Abstract
Purpose:
The incidence of localized prostate cancer has decreased with shifts in prostate cancer screening. While recent population based studies demonstrated a stable incidence of locoregional prostate cancer, they categorized organ confined, extraprostatic and lymph node positive disease together. However, to our knowledge the contemporary incidence of prostate cancer with pelvic lymph node metastases remains unknown.
Materials and Methods:
We used SEER (Surveillance, Epidemiology and End Results) data from 2004 to 2014 to identify men diagnosed with prostate cancer. We analyzed trends in the age standardized prostate cancer incidence by stage. The impact of disease extent on mortality was assessed by adjusted Cox proportional hazard analysis.
Results:
During the study period the annual incidence of nonmetastatic prostate cancer decreased from 5,119.1 to 2,931.9 per million men (IR 0.57, 95% CI 0.56–0.58, p <0.01) while the incidence of pelvic lymph node metastases increased from 54.1 to 79.5 per million men (IR 1.47, 95% CI 1.33–1.62, p <0.01). The incidence of distant metastases in men 75 years old or older reached a nadir in 2011 compared to 2004 (IR 0.81, 95% CI 0.74–0.90, p <0.01) and it increased in 2012 compared to 2011 (IR 1.13, 95% CI 1.02–1.24, p <0.05). The risk of cancer specific mortality significantly increased in men diagnosed with pelvic lymph node metastases (HR 4.5, 95% CI 4.2–4.9, p <0.01) and distant metastases (HR 21.9, 95% CI 21.2–22.7, p <0.01) compared to men with nonmetastatic disease.
Conclusions:
The incidence of pelvic lymph node metastases is increasing coincident with a decline in the detection of localized disease. Whether this portends an increase in the burden of advanced disease or simply reflects decreased lead time remains unclear. However, this should be monitored closely as the increase in N1 disease reflects an increase in incurable prostate cancer at diagnosis.
Keywords: prostatic neoplasms, neoplasm metastasis, SEER Program, practice guidelines as topic, mortality
THE last decade was characterized by major shifts in prostate cancer epidemiology.1–4 The prostate cancer incidence decreased from 175 to 100/100,000 men from 2007 to 20145 with a decrease in the incidence of local/regional prostate cancer.2,6,7 Concurrent with this trend we reported that the decrease in localized disease detection was accompanied by an increase in the incidence of distant metastases in men older than 75 years following a nadir in 2011.6 Others confirmed these findings.2
Using SEER data we also found that the proportion of men with PLNM at radical prostatectomy is increasing.6,8 However, this may be secondary to selection bias in the performance of radical prostatectomy for higher risk features as up to 50% of men with low risk features now elect active surveillance.9
In this study we used the most recent update of SEER through 2014 to examine temporal changes in the incidence of nonmetastatic disease, PLNM and distant metastases. In addition, we studied PCSM in men with nonmetastatic disease and pelvic lymph node metastases to determine the potential consequences of an increase in nonlocalized disease.
METHODS
Study Population
The SEER program of NCI (National Cancer Institute) captures a representative sample of 28% of the American population.10 We identified 573,669 men 50 years old or older who were diagnosed with pathologically confirmed prostate cancer from 2004 to 2014 to examine the incidence by SEER Collaborative Stage with time. Only the 475,153 men with prostate cancer as the only malignancy were included in mortality analysis to avoid potential confounding due to competing cancer specific mortality. Additionally, men with missing stage and missing survival time were excluded, resulting in 443,000 men available for analysis. Incident prostate cancers were categorized into 3 groups by disease extent at diagnosis, including group 1—nonmetastatic disease (T1N0M0, T2N0M0, T3N0M0 and T4N0M0), 2—PLNM (N1M0) and 3—distant metastasis (M1).
Independent Variables
Sociodemographic characteristics included age at diagnosis (50 to 74 or 75 years old or older), race/ethnicity (Caucasian, African American, Hispanic or other), diagnosis year (2004 to 2008 or 2009 to 2014) and United States Census region (Northeast, South, Midwest or West). Clinical characteristics included pathological stage (T1, T2, T3 or T4), N stage (N0 or N1),2,7 M stage (M0 or M1) and Gleason score (6 or less, or 7 or greater).
Outcomes and Statistical Analysis
We evaluated the yearly and quarterly age adjusted incidences of prostate cancer per million men standardized to the 2000 United States Census population with time. The quarterly incidence was assessed graphically and a linear model or a restricted cubic spline model was fitted based on the Akaike information criterion. A restricted cubic splines model was chosen to provide a flexible description of the nonlinear relationship when linearity was inappropriate.11,12 In addition, IRRs by year were calculated to evaluate relative changes in incidence to the beginning of the study period in 2004 and between each consecutive year. To account for missing values in TNM stage the quarterly incidence was derived by applying the proportion of each disease stage to the total number of men diagnosed with prostate cancer.
