Abstract
Interest for the use of oxytocin as a treatment for addiction began over 40years ago. Better known for its roles in parturition, lactation and pair bonding, oxytocin also has anxiolytic properties, reduces immune and inflammatory responses, and has a role in learning and memory. In this chapter, oxytocin effects on addiction processes are described by highlighting research findings that have used oxytocin within current preclinical animal models of addiction, relapse, or craving. First, we provide a brief background of the endogenous oxytocin system followed by descriptions of the behavioral models used to study addiction, including models of drug taking and seeking. Then we review recent preclinical studies that have used oxytocin as a therapeutic intervention throughout multiple stages of the addiction cycle from a behavioral and neurobiological perspective. These models encompass the entire range of the addiction cycle including acquisition and maintenance of drug taking, withdrawal and craving during periods of drug abstinence, and ultimately relapse. We then posit several theories about how oxytocin interacts with both drug and social reward, as well as presenting a mechanistic account of how specific oxytocin receptor localization may contribute to oxytocin’s efficacy as an addiction therapeutic.
1. INTRODUCTION
Over 40years ago reports emerged that neurohypophyseal peptides interact with memory (De Wied, 1971), alcohol tolerance (Hoffman, Ritzmann, Walter, & Tabakoff, 1978), and opiate addiction (Van Ree & De Wied, 1977) leading to a field of investigation implicating use of the hypothalamic neuropeptide, oxytocin, in opioid, psychostimulant, and alcohol addiction (Kovacs, Sarnyai, & Szabo, 1998; Sarnyai & Kovacs, 2014). Interest in oxytocin and addiction waned for a period while research focused on other features and functions of oxytocin, when it became well known for its role in parturition, lactation and pair bonding (Lee, Macbeth, Pagani, & Young, 2009; Waldherr & Neumann, 2007). The seminal work of Carter, Insel, and Young established that oxytocin takes center stage in areas of social attachment (Carter, Williams, Witt, & Insel, 1992), social memory (Young, Lim, Gingrich, & Insel, 2001), and generating trust in humans (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005). In addition to increased trust, oxytocin acts as an anxiolytic, reduces immune and inflammatory responses, and has a role in learning, memory and pain reduction (reviewed in Tops, Koole, H, & Buisman-Pijlman, 2014). Coming full circle, interest in the use of oxytocin as a therapeutic for addiction has risen substantially over the past decade.
In this chapter, we focus on oxytocin’s impact on drug taking, seeking, and relapse by highlighting research findings that have used oxytocin within current models of addiction, relapse, or craving. First, we provide a brief background of the endogenous oxytocin system followed by descriptions of the behavioral models used to study addiction, including models of drug taking and seeking. Then we review recent preclinical studies that use oxytocin as a therapeutic intervention throughout multiple stages of the addiction cycle from a behavioral and neurobiological perspective. We then posit several theories about how oxytocin interacts with both drug and social reward, as well as presenting a mechanistic account of how specific oxytocin receptor localization may contribute to oxytocin’s efficacy as an addiction therapeutic.
1.1. The Endogenous Central Oxytocin System
The central and peripheral oxytocin systems have been recently reviewed (Bowen & Neumann, 2017; Buisman-Pijlman et al., 2014; Knobloch & Grinevich, 2014), so we will only briefly describe the central system here. The neuropeptide oxytocin, produced by the precursor protein oxytocin/neurophysin I prepropeptide, consists of 9 amino acids. Synthesis within the central nervous system takes place in the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus. The majority of oxytocin is transported to the neurohypophysis and thereby released into the bloodstream for peripheral distribution (Leng & Ludwig, 2016; Quirin, Kuhl, & Dusing, 2011). In the periphery, oxytocin release is stimulated by birth, suckling, and sexual stimulation in mammals as well as exposure to physical or psychosocial stress.
Hypothalamic oxytocin projection neurons target the forebrain, limbic and reward systems, including areas of the brain involved in both drug and social reward (Knobloch & Grinevich, 2014). Cells originating in the PVN deliver oxytocin to brain regions such as the amygdala, bed nucleus of the stria terminalis (BNST), lateral septum, hippocampus and nucleus accumbens core (NAcc) (Stoop, 2012). Within these projection areas oxytocin has specific effects on behavior. For example, within the central amygdala oxytocin reduces anxiety and inhibits stress responding (Neumann & Landgraf, 2012; Neumann & Slattery, 2016). Oxytocin within the lateral septum is involved in prosocial behavior including social preference (Lukas, Toth, Veenema, & Neumann, 2013). The impact of central oxytocin on addictive behaviors in rodent models will be discussed in detail in Section 3.
To date, there has been only one oxytocin receptor (OXTR) identified (Busnelli & Chini, 2017). These receptors are abundant throughout the peripheral and central nervous systems and are expressed in a sex dependent and species-specific manner (Dumais & Veenema, 2016; Lee et al., 2009). In the periphery, receptor expression is found in mammary glands, uterus, gastrointestinal tract, heart, and vascular endothelium layer (Gimpl & Fahrenholz, 2001). Centrally, oxytocin receptors are expressed in brain areas that regulate mood, and social and addictive behaviors such as the prefrontal cortex (PFC), ventral tegmental area (VTA), nucleus accumbens (NAc), hippocampus, hypothalamus, ventral pallidum, medial preoptic area, olfactory bulbs, (central) amygdala and brain stem (Gimpl & Fahrenholz, 2001; Sarnyai, 2011). Of these regions, males have increased oxytocin receptor expression mRNA in the NAc, hippocampus, medial preoptic area, and the BNST (an area projecting directly to the amygdala) relative to females (Dumais & Veenema, 2016). However, sex differences are not necessarily consistent across species as female mice show increased oxytocin receptor expression mRNA in the hippocampus and very few sex differences have been observed in other rodent species and humans (reviewed in Dumais & Veenema, 2016).
The OXTR is Gq-coupled and its activation results in changes of activity in phospholipase C and protein kinase C. The oxytocin receptor couples to two different G proteins, Gq/11 at its proximal portion of the C-terminus and to Gi/o depending on brain area and oxytocin concentration (reviewed in Busnelli & Chini, 2017). Activation of these two different signaling pathways may be one explanation for oxytocin’s diverse functions (Chini & Fanelli, 2000; Devost, Wrzal, & Zingg, 2008; Gimpl, Reitz, Brauer, & Trossen, 2008; Rimoldi et al., 2003). Supra-physiological levels of oxytocin also bind receptors for vasopressin, a closely related peptide, that also has effects on social mediated behaviors (Bowen & McGregor, 2014; Sala et al., 2011). Lemaire et al. (2002) proposed that the oxytocin and vasopressin V1a receptors are the predominant receptor subtypes in rat brain and spinal cord with specific distribution patterns. However, it is the vasopressin V1b2 receptor that is co-localized with oxytocin receptors in forebrain areas, with oxytocin receptor gene expression being much more robust than that of V1b2 (Vaccari, Lolait, & Ostrowski, 1998).
Characterization of cell-type specific oxytocin receptor expression has been impeded by the lack of specific antibodies recognizing the oxytocin receptor with levels of specificity to rule out vasopressin binding sites. This absence has limited the use of immunohistochemistry to identify the neurochemical and projection phenotype of oxytocin sensitive cells in the brain (see Mouillac, Manning, & Durroux, 2008). Recently, through the use of genetically modified mice, progress has been made to characterize neuronal phenotypes that co-localize with oxytocin receptors in specific brain areas integral to addiction circuitry (Dolen, Darvishzadeh, Huang, & Malenka, 2013; Nakajima, Gorlich, & Heintz, 2014; Peris et al., 2017; Tan et al., 2017). Magnocellular oxytocin neurons in the PVN express high levels of corticotrophin-releasing factor (CRF) type 2 receptors and these CRF receptor positive neurons express oxytocin receptor mRNA as do CRF receptor positive neurons in the bed nucleus of the striatal terminalis (Dabrowska et al., 2011). Oxytocin receptors have been identified on cortical somatostatin-containing interneurons (Li, Nakajima, Ibanez-Tallon, & Heintz, 2016; Nakajima et al., 2014), cortical glutamatergic and GABAergic neurons (Tan et al., 2017), parvalbumin positive interneurons and astrocytes in the NAcc (Dolen et al., 2013), and on glutamate, GABA and dopamine producing neurons in the VTA (Peris et al., 2017). These three brain areas are crucial to the circuitry regulating drug reward and reinforcement as well as relapse to drug use, and oxytocin in each of these areas may uniquely impact drug seeking.
1.2. Oxytocin and the Blood–Brain Barrier
A current research question being investigated is whether oxytocin administration through systemic delivery methods crosses the blood–brain barrier. Recent evidence suggests that it does to a limited extent and mechanisms underlying such penetration into brain are just recently coming into focus (Veening & Olivier, 2013). Central oxytocin synthesized in the SON is secreted into the bloodstream through neurohemal contact in response to reproductive behaviors, social stimuli, and stress (Bowen & Neumann, 2017). However, only 1–2% of oxytocin synthesized and released by peripheral organs (uterine epithelium, ovary, testis, vascular endothelium cells and heart) (Nishimori et al., 2008) and or by the posterior pituitary cross the blood–brain barrier (Ermisch et al., 1985; Landgraf, Ermisch, & Hess, 1979). Veening and Olivier (2013) discuss possible other ways in which oxytocin can pass from peripheral circulation to the brain and back. One mechanism may be through transport via a saturable carrier (McEwen, 2004). Alternatively, oxytocin may be able to cross a damaged BBB more readily. For example, heavy alcohol/drug use may induce leakage of oxytocin from the blood to the cerebrospinal fluid (Kovacs et al., 1998; Nishimori et al., 2008). Stress, hypertension, or disease may also impact blood–brain barrier permeability (Churchland & Winkielman, 2012).
Administration of central oxytocin activates oxytocin-producing cell bodies in the paraventricular nucleus of the hypothalamus (PVN) (Carson, Cornish, Guastella, Hunt, & McGregor, 2010; Carson, Hunt, et al., 2010). This activation results in local dendritic release of oxytocin and an ensuing positive-feedback effect promoting prolonged activation of the oxytocin system (Ludwig et al., 2002; Rossoni et al., 2008), which is presumed to cause brain wide oxytocin release, including areas associated with addiction. It has been suggested that only a relatively small amount of oxytocin is required to initiate such a feed-forward mechanism to propagate endogenous oxytocin production (Rossoni et al., 2008). Recently, Neumann, Maloumby, Beiderbeck, Lukas, and Landgraf (2013) used microdialysis procedures in rats to demonstrate that systemic (intraperitoneal, ip) administration of oxytocin caused rapid peak levels in brain dialysates 30min post-injection, providing direct evidence that systemic oxytocin increases central oxytocin levels. Consistent with these results, intravenous oxytocin (5IU/kg) increased oxytocin levels in cerebrospinal fluid in rhesus macaques, peaking at 15min post-infusion and gradually returning to baseline by 120min (Freeman et al., 2016). However, Lee et al. (2018) demonstrated that while oxytocin administered through intranasal and intravenous routes of administration increased oxytocin in cerebrospinal fluid, it did not activate a feed-forward mechanism to elevate endogenous oxytocin. Thus, the mechanism by which exogenous oxytocin delivered in the periphery activates oxytocin signaling in the brain remains unclear. Further studies are needed to address this issue as it is relevant to the potential for oxytocin to serve as a therapeutic for alcohol/drug addiction.
2. PRECLINICAL MODELS OF ADDICTION
The most commonly used behavioral tasks to study alcohol/drug reward and reinforcement include intravenous or oral self-administration involving operant conditioning procedures and place conditioning. Although these two measures have some overlap, the general consensus is that these procedures involve dissociable processes (Bardo & Bevins, 2000). Other models to study alcohol addiction involve free-choice drinking and binge-like drinking procedures. This section will describe effects of oxytocin treatment in studies employing these models as well as procedures commonly used to index social reward.
2.1. Intravenous (IV) Drug Self-administration
Drug self-administration models rely on the basic premises of operant conditioning—a response (e.g., lever press, keypeck, nose poke, etc.) results in an outcome (i.e., a drug infusion typically via an intravenous route of administration). In this model rats or mice are typically trained to press a lever, resulting in an IV infusion of drug into an indwelling jugular catheter. A drug infusion is often paired with the presentation of a stimulus (cue light or a tone), which through repeated pairing can become secondary reinforcers themselves. In addition to an “active” lever that results in drug delivery, a second “inactive” lever is typically available, where responses are recorded but do not result in any consequences. Responses on the “inactive” lever are typically considered a measure of non-differentiated responding and reflective of general (nonspecific) motor activation. Subjects can administer drugs on simple schedules of reinforcement including fixed ratio (FR) or variable ratio (VR) schedules, until stable responding is obtained. Alternatively, more complex schedules may be used, such as a progressive ratio (PR), in which the number of active lever presses required to obtain a reinforcer increases along a logarithm for subsequent reinforcers.
Once stable responding is achieved, animals undergo extinction or abstinence based on specific experimental parameters. The extinction–reinstatement model is considered to have high face and construct validity (Markou, Chiamulera, Geyer, Tricklebank, & Steckler, 2008) as drug-seeking behavior is re-established when the animals are re-exposed to the drug, drug-paired cues, or stress (Bossert, Marchant, Calu, & Shaham, 2013; Feltenstein & See, 2008; Yahyavi-Firouz-Abadi & See, 2009), all of which also induce drug relapse in humans (Childress et al., 1993; Sinha, 2001). Animals undergoing extinction are placed in the drug-paired environment in the absence of drug or drug-paired cues. Extinction results in decreased responding with repeated trials, as reinforcement no longer occurs following a response. Reinstatement occurs when rats resume responding on a previously drug-paired lever, though typically no drug is actually delivered. During drug-primed reinstatement rats typically receive a small dose of the self-administered drug prior to the session and typically results in a robust increase in drug seeking. Cue-induced reinstatement occurs when rats are re-exposed to the previously drug-paired cues. Finally, during stress-induced reinstatement, administration of a physiological stressor or anxiogenic drug, such as footshock or yohimbine, can induce reinstatement.
Adaptations to the above procedures have been incorporated to demonstrate time dependent “incubation” of drug craving without extinction procedures. In this model rats undergo self-administration training as described above. Following the maintenance phase, rats are subject to abstinence for approximately 1–8weeks. During abstinence rats are placed in their home cages (Grimm, Hope, Wise, & Shaham, 2001), in an alternative environment (Hearing, Schochet, See, & McGinty, 2010), or handled on a daily basis (Moussawi et al., 2011; Reichel, Moussawi, Do, Kalivas, & See, 2011). Rats are never returned to the drug-taking environment during the abstinence period because “relapse” occurs when rats are placed back into the drug-paired context (Reichel & Bevins, 2009). The extent and degree of relapse varies as a function of the time since the last drug experience (Grimm et al., 2001; Tran-Nguyen et al., 1998), and generally increases over time, and thus has been coined “incubation” (Grimm et al., 2001) of drug craving.
