Abstract
STUDY QUESTION
Does vitamin D attenuate the adverse effects of advanced glycation end products (AGEs) on steroidogenesis by human granulosa cells (GCs)?
SUMMARY ANSWER
AGEs alter the expression of genes important in steroidogenesis while 1,25-dihydroxyvitamin D3 (vit D3) in vitro attenuates some of the actions of AGEs on steroidogenic gene expression, possibly by downregulating the expression of the pro-inflammatory cell membrane receptor for AGEs (RAGE).
WHAT IS KNOWN ALREADY
Vitamin D attenuates the pro-inflammatory effects of AGEs in non-ovarian tissues.
STUDY DESIGN, SIZE, DURATION
Women who were undergoing IVF were enrolled. Follicular fluid samples (n = 71) were collected and cumulus GCs (n = 12) were treated in culture.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Follicular fluid levels of the anti-inflammatory soluble RAGE (sRAGE), AGEs and 25-hydroxyvitamin D (25-OHD) were quantified for possible correlations. GCs of each participant were split equally and treated with either media alone (control) or with human glycated albumin (HGA as a precursor for AGEs) with or without vit D3 after which RT-PCR and immunofluorescence were performed and cell culture media estradiol (E2) levels were compared.
MAIN RESULTS AND THE ROLE OF CHANCE
In follicular fluid, sRAGE levels were positively correlated with 25-OHD levels. HGA treatment (i) increased CYP11A1 (by 48%), 3β-HSD (by 38%), StAR (by 42%), CYP17A1 (by 30%) and LHR (by 37%) mRNA expression levels (P < 0.05 for all) but did not alter CYP19A1 or FSHR mRNA expression levels; and (ii) increased E2 release in cell culture media (P = 0.02). Vit D3 treatment (i) downregulated RAGE mRNA expression by 33% and RAGE protein levels by 44% (P < 0.05); (ii) inhibited the HGA-induced increase in CYP11A1, StAR, CYP17A1 and LHR mRNA levels, but not the increase in 3β-HSD mRNA levels; and (iii) did not inhibit the HGA-induced E2 release in cell culture media.
LIMITATIONS REASONS FOR CAUTION
This study used luteinized GCs that were collected from women who received gonadotropins thus the results obtained may not fully extrapolate to non-luteinized GCs in vivo.
WIDER IMPLICATIONS OF THE FINDINGS
This study suggests that there is a relationship between AGEs and their receptors (RAGE and sRAGE) with vitamin D. Understanding the interaction between AGEs and vitamin D in ovarian physiology could lead to a more targeted therapy for the treatment of ovarian dysfunction.
STUDY FUNDING/COMPETING INTEREST(S)
Funding was received from NIH (R01 NS045940), American Society for Reproductive Medicine, Ferring Pharmaceuticals Inc., and University of Vermont College of Medicine Bridge Funds. All authors have nothing to disclose.
Keywords: advanced glycation end products, vitamin D, RAGE, sRAGE, granulosa, follicular fluid, steroidogenesis
Introduction
Advanced glycation end products (AGEs) are physiologically formed by a non-enzymatic modification of proteins, lipids and nucleic acids by glucose (Monnier and Sell, 2006; Inagi, 2011; Piperi et al., 2012). AGEs constitute a heterogeneous group of compounds of more than 20 members (Monnier and Sell, 2006; Ramasamy et al., 2012). Pentosidine and N-carboxymethyl-lysine (CML) are well characterized AGEs and have been used as markers of AGE accumulation in various tissues (Diamanti-Kandarakis et al., 2007a; Merhi, 2014). Diabetes, insulin resistance, aging and oxidative stress accelerate the generation, or decrease the renal clearance, of AGEs (Bohlender et al., 2005; Uribarri et al., 2007; Unoki and Yamagishi, 2008; Tatone and Amicarelli, 2013; Yan et al., 2007). Additionally, AGEs play a role in the pathogenesis of cardiovascular disease (Yan et al., 2009) and are elevated in obesity and polycystic ovary syndrome (PCOS) (Diamanti-Kandarakis et al., 2008, 2005, 2007b). In addition to endogenous AGEs, serum and ovarian levels of AGEs depend on exogenous sources such as unhealthy diet and smoking (Cerami et al., 1997; Goldberg et al., 2004). Contemporary methods of cooking such as precooked fast-food meals and foods high in protein and fat such as fried eggs, meat and cheese dramatically increase serum concentrations of AGEs (Goldberg et al., 2004; Tantalaki et al., 2014), potentially leading to systemic inflammation. In animals, a high-AGE diet can initiate and provoke insulin resistance, an elevation in serum testosterone levels, an increase in ovarian weight and over-expression of the pro-inflammatory cell membrane receptor for AGEs, RAGE, in the ovaries (Diamanti-Kandarakis et al., 2007a).
Advanced glycation results in irreversible cross-linking of proteins, causing loss of protein structure and function (Monnier and Sell, 2006). Once formed, the protein cross-links damage cellular structures through a number of mechanisms (Inagi, 2011; Piperi et al., 2012). One mechanism of damage to cellular structures is the formation of cross-links between key molecules in the basement membrane of the extracellular matrix, e.g. collagen. Another mechanism involves the interaction of AGEs with RAGE, thus inducing intracellular inflammation and apoptosis. The circulating soluble RAGE (sRAGE) is a truncated form of RAGE and is secreted extracellularly and can be detected in the blood and the follicular fluid (Bonetti et al., 2013; Fujii and Nakayama, 2010; Malickova et al., 2010; Merhi et al., 2014b). sRAGE confers an anti-inflammatory role (Basta, 2008) by binding circulating AGEs, thus preventing the adverse intracellular events of the pro-inflammatory AGE–RAGE interaction (Merhi, 2014). Contrary to RAGE, sRAGE is often considered the good receptor (Basta et al., 2006) and circulating sRAGE levels have been used as a predictive biomarker of the development and progression of several diseases such as obesity, insulin resistance, PCOS, diabetes, cardiovascular disease and endothelial dysfunction (Pertynska-Marczewska and Merhi, 2015). As for ovarian physiology, follicular fluid sRAGE levels positively correlate with markers of ovarian reserve (Merhi et al., 2014b) and follicular fluid levels of sRAGE have been shown to be significantly lower in women with PCOS compared to healthy women (Garg et al., 2017).
Vitamin D (vit D) deficiency remains common worldwide (Holick et al., 2011). In the USA, 20–90% of reproductive-aged women are vit D deficient (Holick et al., 2011). Vit D deficiency is associated with obstetrical and reproductive complications including recurrent pregnancy loss, small for gestational age babies, abnormal puberty and infertility (Dicken et al., 2012; Ozkan et al., 2010; Twig et al., 2012). Emerging data have shown that vit D plays a role in female reproductive physiology (Anifandis et al., 2010; Ozkan et al., 2010; Pal et al., 2016). Vit D binds to vit D receptor (VDR), which is a transcription factor that belongs to the steroid and nuclear hormone receptor superfamily. VDR is expressed in ovarian tissue including granulosa cells (GCs) (Parikh et al., 2010), indicating that VDR plays a role in ovarian function, and it has been shown that vit D affects genes involved in ovarian steroidogenesis and folliculogenesis in human GCs (Merhi et al., 2014a). Several recent data have shown a relationship between vit D and the AGE–RAGE/sRAGE axis (Guo et al., 2016; Irani et al., 2014; Lee et al., 2014). Vit D is considered to have an anti-inflammatory effect and has been shown to exert a protective effect against the inflammatory actions of AGEs; for example, vit D3 and vit D2 downregulate RAGE expression and attenuate the deposition of AGEs in the blood–brain barrier (Guo et al., 2016), the endothelium (Dreyer et al., 2014) and the heart (Lee et al., 2014). Additionally, there is a significant positive correlation between follicular fluid sRAGE and follicular fluid 25-hydroxyvitamin D (25-OHD) (Garg et al., 2017) and administration of 1,25-dihydroxyvitamin D3 (vit D3) to vit D-deficient women with PCOS causes an increase in serum sRAGE levels (Irani et al., 2014).
