Abstract
Objectives:
The clinical experience with tissue-engineered tracheal grafts (TETGs) has been fraught with graft stenosis and delayed epithelialization. A mouse model of orthotopic replacement that recapitulates the clinical findings would facilitate the study of the cellular and molecular mechanisms underlying graft stenosis.
Methods:
Electrospun nanofiber tracheal scaffolds were created using nonresorbable (polyethylene terephthalate + polyurethane) and co-electrospun resorbable (polylactide-co-caprolactone/polyglycolic acid) polymers (n = 10/group). Biomechanical testing was performed to compare load displacement of nanofiber scaffolds to native mouse tracheas. Mice underwent orthotopic tracheal replacement with syngeneic grafts (n = 5) and nonresorbable (n = 10) and resorbable (n = 10) scaffolds. Tissue at the anastomosis was evaluated using hematoxylin and eosin (H&E), K5+ basal cells were evaluated with the help of immunofluorescence testing, and cellular infiltration of the scaffold was quantified. Micro computed tomography was performed to assess graft patency and correlate radiographic and histologic findings with respiratory symptoms.
Results:
Synthetic scaffolds were supraphysiologic in compression tests compared to native mouse trachea (P < .0001). Nonresorbable scaffolds were stiffer than resorbable scaffolds (P = .0004). Eighty percent of syngeneic recipients survived to the study endpoint of 60 days postoperatively. Mean survival with nonresorbable scaffolds was 11.40 ± 7.31 days and 6.70 ± 3.95 days with resorbable scaffolds (P = .095). Stenosis manifested with tissue overgrowth in nonresorbable scaffolds and malacia in resorbable scaffolds. Quantification of scaffold cellular infiltration correlated with length of survival in resorbable scaffolds (R2 = 0.95, P = .0051). Micro computed tomography demonstrated the development of graft stenosis at the distal anastomosis on day 5 and progressed until euthanasia was performed on day 11.
Conclusion:
Graft stenosis seen in orthotopic tracheal replacement with synthetic tracheal scaffolds can be modeled in mice. The wide array of lineage tracing and transgenic mouse models available will permit future investigation of the cellular and molecular mechanisms underlying TETG stenosis.
Keywords: tissue engineered trachea, tracheal stenosis, biomaterials, micro computed tomography
Introduction
The clinical translation of tissue engineered tracheal grafts has been limited due to graft collapse, stenosis, and delayed epithelialization.1 Animal models including sheep, dogs, pigs, rabbits, and rats have been used to study the in vivo performance of tissue-engineered tracheal constructs. Critical to the development of a tissue-engineered trachea is the delineation of the cellular and molecular mechanisms driving regeneration as well as the complications that prohibit translation from the bench to bedside. The development of a mouse model that recapitulates the complications seen in humans would allow for the application of transgenic models that would both define mechanisms and subsequent modification of these mechanisms to attenuate graft stenosis and accelerate regeneration.2 While the feasibility of decellularized tracheal constructs have been reported, orthotopic implantation of biosynthetic tracheal scaffolds in a mouse model has yet to be explored.3
The use of a synthetic scaffold to support tracheal regeneration would avoid the need for donor tissue, spare a lengthy decellularization process, permit off-the-shelf applications and is easily customizable.4–6 Using electrospinning, small tubular scaffolds can be fabricated to mimic native extracellular matrix on a nanoarchitectural level.7–9 In this report, we developed a mouse model of orthotopic tracheal replacement using electrospun scaffolds using both nonresorbable and resorbable polymers, comparing the biomechanical properties of the 2 scaffolds, histologic findings, and overall survival.
Methods
Animal Care and Ethics Statement
Nationwide Children’s Hospital’s (Columbus, Ohio, USA) Institutional Animal Care and Use Committee reviewed, approved, and monitored the protocol (AR15–00090). Humane care was in accordance with standards published by the Public Health Service, National Institutes of Health (Bethesda, Maryland, USA) in the Care and Use of Laboratory Animals (2011), and USDA regulations outlined in the Animal Welfare Act.
Syngeneic mouse trachea harvest
Six- to 8-week-old specific pathogen-free C57BL/6 female mice were euthanized using an overdose cocktail of ketoprofen (10 mg/kg), xylazine (20 mg/kg), and ketamine (200 mg/kg, intraperitoneal) and bilateral pneumothoraces. Once euthanasia was confirmed and the surgical site was prepared, a vertical midline incision was made through the anterior neck skin from the clavicles to the hyoid bone with retraction of the strap muscles using a dissection microscope (Figure 1A). Once the thyroid cartilage, cricoid cartilage, and proximal trachea were identified, circumferential dissection of the trachea was performed at the level of the third tracheal ring in a supraperichondrial plane (Figure 1B). A 22 G needle was inked using a surgical marker to mark the anterior midline portion along the tracheal segment and the graft to assist with maintaining the orientation during the implant surgery (Figure 1B). Transection of the trachea was then performed below the third tracheal ring, and a 5 mm specimen was resected. Specimens were stored in cold phosphate buffered saline (PBS) for approximately 10 minutes until implantation.
