Abstract
Gut microbiota modulates metabolic and immunoregulatory axes and contributes to the pathophysiology of diseases with inflammatory components, such as atherosclerosis, diabetes, and ischemic stroke. Inflammation is emerging as a critical player in the pathophysiology of intracranial aneurysm. Therefore, we hypothesized that the gut microbiota affects aneurysm formation by modulating inflammation. We induced intracranial aneurysms in mice by combining systemic hypertension and a single injection of elastase into the cerebrospinal fluid. Depletion of the gut microbiota was achieved via an oral antibiotic cocktail of vancomycin, metronidazole, ampicillin, and neomycin. Antibiotics were given three weeks before aneurysm induction and either continued until the end of the experiment or stopped one day before aneurysm induction. We also assessed the effects of the gut microbiota depletion on macrophage infiltration and mRNA levels of inflammatory cytokines. Gut microbiota depletion by antibiotics reduced the incidence when antibiotics were started three weeks before aneurysm induction and continued until the end of the experiment (83% vs. 6%, P < 0.001). Even when antibiotics were stopped one day before aneurysm induction, the gut microbiota depletion significantly reduced the incidence of aneurysms (86% vs. 28%, P < 0.05). Both macrophage infiltration and mRNA levels of inflammatory cytokines were reduced with gut microbiota depletion. These findings suggest that the gut microbiota contributes to the pathophysiology of aneurysms by modulating inflammation. Human studies are needed to determine the exact contribution of the gut microbiota to the pathophysiology of aneurysm formation and disease course in humans.
Keywords: stroke, intracranial aneurysm, subarachnoid hemorrhage, intracranial hemorrhage, animal model
Introduction
Gut microbiota is emerging as a critical environmental factor that contributes to human physiology and pathology. 1–4 Recent studies suggest that gut microbiota affects various diseases in which inflammation plays pivotal roles in determining the course and severity of the disease. 4–8 Animal studies suggest that the gut microbiota influences the progression and outcomes of atherosclerotic disease, diabetes, and ischemic stroke, disease processes that involve inflammation. 2, 9–13
Gut microbiota may affect the disease courses through the modulation of metabolic and immunoregulatory axes. 5, 14, 15 Various environmental factors including diet, lifestyle, physical activity, sanitation, alcohol, and smoking determine the composition of the gut microbiota. 3, 16–19 Among those many environmental factors, diet is considered the most significant in determining the diversity and composition of the human gut microbiota. 16, 17, 20, 21
Clinical and animal studies suggest critical roles of inflammation and inflammatory cells in the pathophysiology of intracranial aneurysms. 22–26 By modulating vascular inflammation, the gut microbiota may affect the disease course of intracranial aneurysms. As a first step to study the potential contribution of the gut microbiota to the pathophysiology of intracranial aneurysms, we tested whether the presence of the gut microbiota affects the formation of intracranial aneurysms in mice. We utilized a well-established method of antibiotics-induced depletion of the mice gut microbiota. 6, 27
Materials and methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Experiments were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee. We used 10- to 12-week-old C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine). Intracranial aneurysms were induced by combining induced systemic hypertension and a single injection of elastase (35 milli-units; mU) into the cerebrospinal fluid at the right basal cistern as we previously described. 23, 24, 28, 29 Detailed methods are described in the Online Data Supplement.
