Abstract
Implicit motor learning is essential to the acquisition of motor skills. Examination of implicit motor learning, however, has largely involved single-finger button presses or 2-dimensional movements of a computer mouse or joystick. The purpose of this study was to demonstrate sequence-specific implicit motor learning in individuals that practiced a 3-dimensional (3D) whole-arm reach task. Fifteen young, non-disabled individuals completed two consecutive days of practice of a 3D target task presented in a virtual environment with the dominant, right arm. Stimuli were displayed one at a time and alternated between an 8-target random sequence and an 8-target repeated sequence. Movement of the shoulder and elbow was required to successfully capture a target. Performance was indicated by time to complete a sequence (response time) and analyzed by sequence type (random, repeated). Kinematic data (total distance to complete a sequence, peak velocity, and time to peak velocity) were used to determine how movement changed over time. Results showed significant improvements in performance early in practice, regardless of sequence type. However, individuals completed the repeated sequence faster than the random sequence, indicating sequence-specific implicit motor learning. The difference in response time between the sequence types was driven by the total distance of the hand path; the distance traveled for the repeated sequence was shorter than the distance of the random sequence. Examination of implicit motor learning using 3D reach movements provides the opportunity to study learning using whole-arm movements, an important component of many real-world, functional tasks.
Keywords: Implicit motor learning, sequence learning, reaching, virtual environment
Introduction
Motor learning principles serve as the conceptual framework for certain aspects of rehabilitation (Krakauer 2006; Winstein et al. 2014). Both motor learning and motor recovery after injury are predicated on neuroplastic adaptations which occur as a result of task practice. Explicit motor learning, which requires higher-order cognitive functions such as working memory, results in a declarative knowledge of the learned skill (Orrell et al. 2006). Explicit learning of a motor skill is consequently limited by the cognitive functions that govern its underlying processes. When cognitive resources are limited or diminished, such as in individuals post-stroke (Hochstenbach et al. 1998; Tatemichi et al. 1994), their ability to learn or relearn a motor skill through explicit processes can be impaired. Implicit motor learning occurs when a motor skill is acquired or adapted without explicit awareness of skill performance, and is a fundamental aspect of motor learning and relearning (Maxwell et al. 2000). Compared to explicit motor learning, motor skills learned implicitly are often more robust (Orrell et al. 2006) and result in greater performance at retention (Maxwell et al. 2001). Importantly, implicit motor learning processes are preserved in individuals post-stroke (Boyd and Winstein 2006). Therefore, a greater understanding of implicit motor learning will further promote the application of these concepts in rehabilitation settings. However, traditional investigations of implicit motor learning, which typically involve button presses (Nissen and Bullemer 1987; Nitsche et al. 2003b; Robertson et al. 2001) or 2-dimensional (2D) movements of a computer mouse (Brodie et al. 2014a; Brodie et al. 2014b; Meehan et al. 2011) or joystick (Mang et al. 2014; Wadden et al. 2013), may not translate well to multi-joint, 3-dimensional (3D) movements, which are a large focus of rehabilitation.
Practice of a sequence-specific implicit motor learning task leads to learning of the spatial relationship between the position of the cue and the corresponding movement (Willingham et al. 2000). Completion of a sequence-specific implicit motor learning task in 3D space is not expected to alter the way the task is learned; learning is still presumed to be driven by increased knowledge of the spatial relationship of cues. However, movements of the whole-arm in 3D have increased motor demands compared to 2D tasks as they require greater coordination of muscle recruitment, muscle activation, and kinematic variables such as velocity and force (D’avella and Lacquaniti 2013). Furthermore, a higher number of degrees of freedom must be controlled when completing 3D reach movements compared to 2D movements (Perrot et al. 2012). Additionally, natural, unsupported reach movements require compensation of gravitational forces (Perrot et al. 2012). Research with tasks that require small or 2D movement may minimize or remove these important aspects of functional movement, and may not best represent the whole-arm reach behaviors that are essential to real-world, functional tasks.
Development of a motor learning task that incorporates implicit motor learning concepts with whole-arm reach movements can provide the opportunity to investigate how increased motor control demands affect known motor learning constructs. A computer-based virtual environment (VE) can be used to replicate the design of traditional sequence-specific implicit motor learning tasks previously used in research. In these tasks, stimuli are presented in patterns of random and repeated sequences, however the performer is not made aware that a repeated sequence of stimuli is present (Meehan et al. 2011; Nissen and Bullemer 1987; Nitsche et al. 2003a). Faster reaction times when completing the repeated sequence compared to a random sequence indicates sequence-specific implicit motor learning. Transferring this same task design into a VE would facilitate examination of motor skill learning with whole-arm 3D reach movements. Thus, precise control is maintained over stimuli presentation, while also including more demanding behaviors that incorporate the essential physical components of reach movements.
