nowak PR, Larburu N, Adams CJ, Ali MM. In vitro FRET analysis of IRE1 and BiP association and dissociation upon endoplasmic reticulum stress. eLife 2018; 7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQuiston A, Diehl JA. Recent insights into PERK-dependent signaling from the stressed endoplasmic reticulum. F1000 Res 2017; 6:1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Liu H, Li L, et al. Structural insights into IRE1 functions in the unfolded protein response. Curr Med Chem 2016; 23(41): 4706–4716 [DOI] [PubMed] [Google Scholar]
- 24.Han J, Kaufman RJ. Physiological/pathological ramifications of transcription factors in the unfolded protein response. Genes Dev 2017; 31(14):1417–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8(07): 519–529 [DOI] [PubMed] [Google Scholar]
- 26.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev 1998; 12(12): 1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oikawa D, Kimata Y, Kohno K, Iwawaki T. Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp Cell Res 2009; 315(15):2496–2504 [DOI] [PubMed] [Google Scholar]
- 28.Kosmaczewski SG, Edwards TJ, Han SM, et al. The RtcB RNA ligase is an essential component of the metazoan unfolded protein response. EMBO Rep 2014; 15(12):1278–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jurkin J, Henkel T, Nielsen AF, et al. The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. EMBO J 2014; 33(24):2922–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Liang FX, Wang X. A synthetic biology approach identifies the mammalian UPR RNA ligase RtcB. Mol Cell 2014; 55(05): 758–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tirosh B, Iwakoshi NN, Glimcher LH, Ploegh HL. Rapid turnover of unspliced Xbp-1 as a factor that modulates the unfolded protein response. J Biol Chem 2006; 281(09):5852–5860 [DOI] [PubMed] [Google Scholar]
- 32.Calfon M, Zeng H, Urano F, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 2002; 415(6867):92–96 [DOI] [PubMed] [Google Scholar]
- 33.Oda Y, Okada T, Yoshida H, Kaufman RJ, Nagata K, Mori K. Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J Cell Biol 2006; 172(03):383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han D, Lerner AG, Vande Walle L, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 2009; 138(03):562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 2009; 186(03):323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 2006; 313(5783):104–107 [DOI] [PubMed] [Google Scholar]
- 37.Upton JP, Wang L, Han D, et al. IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science 2012; 338(6108):818–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alphamediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol 2006; 26(08):3071–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000; 287(5453):664–666 [DOI] [PubMed] [Google Scholar]
- 40.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A 2003; 100(05):2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol 1999;19(12): 8469–8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darling NJ, Cook SJ. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta 2014; 1843(10):2150–2163 [DOI] [PubMed] [Google Scholar]
- 43.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999; 397(6716):271–274 [DOI] [PubMed] [Google Scholar]
- 44.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A 2004;101(31):11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol 2004; 167(01):27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ventoso I, Kochetov A, Montaner D, Dopazo J, Santoyo J. Extensive translatome remodeling during ER stress response in mammalian cells. PLoS One 2012; 7(05):e35915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 1999; 339(Pt 1):135–141 [PMC free article] [PubMed] [Google Scholar]
- 48.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 1998; 12(07):982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem 2000; 275 (35):27013–27020 [DOI] [PubMed] [Google Scholar]
- 50.Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 1998; 273(50):33741–33749 [DOI] [PubMed] [Google Scholar]
- 51.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 1999; 10(11):3787–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto K, Sato T, Matsui T, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 2007;13(03): 365–376 [DOI] [PubMed] [Google Scholar]
- 53.Loud AV. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol 1968; 37(01):27–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. The compartmentalization of cells In: Molecular Biology of the Cell. 4th ed. New York, NY: Garland Science; 2002 [Google Scholar]
- 55.Barle H, Nyberg B, Essén P, et al. The synthesis rates of total liver protein and plasma albumin determined simultaneously in vivo in humans. Hepatology 1997; 25(01):154–158 [DOI] [PubMed] [Google Scholar]
- 56.Miller LL, Bale WF. Synthesis of all plasma protein fractions except gamma globulins by the liver; the use of zone electrophoresis and lysine-epsilon-C14 to define the plasma proteins synthesized by the isolated perfused liver. J Exp Med 1954; 99(02):125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fagone P, Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res 2009; 50(Suppl): S311–S316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res 2003; 44(01):22–32 [DOI] [PubMed] [Google Scholar]