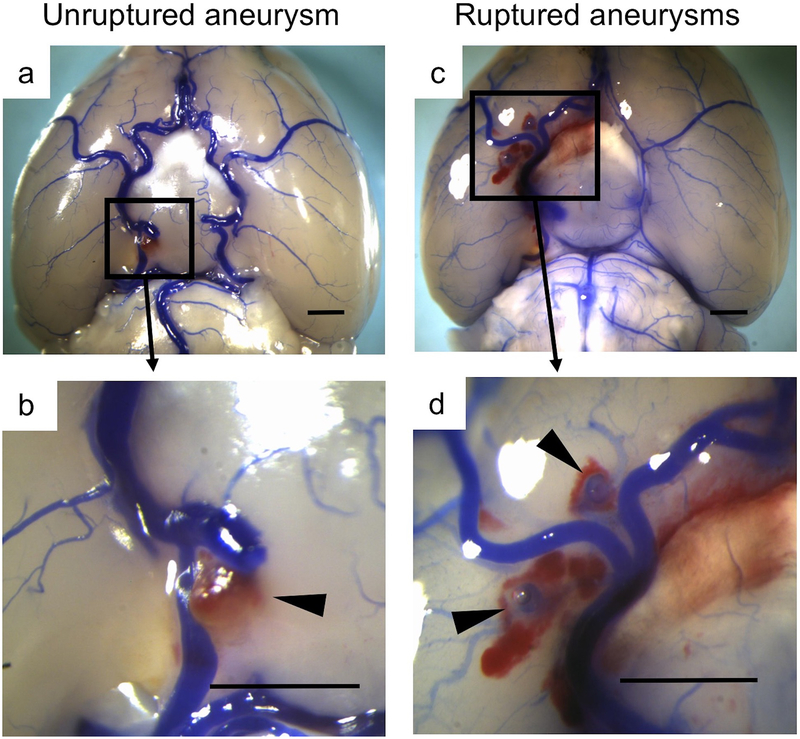
Two blinded observers performed a daily neurological examination. 24 Neurological symptoms were scored as follows: 0, normal function; 1, reduced eating or drinking activity demonstrated by a weight loss >2 g of body weight (10% weight loss) >24 hours; 2, flexion of the torso and forelimbs on lifting the whole animal by the tail; 3, circling to 1 side with a normal posture at rest; 4, leaning to 1 side at rest; and 5, no spontaneous activity. Mice were euthanized three weeks after aneurysm induction or when they developed neurological symptoms associated with aneurysmal subarachnoid hemorrhage (score, 1–5). Two blinded observers assessed the formation of intracranial aneurysms by examining the Circle of Willis and its major branches under a dissecting microscope (10×). Intracranial aneurysms were defined as a localized outward bulging of the vascular wall in the Circle of Willis or its major primary branches, as previously described. 28 Representative aneurysms are shown in Figure 1. We have observed slight dilation of cerebral arteries ipsilateral to the elastase injection, consistent with our previous studies. 23, 28, 30
Figure 1.
Representative aneurysms. Cerebral arteries were visualized by blue dyes dissolved in gelatin. a-b: Unruptured aneurysm in the internal carotid artery. c-d: Ruptured aneurysms in the middle cerebral artery. Bar = 1 mm.
Depletion of the gut microbiota
To deplete the gut microbiota, an antibiotic cocktail composing vancomycin (0.5g/L), metronidazole (1g/L), ampicillin (1g/L) (Sigma-Aldrich Inc.) and neomycin (1g/L) (Life Technologies Corporation) was administered through drinking water. 6, 31, 32 This method has been shown to effectively abolish the intestinal flora in mice. 6, 32 In the first experiment, the antibiotic cocktail or vehicle was started 3 weeks prior to aneurysm induction and continued until the end of the experiment. In the second experiment, the antibiotic cocktail or vehicle was started 3 weeks prior to aneurysm induction and stopped 1 day before aneurysm induction. Fecal samples were tested for aerobic and anaerobic fecal cultures (Charles River Laboratories, Wilmington, MA) every day. In addition, fecal samples were cultured on LB (Luria Broth) agar plate for the rapid detection of bacteria daily. The antibiotic-treated mice whose fecal culture showed a presence of the fecal bacteria before or after aneurysmal induction were excluded from this study.