Sensitivity analysis was done to impute missing values with the clinical range of each cancer group.13–15 We performed a subanalysis to account for potential detection bias secondary to increasing performance of prostatectomy in patients at high risk. The proportion of patients with PLNM who did and did not undergo prostatectomy was assessed by the Cochran-Armitage trend test.
Among men who had only prostate cancer we compared demographic characteristics, overall mortality and PCSM among those diagnosed with nonmetastatic disease, PLNM and distant metastases. Differences were evaluated using the percent of event count, the chi-square test for categorical variables and means, and the Kruskal-Wallis test for continuous variables.
Kaplan-Meier curves were constructed to visualize unadjusted overall mortality and PCSM. Cox regression was used to determine the HR of PCSM adjusting for year of diagnosis, age at diagnosis, race/ethnicity, tumor grade and pathology findings. Men with missing survival time were excluded from survival analysis. Statistical significance was considered at p<0.05. All analysis were performed with SAS®, version 9.4.
RESULTS
Incidence
The incidence of nonmetastatic prostate cancer decreased from 5,119.1 to 2,931.9 per million men from 2004 to 2014 (supplementary figure, http://jurology.com/, table 1 and fig.1). This corresponded to a relative decrease of 43% (IR 0.57, 95% CI 0.56–0.58, p<0.01, table 2). The trend persisted after stratifying by age with the incidence of nonmetastatic prostate cancer decreasing form 4,616.3 to 2,880.3 per million men 50 to74 years old (IR 0.62, 95% CI 0.61–0.63, p<0.01) and from 6,918.4 to 3,116.7 per million men 75 years old or older (IR 0.45, 95% CI 0.44–0.47, p<0.01).
Table 1.
Standardized annual incidence of prostate cancer per 1,000,000 men stratified by age
| Metastasis |
|||
|---|---|---|---|
| Age | None | Pelvic Lymph Node | Distant |
| All 50 or Greater: | |||
| 2004 | 5,119.1 | 54.1 | 226.5 |
| 2005 | 4,770.7 | 52.9 | 222.9 |
| 2006 | 5,186.3 | 53.6 | 217.3 |
| 2007 | 5,361.4 | 61.7 | 213.5 |
| 2008 | 4,869.6 | 60.3 | 207.0 |
| 2009 | 4,724.9 | 60.7 | 204.3 |
| 2010 | 4,475.8 | 66.5 | 207.6 |
| 2011 | 4,412.0 | 65.2 | 206.9 |
| 2012 | 3,546.9 | 65.2 | 220.4 |
| 2013 | 3,309.6 | 71.0 | 230.7 |
| 2014 | 2,931.9 | 79.5 | 235.2 |
| 50–74: | |||
| 2004 | 4,616.3 | 60.2 | 136.0 |
| 2005 | 4,314.1 | 59.6 | 142.2 |
| 2006 | 4,768.0 | 59.4 | 134.5 |
| 2007 | 4,990.1 | 66.3 | 129.2 |
| 2008 | 4,607.0 | 63.1 | 130.4 |
| 2009 | 4,565.7 | 64.3 | 134.2 |
| 2010 | 4,318.8 | 69.7 | 137.0 |
| 2011 | 4,280.4 | 65.9 | 139.7 |
| 2012 | 3,495.5 | 67.4 | 141.3 |
| 2013 | 3,263.8 | 72.0 | 148.2 |
| 2014 | 2,880.3 | 80.5 | 157.3 |
| 75 or Greater: | |||
| 2004 | 6,918.4 | 32.2 | 550.2 |
| 2005 | 6,405.0 | 29.2 | 511.8 |
| 2006 | 6,683.6 | 32.5 | 513.8 |
| 2007 | 6,690.1 | 45.6 | 515.1 |
| 2008 | 5,809.5 | 50.2 | 481.1 |
| 2009 | 5,295.0 | 47.8 | 455.2 |
| 2010 | 5,037.8 | 54.8 | 460.4 |
| 2011 | 4,883.1 | 62.7 | 447.3 |
| 2012 | 3,730.7 | 57.3 | 503.3 |
| 2013 | 3,473.5 | 67.5 | 526.0 |
| 2014 | 3,116.7 | 75.9 | 514.2 |
Figure 1.