- 59.Wang S, Chen Z, Lam V, et al. IRE1α-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab 2012; 16(04):473–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liverspecific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology 2011; 54(01):229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lloyd DJ, Wheeler MC, Gekakis N. A point mutation in Sec61alpha1 leads to diabetes and hepatosteatosis in mice. Diabetes 2010; 59(02):460–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rutkowski DT, Wu J, Back SH, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell 2008; 15(06): 829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 2008; 320 (5882):1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.So JS, Hur KY, Tarrio M, et al. Silencing of lipid metabolism genes through IRE1α-mediated mRNA decay lowers plasma lipids in mice. Cell Metab 2012; 16(04):487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang K, Wang S, Malhotra J, et al. The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis. EMBO J 2011; 30(07):1357–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng L, Lu M, Mori K, et al. ATF6 modulates SREBP2-mediated lipogenesis. EMBO J 2004; 23(04):950–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 2009; 58(03):693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004; 306(5695):457–461 [DOI] [PubMed] [Google Scholar]
- 69.Yang L, Calay ES, Fan J, et al. METABOLISM. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science 2015; 349(6247):500–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang JM, Qiu Y, Yang Z, et al. IRE1α prevents hepatic steatosis by processing and promoting the degradation of select microRNAs. Sci Signal 2018; 11(530):eaao4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herrema H, Zhou Y, Zhang D, et al. XBP1s is an anti-lipogenic protein. J Biol Chem 2016; 291(33):17394–17404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mauer AS, Hirsova P, Maiers JL, Shah VH, Malhi H. Inhibition of sphingosine 1-phosphate signaling ameliorates murine nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol 2017; 312(03):G300–G313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X, Henkel AS, LeCuyer BE, Schipma MJ, Anderson KA, Green RM. Hepatocyte X-box binding protein 1 deficiency increases liver injury in mice fed a high-fat/sugar diet. Am J Physiol Gastrointest Liver Physiol 2015; 309(12):G965–G974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lebeaupin C, Vallée D, Rousseau D, et al. Bax inhibitor-1 protects from nonalcoholic steatohepatitis by limiting inositol-requiring enzyme 1 alpha signaling in mice. Hepatology 2018; 68(02): 515–532 [DOI] [PubMed] [Google Scholar]
- 75.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115(05):1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shan B, Wang X, Wu Y, et al. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol 2017; 18(05): 519–529 [DOI] [PubMed] [Google Scholar]
- 77.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab 2008; 7(06):520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li H, Meng Q, Xiao F, et al. ATF4 deficiency protects mice from high-carbohydrate-diet-induced liver steatosis. Biochem J 2011; 438(02):283–289 [DOI] [PubMed] [Google Scholar]
- 79.Xiao G, Zhang T, Yu S, et al. ATF4 protein deficiency protects against high fructose-induced hypertriglyceridemia in mice. J Biol Chem 2013; 288(35):25350–25361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cazanave SC, Elmi NA, Akazawa Y, Bronk SF, Mott JL, Gores GJ. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol 2010; 299 (01):G236–G243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cazanave SC, Mott JL, Bronk SF, et al. Death receptor 5 signaling promotes hepatocyte lipoapoptosis. J Biol Chem 2011; 286(45): 39336–39348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miyamoto Y, Mauer AS, Kumar S, Mott JL, Malhi H. Mmu-miR-615–3p regulates lipoapoptosis by inhibiting C/EBP homologous protein. PLoS One 2014; 9(10):e109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malhi H, Kropp EM, Clavo VF, et al. C/EBP homologous proteininduced macrophage apoptosis protects mice from steatohepatitis. J Biol Chem 2013; 288(26):18624–18642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rahman K, Liu Y, Kumar P, et al. C/EBP homologous protein modulates liraglutide-mediated attenuation of non-alcoholic steatohepatitis. Lab Invest 2016; 96(08):895–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toriguchi K, Hatano E, Tanabe K, et al. Attenuation of steatohepatitis, fibrosis, and carcinogenesis in mice fed a methioninecholine deficient diet by CCAAT/enhancer-binding protein homologous protein deficiency. J Gastroenterol Hepatol 2014; 29(05):1109–1118 [DOI] [PubMed] [Google Scholar]
