Abstract
In recent years there has been a growing interest in the interactions of fluorophores with metallic nanostructures or nanoparticles. The spectra properties of fluorophores can be dramatically modified by near-field interactions with the electron clouds present in metals. Near-field interactions are those occurring within a wavelength distance of an excited fluorophore. These interactions modify the emission in ways not seen in ensemble fluorescence experiments. In this review we provide an insightful description of the photophysics of metal plasmons and near-field interactions. Additionally, we summarize recent works on single-molecule studies on metal-fluorophore interactions and suggest how these effects will result in new classes of experimental procedures, novel probes, bioassays and devices.
The spectral properties of single fluorophores can be dramatically altered by near-field interactions with the electron clouds presented in metals.
Keywords: Single molecule, metal nanostructures, plasmon, fluorescence
1. Introduction
There is a long history of using fluorescence spectroscopy to study fluorophores, fluorophore-labeled biomolecules and diagnostic protein antigens. Although fluorescence is a highly sensitive technique, detection of single molecules represents the ultimate level of sensitivity and has been a longstanding goal of analytical methods. The detection of a single fluorophore is usually limited by its quantum yield, background signals from the sample. The observation of single molecules is also limited by their photostability and brightness. The observation times for single fluorophores are typically several seconds, after which irreversible photobleaching occurs. The ability to observe single molecules over the background is determined by the number of photons emitted per second. In single-molecule detection background fluorescence can easily obscure the signal from single fluorophores. This is the why single-molecule fluorescence experiments are usually performed on organic fluorophores with high extinction coefficients, high quantum yields and short lifetimes. However, the fluorescence emission of a single molecule can be modified by appropriately tailoring its external electromagnetic environment, providing new opportunities for single-molecule detection. The environment can affect the fluorescence emission in various ways by locally increasing the emitter's radiative decay rate and by modifying its spontaneous emission into a directional emission to enhance the detection efficiency. Metallic surfaces have shown important application in biotechnology due to their light-localization properties, leading to strong absorption features and local field enhancement [1–3]. A moderately extensive physics literature exists on noble-metal nanostructures [4–10]. The most established application to explore these properties has been surface-enhanced Raman scattering (SERS) [11–15], which uses the evanescent field at the surface of nanostructured materials to greatly amplify the weak, but molecule-specific Raman signals. SERS has been used for a wide range of applications in physics, chemistry, and biology. However, SERS is not the only application that has been found. Metal-enhanced fluorescence (MEF) [16–20] or plasmon-controlled fluorescence (PCF) [3] have been reported for noncontinuous noble metallic surfaces. It would be useful to obtain MEF at desired locations in the measurement device (i.e. MEF on demand). The use of fluorophore-metal interactions has the potential to dramatically increase the detectability of single fluorophores for single-molecule detection. Yokota et al. [21] report the first successful detection of single tetramethylrhodamine and Cy5 molecules attached to protein molecules on metal surfaces. The advances in nanotechnologies over the past few years have made the manipulation of the nanometer-scale processes more accessible and desirable. It is highly interesting to investigate the use of metallic nanostructures to favorably modify the spectral properties of fluorophores and to alleviate some of these fluorophore photophysical limitations. For instance, recent studies involve metallic colloid nanoparticles [22–24], core-shell particles [25], noncontinuous silver films [26–28], continuous thin films [29], nanoantennas [30], subwave-length nanoarrays [31], and single nanoapertures [32,33]. Determining the influence of these processes is a crucial issue to characterize nanodevices for enhanced fluorescence, which has been a topic of great interest in recent years. These photophysical behaviors would be further explored and to be used to create the next generation of probes, which could be fluorophores in nanoshells, fluorophores bound to colloids, or fluorophores trapped in confined volumes. The effects may also be used to increase brightness and photostabiltiy of single molecules bound to surfaces that contain metallic structures, for either biophysical studies or high sensitivity assays.
2. Principles of surface plasmons and fluorescence
The first studies of fluorophore-metal interactions appeared in the 1970s [34, 35]. These papers showed that a fluorophore placed within distances of several wavelengths from a reflecting metallic surface, in this case a thick continuous silver film (≥100 nm), resulted in oscillations of the emissive lifetime with distance from the metal surface. This effect could be explained by the reflected field from the fluorophore back on itself, which depends on the distance from the metal surface. When the reflected field amplitude at the fluorophore was increased the lifetime decreased. When the reflected field opposed the fluorophores field the lifetime increased. The data agreed with this reflective model at wavelength distances, but not when the fluorophores was close to the metal. At distances less than 20 nm the lifetimes dropped dramatically and the emission was strongly quenched. This quenching effect was attributed to loss surface waves (LSWs), dissipated losses and similar terms, all of which implied the nonradiative dissipation of energy within the metal. These early reports [34,35] resulted in a number of theoretical and experimental studies of oscillating dipoles with metallic surfaces and particles [1,2,36–39].
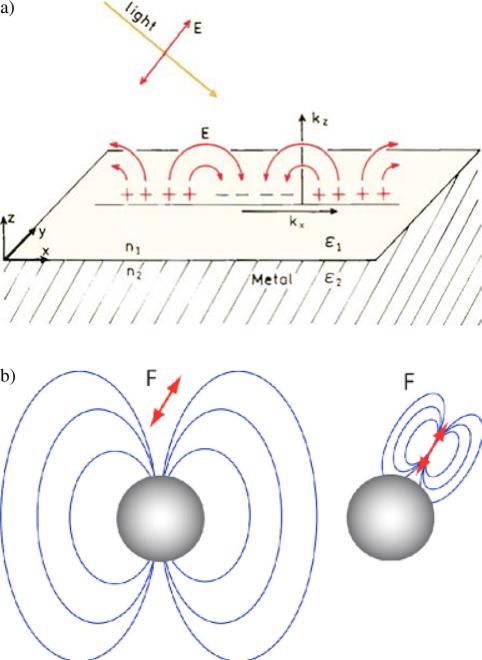
The interaction of fluorophores with metals must depend on the optical properties of both the metal and the fluorophores. The optical properties of metallic surfaces and surfaces plasmons are complex. The term surface plasmons is now commonly used to describe both the spatially limited motion of electrons in metal particles and the motions of electrons on a continuous surface. Plasmons cannot be created when a metallic surface or mirrors are illuminated directly from the air. These surfaces reflect the energy and do not display the plasmon absorption. Creation of surface plasmons requires illumination of a thin metal film through a glass prism or some higher dielectric constant material. The metal film must be thin so that the field penetrates from the prism side to the distal side of the metal. In contrast to a continuous metal surface, metallic colloids interact strongly with light [5]. Localized surface plasmons can be created by direct illumination of metal colloids resulting in rapid oscillation of the spatially bound electrons [40] (Fig. 1a). The metal plasmon resonance can be described as arising from the collective oscillations of a group of electrons that migrate freely on the surface of metal particle. In order to obtain resonance, the in-plane (xy plane) wave vector (kX) of the incident light must match the surface plasmon wave vector.
Figure 1.
(online color at: www.lpr-journal.org) (a) Schematic of surface plasmons on a metal surface; (b) Schematic of plasmonic oscillation from a metal sphere.