Figure 1.
Orthotopic tracheal replacement with synthetic graft. (A) Vertical midline incision and retraction of the strap muscles. (B) Circumferential dissection of the trachea and labeling of the trachea to maintain orientation. (C) Securing the distal trachea to the sternal notch, serving as a temporary tracheostomy (arrow). (D) Graft implantation following removal of segment of syngeneic trachea. Scale bar = 2 mm.
Scaffold Fabrication
Scaffolds were manufactured by Nanofiber Solutions, Inc (Hilliard, Ohio, USA).
Electrospun nanofiber nonresorbable tracheal scaffold: Polyethylene terephthalate + polyurethane.
A polymer nanofiber precursor solution was prepared by (1) dissolving 8 wt% polyethylene terephthalate (PET) in 1,1,1,3,3,3-hexafluoroisopropanol and heating the solution to 60°C and (2) dissolving 3 wt% polyurethane (PU) in 1,1,1,3,3,3-hexafluoroisopropanol. Once cooled, the solutions were combined to create a final polymer mixture of 20 wt% PET and 80 wt% PU. The PET + PU solution was electrospun onto the custom-designed mandrel utilizing a 20-gauge blunt tip needle, a high-voltage DC power supply set to +14 kV, and a 20 cm tip-to-substrate distance. A wall thickness of 300 µm and lumen diameter of 1 mm was designed to approximate native mouse trachea. Scaffolds were sterilized using ultraviolet light prior to implantation.
Co-electrospun nanofiber resorbable tracheal scaffold: Polylactide-co-caprolactone/polyglycolic acid.
Grafts were produced as previously described.10 In summary, 10 wt% polyglycolic acid (PGA) and 5 wt% polylactide-co-caprolactone (PLCL) were separately dissolved in 1,1,1,3,3,3-hexafluoroisopropanol and stirred with a magnetic stir bar for a minimum of 3 hours at room temperature. Using separate syringes for each solution, both solutions were simultaneously electrospun (ie, co-electrospun) onto a rotating grounded mandrel (30 revolutions per minute) positioned 20 cm from the syringe tips, with +25 kV charge applied. The PGA solution (10 wt%) was dispensed at 2.5 mL/hr while the PLCL solution (5 wt%) was dispensed at 5.0 mL/hr to create co-electrospun PGA and PLCL scaffolds with a PGA:PLCL weight ratio of 1:1. After electrospun nanofibers were deposited onto the grounded mandrel, the electrospun scaffold was removed from the mandrel. The wall thickness was then measured with a digital snap gauge by placing the scaffold between 2 glass slides to ensure a desired wall thickness of 300 µm was achieved.
Biomechanical Testing
Uniaxial compression testing was performed to compare the force endured by the nonresorbable scaffold (n = 10), resorbable scaffold (n = 10), and native trachea (n = 10). A 5 Newton load cell was attached to a Mecmesin MultiTest 5-i computer-controlled tensile and compression test system (West Sussex, United Kingdom), and a small block of high-density polyethylene was threaded and attached. Specimens were placed on a larger block of polyethylene just under the small block/load cell assembly. The load cell assembly was manually jogged down until the small block began to touch the top of the sample. Compression was then performed at a rate of 3 mm/min downward until the sample was compressed 0.5 mm (50% luminal obstruction), then returned to its starting position.
Orthotopic tracheal replacement with syngeneic trachea or biosynthetic tracheal scaffold
Implant study design.
Six- to 8-week-old specific pathogen free C57BL/6 female mice (n = 25) were used as graft recipients. Recipients were randomized to receive syngeneic trachea (n = 5), nonresorbable PET + PU scaffolds (n = 10), or resorbable PLCL/PGA scaffolds (n = 10).
Surgical preparation and anesthesia.
Mice were sedated with an intraperitoneal injection including ketoprofen (5 mg/kg), ketamine (100 mg/kg), and xylazine (10 mg/kg). A plane of anesthesia that permits spontaneous ventilation was used and obviates the need for endotracheal intubation.
Resection of the native trachea was then performed as outlined in the “Syngeneic Mouse Trachea Harvest” section. Care was directed to avoid injury or stretch of the recurrent laryngeal nerves located in the tracheoesophageal groove and violation of the esophagus located posteriorly. The trachea was transected below the third ring, entering the airway.