The purpose of the current study was to examine sequence-specific implicit motor learning for a task that involved whole-arm reach movements within a VE. It was hypothesized that an individual’s overall performance of the task, indicated by a reduction in response time, would improve with practice. Additionally, based on previous research that examined sequence specific implicit motor learning, it was expected that the repeated sequence of stimuli would be completed faster than a sequence of randomly presented stimuli, despite the addition of more demanding 3D reach movements.
Methods
Participants
Fifteen nondisabled, neurologically-intact adults (23.5 ± 3.7 years, 6 female) were recruited from the university community. In order to be eligible to participate, individuals had to: 1) be right hand dominant as determined by the Edinburgh Handedness Questionnaire (Oldfield 1971); 2) be between the ages of 18–40; 3) have no current or recent neurological symptoms as determined by a general neurological symptom checklist; and 4) have no pain in the right upper extremity. All participants provided informed consent prior to enrollment in the study. The study was conducted in accordance with the Declaration of Helsinki, and all aspects of the study were approved by the Institutional Review Board at the University of South Carolina.
Experimental Task
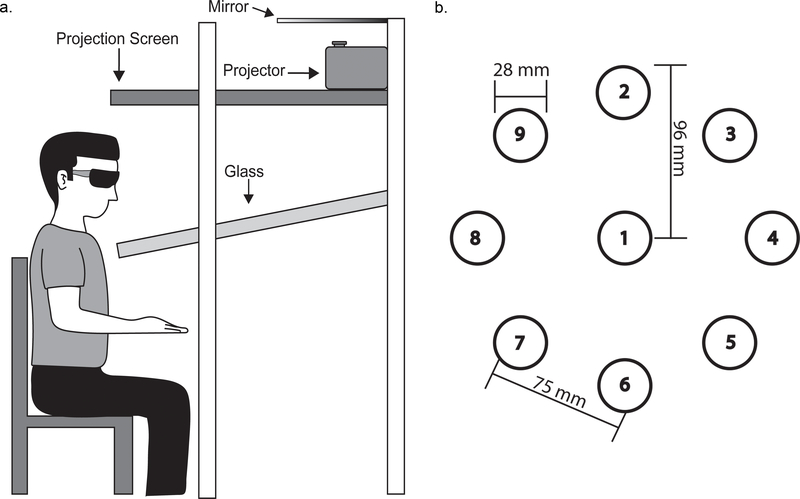
Participants sat facing a virtual display (Innovative Sport Training Inc., Chicago, IL), and the task was projected down into the workspace directly in front of them (Fig. 1a). Stereoscopic glasses were worn to provide 3D visualization of the targets. An electromagnetic marker was secured to the right index finger, and provided position data during reaching. The marker was displayed as a white sphere (25 mm diameter) on a simple black background, which provided visual feedback to the participant on finger position throughout the task; visual feedback of arm position was not provided.
Fig. 1.
Experimental setup. a Side view of a participant sitting at the virtual display. Stereoscopic glasses provided a 3-dimensional view of the virtual environment. Virtual objects were sent from the projector, reflected off the mirror, and presented in the area below the glass. b Representation of the nine possible target locations. Each target was 28 mm in diameter. Targets were presented in a circular array with a radius of 96 mm and a tangent distance between any adjacent targets of 75 mm. The repeated sequence consisted of targets 1, 8, 6, 5, 9, 4, 8, 2.
Task parameters for the current study were adapted from a previous implicit motor learning serial target task that required 2D movements (Brodie et al. 2014a; Brodie et al. 2014b; Meehan et al. 2011). Targets were displayed as red spheres (28 mm diameter) and were presented one at a time. Participants were instructed to reach towards each target as quickly and accurately as possible. Once the center of the cursor was within 5 mm of the center of the target for 500 msec, that target was considered “hit” and would disappear as the next target was displayed. All targets were presented at one of nine pre-determined target locations (Fig. 1b). Eight target locations were placed equidistant in a circular array (96 mm radius), with the remaining target location positioned directly in the center. The tangent distance between any adjacent target locations was 75 mm. The array of targets was positioned to the right of the midline of the trunk, permitting the participant to reach all targets without any trunk flexion or rotation. All targets were in the same Z-plane (up/down direction) but required unsupported 3D movement of the arm for successful capture.
Individuals reached to targets under two sequence conditions: repeated and random. Each sequence consisted of eight targets and was controlled for overall difficulty by keeping the total distance traveled constant (93.8 cm). Individual movements between any two targets were assigned an index of difficulty (ID) based on Fitts’ Law (Fitts and Peterson 1964; Meehan et al. 2011). Calculated values of each ID were 2.42, 2.78, 3.28, 3.66, and 3.78. To simplify, each calculated value was assigned an ID value 1–5, with 1 being the shortest movement (calculated ID 2.42) and 5 being the longest movement (calculated ID 3.78). Each sequence was then assigned targets consisting of the same ID levels such that every eight-target sequence was comprised of one movement at ID levels 1 and 4, and two movements at ID levels 2, 3, and 5 (8 total movements). The repeated sequence (targets: 1, 8, 6, 5, 9, 4, 8, 2) was the same across all trials. For all random sequences, target position and ID level were randomly presented but overall difficulty level for the sequence remained constant.