- 86.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab 2010; 298(05):E1027–E1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakagawa H, Umemura A, Taniguchi K, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 2014; 26(03):331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weglarz TC, Degen JL, Sandgren EP. Hepatocyte transplantation into diseased mouse liver. Kinetics of parenchymal repopulation and identification of the proliferative capacity of tetraploid and octaploid hepatocytes. Am J Pathol 2000; 157 (06):1963–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Usui M, Yamaguchi S, Tanji Y, et al. Atf6α-null mice are glucose intolerant due to pancreatic β-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metabolism 2012; 61(08):1118–1128 [DOI] [PubMed] [Google Scholar]
- 90.Fu S, Yang L, Li P, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011; 473(7348):528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mridha AR, Wree A, Robertson AAB, et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 2017; 66(05):1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lebeaupin C, Proics E, de Bieville CH, et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis 2015;6:e1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Menu P, Mayor A, Zhou R, et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis 2012; 3:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Musso G, Cassader M, Paschetta E, Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology 2018; 155(02): 282.e8–302.e8 [DOI] [PubMed] [Google Scholar]
- 95.Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A 2013; 110(12):4628–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 2011; 13(03): 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodriguez I, Matsuura K, Khatib K, Reed JC, Nagata S, Vassalli P. A bcl-2 transgene expressed in hepatocytes protects mice from fulminant liver destruction but not from rapid death induced by anti-Fas antibody injection. J Exp Med 1996; 183(03): 1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moravcová A, Červinková Z, Kučera O, Mezera V, Rychtrmoc D, Lotková H. The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture. Physiol Res 2015; 64(Suppl 5):S627–S636 [DOI] [PubMed] [Google Scholar]
- 99.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 2003; 100(06):3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akazawa Y, Cazanave S, Mott JL, et al. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol 2010; 52(04):586–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest 2008; 118(01):316–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee W-NP, Lim S, Bassilian S, Bergner EA, Edmond J. Fatty acid cycling in human hepatoma cells and the effects of troglitazone. J Biol Chem 1998; 273(33):20929–20934 [DOI] [PubMed] [Google Scholar]
- 103.Caviglia JM, Gayet C, Ota T, et al. Different fatty acids inhibit apoB100 secretion by different pathways: unique roles for ER stress, ceramide, and autophagy. J Lipid Res 2011; 52(09): 1636–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 2006; 291(02):E275–E281 [DOI] [PubMed] [Google Scholar]
- 105.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner. J Lipid Res 2016; 57(02): 233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dallavilla T, Abrami L, Sandoz PA, Savoglidis G, Hatzimanikatis V, van der Goot FG. Model-driven understanding of palmitoylation dynamics: regulated acylation of the endoplasmic reticulum chaperone calnexin. PLOS Comput Biol 2016; 12(02):e1004774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lakkaraju AK, Abrami L, Lemmin T, et al. Palmitoylated calnexin is a key component of the ribosome-translocon complex. EMBO J 2012; 31(07):1823–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kakisaka K, Cazanave SC, Fingas CD, et al. Mechanisms of lysophosphatidylcholine-induced hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol 2012; 302(01):G77–G84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Han MS, Park SY, Shinzawa K, et al. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res 2008; 49(01):84–97 [DOI] [PubMed] [Google Scholar]
- 110.Drzazga A, Sowińska A, Koziołkiewicz M. Lysophosphatidylcholine and lysophosphatidylinosiol–novel promissing signaling molecules and their possible therapeutic activity. Acta Pol Pharm 2014; 71(06):887–899 [PubMed] [Google Scholar]
- 111.Ojala PJ, Hirvonen TE, Hermansson M, Somerharju P, Parkkinen J. Acyl chain-dependent effect of lysophosphatidylcholine on human neutrophils. J Leukoc Biol 2007; 82(06):1501–1509 [DOI] [PubMed] [Google Scholar]
- 112.Thibault G, Shui G, Kim W, et al. The membrane stress response buffers lethal effects of lipid disequilibrium by reprogramming the protein homeostasis network. Mol Cell 2012;48(01):16–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koh JH, Wang L, Beaudoin-Chabot C, Thibault G. Lipid bilayer stress-activated IRE-1 modulates autophagy during endoplasmic reticulum stress. J Cell Sci 2018. DOI: 10.1242/jcs.217992 [DOI] [PubMed] [Google Scholar]
- 114.Barak AJ, Beckenhauer HC, Tuma DJ. Betaine effects on hepatic methionine metabolism elicited by short-term ethanol feeding. Alcohol 1996; 13(05):483–486 [DOI] [PubMed] [Google Scholar]