Creation of tracheostomy and graft implantation.
To avoid the need for endotracheal intubation, temporary tracheostomy was then performed, securing the distal trachea to the sternal notch. This permitted unobstructed breathing during graft implantation (Figure 1C). A 5 mm long segment of either syngeneic trachea or tracheal scaffold was then anastomosed to the proximal trachea using a 9–0 monofilament nylon suture (Figure 1D). The anterior trachea and scaffold were marked to ensure precise approximation as twisting of the graft or native airway can result in acute obstruction (Figure 1B–1D). The tracheostoma was then released from the sternum, and a distal tracheal transection was made, creating a 5 mm defect in the native mouse trachea. The distal anastomosis was then completed (Figure 1D). The distal trachea then easily approximates the graft in a tension-free fashion. The strap muscles and skin were then reapproximated over the trachea using interrupted nylon sutures. Postoperatively, the animals received 1 dose of buprenorphine (0.1 mg/kg subcutaneous) and 48 hours of ibuprofen (30 mg/kg in drinking water) for pain management.
Euthanasia
Animals were euthanized using an overdose cocktail of ketoprofen (10 mg/kg), xylazine (20 mg/kg), and ketamine (200 mg/kg, intraperitoneal) and removal of vital organs. Indications for euthanasia included significant weight loss (>20% initial weight), increased respiratory rate, increased work of breathing, and stridor. If asymptomatic, euthanasia was performed at the study endpoint of postoperative day 60 (POD 60).
Quantification of cellular infiltration
Axial sections of resorbable (n = 5) and nonresorbable (n = 5) scaffolds were evaluated at the anastomosis for the degree of cellular infiltration using the Zeiss Axio Imager A2 and Zeiss Pro 2. Images were first captured at a high-powered field of 200× magnification. Image processing software was then used to apply a grid (Image J, v. 1.49, NIH, Bethesda, Maryland, USA), and the number of cells within the scaffold were manually counted. Quantification was compared to overall survival.
Histology and Immunofluorescence
The graft was resected in combination with a segment of native trachea proximal and distal to the anastomoses. Specimens were immersed in 10% neutral buffered formalin for at least 48 hours at 4°C and embedded in paraffin. Serial axial and coronal sections (4 µm thick) were stained with hematoxylin and eosin (H&E).
For additional analysis of K5+ basal cells, the tissue sections were deparaffinized, rehydrated, and blocked for nonspecific antibody binding using a buffer containing 5% bovine serum albumin, 1X phosphate-buffered saline (PBS), and 0.1% Triton X100. The sections were incubated overnight at 4°C with rabbit anti-K5 (1:1000, AF-138; Biolegend, San Diego, California, USA). Immunofluorescence was detected using Alexa Fluor 594 donkey anti-rabbit IgG (1:500, A21207; Invitrogen, Carlsbad, California, USA). To counterstain and identify cell nuclei, 4’, 6-diamidino-2- phenylindole (DAPI) was used.
Images of H&E-stained axial sections at the anastomosis of nonresorbable and resorbable scaffolds were acquired at 100× and 200× magnification using a Zeiss Axio Imager A2 or Zeiss 2 Pro, and the immunofluorescent images were obtained using Zeiss Axio Imager M2 or Zeiss 2 Pro at 200× magnification.
Micro Computed Tomography
Under inhalational anesthesia (1%−3% isoflurane in room air at 1–3 L/min), in vivo imaging of the native airway and graft was performed with the Trifoil eXplore Locus RS 80 micro computed tomography (CT). Data on days 0, 3, 5, and 7 were acquired with parameters to include 720 views, 0.5° angle of increment, 1 frame to average, 80 kVp, 450 uA, 400 ms exposure time, 360° rotation, and 45 um detector resulting in a 17- to 20-minute overall scan time. All scans had a full resolution reconstruction completed producing a 45-micron image result. The terminal scan on day 11 was acquired with parameters to include 900 views, 0.4° angle of increment, 2 frames to average, 80 kVp, 450 uA, 2000 ms exposure time, 360° rotation, and 27 um detector. This scan resulted in a 1 hour and 40-minute overall scan time for half resolution reconstruction producing a 40-micron image result. Data were transferred to GEHC MicroView.
Statistical Analysis
Comparison of survival between the 3 groups was performed by plotting Kaplan-Meier survival curves followed by curve comparisons via Log-rank (Mantel-Cox) testing. Comparison between the forces endured during uniaxial compression testing and cellular infiltration in scaffolds relative to survival days and graft types among the 3 groups was performed using 1-way ANOVA and Welch’s t test. Significance of the relationship between cellular infiltration and implant time was determined via calculation of the Pearson correlation coefficient. All statistical analyses were performed in GraphPad Prism 7.03 (La Jolla, California, USA).