The interpretation of radiating and nonradiating plasmons would provide an intuitive model for metal-enhanced fluorescence. Suppose an excited fluorophore is near to 50 nm or distant from (> 500 nm) the metal. The fluorophores at large distance from the surface can only interact with the metal by the far-field radiation. The charge distributions induced on the metal surface will be the same for the incident light. Thus, the field is reflected, resulting in lifetimes that oscillate as a function of distance from the metal [34,35]. If the fluorophore is close to the metal, the interactions are via the near field, and the charges are more closely spaced. These plasmons cannot radiate into free space and the emission is to be quenched. Justification for the radiating plasmon model can be found in electrodynamic theory [41,42]. Considering an oscillating dipole (μ) above a metallic surface, the ratio of the decay rates in the absence (b0 = 1/τ0) and presence (b = 1/τ) of metal is given by
| (1) |
where q is the quantum yield, n1 is the refractive index surrounding the dipole, k1 is the wavevector for frequency ω in medium 1 surrounding the fluorophore and Im(ẼR) is the refractive field at the dipole. Eq. (1) can be used to calculate the relative decay rate for dipoles parallel (∥) or perpendicular (−) to the metal surface. For a fluorophore in front of the metal the total decay rate is given by b = b− +2b∥. Classical electrodynamic theory has been used to calculate the radiative decay rate of oscillating dipoles near metallic spheres and ellipsoids [36,43,44]. Depending on the distance from the surface and dipole orientation the decay rates can be increased or decreased. Overall, the decay rates are increased and the effect can be dramatic, up to 100 000-fold. Such an increase in the radiative decay rate should result in quantum yields near unity and very short lifetimes. Additionally, the shorter lifetimes can improve photostability because the molecules spend less time in the excite state per excitation cycle. As a result, the fluorophores will be less prone to optical saturation and can emit more photons per second than an equivalent fluorophore with a longer lifetime.
Mie theory is an alternative tool to describe the interaction of fluorophore with metal particle [4,5,45]. According to Mie theory, the extinction coefficient of metal particles is due to both light absorption and light scattering. The extinction, scattering and absorption cross sections of metal nanoparticles when excited by electromagnetic radiation can be calculated by series expansions of the involved fields into partial waves of different spherical symmetries. The absorption component CA represents the cross section due to absorption. The scattering component CS represents the ability of a metal particle to be an oscillating dipole that radiates light and propagates to far-field. The extinction cross section CE is given by CE = CA + CS. To a first approximation, these spectra can be used to predict the effect of a nearby particle on a fluorophore. If a fluorophore interacts with the absorption component CA, it will result in the metal plasmons. The scattering component CS allows the fluorophore-induced plasmons to radiate to far-field showing as fluorescence (Fig. 1b). The lifetime of a fluorophore-metal particle complex is decreased because of a high extinction coefficient. The extinction properties of metal particles with the subwavelength sizes can be expressed by a combination of both absorption (CA) and scattering (CS) factors,
| (2) |
where k1 is the wavevector of the incident light in medium 1 and α is the polarizability of the sphere of radius r,
| (3) |
and εm is the complex dielectric constant of metal. The first term represents the cross section due to absorption and the second term represents the cross section due to scattering. It is expected CA to cause quenching and CS to cause enhancement. The quenching term increases as the r3 factor, and the enhancement term increases as the r6 factor. Hence, a small particle can quench fluorescence because the absorption dominates over the scattering. However, Mie theory is limited to spheres, and the extensions to shells and a few other structures are less frequently used [46].
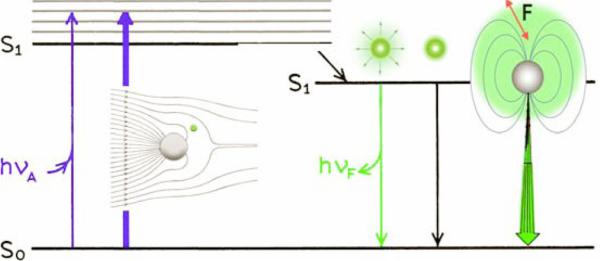
Compared with the spontaneous isotropic emission of the excited fluorophore, the photophysical properties of fluorophore near the metal surface can be interpreted by a modified Jablonski diagram [47] (Fig. 2). The fluorophore-induced plasmons can radiate to the far-field and create observable emission. The usefulness of an increase in the radiative decay rate can be understood by considering the definitions of the quantum yield (Q0) and the fluorescence lifetimes (τ0). The fluorescence lifetime and quantum yield (Q0) of a fluorophore in the free-space emission can be given by
| (4) |
| (5) |
In traditional fluorescence experiments, the change in quantum yield or lifetime is due to the change in the nonradiative decay rate (knr), which results from the change in environment of a fluorophore, quenching, or FRET. The values of Q0 and τ0 can both increase or both decrease, but do not change in the opposite direction. Unique spectral changes are possible for fluorophores near metal particles or surfaces. Suppose this radiative decay rate near the metal is increased and is given by Γ +Γm, when Γm is the additional rate due to the metal substrate, the quantum yield Qm and lifetime Γm of the fluorophores near the metal substrate thus become
| (6) |
| (7) |
The metal-induced change to Γ + Γm results in unusual effects, the quantum yield increases and the lifetime decreases, which is different from the change in knr where both Q and Γ change in the same direction. A decrease in lifetime has several favorable consequences. Fluorophores can become more photostable because they spend less time in the excited state where the photochemistry occurs. Hence, a fluorophore can undergo more excitation and emission cycles prior to photobleaching. The maximum emission rate of a fluorophore is approximately equal to the inverse of the lifetime. Hence, a fluorophore with a shorter lifetime will be less prone to optical saturation and have higher maximum emission rates. These changes result in an increasing detectability of single or multiple fluorophores.
Figure 2.
(online color at: www.lpr-journal.org) Modified Jablonski diagram that includes metal-fluorophore interactions. The thicker arrows represent increased rates of excitation and emission.
3. Single-molecule approaches on fluorophore-metal interactions
Early experimental efforts on fluorophore-metal interaction used ensembles of molecules and nanoparticles and revealed ensemble-averaged data. The main obstacle is that the spectral properties of a fluorophore are very sensitive to its surrounding nanoenvironments such as the shape, size, and material properties of the nanostructures. Distance to the metal surface and the dipole orientation also play a role when fluorophores are near structured surfaces. For planar metallic structures the fluorophores can be at various distances and various orientations relative to the surface. For noncontinuous thin films (i.e. silver island films) there exist a range of particles sizes and shapes, and the fluorophores may be localized on the metal or on the glass. Metal colloids in suspension can have a distribution of sizes, which can also contribute to sample heterogeneity. The inherent inhomogeneity in such experiments overwhelmed clear comparisons with the theoretical predictions. Single-molecule studies thus become valuable for studies of fluorophore-metal interactions. Optical spectroscopy of single molecules in condensed phases provides a tool to sense local nanoenvironments that complements and extends conventional bulk ensemble-averaged measurements. The distribution of environments can be determined by investigating a number of individual molecules. Since typical single-molecule fluorophores are on the order of a few nm in size, detecting the state of the single molecule allows exploration of several features of the interaction of the molecule with its immediate neighbors in the material or biomolecule. Additional information is obtained because single-molecule measurements reveal the real-time behavior of single molecules, rather than the time and ensemble average. For instance, single-molecule detection (SMD) can be used to determine if a particular protein folds by a continuous or one-step mechanism. There is a great deal to be learned from single-molecule or few-molecule spectroscopy (i.e. fluorescence correlation spectroscopy, FCS), for fluorophores near metal particles. In some cases it may be preferable to resolve the underlying heterogeneity using SMD or FCS, rather than trying to prepare a chemically homogeneous sample. Additionally, it is vital to synthesize metal-fluorophore structures with precisely defined spatial parameters, and a large number of such structures would need to be fabricated to identify the preferred structure for brightness, photostability, or other desired properties. Single-molecule experiments can show what is possible in the best structure by finding several of these fluorophores among the observed population.
Advances in nano-optics have brought about various new tools for studying single molecules and single nanoparticles both spatially and spectrally. These developments motivated the investigation of the Raman enhancement at the level of a single molecule and a single nanoparticle, clusters, and aggregates. In the past few years, using simple nanostructures above have also been performed in modification of the spontaneous emission into a controlled way in nano-optics. Metallic tips or particles are expected to enhance the local field at the molecule location. This results in an increase of the exciting intensity and a modification of the spontaneous emission rate. Some of these investigations are described below.