2.2. Conditioned Place Preference
In contrast to the behavior-outcome schedules of reinforcement that maintain responding for the drug in self-administration experiments, the place conditioning task relies on the outcome of stimulus–stimulus associations that come to control behavior. In the place conditioning task, one environment or discrete cue (conditioned stimulus, CS) is paired with a biologically and motivationally rewarding stimulus (unconditioned stimulus, US; e.g., cocaine, novelty, water, conspecific), whereas another environment remains unpaired or paired with only a neutral stimulus such as saline administration. On the test day, the animal is given a choice between the two environments. In most place conditioning preparations, exteroceptive cues of the paired environment (CS) become associated with the rewarding aspects of the stimuli of interest (i.e., the US). Accordingly, the paired environment acquires an appetitive value, thus, eliciting approach behaviors (Bardo & Bevins, 2000; Panksepp, Nocjar, Burgdorf, Panksepp, & Huber, 2004; Reichel, Wilkinson, & Bevins, 2010). This approach behavior is expressed as an increase in the amount of time spent in the paired environment on the choice test day.
Compartment preferences motivated by associations with drug reward are also subject to extinction and reinstatement procedures (Mueller & Stewart, 2000). In this case, extinction procedures refer to re-exposure to the drug-associated chamber without the US (i.e., drug effects) (Rescorla, 2004). Accordingly, conditioned responding (i.e., time spent on the cocaine-paired side) decreases on subsequent test days. After conditioned responding is no longer evident, re-exposure US can reinstate conditioned responding (Rescorla, 2004; Shaham, Shalev, Lu, de Wit, & Stewart, 2003). With regards to place conditioning, cocaine-associated cues maintain effectiveness over a considerable length of time (Mueller & Stewart, 2000; Reichel & Bevins, 2008). Notably, this extinguished preference can be reinstated by a priming dose of the drug (Mueller & Stewart, 2000; Reichel & Bevins, 2008).
2.3. Drinking in the Dark
A number of preclinical models have been employed to study the neurobiological consequences of alcohol drinking, however, in many models of voluntary consumption rodents typically consume low levels of alcohol that do not generate blood ethanol concentrations (BECs) considered to be pharmacologically meaningful or the ability to induce dependence. The drinking in the dark (DID) procedure promotes high levels of ethanol drinking in ethanol-preferring strains of mice by taking advantage of time in the animals dark cycle in which activity and nocturnal ingestive behaviors are high (Thiele & Navarro, 2014). A “binge” session, as defined by the National Institutes on Alcohol Abuse and Alcoholism (NIAAA), as a pattern of drinking that produces BECs exceeding 0.08% (80mg/dL). Using this limited access procedure, mice typically consume enough ethanol to reach BECs >100mg/dL and resulting in behavioral evidence of intoxication in a short period of time (Sprow & Thiele, 2012).
In the most common variation of the DID procedure, first implemented by Rhodes et al., C57BL/6J mice are presented with a single bottle containing 20% (v/v) ethanol in place of the home cage water bottle for 2h, 3h after the beginning of the dark cycle, for 3 consecutive days. On the 4th day, the drinking session is extended to 4h during which mice exhibit binge-like behavior, consuming significant and physiologically relevant amounts of ethanol (Rhodes, Best, Belknap, Finn, & Crabbe, 2005; Thiele, Crabbe, & Boehm, 2014). Several studies have characterized DID and have shown that BECs of <100mg/dL produce significant motor incoordination (Moore et al., 2007; Rhodes et al., 2007), tolerance to this effect after repeated binge-like episodes (Linsenbardt, Moore, Griffin, Gigante, & Boehm, 2011) and increases in subsequent voluntary ethanol drinking (Cox, Olney, et al., 2013; Cox, Young, See, & Reichel, 2013). The DID model does not require training prior exposure to ethanol or inclusion of sweet compounds to motivate high levels of ethanol intake. Further, in contrast to other models of binge-like drinking, DID does not require ethanol to be administered by the experimenter as by gavage, injection or ethanol vapor exposure (Becker & Lopez, 2004), or by fluid deprivation or inclusion of ethanol into an animals source of nutrients. Such procedures may cause stress to the animal or other confounding variables, calling into the question the motivation to drink ethanol (Thiele & Navarro, 2014). Thus, DID provides a simple and effective high throughput tool for characterization of potential pharmacotherapies and may provide a useful model for studying binge patterns of consumption and the transition to ethanol dependence.
2.4. Social Interaction and Conditioned Social Reward
Environments that have been associated with social interaction can also engender place preferences. In general, conditioned social reward is evaluated by placing the animal into one compartment of the place conditioning apparatus with their social partner (US), and during the other half of the sessions the animals are placed alone in the alternate compartment (Douglas, Varlinskaya, & Spear, 2004; Thiel, Okun, & Neisewander, 2008; Trezza, Damsteegt, & Vanderschuren, 2009). On test, animals are given a choice to spend time between the two compartments. More time in the side that was associated with social interaction is interpreted as conditioned social reward. Interestingly, conditioned social reward can be extinguished and reinstated by a single re-exposure to the social partner in the social-paired compartment (Trezza et al., 2009).
In mice, cocaine (15mg/kg) and social interaction (15 min) engender similar preferences for the paired environments (Kummer et al., 2014; Panksepp & Lahvis, 2007) suggesting similar conditioned rewarding value of the US. In a variation of a place conditioning procedure developed by Reichel and Bevins (2008, 2010) competition between conditioned rewards can define the relative competitive value of each reward. Conditioned social reward can directly compete with cocaine conditioned reward when one side was paired with social interaction and the other with cocaine (Fritz et al., 2011). Furthermore, after establishing a place preference to cocaine, extinction of the cocaine CS with social reward conditioning in the alternate side inhibits cocaine-primed reinstatement of the cocaine CR (Fritz et al., 2011).
3. OXYTOCIN EFFECTS ON PRECLINICAL MODELS OF ADDICTION
The previous section described some of the most common behavioral assays employed to study alcohol and drug addiction. Within each of these models, oxytocin decreases the tendency to take drugs and reduces the propensity relapse. This next section will detail the oxytocin effects on methamphetamine, cocaine, heroin and alcohol intake and seeking within the models previously described.
3.1. Methamphetamine
Methamphetamine (N-methyl-O-phenylisopropylamine; meth) is a highly addictive illicit stimulant. According to the United Nations Office on Drugs and Crime (2017) meth and other amphetamine-like stimulants are second only to opioids in the burden of disease risk. And, within the United States, there is a rising trend of meth-related visits at publicly funded treatment facilities (Gonzales, Mooney, & Rawson, 2010) and emergency departments (Dobkin & Nicosia, 2009). The physiological and cognitive consequences of meth use are detailed in Barr et al. (2006), in which the authors highlight the high abuse potential of meth and its associated impairments in working memory, attention, and executive function. Deficits in long-term memory have also been reported following more chronic meth use (reviewed in Bernheim, See, & Reichel, 2016).
Physiologically, the short-term effects of meth use include enhanced energy, alertness, elation, and elevated heart rate/blood pressure (Rawson, Gonzales, & Brethen, 2002). Meth facilitates rapid release of norepinephrine, serotonin, and dopamine at synapses (Rothman et al., 2001). Meth reverses mechanisms that regulate homeostatic levels of these monoamines leading to a sustained increase in synaptic levels through its action as an indirect agonist (Elkashef et al., 2008). Chronic meth users often have various cardiovascular impairments such as arrhythmias, hypertension, cardiomyopathy, and acute myocardial infarction (Kaye, McKetin, Duflou, & Darke, 2007; Turnipseed, Richards, Kirk, Diercks, & Amsterdam, 2003; Westover, Nakonezny, & Haley, 2008). It is not uncommon for users to suffer from accelerated coronary artery disease and cardiac hypertrophy. Meth users also present with severe mental health problems, skin infections, and dental pathology (Hendrickson, Cloutier, & McConnell, 2008). The abuse potential of meth, taken together with the high abuse rate and subsequent physical and mental health consequences highlights the need for effective pharmacotherapies. Preclinical data suggest that oxytocin is a viable therapeutic target for the treatment of meth addiction at various stages of the addiction cycle.
3.1.1. Oxytocin Effects on Acquisition and Maintenance of Meth Addiction
Significant progress has been made examining the therapeutic effect of oxytocin on the acquisition of meth-seeking behavior. For example, Qi et al. (2009) demonstrated that intracerebroventricular (icv) infusions of oxytocin (2.5μg) given 30min before meth impaired acquisition of a place preference in mice. Here the authors infused oxytocin during training in a conditioned place preference (CPP) task and found that oxytocin-injected animals showed less preference for the meth-associated chamber relative to controls. However, when oxytocin was infused after training, prior to testing, there was no effect on meth place preference. Combined this data shows that oxytocin impacts the induction but not the expression of meth-induced CPP (Qi et al., 2009). Consistently, systemic oxytocin administration (0.3 and 1mg/kg; ip) attenuated active lever presses and meth infusions in self-administering male rats (Carson, Cornish, et al., 2010). However, oxytocin did not block acquisition or maintenance of self-administration behaviors, as all rats self-administered meth and responded on PR schedule of reinforcement. Plasma oxytocin concentrations were also increased 10days following the last administration of oxytocin, providing evidence that systemic oxytocin administration produces an up-regulation of the endogenous oxytocin system (Carson, Guastella, Taylor, & McGregor, 2013).
3.1.2. Oxytocin Effects on Motivation to Seek Meth
The effect of oxytocin on motivation to seek meth is dependent on experimental procedures, but overall studies indicate that oxytocin decreases motivation to take meth in male and female rats. Our initial report found that peripheral oxytocin administration (1mg/kg; ip) decreased motivation to seek meth only in females as indicated by reduction of active lever presses, break point (i.e., number of lever presses before reward is obtained), and infusions during a PR test (Cox, Young, et al., 2013). Baseline levels of motivation for meth differed significantly between males and females, with female rats showing greater levels of active lever presses and breakpoints. This is consistent with previous studies showing that females typically display greater motivated meth taking relative to males (Roth & Carroll, 2004). Although oxytocin did not impact male responding on the PR tests, a similar study with males found that oxytocin (1mg/kg; ip) decreased motivated responding for meth as indicated by lower break points (Carson, Cornish, et al., 2010; Carson, Hunt, et al., 2010). These contrasting results can be attributed to methodological differences. In Cox, Young, et al. (2013), rats were maintained on an FR 5 schedule of reinforcement prior to PR testing. In Carson, Cornish, et al. (2010) and Carson, Hunt, et al. (2010), rats were maintained along a PR schedule of reinforcement and received escalating doses of oxytocin before testing over 5 consecutive days. Consecutive administration of oxytocin may have resulted in increased sensitivity to oxytocin in male rats leading to a greater effect on motivated meth seeking.
Perhaps shedding some light on oxytocin’s impact on motivated meth taking comes from a follow up study in our laboratory using a newly developed behavioral economics (BE) paradigm (Cox et al., 2017). Using this task, comprised of a within-session BE procedure (Bentzley, Jhou, & Aston-Jones, 2014), we determined that greater motivation to seek meth was predictive of an increased likelihood to reinstate meth-seeking behavior in both males and females. Furthermore, oxytocin (1mg/kg; ip) decreased motivation for meth in both male and female rats as indicated by an increase in alpha score (the task-specific measure of motivation). It is important to note that although oxytocin was effectively reduced motivation to seek meth, drug consumption at low effort (consumption when cost to obtain reward requires little to no effort, i.e., “hedonic set point”) was not affected. The effects were centrally-driven as icv and intra-NAc infusions of an oxytocin antagonist blocked the attenuating effect of peripheral oxytocin. In general, studies assessing oxytocin’s effects on motivation to take meth show decreases in responding for both males and females, which may subsequently affect the likelihood for relapse.
3.1.3. Oxytocin Effects on Extinction of Meth Seeking
Few studies have looked at the direct effect of oxytocin on the extinction of meth-seeking behaviors. In a CPP paradigm, multiple doses of central oxytocin infusions (0.1, 0.5, and 2.5μg; icv) enhanced the extinction of meth-induced conditioned place preference (Qi et al., 2009). Mice went through CPP and were subsequently extinguished after 10days. During extinction, mice were centrally infused with oxytocin before chamber placement in the absence of meth. Extinction criteria was set as a lack of significant difference between exploration in either the previously meth-paired chamber and control chamber. Oxytocin facilitated extinction indexed by less time spent in the meth associated side by the 7th day of extinction training. While no studies to our knowledge have investigated the effect of oxytocin on extinction following meth self-administration, Carson, Cornish, et al. (2010) and Carson, Hunt, et al. (2010) report that multiple injections of oxytocin during the self-administration period do not impact lever responding during extinction.
3.1.4. Oxytocin Effects on Reinstated Meth-Seeking Behavior
A number of preclinical studies have shown that oxytocin is a viable therapeutic target for the treatment of reinstated meth seeing in response to conditioned cues, a drug or a stress prime. Meth-primed reinstatement is reduced by systemic oxytocin (1mg/kg; ip) in both male and female rats (Carson, Cornish, et al., 2010; Carson, Hunt, et al., 2010; Cox, Olney, et al., 2013; Cox, Young, et al., 2013). When directly compared, females in general respond to a greater extent to a priming injection of meth, but oxytocin is effective in both sexes (Cox, Young, et al., 2013). Repeated treatment in adolescent females also has an inhibitory effect on meth-primed reinstatement during adulthood (Hicks, Cornish, Baracz, Suraev, & McGregor, 2016). Oxytocin directly applied to the NAcc blocked meth-induced CPP (Baracz et al., 2012) and attenuated meth-primed reinstatement (Baracz, Everett, & Cornish, 2015; Baracz, Everett, McGregor, & Cornish, 2016). Co-administration of an oxytocin receptor antagonist into the NAcc only partially blocked the attenuating effect of intra-NAc oxytocin (Baracz et al., 2016; Everett, McGregor, Baracz, & Cornish, 2018) in contrast to a V1a antagonist, which sufficiently blocked the attenuating effect of systemic and intra-NAc oxytocin on meth-primed reinstatement (Everett et al., 2018). In regard to meth-primed reinstatement, the ability of oxytocin to reduce responding may be through vasopressin receptors driving the therapeutic effect. In contrast, NAcc oxytocin receptor antagonism blocked the attenuating effect of systemic oxytocin on motivation to seek meth and in response to meth associated cues (Cox et al., 2017). Taken together, it appears that oxytocin receptors in the NAcc may have a different roll depending on the phase of addiction pathology or the events that precipitate relapse.