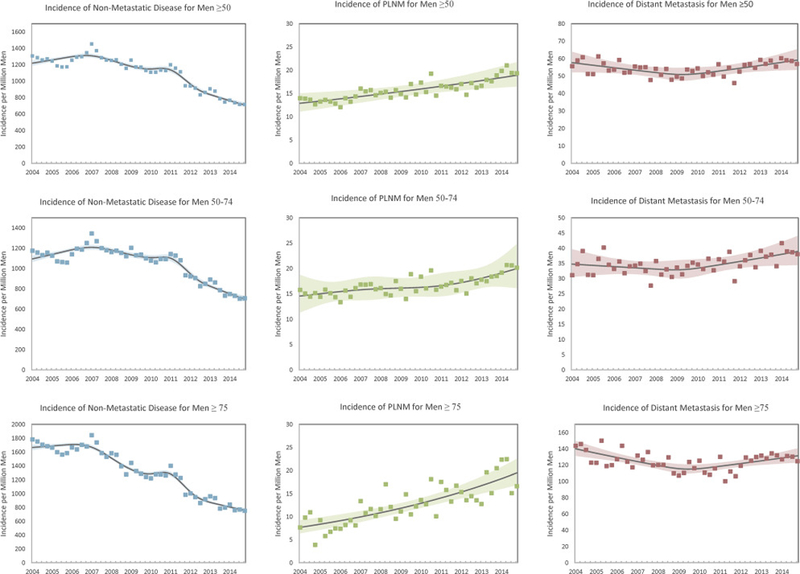
Standardized incidence of nonmetastatic, pelvic lymph node metastatic and distantly metastatic prostate cancer at diagnosis by quarter between 2004 and 2014
Table 3.
Baseline cohort characteristics
| Metastasis |
|||
|---|---|---|---|
| None | Pelvic Lymph Node |
Distant | |
| No. pts | 417,893 | 6,416 | 18,691 |
| Age: | |||
| Mean ± SD* | 66.0 ± 8.5 | 64.1 ± 7.8 | 70.6 ± 10.5 |
| No. 50–74 | 348,025 (83.3) | 5,784 (90.1) | 11,788 (63.1) |
| No. 75 or greater | 69,868 (16.7) | 632 (9.9) | 6,903 (36.9) |
| No. diagnosis yr (%): | |||
| 2004–2008* | 191,911 (45.9) | 2,335 (36.4) | 7,749 (41.5) |
| 2009–2014 | 225,982 (54.1) | 4,081 (63.6) | 10,942 (58.5) |
| No. race/ethnicity (%): | |||
| Caucasian, | 288,532 (69.0) | 4,429 (69.0) | 11,664 (62.4) |
| nonHispanic* | |||
| African American, | 37,218 (8.9) | 696 (10.8) | 2,217 (11.9) |
| nonHispanic | |||
| Hispanic | 60,923 (14.6) | 909 (14.2) | 3,493 (18.7) |
| Other | 31,220 (7.5) | 382 (6.0) | 1,317 (7.0) |
| No. region (%): | |||
| Northeast* | 71,730 (17.2) | 953 (14.9) | 2,818 (15.1) |
| South | 95,745 (22.9) | 1,142 (17.8) | 3,961 (21.2) |
| West | 208,333 (49.9) | 3,614 (56.3) | 10,038 (53.7) |
| Midwest | 42,085 (10.1) | 707 (11.0) | 1,874 (10.0) |
| No. pathology finding (%): | |||
| T1* | 264,507 (63.3) | 2,388 (37.2) | 4,591 (24.6) |
| T2 | 144,458 (34.6) | 2,552 (39.8) | 5,848 (31.3) |
| T3 | 7,841 (1.9) | 1,019 (15.9) | 1,766 (9.4) |
| T4 | 1,087 (0.3) | 328 (5.1) | 2,333 (12.5) |
| Missing | — | 129 (2.0) | 4,153 (22.2) |
| No. N1 stage (%): | |||
| N0* | 417,893 (100.0) | — | 9,606 (51.4) |
| N1 | — | 6,416 (100.0) | 4,256 (22.8) |
| Missing | — | — | 4,829 (25.8) |
| No. M1 stage (%): | |||
| No | 417,893 (100.0) | 6,416 (100.0) | — |
| Yes | — | — | 18,691 (100.0) |
| No. Gleason grade (%): | |||
| 6 or Less* | 201,969 (48.3) | 559 (8.7) | 1,576 (8.4) |
| 7 or Greater | 208,898 (50.0) | 5,705 (88.9) | 13,887 (74.3) |
| Unknown | 7,026 (1.7) | 152 (2.4) | 3,228 (17.3) |
p <0.01.
In contrast, we observed an increase in the incidence of PLNM from 2004 to 2014 from 54.1 to 79.5 per million among all men 50 years old or older, representing a 47% relative increase (IR 1.47, 95% CI 1.33–1.62, p <0.01). When stratified by age, the incidence increased from 60.2 to 80.5 per million men 50 to 74 years old (IR 1.34, 95% CI 1.20–1.49, p <0.01) and from 32.2 to 75.9 per million men 75 years old or older (IR 2.36, 95% CI 1.71–3.26, p <0.01). When stratified by initial therapy, the proportion of men with PLNM increased significantly in those who did and did not undergo prostatectomy, that is from 2.0% to 4.7% and from 0.7% to 1.4%, respectively, from 2004 to 2014 (each trend p <0.05, supplementary table, http://iurology.com/).