4. Single organic fluorophores near various metallic nanostructures
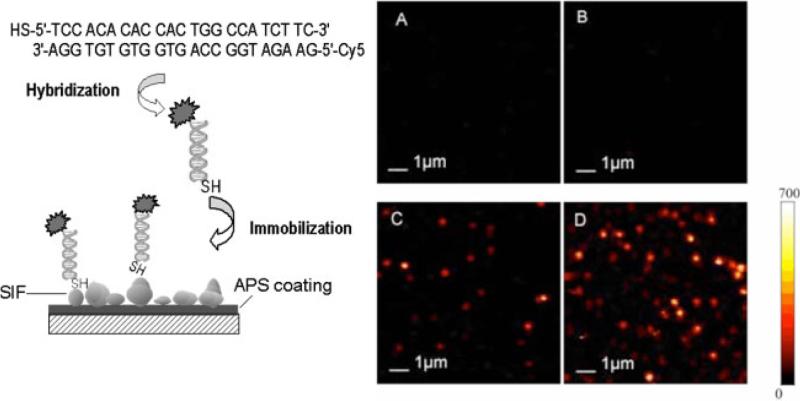
A widely used surface immobilization technique is the self-assembly of monolayers of thiol molecules by covalent bonding of their sulfur groups on metals such as gold or silver. Recent studies on MEF have reported the use of a relatively facile deposition of metal nanoparticles onto glass slides (i.e. silver island films, SIFs [19, 20]). In addition to varying particle shapes, different techniques for silver deposition, such as wet chemical deposition and electroplating of silver and fractal-like silver nanostructures have also been deposited onto glass substrates onto electrodes [48] and/or glass slides [49, 50], have also been employed to obtain localized enhancement of fluorescence. Surface-feature studies have revealed that the SIFs are 30–80 nm in size with approximately 40% surface coverage. The deposited SIFs display plasmon absorption maxima near 430 nm. All of these recent silver nanostructure preparations have resulted in attractive silvered surfaces for MEF applications. Single dye-labeled thiolated DNA molecules tethered to SIFs are brighter than their free counterparts as observed [27]. Time profiles also indicate higher photostability in these tethered samples (Fig. 3). Most of the photons detected on bare glass coverslips range from 104 to 105 photons. By contrast, measured counts for single molecules on silvered surface are in the range of a few 106, and these values show that dye molecules tethered to SIF are very efficient emitters. The broad distribution of photon counts is in agreement with a larger heterogeneity of the geometric shapes of a SIF nanostructure, various locations of the molecule relative to the particle and the random orientation of the probe molecules. In addition to stable fluorescing emitters, a rather weak fluorescence such as light-harvesting complexes deposited on SIF can be enhanced up to 18-fold due to plasmonic interactions [28].
Figure 3.
(online color at: www.lpr-journal.org) The Cy5-labeled oligonucleotide is hybridized to the complementary thiolated oligonucleotide. After incubation, labeled DNA molecules are tethered to silver island nanostructures on an APS-treated glass substrate. A) Cy5-labeled dsDNA molecules spin-dispersed on a glass coverslip; B) Silver island film deposited on a glass coverslip; C) Cy5-labeled dsDNA-SH molecules tethered to silver island film deposited on a glass coverslip; D) Cy5-labeled dsDNA-SH molecules tethered to silver island film deposited on a glass coverslip. From [27].
The single-molecule spectroscopic method has also been employed to study the distance effect of MEF near silver nanostructures [26]. Dye-labeled oligomers were attached to an avidin layer and were inferred from the surface properties of the protein monolayer. The controlled pattern of ordered macromolecules has the potential application in the field of biotechnology. Single-molecule spectroscopic methods assure detailed new information is provided on the emission of a single fluorophore and its interaction with interfaces. Intensity and lifetime distributions reveal a mixture of spectral properties of dye-labeled oligonucleotides near silver nanostructures. It is believed that these are caused by the sample heterogeneity. The immobilized dye probes are located randomly and distantly near metallic surface, which could lead to such variations. By the use of protein layers, it is found that the maximum increase (~ 100-fold) in intensity and maximum decrease in lifetime from a single BSA-avidin layer that positions the fluorophore about 10 nm from the surface. Hence, the optimal distance for MEF can be readily obtained using a protein monolayer. More uniform intensity and lifetime distributions are observed with multiple protein layers on silvered surfaces. The nanometer changes in distance can be used to adjust emission properties of the fluorophore.
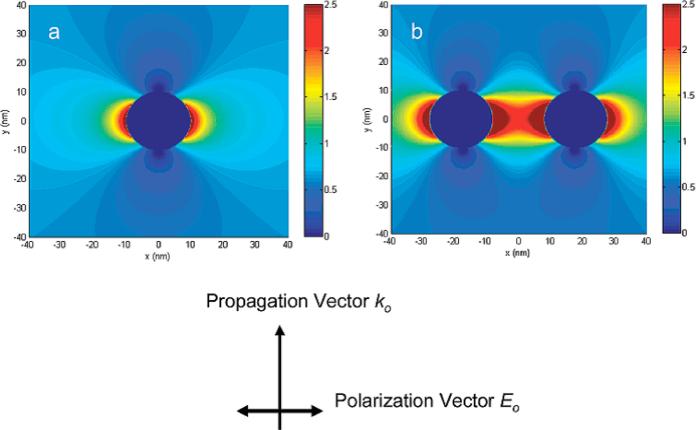
In contrast to SIFs, the preparation of silver colloid suspensions yields spherical particles of homogeneous size. The preparation of water soluble metal nanoparticles is well established [51–54]. Colloids are prepared as suspensions by citrate reduction of silver or gold ions. One interesting application of metal nanoparticles is their use in combination with conjugated dye molecules. Such hybrid systems are attractive because they can be used as sensitive imaging probes in biological studies. A recent study demonstrated the enhanced fluorescence and shortened lifetime of a fluorophore attached a metal nanoparticle with an approximate distance of 8 nm [22], the bound fluorophore was spatially separated from the metal core by the hybridized DNA duplex chain to control the distance between the particle and the probe. The work suggests potential of increasing detectability of single molecules coupling to metal nanoparticles. Similarly, another publication by this group reveals the coupling effect of single fluorophore localized between silver dimer nanoparticle and fluorophore [55]. In this case, the dimers were formed by hybridization with double-length single-stranded oligonucleotides that contained single Cy5 molecules. The study revealed that the single-molecule fluorescence was enhanced 7-fold on the metal monomer and 13-fold on the metal dimer relative to the free Cy5-labeled oligonucleotide in the absence of metal. The lifetimes were shortened on the silver monomers and further shortened on the silver dimers, demonstrating the near-field interaction mechanism of fluorophore with the metal substrate. FDTD calculations were employed to study the distribution of the electric field near the metal monomer and dimer (Fig. 4). The finite-difference time-domain (FDTD) technique is an implementation of Maxwell's time-dependent curl equations for solving the temporal variation of electromagnetic waves within a finite space that contains a target of arbitrary shape and has recently become the state-of- the-art method for solving Maxwell's equations for complex geometries [56]. Herein, the distribution of electric field near the metal monomer and dimer can be estimated to explain single-molecule MEF of these metal particles. The correlation between the emission enhancement and the field density indicates that the metal particle affects the intrinsic decay rate of fluorophore. The near-field interaction between the fluorophore and metal particle is reflected by a change of lifetime. The results revealed that the lifetimes of all samples with the metal were shortened significantly relative to that of free Cy5 in the absence of metal, indicating the near-field interactions for all metal particles. The lifetimes of fluorophore on the smallest silver particle (5 nm) and largest silver particle (100 nm) were obviously longer than those on the intermediate size of metal particles such as 20, 50, and 70 nm, supporting theoretical calculations and experimental results that the fluorophore could interact most efficiently with the intermediate size metal particles at an optimized distance from the metal core.
Figure 4.