In regard to cued reinstatement of meth seeking, oxytocin decreases responding in males and females (Bernheim, Leong, Berini, & Reichel, 2017; Cox et al., 2017; Cox, Young, et al., 2013). This decrease may involve additional behavioral parameters because in one study (Cox, Young, et al., 2013), rats were reinstated on an FR5 schedule of reinstatement and responding was greater female rats. When rats were reinstated on an FR1, both sexes robustly reinstated on the active lever and oxytocin decreased this reinstatement (Bernheim et al., 2017; Cox et al., 2017). Recently, we have proposed that the ability of oxytocin to reduce cued reinstatement is through interactions with the glutamatergic system in the NAcc (discussed in Section 4.1).
Consistent with other reinstatement modalities, oxytocin is also equally effective in treating stress-induced meth-reinstatement. A pharmacological stressor yohimbine, which acts an alpha-2-adrenoceptor antagonist, facilitated meth seeking after extinction to a greater degree in females relative to males (Cox, Young, et al., 2013). Systemic oxytocin administered before yohimbine prevented reinstatement (Cox, Young, et al., 2013). Oxytocin also decreased reinstatement in response to an ethologically-relevant predator odor (trimethylthiazoline; TMT) (Ferland, Reichel, & McGinty, 2016). Male rats underwent exposure to TMT (or a neutral odor) in locomotor chambers for 5days before self-administration training. During testing rats were re-exposed to TMT in the testing chamber, and rats reinstated meth seeking. An acute injection of oxytocin given before reinstatement testing was sufficient to attenuate meth seeking in both conditions. As well, in a separate experiment, repeated oxytocin doses following TMT pre-exposure but prior to self-administration training blocked TMT-induced reinstatement, suggesting a protective effect of oxytocin administration on future meth-seeking behavior (Ferland et al., 2016; Hicks et al., 2016). Centrally administered oxytocin (0.1, 0.5, and 2.5μg; icv) reduced the effect of restraint stress on reinstatement of meth CPP but did not block meth-primed CPP (Qi et al., 2009).
3.2. Cocaine
The number of cocaine users in the United States rose exponentially in the mid-1990s, where there was an 82% increase within a 4year period. As of 2012, there were 4.7 million individuals above the age of 12 reported current or past use of cocaine within the United States (Palamar, Davies, Ompad, Cleland, & Weitzman, 2015). Approximately 5–10% of all emergency room visits in the country can be attributed to cocaine usage, resulting in an estimated cost of $83 million dollars a year for treatment of cocaine users (Maraj, Figueredo, & Lynn Morris, 2010). Some of the acute toxic effects of cocaine use include cardiovascular complications, anxiety, panic, and paranoia. Chronic use of cocaine can result in fatigue, blood borne disease, stroke, seizures, and sudden death. Despite the prevalence of cocaine as a prominent drug of abuse, and its associated negative health outcomes, there are no well-established pharmacological treatment options for cocaine addiction. Preclinical studies have found that oxytocin might be a viable therapeutic target (Sarnyai & Kovacs, 2014) through blockade of cocaine-associated behaviors such as hypervocalization (Kovacs, Sarnyai, Barbarczi, Szabo, & Telegdy, 1990) and cocaine-induced stereotypy (Sarnyai & Kovacs, 1994). Here we will cover the effect of oxytocin administration on various stages of the cocaine addiction cycle and its potential mechanisms.
3.2.1. Oxytocin Effects on Cocaine Acquisition and Maintenance
One of the earliest studies investigating the effect of oxytocin administration on cocaine self-administration determined that a systemic injection of oxytocin reduced the amount of lever presses during the maintenance phase of cocaine self-administration (Sarnyai & Kovacs, 1994). Other preclinical studies have found that oxytocin dose dependently (0.3, 1, and 3mg/kg; ip) reduced active lever pressing and cocaine intake on both an FR1 and FR5 schedule of reinforcement in male rats (Zhou et al., 2014). In females, similar doses of systemic oxytocin (0.3, 1, and 3mg/kg; ip) attenuated lever pressing and cocaine intake on an FR1 schedule of reinforcement (Leong, Zhou, Ghee, See, & Reichel, 2016). Interestingly, despite receiving multiple oxytocin injections over the course of self-administration, female rats displayed no sustained reduction in overall cocaine intake between self-administration test days (Leong et al., 2016).
3.2.2. Oxytocin Effects on Motivation of Cocaine Seeking
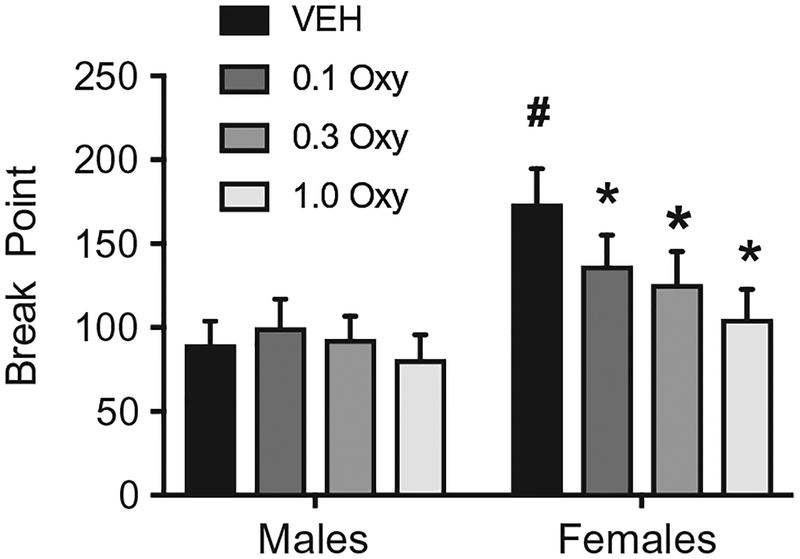
Consistent with meth, oxytocin decreases motivation to take cocaine in male and female rats. Zhou et al. (2014) discovered that peripheral administration of oxytocin (1mg/kg) was effective in reducing active lever presses and break points in a PR test of cocaine seeking in male rats. This suggests that, in males, oxytocin is effective in suppressing the motivational limit of cocaine. These results were further supported in a recent study that employed a within-session model of economic demand of cocaine (Bentzley et al., 2014). Here, oxytocin reduced demand and motivation for cocaine in male rats with a history of cocaine self-administration. Through this novel BE paradigm, the effectiveness of oxytocin in suppressing demand was proportional to their initial baseline demand, which provides translational value for the use of oxytocin as a pharmacological treatment option of cocaine use. In females, oxytocin proved to be effective in reducing breakpoint in a PR test of cocaine seeking in female rats (Fig. 1). However, in contrast to Zhou et al. (2014) which found that oxytocin attenuates motivation in male rats, oxytocin did not impact break point values in male rats in our unpublished study (Fig. 1). One explanation for this sex difference is that oxytocin has sex-specific effects on motivation to take cocaine. However, the difference could also be due to innate differences in motivational properties between males and females assessed with a PR procedure. Females displayed significantly greater motivation to take cocaine leading to a floor effect in male rats that might render oxytocin ineffective relative to females. Indeed, oxytocin did reduce motivation for cocaine in the behavior economics study that accounts for both the demand and motivation for drug (Bentzley et al., 2014).
Fig. 1.
Oxytocin decreased the breakpoint for cocaine self-administration in female rats. # Females significantly greater than males. *Significantly reduced relative to vehicle-treated animals of the same sex vehicle.
3.2.3. Oxytocin Effects on Cocaine Extinction
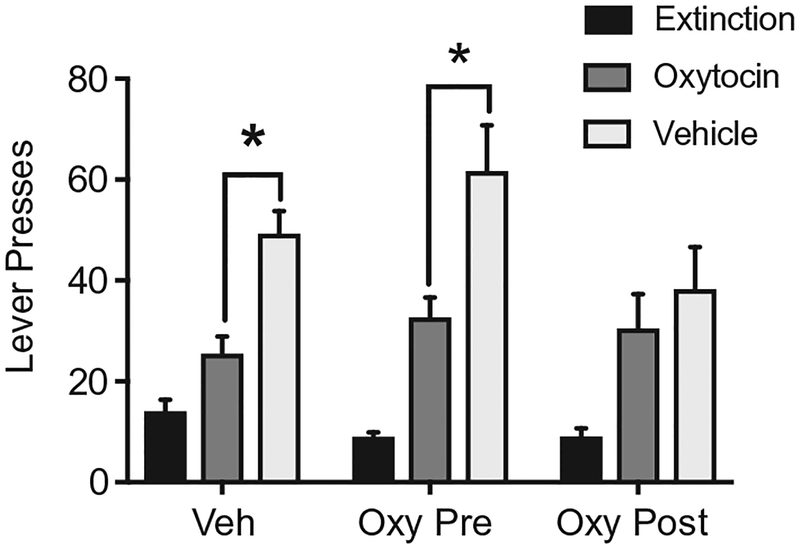
To our knowledge, few studies have investigated the effect of oxytocin on cocaine extinction behavior. A recent study revealed that oxytocin reduces lever responding on the first day of extinction (Ext Day 1) in male rats (Bentzley et al., 2014). However, it is uncertain whether this single dose of oxytocin on Day 1 of extinction is sufficient to produce long-lasting changes on subsequent extinction sessions or on measures of relapse. We gave oxytocin to male rats 30min before daily extinction sessions (Oxy Pre) or immediately after the session (Oxy Post). A separate group of rats received vehicle (Fig. 2). There were no differences in lever responding over 8days, all rats then underwent cue-induced reinstatement tests with oxytocin or vehicle injected 30min before chamber placement. Oxytocin attenuated lever responses in Veh and Oxy Pre-rats relative to vehicle injected rats in the same group. Interestingly, Oxy Post rats given vehicle had reduced reinstatement responding consistent with rats that received oxytocin on test. Despite not having an effect of extinction responding, subsequent reinstatement was blunted when oxytocin was given after the daily session, future work will need to determine mechanisms by which oxytocin enhanced learning about the extinction session. Early studies have found that oxytocin infused icv or directly into the hippocampus facilitated extinction of an active avoidance task (Bohus, Urban, van Wimersma Greidanus, & de Wied, 1978; Ibragimov, 1990). However, future work will need to determine mechanisms by which oxytocin enhanced learning about the extinction session within a self-administration paradigm.
Fig. 2.
Daily oxytocin administered following extinction sessions deceased cue-induced reinstatement of cocaine seeking. Vehicle-treated animals received daily saline injections, Oxy Pre animals received oxytocin (1 mg/kg) before the extinction session and Oxy Post animals had oxytocin after the session. On test days rats were injected with vehicle or oxytocin. *Significant difference between oxytocin and vehicle treatments.
3.2.4. Oxytocin Effects on Cocaine Relapse/Reinstatement
Overwhelming preclinical evidence suggests that oxytocin may have therapeutic potential for the treatment of cocaine addiction through preclinical assessment of reinstatement of cocaine seeking. In general, peripheral oxytocin administration attenuates lever pressing during a cocaine-primed reinstatement test and cue-induced reinstatement tests in male (Bentzley et al., 2014; Zhou et al., 2014) and female rats and this reduction is not dependent on cycle (Leong et al., 2016; Leong et al., 2017; Weber et al., 2018). Central infusions of oxytocin (icv) also attenuate cue-induced cocaine-seeking behavior in both sexes (Leong et al., 2017; Morales-Rivera et al., 2014). Recent studies have begun to highlight specific neural regions that may be mediating oxytocin’s central effect on cocaine-seeking behavior.
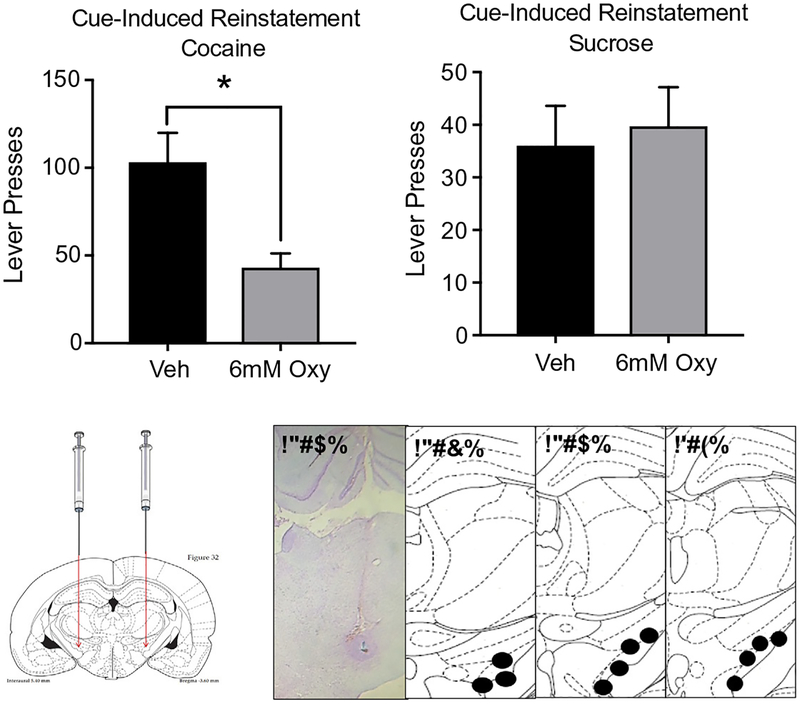
Few studies have directly compared oxytocin’s effect on neuronal function following cocaine self-administration. Recently, we demonstrated that cue-induced fos activation in various brain regions was normalized (returned to extinction values) by a systemic injection of oxytocin (Leong et al., 2017). Specifically, in the medial prefrontal cortex (mPFC), NAc, and subthalamic nucleus (STN) fos levels were elevated in response to a cocaine-associated cue and oxytocin brought this expression back down to control levels. In contrast, oxytocin enhanced central amygdala (CeA) fos expression. The CeA is a region critical in mediating emotional responses and the mPFC, NAc, and STN are critical components of the reward circuit. Direct oxytocin infusions into the aforementioned brain areas result in regionally specific changes in cued reinstatement. Intra-accumbal oxytocin reduced whereas intra-PFC oxytocin increased reinstatement of cocaine seeking (Weber et al., 2018). The STN, a downstream projection of the NAc, is also an important region mediating the effect of oxytocin on drug seeking (Baracz & Cornish, 2016). Fig. 3 shows that intra-STN infusions of oxytocin reduced cued reinstatement of cocaine seeking, but not sucrose seeking. The involvement of the STN in mediating oxytocin’s effect on cocaine seeking is similar to its involvement in mediating oxytocin’s effect on meth-seeking behavior (Baracz et al., 2012; Baracz et al., 2015).