Experimental Procedure
Participants completed the 3D reach task over two consecutive days. On Day 1, individuals practiced 144 sequences in an alternating random-repeated sequence order, such that every other sequence of eight targets was the repeated sequence. Participants were not made aware of the presence of the repeated sequence. A 10 second rest was provided after every third sequence to prevent fatigue. All participants returned on Day 2 (24 ± 2 hours) for a retention test, and completed 72 alternating random-repeated sequences. All other task procedures were identical to Day 1.
After completing the retention test on Day 2, explicit awareness of the repeated sequence was assessed. Participants were asked if they noticed the presence of a repeated sequence. If the individual answered ‘Yes’, he or she was asked to recall the sequence. All participants then completed six explicit awareness tests. For each test, the participant viewed three eight-target sequences presented in the VE. After each explicit test, the participant was asked if the repeated sequence was present. Three of the six explicit tests contained the repeated sequence.
Data Analysis
The position of the electromagnetic marker was sampled at a rate of 120 Hz throughout the task and data were analyzed with a custom MATLAB script (Mathworks, Inc., Natick, MA). Total time to complete an eight-target sequence (response time) was the primary measure of task performance consistent with previous studies that used a similar task (Brodie et al. 2014a; Brodie et al. 2014b; Mang et al. 2016). To determine how performance changed over time, kinematic variables of both spatial and temporal components of performance were evaluated. Spatial aspects of performance were indicated by total length of the hand path (sum of total distance moved) when completing a sequence. A shorter distance moved indicates a straighter hand path between the targets. Temporal aspects of performance were assessed using peak velocity and time to peak velocity; both values were extracted for each reach movement and averaged across each eight-target sequence. A higher peak velocity indicates faster reach speed, and an earlier time to peak velocity suggests heavier reliance on feedforward control (Sainburg and Schaefer 2004; Schmidt 1975).
SPSS 22.0 (IBM Corp., Armonk, NY) was used for all statistical analyses (α = 0.05). Data from each sequence type (random and repeated) were combined and averaged into blocks of nine sequences for analysis (Day 1 = 8 blocks of 9 sequences, Day 2 = 4 blocks of 9 sequences). Changes across the eight blocks of Day 1 were assessed to examine motor skill acquisition. A within-subject 2×8 repeated-measures analysis of variance (ANOVA) with factors for sequence type (repeated, random) and block (Day 1 blocks 1–8) was run for response time and each kinematic variable. Retention was examined as the amount of forgetting between the end of Day 1 (block 8) and the start of Day 2 (block 9) with a within-subject 2×2 repeated-measures ANOVA with factors for sequence (random, repeated) and time (block 8, block 9). Post-hoc pairwise comparisons with Bonferroni corrections were used to further assess any significant effects.
Results
Acquisition
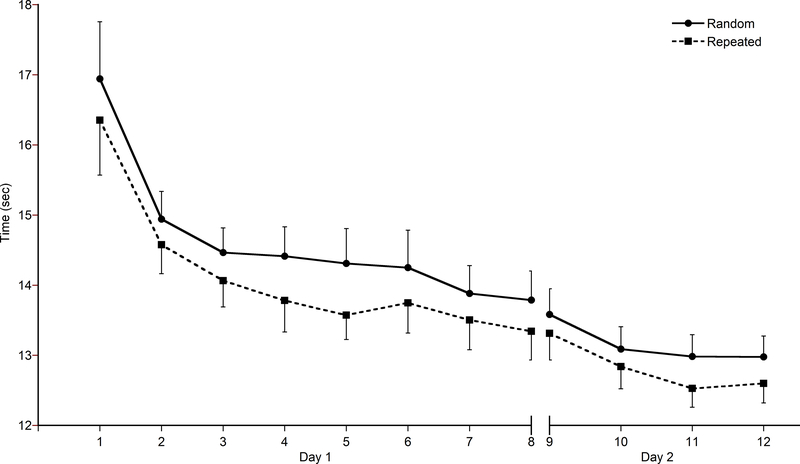
Figure 2 shows response time for the random (solid line) and repeated (dashed line) sequences over practice on Day 1. As expected, response time was significantly reduced by the end of task practice, regardless of sequence (main effect of time F (7, 8) = 12.66, p = 0.001). Pairwise comparisons indicated that by the second block, participants were already moving significantly faster than the first block (mean difference = 1.89 sec, p = 0.04). A subsequent 2×9 repeated-measures ANOVA on the first block only (first nine sequences of each sequence type) was performed to investigate how quickly a significant change in response time occurred. A main effect of time (F (1,14) = 5.32, p = 0.02) was evident and revealed that, compared to the first sequence, response time was significantly faster by the sixth sequence of practice (mean difference = 1.79 sec, p = 0.034). In addition to changes over time, a difference in response time by sequence type was found (main effect of sequence F (1, 14) = 57.76, p < 0.001), and revealed that the repeated sequence was completed significantly faster than the random sequence throughout the acquisition period. When examining the first block only, the repeated sequence was completed significantly faster than the random sequence by the eighth trial (mean difference at sequence 8 = 1.15 sec, p = 0.001). Performance up to that point (through the first seven trials) was similar for both sequences types.