Results
Biomechanical testing
Mouse tracheal scaffolds of electrospun PET + PU (nonresorbable), PLCL/PGA (resorbable), and native mouse trachea (n = 10/group) were subjected to compression testing via uniaxial loading (Figure 2). Average maximum force for 50% compression of native mouse trachea was 6.71 millinewtons (mN) (SE ± 0.79). Average maximum force for 50% compression was 97.69 mN (SE ± 6.93) for PET + PU grafts and 452.49 mN (SE ± 66.37) for PLCL/PGA grafts. When compared to native mouse trachea, both biosynthetic constructs were supraphysiologic in response to uniaxial compression (P < .0001). Pairwise comparisons between the 2 grafts showed that PLCL/PGA grafts were more rigid than PET + PU (P = .0004).
Figure 2.
Uniaxial compression testing of native mouse and synthetic tracheas. Compared to the native trachea, the nonresorbable (P < .0001) and resorbable (P < .0001) grafts demonstrated supraphysiologic properties. Compression testing showed a significant difference between the resorbable and nonresorbable grafts (P = .0004).
Outcomes of orthotopic segmental tracheal replacement
All mice successfully tolerated orthotopic tracheal replacement. Both syngeneic and synthetic tracheas were similar in handling during the implant—there were no tears or injury of the grafts during the procedure. One of the syngeneic trachea recipients was euthanized on POD 2 due to respiratory distress, and the remaining 4 animals survived until the study endpoint of POD 60 with a mean survival of 48.4 days (SD ± 25.9) (Figure 3A).
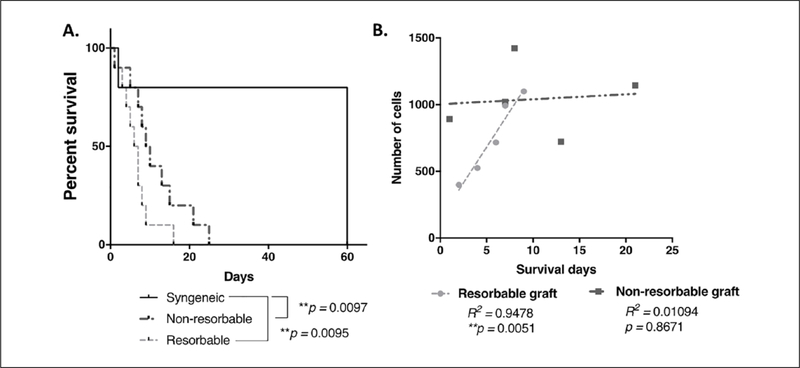
Figure 3.
Survival and cellular infiltration of syngeneic and synthetic tracheal implants. (A) Kaplan-Meier survival curve comparing animals which received resorbable and nonresorbable grafts to those with syngeneic transplants. Log rank (Mantel-Cox) testing revealed statistically significant differences between each biosynthetic scaffold and the syngeneic implant group (nonresorbable **P = .0097, resorbable **P = .0095). Differences between the survival curves for the biosynthetic approached but did not achieve statistical significance (P = .0691). (B) Scaffold cellular infiltration plotted against survival days for resorbable and nonresorbable grafts. Cellular infiltration was significantly correlated with survival in the resorbable cohort, and this relationship was linear (R2 = 0.9478, **P = .0051). This relationship was not observed in nonresorbable grafts (R2 = 0.01094, P = .8671).
All biosynthetic scaffold recipients that required euthanasia demonstrated signs of respiratory distress and more than 20% weight loss. Mean survival with nonresorbable scaffolds was 11.40 ± 7.31 days and 6.70 ± 3.95 days with resorbable scaffolds (P = .095). Syngeneic graft recipient survival was significantly longer than nonresorbable (P = .03) and resorbable (P = .02) grafts. Comparison of Kaplan-Meier survival plots indicated that curves for either the resorbable and nonresorbable scaffolds were significantly different than that of the syngeneic grafts (P = .0097 and P = .0095, respectively). A difference between the resorbable and nonresorbable scaffold cohorts approached but did not reach statistical significance (P = .0691).
Findings at Necropsy
Necropsy revealed intact grafts without evidence of graft failure, dislodgement, or fistula. Syngeneic implants demonstrated an intact lining of respiratory epithelium in the lumen at the study endpoint without evidence of graft stenosis. In graft recipients at early time points (<1 week), no neotissue formation was observed in the lumen. Neotissue ingrowth was observed in both types of synthetic grafts by day 7 with progressive stenosis observed at variable time points up to 21 days. Resorbable grafts showed signs of collapse by the end of 2 weeks postoperatively.