Data on the relationship between vit D and AGEs and their receptors are scarce. Since in-vitro and animal studies have shown that vit D application could lower the toxic effects of AGEs (Salum et al., 2013; Talmor et al., 2008), we were interested in assessing the relationship between follicular fluid sRAGE and follicular fluid 25-OHD in a relatively large sample of women without PCOS. We also wanted to study the role of AGEs in vitro, in the absence or presence of vit D3, on GC function, which represents a long-standing effective tool in characterizing ovarian physiology in humans (Catteau-Jonard et al., 2008; Cloix et al., 2014; Merhi et al., 2014a, 2014b). We hypothesized that there is a positive correlation between follicular fluid sRAGE and follicular fluid 25-OHD and that AGEs affect GC steroidogenic function while vit D3 treatment attenuates the AGE-induced GC alterations.
Materials and Methods
Subjects
Among 76 women who were offered participation, 71 infertile women aged between 26 and 42 years old undergoing fresh IVF cycles agreed to participate in the study and were prospectively enrolled. Inclusion criteria included women with normal ovarian reserve defined as Day 3 FSH <10 mIU/mL and Day 3 estradiol (E2) <294 pmol/L. Women with PCOS as defined by the Rotterdam criteria (Franks, 2006) were excluded from the study. All patients gave informed consent and the study was approved by the Institutional Review Board (IRB# M13-062 and i6902 from University of Vermont and NYU School of Medicine; respectively).
Participants underwent ovarian stimulation with a combination of gonadotropins (Follistim, Merck, Whitehouse Station, NJ; Gonal-F, EMD-Serono, Rockland, MA; Menopur and Bravelle, Ferring, Parsippany, NJ) using either a long agonist (Lupron, AbbVie, North Chicago, IL) or antagonist (Ganirelix acetate, Merck, Whitehouse Station, NJ, or Cetrorelix acetate, EMD-Serono, Rockland, MA) protocol. When two or more follicles reached a diameter of ≥17 mm, hCG (ovidrel, EMD-Serono, Rockland, MA; or Novarel, Ferring, Parsippany, NJ) was administered for oocyte maturation, followed by transvaginal ultrasound-guided oocyte retrieval 34–36 h later. All the participants were fasting from the night before the day of the oocyte retrieval.
Follicular fluid collection for measurement of sRAGE, AGEs (pentosidine and CML), 25-OHD, vit D binding protein, total and free testosterone, sex hormone binding globulin, insulin and glucose
Follicle size was estimated immediately at the time of oocyte retrieval by ultrasound. Follicular fluid from the first large (>14 mm) aspirated follicle was used for protein measurement in order to avoid blood contamination. Follicular fluid was then centrifuged at 5000g for 5 min at 20°C to pellet the cells and debris, and the supernatant was stored at −80°C for protein analysis.
sRAGE was measured by ELISA using reagents from R&D Systems (Human RAGE Immunoassay kit; Minneapolis, MN). The assay sensitivity was 0.1 nmol/L and the interassay coefficient of variation (CV) was 8.3, 8.2 and 6.6% at concentrations of 14.8, 41.2 and 82.1 nmol/L, respectively. The two AGEs, CML and pentosidine, were quantified. CML was quantified by a competitive enzyme immunoassay using reagents (CML Competitive ELISA Kit) from Cell Biolabs, Inc. (San Diego, CA). The assay sensitivity was 10.7 μmol/L and the interassay CV was <10%. Pentosidine was measured by ELISA using reagents from Antibody Research (St. Charles, MO). The assay sensitivity was 2 nmol/L and the interassay CV was <10%.
25-OHD was measured by radioimmunoassay (RIA) using reagents (25-OHD 125I RIA Kit) from DiaSorin (Stillwater, MN). The RIA of 25-OHD involved a preceding extraction step with acetonitrile. The assay sensitivity was 3.7 nmol/L and the interassay CV was 9.4, 8.2 and 9.1% at concentrations of 21.5, 56.7 and 82.4 nmol/L, respectively. Vit D binding protein (VDBP) was quantified by ELISA using reagents (Human Vit D BP Immunoassay kit) from R&D Systems (Minneapolis, MN). The assay sensitivity was 12.3 nmol/L, and the interassay CV was 5.1, 6.0 and 7.4% at concentrations of 999.4 , 1983.8 and 3098.6 nmol/L, respectively.
Testosterone was measured by RIA with preceding organic solvent extraction and Celite column partition chromatography. It was eluted off the column with 40% toluene in isooctane. The assay sensitivity was 0.05 nmol/L and the interassay CV was 8, 12 and 12% at 0.45, 1 and 3.3 nmol/L, respectively. Free and bioavailable (non-SHBG-bound) testosterone were calculated using the measured total testosterone levels and sex hormone binding globulin (SHBG) concentrations as well as an average assumed concentration for albumin (Sodergard et al., 1982; Vermeulen et al., 1999)—this method has been shown to have high validity (Rinaldi et al., 2002). SHBG was measured by direct immunoassay on the Immulite analyzer (Siemens Healthcare Diagnostics, Deerfield, TX). The assay sensitivity was 1 nmol/L and the interassay CV was 9.1% at 69 nmol/L.
Insulin was measured by a solid-phase chemiluminescent immunometric assay using the Immulite 2000 analyzer (Siemens Healthcare Diagnostics, Deerfield, IL). The assay sensitivity was 2 μIU/mL. The interassay CV was 4.2 and 2.9% at 10.0 and 47.8 μIU/mL, respectively. Glucose was measured by a standard procedure using the Vitros Chemistry System.
Cumulus GC collection and culture for RNA extraction, reverse transcription and RT-PCR for steroid gene expression
Cumulus GCs of 4 out of the 71 participants were collected. After identification of the cumulus–oocyte complex in the aspirate, cumulus GCs were mechanically collected by cutting the cumulus layer from each oocyte. Cumulus GCs collected from each participant were pooled and then suspended in phosphate buffered saline (PBS), centrifuged for 5 min at 325g and re-suspended in fresh PBS at 4°C, as we previously described (Merhi et al., 2014a). Cells were briefly treated with 0.614 U/mL hyaluronidase (Sigma, MO), followed by 2× wash with PBS and centrifugation. The pellet of GCs of each participant was washed with fresh medium (DMEM-F12 and 1% fetal bovine serum, FBS). Since naturally occurring glycated albumin presents a sensitive glycemic indicator (Stensen et al., 2014), commercially available human glycated albumin (HGA) was used as a precursor for AGEs (Diamanti-Kandarakis et al., 2016) throughout the study.