Fig. 3.
Oxytocin infused into the STN deceased cued-induced reinstatement of cocaine but not sucrose seeking. Vehicle or oxytocin was infused into the STN before reinstatement testing. Images depict the terminal point of the injectors used to infuse compounds, with numbers next to brain sections detailing the distance in mm to Bregma. *Significantly decreased compared to vehicle.
Another similarity with meth, a concurrent intra-NAcc infusion of an mGluR2/3 antagonist blocked the oxytocin reduction of cued reinstatement suggesting presynaptic mechanisms in the NAcc (Bernheim et al., 2017; Weber et al., 2018). Similar findings were uncovered with peripheral administration of both compounds. This suggests that the mechanisms underlying oxytocin’s therapeutic effect on relapse behavior are pharmacologically and neuroanatomically similar in both cocaine and meth addiction. Overall, there is strong evidence that oxytocin is a viable pharmacological therapeutic target for the treatment of cocaine addiction throughout various stages of the addiction cycle. Recent studies have provided insight into the mechanisms underlying oxytocin’s impact on cocaine-seeking behavior. Importantly, few sex differences have been documented thus far, at the behavioral and neuronal level suggesting that it might be equally as effective in both males and females.
3.3. Heroin
There has been a substantial increase in prevalence of heroin and prescription opioid use in the United States over the last few years, with users ranging from affluent suburban backgrounds to low-income minority populations (Cicero, Ellis, Surratt, & Kurtz, 2014). Nationwide, there has been a stark increase in documented overdose-related hospitalizations within the past 10years (Unick, Rosenblum, Mars, & Ciccarone, 2013). The rise in heroin users is attributed to the increase in prescription opioid abuse in the last 20years (Unick et al., 2013), accelerated by the frequently prescribed oxycodone in the 1990s (Siegal, Carlson, Kenne, & Swora, 2003). In 2014 alone, opioid overdose accounted for 28,647 deaths in the United States (Rudd, Rudd, Seth, David, & Scholl, 2016). Various medical complications are associated with heroin use including cardiac infections, kidney and liver disease, autoimmune disorders, and depression. Furthermore, the threat of heroin overdose is immensely high as large doses of heroin can quickly cause respiratory arrest.
Despite the availability of drugs to counteract the immediate effects of heroin overdose, such as the opioid receptor antagonist naloxone and buprenorphine (Sung & Conry, 2006), few other pharmacological options exist to combat heroin’s significant addictive properties. Preliminary findings suggest that oxytocin might act as a potential therapeutic target or adjunct treatment for heroin addiction at various stages of the addiction cycle. Chronic opioid administration reduced oxytocin immunoreactivity in the hippocampus and decreased oxytocin mRNA levels in the hypothalamus and SON, the primary area of oxytocinergic innervation within the brain (for review see Zanos et al., 2017). It is possible that endogenous administration of oxytocin can provide therapeutic value through restoration of oxytocin homeostasis.
3.3.1. Oxytocin Effects on Heroin Self-Administration and Maintenance
Most early studies investigating the effect of oxytocin on heroin addiction have focused on its effect during self-administration. In one study, daily peripheral oxytocin administration diminished acquisition of heroin self-administration in heroin-tolerant male rats (Kovacs, Borthaiser, & Telegdy, 1985). Oxytocin had no effect on heroin-naïve animals during self-administration. Similarly, oxytocin reduced heroin intake in heroin-tolerant rats but not in naïve rats. Site specific application intra-hippocampal and intra-accumbal oxytocin impaired acquisition of lever pressing responses (Ibragimov, Kovacs, Szabo,&Telegdy, 1987). These findings suggest that oxytocin attenuated the development of heroin tolerance. Support for this idea comes from a follow up study showing that oxytocin pre-treatment diminished heroin tolerance in mice as measured by its anti-nociceptive effects (Kovacs, Faludi, & Telegdy, 1985).
3.3.2. Oxytocin Effects on Heroin Reinstatement
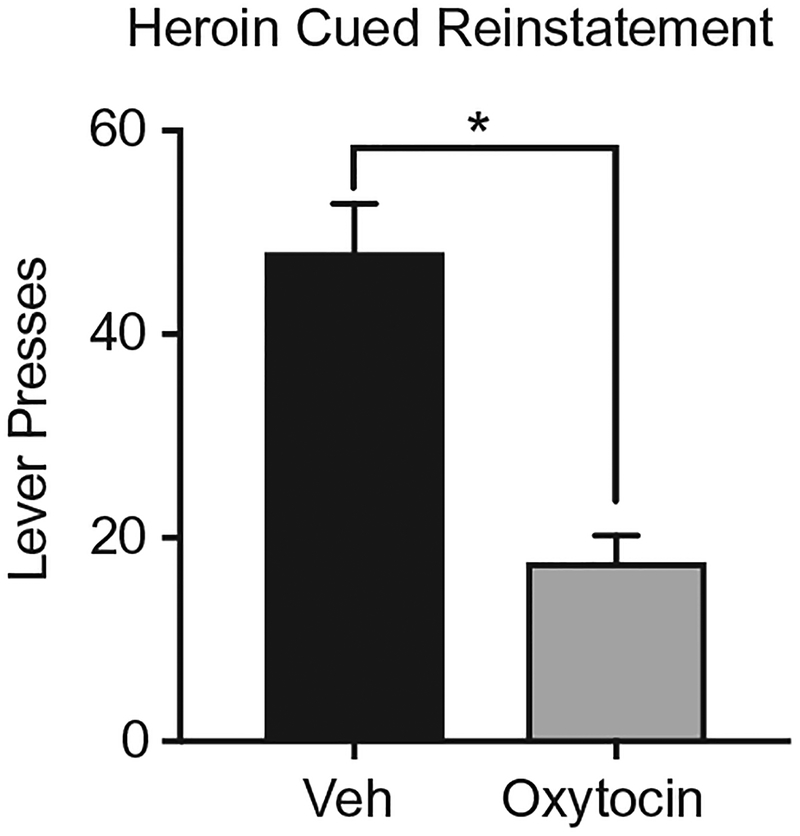
Oxytocin’s effect on reinstatement of heroin seeking has not been as extensively studied relative to psychostimulants. To our knowledge, no studies have investigated the impact of oxytocin during the extinction phase of heroin addiction. Furthermore, no studies have been published showing that oxytocin impacts heroin reinstatement. Related, carbetocin, an oxytocin analogue, reduced stress-induced (Zanos et al., 2014) and drug-primed (Georgiou et al., 2015) reinstatement of morphine-seeking. Preliminary data from our laboratory (Fig. 4) shows that oxytocin also blocks cued reinstatement of heroin seeking. Male and female rats self-administered heroin for 14days, followed by extinction, and subsequent cued reinstatement testing. So, consistent with cocaine and methamphetamine, oxytocin and/or its analogs decrease stress, drug, and cued reinstatement of opioid seeking.
Fig. 4.
Oxytocin decreased cued reinstatement of heroin seeking. *Significant difference between vehicle and oxytocin treatments.
3.4. Alcohol
Alcohol use disorder is a substantial and disabling public health concern, with lifetime prevalence rates of 29.1% reported in the United States (Grant et al., 2015). Excessive alcohol consumption is linked to a number of diseases, including cancers, cardiovascular disease, liver cirrhosis, neuropsychiatric disorders and fetal alcohol syndrome (Rehm et al., 2009). The Center for Disease Control places excessive alcohol use/abuse as the third leading cause of preventable deaths in the United States (Mokdad, Marks, Stroup, & Gerberding, 2004). Economically, alcohol misuse is estimated to cost the U.S. roughly $223.5 billion per year. Despite the significant personal, social and economic burden alcohol use disorders present, there are few effective pharmacotherapies available to help curb alcohol addiction. Preclinical evidence indicates that oxytocin influences a number of behavioral and physiological effects of alcohol (Lee & Weerts, 2016). Here we discuss the findings that support oxytocin as a promising novel therapeutic target in the treatment of alcohol use disorders.
3.4.1. Oxytocin Effects on Alcohol Self-Administration Behavior
The early seminal body of research in this area focused on the effect of oxytocin on tolerance to the physiological effects of alcohol. Repeated systemic administration (ip) of oxytocin prevented the development of tolerance to the hypothermic, hypnotic, and ataxic effects of alcohol (Jodogne, Tirelli, Klingbiel, & Legros, 1991; Kovacs et al., 1998; Szabo, Kovacs, Szekeli, & Telegdy, 1985; Szabo, Kovacs, & Telegdy, 1989). Oxytocin treatment was also found to modulate the severity of alcohol withdrawal symptoms (Szabo, Kovacs, Szekeli, Balaspiri, & Telegdy, 1987). Additionally, central administration of oxytocin (icv) was shown to block rapid tolerance to the effects of alcohol (Szabo et al., 1989). Central administration was found to be more efficacious than peripheral administration, pointing to a centrally mediated mechanism. In this regard, interactions with dopamine and serotonin neurotransmission were postulated to mediate this effect (Szabo et al., 1989).
In more recent reports, systemic administration of oxytocin has been shown to reduce alcohol preference and intake in a variety of voluntary drinking models in rats (Bowen, Carson, Spiro, Arnold, & McGregor, 2011; MacFadyen et al., 2016; McGregor & Bowen, 2012) and mice (King et al., 2017; Peters, Slattery, Flor, Neumann, & Reber, 2013). Specifically, McGregor and Bowen (2012) found that a single dose of oxytocin (1mg/kg) produced a long-lasting reduction in preference for an alcohol-containing solution compared with a nonalcoholic sweet solution. Additionally, treatment with oxytocin for 2weeks prior to the induction of a two-bottle free-choice paradigm resulted in lower alcohol preference in oxytocin-treated rats compared to controls. MacFadyen et al. (2016) reported that systemic administration of a lower dose range of oxytocin (0.1–0.5mg/kg; ip) reduced operant alcohol self-administration in rats. Further, in alcohol-preferring C57BL/6J mice, systemic administration of oxytocin decreased alcohol self-administration and binge-like alcohol consumption in a dose-related manner (0.3, 1, 3 or 10 mg/kg; ip), as well as reduced motivation for alcohol reward as measured by responding under a PR schedule (King et al., 2017). Peripheral oxytocin treatment in a similar dose range (1–10mg/kg) reduced alcohol consumption in male and female prairie voles that had two-bottle choice free-access to 15% alcohol (Stevenson, Wenner, et al., 2017). Additionally, in a continuous access paradigm, systemic oxytocin administration decreased alcohol intake in the first hour after treatment but had no significant effect on consumption over a 24-h period (Stevenson, Wenner, et al., 2017).
While there is strong preclinical evidence to suggest a role for oxytocin in alcohol use disorders, the mechanisms by which oxytocin reduces alcohol consumption is not fully understood. In a recent study, direct (icv) administration of oxytocin reduced alcohol consumption and alcohol-induced dopamine efflux in the NAc (Peters, Bowen, Bohrer, McGregor, & Neumann, 2017). Additionally, oxytocin receptors have been identified on neurons in the ventral tegmental area projecting to the NAc (Peris et al., 2017). Thus, it is possible that oxytocin reduces alcohol self-administration by altering mesolimbic dopamine activity that ordinarily signals alcohol-related reward. However, few studies have examined the role of oxytocin receptors in mediating the neuropeptide’s effect on the motivational actions of alcohol. Recent reports showed that viral-mediated overexpression of oxytocin receptors in NAc reduced alcohol-induced conditioned place preference and alcohol consumption in mice (Bahi, 2015; Bahi, Al Mansouri, & Al Maamari, 2016). Additionally, the ability of oxytocin to reduce binge-like alcohol drinking was blocked by pre-treatment with an oxytocin receptor antagonist, suggesting that oxytocin reduced alcohol consumption via interaction with its own receptor (King et al., 2017). While the oxytocin receptor appears to be involved in the rewarding effects of alcohol, it is important to note that Bowen and colleagues showed that attenuation of sedative and ataxic effects by central oxytocin administration was mediated by GABAA receptors (Bowen et al., 2015). Although additional studies are necessary to further investigate the conditions and mechanisms by which oxytocin reduces alcohol consumption, the growing body of literature investigating the effect of oxytocin on the neurobehavioral response to alcohol points to the oxytocin system as a promising target for therapeutic intervention of alcohol use disorders.
3.4.2. Oxytocin Effects on Alcohol Relapse/Reinstatement Behaviors
To our knowledge, only one published study has investigated the effects of oxytocin administration on relapse-like behavior in animal models. Following repeated cycles of chronic intermittent alcohol vapor exposure to induce dependence in rats, Hansson and colleagues injected (icv) oxytocin in alcohol-dependent and non-dependent rats trained to orally self-administer alcohol using operant conditioning procedures. Central administration of oxytocin reduced cue-induced alcohol relapse-like behavior in alcohol-dependent rats, but not in non-dependent rats (Hansson et al., 2017). Additionally, the authors demonstrated that oxytocin mRNA was reduced in the NAc by acute alcohol and during early withdrawal, but increased after prolonged (3weeks) abstinence, suggesting dynamic dependence-related neuroadaptations of the oxytocin system. After 3weeks of abstinence, dependent rats showed increases in oxytocin receptor mRNA and protein levels in PFC, striatal, amygdala and hippocampal regions, and a reduction in oxytocin mRNA and peptide expression in hypothalamic nuclei. In congruence with this, a loss of hypothalamic oxytocin neurons after prolonged alcohol intake has been reported in other studies (Stevenson, Young, et al., 2017).
4. OXYTOCIN AS A TREATMENT FOR ADDICTION
The mechanisms by which oxytocin provides therapeutic value for the treatment of addiction are just now beginning to be identified. This section will present a mechanistic account that illustrates oxytocin’s interactions with other neurotransmitter systems within the addiction circuitry. Following, we present prominent theories hypothesizing that oxytocin attenuates drug-seeking behavior through its anxiolytic and prosocial properties.