(online color at: www.lpr-journal.org) Electric fields near silver (a) monomer and (b) dimer were calculated by FDTD model under an incident light of 635 nm. Note the incident light is propagating along the y-axis and is polarized along the x-axis. From [55].
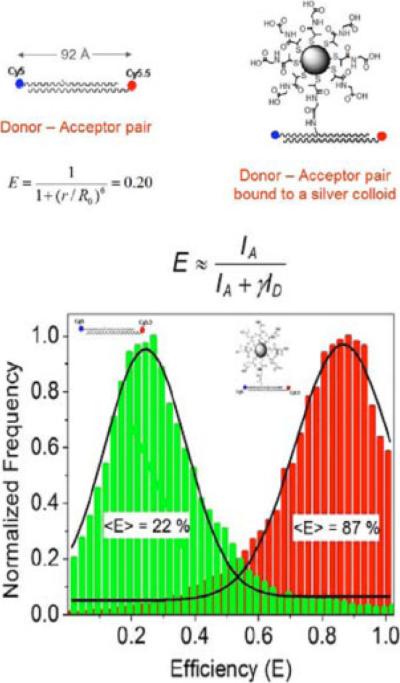
Beside the fluorescence intensity, it is found that the Förster resonance energy transfer (FRET) could be enhanced significantly when the donor-acceptor pair was placed adjacent to the metal particle [57,58] (Fig. 5). This result could be utilized as a ruler to measure the energy-transfer distance. In the experiment, a donor-labeled DNA was chemically bound to a single 20-nm silver particle and then an acceptor-labeled DNA was coupled by hybridization. Both the emission intensity and lifetime obtained from the bulk emission measurement and single-molecule fluorescence measurement indicated that FRET was enhanced on the metal particle, which resulted in increasing the efficiency and lengthening the distance of energy transfer to 1.4 times. The result is consistent with the FDTD simulation. The enhanced rate constant for FRET was 21 times faster than the free donor-acceptor pair in solution. The metal-enhanced FRET was also found to depend on the size of the metal core. The Förster distance was extended to 1.4 times for the donor-acceptor pair was bound on 20-nm silver particles, 1.6 times on 50-nm silver particles, and 1.7 times on 100-nm silver particles. The enhancement of FRET was dependent on the distance of the spacer between the donor-acceptor pair and the metal particle. The Förster distance was noted to lengthen from 1.4 R0 to 1.6 R0 when the spacer increased from 2 to 10 nm.
Figure 5.
(online color at: www.lpr-journal.org) Tiopronin-coated silver particle succinimidylated via ligand exchange, followed by binding of the aminated donor-labeled oligonucleotide and hybridization with the complementary acceptor-labeled oligonucleotide (top). Histograms of FRET on the free donor-acceptor pairs and that of the donor-acceptor pairs bound to the silver particles (bottom). Modified from [57].
A similar demonstration of enhanced fluorescence near single metal particle uses an optical antenna in the proximity of the substrate surface [59–64]. Schematic antenna configuration is shown in Fig. 6. The antenna in the form of a spherical metal nanoparticle is attached to a glass tip and raster scanned across a dye layer at variable heights. The near-field optical microscopy (NSOM) has been widely used in this respect due to its exceptional resolution to investigate electromagnetic field that localizes in the close proximity of nano-objectives and surfaces. Simulation and theoretical considerations have shown how separation distance and particle size strongly affect the enhancement properties of gold or silver antenna. An optical antenna scatters electromagnetic field around the particle and therefore, when placed close to a single fluorophore, increase the molecule's excitation rate [59, 60]. However, at very small distances between the antenna and the single molecule, the nonradiative decay component becomes dominant and lowers the fluorescent rate. In the publications of Bharadwaj et al. [61, 62], the authors reveal that the enhancement is strongly particle-sample separation dependent. The fluorescence quenching dominates over the enhancement of the excitation rate at particle-sample distances shorter than 2 nm, leading to a drop in the overall fluorescence rate. The competition between quenching and plasmonic enhancement of fluorescence gives an enhancement factor of approximately 10 when the 80 nm nanoparticle is about 5 nm separated from the surface. The experiments [61,62] using individual gold and silver nanoparticles demonstrate that plasmonic-enhanced single-molecule fluorescence is strongly frequency dependent, the maximum fluorescence enhancement is obtained for wavelengths red-shifted from the surface plasmon resonance.
Figure 6.
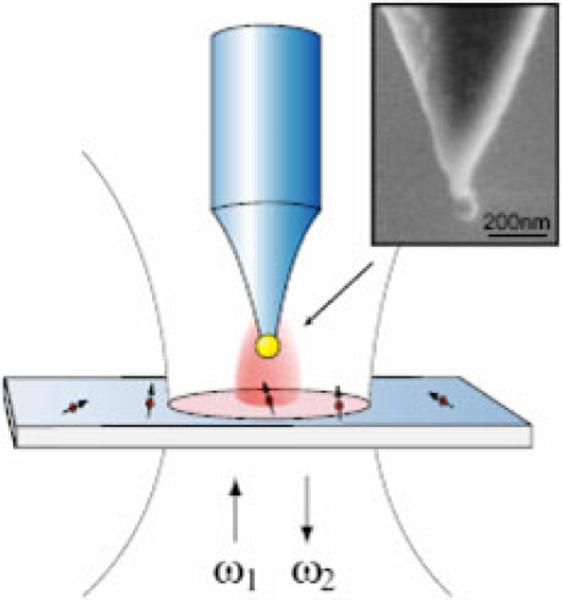
(online color at: www.lpr-journal.org) An optical antenna in the form of a gold or silver nanoparticle attached to the end of a pointed glass tip is interacting with individual fluorescent molecules excited at frequency ω1 and emitting at frequency ω2. From [59].
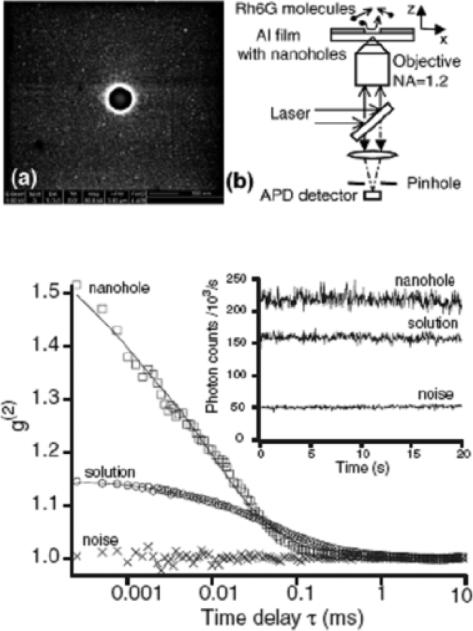
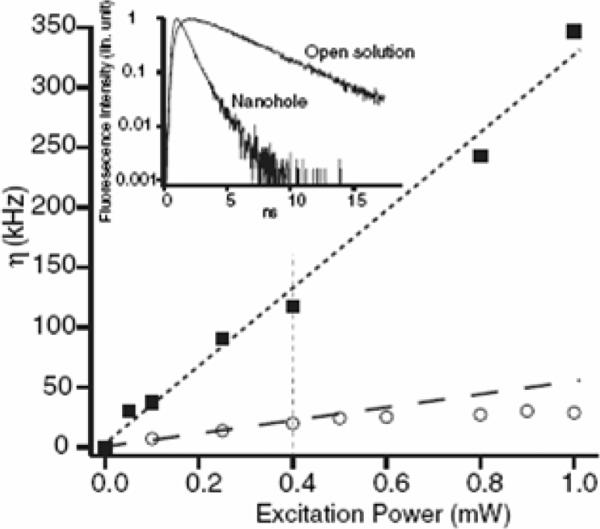
Other common nano-objectives are subwavlength apertures or nanoholes [65–67] (Fig. 7). The isolated nanometric circular holes or rectangular apertures are milled in metallic films with various aspect ratios. In these experiments, nanometric apertures/holes act as tiny reaction chambers containing a small number of molecules. This allows reduction of the effective observation volume Veff from one femtoliter in a standard confocal microscope to the attoliter (10−18 L) or even down to zeptoliter (10−21 L) range, allowing techniques such as fluorescence correlation spectroscopy (FCS) that only work while observing a few molecules to study chemical reactions at relative high molecular concentrations. This aspect is of interesting when studying biologically relevant enzyme kinetics that generally require concentrations in the micro- to millimolar range [68,69]. Rigneault et al. [66] observed enhanced molecular count rate and reduced lifetime for single fluorophores diffusing in single nanoholes drilled in an Al film (Fig. 8). The authors demonstrated that the overall enhanced fluorescence is due to a combined effect: a decrease in the effective observation volume delays the fluorescence saturation and also an increase in the local excitation intensity induced by the metal nanoholes. In another experiment described by Wenger et al. [67], the rectangular nanoapertures were milled in a metal film that allows tailoring of the penetration depth of the excitation light within the aperture. This offers new possibilities to adjust the effective observation volume and to further reduce volumes for the evanescent excitation field. Such rectangular nanoapertures do not indicate any fluorescence enhancement with a high transmission of the excitation field (longitudinal mode excitation), whereas an evanescent coupling of the pump field (the transverse mode with small edges) induces a significant increase in fluorescence count rates. These results suggest that the observed molecular fluorescence enhancement is mainly contributed to the near-field excitation within the subwavelength aperture and not much related to radiation-pattern modification.