Neotissue Formation and Scaffold Characteristics
Syngeneic implants demonstrated normal cartilaginous architecture and normal respiratory epithelium (Figure 4A). There was no stenosis seen in syngeneic graft recipients. In synthetic scaffolds recipients at early time points (less than 7 days), there was minimal tissue formation on the luminal surface of the graft. Red blood cells and mixed inflammatory cells were observed luminal and abluminal to the scaffold. K5+ respiratory epithelium was seen lining the scaffold at day 21 (Figures 4B–4D). There was marked thickening of the lamina propria and submucosal region with dilated neovessels and inflammatory cell infiltration within synthetic grafts when compared to syngeneic grafts (Figures 4B–4D). PLCL/PGA grafts demonstrated degradation and scaffold distortion at 16 days and evidence of graft malacia and collapse on day 16 (Figure 4C). PET + PU grafts maintained normal graft architecture throughout all endpoints (Figure 4D). Graft stenosis was seen in both scaffold types but was represented by overgrowth of neotissue in PET + PU grafts versus graft degradation and collapse in PLCL/PGA grafts. There was incomplete epithelialization of the grafts even at late time points.
Figure 4. Graft histology and immunofluorescence.
(A.1-A.2). Low and high magnification (100×, 200×) of axial section through anastomosis in syngeneic tracheal replacement. (A.3). K5+ basal cells represent an intact epithelium. (B.1-B.2). Low and high magnification (100×, 200×) of axial section through anastomosis in resorbable graft implantation with (B.3) redemonstration of epithelium. (C.1-C.2). Low and high magnification (100×, 200×) of coronal section through anastomosis of resorbable scaffold; (C.3) anastomoses demonstrated evidence of respiratory epithelialization (black arrow) and K5+. (D.1-D.2). Nonresorbable grafts with dilated vessels, inflammatory cell infiltrate, and thickened sub-epithelium (*) leading to graft stenosis when compared to syngeneic grafts (open arrow). (C.1–C.2). Graft resorption and collapse in resorbable scaffolds (double open arrow). (C.3, D.3). Immunostaining demonstrates incomplete migration of epithelium onto the scaffold. Example suture artifact = red arrow. Scale bars: Low magnification (A.1–D.1): 200 µm. High magnification (A.2–D.2) and K5+ basal cells (A.3–D.3): 50 µm.
Quantification of cellular infiltration at the anastomosis was compared to overall survival. Gradual degradation of PLCL/PGA grafts resulted in a positive linear relationship, and implant time was significantly correlated with scaffold cellular infiltration (R2 = 0.95, P = .0051). In the nonresorbable scaffold cohort, cellular infiltration was not correlated with implant time, and the relationship between these two variables was not linear (R2 = 0.01, P = .8671) (Figure 3B).
Micro CT
General anesthesia and micro CT were performed on 1 nonresorbable scaffold recipient. Sagittal images obtained demonstrated graft patency following implantation (Figures 5A–5B). Graft stenosis was observed at the distal anastomosis at day 5 (Figure 5C). The onset of increased work of breathing was noted on day 7, which correlated with progressive distal stenosis. Respiratory distress was noted on day 11, and euthanasia was performed. Histologic analysis correlated with radiographic findings, demonstrating graft patency at the proximal anastomosis and near complete stenosis at the distal anastomosis (Figures 5E–5F).
Figure 5.
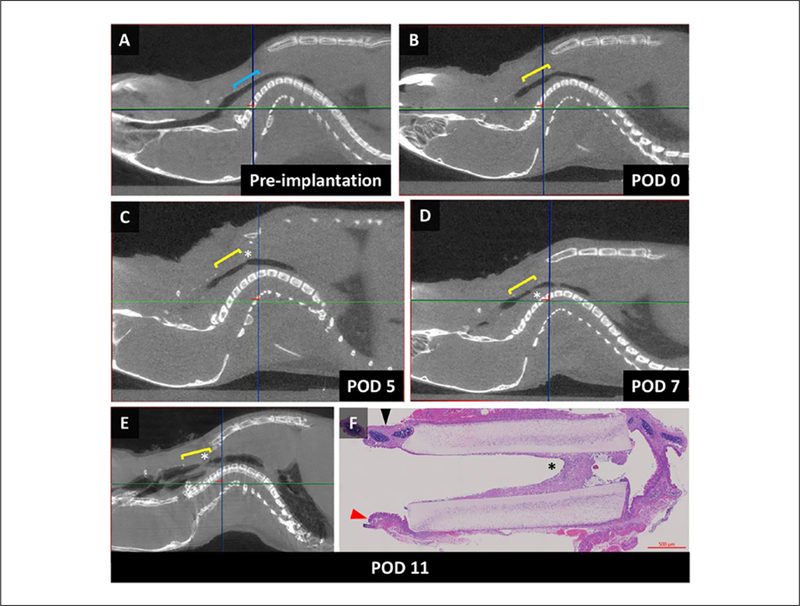
Serial imaging of implanted synthetic tracheal scaffold. (A) Sagittal micro computed tomography (CT) image of the airway before implantation; blue bracket defines the implantation site (cephalad – left border, anterior – superior border). (B) Following synthetic graft implantation with graft patency, yellow bracket defines the implanted scaffold. (C) Development of graft narrowing at the distal anastomosis (asterisk). (D) Progressive graft narrowing, correlating with the onset of increased work of breathing seen in the animal. (E) Near complete stenosis of the distal anastomosis on day of euthanasia. (F) Sagittal section through the native airway and scaffold redemonstrating radiologic findings of stenosis at the distal anastomosis (black arrow – anterior tracheal wall, red arrow – posterior tracheal wall, asterisk – graft stenosis).