4.1. Interactions Within Addiction Circuitry
As mentioned in a previous section, oxytocin receptors are ubiquitous in the brain and in particular throughout the addiction circuitry. Within this circuitry, oxytocin directly interacts with dopamine, glutamate and GABA neurotransmission. Oxytocin projection neurons within the PVN synapse on dopaminergic cells within the NAc (Knobloch & Grinevich, 2014) and oxytocin receptors are also present on dopamine neurons projecting from the VTA to the NAc and mPFC (Knobloch & Grinevich, 2014; Peris et al., 2017). Intra-NAc oxytocin infusions block cocaine-induced increases in dopamine utilization (Kovacs et al., 1998) and ICV infusion of oxytocin inhibited meth-induced dopamine turnover (Qi et al., 2008). Similarly, ICV oxytocin blocks dopamine release induced by ethanol administration within the NAc (Peters et al., 2017), which corresponds with reduced ethanol seeking behavior. Recent studies have characterized the presence of oxytocin/dopamine receptor complexes (OXTR-D2R) within the NAc in which oxytocin acts as an allosteric agonist to increase D2R affinity (de la Mora et al., 2016; Fuxe et al., 2012; Romero-Fernandez, Borroto-Escuela, Agnati, & Fuxe, 2013). Interestingly, activation of D2R reduces drug-seeking behavior and this receptor subtype is down-regulated following chronic drug exposure (for review see Koob & Mason, 2016; Volkow & Morales, 2015). Therefore, oxytocin may reduce drug-seeking behavior via interactions with D2Rs in the NAc.
Alternatively, oxytocin may impact drug seeking and dopaminergic signaling through GABAergic interneurons. A number of studies have found that excitatory Gq-coupled oxytocin receptors are localized on GABAergic interneurons in the NAc (Dolen et al., 2013), hippocampus (Zaninetti & Raggenbass, 2000), and PFC (Li et al., 2016; Nakajima et al., 2014). Oxytocin signaling in these neurons would increase inhibitory tone within these regions. Along these lines, an oxytocin receptor agonist suppressed hippocampal pyramidal cell firing via GABAergic interneurons within the CA1 layer of the hippocampus (Owen et al., 2013; Zaninetti & Raggenbass, 2000). Given that oxytocin receptors are also present on parvalbumin-containing GABA interneurons in the NAc (Dolen et al., 2013) and that these interneurons regulate the expression of psychostimulant-induced behavioral adaptations (Wang et al., 2018), it is possible that oxytocin impacts drug-seeking behavior directly through interactions with its own receptor on GABAergic interneurons in areas that are critical to dopamine signaling and addiction processes.
A recent model from our laboratory postulates that oxytocin receptors located on astrocytes (Dolen et al., 2013) within the NAc modulate drug-seeking behavior (Bernheim et al., 2017; Weber et al., 2018). More specifically, oxytocin activation of Gq-coupled oxytocin receptors on astrocytes results in astrocytic glutamate release. Extra-synaptic glutamate can thereby activate type 2 and 3 metabotropic (mGlu2/3) glutamate receptors located presynaptically on cortical glutamatergic axons, inhibiting synaptic release of glutamate thus, restoring glutamatergic tone (Moussawi & Kalivas, 2010). Scofield et al. (2015) demonstrated that chemogenetic activation of astrocytes in NAc core decreased cue reinstatement of cocaine (Scofield et al., 2015) seeking through a mGlu2/3 receptor dependent mechanism. Support for this notion, comes from work showing concurrent mGlu2/3 receptor antagonism reversed the attenuating effect of oxytocin on cocaine and meth-seeking behavior (Bernheim et al., 2017; Weber et al., 2018).
4.2. Relief of Stress
Due to the strong link between stress, drug use and relapse, the ability of oxytocin to modulate stress processes has generated growing interest in its potential as a treatment for substance use disorders. Stress is recognized as a significant risk factor that promotes the development of addictive behavior, and in particular, triggering relapse (Sinha, 2001, 2008, 2012), and may be a motivator to use drugs (Koob, 2014). However, drugs themselves can also serve as stressors by activating the hypothalamic–pituitary–adrenocortical (HPA) axis, the primary mediator of neuroendocrine response to stress. This includes stimulating the release of CRF from the hypothalamus (Sinha, 2001; Smith & Vale, 2006), norepinephrine from the locus coeruleus (Koob, 2008) as well as other stress-related neuropeptides in extrahypothalamic stress circuits in the brain (Koob, 2009; Koob & Mason, 2016). Repeated drug exposure followed by withdrawal can lead to profound dysregulation of the HPA axis as well as increased CRF activity within brain stress-reward pathways, which contribute to negative affective states related to withdrawal (e.g., anxiety, dysphoria, irritability) along with increased drive to use or drink (Koob & Volkow, 2010).
Oxytocin is known to exert potent anti-stress and anxiolytic effects (Jurek et al., 2015; Peters, Slattery, Uschold-Schmidt, Reber, & Neumann, 2014; Slattery & Neumann, 2010). Oxytocin administration has been shown to dampen increases in corticosterone (CORT) levels and stress-induced HPA-axis activation, as well as reduce behavioral responses in animal models ofanxiety and depression (Neumann, Kromer,Toschi,&Ebner, 2000;Windle et al., 2004; Windle, Shanks, Lightman, & Ingram, 1997). Conversely, mice genetically engineered to lack the gene for oxytocin (OTKO) display more anxiety-related behavior and greater CORT response following exposure to stress (Amico, Mantella, Vollmer, & Li, 2004; Mantella, Vollmer, & Amico, 2005). Further, high levels of oxytocin and oxytocin receptor mRNA expression are localized to forebrain regions, such as the extended amygdala, that are critically involved in anxiety and stress-responsiveness (Dabrowska et al., 2011; Gimpl & Fahrenholz, 2001; Veinante & Freund-Mercier, 1997), thereby enabling oxytocin to contribute to the regulation of anxiety, stress and reward-related behaviors.
While endogenous oxytocin appears to be intricately involved in the homeostatic regulation of stress responses (Lee & Weerts, 2016), oxytocin signaling in brain has been shown to be altered by both acute and chronic exposure to alcohol and/or drugs of abuse. Thus, exogenous oxytocin administration may help to attenuate the effects of stress associated with heavy drinking and drug use. In support of this hypothesis, Peters and colleagues demonstrated that oxytocin administration attenuated stress-induced increase in alcohol consumption in rats (Peters et al., 2013). Additionally, systemic administration of the oxytocin analogue carbetocin reversed anxiety- and depressive-like behaviors in morphine-withdrawn mice to levels similar to control groups (Zanos et al., 2014). Clinically, a single dose of intranasal oxytocin was shown to reduce stress-induced craving and anxiety in cannabis-dependent individuals (McRae-Clark, Baker, Maria, & Brady, 2013). Further, intranasal oxytocin treatment decreased alcohol withdrawal symptoms in treatment-seeking patients compared to placebo and significantly reduced anxiety in dependent subjects following cessation of drinking (Pedersen, 2017; Pedersen et al., 2013). Taken together, oxytocin treatment may be a useful therapeutic to reduce stress-related physiological responses (e.g., anxiety, craving) relevant to drug and alcohol use and relapse.
4.3. Social Affiliation
Oxytocin may amplify the reinforcing or rewarding properties of social engagement. Often people begin using drugs or alcohol in social contexts and the reinforcing value of some drugs are enhanced when used in the presence of others (McGregor & Bowen, 2012). Social factors can also have a profound impact on maintaining abstinence from drug use for some people. It is suggested that parallel brain circuits mediate social vs. drug reward-related behaviors and that oxytocin may act to redirect behaviors between these reward circuits (McGregor & Bowen, 2012), shift the preference for novel situations and experiences to one for familiarity (Tops et al., 2014), and rebalances the addicted brain (Bowen & Neumann, 2017).
McGregor and Bowen (2012) posit that oxytocin administration can break the behavioral “loops” that maintain the cyclical patterns of drug use–abstinence–relapse. Specifically, they hypothesize that excessive dopaminergic signaling within basal ganglia circuitry resulting from high dose stimulant use lead to a bias in behaviors directed toward object-oriented rewards (food, drugs, alcohol) and the execution of these behavioral loops to seek the object (McGregor & Bowen, 2012). Oxytocin may interfere with this biased behavior by shifting a focus from object-oriented behavioral seeking to one of preference for social interaction. As a treatment for addiction, oxytocin may increase the proclivity towards social rewards thereby strengthening social bonds that may be essential for maintaining abstinence.
Along these same lines Tops et al. (2014) postulate that oxytocin involves overlapping mechanisms between attachment formation and stress by shifting preferences from novel experiences and reward to one of familiarity by facilitating a ventral-to-dorsal shift in activation of cortical striatal loops. This hypothesis is in line with a ventral-to-dorsal shift in striatal control of behavior in addiction. That is, early in drug use, behavior is driven by dopamine release in the ventral striatum mediated by the acute rewarding effects of the drug. With repeated use habitual responding for drugs comes under control of the dorsal striatum (Belin & Everitt, 2008; Gabriele, Pacchioni, & See, 2012). Perhaps oxytocin given as an adjunct for addiction increases the preference for familiarity thereby enhancing social bonds that may help maintain abstinence.
5. OXYTOCIN, SOCIAL REWARD, AND ADDICTION
Prosocial interactions are necessary for the promotion and enhancement of cooperation between group members in many diverse animal species. Although these behaviors serve a critical function, the underlying neural circuitry is understudied. Mounting evidence suggests prosocial behaviors are reinforcing (Song, Borland, Larkin, O’Malley, & Albers, 2016) and the reinforcing properties of prosocial behavior are mediated by the activation of the dopaminergic mesocorticolimbic (MCL) circuit (Insel, 2003). Over the past few decades, oxytocin has attracted interest for its role in prosocial behaviors (Dolen et al., 2013; Hung et al., 2017). As discussed previously in this chapter, both the MCL and oxytocin systems play crucial roles in encoding the rewarding and reinforcing properties of drugs of abuse. In the following sections, the current understanding of the neural circuitry of social rewards will be discussed, focusing in particular on oxytocin’s regulation of these behaviors. In addition, the literature describing the bidirectional modulation of social reward and drugs of abuse will be described with a special interest in the role oxytocin may play in these competing reward signals.
5.1. The Neural Circuitry of Social Reward and Oxytocin
Prosocial behaviors, such as pair bonding and social interaction, have rewarding properties that are encoded by the MCL pathway (Becker, Rudick, & Jenkins, 2001; Insel, 2003), similar to drugs of abuse. For example, dopamine is released in the NAc following pup exposure in maternal female rats (Hansen, Bergvall, & Nyiredi, 1993), and lesions in either the VTA or the NAc will disrupt maternal approach and interaction with pups (Lee, Li, Watchus, & Fleming, 1999; Numan & Smith, 1984). The dopaminergic reinforcement of social reward alone is an incomplete picture of the complexity of these behaviors. Oxytocin has gained growing interest for its role in social reward since Pedersen and Prange (1979) demonstrated that oxytocin induced maternal behavior in virgin female rats. Since this seminal study, it has been established that oxytocin modulates social interaction (Young & Wang, 2004) and pair bonding (Carter et al., 1992), as well as enhanced social memory (Ferguson et al., 2000). The interplay between the MCL and oxytocin systems that was first observed in studies of monogamous prairie voles, concluded oxytocin receptors in the NAc are required for pair bonding formation (Young & Wang, 2004). Similar to the rat model, dopamine is released in the NAc during social rewards in prairie voles. More specifically, D2-specific activity induces mating-induced partner preference in female prairie voles (Young & Wang, 2004). Moreover, Young et al. (2001) demonstrated an oxytocin receptor antagonist directly infused into the NAc of these voles reduced D2-mediated partner preference.
While high densities of oxytocin receptor have been observed in the NAc, they have also been detected in other areas related to reward signaling. For example, oxytocin receptors have been discovered in high density in the PFC of female prairie voles, and direct infusion of an oxytocin receptor antagonist into this brain region blocked partner preference (Young et al., 2001). The role of oxytocin in social reward in non-monogamous species is also becoming more apparent. Oxytocin projections to the PFC have been identified in rats (reviewed in Buisman-Pijlman et al., 2014) but there is no study to date that has examined these projections in relation to social reward. Further, dopamine administration induces central release of oxytocin, while oxytocin administration potentiates central dopamine in the rat (Melis et al., 2007). Rodents that do not demonstrate monogamous mating behavior seem to have similar levels of circulating oxytocin as that of prairie voles, but oxytocin receptor densities vary across species (Buisman-Pijlman et al., 2014), which may contribute to the differences in their mating preferences (Young & Wang, 2004). Regardless of social and mating structure, oxytocin modulates reward signals within the NAc as direct administration of an oxytocin receptor antagonist eliminated social reward preference in a social CPP test in mice without altering cocaine CPP (Dolen et al., 2013).
Dolen et al. (2013) additionally discovered oxytocin within the NAc induced long-term depression (LTD) on presynaptic excitatory synapses of medium spiny neurons (MSNs) in a mouse model. The oxytocin-induced LTD was significantly attenuated if the mice had been previously socially conditioned. The prosocial behavior did not preferentially act on oxytocin-induced LTD within the D1 or D2 MSN pathways, suggesting another possible mechanistic difference between monogamous and non-monogamous rodents. Finally, social reward and oxytocin-induced LTD requires inputs into the NAc from the dorsal raphe nucleus (DRN). The pertinent afferents from the DRN were shown to be serotonergic (5-HT), acting on 5-HT-1b receptors on glutamate neurons. These DRN afferents have oxytocin receptors that mediate 5-HT release within the NAc. During social reward, oxytocin is released into the NAc via PVN neurons, which binds oxytocin receptor on serotonergic inputs from the DRN, causing a release of 5-HT. Subsequently, it binds to 5-HT-1b receptors on presynaptic excitatory synapses onto MSNs, inducing LTD. Taken together, these experiments eloquently demonstrate the mechanistic impact of oxytocin within NAc during prosocial behaviors.
The VTA is another region that has long been characterized within the MCL pathway as a critical brain region for reward signals related to drugs of abuse. Recently, studies have implicated the VTA in social reward cues (Song et al., 2016) via increases in dopaminergic NAc-projecting VTA neuron activity following social interactions in mice (Gunaydin et al., 2014). Exciting research from Hung et al. (2017) explored the role oxytocin plays in this change in dopamine concentrations following social reward. They provide evidence that there are oxytocin projections from the PVN to the VTA in mice. These oxytocin projections within the VTA were often co-localized to oxytocin receptor-containing dopamine neurons. Mice lacking oxytocin receptor specifically on dopaminergic neurons within the VTA did not express social CPP behavior compared to controls. These mice did, however, express CPP in response to cocaine. They also determined c-fos levels in VTA-projecting PVN oxytocin neurons were significantly potentiated early on during a bout of social interaction, but activation of these oxytocin neurons alone was not rewarding. Instead, temporally-dependent oxytocin release into the VTA during social reward seems to directly enhance the excitability of NAc-projecting VTA neurons, potentiating dopamine levels within the NAc, thereby reinforcing the prosocial behavior (Hung et al., 2017). In summary, there is clear evidence from these rodent models for the concomitant role of the dopaminergic MCL and oxytocin systems in the control and regulation of social reward. In addition, a growing body of research indicates oxytocin gates dopamine’s reinforcing effects of social reward via indirect modulation of other neurotransmitters including, but not limited to, 5-HT.