Figure 7.
An isolated subwavelength aperture milled in an Al film coated on a microscope slip (top). Line traces and autocorrelation functions in open solution (○) in a 150 nm diameter aperture () and for the noise within the aperture (×) (bottom). From [66].
Figure 8.
Relation of enhancement with the excitation laser power in a 150-nm diameter aperture (solid square) and in open solution (open circle). The inset shows the fluorescence radiative decay curve in open solution and into a 150-nm aperture. From [66].
5. Single quantum dots near metallic nanostructure
The emission properties of quantum dots, especially high-absorption cross section, exceptional photostability, wide excitation spectra and narrow emission bands, are important in biological tagging applications [70,71]. The fluorescence from individual QDs shows strong blinking in the time scale ranging from milliseconds to many minutes [72–74]. The long “off” periods are the main restriction for the use of QDs in imaging applications and limits their applicability in single-molecule detection. The modification of spectral properties from QDs located near metallic nanostructure surfaces and nano-objectives is an interesting subject in nanoscience, and its study gives new insights into the basic aspects of field-matter interaction. In a QD-metal system, the exact schemes under which enhancement or quenching of the fluorescence emission will occur are still under investigation [75–80]. Recent progresses in single-molecule detection offer an opportunity to explore and understand the details of optical quenching or enhancement. Using a layer-by-layer polyelectrolyte deposition technique, Kulakovich et al. [79] observed a distance-dependent enhancement and quenching of quantum dot separated from gold nanoparticles. This observation is in agreement with recent reports [80] about a quenching of luminescence due to resonant energy transfer (RET) observed for molecular systems at metallic surfaces. Resonant energy transfer (RET) is very efficient at very short distances between QDs and gold nano-objective, thus is mainly responsible to a quenching of QD emission, and can compensate the effect of enhanced emission due to coupling surface plasmons between QDs and gold surfaces [79, 81]. Furthermore, at shorter distance electron tunneling may contribute to nonradiative recombination with remaining electron-hole pairs at the QD/Au interface [79]. As the separation distance increases, the potential barrier between the QDs and gold colloids increases and significantly weaken the probability of photoelectron tunneling into gold surfaces.
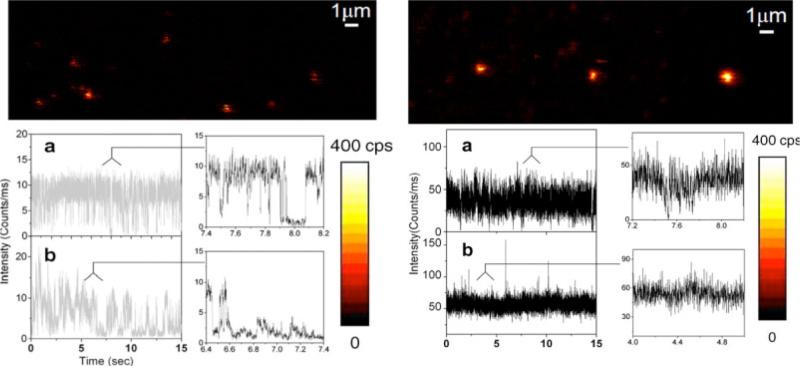
The enhancement in fluorescence emission of QDs near metallic surfaces is also accompanied by remarkable changes in dynamic optical behaviors (i.e. lifetimes, blinking, spectral shift, emission polarization etc.) [75, 77, 82]. Single-molecule experiments performed at cryogenic temperatures on single QDs reveal that blinking effects are noticeably reduced on a rough gold film [75]. Shimizu proposed that the combination of an increased radiative and absorption rate near metal surfaces accelerates the radiative relaxation process and results the emission from the charged nanocrystals. The suppressed blinking of a single quantum dot immobilized near silver island films is also reported [82] (Fig. 9). The “off” time distribution of quantum dots near SIFs displayed power-law dependence with a dissimilar exponent compared with that of electromagnetic inert surface. It is ascribed to the modification of fluorescence by the surface-plasmon resonance from the metallic nanostructure. The radiating energy from the emitter is dramatically altered through coupling with the metal plasmon resonance to cause a change of the fluorescence properties. The strong interaction of the excited molecules with the metal nanostructures dramatically shortens the lifetime of the nonemissive state. The individual QDs showed the extended “on” time and overall increased intensity near metallic nanostructures.
Figure 9.
(online color at: www.lpr-journal.org) Single-molecule fluorescence images of and representative fluorescence time traces (a,b) and their expanded sections recorded for single QDs immobilized on glass (left) and SIF substrates (right). Single streptavidin-conjugated quantum dots are immobilized on biotinylated BSA-modified surfaces. From [82].
In the cases discussed above, the fluorophores were located in external environments over metallic layers or from the metallic nanostructures at nanometer scales. Enderlein [83] has proposed a theoretical study of embedded single fluorophores within a metal shell. The simulation presented a dramatic decrease of the excited state lifetime and enhancement of the fluorescence properties. These results may lead to new classes of fluorescent probes with extensively higher photostability and amplified fluorescence intensity, and for improving the optical properties of semiconductor nanocrystals. However, it is not practical to cite all of the many authors who have contributed to these novel approaches.
6. Outlook
The ability to improve emission of a single emitter is crucial to single-molecule detection. The effects of metallic nanostructured surfaces on the spectra properties of single fluorophores have been demonstrated though the alternation of the local electromagnetic field. Metallic nanostructures affect both absorption and emission. The fluorophores are excited by the evanescent fields due to surface plasmons, rather than by propagating light. The emission is modified by the presence of nearby metal particles or structured metal surfaces. The metal can result in more rapid emission of the fluorophore, or may change the normally isotropic emission into directional emission. The near-field interaction of the fluorophore with evanescent surface plasmons result in a number of important spectral changes, including increases in intensity and photostability, decreased lifetimes due to increased rates of radiative decay, and increased distances for FRET.