GCs of each participant were split into three equal groups and then cultured in 24 well-culture plates (pretreated with poly-l-lysine for 5 min). All cells were plated 2–4 h after the participants underwent oocyte collection. From each participant, one-third of GCs was treated with media alone (control), another third of GCs was treated with HGA (0.4 mg/mL, Sigma Aldrich St. Louis, MO), and the last third of cells was treated with HGA (0.4 mg/mL) and vit D3 (100 nM, Sigma Aldrich St. Louis, MO) together. The treatment was for 48 h after which RT-PCR was performed. Since vit D3 was prepared with ethanol, ethanol was used as a vehicle control thus it was added to the all control groups of cells in all experiments. The doses of vit D3 (Merhi et al., 2014a) and HGA (Diamanti-Kandarakis et al., 2016; Stensen et al., 2014, Xu et al., 2014) used were chosen based on previous studies related to their effects on human GC function. Plates were incubated under a humidified atmosphere of 95% air and 5% CO2 at 37°C.
RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) and chloroform extraction according to the manufacturer’s instructions. RNA quality was assessed by a Nanodrop Spectrophotometer and Agilent Bioanalyzer (Santa Clara, CA). Only samples with a minimum concentration of 10 ng/μL and with an optical density (OD) 260/280 ratio of 1.8–2.0 were used for evaluation of RAGE, CYP19A1 (aromatase), 17α-hydroxylase/17,20 lyase (CYP17A1), P450 side-chain cleavage enzyme (CYP11A1), steroidogenic acute regulatory protein (StAR), 3 beta-hydroxysteroid dehydrogenase (3β-HSD), FSH receptor (FSHR) and LH receptor (LHR) mRNA expression levels. Although CYP17A1 is usually suppressed in non-luteinized GCs (Patel et al., 2009), luteinized GCs do express CYP17A1 (Moran et al., 2000). RT-PCR kinetics was achieved by using the SYBR Green I chemistry as we described elsewhere (Merhi et al., 2013). The primers used (Table I) were synthesized by Fisher (Pittsburg, PA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were used as a loading control and the levels of mRNA for each gene relative to GAPDH was calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001).
Table I.
Primers used for RT-PCR in the study.
| Gene | Sequence primers (5′–3′) |
|---|---|
| CYP19A1 | Forward: GAC GGA AGG TCC TGT GCT C |
| Reverse: GGG GGC AAT TTA GAG TCC ACA | |
| CYP17A1 | Forward: TGGGCACCAAGACTACAGTG |
| Reverse: CAGAGTCAGCGAAGGCGATA | |
| CYP11A1 | Forward: TGGGTCGCCTATCACCAGTA |
| Reverse: TGCAGGACACTGACGAAGTC | |
| StAR | Forward: AGGACGAAGAACCACCCTTG |
| Reverse: CATCACAGCCTGTTGCCTCA | |
| 3β-HSD | Forward: GCTTCTGGGTCAGAGGATCG |
| Reverse: CTGGCAGGCTCTTTTCAGGA | |
| FSHR | Forward: CCA GAA CCT TCC CAA CCT TCA |
| Reverse: TTT CAA AGC TCA GCC CCA CG | |
| LHR | Forward: GTTGACTTACCCCAGCCACT |
| AGTCCCAGCCACTCAGTTCA | |
| RAGE | Forward: AGCCTCCCCCTCAAATCCACT |
| Reverse: CAGCCCAGACCCATCCACAG | |
| GAPDH | Forward: ACC CAC TCC TCC ACC TTT GA |
| Reverse: TGT TGC TGT AGC CAA ATT CGT T |
Immunofluorescence for RAGE in luteinized GCs
Cumulus GCs obtained from 4 out of the 71 participants were used for immunofluorescence. GCs of each participant were split into two equal groups and then cultured on eight-well culture slides (Permanox® slide, 0.8 cm2/well, sterile, 96/cs Nunc® Lab-Tek® Chamber Slide™ system; Sigma, MO). From each participant, one half of GCs were treated with media alone (control) and the other half of GCs were treated with vit D3 (100 nM) for 24 h. GCs were fixed in 3.7% formaldehyde and permeabilized in 0.2% Triton X-100 for 10 min at room temperature, and nonspecific binding was inhibited by blocking with 1% bovine serum albumin (BSA) in PBS for 1 h. Fixed GCs were incubated with primary antibody for RAGE (1:100 dilution; Cell Signaling Technology Inc., Danvers, MA) overnight at 4°C, washed and then incubated in secondary antibody (1:200 dilution; Alexa Fluor 488 goat anti-rabbit IgG; Invitrogen) for 1–2 h at room temperature. Nuclear staining was performed using DAPI mounting media (Vector Labs, Inc., Burlingame CA) and observed on a Zeiss 510 META Laser Scanning Confocal microscope. Cellular images were captured using the Plane-NEOFLUAR 25× Immersion objective lens. Using MetaMorph software analysis, a threshold of 62 (0–255 scale of intensity) was used to exclude any background produced by DAPI. RAGE intensity on individual cells was performed by subtracting the nuclear staining from the cell surface staining.
Measurement of E2 concentration in cell culture media
Cumulus GCs obtained from 4 out of the 71 participants were used for measuring E2. GCs of each participant were split into three equal groups and then cultured in 24 well-culture plates (pretreated with poly-l-lysine for 5 min) in media for 24 h after which the culture media was collected for E2 concentrations (baseline levels). Thus baseline E2 levels reflect 24-h accumulation of E2 in the culture media. From each participant, one-third of GCs were treated with media alone (control), another third of GCs were treated with HGA (0.4 mg/mL), and the last third of GCs were treated with HGA (0.4 mg/mL) and vit D3 (100 nM) together for another 48 h after which culture media was collected for E2 concentrations (48 h levels). Cell culture media E2 levels were quantified by RIA. Prior to the RIA, E2 was extracted with ethylacetate:hexane (3:2) and was then separated by Celite column partition chromatography, using ethylene glycol as the stationary phase. E2 was eluted with 40% ethylacetate in isooctane. The assay sensitivity was 7.3 pmol/L and the interassay CV ranged from 9 to 14%.
Statistics
In order to determine the normality of the data, sktest in STATA 10.0 program that assesses both the skewness and kurtosis of the data simultaneously was used. Comparisons were performed using paired, unpaired t-test or ANOVA with a post hoc Bonferroni test for multiple comparisons if the data were normally distributed and Wilcoxon matched-pairs signed rank test, Mann–Whitney U test or Kruskal–Wallis test if the data were not normally distributed. Demographic and clinical data of the 71 participants were expressed as mean ± SD. Pearson correlation analyses between follicular fluid sRAGE versus 25-OHD and between follicular fluid sRAGE versus other relevant markers (VDBP, pentosidine, CML, total and free testosterone, SHBG, insulin and glucose) were performed.
For cell culture experiments, comparisons were performed using multilevel (hierarchical) analysis to account for cells in different conditions being from the same participant. This approach adjusts standard errors for clustering of cells within participant so the type I error rate was not inflated. Condition was the fixed effect in the model and participant was the random effect. For RT-PCR and immunofluorescence, a sample size of n = 4 has been shown to be adequate to produce statistical difference with 80% power and two-tailed α error of 0.05 (Merhi et al., 2015). RT-PCR results were expressed as relative number of copies ± SEM in cell culture experiments with controls being set at 1. Immunofluorescence was determined by taking the mean densitometry values of each treated cell group. All statistical procedures, except testing for normality of the data, were run on GraphPad Prism 7. P ≤ 0.05 was considered statistically significant.