Fig. 2.
Average time (sec) to complete a sequence across acquisition on Day 1 and at retention on Day 2. Each block (1–8 on Day 1 and 9–12 on Day 2) consists of nine sequences. The solid line represents the sequences of randomly presented stimuli and the dashed line represents the repeated sequence. Error bars represent standard error.
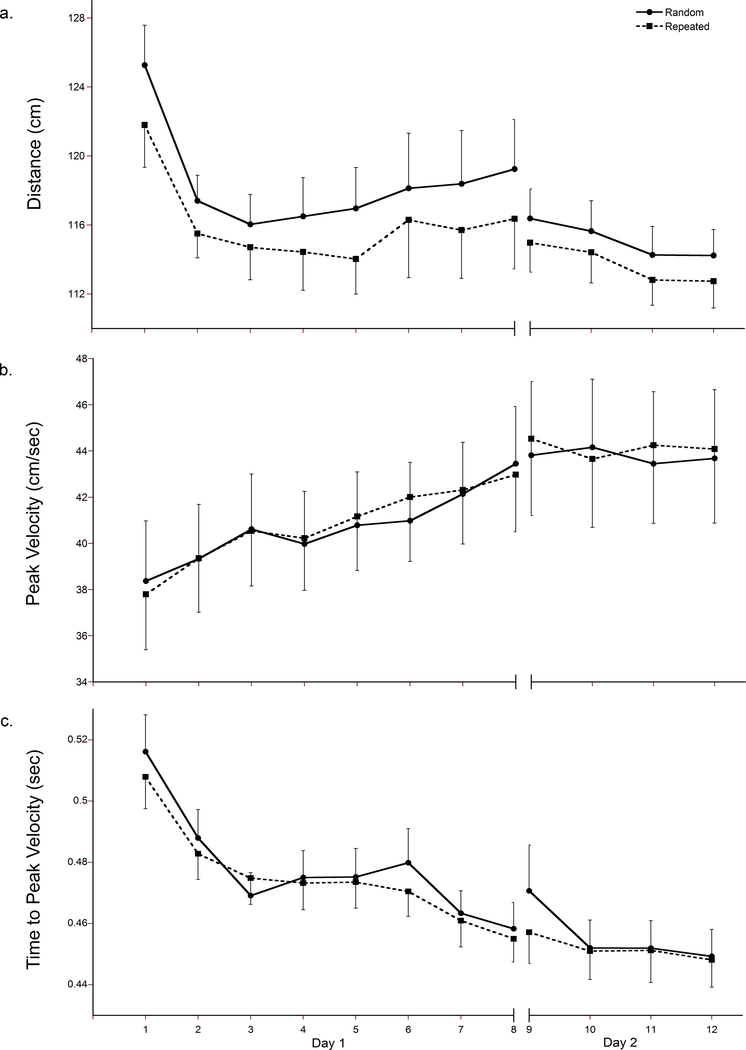
Total distance moved, as determined by the length of the hand path, was examined to represent spatial aspects of task performance. Figure 3a demonstrates that, irrespective of sequence, there was a significant decrease in total distance over practice (main effect of time F (7, 8) = 5.67, p = 0.013), suggesting a straighter, more efficient hand path was used while traveling between the targets. Pairwise comparisons indicated that total distance significantly decreased as early as block 2 (mean difference = 7.08 cm; p = 0.029). A 2×9 repeated-measures ANOVA was completed on the first block of task practice and revealed that, when compared to the first sequence, the distance of the hand path was significantly reduced by the seventh sequence (main effect of time F (1,14) = 4.863, p = 0.025; mean difference 11.07 cm, p = 0.019). Like response time, total distance of the hand path also differed by sequence type (main effect of sequence F (1, 14) = 44.72, p < 0.001). The distance travelled for the repeated sequence type was shorter than the random sequence type.
Fig. 3.
Distance of the hand path (a), peak velocity (b), and time to peak velocity (c) across acquisition on Day 1 and at retention on Day 2. Each block (1–8 on Day 1 and 9–12 on Day 2) consists of nine sequences. The solid line represents the sequences of randomly presented stimuli and the dashed line represents the repeated sequence. Error bars represent standard error.
Neither peak velocity (Fig. 3b) nor time to peak velocity (Fig. 3c), both temporal components of performance, differed by sequence type (no main effect of sequence: peak velocity, p = 0.72; time to peak velocity, p = 0.075). Peak velocity did not significantly change during practice (no main effect of time, p = 0.368), however time to peak velocity was significantly shortened over practice regardless of sequence type (main effect of time F (7, 8) = 7.44, p = 0.006), indicating participants adopted more feedforward control as practice progressed. Pairwise comparisons indicated that, when compared to the first block, a significant temporal shift occurred as early as block 2 (mean difference = 0.03 sec, p = 0.001). Closer examination of the first practice block revealed that, unlike response time and distance of the hand path, no significant change was evident during the first nine trials (no main effect of time, p = 0.184).
