Abstract
Purpose
Despite documented oncologic benefit, use of postoperative adjuvant radiotherapy (aRT) in patients with prostate cancer is still limited in the United States. We aimed to develop and internally validate a risk-stratification tool incorporating the Decipher score, along with routinely available clinicopathologic features, to identify patients who would benefit the most from aRT.
Patient and Methods
Our cohort included 512 patients with prostate cancer treated with radical prostatectomy at one of four US academic centers between 1990 and 2010. All patients had ≥ pT3a disease, positive surgical margins, and/or pathologic lymph node invasion. Multivariable Cox regression analysis tested the relationship between available predictors (including Decipher score) and clinical recurrence (CR), which were then used to develop a novel risk-stratification tool. Our study adhered to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis guidelines for development of prognostic models.
Results
Overall, 21.9% of patients received aRT. Median follow-up in censored patients was 8.3 years. The 10-year CR rate was 4.9% vs. 17.4% in patients treated with aRT versus initial observation (P < .001). Pathologic T3b/T4 stage, Gleason score 8-10, lymph node invasion, and Decipher score > 0.6 were independent predictors of CR (all P < .01). The cumulative number of risk factors was 0, 1, 2, and 3 to 4 in 46.5%, 28.9%, 17.2%, and 7.4% of patients, respectively. aRT was associated with decreased CR rate in patients with two or more risk factors (10-year CR rate 10.1% in aRT v 42.1% in initial observation; P = .012), but not in those with fewer than two risk factors (P = .18).
Conclusion
Using the new model to indicate aRT might reduce overtreatment, decrease unnecessary adverse effects, and reduce risk of CR in the subset of patients (approximately 25% of all patients with aggressive pathologic disease in our cohort) who benefit from this therapy.
INTRODUCTION
Prostate cancer (PCa) is the most common noncutaneous malignancy and the second most common cause of cancer-specific mortality in North American men. Despite widespread prostate-specific antigen (PSA) screening and the consequent stage migration,1 up to 40% of contemporary men undergoing radical prostatectomy (RP) may harbor aggressive disease at pathology,2,3 These men are at the highest risk of recurrence and would benefit from postoperative radiation therapy (RT) with or without androgen deprivation therapy (ADT).4
Three randomized clinical trials from North America and Europe showed better oncologic outcomes in men treated with adjuvant RT (aRT) when harboring adverse pathologic characteristics at surgery.5-7 However, the use of aRT in contemporary American patients remains limited at best (approximately 10% to 12%).8,9 This might be due to the concern of overtreatment, because up to 40% of patients in the control arms of these trials remained disease free.5-7 Moreover, the beneficial impact of aRT on oncologic outcomes is accompanied by an important risk of compromising functional outcomes after surgery.10 As such, there is a critical need to identify patients who are most likely to accrue curative benefit from aRT. In this context, genomic tests such as the Decipher (GenomeDx Biosciences, Vancouver, British Columbia, Canada) have recently shown promising results in prognosticating 5- and 10-year metastases rates after RP,11-18 with Den et al13 reporting on the ability of the Decipher score to potentially identify men likely to benefit from adjuvant versus salvage RT. However, comparative outcomes for men who did not develop biochemical recurrence or received aRT after surgery (which might be the case in a substantial proportion of men with aggressive disease5-7) were not reported in the Den et al13 study. Additionally, although the reported outcomes were stratified based on Decipher score (< 0.4 v ≥0.4),13 pathologic tumor characteristics were not taken into account. The number and nature of these characteristics are considered a good indicator of who would benefit from aRT.19
To address these limitations, we combined genomic data (ie, the Decipher score) and pathologic tumor characteristics to identify patients who would derive the most oncologic benefit from aRT versus initial observation (with or without subsequent salvage RT). We adhered to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines for development and internal validation of a prognostic model during the conduct of the study.20
PATIENTS AND METHODS
Patient Cohort
We focused on a total of 512 patients with PCa treated with RP between 1990 and 2010 at one of four academic institutions: Mayo Clinic (n = 141), Durham Veterans Affairs (n = 104), Johns Hopkins Medical Institution (n = 141), and Thomas Jefferson University (n = 126).12,15,17,21 All patients had at least one adverse pathologic feature at the time of surgery (ie, ≥ pT3a stage, positive surgical margins [PSMs], and/or lymph node invasion [LNI]) and reached undetectable PSA levels after surgery. Postoperatively, these individuals received either aRT or initial observation (with salvage RT [sRT] offered in case of biochemical recurrence).
Details of inclusion and exclusion criteria are reported in Figure 1. Patient tumors (ie, RP specimens) were collected under institutional review board-approved studies of the Decipher test and data were stored in the GenomeDx PCa Genomic Resource Information Database.
Fig 1.
Flow diagram illustrating the study cohort selection. PSA, prostate-specific antigen; PSM, positive surgical margin.