As reported previously, the incidence of distant metastasis decreased from 2004 to 2011 but increased afterward in men 50 years old or older.6 This represented a relative decline of 9% from 2004 to 2011 (2011 vs 2004 IR 0.91,95% CI 0.87–0.96, p <0.01) and a 14% relative increase from 2011 to 2014 (2014 vs 2011 IR 1.14, 95% CI 1.08–1.20, p <0.01).6 Temporal variation was more profound in men 75 years old or older. A significant increase in the incidence of distant metastases was found between 2011 and 2012. This corresponded to an increase from 447.3 to 503.3 per million men (IR 1.13, 95% CI 1.02–1.24, p <0.05).
Mortality
We identified 417,893 men (94.3%) with nonmetastatic disease, 6,416 (1.4%) with PLNM and 18,691 (4.2%) with distant metastases. Mean age at diagnosis was 66.0,64.1 and 70.6 years, respectively (p <0.01, table 3). Nonmetastatic disease was more prevalent in Caucasian than in African American men (94.7% vs. 93.3%, p <0.01) while distant metastases were more prevalent in African American men relative to Caucasian men (5.4% vs. 3.8%, p <0.01).
Table 4.
Prostate cancer specific mortality by pelvic lymph node and distant metastases vs nonmetastatic prostate cancer controlling for diagnosis year and age, race/ethnicity, tumor grade and pathology findings (all p <0.01)
| Age | HR (95% CI) |
|---|---|
| All: | |
| None | Referent |
| Pelvic lymph node | 4.5 (4.2—4.9) |
| Distant | 21.9 (21.2—22.7) |
| 50–74: | |
| None | Referent |
| Pelvic lymph node | 5.6 (5.2—6.1) |
| Distant | 35.5 (33.8—37.1) |
| 75 or Greater: | |
| None | Referent |
| Pelvic lymph node | 2.9 (2.4—3.5) |
| Distant | 10.7 (10.1—11.3) |
Median followup was 4.9 (IQR 2.5–7.5), 3.6 (IQR 1.5–6.2) and 1.6 years (IQR 0.8–3.2) for nonmetastatic disease, PLNM and distant metastasis, respectively. On adjusted analyses among men 50 years old or older PLNM was associated with worse PCSM than nonmetastatic disease (HR.5, 95% CI 4.2–4.9, p <0.01, table 4). Distant metastases were associated with worse PCSM compared to nonmetastatic disease (HR 21.9, 95% CI 21.2–22.7, p <0.01). Among men 75 years old or older the HR of PLNM and distant metastasis compared to nonmetastatic disease was 2.9 (95% CI 2.4–3.5, p <0.01) while for PCSM it was 10.7 (95% CI 10.1–11.3, p <0.01). Figure 2 shows survival by stage at diagnosis.
Figure 2.
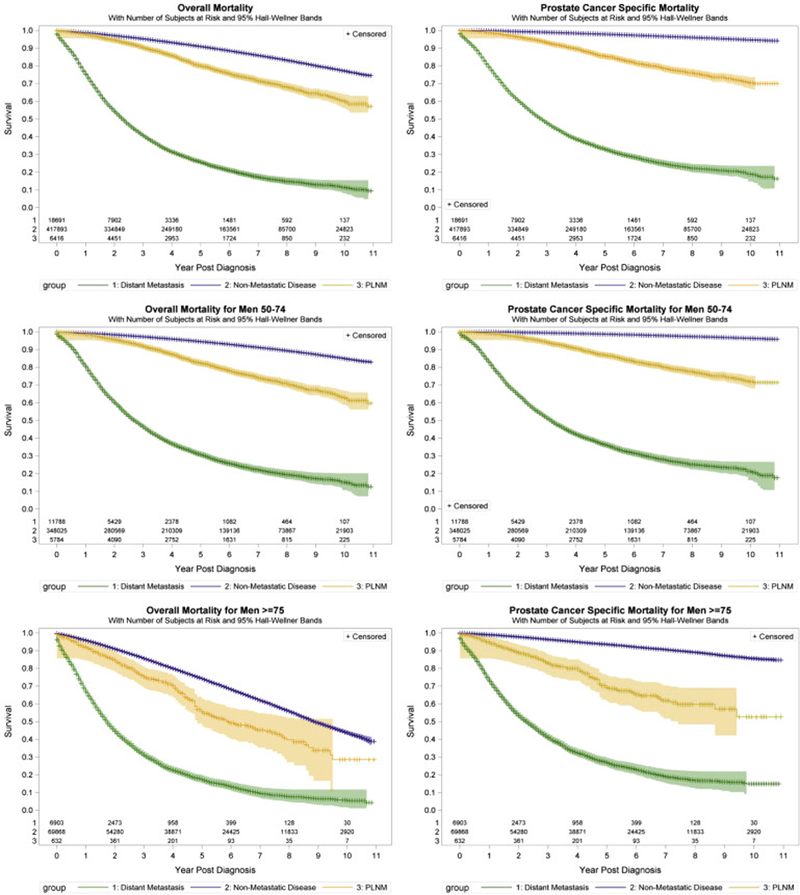
Kaplan-Meier curves of prostate cancer specific mortality in men with nonmetastatic, pelvic lymph node metastatic and distantly metastatic prostate cancer at diagnosis.