Discussion
While segmental orthotopic tracheal implant in a mouse model has been applied in tracheal transplant models, our report represents the first report of orthotopic segmental tracheal replacement in a mouse model using synthetic tracheal scaffolds. Despite the challenges from scale, a functioning mouse model is critical to define the mechanisms of airway regeneration as it offers the application of transgenic and lineage tracing animals to characterize and modulate neotissue formation.
An ideal tracheal scaffold for the management of long segment tracheal defects would be biocompatible, have low risk of complication, and seamlessly integrate with the host. As a surrogate for the ideal tracheal scaffold, we used fresh syngeneic tracheal grafts for orthotopic replacement to validate the technical approach to implantation. Of the 5 implanted animals, 4 survived to the 60-day study endpoint. This is similar to outcomes observed in prior reports of syngeneic tracheal replacement.3,11,12
Numerous biomaterials have been examined as candidates for tracheal tissue engineering. Polyethylene terephthalate (PET) and polyurethane (PU) are FDA-approved polymers that have been used in humans for tracheal tissue engineering.4–6,13 Using a lamb model, we demonstrated that seeding a PET + PU scaffold with autologous bone marrow-derived mononuclear cells (BM-MNC) attenuates stenosis and accelerates epithelialization.8 However, graft stenosis resulting in airway obstruction is seen in all cases and must be managed with endoscopic interventions.14 We developed a miniaturized version of this scaffold that recapitulates stenosis seen in the clinic and in our large animal work to better understand the mechanisms driving graft incorporation, remodeling, and stenosis.
We first examined the biomechanical properties of our miniaturized graft compared to native trachea and a resorbable (PLCL/PGA) graft.10,15,16 Lack of mechanical compatibility, or compliance mismatch, is a known source of complications in other implanted biosynthetic constructs, as demonstrated by increased intimal hyperplasia seen in mismatched arterial bypass grafts and synthetic hemodialysis grafts as well as hernia mesh failure.17–19 This poses a unique challenge in airway tissue engineering where relative weakness of the scaffold can result in graft malacia and supraphysiologic properties can contribute to graft stenosis and compromise graft integration. Both scaffolds demonstrated mechanical properties of greater rigidity than native mouse trachea. Despite the initial supraphysiologic properties seen in PLCL/PGA before implantation, there is histologic evidence of scaffold degradation and malacia after implantation. Further work is needed to define the degradation rate of resorbable grafts as well as the role of compliance mismatch on scaffold performance.
There were 2 types of graft compromise seen in this study. Nonresorbable scaffolds maintained normal graft architecture, but an overgrowth of neotissue leads to the development of graft stenosis, most notably at the anastomosis. Resorbable scaffolds demonstrated loss of mechanical integrity and resulted in collapse more prominent in the midgraft region.
In both scaffolds, neotissue formation was limited to the anastomosis region; there was no evidence of respiratory epithelialization in the midgraft region. This rate of re-epithelialization is consistent with other experimental reports, where reconstitution of airway epithelium is not observed in the first month after implantation and can take several years to regenerate.20–22
We hypothesized that a resorbable scaffold would result in greater cellular infiltration than a nonresorbable scaffold. We demonstrated a relationship between cellular infiltration with time in resorbable scaffolds, which was not seen with the nonresorbable scaffold. This demonstrates feasibility of the scaffold replacement with native tissue over time; however, further explorations in the ideal resorption time and characterization of the infiltrating cell types are necessary.