Results
Correlation between follicular fluid sRAGE versus 25-OHD and other relevant markers
Table II shows the demographics and clinical characteristics of all the participants including follicular fluid levels of sRAGE, 25-OHD, VDBP, pentosidine, CML, total and free testosterone, SHBG, insulin and glucose. As seen in Table III, follicular fluid sRAGE levels positively correlated with 25-OHD, pentosidine, CML and SHBG. There was a negative correlation between follicular fluid sRAGE levels and follicular fluid insulin and glucose levels. No correlation was found between follicular fluid sRAGE levels and follicular fluid VDBP, total testosterone or free testosterone levels.
Table II.
Demographics and clinical characteristics of all the 71 participants.
| Mean ± SD | |
|---|---|
| Age (years) | 33.4 ± 2.7 |
| BMI (kg/m2) | 25.8 ± 2.8 |
| Serum Day 3 FSH (mIU/mL) | 6.9 ± 2.3 |
| Serum Day 3 E2 (pmol/L) | 171.1 ± 40.8 |
| Antral follicle count by ultrasound | 15.9 ± 4.9 |
| Number of days of stimulation | 9.8 ± 3.3 |
| Total gonadotropin IUs per cycle | 3481.5 ± 956.5 |
| Serum peak E2 level (pmol/L) on day of hCG | 6689.2 ± 2892.3 |
| Number of oocytes retrieved | 10.3 ± 4.9 |
| Follicular fluid sRAGE (nmol/L) | 155.7 ± 62.3 |
| Follicular fluid 25-hydroxyvitamin D (nmol/L) | 95.1 ± 26 |
| Follicular fluid VDBP (nmol/L) | 4553.4 ± 1481.3 |
| Follicular fluid pentosidine (nmol/L) | 20.3 ± 18.6 |
| Follicular fluid CML (μmol/L) | 0.34 ± 0.1 |
| Follicular fluid total testosterone (nmol/L) | 9.7 ± 18.9 |
| Follicular fluid free testosterone (nmol/L) | 1.2 ± 2.5 |
| Follicular fluid SHBG (nmol/L) | 1297.9 ± 466.3 |
| Follicular fluid insulin (μIU/mL) | 5.9 ± 6.9 |
| Follicular fluid glucose (mmol/L) | 2.92 ± 0.82 |
E2, estradiol; sRAGE, soluble receptor for advanced glycation end products; CML, N-(carboxymethyl) lysine; SHBG, sex hormone binding globulin.
Table III.
Correlation between follicular fluid sRAGE versus 25-hydroxyvitamin D (25-OHD) and other relevant markers.
| n = 71 participants | Variable | R-value | P-value |
|---|---|---|---|
| sRAGE | |||
| 25-OHD | 0.27 | 0.02 | |
| VDBP | 0.02 | 0.8 | |
| Pentosidine | 0.24 | 0.04 | |
| CML | 0.32 | 0.006 | |
| SHBG | 0.37 | 0.001 | |
| Insulin | −0.28 | 0.02 | |
| Glucose | −0.39 | 0.0007 | |
| Total testosterone | 0.0002 | 0.9 | |
| Free testosterone | −0.04 | 0.7 |
sRAGE, soluble receptor for advanced glycation end products; VDBP, vitamin D-binding protein; CML, N-(carboxymethyl) lysine; SHBG, sex hormone binding globulin.
Vit D3 treatment in vitro downregulates RAGE mRNA and protein expression levels in human luteinized GCs
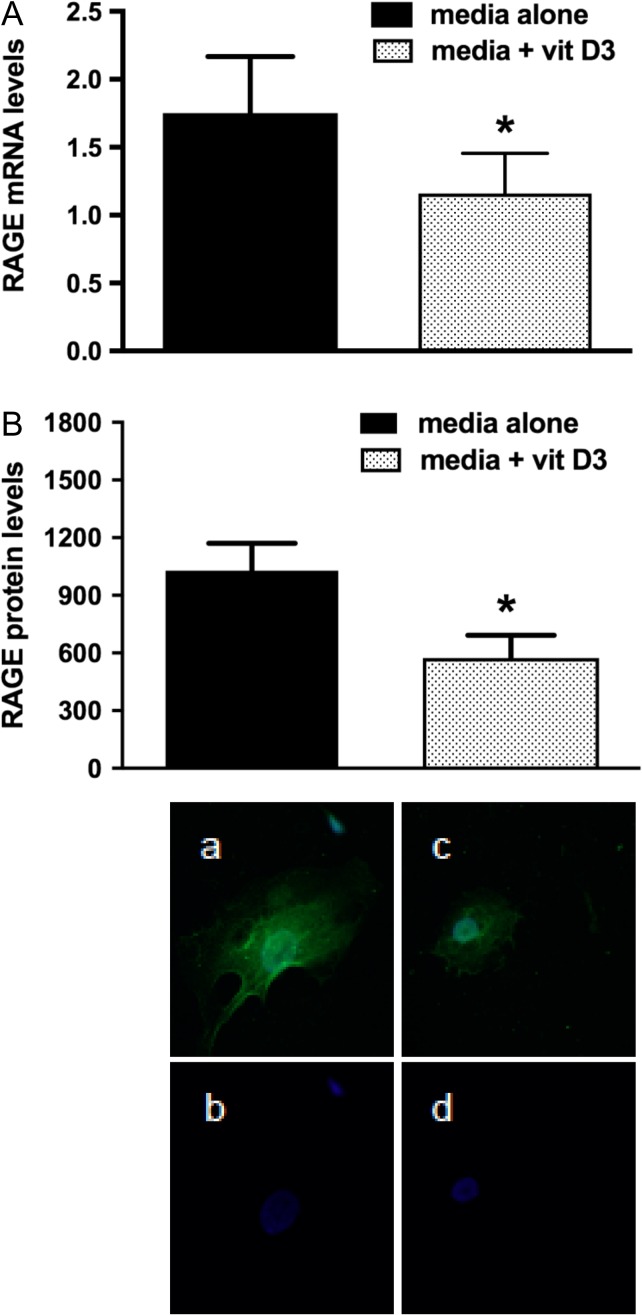
In order to evaluate whether vit D3 alters RAGE expression in human GCs, we treated human GCs with vit D3 after which RT-PCR for RAGE mRNA and immunofluorescence for RAGE protein were performed. Compared to GCs treated with media alone (control), the addition of vit D3 significantly suppressed RAGE mRNA (by 33%) and protein (by 44%) levels (P < 0.05) (Fig. 1) indicating that vit D3 might attenuate the effects of AGEs by suppressing RAGE expression.
Figure 1.
Real-time PCR and immunofluorescence for RAGE in cumulus granulosa cells treated with media alone or media with 1,25 dihydroxyvitamin D3 (vit D3). Pooled cumulus granulosa cells of women undergoing oocyte retrieval following ovarian stimulation for IVF were mechanically collected. Cells were treated with media alone or media + vit D3 (100 nM) for 24 h after which (A) RT-PCR for RAGE mRNA (n = 4) and (B) immunofluorescence for RAGE protein (n = 4) were performed. The addition of vit D3 significantly suppressed RAGE mRNA and protein levels. For immunofluorescence, the positive signal is seen in green and the blue signal represents DAPI; (a, b) are for cells treated with media alone (control) and (c, d) are for cells treated with media + vit D3. *P < 0.05 for media + vit D3 versus media alone.