DISCUSSION
We report that the incidence of PLNM has been increasing in the last decade, particularly in men 75 years old or older, in whom the incidence has more than doubled. This is notable because of the coincident decrease in prostate cancer screening, detection of localized disease and treatment.7,16,17 Despite an overall decrease in the incidence of prostate cancer the increase in PLNM raises concern for an increase in disease severity at presentation in the current era, which could portend decreased disease specific survival.
These results are more striking when considering 2 concurrent trends which would tend to reduce PLNM detection. 1) Pelvic lymph node dissection during radical prostatectomy has decreased in recent years, coincident with the increased performance of minimally invasive surgical approaches.17 2) This coincides with recommendations against lymph node dissection for low risk features at diagnosis.18 These factors would tend to bias our results toward the null.
Alternatively the increased incidence of PLNM may be secondary to detection bias which is surgery or imaging based. To that end we performed a sensitivity analysis using SEER-Medicare data during the study period and found low and unchanging utilization of positron emission tomography-computerized tomography.19 Pelvic imaging is also under performed in men with high risk disease, likely resulting in underestimation of the true incidence of PLNM.20 Additionally, recent studies demonstrated that the rate of surgery of high risk disease has not increased in men older than 75 years, in whom the increased PLNM incidence is most pronounced.9 Furthermore, our supplemental analysis, which was stratified by initial treatment, demonstrated an increase in PLNM even in men who had not undergone prostatectomy.
According to the classic Halstedian model of cancer progression an increased incidence of lymph node metastases may be the first indication of an increased burden of advanced disease.21 Moreover, this incurable pattern of stepwise cancer spread is thought to be containable by early detection.21 While there may be other contributing factors, the progression of undetected localized disease is a plausible explanation of this finding. However, we note that changes in disease characteristics at diagnosis do not necessarily imply that earlier detection or treatment would be beneficial or could have prevented disease progression. Furthermore, we cannot rule out that other epidemiological trends may have contributed to our findings. For instance, obesity, which increased during the study period in the United States, has been linked to more aggressive prostate cancer.22,23
Consistent with prior studies,24–26 our contemporary assessment of the impact of stage at diagnosis of survival demonstrates that PLNM carries a 4.5-fold higher risk of PCSM compared to nonmetastatic disease. Although the risk of PCSM conferred by PLNM is lower in men who are 75 years old or older, it remains significant and threefold higher than the risk of nonmetastatic disease.
It is important to note that lead time bias may have significantly contributed to these findings, leading to a perceived decrease in survival following diagnosis solely due to men being diagnosed later in the disease process. However, interestingly men with PLNM were younger than those diagnosed with nonmetastatic disease and so they faced reduced overall survival, mirroring prior findings.
Clinically PLNM is a distinct entity with guidelines recommending androgen deprivation therapy based on level 1 evidence and some studies suggesting that these men also benefit from radiotherapy.24,27 In these men androgen deprivation therapy is associated with significant morbidity, including hot flashes, sexual dysfunction, osteoporosis, clinical fractures, cardiovascular events, obesity and insulin resistance.28 Indeed, the morbidity of metastatic disease combined with the prolonged clinical course of prostate cancer has led to the recommendation that metastasis should be used as a new primary end point of prostate cancer clinical trials.29
Our findings must be interpreted in the context of the study design. 1) The increased incidence of PLNM may be related to decreased prostate cancer screening. However, the implications of this association are unclear as an increased incidence of advanced disease at diagnosis would be expected with decreased detection of localized disease. It may not reflect an increased overall burden of advanced disease or imply a benefit to earlier detection. 2) Other concurrent trends in imaging, management and epidemiology may have contributed to our findings, as discussed. 3) We analyzed the most contemporary data to provide a current analysis of temporal variations. This limited our median followup, potentially underestimating the impact of PLNM and distant metastasis on PCSM.
CONCLUSIONS
The incidence of PLNM in the United States is increasing. Given the distinct clinical course and prognosis of PLNM compared to other disease stages, our findings warrant continued monitoring and observation.
Supplementary Material
Table 2.