Retention
Performance, indicated by response time, was maintained on Day 2 (no main effect of time, p = 0.386), regardless of sequence. While overall performance was retained for both sequences, the repeated sequence was completed significantly faster than the random sequence at both time points (main effect of sequence F (1,14) = 24.999, p < .01, mean difference = 0.358 seconds).
Like acquisition, differences in response time between the repeated and random sequences on retention appeared to be driven by differences in the spatial component of task performance. Total distance moved was significantly less for the repeated sequence than for the random sequence (main effect of sequence F (1, 14) = 17.831, p < .01) at both the end of Day 1 and the start of Day 2. However, regardless of sequence, total distance was not significantly different at retention (no main effect of time, p = .301).
Temporal aspects of performance were also maintained at retention, regardless of sequence (no main effect of time for: peak velocity, p = 0.491; time to peak velocity, p = 0.382). No differences between the sequences for either temporal component were present (no main effect of sequence for: peak velocity, p = 0.714; time to peak velocity, p = 0.073).
Explicit Awareness
Five participants stated they recognized some repetition, but none were able to recall the repeated sequence from memory when provided a template of target position. Recognition of the repeated sequence was assessed as a measure of sensitivity and specificity to the explicit awareness tests. Individuals who correctly identified two out of the three positive tests, while correctly rejecting two out of three negative tests were considered to have recognition of the repeated sequence (n = 6). A Group X Time repeated measures ANOVA was performed for each sequence type to examine differences in response time across task practice between participants who recognized the sequence and participants who did not. Results indicated that individuals who recognized the sequence did not improve response time differently than individuals who did not recognize the sequence (no main effect of group: random, p = 0.655; repeated, p = 0.702). The results suggest that recognizing the repeated sequence did not influence task performance.
Discussion
This study examined sequence-specific implicit motor learning with a whole-arm 3D reach task. Improvements in performance, indicated by faster response times, were evident regardless of sequence type. However, the repeated sequence was completed faster throughout the acquisition and retention phases, suggesting implicit motor learning of the sequence occurred. Examination of temporal and spatial kinematic variables revealed that the faster response times during the repeated sequence were driven by a shorter, more direct hand path. The current 3D reach task demonstrates sequence-specific implicit motor learning with whole-arm functional movements. Results from studies using this task may inform rehabilitation methods, which often include the practice of functional tasks that require 3D, whole-arm movements.
Results of the current study completed in 3D space are comparable to experiments where a similar 2D task was used to examine implicit motor learning (Boyd and Linsdell 2009; Brodie et al. 2014a; Mang et al. 2016; Vidoni et al. 2010). Regardless of sequence type, generalized improvements of motor performance were observed during acquisition (Day1) with changes in performance evident early in practice (within Block 1). The rapid improvement in performance was supported by quick changes in both spatial (distance of the hand path) and temporal (time to peak velocity) kinematic variables. Increased trajectory accuracy, indicated by a shorter hand path, is an integral aspect of movement optimization and sequence learning (Moisello et al. 2009), and signifies greater coordination of muscle activity (Diedrichsen et al. 2010). Earlier time to peak velocity indicates increased reliance of feedforward control of movement, which facilitates faster and more accurate movements (Adams 1971; Sainburg and Schaefer 2004; Seidler-Dobrin and Stelmach 1998; Seidler et al. 2004). The changes in hand path distance and time to peak velocity occurred in parallel with response time, which suggests that improvements in response time were driven by these kinematic variables.
The rapid decrease in response time early in practice was not unexpected. This is likely supported by three factors: the level of task complexity, visuospatial adaptation to the VE, and redundant sensory feedback. While the motor demands for the current task were greater than 2D tasks, the relative simplicity of the task allowed for large gains in performance to occur after only minutes of practice (Dayan and Cohen 2011). In addition, while not strictly a motor adaptation task, the need to transfer reach movements from the real-world into the VE necessitates adaptation of the visuospatial aspects of the reach behavior (Levin et al. 2009) which may have occurred early in practice. Given that the current task provided multimodal sensory feedback and information about motor accuracy, quick adjustments could be made to meet the demands of the novel environment (Krakauer et al. 1999; Wolpert et al. 2011). Further promoting quick improvements in performance, the current 3D reach task places a higher demand on proprioceptive feedback compared to 2D laboratory tasks, as the arm is unsupported and the performers needed to control more degrees of freedom (Mongeon et al. 2013). Proprioceptive feedback is thought to be especially important in the execution of sequential movements (Vidoni and Boyd 2008), and therefore may have provided additional feedback that supported fast motor learning.