5.2. The Overlap Between Oxytocin Effects on Social Reward and Drug Reward
Numerous studies described previously in this chapter have worked to explain how oxytocin is able to affect addictive processes associated with drugs of abuse. The overlap between oxytocin’s role in the shared neural circuitry of addiction and social reward has been postulated (Insel, 2003), and there is evidence to suggest oxytocin may facilitate the rewarding effects of prosocial behavior at the expense of drug-related rewards (Sarnyai & Kovacs, 2014; Thompson, Callaghan, Hunt, Cornish, & McGregor, 2007).
The method by which oxytocin does this, however, is still under investigation. In this section, the current hypotheses of the role of oxytocin in mediating the differing reward signals will be discussed.
It has long been hypothesized that drug-mediated and social reward signals compete within the reward circuitry of the brain (Buisman-Pijlman et al., 2014; Insel, 2003). Several rodent studies have shown that social rewards, such as conspecific interaction, have the ability to completely reverse cocaine CPP (Fritz et al., 2011; Kummer et al., 2014). In a comparative CPP paradigm, mice that were given a choice between cocaine and social interaction with a conspecific did not develop a significant preference for either compared to mice that underwent cocaine or social interaction CPP alone (Liu, Wang, Zhan, & Cheng, 2016). Interestingly, when the experimenters evaluated oxytocin expression in the PVN, CPP of cocaine alone, social interaction alone, and social interaction and cocaine combined all had elevated levels compared to controls. However, while social interaction alone had the largest increase and cocaine had the lowest, the animals forced to choose between the two rewards had oxytocin expression in the PVN that fell in between the levels quantified in the two groups that only experienced a single reward (Liu et al., 2016). It is evident the brain’s oxytocin system is extremely plastic, especially in response to these differing rewards. Drugs of abuse may cause lasting downregulation in oxytocin expression or function, which explains social behavior deficits observed in animals repeatedly exposed to them (McGregor & Bowen, 2012). Conversely, during prosocial behavior, oxytocin may promote reward value associated with prosocial behavior to compete with that of the drugs (Campbell, 2008).
Addiction has long been understood as a neuroadaptive process of “pathological learning” to drugs of abuse (Koob & Volkow, 2016). For example, they can alter or induce long-term potentiation (LTP), a cellular prerequisite for learning and memory, by altering the strength of functional neuronal connections (Berretta, Nisticò, Bernardi, & Mercuri, 2008; Sarnyai & Kovacs, 2014). Additionally, drugs cause dramatic changes in synaptic density within the MCL regions of the brain, which play a critical role in motivation and learning (Koehl & Abrous, 2011). Social reward may negate or reverse these changes through an oxytocin-mediated neuroplasticity in some of the same brain regions. The LTD induced in the NAc by oxytocin during social reward (Dolen et al., 2013) may help reverse drug-induced LTP and shift the addictive bias from habitual behavior loops associated with drug towards social stimuli (McGregor & Bowen, 2012).
Further, Dölen and colleague’s study established a critical interaction between oxytocin and serotonergic transmission in the NAc. Serotonin release in the NAc has been implicated in the regulation of psychostimulant drug-related memories (Cunningham & Anastasio, 2014) and the reinforcing effects of methamphetamine (Chiu & Schenk, 2012; Sarnyai & Kovacs, 2014). It may be possible that oxytocinergic modulation of this serotonergic pathway can reduce these pathological changes in addiction. Further research is needed to examine whether endogenous changes to oxytocin via prosocial activity is sufficient to significantly alter these drug-related changes in learning and memory.
The hippocampus is a critical brain structure in addiction, providing contextual learning components to drugs of abuse (Robbins & Everitt, 2002) and oxytocin was shown to attenuate drug-mediated learning by specifically targeting fast-spiking inhibitory interneurons within the hippocampus to sculpt its inhibitory signals (Owen et al., 2013). It is hypothesized this alteration in hippocampal excitability may, in turn, influence regions in the MCL circuit, such as the NAc and PFC, thereby mediating drug-induced neuroplasticity (Sarnyai & Kovacs, 2014). A recent study has also established that oxytocin receptors within the hippocampus (notably the anterior dentate gyrus, and anterior CA2/CA3) of mice are necessary for social stimuli discrimination and social recognition (Raam, McAvoy, Besnard, Veenema, & Sahay, 2017). It is possible, therefore, that prosocial memories, mediated by oxytocin in the hippocampus, inhibit the drug-related neuroplasticity, or alter the engrams associated with drug cues and reward. Despite these advancements, further evidence evaluating the competing influence social and drug reward on neuroplasticity are still needed.
6. CONCLUSIONS
Very little research has focused on the effect of prosocial behavior on neural changes associated with drugs of abuse. From these few studies, it is clear that a potentiation in oxytocin activity during social reward attenuates or reverses the reinforcing effects of drugs of abuse (Fritz et al., 2011; Kummer et al., 2014; Liu et al., 2016). Yet, important questions still remain. At present, there is no definitive understanding of the causality of these changes in neuroplasticity. In our previous sections we have presented strong preclinical evidence from our laboratories and others to suggest that exogenous administration of oxytocin reduces drug seeking and self-administration, tolerance, and reinstatement. Importantly, these effects occur across multiple drug classes and impact multiple neurotransmitter systems (dopamine, GABA, serotonin) indicating that oxytocin may be rebalancing an addicted brain through multiple dependent and interdependent mechanisms (Bowen & Neumann, 2017). Additionally, burgeoning research has exemplified the impact of oxytocin on social reward alone (Dolen et al., 2013; Hung et al., 2017). Future studies must work to elucidate the direct interaction of these two reward systems in hopes of understanding how prosocial behaviors aid in the recovery of those with substance abuse disorders. Adaptations to current methodologies to provide new information on the neurobiology or function of oxytocin signaling should include several different approaches. First, continued development of oxytocin receptor specific pharmaceuticals to determine specificity of the neuropeptide will aid understanding the neurobiology and treatment approaches. Second, adaptation of intranasal oxytocin as route of administration will add consistency with addiction studies in humans, which will directly increase the translational relevance of the basic science models (Ditzen et al., 2009; Guastella et al., 2010; Pedersen et al., 2013). And, third, recent technological advances in viral mediated gene transfer approaches will increase understanding brain region specific roles (through receptor over expression or knockdown) and functional circuitry (through optogenetics and/or chemogenetics) of oxytocin from a basic science level.
REFERENCES
- Amico JA, Mantella RC, Vollmer RR, & Li X (2004). Anxiety and stress responses in female oxytocin deficient mice. Journal of Neuroendocrinology, 16(4), 319–324. 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Bahi A (2015). The oxytocin receptor impairs ethanol reward in mice. Physiology & Behavior,139, 321–327. 10.1016/j.physbeh.2014.11.046. [DOI] [PubMed] [Google Scholar]
- Bahi A, Al Mansouri S, & Al Maamari E (2016). Nucleus accumbens lentiviral-mediated gain of function of the oxytocin receptor regulates anxiety- and ethanol-related behaviors in adult mice. Physiology & Behavior, 164(Pt. A), 249–258. 10.1016/j.physbeh.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, & Cornish JL (2016). The neurocircuitry involved in oxytocin modulation of methamphetamine addiction. Frontiers in Neuroendocrinology, 43, 1–18. 10.1016/j.yfrne.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, & Cornish JL (2015). The involvement of oxytocin in the subthalamic nucleus on relapse to methamphetamine-seeking behaviour. PLoS One, 10(8), e0136132 10.1371/journal.pone.0136132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, McGregor IS, & Cornish JL (2016). Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addiction Biology, 21(2), 316–325. 10.1111/adb.12198. [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, & Cornish JL (2012). Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference. Behavioural Brain Research, 228(1), 185–193. 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Bardo MT, & Bevins RA (2000). Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology, 153(1), 31–43. [DOI] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, et al. (2006). The need for speed: An update on methamphetamine addiction. Journal of Psychiatry & Neuroscience, 31(5), 301–313. [PMC free article] [PubMed] [Google Scholar]
- Becker HC, & Lopez MF (2004). Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcoholism, Clinical and Experimental Research, 28(12), 1829–1838. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, & Jenkins WJ (2001). The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. The Journal of Neuroscience, 21(9), 3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, & Everitt BJ (2008). Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron, 57(3), 432–441. 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, & Aston-Jones G (2014). Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proceedings of the National Academy of Sciences of the United States of America, 111(32), 11822–11827. 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A, Leong KC, Berini C, & Reichel CM (2017). Antagonism of mGlu2/3 receptors in the nucleus accumbens prevents oxytocin from reducing cued methamphetamine seeking in male and female rats. Pharmacology, Biochemistry, and Behavior, 161, 13–21. 10.1016/j.pbb.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim A, See RE, & Reichel CM (2016). Chronic methamphetamine self-administration disrupts cortical control of cognition. Neuroscience and Biobehavioral Reviews, 69, 36–48. 10.1016/j.neubiorev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta N, Nisticò R, Bernardi G, & Mercuri NB (2008). Synaptic plasticity in the basal ganglia: A similar code for physiological and pathological conditions. Progress in Neurobiology, 84(4), 343–362. 10.1016/j.pneurobio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Bohus B, Urban I, van Wimersma Greidanus TB, & de Wied D (1978). Opposite effects of oxytocin and vasopressin on avoidance behaviour and hippocampal theta rhythm in the rat. Neuropharmacology, 17(4–5), 239–247. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, & Shaham Y (2013). The reinstatement model of drug relapse: Recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology, 229(3), 453–476. 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, & McGregor IS (2011). Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PLoS One, 6(11), e27237 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, & McGregor IS (2014). Oxytocin and vasopressin modulate the social response to threat: A preclinical study. The International Journal of Neuropsychopharmacology, 17(10), 1621–1633. 10.1017/s1461145714000388. [DOI] [PubMed] [Google Scholar]
- Bowen MT, & Neumann ID (2017). Rebalancing the addicted brain: Oxytocin interference with the neural substrates of addiction. Trends in Neurosciences, 40(12), 691–708. 10.1016/j.tins.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Bowen MT, Peters ST, Absalom N, Chebib M, Neumann ID, & McGregor IS (2015). Oxytocin prevents ethanol actions at delta subunit-containing GABAA receptors and attenuates ethanol-induced motor impairment in rats. Proceedings of the National Academy of Sciences of the United States of America, 112(10), 3104–3109. 10.1073/pnas.1416900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisman-Pijlman FT, Sumracki NM, Gordon JJ, Hull PR, Carter CS, & Tops M (2014). Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacology, Biochemistry, and Behavior, 119, 22–38. 10.1016/j.pbb.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Busnelli M, & Chini B (2017). Molecular basis of oxytocin receptor signalling in the brain: What we know and what we need to know. Current Topics in Behavioral Neurosciences, 35, 3–29. 10.1007/7854_2017_6. [DOI] [PubMed] [Google Scholar]
- Campbell A (2008). Attachment, aggression and affiliation: The role of oxytocin in female social behavior. Biological Psychology, 77(1), 1–10. 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Carson D, Cornish J, Guastella A, Hunt G, & McGregor I (2010). Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology, 58(1), 38–43. 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Carson DS, Guastella AJ, Taylor ER, & McGregor IS (2013). A brief history of oxytocin and its role in modulating psychostimulant effects. Journal of Psychopharmacology, 27(3), 231–247. 10.1177/0269881112473788. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, et al. (2010). Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addiction Biology, 15(4), 448–463. 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Williams JR, Witt DM, & Insel TR (1992). Oxytocin and social bonding. Annals of the New York Academy of Sciences, 652, 204–211. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, & O’Brien CP (1993). Cue reactivity and cue reactivity interventions in drug dependence. NIDA Research Monograph, 137, 73–95. [PubMed] [Google Scholar]
- Chini B, & Fanelli F (2000). Molecular basis of ligand binding and receptor activation in the oxytocin and vasopressin receptor family. Experimental Physiology, 85, 59s–66s. Spec No. [DOI] [PubMed] [Google Scholar]
- Chiu VM, & Schenk JO (2012). Mechanism of action of methamphetamine within the catecholamine and serotonin areas of the central nervous system. Current Drug Abuse Reviews, 5(3), 227–242. [DOI] [PubMed] [Google Scholar]
- Churchland PS, & Winkielman P (2012). Modulating social behavior with oxytocin: How does it work? What does it mean? Hormones and Behavior, 61(3), 392–399. 10.1016/j.yhbeh.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL, & Kurtz SP (2014). The changing face of heroin use in the United States: A retrospective analysis of the past 50 years. JAMA Psychiatry, 71(7), 821–826. 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- Cox B, Bentzley B, Regen-Tuero H, See R, Reichel C, & Aston-Jones G (2017).Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biological Psychiatry, 81(11), 949–958. 10.1016/j.biopsych.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, et al. (2013). Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcoholism, Clinical and Experimental Research, 37(10), 1688–1695. 10.1111/acer.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B, Young A, See R, & Reichel C (2013). Sex differences in methamphetamine seeking in rats: Impact of oxytocin. Psychoneuroendocrinology, 38(10), 2343–2353. 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, & Anastasio NC (2014). Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology, 76, 460–478. 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, et al. (2011). Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology, 36(9), 1312–1326. 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mora MP, Perez-Carrera D, Crespo-Ramirez M, Tarakanov A, Fuxe K, & Borroto-Escuela DO (2016). Signaling in dopamine D2 receptor-oxytocin receptor heterocomplexes and its relevance for the anxiolytic effects of dopamine and oxytocin interactions in the amygdala of the rat. Biochimica et Biophysica Acta, 1862(11), 2075–2085. 10.1016/j.bbadis.2016.07.004. [DOI] [PubMed] [Google Scholar]
- De Wied D (1971). Long term effect of vasopressin on the maintenance of a conditioned avoidance response in rats. Nature, 232(5305), 58–60. [DOI] [PubMed] [Google Scholar]
- Devost D, Wrzal P, & Zingg HH (2008). Oxytocin receptor signalling. Progress in Brain Research, 170, 167–176. 10.1016/s0079-6123(08)00415-9. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, & Heinrichs M (2009). Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry, 65(9), 728–731. [DOI] [PubMed] [Google Scholar]