Single fluorophores immobilized on metallic nanosurfaces (i.e. SIFs, nanohole arrays) can achieve higher amplification in fluorescence emission. Nanoparticles can be favorably coupled to single fluorophores to locally enhance fluorescence as free imaging probes. Plasmon-fluorophore interactions will result in a new generation of formats for clinical testing and in a new generation of ultrabright probes such as plasmon hybrid complexes [24,84]. The nanotechnology development in design metallic nanostructures with well-defined patterns and novel structures on the nanometric scales creates new opportunities to dramatically improve the fluorescence detection efficiency. An ability to control the flow of optical energy in two dimensions would have a profound impact on the design of devices for fluorescence sensing. Instead of using optical components to manipulate light, the fluorescence could be trapped in the form of plasmons and moved to different locations on the surface. This capability would be useful in nucleic acid and protein arrays, where the chemistry is already on a surface, which can easily be a metal surface.
Biography
 Joseph R. Lakowicz is the Director of the Center for Fluorescence Spectroscopy (CFS), a professor in the Department of Biochemistry and Molecular Biology, University of Maryland at Baltimore, School of Medicine. Dr. Lankowicz’ research is focused on advancing the field of fluorescence spectroscopy. This involves novel plasmon-controlled fluorescence techniques, development of novel fluorophores, development of novel fluorescence measurements, development of instrumentation for time-resolved fluorescence, and the chemical applications of fluorescence sensing.
Joseph R. Lakowicz is the Director of the Center for Fluorescence Spectroscopy (CFS), a professor in the Department of Biochemistry and Molecular Biology, University of Maryland at Baltimore, School of Medicine. Dr. Lankowicz’ research is focused on advancing the field of fluorescence spectroscopy. This involves novel plasmon-controlled fluorescence techniques, development of novel fluorophores, development of novel fluorescence measurements, development of instrumentation for time-resolved fluorescence, and the chemical applications of fluorescence sensing.
 Yi Fu achieved his Ph. D degree at West Virginia University in 2003. Currently he holds a research position in the Center for Fluorescence Spectroscopy (CFS) at the University of Maryland School of Medicine. Dr. Fu's current research is focused on single-molecule fluorescence studies on condensed materials and biomolecules.
Yi Fu achieved his Ph. D degree at West Virginia University in 2003. Currently he holds a research position in the Center for Fluorescence Spectroscopy (CFS) at the University of Maryland School of Medicine. Dr. Fu's current research is focused on single-molecule fluorescence studies on condensed materials and biomolecules.
References
- 1.Ford GW, Weber WH. Electromagnetic effects on a molecule at a metal surface. Surf. Sci. 1981;109:451–481. [Google Scholar]
- 2.Korzeniewski GE, Maniv T, Metiu H. Electrodynamics at metal surfaces. IV. The electric fields caused by the polarization of a metal surface by an oscillating dipole. J. Chem. Phys. 1982;76(3):1564–1573. [Google Scholar]
- 3.Lakowicz JR. Plasmonics in biology and plasmon-controlled fluorescence. Plasmonics. 2006;1(1):5–33. doi: 10.1007/s11468-005-9002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly KL, Jensen TR, Lazarides AA, Schatz GC. Metal Nanoparticles. Synthesis, Characterization, and Application. Marcel Dekker, Inc.; New York: 2002. Modeling metal nanoparticle optical properties; pp. 89–118. [Google Scholar]
- 5.Kelly KL, Coronada E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. B. 2003;107:668–677. [Google Scholar]
- 6.Zou SL, Schatz GC. Narrow plasmonic/photonic extinction and scattering line shapes for one and two dimensional silver nanoparticle arrays. J. Chem. Phys. 2004;121(24):12606–12612. doi: 10.1063/1.1826036. [DOI] [PubMed] [Google Scholar]
- 7.Yguerabide J, Yguerabide EE. Light-scattering sub-microscopic particles as highly fluorescence analog as their use as tracer labels in clinical and biological applications. I. Theory, Anal. Biochem. 1998;262:137–156. doi: 10.1006/abio.1998.2759. [DOI] [PubMed] [Google Scholar]
- 8.Yguerabide J, Yguerabide EE. Light-scattering sub-microscopic particles as highly fluorescence analog as their use as tracer labels in clinical and biological applications. II. Experimental characterization. Anal. Biochem. 1998;262:157–176. doi: 10.1006/abio.1998.2760. [DOI] [PubMed] [Google Scholar]
- 9.Hao E, Schatz GC. Electromagetic fields around silver nanoparticles and dimers. J. Chem. Phys. 2004;120(1):357–366. doi: 10.1063/1.1629280. [DOI] [PubMed] [Google Scholar]
- 10.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B. 2006;110:7238–7248. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 11.Cui B, Clime L, Li K, Veres T. Fabrication of large area nanoprism arrays and their application for surface enhanced Raman spectroscopy. Nanotechnology. 2008;19(14):145302. doi: 10.1088/0957-4484/19/14/145302. [DOI] [PubMed] [Google Scholar]
- 12.Li HG, Baum CE, Sun J, Cullum BM. Multilayer enhanced gold film over nanostructure surface-enhanced Raman substrates. Appl. Spectrosc. 2006;60(12):1377–1385. doi: 10.1366/000370206779321562. [DOI] [PubMed] [Google Scholar]
- 13.Aroca RF, Alvarez-Puebla RA, Pieczonka N, Sanchez-Cortez S, Garcia-Ramos JV. Surface-enhanced Raman scattering on colloidal nanostructures. Adv. Colloid Interf. Sci. 2005;116(1–3):45–61. doi: 10.1016/j.cis.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi Y, Ishikawa M, Maruyama Y, Futamata M. Trigonal silver nanostructure for single-molecule detection with surface enhanced Raman scattering. J. Korean Phys. Soc. 2005;47:S56–S62. [Google Scholar]
- 15.Eggeling C, Schaffer J, Seidel CAM, Korte J, Brehm G, Schneider S, Schrof W. Homogeneity, transport, and signal properties of single Ag particles studied by single-molecule surface-enhanced resonance Raman scattering. J. Phys. Chem. A. 2001;105(15):3673–3679. [Google Scholar]
- 16.Lukomska J, Malicka J, Gryczynski I, Leonenko Z, Lakowicz JR. Fluorescence enhancement of fluorophores tethered to different sized silver colloids deposited on glass substrate. Biopolymers. 2005;77:31–37. doi: 10.1002/bip.20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sokolov K, Chumanov G, Cotton TM. Enhancement of Molecular Fluorescence Near the Surface of Colloid Metal Films. Anal. Chem. 1998;70:3898–3905. doi: 10.1021/ac9712310. [DOI] [PubMed] [Google Scholar]
- 18.Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR, Geddes CD. Metal-enhanced fluorescence: an emerging tool in biotechnology. Curr. Opin. Biotechnol. 2005;16(1):55–62. doi: 10.1016/j.copbio.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geddes CD, Lakowicz JR. Metal-enhanced fluorescence. J. Fluoresc. 2002;12(2):121–129. doi: 10.1007/s10895-005-2515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geddes CD, Roll D, Parfenov A, Lakowicz JR. Noble-metal surfaces for use in metal-enhanced fluorescence. Biophys. J. 2003;84(2):477A–477A. [Google Scholar]