Standardized incidence ratio stratified by age in consecutive years and compared to 2004
| All Ages 50 or Greater |
Ages 50–74 |
Ages 75 or Greater |
||||
|---|---|---|---|---|---|---|
| Metastasis | Yr to Yr | 2004 | Yr to Yr | 2004 | Yr to Yr | 2004 |
| None: | ||||||
| 2005 | 0.93 (0.92–0.94)* | 0.93 (0.92–0.94)* | 0.93 (0.92–0.95)* | 0.93 (0.92–0.95)* | 0.93 (0.90–0.95)* | 0.93 (0.90–0.95)* |
| 2006 | 1.09 (1.07–1.10)* | 1.01 (1.00–1.02)† | 1.11 (1.09–1.12)* | 1.03 (1.02–1.05)* | 1.04 (1.02–1.07)* | 0.97 (0.94–0.99)* |
| 2007 | 1.03 (1.02–1.05)* | 1.05 (1.04–1.06)* | 1.05 (1.03–1.06)* | 1.08 (1.07–1.10)* | 1.00 (0.97–1.03) | 0.97 (0.94–0.99)† |
| 2008 | 0.91 (0.90–0.92)* | 0.95 (0.94–0.96)* | 0.92 (0.91–0.94)* | 1.00 (0.98–1.01) | 0.87 (0.84–0.89)* | 0.84 (0.82–0.86)* |
| 2009 | 0.97 (0.96–0.98)* | 0.92 (0.91–0.93)* | 0.99 (0.98–1.00) | 0.99 (0.98–1.00) | 0.91 (0.89–0.94)* | 0.77 (0.74–0.79)* |
| 2010 | 0.95 (0.94–0.96)* | 0.87 (0.86–0.88)* | 0.95 (0.93–0.96)* | 0.94 (0.92–0.95)* | 0.95 (0.92–0.98)* | 0.73 (0.71–0.75)* |
| 2011 | 0.99 (0.97–1.00)† | 0.86 (0.85–0.87)* | 0.99 (0.98–1.00) | 0.93 (0.91–0.94)* | 0.97 (0.94–1.00)† | 0.71 (0.69–0.73)* |
| 2012 | 0.80 (0.79–0.81)* | 0.69 (0.68–0.70)* | 0.82 (0.80–0.83)* | 0.76 (0.75–0.77)* | 0.76 (0.74–0.79)* | 0.54 (0.52–0.56)* |
| 2013 | 0.93 (0.92–0.95)* | 0.65 (0.64–0.65)* | 0.93 (0.92–0.95)* | 0.71 (0.70–0.72)* | 0.93 (0.90–0.97)* | 0.50 (0.49–0.52)* |
| 2014 | 0.89 (0.87–0.90)* | 0.57 (0.56–0.58)* | 0.88 (0.87–0.90)* | 0.62 (0.61–0.63)* | 0.90 (0.86–0.93)* | 0.45 (0.44–0.47)* |
| Pelvic lymph node: | ||||||
| 2005 | 0.98 (0.88–1.09) | 0.98 (0.88–1.09) | 0.99 (0.88–1.11) | 0.99 (0.88–1.11) | 0.91 (0.62–1.34) | 0.91 (0.62–1.34) |
| 2006 | 1.01 (0.91–1.13) | 0.99 (0.89–1.10) | 1.00 (0.89–1.12) | 0.99 (0.88–1.11) | 1.11 (0.76–1.64) | 1.01 (0.69–1.48) |
| 2007 | 1.15 (1.04–1.28)* | 1.14 (1.03–1.27)† | 1.11 (1.00–1.25) | 1.10 (0.98–1.23) | 1.40 (0.99–1.99) | 1.42 (0.99–2.02) |
| 2008 | 0.98 (0.88–1.08) | 1.11 (1.00–1.24)† | 0.95 (0.85–1.07) | 1.05 (0.93–1.18) | 1.10 (0.80–1.51) | 1.56 (1.10–2.21)† |
| 2009 | 1.01 (0.91–1.12) | 1.12 (1.01–1.25)† | 1.02 (0.91–1.14) | 1.07 (0.95–1.20) | 0.95 (0.70–1.30) | 1.49 (1.05–2.11)† |
| 2010 | 1.09 (0.99–1.21) | 1.23 (1.11–1.36)* | 1.08 (0.97–1.21) | 1.16 (1.04–1.29)† | 1.15 (0.84–1.56) | 1.70 (1.21–2.40)* |
| 2011 | 0.98 (0.89–1.08) | 1.21 (1.09–1.34)* | 0.94 (0.85–1.05) | 1.09 (0.98–1.23) | 1.15 (0.86–1.52) | 1.95 (1.40–2.72)* |
| 2012 | 1.00 (0.91–1.10) | 1.21 (1.09–1.34)* | 1.02 (0.92–1.14) | 1.12 (1.00–1.25) | 0.91 (0.69–1.21) | 1.78 (1.27–2.50)* |
| 2013 | 1.09 (0.99–1.20) | 1.31 (1.18–1.45)* | 1.07 (0.96–1.19) | 1.20 (1.07–1.34)* | 1.18 (0.89–1.56) | 2.10 (1.51–2.92)* |
| 2014 | 1.12 (1.02–1.23)† | 1.47 (1.33–1.62)* | 1.12 (1.01–1.24)† | 1.34 (1.20–1.49)* | 1.12 (0.87–1.46) | 2.36 (1.71–3.26)* |
| Distant: | ||||||
| 2005 | 0.98 (0.93–1.04) | 0.98 (0.93–1.04) | 1.05 (0.97–1.13) | 1.05 (0.97–1.13) | 0.93 (0.85–1.02) | 0.93 (0.85–1.02) |
| 2006 | 0.97 (0.92–1.03) | 0.96 (0.91–1.01) | 0.95 (0.88–1.02) | 0.99 (0.91–1.07) | 1.00 (0.91–1.10) | 0.93 (0.85–1.03) |
| 2007 | 0.98 (0.93–1.04) | 0.94 (0.89–1.00)† | 0.96 (0.89–1.04) | 0.95 (0.88–1.03) | 1.00 (0.91–1.10) | 0.94 (0.85–1.03) |
| 2008 | 0.97 (0.92–1.02) | 0.91 (0.87–0.97)* | 1.01 (0.93–1.09) | 0.96 (0.89–1.04) | 0.93 (0.85–1.03) | 0.87 (0.79–0.96)* |