We also demonstrate the feasibility of using micro CT as an instrument to interrogate in vivo tracheal graft performance. Micro CT has been used in mouse models to evaluate in vivo tissue engineered vascular graft performance as well as airway pathology.23,24 We explored the feasibility of micro CT to further characterize graft patency in time without euthanasia. We demonstrate that a synthetic trachea recipient tolerated serial imaging on days 0, 3, 5, 7, and 11 with demonstration of progressive graft stenosis. Of note, there was no obvious signs of impaired secretion clearance or mucous plugging on the scan, a potential concern when interposing a long-segment synthetic scaffold.
Of note, we deliberately avoided cell seeding and prelamination of the scaffolds for several reasons. First, while cell seeding has been proven to be instrumental in the integration of other tissue engineered constructs, the role of cell seeding in airway tissue engineering remains unclear.25,26 Second, cell seeding introduces numerous levels of heterogeneity (seeding capacity of the scaffold, cell viability, cell type) that will require further preclinical testing prior to implementation. Third, prelamination with an intact epithelium at the time of implantation does not ensure regenerative capacity. Prior animal work with tracheal transplant in the mouse model demonstrates that the graft is repopulated with host-derived epithelium.27
There are several limitations of this study. First, segmental tracheal replacement in a mouse is technically very challenging because anesthesia- and surgical-related complications are magnified in this model, specifically trachea-into-graft telescoping at the anastomotic site, which can result in early airway obstruction and frequently responsible for early term (less than 7 days) deaths (data not shown). Despite an appearance of appropriate trachea + scaffold approximation during implantation, we still observe histologic evidence of ring distortion, graft telescoping, and asymmetry. Despite its challenges, we believe that segmental replacement is a rigorous model for us to study the complications of tracheal replacement such as graft stenosis. Second, as we examined overall survival, our histologic findings do not represent a natural history of graft regeneration. Third, due to analysis of both axial and coronal planes, histologic quantification was not possible. Further characterization of cellular infiltration, immune response, and the epithelium is needed. Next, this preliminary work demonstrates a proof of concept applying synthetic scaffolds for airway replacement and does not involve graft seeding, thus not fully recapitulating constructs used in humans. Future work will be directed to planned euthanasia time points and will examine the impact of BM-MNC seeding of biosynthetic scaffolds. BM-MNC seeding in other tissue-engineered constructs prevents host macrophage infiltration and expression of pro-inflammatory markers, and this effect has been suggested to be scaffold-independent.10,28
Finally, a consideration should be made regarding the differences in scale when comparing mouse models to large animal and human studies. Given that a mouse trachea has a 1 mm inner lumen diameter, inflammation and narrowing can have a much greater clinical impact than in a large animal model with airway dimensions more consistent with a human. Conversely, diffusion of nutrients, neovascularization, and graft incorporation may be more easily accomplished in a mouse model than in a large animal or human model. Despite these limitations, a viable mouse model would let us apply well-established molecular reagents to define the mechanisms driving both regeneration and graft stenosis.
Conclusion
A mouse model utilizing an electrospun synthetic scaffold as a tracheal graft is feasible and allows the evaluation of epithelialization, neovascularization, and the development of stenosis. This model provides the fundamental groundwork on which further testing and analysis may be performed.
Acknowledgments
We would like to express our gratitude to the animal care and veterinary staff at the Research Institute at Nationwide Children’s Hospital. In addition, we would like to acknowledge the contribution of the Morphology Core and Ms Terri Shaffer, MLAS, RLATG, from the Small Animal Imaging Facility (micro computed tomography) at the Research institute at Nationwide Children’s Hospital.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Tendy Chiang received support from the NIH NHLBI K08HL138460.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jed Johnson is a co-founder and chief technology officer of Nanofiber Solutions, Inc. Christopher Breuer receives research support from Cook Medical (Bloomington, Indiana, USA) and Gunze Ltd (Kyoto, Japan). Christopher Breuer and Cameron Best are co-founders of LYST Therapeutics, LLC (Columbus, Ohio, USA). The remaining authors have no disclosures.