In-vitro effect of AGEs (HGA) with or without vit D3 on steroid gene expression in human luteinized GCs
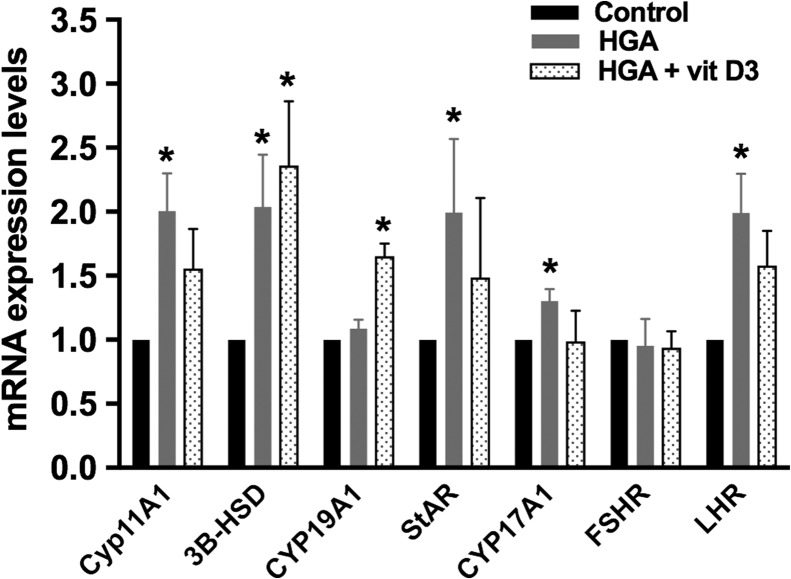
HGA, a precursor for AGEs, binds and activates the pro-inflammatory cell membrane receptor RAGE. Compared to GCs cultured in media alone (control), HGA treatment significantly increased CYP11A1 (by 48%), 3β-HSD (by 38%), StAR (by 42%), CYP17A1 (by 30%) and LHR (by 37%) mRNA expression levels (P < 0.05) but did not alter CYP19A1 or FSHR mRNA levels (Fig. 2). The addition of vit D3 to HGA inhibited the HGA-induced increase in CYP11A1, StAR, CYP17A1 and LHR mRNA levels, but not 3β-HSD mRNA levels, which remained 48% higher than controls (P < 0.05). Although HGA alone did not increase CYP19A1 mRNA expression, the addition of vit D3 to HGA significantly increased CYP19A1 mRNA expression as compared to controls (P < 0.05).
Figure 2.
Real-time PCR for steroid gene expression following treatment of cumulus granulosa cells with human glycated albumin (HGA), as a precursor for AGEs, with or without 1,25 dihydroxyvitamin D3 (vit D3). Pooled cumulus granulosa cells of women (n = 4) undergoing oocyte retrieval following ovarian stimulation for IVF were mechanically collected by cutting the cumulus layer from each oocyte. Cells were treated with HGA (0.4 mg/mL) with or without vit D3 (100 nM) for 24 h after which real-time PCR was performed. HGA significantly increased CYP11A1 (side chain cleavage), 3β-HSD, StAR, CYP17A1 and LHR mRNA expression levels but did not alter CYP19A1 or FSHR mRNA levels. The addition of vit D3 to HGA inhibited the HGA-induced increase in CYP11A1, StAR, CYP17A1 and LHR but not increase in 3-βHSD mRNA levels. The combination of HGA + vit D3 significantly increased CYP19A1 mRNA expression levels. Controls cells were set at 1. *P < 0.05 for HGA versus control, and *P < 0.05 for HGA + vit D3 versus control.
In-vitro effect of AGEs (HGA) with or without vit D3 on E2 release by human luteinized GCs
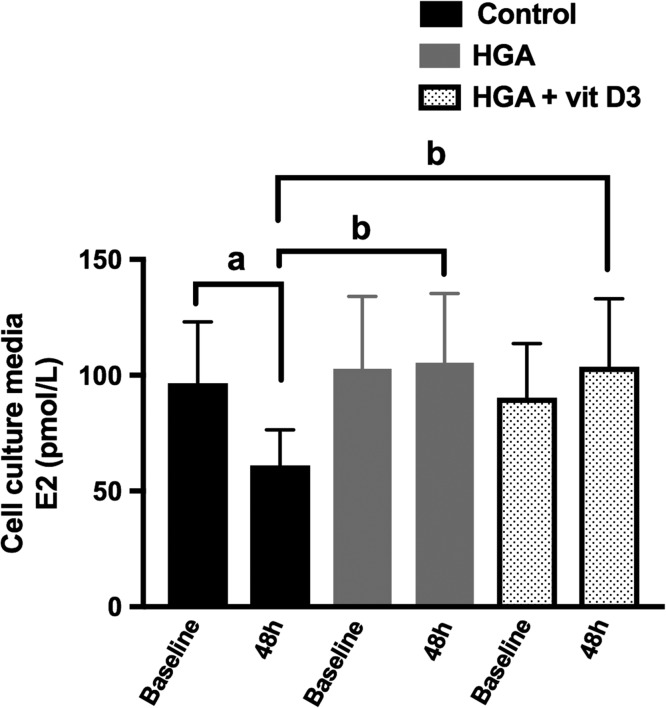
To further study the effect of HGA in the presence of absence of vit D3 on E2 release by GCs, we treated GCs with media alone (control) or with HGA with or without vit D3. It has been shown that E2 production of cultured human luteinized GCs gradually decreases during culture in media alone (Foldesi et al., 1998). Similarly in our results as seen in Fig. 3 in control cells, E2 in cell culture media of control GCs decreased from 356 ± 99 pmol/L at baseline to 224 ± 55 pmol/L following 48 h of culture (P = 0.03). Baseline E2 concentration levels in culture media of GCs treated with media alone, HGA alone or HGA + vit D3 were identical (P = 0.42) (Fig. 3). GCs treated for 48 h with HGA alone or HGA + vit D3 had significantly higher E2 concentration levels compared to GCs treated with media alone for 48 h (P = 0.02). In GCs treated with HGA alone, E2 in cell culture media remained stable between baseline (378 ± 114 pmol/L) and 48 h following culture (385 ± 110 pmol/L, P = 0.84). Similarly in GCs treated with HGA + vit D3, E2 in cell culture media remained stable between baseline (330 ± 84 pmol/L) and 48 h following culture (382 ± 106 pmol/L, P = 0.22).
Figure 3.
Effect of human glycated albumin (HGA), as a precursor for AGEs, with or without 1,25 dihydroxyvitamin D3 (vit D3) on estradiol (E2) release by cumulus granulosa cells. Pooled cumulus granulosa cells of women (n = 4) undergoing oocyte retrieval following ovarian stimulation for IVF were mechanically collected by cutting the cumulus layer from each oocyte. Cells were treated with HGA (0.4 mg/mL) with or without vit D3 (100 nM) for 24 h after which cell culture media was collected for E2 radioimmunoassay. Between baseline and following 48 h of culture, E2 release significantly decreased in control cells (P = 0.03) but remained unchanged in cells treated with HGA alone or with HGA + vit D3 (P = 0.84). Following 48 h of culture, E2 in cell culture media was significantly higher with HGA alone or with HGA + vit D3 compared to control cells. aP < 0.05 for baseline versus 48 h levels in the control cells, and bP < 0.05 for HGA versus control at 48 h of culture and bP < 0.05 for HGA + vit D3 versus control at 48 h of culture.