Regardless of sequence, the observed improvement in performance across Day 1 was maintained at retention on Day 2. In addition, none of the measured kinematic variables were significantly different between the end of Day 1 and the start of Day 2. The lack of forgetting between days is evidence of motor learning, rather than a transient change in motor performance (Kantak and Winstein 2012). Motor learning is evident in many 2D motor tasks (Boyd and Winstein 2004; Mang et al. 2014; Roig et al. 2012), and results from such studies have been used to support conclusions concerning complex, 3D movements. The current task, which demonstrates motor learning with whole-arm reach movements, may be more ecologically valid, and results may be more directly transferable to real-world settings.
In addition to generalized motor learning, individuals demonstrated sequence-specific implicit motor learning. Throughout practice (Day1) and at retention (Day 2), participants completed the repeated sequence faster than the random sequence. The difference in response time between the two sequence types was evident as early as the first block of task practice. Further examination of the kinematic variables identified a shorter hand path as the driver of this difference. It is unclear why sequence-specific differences were only present for hand path distance, a spatial component of performance, and not for either of the temporal components examined (peak velocity, time to peak velocity). Given that task performance was limited by spatial accuracy (cursor required to be within 5 mm of the center of the target), and not by any temporal constraints, participants likely adopted a movement strategy that prioritized spatial aspects of performance. In addition, similar to other implicit motor learning tasks, improved performance of the repeated sequence is likely improved as a spatial relationship between the targets and the reach movement is developed (Willingham et al. 2000). The development if this spatial relationship supports straighter, more efficient movement to the targets.
Previous research that utilized a continuous tracking task to examine implicit motor learning demonstrated that changes in temporal, rather than spatial, components of performance facilitated improved tracking of a repeated sequence compared to randomly presented sequences (Mang et al. 2014). Contrasting results in the current study and this previous work are likely driven by differences in the demands of the task. A continuous tracking task requires the performer to meet both spatial and temporal demands to successfully follow the target. Improvement in either the temporal or spatial domains could enhance task performance. However, in the serial target task there is no temporal restriction that limits performance. The performer’s ability to navigate 3D space is the major requirement in this task, and therefore changes in the spatial domain are necessary for performance to improve. Continued investigation of both serial discrete motor tasks and continuous motor tasks are necessary as they not only present different behavioral demands, but the underlying neuroanatomical processes associated with each type of task may differ (Doya 2000; Mang et al. 2016; Vakil et al. 2000).
Sequential motor skill learning may require both explicit and implicit processes working in parallel to learn both the sequence of elements which comprise a task, and the sequence of movements required to complete the task (Ghilardi et al. 2009). However, the current task was designed to limit the explicit processes associated with sequential motor skill learning. Participants exhibited faster performance of the repeated sequence compared to the random sequence without explicit awareness, which indicates that implicit processes alone may be enough to facilitate some sequence learning tasks (Willingham et al. 2002). Therefore, results of the current study may be especially relevant in clinical populations, such as individuals post-stroke, where implicit processes are often preserved and explicit processes may be limited (Boyd and Winstein 2006).
A variety of tasks have been used to examine implicit motor learning, such as sequential button presses (Nissen and Bullemer 1987), computer based continuous tracking tasks (Boyd and Winstein 2001), and 2D serial target tasks (Mang et al. 2016). However, it is important to understand how implicit motor learning translates to tasks requiring whole-arm, 3D movements. An increased understanding of implicit motor learning may better inform learning, or relearning, of real-world functional tasks. Examination of implicit motor learning is specifically important as it is a fundamental aspect of motor skill learning (Maxwell et al. 2000), and often leads to motor skills that are more durable and less prone to forgetting (Baars et al. 1998; Kahneman 1973). Our finding that implicit motor learning is evident in a whole-arm reach task may better translate to future work in older adults or individuals with clinical diagnoses such as stroke, who often practice functional tasks that require whole-arm movement in rehabilitation.
While the virtual environment allows 3D reach movements that are closer to real-world movements than many previously studied laboratory tasks, the current task was not performed in an actual “real-world” environment. However, reach kinematics have been found to be similar when comparing movements made in a virtual reality system and a real-world setting (Stewart et al. 2013; Viau et al. 2004). Furthermore, while the random and repeated sequences were matched for difficulty based on the distance between the targets, the resultant spatial configuration produced by reaching to the targets in a specific order was not controlled for between sequence types. It is possible that the participants implicitly learned the spatial configuration of the targets rather than the sequence of targets. In addition, the current work examined implicit motor learning as a series of discrete movements. A continuous motor task designed to examine implicit motor learning in a 3D virtual environment may yield differing results (Mang et al. 2016) and warrants future investigation. Results may also differ when examining the non-dominant arm. Previous work examining the scaling of reach movements has demonstrated different control mechanisms for the dominant vs non-dominant arm (Sainburg and Schaefer 2004). Future work examining 3D reach movements could investigate interlimb differences in implicit learning using a whole-arm reach task.