References
- 1.Chiang T, Pepper V, Best C, Onwuka E, Breuer CK. Clinical translation of tissue engineered trachea grafts. Ann Otol Rhinol Laryngol 2016;125(11):873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jungebluth P, Alici E, Baiguera S, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: a proof-of-concept study. Lancet 2011;378: 1997–2004. [DOI] [PubMed] [Google Scholar]
- 3.Kutten JC, McGovern D, Hobson CM, et al. Decellularized tracheal extracellular matrix supports epithelial migration, differentiation, and function. Tissue Eng Part A 2015;21:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajalloueian F, Lim ML, Lemon G, et al. Biomechanical and biocompatibility characteristics of electrospun polymeric tracheal scaffolds. Biomaterials 2014;35:5307–5315. [DOI] [PubMed] [Google Scholar]
- 5.Gilevich IV, Polyakov IS, Porkhanov VA, Chekhonin VP. Morphological analysis of biocompatibility of autologous bone marrow mononuclear cells with synthetic polyethylene terephthalate scaffold. Bull Exp Biol Med 2017;163: 400–404. [DOI] [PubMed] [Google Scholar]
- 6.Jungebluth P, Macchiarini P. Airway transplantation. Thorac Surg Clin 2014;24:97–106. [DOI] [PubMed] [Google Scholar]
- 7.Best CA, Pepper VK, Ohst D, et al. Designing a tissue-engineered tracheal scaffold for preclinical evaluation. Int J Pediatr Otorhinolaryngol 2018;104:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark ES, Best C, Onwuka E, et al. Effect of cell seeding on neotissue formation in a tissue engineered trachea. J Pediatr Surg 2016;51(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 2008;29: 1989–2006. [DOI] [PubMed] [Google Scholar]
- 10.Fukunishi T, Best CA, Ong CS, et al. Role of bone marrow mononuclear cell seeding for nanofiber vascular grafts. Tissue Eng Part A 2018;24(1–2):135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genden EM, Boros P, Liu J, Bromberg JS, Mayer L. Orthotopic tracheal transplantation in the murine model. Transplantation 2002;73:1420–1425. [DOI] [PubMed] [Google Scholar]
- 12.Jungraithmayr W, Jang JH, Schrepfer S, Inci I, Weder W. Small animal models of experimental obliterative bronchiolitis. Am J Respir Cell Mol Biol 2013;48:675–684. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Park HS, Oh SH, et al. Triple-layered polyurethane prosthesis with wrinkles for repairing partial tracheal defects. Laryngoscope 2014;124:2757–2763. [DOI] [PubMed] [Google Scholar]
- 14.Pepper VK, Onwuka EA, Best CA, et al. Endoscopic management of tissue-engineered tracheal graft stenosis in an ovine model. Laryngoscope 2017;127(10):2219–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukunishi T, Best CA, Sugiura T, et al. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J Thorac Cardiovasc Surg 2017;153:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong CS, Fukunishi T, Liu RH, et al. Bilateral arteriovenous shunts as a method for evaluating tissue-engineered vascular grafts in large animal models. Tissue Eng Part C Methods 2017;23:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Terry CM, Shiu YT, Cheung AK. Neointimal hyperplasia associated with synthetic hemodialysis grafts. Kidney Int 2008;74:1247–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deeken CR, Lake SP. Mechanical properties of the abdominal wall and biomaterials utilized for hernia repair. J Mech Behav Biomed Mater 2017;74:411–427. [DOI] [PubMed] [Google Scholar]
- 19.Ballyk PD, Walsh C, Butany J, Ojha M. Compliance mismatch may promote graft-artery intimal hyperplasia by altering suture-line stresses. J Biomechanics 1998;31:229–237. [DOI] [PubMed] [Google Scholar]
- 20.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet 2008;372:2023–2030. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton NJ, Kanani M, Roebuck DJ, et al. Tissue-engineered tracheal replacement in a child: a 4-year follow-up study. Am J Transplant 2015;15:2750–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nomoto M, Nomoto Y, Tada Y, et al. Bioengineered trachea using autologous chondrocytes for regeneration of tracheal cartilage in a rabbit model. Laryngoscope 2013;123: 2195–2201. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Yi T, Shinoka T, et al. Pilot mouse study of 1 mm inner diameter (ID) vascular graft using electrospun poly(ester urea) nanofibers. Adv Healthc Mater 2016;5:2427–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford NL, Martin EL, Lewis JF, Veldhuizen RA, Holdsworth DW, Drangova M. Quantifying lung morphology with respiratory-gated micro-CT in a murine model of emphysema. Phys Med Biol 2009;54:2121–2130. [DOI] [PubMed] [Google Scholar]
- 25.Warren MUK trials of airway transplants are in limbo. Science 2018;359:1448–1450. [DOI] [PubMed] [Google Scholar]
- 26.Breuer CK. The development and translation of the tissue-engineered vascular graft. J Pediatr Surg 2011;46:8–17. [DOI] [PubMed] [Google Scholar]
- 27.Genden EM, Iskander AJ, Bromberg JS, Mayer L. Orthotopic tracheal allografts undergo reepithelialization with recipient-derived epithelium. Arch Otolaryngol Head Neck Surg 2003;129:118–123. [DOI] [PubMed] [Google Scholar]
- 28.Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 2010;107:4669–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]