Histology
For quantification of macrophages infiltration into cerebral arteries, additional 8-10 mice in each group were sacrificed 4 days after the induction of aneurysms. From each mouse, one cross-sectional slice of the middle cerebral artery, immediately distal to the bifurcation from the internal carotid artery, was used for staining with anti-mouse CD68 to detect macrophages. 23 Macrophage infiltration was assessed by counting the number of the positive cells per unit of area (0.01 square of millimeter) as previously described. 33 From 15 - 20 sections of cerebral arteries, a blinded observer randomly chosen two sections. Then, two blinded investigators counted macrophages independently. Average values of measurements from two blinded observers were used for the final analysis.
Real-time PCR analysis
In separate sets of animals, we collected the total RNA samples from cerebral arteries (Circle of Willis including aneurysms) at 14 days after aneurysm induction. We measured mRNA expression levels of inflammation-related cytokines (monocyte chemoattractant protein-1: MCP-1, interleukin-1β: IL-1β, interleukin-6: IL-6, and inducible nitric oxide synthase: iNOS, tumor necrosis factor-α: TNF-α). The whole cerebral arteries, including aneurysms, were isolated and total RNA was extracted using the RNeasy Mini Kit (Qiagen, CA, USA). The total RNAs were transcribed to cDNA using QuantiTect reverse transcription kit (Qiagen). The mRNA expression levels were determined using SYBR Green technology (Applied Biosystems, CA, USA). Quantitative values were obtained from the threshold cycle value (Ct), and the data were analyzed by the 2-⊿⊿CT method. The transcript amount of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was quantified as an internal RNA control.
Statistical analysis
For the primary outcome (incidence of aneurysms), we used Fisher’s exact test. As an exploratory analysis, the survival analysis was performed using the log-rank test. Mice that did not develop aneurysms were excluded from the survival analysis. For systolic blood pressure, cell counts, and mRNA expression, Wilcoxon signed rank test was used. P-values < 0.05 were considered statistically significant. Data are expressed as means ± SD.
Results
Depletion of gut microbiota by antibiotics treatment reduced the formation of intracranial aneurysms.
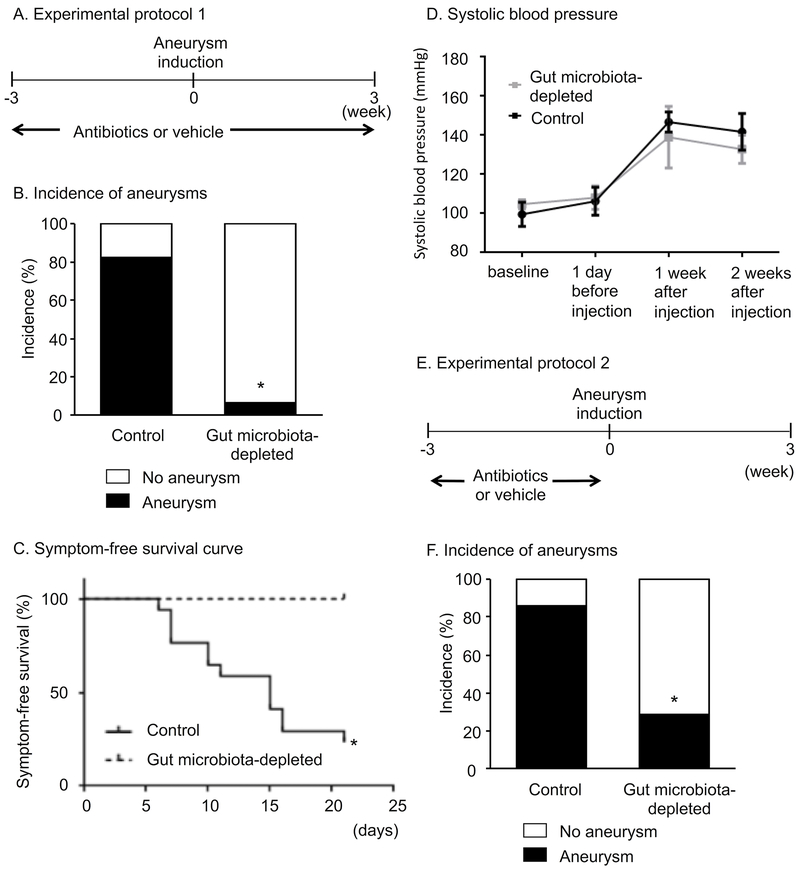