Conclusion
Results from the current study indicate that a motor task requiring whole-arm 3D reach movements demonstrate sequence-specific implicit motor learning. Compared to previously researched 2D laboratory tasks, results from the current task may be more applicable to the learning of functional tasks that often require whole-arm movement. Furthermore, the current task enables researchers to examine specific kinematic variables that may be important in understanding how reach movements are learned over time. Future research utilizing this novel task may better inform rehabilitation practice, where similar functional movements are often an important component of motor practice.
Acknowledgments
This work was supported in part by grant 15SDG24970011 from the American Heart Association and the Normal J. Arnold Doctoral Fellowship from the Arnold School of Public Health at the University of South Carolina.
Funding
This work was supported in part by grant 15SDG24970011 from the American Heart Association and the Normal J. Arnold Doctoral Fellowship from the Arnold School of Public Health at the University of South Carolina.
Footnotes
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Adams JA (1971) A closed-loop theory of motor learning Journal of motor behavior 3:111–150 [DOI] [PubMed] [Google Scholar]
- Baars B, Newman J, Taylor J (1998) Neuronal mechanisms of consciousness: A Relational Global Workspace framework In: Toward a Science of Consciousness II: The second Tucson discussions and debates. MIT Press, pp 269–278 [Google Scholar]
- Boyd LA, Linsdell MA (2009) Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills BMC neuroscience 10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ (2001) Implicit motor-sequence learning in humans following unilateral stroke: the impact of practice and explicit knowledge Neuroscience letters 298:65–69 [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ (2004) Cerebellar stroke impairs temporal but not spatial accuracy during implicit motor learning Neurorehabilitation and neural repair 18:134–143 [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ (2006) Explicit information interferes with implicit motor learning of both continuous and discrete movement tasks after stroke Journal of Neurologic Physical Therapy 30:46–57 [DOI] [PubMed] [Google Scholar]
- Brodie SM, Borich MR, Boyd LA (2014a) Impact of 5-Hz rTMS over the primary sensory cortex is related to white matter volume in individuals with chronic stroke European Journal of Neuroscience 40:3405–3412 [DOI] [PubMed] [Google Scholar]
- Brodie SM, Meehan SK, Borich M, Boyd LA (2014b) 5 Hz repetitive transcranial magnetic stimulation over the ipsilesional sensory cortex enhances motor learning after stroke Frontiers in human neuroscience 8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’avella A, Lacquaniti F (2013) Control of reaching movements by muscle synergy combinations Frontiers in computational neuroscience 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Cohen LG (2011) Neuroplasticity subserving motor skill learning Neuron 72:443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R, Ivry RB (2010) The coordination of movement: optimal feedback control and beyond Trends in cognitive sciences 14:31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K (2000) Complementary roles of basal ganglia and cerebellum in learning and motor control Current opinion in neurobiology 10:732–739 [DOI] [PubMed] [Google Scholar]
- Fitts PM, Peterson JR (1964) Information capacity of discrete motor responses Journal of experimental psychology 67:103. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Moisello C, Silvestri G, Ghez C, Krakauer JW (2009) Learning of a sequential motor skill comprises explicit and implicit components that consolidate differently Journal of neurophysiology 101:2218–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach J, Mulder T, van Limbeek J, Donders R, Schoonderwaldt H (1998) Cognitive decline following stroke: a comprehensive study of cognitive decline following stroke Journal of clinical and experimental neuropsychology 20:503–517 [DOI] [PubMed] [Google Scholar]
- Kahneman D (1973) Attention and effort. Englewood Cliffs, NJ: Prentice-Hall [Google Scholar]
- Kantak SS, Winstein CJ (2012) Learning–performance distinction and memory processes for motor skills: A focused review and perspective Behavioural brain research 228:219–231 [DOI] [PubMed] [Google Scholar]
- Krakauer JW (2006) Motor learning: its relevance to stroke recovery and neurorehabilitation Current opinion in neurology 19:84–90 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi M-F, Ghez C (1999) Independent learning of internal models for kinematic and dynamic control of reaching Nature neuroscience 2:1026–1031 [DOI] [PubMed] [Google Scholar]
- Levin MF, Knaut L, Magdalon EC, Subramanian S (2009) Virtual reality environments to enhance upper limb functional recovery in patients with hemiparesis Stud Health Technol Inform 145:108. [PubMed] [Google Scholar]
- Mang CS, Snow NJ, Campbell KL, Ross CJ, Boyd LA (2014) A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning Journal of Applied Physiology 117:1325–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CS, Snow NJ, Wadden KP, Campbell KL, Boyd LA (2016) High-Intensity Aerobic Exercise Enhances Motor Memory Retrieval Medicine and science in sports and exercise 48:2477. [DOI] [PubMed] [Google Scholar]