- 21.Yokota H, Saito K, Yanagida T. Single molecule imaging of fluorescently labeled proteins on metal by surface plasmons in aqueous solution. Phys. Rev. Lett. 1998;80(20):4606–4609. [Google Scholar]
- 22.Fu Y, Zhang J, Lakowicz JR. Plasmonic enhancement of single-molecule fluorescence near a silver nanoparticle. J. Fluoresc. 2007;17(6):811–816. doi: 10.1007/s10895-007-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Fu Y, Chowdhury MH, Lakowicz JR. Single-molecule studies on fluorescently labeled silver particles: Effects of particle size. J. Phys. Chem. C. 2008;112(1):18–26. doi: 10.1021/jp074938r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Fu Y, Liang D, Nowaczyk K, Zhao RY, Lakowicz JR. Single-cell fluorescence imaging using metal plasmon-coupled probe 2: Single-molecule counting on lifetime image. Nano Lett. 2008;8(4):1179–1186. doi: 10.1021/nl080093z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam F, Goodrich GP, Johnson BR, Halas NJ. Plasmonic Enhancement of Molecular Fluorescence. Nano Lett. 2007;7:496–501. doi: 10.1021/nl062901x. [DOI] [PubMed] [Google Scholar]
- 26.Fu Y, Lakowicz JR. Enhanced fluorescence of Cy5-labeled oligonucleotides near silver island films: A distance effect study using single molecule spectroscopy. J. Phys. Chem. B. 2006;110(45):22557–22562. doi: 10.1021/jp060402e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu Y, Lakowicz JR. Enhanced fluorescence of Cy5-labeled DNA tethered to silver island films: Fluorescence images and time-resolved studies using single-molecule spectroscopy. Anal. Chem. 2006;78(17):6238–6245. doi: 10.1021/ac060586t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackowski S, Wormke S, Maier AJ, Brotosudarmo THP, Harutyunyan H, Hartschuh A, Govorov AO, Scheer H, Brauchle C. Metal-enhanced fluorescence of chlorophylls in single light-harvesting complexes. Nano Lett. 2008;8(2):558–564. doi: 10.1021/nl072854o. [DOI] [PubMed] [Google Scholar]
- 29.Stefani FD, Vasilev K, Bocchio N, Stoyanova N, Kreiter M. Surface-plasmon-mediated single-molecule fluorescence through a thin metallic film. Phys. Rev. Lett. 2005;94(2):4. doi: 10.1103/PhysRevLett.94.023005. [DOI] [PubMed] [Google Scholar]
- 30.Giannini V, Sanchez-Gil JA. Excitation and emission enhancement of single-molecule fluorescence through multiple surface-plasmon resonances on metal trimer nanoantennas. Opt. Lett. 2008;33(9):899–901. doi: 10.1364/ol.33.000899. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Blair S. Fluorescence enhancement from an array of subwavelength metal apertures. Opt. Lett. 2003;28:507–509. doi: 10.1364/ol.28.000507. [DOI] [PubMed] [Google Scholar]
- 32.Wenger J, Gerard D, Dintinger J, Mahboub O, Bonod N, Popov E, Ebbesen TW, Rigneault HE. Emission and excitation contributions to enhanced single-molecule fluorescence by gold nanometric apertures. OptĖxpress. 2008;16(5):3008–3020. doi: 10.1364/oe.16.003008. [DOI] [PubMed] [Google Scholar]
- 33.Gerard D, Wenger J, Bonod N, Popov E, Rigneault H, Mahdavi F, Blair S, Dintinger J, Ebbesen TW. Nanoaperture-enhanced fluorescence: Towards higher detection rates with plasmonic metals. Phys. Rev. B. 2008;77(4):8. [Google Scholar]
- 34.Drexhage KH. Influence of a dielectric interface on fluorescence decay time. J. Lumin. 1970;1(2):693–701. [Google Scholar]
- 35.Persson BNJ. Theory of the damping of excited molecules located above a metal surface. J. Phys. C: Solid State Phys. 1978;11:4251–4269. [Google Scholar]
- 36.Gersten J, Nitzan A. Spectroscopic properties of molecules interacting with small dielectric particles. J. Chem. Phys. 1981;75(3):1139–1152. [Google Scholar]
- 37.Das PC, Puri A. Energy flow and fluorescence near a small metal particle. Phys. Rev. B. 2002;65:155416/1–155416/9. [Google Scholar]
- 38.Enderlein J. Single-molecule fluorescence near a metal layer. Chem. Phys. 1999;247(1):1–9. [Google Scholar]
- 39.Enderlein J. A theoretical investigation of single-molecule fluorescence detection on thin metallic layers. Biophys. J. 2000;78(4):2151–2158. doi: 10.1016/S0006-3495(00)76761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Link S, El-Sayed MA. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int. Rev. Phys. Chem. 2000;19(3):409–453. [Google Scholar]
- 41.Chance RR, Prock A, Sibey R. Molecular fluorescence and energy transfer near interface. Adv. Chem. Phys. 1973;37:1–65. [Google Scholar]
- 42.Ford GW, Weber WH. Electromagnetic interactions of molecules with metal surfaces. Phys. Rep. 1984;113(4):195–287. [Google Scholar]
- 43.Gersten JI. Topics in Fluorescence Spectroscopy. Springer Science + Business Medica Inc.; New York: 2005. Theory of fluorophore-metallic surface interaction; pp. 197–221. [Google Scholar]
- 44.Guzatov DV, Klimov VV. Radiative decay engineering by triaxial nanoellopsoids. Chem. Phys. Lett. 2005;412:341–346. [Google Scholar]
- 45.Bohren CF, Huffman DR. Absorption and Scattering of Light by Small Particles. John Wiley & Sons, Inc.; New York: 1983. p. 530. [Google Scholar]
- 46.Kreibig U, Vollmer M. Optical Properties of Metal Clusters. Springer-Verlag; New York: 1995. [Google Scholar]
- 47.Lakowicz JR. Radiative decay engineering: biophysical and biomedical applications. Anal. Biochem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shang L, Chen HJ, Dong SJ. Electrochemical preparation of silver nanostructure on the planar surface for application in metal-enhanced fluorescence. J. Phys. Chem. C. 2007;111(29):10780–10784. [Google Scholar]
- 49.Goldys EM, Drozdowicz-Tomsia K, Xie F, Shtoyko T, Matveeva E, Gryczynski I, Gryczynski Z. Fluorescence amplification by electrochemically deposited silver nanowires with fractal architecture. J. Am. Chem. Soc. 2007;129(40):12117–12122. doi: 10.1021/ja071981j. [DOI] [PubMed] [Google Scholar]
- 50.Goldys EM, Barnett A, Xie F, Drozdowicz-Tomsia K, Gryczynski I, Matveeva EG, Gryczynski Z, Shtoyko T. Plasmon-enhanced fluorescence near metallic nanostructures: biochemical applications. Appl. Phys A – Mater. Sci. Proc. 2007;89(2):265–271. [Google Scholar]
- 51.Haynes CL, Duyne Van R. P. Nanosphere lithography: A versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J. Phys. Chem. B. 2001;105(24):5599–5611. [Google Scholar]
- 52.Hulteen JC, Treichel DA, Smith MT, Duval ML, Jensen TR, Duyne Van R. P. Nanosphere lithography: Size-tunable silver nanoparticle and surface cluster arrays. J. Phys. Chem. B. 1999;103(19):3854–3863. [Google Scholar]
- 53.Wiley B, Sun Y, Xia Y. Synthesis of silver nanostructures with controlled shapes and properties. Acc. Chem. Res. 2007;40(10):1067–1076. doi: 10.1021/ar7000974. [DOI] [PubMed] [Google Scholar]