As a first step, we assessed the effects of the gut microbiota depletion on the formation of aneurysms. The antibiotic cocktail or vehicle was started 3 weeks prior to aneurysm induction and continued until 3 weeks after aneurysm induction (Figure 2A) as previously described. 6, 32, 34 As shown in Figure 2B, the depletion of the gut microbiota significantly reduced the incidence of aneurysms (gut microbiota-depleted vs. intact gut microbiota: 6% vs. 83%, P < 0.001). In the intact gut microbiota group, 17 of 19 aneurysms presented as ruptured aneurysms. In the gut microbiota depleted group, there was no ruptured aneurysm. For the purpose of exploratory analysis, a symptom-free curve (Kaplan-Meier analysis curve) was plotted after excluding mice that did not have aneurysms. A log-rank test revealed the higher symptom-free rate in the antibiotics treated group compared to the vehicle-treated groups (Figure 2C) (6% vs. 76% at 21 days, P < 0.001). There was no difference in systolic blood pressure between gut microbiota-depleted mice and control mice (Figure 2D).
Figure 2.
A. Experimental protocol 1. Gut microbiota was depleted by the oral antibiotics treatment that was started 3 weeks before aneurysm induction and continued for 6 weeks. B. Incidence of aneurysms. Depletion of gut microbiota by antibiotics reduced the formation of aneurysms. C. Symptom-free curve (Kaplan-Meier analysis curve). Depletion of gut microbiota by antibiotics improved the symptom-free survival. D. Systolic blood pressure in the mice that induced DOCA-salt hypertension, and the mice that received antibiotics or vehicle. Antibiotics treatment did not affect blood pressure levels in our model. E. Experimental protocol 2. To exclude the possibility of direct effects of antibiotics on aneurysm formation, the antibiotics treatment was started three weeks before aneurysm induction and stopped one day before aneurysm induction. F. Even when the antibiotics treatment was stopped one day before aneurysm induction, the depletion of the gut microbiota resulted in reduced aneurysm formation.
The depletion of gut microbiota by antibiotics reduced aneurysm formations even when the antibiotics treatment was stopped before the induction of aneurysms.
As a next step, to exclude the possibility of direct effects of antibiotics on aneurysm formation, we started the antibiotics cocktail three weeks before aneurysm induction and stopped it one day before aneurysm induction (Figure 2E). Those mice that developed positive fecal culture during the experimental period were excluded from the analysis. As shown in Figure 2F, even when the antibiotics treatment was stopped one day before aneurysm induction, the depletion of the gut microbiota resulted in reduced aneurysm formation (gut microbiota-depleted vs. intact gut microbiota: 28% vs. 86%, P < 0.05).
Effects of the depletion of gut microbiota on the inflammation of cerebral arteries during aneurysm formation.