- Dobkin C, & Nicosia N (2009). The war on drugs: Methamphetamine, public health, and crime. The American Economic Review, 99(1), 324–349. 10.1257/aer.99.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, & Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179–184. 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, & Spear LP (2004). Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Developmental Psychobiology, 45(3), 153–162. 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Dumais KM, & Veenema AH (2016). Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Frontiers in Neuroendocrinology, 40, 1–23. 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Hanson G, White J, Wickes W, & Tiihonen J (2008). Pharmacotherapy of methamphetamine addiction: An update. Substance Abuse, 29(3), 31–49. 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermisch A, Barth T, Ruhle HJ, Skopkova J, Hrbas P, & Landgraf R (1985). On the blood-brain barrier to peptides: Accumulation of labelled vasopressin, DesGlyNH2-vasopressin and oxytocin by brain regions. Endocrinologia Experimentalis, 19(1), 29–37. [PubMed] [Google Scholar]
- Everett NA, McGregor IS, Baracz SJ, & Cornish JL (2018). The role of the vasopressin V1A receptor in oxytocin modulation of methamphetamine primed reinstatement. Neuropharmacology, 133, 1–11. 10.1016/j.neuropharm.2017.12.036. [DOI] [PubMed] [Google Scholar]
- Feltenstein M, & See R (2008). The neurocircuitry of addiction: An overview. British Journal of Pharmacology, 154(2), 261–274. 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, & Winslow JT (2000). Social amnesia in mice lacking the oxytocin gene. Nature Genetics, 25, 284 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferland CL, Reichel CM, & McGinty JF (2016). Effects of oxytocin on methamphetamine-seeking exacerbated by predator odor pre-exposure in rats. Psychopharmacology, 233(6), 1015–1024. 10.1007/s00213-015-4184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GG, et al. (2016). Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology, 66, 185–194. 10.1016/j.psyneuen.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, et al. (2011). Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addiction Biology, 16(2), 273–284. 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, et al. (2012). On the role of volume transmission and receptor-receptor interactions in social behaviour: Focus on central catecholamine and oxytocin neurons. Brain Research, 1476, 119–131. 10.1016/j.brainres.2012.01.062. [DOI] [PubMed] [Google Scholar]
- Gabriele A, Pacchioni AM, & See RE (2012). Dopamine and glutamate release in the dorsolateral caudate putamen following withdrawal from cocaine self-administration in rats. Pharmacology, Biochemistry, and Behavior, 103(2), 373–379. 10.1016/j.pbb.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Garcia-Carmona JA, Hourani S, Kitchen I, Kieffer BL, et al. (2015). The oxytocin analogue carbetocin prevents priming-induced reinstatement of morphine-seeking: Involvement of dopaminergic, noradrenergic and MOPr systems. European Neuropsychopharmacology, 25(12), 2459–2464. 10.1016/j.euroneuro.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Gimpl G, & Fahrenholz F (2001). The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews, 81(2), 629–683. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Reitz J, Brauer S, & Trossen C (2008). Oxytocin receptors: Ligand binding, signalling and cholesterol dependence. Progress in Brain Research, 170, 193–204. 10.1016/s0079-6123(08)00417-2. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, & Rawson RA (2010). The methamphetamine problem in the United States. Annual Review of Public Health, 31, 385–398. 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Smith SM, Jung J, Zhang H, Chou SP, et al. (2015). The Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5): Reliability of substance use and psychiatric disorder modules in a general population sample. Drug and Alcohol Dependence, 148, 27–33. 10.1016/j.drugalcdep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, & Shaham Y (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature, 412(6843), 141–142. 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. (2010). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry, 67(7), 692–694. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. (2014). Natural neural projection dynamics underlying social behavior. Cell, 157(7), 1535–1551. 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, & Nyiredi S (1993). Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: A microdialysis study. Pharmacology, Biochemistry, and Behavior, 45(3), 673–676. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Koopmann A, Uhrig S, Buhler S, Domi E, Kiessling E, et al. (2017). Oxytocin reduces alcohol cue-reactivity in alcohol-dependent rats and humans. Neuropsychopharmacology, 43(6), 1235–1246. 10.1038/npp.2017.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Schochet TL, See RE, & McGinty JF (2010). Context-driven cocaine-seeking in abstinent rats increases activity-regulated gene expression in the basolateral amygdala and dorsal hippocampus differentially following short and long periods of abstinence. Neuroscience, 170(2), 570–579. 10.1016/j.neuroscience.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson RG, Cloutier R, & McConnell KJ (2008). Methamphetamine-related emergency department utilization and cost. Academic Emergency Medicine, 15(1), 23–31. 10.1111/j.1553-2712.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- Hicks C, Cornish JL, Baracz SJ, Suraev A, & McGregor IS (2016). Adolescent pre-treatment with oxytocin protects against adult methamphetamine-seeking behavior in female rats. Addiction Biology, 21(2), 304–315. 10.1111/adb.12197. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Ritzmann RF, Walter R, & Tabakoff B (1978). Arginine vasopressin maintains ethanol tolerance. Nature, 276(5688), 614–616. [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, et al. (2017). Gating of social reward by oxytocin in the ventral tegmental area. Science, 357(6358), 1406–1411. 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibragimov R (1990). Influence of neurohypophyseal peptides on the formation of active avoidance conditioned reflex behavior. Neuroscience and Behavioral Physiology, 20(3), 189–193. [DOI] [PubMed] [Google Scholar]
- Ibragimov R, Kovacs GL, Szabo G, & Telegdy G (1987). Microinjection of oxytocin into limbic-mesolimbic brain structures disrupts heroin self-administration behavior: A receptor-mediated event? Life Sciences, 41(10), 1265–1271. [DOI] [PubMed] [Google Scholar]
- Insel TR (2003). Is social attachment an addictive disorder? Physiology & Behavior, 79(3),351–357. [DOI] [PubMed] [Google Scholar]
- Jodogne C, Tirelli E, Klingbiel P, & Legros JJ (1991). Oxytocin attenuates tolerance not only to the hypothermic but also to the myorelaxant and akinesic effects of ethanol in mice. Pharmacology, Biochemistry, and Behavior, 40(2), 261–265. [DOI] [PubMed] [Google Scholar]
- Jurek B, Slattery DA, Hiraoka Y, Liu Y, Nishimori K, Aguilera G, et al. (2015). Oxytocin regulates stress-induced Crf gene transcription through CREB-regulated transcription coactivator 3. The Journal of Neuroscience, 35(35), 12248–12260. 10.1523/JNEUROSCI.1345-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye S, McKetin R, Duflou J, & Darke S (2007). Methamphetamine and cardiovascular pathology: A review of the evidence. Addiction, 102(8), 1204–1211. 10.1111/j.1360-0443.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, & Becker HC (2017). Oxytocin reduces ethanol self-administration in mice. Alcoholism, Clinical and Experimental Research, 41(5), 955–964. 10.1111/acer.13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, & Grinevich V (2014). Evolution of oxytocin pathways in the brain of vertebrates. Frontiers in Behavioral Neuroscience, 8, 31 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M, & Abrous DN (2011). A new chapter in the field of memory: Adult hippocampal neurogenesis. The European Journal of Neuroscience, 33(6), 1101–1114. 10.1111/j.1460-9568.2011.07609.x. [DOI] [PubMed] [Google Scholar]
- Koob GF (2008). A role for brain stress systems in addiction. Neuron, 59(1), 11–34. 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2009). Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology, 56(Suppl. 1), 18–31. 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2014). Neurocircuitry of alcohol addiction: Synthesis from animal models. Handbook of Clinical Neurology, 125, 33–54. 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Mason BJ (2016). Existing and future drugs for the treatment of the dark side of addiction. Annual Review of Pharmacology and Toxicology, 56, 299–322. 10.1146/annurev-pharmtox-010715-103143. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology,35(1), 217–238. 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet. Psychiatry, 3(8), 760–773. 10.1016/s2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, & Fehr E (2005). Oxytocin increases trust in humans. Nature, 435(7042), 673–676. 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Borthaiser Z, & Telegdy G (1985). Oxytocin reduces intravenous heroin self-administration in heroin-tolerant rats. Life Sciences, 37(1), 17–26. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Faludi M, & Telegdy G (1985). Oxytocin diminishes heroin tolerance in mice. Psychopharmacology, 86(3), 377–379. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Barbarczi E, Szabo G, & Telegdy G (1990). The role of oxytocin-dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology, 29(4), 365–368. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, & Szabo G (1998). Oxytocin and addiction: A review. Psychoneuroendocrinology, 23(8), 945–962. [DOI] [PubMed] [Google Scholar]
- Kummer KK, Hofhansel L, Barwitz CM, Schardl A, Prast JM, Salti A, et al. (2014). Differences in social interaction- vs. cocaine reward in mouse vs. rat. Frontiers in Behavioral Neuroscience, 8, 363 10.3389/fnbeh.2014.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Ermisch A, & Hess J (1979). Indications for a brain uptake of labelled vasopressin and ocytocin and the problem of the blood-brain barrier. Endokrinologie, 73(1), 77–81. [PubMed] [Google Scholar]
- Lee A, Li M, Watchus J, & Fleming AS (1999). Neuroanatomical basis of maternal memory in postpartum rats: Selective role for the nucleus accumbens. Behavioral Neuroscience, 113(3), 523–538. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, & Young WS 3rd (2009). Oxytocin: The great facilitator of life. Progress in Neurobiology, 88(2), 127–151. 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Scheidweiler KB, Diao XX, Akhlaghi F, Cummins A, Huestis MA, et al. (2018). Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: Determination using a novel oxytocin assay. Molecular Psychiatry, 23(1), 115–122. 10.1038/mp.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, & Weerts EM (2016). Oxytocin for the treatment of drug and alcohol use disorders. Behavioural Pharmacology, 27(8), 640–648. 10.1097/FBP.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire W, O’Brien JA, Burno M, Chaudhary AG, Dean DC, Williams PD, et al. (2002). A nonpeptide oxytocin receptor antagonist radioligand highly selective for human receptors. European Journal of Pharmacology, 450(1), 19–28. [DOI] [PubMed] [Google Scholar]
- Leng G, & Ludwig M (2016). Intranasal oxytocin: Myths and delusions. Biological Psychiatry, 79(3), 243–250. 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Leong K, Freeman L, Berini C, Ghee S, See R, & Reichel C (2017). Oxytocin reduces cocaine cued Fos activation in a regionally specific manner. The International Journal of Neuropsychopharmacology, 20(10), 844–854. 10.1093/ijnp/pyx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong K, Zhou L, Ghee S, See R, & Reichel C (2016). Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Experimental and Clinical Psychopharmacology, 24(1), 55–64. 10.1037/pha0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Nakajima M, Ibanez-Tallon I, & Heintz N (2016). A cortical circuit for sexually dimorphic oxytocin-dependent anxiety behaviors. Cell, 167(1). 60–72.e11 10.1016/j.cell.2016.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Griffin KD, Gigante ED, & Boehm SL 2nd (2011). Tolerance to ethanol’s ataxic effects and alterations in ethanol-induced locomotion following repeated binge-like ethanol intake using the DID model. Alcoholism, Clinical and Experimental Research, 35(7), 1246–1255. 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Wang J, Zhan B, & Cheng G (2016). Neuronal activity and the expression of hypothalamic oxytocin and vasopressin in social versus cocaine conditioning. Behavioural Brain Research, 310, 84–92. 10.1016/j.bbr.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, & Leng G (2002). Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature, 418(6893), 85–89. 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Lukas M, Toth I, Veenema AH, & Neumann ID (2013). Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: Male juvenile versus female adult conspecifics. Psychoneuroendocrinology, 38(6), 916–926. 10.1016/j.psyneuen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, et al. (2016). Peripheral oxytocin administration reduces ethanol consumption in rats. Pharmacology, Biochemistry, and Behavior, 140, 27–32. 10.1016/j.pbb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Vollmer RR, & Amico JA (2005). Corticosterone release is heightened in food or water deprived oxytocin deficient male mice. Brain Research, 1058(1–2), 56–61. 10.1016/j.brainres.2005.07.062. [DOI] [PubMed] [Google Scholar]
- Maraj S, Figueredo VM, & Lynn Morris D (2010). Cocaine and the heart. Clinical Cardiology, 33(5), 264–269. 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, & Steckler T (2008). Removing obstacles in neuroscience drug discovery: The future path for animal models. Neuropsychopharmacology, 34(1), 74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BB (2004). Closing remarks: Review and commentary on selected aspects of the roles of vasopressin and oxytocin in memory processing. Advances in Pharmacology, 50(593–654), 655–708. 10.1016/s1054-3589(04)50015-7. [DOI] [PubMed] [Google Scholar]
- McGregor IS, & Bowen MT (2012). Breaking the loop: Oxytocin as a potential treatment for drug addiction. Hormones and Behavior, 61(3), 331–339. 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MM, & Brady KT (2013). Effect of oxytocin on craving and stress response in marijuana-dependent individuals: A pilot study. Psychopharmacology, 228(4), 623–631. 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, et al. (2007). Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. The European Journal of Neuroscience, 26(4), 1026–1035. 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, & Gerberding JL (2004). Actual causes of death in the United States, 2000. JAMA, 291(10), 1238–1245. 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, & Boehm SL 2nd (2007). GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacology, Biochemistry, and Behavior, 88(1), 105–113. 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Rivera A, Hernandez-Burgos MM, Martinez-Rivera A, Perez-Colon J, Rivera R, Montalvo J, et al. (2014). Anxiolytic effects of oxytocin in cue-induced cocaine seeking behavior in rats. Psychopharmacology, 231, 4145–4155. 10.1007/s00213-014-3553-y. [DOI] [PubMed] [Google Scholar]
- Mouillac B, Manning M, & Durroux T (2008). Fluorescent agonists and antagonists for vasopressin/oxytocin G protein-coupled receptors: Usefulness in ligand screening assays and receptor studies. Mini Reviews in Medicinal Chemistry, 8(10), 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, & Kalivas PW (2010). Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. European Journal of Pharmacology, 639(1–3), 115–122. 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel C, See R, Carr D, et al. (2011). Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences of the United States of America, 108(1), 385–390. 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, & Stewart J (2000). Cocaine-induced conditioned place preference: Reinstatement by priming injections of cocaine after extinction. Behavioural Brain Research, 115(1), 39–47. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Gorlich A, & Heintz N (2014). Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell, 159(2), 295–305. 10.1016/j.cell.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Kromer SA, Toschi N, & Ebner K (2000). Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: Involvement of hypothalamic and limbic brain regions. Regulatory Peptides, 96(1–2), 31–38. [DOI] [PubMed] [Google Scholar]
- Neumann ID, & Landgraf R (2012). Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends in Neurosciences, 35(11), 649–659. 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, & Landgraf R (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology, 38(10), 1985–1993. 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Neumann ID, & Slattery DA (2016). Oxytocin in general anxiety and social fear: A translational approach. Biological Psychiatry, 79(3), 213–221. 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Takayanagi Y, Yoshida M, Kasahara Y, Young LJ, & Kawamata M (2008). New aspects of oxytocin receptor function revealed by knockout mice: Sociosexual behaviour and control of energy balance. Progress in Brain Research, 170, 79–90. 10.1016/s0079-6123(08)00408-1. [DOI] [PubMed] [Google Scholar]