- 115.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res 2005; 29(08):1496–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 2003; 124(05):1488–1499 [DOI] [PubMed] [Google Scholar]
- 117.Fernandez A, Matias N, Fucho R, et al. ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading. J Hepatol 2013; 59(04): 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.French SW, Masouminia M, Samadzadeh S, Tillman BC, Mendoza A, French BA. Role of protein quality control failure in alcoholic hepatitis pathogenesis. Biomolecules 2017; 7(01):E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang JX, Török NJ. Liver injury and the activation of the hepatic myofibroblasts. Curr Pathobiol Rep 2013; 1(03):215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dorner AJ, Wasley LC, Kaufman RJ. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem 1989; 264(34):20602–20607 [PubMed] [Google Scholar]
- 121.Hernández-Gea V, Hilscher M, Rozenfeld R, et al. Endoplasmic reticulum stress induces fibrogenic activity in hepatic stellate cells through autophagy. J Hepatol 2013; 59(01):98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heindryckx F, Binet F, Ponticos M, et al. Endoplasmic reticulum stress enhances fibrosis through IRE1α-mediated degradation of miR-150 and XBP-1 splicing. EMBO Mol Med 2016; 8(07): 729–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim RS, Hasegawa D, Goossens N, et al. The XBP1 arm of the unfolded protein response induces fiThe XBP1 activity in hepatic stellate cells through autophagy. Sci Rep 2016;6:39342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maiers JL, Kostallari E, Mushref M, et al. The unfolded protein response mediates fibrogenesis and collagen I secretion through regulating TANGO1 in mice. Hepatology 2017;65(03):983–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol 2010; 189(05):783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Galarreta MR, Navarro A, Ansorena E, et al. Unfolded protein response induced by Brefeldin A increases collagen type I levels in hepaticstellatecells through an IRE1α, p38MAPKandSmad-dependent pathway. Biochim Biophys Acta 2016; 1863(08):2115–2123 [DOI] [PubMed] [Google Scholar]
- 127.Wei W, Zhang F, Chen H, et al. Toxoplasma gondii dense granule protein 15 induces apoptosis in choriocarcinoma JEG-3 cells through endoplasmic reticulum stress. Parasit Vectors 2018; 11(01):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sato H, Shiba Y, Tsuchiya Y, Saito M, Kohno K. 4μ8C inhibits insulin secretion independent of IRE1α RNase activity. Cell Struct Funct 2017; 42(01):61–70 [DOI] [PubMed] [Google Scholar]
- 129.Huang Y, Li X, Wang Y, Wang H, Huang C, Li J. Endoplasmic reticulum stress-induced hepatic stellate cell apoptosis through calcium-mediated JNK/P38 MAPK and Calpain/Caspase-12 pathways. Mol Cell Biochem 2014; 394(1–2):1–12 [DOI] [PubMed] [Google Scholar]
- 130.Zhao G, Hatting M, Nevzorova YA, et al. Jnk1 in murine hepatic stellate cells is a crucial mediator of liver fibrogenesis. Gut 2014; 63(07):1159–1172 [DOI] [PubMed] [Google Scholar]
- 131.Shih YC, Chen CL, Zhang Y, et al. Endoplasmic reticulum protein TXNDC5 augments myocardial fibrosis by facilitating extracellular matrix protein folding and redox-sensitive cardiac fibroblast activation. Circ Res 2018; 122(08):1052–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Groenendyk J, Lee D, Jung J, et al. Inhibition of the unfolded protein response mechanism prevents cardiac fibrosis. PLoS One 2016; 11(07):e0159682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang C, Zhang F, Cao Y, et al. Etoposide induces apoptosis in activated human hepatic stellate cells via ER stress. Sci Rep 2016; 6:34330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y, Chen Y, Huang H, et al. Autophagy mediated by endoplasmic reticulum stress enhances the caffeine-induced apoptosis of hepatic stellate cells. Int J Mol Med 2017; 40(05):1405–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.He L, Hou X, Fan F, Wu H Quercetin stimulates mitochondrial apoptosis dependent on activation of endoplasmic reticulum stress in hepatic stellate cells. Pharm Biol 2016; 54(12): 3237–3243 [DOI] [PubMed] [Google Scholar]
- 136.Tsubouchi K, Araya J, Minagawa S, et al. Azithromycin attenuates myofibroblast differentiation and lung fibrosis development through proteasomal degradation of NOX4. Autophagy 2017; 13(08):1420–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kawamura K, Ichikado K, Yasuda Y, Anan K, Suga M. Azithromycin for idiopathic acute exacerbation of idiopathic pulmonary fibrosis: a retrospective single-center study. BMC Pulm Med 2017; 17(01):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maiers JL, Kostallari E, Mushref M, et al. The unfolded protein response mediates fibrogenesis and collagen I secretion through regulating TANGO1 in mice. Hepatology 2017; 65(03):983–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang TK, Lawrence DA, Lu M, et al. Coordination between two branches of the unfolded protein response determines apoptotic cell fate. Mol Cell 2018; 71(04):629–636.e5 [DOI] [PubMed] [Google Scholar]