| 2009 | 0.99 (0.93–1.04) | 0.90 (0.85–0.95)* | 1.03 (0.95–1.11) | 0.99 (0.91–1.07) | 0.95 (0.85–1.05) | 0.83 (0.75–0.91)* |
| 2010 | 1.02 (0.96–1.07) | 0.92 (0.87–0.97)* | 1.02 (0.95–1.10) | 1.01 (0.93–1.09) | 1.01 (0.91–1.12) | 0.84 (0.76–0.92)* |
| 2011 | 1.00 (0.94–1.05) | 0.91 (0.87–0.96)* | 1.02 (0.94–1.10) | 1.03 (0.95–1.11) | 0.97 (0.88–1.08) | 0.81 (0.74–0.90)* |
| 2012 | 1.07 (1.01–1.13)† | 0.97 (0.92–1.03) | 1.01 (0.94–1.09) | 1.04 (0.96–1.12) | 1.13 (1.02–1.24)† | 0.91 (0.83–1.01) |
| 2013 | 1.05 (0.99–1.10) | 1.02 (0.97–1.07) | 1.05 (0.97–1.13) | 1.09 (1.01–1.18)† | 1.05 (0.95–1.15) | 0.96 (0.87–1.05) |
| 2014 | 1.02 (0.97–1.07) | 1.04 (0.99–1.10) | 1.06 (0.99–1.14) | 1.16 (1.07–1.25)* | 0.98 (0.89–1.08) | 0.93 (0.85–1.03) |
p <0.01.
p <0.05.
Acknowledgments
Supported by The Fredrick J. and Theresa Dow Wallace Fund of the New York Community Trust.
Abbreviations and Acronyms
- PCSM
prostate cancer specific mortality
- PLNM
pelvic lymph node metastases
- SEER
Surveillance, Epidemiology and End Results
Contributor Information
Adrien N. Bernstein, Department of Urology, New York Presbyterian Hospital, Weill Cornell Medical College, New York, New York.
Jonathan E. Shoag, Department of Urology, New York Presbyterian Hospital, Weill Cornell Medical College, New York, New York.
Ron Golan, Department of Urology, New York Presbyterian Hospital, Weill Cornell Medical College, New York, New York.
Joshua A. Halpern, Department of Urology, New York Presbyterian Hospital, Weill Cornell Medical College, New York, New York
Edward M. Schaeffer, Department of Urology, Northwestern University Feinberg School of Medicine, University of Chicago Medicine, Chicago, Illinois
Wei-Chun Hsu, Department of Healthcare Policy and Research, Weill Cornell Medical College, New York, New York.
Paul L. Nguyen, Department of Radiation Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, Massachusetts
Art Sedrakyan, Department of Healthcare Policy and Research, Weill Cornell Medical College, New York, New York.
Ronald C. Chen, Department of Radiation Oncology, University of North Carolina, Chapel Hill, North Carolina
Scott E. Eggener, Division of Urology, University of Chicago Medicine, Chicago, Illinois
Jim C. Hu, Department of Urology, New York Presbyterian Hospital, Weill Cornell Medical College, New York, New York.
REFERENCES
- 1.Ong MS and Mandl KD: Trends in prostate-specific antigen screening and prostate cancer interventions 3 years after the U.S. Preventive Services Task Force recommendation. Ann Intern Med 2017; 166: 451. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ma J, Siegel R et al. : Prostate cancer incidence rates 2 years after the US Preventive Services Task Force recommendations against screening. JAMA Oncol 2016; 2: 1657. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute Surveillance, Epidemiology and End Results Program: Cancer of the Prostate Cancer Stat Facts. Available at https://seer.cancer.gov/statfacts/html/prost.html. Accessed April 19, 2017.
- 4.Lee DJ, Mallin K, Graves AJ et al. : Recent changes in prostate cancer screening practices and epidemiology. J Urol 2017; 198: 1230. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute Surveillance, Epidemiology and End Results Program: SEER Cancer Statistics Review, 2016. Available at http://seer.cancer.gov/csr/1975_2013/. Accessed August 2016.