- Maxwell J, Masters R, Eves F (2000) From novice to no know-how: A longitudinal study of implicit motor learning Journal of Sports Sciences 18:111–120 [DOI] [PubMed] [Google Scholar]
- Maxwell J, Masters R, Kerr E, Weedon E (2001) The implicit benefit of learning without errors The Quarterly Journal of Experimental Psychology: Section A 54:1049–1068 [DOI] [PubMed] [Google Scholar]
- Meehan SK, Dao E, Linsdell MA, Boyd LA (2011) Continuous theta burst stimulation over the contralesional sensory and motor cortex enhances motor learning post-stroke Neuroscience letters 500:26–30 [DOI] [PubMed] [Google Scholar]
- Moisello C, Crupi D, Tunik E, Quartarone A, Bove M, Tononi G, Ghilardi MF (2009) The serial reaction time task revisited: a study on motor sequence learning with an arm-reaching task Experimental brain research 194:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeon D, Blanchet P, Messier J (2013) Impact of Parkinson’s disease and dopaminergic medication on adaptation to explicit and implicit visuomotor perturbations Brain and cognition 81:271–282 [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P (1987) Attentional requirements of learning: Evidence from performance measures Cognitive psychology 19:1–32 [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W (2003a) Safety criteria for transcranial direct current stimulation (tDCS) in humans Clinical Neurophysiology 114:2220–2222 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F (2003b) Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human Journal of cognitive neuroscience 15:619–626 [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory Neuropsychologia 9:97–113 [DOI] [PubMed] [Google Scholar]
- Orrell AJ, Eves FF, Masters RS (2006) Motor learning of a dynamic balancing task after stroke: implicit implications for stroke rehabilitation Physical therapy 86:369–380 [PubMed] [Google Scholar]
- Perrot A, Bherer L, Messier J (2012) Preserved Spatial Memory for Reaching to Remembered Three-Dimensional Targets in Aging Experimental aging research 38:511–536 [DOI] [PubMed] [Google Scholar]
- Robertson E, Tormos J, Maeda F, Pascual-Leone A (2001) The role of the dorsolateral prefrontal cortex during sequence learning is specific for spatial information Cerebral Cortex 11:628–635 [DOI] [PubMed] [Google Scholar]
- Roig M, Skriver K, Lundbye-Jensen J, Kiens B, Nielsen JB (2012) A single bout of exercise improves motor memory PloS one 7:e44594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Schaefer SY (2004) Interlimb differences in control of movement extent Journal of neurophysiology 92:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA (1975) A schema theory of discrete motor skill learning Psychological review 82:225 [Google Scholar]
- Seidler-Dobrin RD, Stelmach G (1998) Persistence in visual feedback control by the elderly Experimental Brain Research 119:467–474 [DOI] [PubMed] [Google Scholar]
- Seidler R, Noll D, Thiers G (2004) Feedforward and feedback processes in motor control Neuroimage 22:1775–1783 [DOI] [PubMed] [Google Scholar]
- Stewart JC, Gordon J, Winstein CJ (2013) Planning and adjustments for the control of reach extent in a virtual environment Journal of neuroengineering and rehabilitation 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemichi T, Desmond D, Stern Y, Paik M, Sano M, Bagiella E (1994) Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities Journal of Neurology, Neurosurgery & Psychiatry 57:202–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil E, Kahan S, Huberman M, Osimani A (2000) Motor and non-motor sequence learning in patients with basal ganglia lesions: the case of serial reaction time (SRT) Neuropsychologia 38:1–10 [DOI] [PubMed] [Google Scholar]
- Viau A, Feldman AG, McFadyen BJ, Levin MF (2004) Reaching in reality and virtual reality: a comparison of movement kinematics in healthy subjects and in adults with hemiparesis Journal of neuroengineering and rehabilitation 1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni E, Acerra N, Dao E, Meehan S, Boyd L (2010) Role of the primary somatosensory cortex in motor learning: An rTMS study Neurobiology of learning and memory 93:532–539 [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Boyd LA (2008) Motor sequence learning occurs despite disrupted visual and proprioceptive feedback Behavioral and Brain Functions 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden K, Brown K, Maletsky R, Boyd LA (2013) Correlations between brain activity and components of motor learning in middle-aged adults: an fMRI study Frontiers in human neuroscience 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham DB, Salidis J, Gabrieli JD (2002) Direct comparison of neural systems mediating conscious and unconscious skill learning Journal of Neurophysiology 88:1451–1460 [DOI] [PubMed] [Google Scholar]
- Willingham DB, Wells LA, Farrell JM, Stemwedel ME (2000) Implicit motor sequence learning is represented in response locations Memory & Cognition 28:366–375 [DOI] [PubMed] [Google Scholar]
- Winstein C, Lewthwaite R, Blanton SR, Wolf LB, Wishart L (2014) Infusing motor learning research into neurorehabilitation practice: a historical perspective with case exemplar from the accelerated skill acquisition program Journal of Neurologic Physical Therapy 38:190–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR (2011) Principles of sensorimotor learning Nature Reviews Neuroscience 12:739–751 [DOI] [PubMed] [Google Scholar]