Discussion
This study evaluated the correlation between follicular fluid sRAGE, a decoy receptor that captures the circulating AGEs thus preventing the activation of the pro-inflammatory RAGE signaling pathway, and follicular fluid 25-OHD in a relatively large sample of women without PCOS. Our results showed that there is a positive correlation between follicular fluid sRAGE and 25-OHD. We also used human luteinized GCs as a model to assess whether treatment with AGEs alters genes involved in steroid production and to assess whether vit D3 application attenuates the effect of AGEs. The results demonstrated that HGA (AGEs) significantly increased CYP11A1, 3β-HSD, StAR, CYP17A1 and LHR mRNA levels but did not change CYP19A1 or FSHR mRNA levels. The addition of vit D3 inhibited most of the HGA-induced changes in these genes. Additionally, HGA (AGEs), in the presence or absence of vit D3, significantly increased E2 release by GCs in culture media. We also found that vit D3 downregulated RAGE mRNA and protein expression, which could constitute a mechanism by which vit D3 inhibited the HGA-induced changes in steroid gene expression.
The functions of sRAGE in the follicular fluid are still unclear, but it may impact the activities of the AGE–RAGE system in ovarian follicles, and it has been shown to correlate with markers of ovarian reserve, such as follicular fluid anti-Mullerian hormone and number of oocytes retrieved following oocyte retrieval for IVF (Merhi et al., 2014b). Similar to what has been reported in the follicular fluid of women with PCOS (Garg et al., 2017), we found, in women without PCOS, that there is a weak positive correlation (R-value was only 0.27) between follicular fluid sRAGE and 25-OHD. Additionally, Sung et al. (2013) reported a positive correlation between serum sRAGE and serum 1,25 dihydroxyvitamin D3 in hemodialysis patients who received vit D3 supplementation. Interestingly, vit D3 replacement to vit D-deficient women with PCOS increases serum sRAGE levels, potentially suggesting a direct effect of vit D3 on sRAGE production (Irani et al., 2014). Both sRAGE and vit D have anti-inflammatory actions, which could explain this positive relationship between the two molecules. Our findings also showed that follicular fluid sRAGE levels correlated positively with follicular fluid levels of the two AGEs: pentosidine and CML. Similarly, Willemsen et al. (2012) showed that serum pentosidine and CML levels were positively correlated with serum sRAGE levels in patients hospitalized for heart failure. Similarly, Kerkeni et al. (2012) observed that sRAGE serum levels were positively correlated with serum AGEs in patients with diabetic retinopathy. An explanatory hypothesis for this correlation is that as the pro-inflammatory AGEs increase, there is a counter-effect of anti-inflammatory response as reflected by more sRAGE production, i.e. increased AGEs stimulate sRAGE expression as a feedback mechanism. Finally, serum sRAGE is related to the insulin resistance state; e.g. it has been shown that there is an inverse relationship between serum sRAGE and serum glucose, insulin and HOMA-IR levels (Basta et al., 2006). Our data showed similar findings in the follicular fluid where sRAGE was negatively correlated with follicular fluid insulin and glucose and positively correlated with SHBG. The relationship between sRAGE, glucose and insulin in the follicular fluid environment might be important in the dynamics of the follicle development and ultimately in oocyte maturation. More studies pertaining to this topic are needed.
Steroidogenesis is crucial for the synchronization of follicle growth and oocyte development (Dumesic et al., 2015). Our current findings demonstrated that HGA, a precursor for AGEs, altered steroidogenic enzymes involved in E2 production and maintained a significantly higher level of E2 in the culture media compared to control cells, despite having no effect on aromatase (CYP19A1) mRNA levels. This was reflected by an upregulation in CYP11A1, 3β-HSD, StAR, CYP17A1 and LHR (Fig. 4). One explanation could be that AGEs, along with the upregulation of these genes, increase E2 ‘release’ from GCs rather than just increasing production by induction of aromatase. Also there could have been a change in aromatase enzymatic activity/protein expression without a change in mRNA levels. Another hypothesis is that HGA could be blocking E2 metabolism rather than directly affecting directly E2. Vit D3 has been shown to downregulate RAGE expression in non-ovarian cells (Dreyer et al., 2014, Guo et al., 2016; Lee et al., 2014). In cell culture studies, calcitriol was shown to attentuate AGE-induced upregulation of RAGE mRNA and protein and counteracted the stimulating effect of AGEs on NF-κB pathway, which is a common pathway between vit D and AGEs (Talmor et al., 2008). In streptozotocin-induced diabetic rats, the increased expression of cardiac RAGE was attentuated by calcitriol (Lee et al., 2014). Also in an in-vitro blood–brain barrier model, RAGE expression was reduced following vit D3 application (Guo et al., 2016). Similarly, our current findings demonstrated that vit D3 suppressed RAGE mRNA and protein expression in human GCs, which could constitute a mechanism by which vit D3 inhibited the HGA-induced changes in steroid gene expression. The mechanisms of this relationship require further studies in order to better elucidate their role in ovarian function and female reproduction.
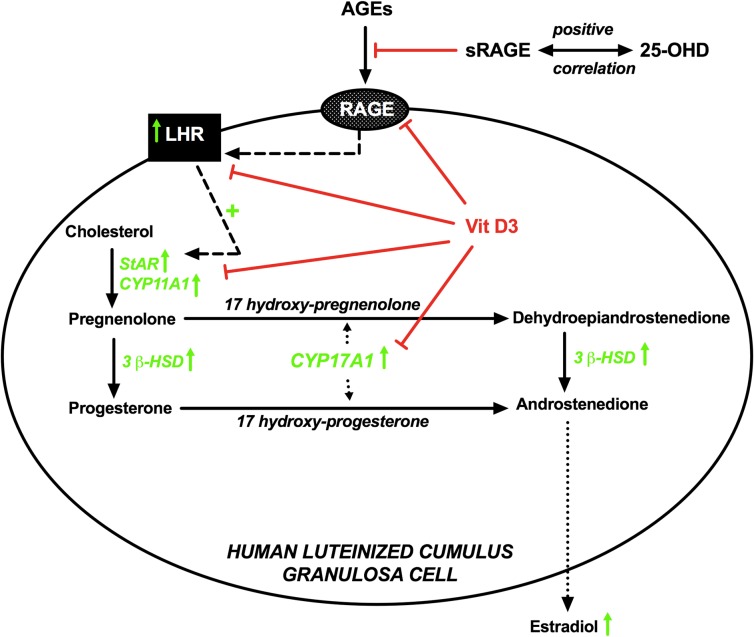
Figure 4.
Schematic diagram illustrating the relationship between vitamin D and AGEs in follicular fluid and human luteinized granulosa cells. A positive correlation exists between follicular fluid 25-hydoxyvitamin D (25-OHD) and follicular fluid sRAGE, which inhibits the circulating AGEs from activating their pro-inflammatory RAGE signaling pathway. AGEs affect steroidogenesis by upregulating luteinizing hormone receptor (LHR), which is known to induce steroidogenic acute regulatory protein (StAR) and P450 side-chain cleavage enzyme (CYP11A1). AGEs also upregulate 17α-hydroxylase/17,20 lyase (CYP17A1) and 3 beta-hydroxysteroid dehydrogenase (3β-HSD) ultimately leading to more production and release of estradiol. Upward green arrows indicate upregulation by AGEs. 1,25-dihydroxyvitamin D3 (vit D3) downregulates RAGE expression and inhibits the effect of AGEs on LHR, StAR, CYP11A1 and CYP17A1, but not the effects on 3β-HSD.