- 6.Hu JC, Nguyen P, Mao J et al. : Increase in prostate cancer distant metastases at diagnosis in the United States. JAMA Oncol 2017; 3: 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jemal A, Fedewa SA, Ma J et al. : Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA 2015; 314: 2054. [DOI] [PubMed] [Google Scholar]
- 8.Hu JC, Nanus DM and Sedrakyan A: Increase in prostate cancer metastases at radical prostatectomy in the United States. Eur Urol 2017; 71: 147. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR and Carroll PR: Trends in management for patients with localized prostate cancer, 1990–2013. JAMA 2015; 314: 80. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute Surveillance, Epidemiology and End Results Program: Overview of the SEER Program. Available at https://seer.cancer.gov/about/overview.html. Accessed April 5, 2017.
- 11.Vanderbilt University: SAS Macros for Assisting with Survival and Risk Analysis, and Some SAS Procedures Useful for Multivariable Modeling. Available at http://biostat.mc.vanderbilt.edu/wiki/Main/SasMacros. Accessed April 19, 2017.
- 12.Durrleman S and Simon R: Flexible regression models with cubic splines. Stat Med 1989; 8: 551. [DOI] [PubMed] [Google Scholar]
- 13.Tosoian JJ, Chappidi M, Feng Z et al. : Prediction of pathological stage based on clinical stage, serum prostate-specific antigen, and biopsy Gleason score: Partin tables in the contemporary era. BJU Int 2017; 119: 676. [DOI] [PubMed] [Google Scholar]
- 14.Buzzoni C, Auvinen A, Roobol MJ et al. : Metastatic prostate cancer incidence and prostate- specific antigen testing: new insights from the European Randomized Study of Screening for Prostate Cancer. Eur Urol 2015; 68: 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu JC, Prasad SM, Gu X et al. : Determinants of performing radical prostatectomy pelvic lymph node dissection and the number of lymph nodes removed in elderly men. Urology 2011; 77: 402. [DOI] [PubMed] [Google Scholar]
- 16.Shoag J, Halpern JA, Lee DJ et al. : Decline in prostate cancer screening by primary care physicians: an analysis of trends in the use of digital rectal examination and prostate specific antigen testing. J Urol 2016; 196: 1047. [DOI] [PubMed] [Google Scholar]
- 17.Halpern JA, Shoag JE, Artis AS et al. : National trends in prostate biopsy and radical prostatectomy volumes following the US Preventive Services Task Force guidelines against prostate- specific antigen screening. JAMA Surg 2017; 152: 192. [DOI] [PubMed] [Google Scholar]
- 18.Gandaglia G, Trinh QD, Hu JC et al. : The impact of robot-assisted radical prostatectomy on the use and extent of pelvic lymph node dissection in the “post-dissemination” period. Eur J Surg Oncol 2014; 40: 1080. [DOI] [PubMed] [Google Scholar]
- 19.Hu JC, O’Malley P, Chughtai B et al. : Comparative effectiveness of cancer control and survival after robot-assisted versus open radical prostatectomy. J Urol 2017; 197: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad SM, Gu X, Lipsitz SR et al. : Inappropriate utilization of radiographic imaging in men with newly diagnosed prostate cancer in the United States. Cancer 2012; 118: 1260. [DOI] [PubMed] [Google Scholar]
- 21.Welch HG, Gorski DH and Albertsen PC: Trends in metastatic breast and prostate cancer— lessons in cancer dynamics. N Engl J Med 2015; 373: 1685. [DOI] [PubMed] [Google Scholar]
- 22.Freedland SJ, Aronson WJ, Kane CJ et al. : Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol 2004; 22: 446. [DOI] [PubMed] [Google Scholar]
- 23.Discacciati A, Orsini N and Wolk A: Body mass index and incidence of localized and advanced prostate cancer—a dose-response meta-analysis of prospective studies. Ann Oncol 2012; 23: 1665. [DOI] [PubMed] [Google Scholar]
- 24.Messing EM, Manola J, Sarosdy M et al. : Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with nodepositive prostate cancer. N Engl J Med 1999; 341: 1781. [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, Zincke H, Blute ML et al. : Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer 2001; 91: 66. [DOI] [PubMed] [Google Scholar]
- 26.Poleszczuk JT, Johnstone PA and Enderling H: Stratifying prostate cancer patients by relative lymph node involvement: population- and modeling-based study. Cancer Med 2016; 5: 1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James ND, Spears MR, Clarke NW et al. : Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: data from patients in the control arm of the STAMPEDE Trial. JAMA Oncol 2016; 2: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keating NL, Liu PH, O’Malley AJ et al. : Androgendeprivation therapy and diabetes control among diabetic men with prostate cancer. Eur Urol 2014; 65: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Amico AV: Active surveillance versus treatment of prostate cancer: should metastasis be the primary end point? J Clin Oncol 2017; 35: 1638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