- 54.Wiley BJ, Chen YC, McLellan JM, Xiong YJ, Li ZY, Ginger D, Xia YN. Synthesis and optical properties of silver nanobars and nanorice. Nano Lett. 2007;7(4):1032–1036. doi: 10.1021/nl070214f. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Fu Y, Chowdhury MH, Lakowicz JR. Metal-enhanced single-molecule fluorescence on silver particle monomer and dimer: Coupling effect between metal particles. Nano Lett. 2007;7(7):2101–2107. doi: 10.1021/nl071084d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang P, Liou KN. Finite-difference time domain method for light scattering by small ice crystals in three-dimensional space. J. Opt. Soc. Am. A – Opt. Image Sci. Vision. 1996;13(10):2072–2085. [Google Scholar]
- 57.Zhang J, Fu Y, Lakowicz JR. Enhanced Forster resonance energy transfer (FRET) on a single metal particle. J. Phys. Chem. C. 2007;111(1):50–56. doi: 10.1021/jp062665e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Fu Y, Chowdhury MH, Lakowicz JR. Enhanced Forster resonance energy transfer on single metal particle. 2. Dependence on donor-acceptor separation distance, particle size, and distance from metal surface. J. Phys. Chem. C. 2007;111(32):11784–11792. doi: 10.1021/jp067887r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anger P, Bharadwaj P, Novotny L. Enhancement and quenching of single-molecule fluorescence. Phys. Rev. Lett. 2006;96(11):113002. doi: 10.1103/PhysRevLett.96.113002. [DOI] [PubMed] [Google Scholar]
- 60.Kuhn S, Hakanson U, Rogobete L, Sandoghdar V. Enhancement of single-molecule fluorescence using a gold nanoparticle as an optical nanoantenna. Phys. Rev. Lett. 2006;97(1):017402. doi: 10.1103/PhysRevLett.97.017402. [DOI] [PubMed] [Google Scholar]
- 61.Bharadwaj P, Anger P, Novotny L. Nanoplasmonic enhancement of single-molecule fluorescence. Nanotechnology. 2007;18(4):044017. [Google Scholar]
- 62.Bharadwaj P, Novotny L. Spectral dependence of single-molecule fluorescence enhancement. OptĖxpress. 2007;15(21):14266–14274. doi: 10.1364/oe.15.014266. [DOI] [PubMed] [Google Scholar]
- 63.Encina ER, Coronado EA. Plasmonic nanoantennas: Angular scattering properties of multipole Resonances in noble metal nanorods. J. Phys. Chem. C. 2008;112(26):9586–9594. [Google Scholar]
- 64.Rogobete L, Kaminski F, Agio M, Sandoghdar V. Design of plasmonic nanoantennae for enhancing spontaneous emission. Opt. Lett. 2007;32(12):1623–1625. doi: 10.1364/ol.32.001623. [DOI] [PubMed] [Google Scholar]
- 65.Gerard D, Wenger J, Bonod N, Popov E, Rigneault H, Mahdavi F, Blair S, Dintinger J, Ebbesen TW. Nanoaperture-enhanced fluorescence: Towards higher detection rates with plasmonic metals. Phys. Rev. B. 2008;77(4):045413. [Google Scholar]
- 66.Rigneault H, Capoulade J, Dintinger J, Wenger J, Bonod N, Popov E, Ebbesen TW, Lenne PF. Enhancement of single-molecule fluorescence detection in subwavelength apertures. Phys. Rev. Lett. 2005;95(11):117401. doi: 10.1103/PhysRevLett.95.117401. [DOI] [PubMed] [Google Scholar]
- 67.Wenger J, Gerard D, Dintinger J, Mahhoub O, Bonod N, Popov E, Ebbesen TW, Rigneault H. Emission and excitation contributions to enhanced single-molecule fluorescence by gold nanometric apertures. OptĖxpress. 2008;16(5):3008–3020. doi: 10.1364/oe.16.003008. [DOI] [PubMed] [Google Scholar]
- 68.Wenger J, Gerard D, Lenne PF, Rigneault H, Dintinger J, Ebbesen TW, Boned A, Conchonaud F, Marguet D. Dual-color fluorescence cross-correlation spectroscopy in a single nanoaperture: towards rapid multi-component screening at high concentrations. OptĖxpress. 2006;14(25):12206–12216. doi: 10.1364/oe.14.012206. [DOI] [PubMed] [Google Scholar]
- 69.Leutenegger M, Gosch M, Perentes A, Hoffmann P, Martin OJF, Lasser T. Confining the sampling volume for Fluorescence Correlation Spectroscopy using a sub-wavelength sized aperture. OptĖxpress. 2006;14(2):956–969. doi: 10.1364/opex.14.000956. [DOI] [PubMed] [Google Scholar]
- 70.Pinaud F, Michalet X, Bentolila LA, Tsay JM, Doose S, Li JJ, Iyer G, Weiss S. Advances in fluorescence imaging with quantum dot bio-probes. Biomaterials. 2006;27:1679–1687. doi: 10.1016/j.biomaterials.2005.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Efros AL, Rosen M, Kuno M, Nirmal M, Noris DJ, Bawendi M. Band-edge exciton in quantum dots of semiconductors with a degenerate valence band: Dark and bright exciton states. Phys. Rev. B. 1996;54(7):4843–4856. doi: 10.1103/physrevb.54.4843. [DOI] [PubMed] [Google Scholar]
- 72.Yoew EKL, Melnikov SM, Bell TDM, Schryver FCD, Hofkens J. Characterizing the fluorescence intermittency and photobleaching kinetics of dye immobilized on a glass surface. J. Phys. Chem. A. 2006;110:1726–1734. doi: 10.1021/jp055496r. [DOI] [PubMed] [Google Scholar]
- 73.Tittel J, Gohde W, Koberling F, Basche T, Kornowski A, Weller H, Eychmuller A. Fluorescence spectroscopy on single CdS nanocrystals. J. Phys. Chem. A. 1997;101(16):3013–3016. [Google Scholar]
- 74.Stefani FD, Zhong X, Knoll W, Han M, Kreiter M. Memory in quantum-dot photoluminescence blinking. J. New Phys. 2005;7(197):1–17. [Google Scholar]
- 75.Shimizu KT, Woo WK, Fisher BR, Eisler HJ, Bawendi MG. Surface-enhanced emission from single semiconductor nanocrystals. Phys. Rev. Lett. 2002;89(11):117401–1–4. doi: 10.1103/PhysRevLett.89.117401. [DOI] [PubMed] [Google Scholar]
- 76.Okamoto K, Vyawahare S, Scherer A. Surface-plasmon enhanced bright emission from CdSe quantum-dot nanocrystals. J. Opt. Soc. Am. B. 2006;23(8):1674–1677. [Google Scholar]
- 77.Ray K, Badugu R, Lakowicz JR. Metal-enhanced fluorescence from CdTe nanocrystals: A Single-molecule fluorescence study. J. Am. Chem. Soc. 2006;128:8898–8999. doi: 10.1021/ja061762i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song J-H, Atray T, Shi S, Urabe H, Nurmikko AV. Large enhancement of fluorescence efficiency from CdSe/ZnS quantum dots induced by resonant coupling to spatially controlled surface plasmons. Nano Lett. 2005;5(8):1557–1561. doi: 10.1021/nl050813r. [DOI] [PubMed] [Google Scholar]
- 79.Kulakovich O, Strekal N, Yaroshevich A, Maskevich S, Gaponenko S, Nabiev I, Woggon U, Artemyev M. Enhanced luminescence of CdSe quantum dots on gold colloids. Nano Lett. 2002;2(12):1449–1452. [Google Scholar]
- 80.Gueroui Z, Libchaber A. Single-molecule measurements of gold-quenched quantum dots. Phys. Rev. Lett. 2004;93(16):166108. doi: 10.1103/PhysRevLett.93.166108. [DOI] [PubMed] [Google Scholar]
- 81.Pompa PP, Martiradonna L, Della A, Torre, Della F, Sala, Manna L, Vittorio De M., Calabi F, Cingolani R, Rinaldi R. Metal-enhanced fluorescence of colloidal nanocrystals with nanoscale control. Nature Nanotechnol. 2006;1(2):126–130. doi: 10.1038/nnano.2006.93. [DOI] [PubMed] [Google Scholar]
- 82.Fu Y, Zhang J, Lakowicz JR. Suppressed blinking in single quantum dots (QDs) immobilized near silver island films (SIFs) Chem. Phys. Lett. 2007;447(1–3):96–100. doi: 10.1016/j.cplett.2007.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Enderlein J. Theoretical study of single-molecule fluorescence in a metallic nanocavity. Appl. Phys. Lett. 2002;80(2):315–317. [Google Scholar]
- 84.Zhang J, Fu Y, Lakowicz JR. Single cell fluorescence imaging using metal plasmon-coupled probe. Bioconjugate Chem. 2007;18(3):800–805. doi: 10.1021/bc0603384. [DOI] [PMC free article] [PubMed] [Google Scholar]