There are several limitations for this study. We did not have serum levels for the markers measured in the follicular fluid. Additionally, several types of ovarian cells play a role in steroidogenesis, such as mural GCs and theca cells. In this study, we focused on cumulus GCs that express characteristics distinct from mural GCs (Eppig et al., 1997, Vanderhyden and Tonary, 1995). Cumulus cells communicate with each other and with the oocyte through specialized gap junctions which allow metabolic exchange and transport of signaling molecules appropriate for follicular development (Dumesic et al., 2015, Simerman et al., 2015, Uyar et al., 2013). Thus, cumulus GCs, arguably, play a more important role in regulating oocyte maturation and in reflecting oocyte quality (Dumesic et al., 2015; Uyar et al., 2013). Another limitation is that our GCs were luteinized, as they were collected from women who were stimulated with gonadotropins. However, although these luteinized GCs may not be the best tool for studying steroidogenesis, we (Merhi et al., 2013) and others (Catteau-Jonard et al., 2008) have shown that luteinized GCs are responsive to several hormonal treatments in vitro. Another potential limitation is that we have pooled cumulus GCs of all small and large follicles retrieved during IVF from each participant. However, large follicles are more mature than smaller follicles, and thus AGEs and vit D3 in vitro could act differently on GCs obtained from large versus small follicles. Finally, the doses of HGA and vit D3 and the culture time used in the study were based on published studies but may not be ideal to reflect changes that occur physiologically.
In summary, understanding the role of the AGE–RAGE system in ovarian dysfunction, particularly for women who have elevated levels of these AGEs, such as obese or PCOS women or women who eat unhealthy diets, may help in our understanding of the mechanisms involved ovarian function. The results of this study provide a proof-of-principle for the potential for vit D supplementation in preventing some ovarian dysfunction and provide justification for the continued development of clinical trials of vit D as a cost-effective strategy to improving function of the ovaries. Additionally, the development of new therapeutic agents, such as AGE blockers, could represent a novel strategy to treat and/or prevent ovarian dysfunction.
Authors’ roles
Z.M. designed the study, performed the experiments and wrote major parts of the article. E.B. helped with the study design, performed some of the experiments and wrote parts of the article. M.J.C. supervised all of the experiments, provided feedback on the progress of the study and wrote parts of the article.
Funding
NIH (R01 NS045940), American Society for Reproductive Medicine (ASRM), Ferring Pharmaceuticals Inc., and University of Vermont College of Medicine Bridge Funds.
Conflict of interest
All authors have nothing to disclose.
References
- Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, Messinis IE. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol 2010;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis 2008;196:9–21. [DOI] [PubMed] [Google Scholar]
- Basta G, Sironi AM, Lazzerini G, Del Turco S, Buzzigoli E, Casolaro A, Natali A, Ferrannini E, Gastaldelli A. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J Clin Endocrinol Metab 2006;91:4628–4634. [DOI] [PubMed] [Google Scholar]
- Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol 2005;289:F645–F659. [DOI] [PubMed] [Google Scholar]
- Bonetti TC, Borges E Jr., Braga DP, Iaconelli A Jr., Kleine JP, Silva ID. Intrafollicular soluble receptor for advanced glycation end products (sRAGE) and embryo quality in assisted reproduction. Reprod Biomed Online 2013;26:62–67. [DOI] [PubMed] [Google Scholar]
- Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:4456–4461. [DOI] [PubMed] [Google Scholar]
- Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucala R et al. Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci USA 1997;94:13915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloix L, Reverchon M, Cornuau M, Froment P, Rame C, Costa C, Froment G, Lecomte P, Chen W, Royere D et al. Expression and regulation of INTELECTIN1 in human granulosa-lutein cells: role in IGF-1-induced steroidogenesis through NAMPT. Biol Reprod 2014;91:50. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Chatzigeorgiou A, Papageorgiou E, Koundouras D, Koutsilieris M. Advanced glycation end-products and insulin signaling in granulosa cells. Exp Biol Med (Maywood, NJ) 2016;241:1438–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Katsikis I, Piperi C, Kandaraki E, Piouka A, Papavassiliou AG, Panidis D. Increased serum advanced glycation end-products is a distinct finding in lean women with polycystic ovary syndrome (PCOS). Clin Endocrinol 2008;69:634–641. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol 2005;62:37–43. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Piperi C, Korkolopoulou P, Kandaraki E, Levidou G, Papalois A, Patsouris E, Papavassiliou AG. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. J Mol Med (Berl) 2007. a;85:1413–1420. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Piperi C, Patsouris E, Korkolopoulou P, Panidis D, Pawelczyk L, Papavassiliou AG, Duleba AJ. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem Cell Biol 2007. b;127:581–589. [DOI] [PubMed] [Google Scholar]
- Dicken CL, Israel DD, Davis JB, Sun Y, Shu J, Hardin J, Neal-Perry G. Peripubertal vitamin D(3) deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biol Reprod 2012;87:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G, Tucker AT, Harwood SM, Pearse RM, Raftery MJ, Yaqoob MM. Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin d deficiency: an exploratory, double blind, randomised controlled trial. PLoS One 2014;9:e99461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril 2015;103:303–316. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Chesnel F, Hirao Y, O’Brien MJ, Pendola FL, Watanabe S, Wigglesworth K. Oocyte control of granulosa cell development: how and why. Hum Reprod (Oxford, England) 1997;12:127–132. [PubMed] [Google Scholar]
- Foldesi I, Breckwoldt M, Neulen J. Oestradiol production by luteinized human granulosa cells: evidence of the stimulatory action of recombinant human follicle stimulating hormone. Hum Reprod (Oxford, England) 1998;13:1455–1460. [DOI] [PubMed] [Google Scholar]
- Franks S. Controversy in clinical endocrinology: diagnosis of polycystic ovarian syndrome: in defense of the Rotterdam criteria. J Clin Endocrinol Metab 2006;91:786–789. [DOI] [PubMed] [Google Scholar]
- Fujii EY, Nakayama M. The measurements of RAGE, VEGF, and AGEs in the plasma and follicular fluid of reproductive women: the influence of aging. Fertil Steril 2010;94:694–700. [DOI] [PubMed] [Google Scholar]
- Garg D, Grazi R, Lambert-Messerlian GM, Merhi Z. Correlation between follicular fluid levels of sRAGE and vitamin D in women with PCOS. J Assist Reprod Genet 2017;34:1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H. Advanced glycoxidation end products in commonly consumed foods. J Am Diet Assoc 2004;104:1287–1291. [DOI] [PubMed] [Google Scholar]
- Guo YX, He LY, Zhang M, Wang F, Liu F, Peng WX. 1,25-Dihydroxyvitamin D3 regulates expression of LRP1 and RAGE in vitro and in vivo, enhancing Abeta1-40 brain-to-blood efflux and peripheral uptake transport. Neuroscience 2016;322:28–38. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- Inagi R. Inhibitors of advanced glycation and endoplasmic reticulum stress. Methods Enzymol 2011;491:361–380. [DOI] [PubMed] [Google Scholar]