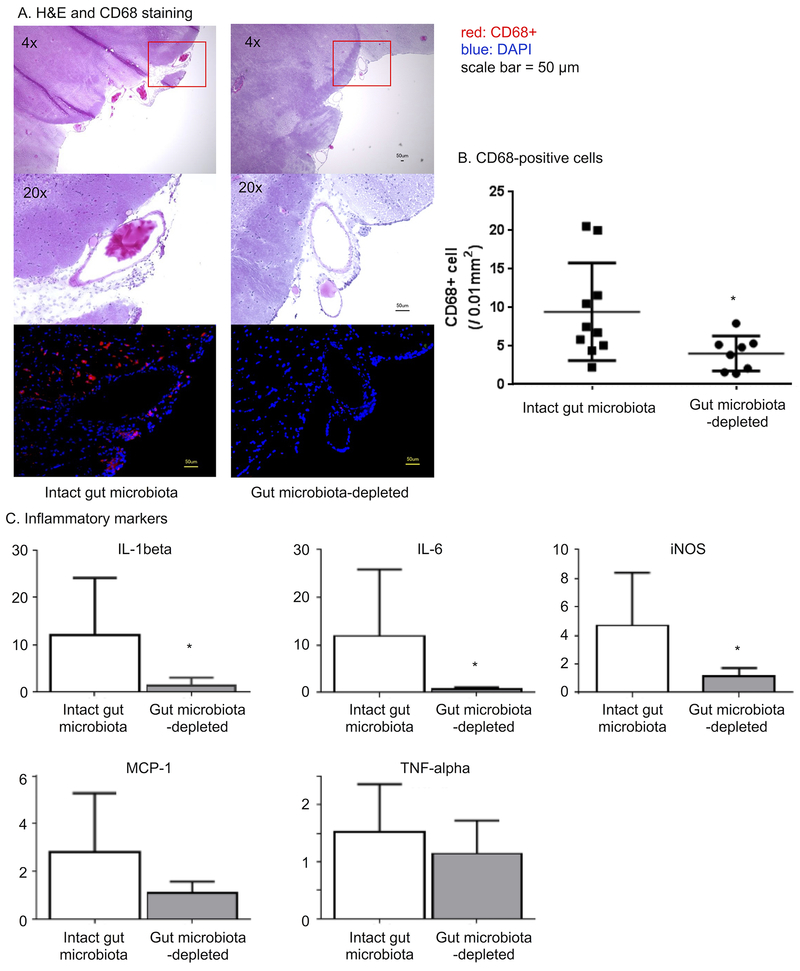
Figure 3A shows representative aneurysms from the gut microbiota-depleted group and the intact gut microbiota group. Hematoxylin-eosin staining revealed the disruption of elastic lamina in both groups, which is a typical histological finding in human intracranial aneurysms. 35, 36 Since both clinical and experimental studies strongly indicated the key roles of macrophages and inflammation in the intracranial aneurysm formation and growth, 23, 26, 37, 38 we assessed macrophage infiltration in aneurysms.
Figure 3.
Effects of the gut microbiota depletion on the inflammation during the formation of aneurysms. A. Hematoxylin-eosin staining and CD68 staining. B. Quantification of macrophage infiltration. Depletion of gut microbiota reduced macrophage infiltration. C. Gut microbiota depletion reduced the mRNA of inflammatory cytokines in the Circle of Willis.
In the intact-gut microbiota group (vehicle group), macrophages (CD68-positive cells) were abundant in the aneurysmal walls but were scarce in the gut microbiota-depleted group (Figure 3A). The quantification of macrophages showed a reduced number of macrophages in the gut microbiota-depleted group compared to the number in intact-gut microbiota group (4.0 ± 2.3 vs. 9.4 ± 6.4 / 0.01 mm2, P < 0.05) (Figure 3B).
We have also assessed mRNA expression of inflammatory cytokines that are mainly produced by macrophages using the real-time PCR (Figure 3C). We found that the depletion of the gut microbiota significantly reduced the mRNA levels of IL (interleukin)-1 beta, IL-6, and iNOS (inducible nitric oxide synthase) (all P < 0.05). There was a trend for MCP-1 (monocyte chemotactic protein-1) to be lower in the gut microbiota-depleted group than in the intact-gut microbiota group (2.8 vs. 1.1, P = 0.06). There was no difference in TNF-αbetween the two groups (TNF-α: 1.5 vs. 1.1, P = 0.5).