- Numan M, & Smith HG (1984). Maternal behavior in rats: Evidence for the involvement of preoptic projections to the ventral tegmental area. Behavioral Neuroscience, 98(4), 712–727. [DOI] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, & Tsien RW (2013). Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature, 500(7463), 458–462. 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar JJ, Davies S, Ompad DC, Cleland CM, & Weitzman M (2015). Powder cocaine and crack use in the United States: An examination of risk for arrest and socioeconomic disparities in use. Drug and Alcohol Dependence, 149, 108–116. 10.1016/j.drugalcdep.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, & Lahvis GP (2007). Social reward among juvenile mice. Genes, Brain, and Behavior, 6(7), 661–671. 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Nocjar C, Burgdorf J, Panksepp JB, & Huber R (2004). The role of emotional systems in addiction: A neuroethological perspective. Nebraska Symposium on Motivation, 50, 85–126. [PubMed] [Google Scholar]
- Pedersen CA (2017). Oxytocin, tolerance, and the dark side of addiction. International Review of Neurobiology, 136, 239–274. 10.1016/bs.irn.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, & Prange AJ Jr. (1979). Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proceedings of the National Academy of Sciences of the United States of America, 76(12), 6661–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, et al. (2013). Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism, Clinical and Experimental Research, 37(3), 484–489. 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, & Krause EG (2017). Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. The Journal of Comparative Neurology, 525(5), 1094–1108. 10.1002/cne.24116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters ST, Bowen MT, Bohrer K, McGregor IS, & Neumann ID (2017). Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addiction Biology, 22(3), 702–711. 10.1111/adb.12362. [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Flor PJ, Neumann ID, & Reber SO (2013). Differential effects of baclofen and oxytocin on the increased ethanol consumption following chronic psychosocial stress in mice. Addiction Biology, 18(1), 66–77. 10.1111/adb.12001. [DOI] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, & Neumann ID (2014). Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology, 42, 225–236. 10.1016/j.psyneuen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Song M, Li Y, Wang F, & Wu CF (2008). Inhibition by oxytocin of methamphetamine-induced hyperactivity related to dopamine turnover in the mesolimbic region in mice. Naunyn-Schmiedeberg’s Archives of Pharmacology, 376(6), 441–448. 10.1007/s00210-007-0245-8. [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, & Wu CF (2009). Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology, 56(5), 856–865. 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, & Dusing R (2011). Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology, 36(6), 898–904. 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Raam T, McAvoy KM, Besnard A, Veenema AH, & Sahay A (2017). Hippocampal oxytocin receptors are necessary for discrimination of social stimuli. Nature Communications, 8(1), 2001 10.1038/s41467-017-02173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, & Brethen P (2002). Treatment of methamphetamine use disorders: An update. Journal of Substance Abuse Treatment, 23(2), 145–150. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, & Patra J (2009). Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet, 373(9682), 2223–2233. 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Reichel CM, & Bevins RA (2008). Competition between the conditioned rewarding effects of cocaine and novelty. Behavioral Neuroscience, 122(1), 140–150. 10.1037/0735-7044.122.1.140. [DOI] [PubMed] [Google Scholar]
- Reichel CM, & Bevins RA (2009). Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Current Drug Abuse Reviews, 2(2), 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, & Bevins RA (2010). Competition between novelty and cocaine conditioned reward is sensitive to drug dose and retention interval. Behavioral Neuroscience, 124(1), 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, & See RE (2011). Chronic N-acetylcysteine during abstinence or extinction following cocaine self-administration produces enduring reductions in drug-seeking. The Journal of Pharmacology and Experimental Therapeutics, 337(2), 487–493. 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Wilkinson JL, & Bevins RA (2010). Reference place conditioning procedure with cocaine: Increased sensitivity for measuring associatively motivated choice behavior in rats. Behavioural Pharmacology, 21(4), 323–331. 10.1097/FBP.0b013e32833b110b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA (2004). Spontaneous recovery. Learning & Memory, 11(5), 501–509. 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, & Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior, 84(1), 53–63. 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T Jr., et al. (2007). Mouse inbred strain differences in ethanol drinking to intoxication. Genes, Brain, and Behavior, 6(1), 1–18. 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Rimoldi V, Reversi A, Taverna E, Rosa P, Francolini M, Cassoni P, et al. (2003). Oxytocin receptor elicits different EGFR/MAPK activation patterns depending on its localization in caveolin-1 enriched domains. Oncogene, 22(38), 6054–6060. 10.1038/sj.onc.1206612. [DOI] [PubMed] [Google Scholar]
- Robbins TW, & Everitt BJ (2002). Limbic-striatal memory systems and drug addiction. Neurobiology of Learning and Memory, 78(3), 625–636. [DOI] [PubMed] [Google Scholar]
- Romero-Fernandez W, Borroto-Escuela DO, Agnati LF, & Fuxe K (2013). Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Molecular Psychiatry, 18(8), 849–850. 10.1038/mp.2012.103. [DOI] [PubMed] [Google Scholar]
- Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, & Moos F (2008). Emergent synchronous bursting of oxytocin neuronal network. PLoS Computational Biology, 4(7), e1000123 10.1371/journal.pcbi.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, & Carroll ME (2004). Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology, 172(4), 443–449. 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. (2001). Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse, 39(1), 32–41. . [DOI] [PubMed] [Google Scholar]
- Rudd RA, Rudd RA, Seth P, David F, & Scholl L (2016). Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR. Morbidity and Mortality Weekly Report, 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, et al. (2011). Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: A neurobehavioral model of autism. Biological Psychiatry, 69(9), 875–882. 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z (2011). Oxytocin as a potential mediator and modulator of drug addiction. Addiction Biology, 16(2), 199–201. 10.1111/j.1369-1600.2011.00332.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, & Kovacs GL (1994). Role of oxytocin in the neuroadaptation to drugs of abuse. Psychoneuroendocrinology, 19(1), 85–117. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, & Kovacs GL (2014). Oxytocin in learning and addiction: From early discoveries to the present. Pharmacology, Biochemistry, and Behavior, 119, 3–9. 10.1016/j.pbb.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, & Kalivas PW (2015). Gq-DREADD selectively initiates glial glutamate release and inhibits cue-induced cocaine seeking. Biological Psychiatry, 78(7), 441–451. 10.1016/j.biopsych.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, & Stewart J (2003). The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology, 168(1–2), 3–20. 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Siegal HA, Carlson RG, Kenne DR, & Swora MG (2003). Probable relationship between opioid abuse and heroin use. American Family Physician, 67(5), 942 945. [PubMed] [Google Scholar]
- Sinha R (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology,158(4), 343–359. 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R (2008). Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences, 1141, 105–130. 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2012). How does stress lead to risk of alcohol relapse? Alcohol Research: Current Reviews, 34(4), 432–440. [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, & Neumann ID (2010). Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology, 58(1), 56–61. 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- Smith SM, & Vale WW (2006). The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in Clinical Neuroscience, 8(4), 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Borland JM, Larkin TE, O’Malley M, & Albers HE (2016). Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology, 74, 164–172. 10.1016/j.psyneuen.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow GM, & Thiele TE (2012). The neurobiology of binge-like ethanol drinking: Evidence from rodent models. Physiology & Behavior, 106(3), 325–331. 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Wenner SM, Freestone DM, Romaine CC, Parian MC, Christian SM, et al. (2017). Oxytocin reduces alcohol consumption in prairie voles. Physiology & Behavior, 179, 411–421. 10.1016/j.physbeh.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Young KA, Bohidar AE, Francomacaro LM, Fasold TR, Buirkle JM, et al. (2017). Alcohol consumption decreases oxytocin neurons in the anterior paraventricular nucleus of the hypothalamus in prairie voles. Alcoholism, Clinical and Experimental Research, 41(8), 1444–1451. 10.1111/acer.13430. [DOI] [PubMed] [Google Scholar]
- Stoop R (2012). Neuromodulation by oxytocin and vasopressin. Neuron, 76(1), 142–159. 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Sung S, & Conry JM (2006). Role of buprenorphine in the management of heroin addiction. The Annals of Pharmacotherapy, 40(3), 501–505. 10.1345/aph.1G276. [DOI] [PubMed] [Google Scholar]
- Szabo G, Kovacs GL, Szekeli S, Balaspiri L, & Telegdy G (1987). C-terminal fragments of oxytocin (prolyl-leucyl-glycinamide and Z-prolyl-D-leucine) attenuate the development of tolerance to ethanol. Acta Physiologica Hungarica, 69(1), 115–122. [PubMed] [Google Scholar]
- Szabo G, Kovacs GL, Szekeli S, & Telegdy G (1985). The effects of neurohypophyseal hormones on tolerance to the hypothermic effect of ethanol. Alcohol, 2(4), 567–574. [DOI] [PubMed] [Google Scholar]
- Szabo G, Kovacs GL, & Telegdy G (1989). Intraventricular administration of neurohypophyseal hormones interferes with the development of tolerance to ethanol. Acta Physiologica Hungarica, 73(1), 97–103. [PubMed] [Google Scholar]
- Tan Y, Singhal S, Hiller H, Harden S, Nguyen D, Colon-Perez C, et al. (2017). Optogenetic excitation of neurons in the prefrontal cortex that express oxytocin receptors eliminates preference for social novelty in male mice. Society for Neurosciece Abstract. 2017. [Google Scholar]
- Thiel KJ, Okun AC, & Neisewander JL (2008). Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug and Alcohol Dependence, 96(3), 202–212. 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, & Boehm SL 2nd. (2014). “Drinking in the dark” (DID): A simple mouse model of binge-like alcohol intake. Current Protocols in Neuroscience, 68.9.49.1–9.49–12 10.1002/0471142301.ns0949s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, & Navarro M (2014). “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol, 48(3), 235–241. 10.1016/j.alcohol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Callaghan PD, Hunt GE, Cornish JL, & McGregor IS (2007). A role for oxytocin and 5-HT1A receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience, 146(2), 509–514. 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Tops M, Koole SL, H IJ, & Buisman-Pijlman FT (2014). Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacology, Biochemistry, and Behavior, 119, 39–48. 10.1016/j.pbb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, & Neisewander JL (1998). Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology, 19(1), 48–59. [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, & Vanderschuren LJ (2009). Conditioned place preference induced by social play behavior: Parametrics, extinction, reinstatement and disruption by methylphenidate. European Neuropsychopharmacology, 19(9), 659–669. 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnipseed SD, Richards JR, Kirk JD, Diercks DB, & Amsterdam EA (2003). Frequency of acute coronary syndrome in patients presenting to the emergency department with chest pain after methamphetamine use. The Journal of Emergency Medicine, 24(4), 369–373. [DOI] [PubMed] [Google Scholar]
- Unick GJ, Rosenblum D, Mars S, & Ciccarone D (2013). Intertwined epidemics: National demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993–2009. PLoS One, 8(2), e54496 10.1371/journal.pone.0054496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. 2017. World Drug Report, June 16 Vienna. [Google Scholar]
- Vaccari C, Lolait SJ, & Ostrowski NL (1998). Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology, 139(12), 5015–5033. 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, & De Wied D (1977). Modulation of heroin self-administration by neurohypophyseal principles. European Journal of Pharmacology, 43(2), 199–202. [DOI] [PubMed] [Google Scholar]
- Veening JG, & Olivier B (2013). Intranasal administration of oxytocin: Behavioral and clinical effects, a review. Neuroscience and Biobehavioral Reviews, 37(8), 1445–1465. 10.1016/j.neubiorev.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, & Freund-Mercier MJ (1997). Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: A histoautoradiographic study. The Journal of Comparative Neurology, 383(3), 305–325. [PubMed] [Google Scholar]
- Volkow ND, & Morales M (2015). The brain on drugs: From reward to addiction. Cell,162(4), 712–725. 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Waldherr M, & Neumann ID (2007). Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proceedings of the National Academy of Sciences of the United States of America, 104(42), 16681–16684. 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gallegos DA, Pogorelov VM, O’Hare JK, Calakos N, Wetsel WC, et al. (2018). Parvalbumin interneurons of the mouse nucleus accumbens are required for amphetamine-induced locomotor sensitization and conditioned place preference. Neuropsychopharmacology, 43(5), 953–963. 10.1038/npp.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Logan CN, Leong KC, Peris J, Knackstedt L, & Reichel CM (2018). Regionally specific effects of oxytocin on reinstatement of cocaine seeking in male and female rats. The International Journal of Neuropsychopharmacology, 21(7), 677–686. 10.1093/ijnp/pyy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westover AN, Nakonezny PA, & Haley RW (2008). Acute myocardial infarction in young adults who abuse amphetamines. Drug and Alcohol Dependence, 96(1–2), 49–56. 10.1016/j.drugalcdep.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, & Ingram CD (2004). Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. The Journal of Neuroscience, 24(12), 2974–2982. 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, & Ingram CD (1997). Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology, 138(7), 2829–2834. 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Yahyavi-Firouz-Abadi N, & See RE (2009). Anti-relapse medications: Preclinical models for drug addiction treatment. Pharmacology & Therapeutics, 124(2), 235–247. 10.1016/j.pharmthera.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, & Insel TR (2001). Cellular mechanisms of social attachment. Hormones and Behavior, 40(2), 133–138. 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, & Wang Z (2004). The neurobiology of pair bonding. Nature Neuroscience, 7,1048 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zaninetti M, & Raggenbass M (2000). Oxytocin receptor agonists enhance inhibitory synaptic transmission in the rat hippocampus by activating interneurons in stratum pyramidale. The European Journal of Neuroscience, 12(11), 3975–3984. [DOI] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Weber C, Robinson F, Kouimtsidis C, Niforooshan R, et al. (2017). Oxytocin and opioid addiction revisited: Old drug, new applications. British Journal of Pharmacology, 175(14), 2809–2824. 10.1111/bph.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, et al. (2014). The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology, 39(4), 855–865. 10.1038/npp.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Sun WL, Young AB, Lee K, McGinty JF, & See RE (2014). Oxytocin reduces cocaine seeking and reverses chronic cocaine-induced changes in glutamate receptor function. The International Journal of Neuropsychopharmacology, 18(1), 677–686. 10.1093/ijnp/pyu009. [DOI] [PMC free article] [PubMed] [Google Scholar]