- Irani M, Minkoff H, Seifer DB, Merhi Z, Vitamin D. Increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J Clin Endocrinol Metab 2014;99:E886–E890. [DOI] [PubMed] [Google Scholar]
- Kerkeni M, Saidi A, Bouzidi H, Ben Yahya S, Hammami M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvasc Res 2012;84:378–383. [DOI] [PubMed] [Google Scholar]
- Lee TW, Kao YH, Lee TI, Chang CJ, Lien GS, Chen YJ. Calcitriol modulates receptor for advanced glycation end products (RAGE) in diabetic hearts. Int J Cardiol 2014;173:236–241. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- Malickova K, Jarosova R, Rezabek K, Fait T, Masata J, Janatkova I, Zima T, Kalousova M. Concentrations of sRAGE in serum and follicular fluid in assisted reproductive cycles—a preliminary study. Clin Lab 2010;56:377–384. [PubMed] [Google Scholar]
- Merhi Z. Advanced glycation end products and their relevance in female reproduction. Hum Reprod (Oxford, England) 2014;29:135–145. [DOI] [PubMed] [Google Scholar]
- Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua S Jr., Jindal S. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod (Oxford, England) 2013;28:1661–1669. [DOI] [PubMed] [Google Scholar]
- Merhi Z, Doswell A, Krebs K, Cipolla M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab 2014. a;99:E1137–E1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merhi Z, Irani M, Doswell AD, Ambroggio J. Follicular fluid soluble receptor for advanced glycation end-products (sRAGE): a potential indicator of ovarian reserve. J Clin Endocrinol Metab 2014. b;99:E226–E233. [DOI] [PubMed] [Google Scholar]
- Merhi Z, Polotsky AJ, Bradford AP, Buyuk E, Chosich J, Phang T, Jindal S, Santoro N. Adiposity alters genes important in inflammation and cell cycle division in human cumulus granulosa cell. Reprod Sci (Thousand Oaks, Calif) 2015;22:1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM, Sell DR. Prevention and repair of protein damage by the Maillard reaction in vivo. Rejuvenation Res 2006;9:264–273. [DOI] [PubMed] [Google Scholar]
- Moran FM, Conley AJ, Corbin CJ, Enan E, VandeVoort C, Overstreet JW, Lasley BL. 2,3,7,8-Tetrachlorodibenzo-p-dioxin decreases estradiol production without altering the enzyme activity of cytochrome P450 aromatase of human luteinized granulosa cells in vitro. Biol Reprod 2000;62:1102–1108. [DOI] [PubMed] [Google Scholar]
- Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril 2010;94:1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, Coutifaris C, Carson SA, Steinkampf MP, Carr BR et al. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: secondary analysis of a multicenter randomized controlled trial. J Clin Endocrinol Metab 2016;101:3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh G, Varadinova M, Suwandhi P, Araki T, Rosenwaks Z, Poretsky L, Seto-Young D. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm Metab Res 2010;42:754–757. [DOI] [PubMed] [Google Scholar]
- Patel SS, Beshay VE, Escobar JC, Suzuki T, Carr BR. Molecular mechanism for repression of 17alpha-hydroxylase expression and androstenedione production in granulosa cells. J Clin Endocrinol Metab 2009;94:5163–5168. [DOI] [PubMed] [Google Scholar]
- Pertynska-Marczewska M, Merhi Z. Relationship of advanced glycation end products with cardiovascular disease in menopausal women. Reprod Sci (Thousand Oaks, Calif) 2015;22:774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperi C, Adamopoulos C, Dalagiorgou G, Diamanti-Kandarakis E, Papavassiliou AG. Crosstalk between advanced glycation and endoplasmic reticulum stress: emerging therapeutic targeting for metabolic diseases. J Clin Endocrinol Metab 2012;97:2231–2242. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Yan SF, Schmidt AM. The diverse ligand repertoire of the receptor for advanced glycation endproducts and pathways to the complications of diabetes. Vascul Pharmacol 2012;57:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 2002;11:1065–1071. [PubMed] [Google Scholar]
- Salum E, Kals J, Kampus P, Salum T, Zilmer K, Aunapuu M, Arend A, Eha J, Zilmer M. Vitamin D reduces deposition of advanced glycation end-products in the aortic wall and systemic oxidative stress in diabetic rats. Diabetes Res Clin Pract 2013;100:243–249. [DOI] [PubMed] [Google Scholar]
- Simerman AA, Hill DL, Grogan TR, Elashoff D, Clarke NJ, Goldstein EH, Manrriquez AN, Chazenbalk GD, Dumesic DA. Intrafollicular cortisol levels inversely correlate with cumulus cell lipid content as a possible energy source during oocyte meiotic resumption in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril 2015;103:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–810. [DOI] [PubMed] [Google Scholar]
- Stensen MH, Tanbo T, Storeng R, Fedorcsak P. Advanced glycation end products and their receptor contribute to ovarian ageing. Hum Reprod (Oxford, England) 2014;29:125–134. [DOI] [PubMed] [Google Scholar]
- Sung JY, Chung W, Kim AJ, Kim HS, Ro H, Chang JH, Lee HH, Jung JY. Calcitriol treatment increases serum levels of the soluble receptor of advanced glycation end products in hemodialysis patients with secondary hyperparathyroidism. Tohoku J Exp Med 2013;230:59–66. [DOI] [PubMed] [Google Scholar]
- Talmor Y, Bernheim J, Klein O, Green J, Rashid G. Calcitriol blunts pro-atherosclerotic parameters through NFkappaB and p38 in vitro. Eur J Clin Invest 2008;38:548–554. [DOI] [PubMed] [Google Scholar]
- Tantalaki E, Piperi C, Livadas S, Kollias A, Adamopoulos C, Koulouri A, Christakou C, Diamanti-Kandarakis E. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones (Athens) 2014;13:65–73. [DOI] [PubMed] [Google Scholar]
- Tatone C, Amicarelli F. The aging ovary—the poor granulosa cells. Fertil Steril 2013;99:12–17. [DOI] [PubMed] [Google Scholar]
- Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun 2012;38:J275–J281. [DOI] [PubMed] [Google Scholar]
- Unoki H, Yamagishi S. Advanced glycation end products and insulin resistance. Curr Pharm Des 2008;14:987–989. [DOI] [PubMed] [Google Scholar]
- Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci 2007;62:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril 2013;99:979–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhyden BC, Tonary AM. Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by A factor(s) secreted by the oocyte. Biol Reprod 1995;53:1243–1250. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–3672. [DOI] [PubMed] [Google Scholar]
- Willemsen S, Hartog JW, van Veldhuisen DJ, van der Meer P, Roze JF, Jaarsma T, Schalkwijk C, van der Horst IC, Hillege HL, Voors AA. The role of advanced glycation end-products and their receptor on outcome in heart failure patients with preserved and reduced ejection fraction. Am Heart J 2012;164:742–749.e743. [DOI] [PubMed] [Google Scholar]
- Xu L, Zang P, Feng B, Qian Q. Atorvastatin inhibits the expression of RAGE induced by advanced glycation end products on aortas in healthy Sprague-Dawley rats. Diabetol Metab Syndr 2014;6:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SF, D’Agati V, Schmidt AM, Ramasamy R. Receptor for Advanced Glycation Endproducts (RAGE): a formidable force in the pathogenesis of the cardiovascular complications of diabetes & aging. Curr Mol Med 2007;7:699–710. [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev Mol Med 2009;11:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]