Discussion
This proof-of-concept study strongly suggests contributions of the gut microbiota to the pathophysiology of intracranial aneurysms. Our findings showed that gut microbiota can affect the formation of intracranial aneurysms through the modulation of inflammation within the aneurysmal walls in mice. As the initial experiment, we abolished the entire gut microbiota as a simplistic method to test whether the presence or absence of the entire gut microbiota affects the formation of aneurysms. The experiment was not intended to test whether the gut microbiota has beneficial or harmful roles in the context of the pathophysiology of aneurysms. The depletion of the entire gut microbiota represents a highly artificial scenario. While some enteric bacteria may have beneficial roles, others may have harmful roles.
Our findings indicate that the gut microbiota affect the pathophysiology of intracranial aneurysm by modulation of the local inflammation. The transmigration of gut bacteria into the cerebral arteries may represent another potential mechanism. There are reports that suggest a potential link between odontogenic bacteria and the pathophysiology of intracranial aneurysms. 39, 40 In our study, however, we did not detect bacterial DNA in the cerebral arteries in our model; the migration of bacteria into the cerebral artery does not explain our findings. The manipulation of the gut microbiota may affect the induction of aneurysms by affecting blood pressure or the elastase activity in the CSF. However, we did not detect any effects on blood pressure or the elastase activity by the antibiotic treatment.
Environmental factors may play a highly significant role in the pathophysiology of intracranial aneurysms. 41–43 A large twin study that analyzed approximately 80,000 twin pairs revealed that the contribution of environmental factors to the development of aneurysmal rupture was far more important than the contribution of genetic factors. 44 The familial aggregation of aneurysmal subarachnoid rupture may be due to the familial aggregation of environmental factors. 41 The notion that environmental factors play far more important roles in the pathophysiology of intracranial aneurysms than genetic factors has been increasingly recognized. 41, 45 Mechanistically, the gut microbiota may directly affect the pathophysiology of intracranial aneurysms, but clinically, the gut microbiota profile may represent a biomarker that reveals collective effects of environmental factors. While animal studies may provide the proof-of-concept, comparative analysis of human gut microbiota from subjects with and without intracranial aneurysms would be needed to identify the exact contribution of gut microbiota to the pathophysiology of intracranial aneurysms, as the composition of gut microbiota may be fundamentally different between humans and laboratory animals.
There are several limitations of this study. First, our experiments relied on antibiotics treatment for the depletion of gut microbiota. And, we have tested feces only for cultivatable gut microbiota. An experimental approach using the gnotobiotic mice—mice that are raised in germ-free condition— may complement our approach. However, because of the surgical complexities of the mouse model of intracranial aneurysm, the use of gnotobiotic mice is highly challenging at this point. Second, we could not assess the potential roles of gut microbiota to the development of aneurysm rupture, because the significant difference in the formation rate made it difficult to compare the rupture rate. Third, we did not assess the plasma levels of inflammatory mediators or the structural changes in the cerebral arteries in this study. Clearly, further studies are needed to identify the mechanisms by which the gut microbiota modulate the pathophysiology of intracranial aneurysms.
Our animal model may not completely replicate biological events that lead to intracranial aneurysm formation and growth in humans. However, our model recapitulates key features of human intracranial aneurysms, including the association between hypertension and the development of aneurysm rupture and sex dimorphism. 23, 24, 28, 46, 47 Another limitation of this study is that we used relatively young male mice to conduct the initial study. Ultimately, human studies are needed to further confirm the contribution of gut microbiota to the natural course of intracranial aneurysm.
Perspective
This study showed a potential contribution of gut microbiota to the pathophysiology of intracranial aneurysm. As the roles of gut microbiota in human intracranial aneurysms would be undoubtedly complex and, perhaps, context-dependent, this study warrants future human studies to firmly establish the contribution of gut microbiota to the pathophysiology of intracranial aneurysms.
Supplementary Material
Novelty and Significance.
1. What is new?
This study provides a first evidence suggesting the link between the gut microbiota and the pathophysiology of intracranial aneurysm.
2. What is relevant?
This study provides a basis for future human studies to firmly establish the contribution of gut microbiota to the pathophysiology of intracranial aneurysms.
3. Summary
Using a mouse model of intracranial aneurysm, this study showed the potential contribution of gut microbiota to the pathophysiology of intracranial aneurysm. As the roles of gut microbiota in human intracranial aneurysms would be undoubtedly complex and, perhaps, context-dependent, this study warrants future human studies to firmly establish the contribution of gut microbiota to the pathophysiology of intracranial aneurysms.
Acknowledgments
Sources of funding
The project was supported by grant number R01NS055876 (TH) and R01NS082280 (TH) from the National Institute of Neurological Disorders and Stroke (NIH/NINDS), Barrow Neurological Foundation (TH, MTL), and Brain Aneurysm Foundation (KS, DK). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Footnotes
Conflicts of Disclosures
None.
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backhed F Meat-metabolizing bacteria in atherosclerosis. Nat Med. 2013;19:533–534. [DOI] [PubMed] [Google Scholar]
- 3.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes E, Li JV, Marchesi JR, Nicholson JK. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;16:559–564. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Meta HITc, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. [DOI] [PubMed] [Google Scholar]
- 8.Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. [DOI] [PubMed] [Google Scholar]
- 9.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell host & microbe. 2008;3:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. [DOI] [PubMed] [Google Scholar]
- 12.Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, Dames C, Kershaw O, Gruber AD, Curato C, Oyama N, Meisel C, Meisel A, Dirnagl U. Depletion of Cultivatable Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Worsens Outcome After Murine Stroke. Stroke. 2016;47:1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. 2016;22:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malkki H Neurodevelopmental disorders: human gut microbiota alleviate behavioural symptoms in a mouse model of autism spectrum disorder. Nature reviews. Neurology. 2014;10:60. [DOI] [PubMed] [Google Scholar]
- 15.Ridler C Gut microbiota: Gut bacteria affect post-ischaemic inflammation in stroke by modulating intestinal T cells. Nat Rev Gastroenterol Hepatol. 2016;13:250. [DOI] [PubMed] [Google Scholar]
- 16.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin JL, Hedin CR, Koutsoumpas A, Ng SC, McCarthy NE, Prescott NJ, Pessoa-Lopes P, Mathew CG, Sanderson J, Hart AL, Kamm MA, Knight SC, Forbes A, Stagg AJ, Lindsay JO, Whelan K. Smokers with active Crohn’s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm Bowel Dis. 2012;18:1092–1100. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Fouts DE, Starkel P, Hartmann P, Chen P, Llorente C, DePew J, Moncera K, Ho SB, Brenner DA, Hooper LV, Schnabl B. Intestinal REG3 Lectins Protect against Alcoholic Steatohepatitis by Reducing Mucosa-Associated Microbiota and Preventing Bacterial Translocation. Cell host & microbe. 2016;19:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frosen J, Tulamo R, Paetau A, Laaksamo E, Korja M, Laakso A, Niemela M, Hernesniemi J. Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol. 2012;123:773–786. [DOI] [PubMed] [Google Scholar]
- 23.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y, Hashimoto T. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, Kanematsu Y, Kurihara C, Palova E, Kanematsu M, Kitazato K, Hashimoto T. Pharmacological stabilization of intracranial aneurysms in mice: a feasibility study. Stroke. 2012;43:2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gounis MJ, Vedantham S, Weaver JP, Puri AS, Brooks CS, Wakhloo AK, Bogdanov AA. Myeloperoxidase in human intracranial aneurysms: preliminary evidence. Stroke. 2014;45:1474–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasan DM, Mahaney KB, Brown RD, Meissner I, Piepgras DG, Huston J, Capuano AW, Torner JC, International Study of Unruptured Intracranial Aneurysms I. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rak K, Rader DJ. Cardiovascular disease: the diet-microbe morbid union. Nature. 2011;472:40–41. [DOI] [PubMed] [Google Scholar]
- 28.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamio Y, Miyamoto T, Kimura T, Mitsui K, Furukawa H, Zhang D, Yokosuka K, Korai M, Kudo D, Lukas RJ, Lawton MT, Hashimoto T. Roles of Nicotine in the Development of Intracranial Aneurysm Rupture. Stroke. 2018;49:2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tada Y, Kanematsu Y, Kanematsu M, Nuki Y, Liang EI, Wada K, Makino H, Hashimoto T. A mouse model of intracranial aneurysm: technical considerations. Acta Neurochir Suppl. 2011;111:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. [DOI] [PubMed] [Google Scholar]
- 32.Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Chopp M, Zhang RL, Goussev A. A mouse model of embolic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:1081–1088. [DOI] [PubMed] [Google Scholar]
- 34.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. [DOI] [PubMed] [Google Scholar]
- 35.Cajander S, Hassler O. Enzymatic destruction of the elastic lamella at the mouth of cerebral berry aneurysm? An ultrastructural study with special regard to the elastic tissue. Acta Neurol Scand. 1976;53:171–181. [DOI] [PubMed] [Google Scholar]
- 36.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. [DOI] [PubMed] [Google Scholar]
- 37.Kim SC, Singh M, Huang J, Prestigiacomo CJ, Winfree CJ, Solomon RA, Connolly ES. Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery. 1997;41:642–666; discussion 646–647. [DOI] [PubMed] [Google Scholar]
- 38.Frosen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, Jaaskelainen J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293. [DOI] [PubMed] [Google Scholar]
- 39.Pyysalo MJ, Pyysalo LM, Pessi T, Karhunen PJ, Lehtimaki T, Oksala N, Ohman JE. Bacterial DNA findings in ruptured and unruptured intracranial aneurysms. Acta Odontol Scand. 2016;74:315–320. [DOI] [PubMed] [Google Scholar]
- 40.Inenaga C, Hokamura K, Nakano K, Nomura R, Naka S, Ohashi T, Ooshima T, Kuriyama N, Hamasaki T, Wada K, Umemura K, Tanaka T. A Potential New Risk Factor for Stroke: Streptococcus Mutans With Collagen-Binding Protein. World Neurosurg. 2018;113:e77–e81. [DOI] [PubMed] [Google Scholar]
- 41.Juvela S Risk factors for aneurysmal subarachnoid hemorrhage. Stroke. 2002;33:2152–2153; author reply 2152–2153. [DOI] [PubMed] [Google Scholar]
- 42.Akiyama K, Narita A, Nakaoka H, Cui T, Takahashi T, Yasuno K, Tajima A, Krischek B, Yamamoto K, Kasuya H, Hata A, Inoue I. Genome-wide association study to identify genetic variants present in Japanese patients harboring intracranial aneurysms. J Hum Genet. 2010;55:656–661. [DOI] [PubMed] [Google Scholar]
- 43.Fukuhara T Geographical analysis of aneurysmal subarachnoid hemorrhage in Japan utilizing publically-accessible DPC database. PLoS One. 2015;10:e0122467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korja M, Silventoinen K, McCarron P, Zdravkovic S, Skytthe A, Haapanen A, de Faire U, Pedersen NL, Christensen K, Koskenvuo M, Kaprio J, Genom EP. Genetic epidemiology of spontaneous subarachnoid hemorrhage: Nordic Twin Study. Stroke. 2010;41:2458–2462. [DOI] [PubMed] [Google Scholar]
- 45.Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10:626–636. [DOI] [PubMed] [Google Scholar]
- 46.Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Shikata F, Pena Silva RA, Kitazato KT, Hasan DM, Kanematsu Y, Nagahiro S, Hashimoto T. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension. 2014;63:1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tada Y, Makino H, Furukawa H, Shimada K, Wada K, Liang EI, Murakami S, Kudo M, Kung DK, Hasan DM, Kitazato KT, Nagahiro S, Lawton MT, Hashimoto T. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery. 2014;75:690–695; discussion 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.