Specimen Collection and Handling
Specimen selection and processing has been described in detail.14 Briefly, after microarray quality control using the Affymetrix Power Tools packages (Thermo Fisher Scientific, Waltham, MA),22 probe set summarization and normalization were performed using the single-channel array-normalization algorithm.23 None of these samples were used in the development of the Decipher genomic classifier. The study adheres to the Reporting Recommendations for Tumor Marker Prognostic Studies criteria for evaluation of prognostic biomarkers.24
Calculation of Genomic Risk of Metastasis
Genomic risk of metastasis was calculated with the Decipher test, which has been validated as an independent predictor of clinical metastatic progression after RP in different studies across multiple cohorts.11-18 The Decipher score was calculated from RP specimens. The expression values for the 22 prespecified biomarkers that constitute Decipher were extracted from the normalized data matrix and entered into the locked random forest algorithm with tuning and weighting parameters defined as reported previously.13,14 The Decipher read-out is a continuous risk score between 0 and 1, with higher scores indicating a greater probability of metastasis at 5 and 10 years after RP.14,16,17 The Decipher score was also categorized into low, intermediate, and high risk (< 0.45, 0.45 to 0.6, and > 0.6, respectively) based on previously reported methodology.17,21
Clinicopathologic Variables
Key preoperative variables of interest were age (in years) at RP and preoperative PSA level; postoperatively, the pathologic variables included pathologic Gleason score, stage, PSM status, and LNI status. aRT was defined as receiving radiotherapy within 12 months from RP, with postoperative PSA levels of < 0.2 ng/mL. Similar conditions were used to identify adjuvant ADT. Patients who did not receive any adjuvant treatment after surgery were categorized as the initial observation group. Salvage treatments were identified as receiving RT and/or ADT after documented biochemical recurrence (PSA levels ≥ 0.2 ng/mL confirmed on two separate occasions), or RT and/or ADT administered ≥ 12 months after RP.
Outcome Definition
The primary end point of the study was time to clinical recurrence (CR; as documented from prostatic fossa biopsy specimen, and/or radiographically on computed tomography scan, bone scan, and/or other imaging modalities). Follow-up time was calculated from time of surgery to time of CR, or time of last available contact. Patients who died before CR were considered to be censored. Of note, GenomeDx Biosciences staff and personnel were blinded to the CR status during the laboratory processing and calculation of Decipher for all patients in the study.
Statistical Analyses
Frequencies and proportions were reported for categorical variables, whereas median and interquartile ranges (IQRs) were reported for continuous variables. Differences in categorical and continuous variables were examined using the Fisher exact test and analysis of variance F test, respectively. The median follow-up time was reported only for censored patients25 and was determined by considering time of surgery and time to last available contact. The Gray test was used to compare cumulative incidence curves.26
Our statistical analyses consisted of two main steps. First, univariable and multivariable Cox proportional hazards models were used to test the relationship between pathologic features/genomic feature (namely, the Decipher score) and CR after surgery. The predictors for the models were chosen a priori to performing the analyses. To account for the small number of events, the results of the multivariable models were confirmed using the Firth penalized likelihood method,27 which showed no substantive change in hazard ratios or P values, and ensured robustness of the analyses. The coefficients of independent predictors from the multivariable model were used to develop a novel nomogram predicting individual probabilities of CR-free survival 5 and 10 years after RP. The discriminant accuracy of the novel nomogram was quantified using time-dependent concordance index (constructed using the nearest neighbor estimator with a span parameter of 0.001 as described by Heagerty et al28) and adjusted for optimism using bootstrapping with 1,000 resamples. Calibration plots assessed the overall extent of over- or underestimation of CR rates compared with nomogram-predicted probability of CR, and calibration was tested using the D’Agostino-Nam29 version of the Hosmer-Lemeshow test for survival data obtained from bootstrapped resamples.
Second, all independent predictors of CR from the aforementioned multivariable model were assigned a score of 1 and summed to generate a novel risk score. Next, cumulative incidence curves, stratified according to the novel risk score, were used to depict CR-rate estimates in men treated with aRT versus initial observation after surgery. Estimation of the number needed to treat (NNT) to prevent one episode of CR was calculated using the method described by Altman and Anderson.30
The accompanying checklist highlights the adherence to the TRIPOD statement guidelines (Appendix Table A1, online only). All statistical tests were two-sided and analyses were performed in R version 3.1 (R Foundation, Vienna, Austria).
RESULTS
Overall, median age (IQR) was 61 years (57 to 65 years). Pathologic Gleason score was ≥ 4+3 in 48.4% of patients, and the majority (72.3%) harbored extraprostatic disease (Table 1). Only 112 (21.9%) of patients received aRT. These men harbored PSMs more frequently (85.7% v 62.0%) and were less likely to have LNI (0.9% v 10.8%) compared with men managed with initial observation (both P < .001). In the initial observation group, 168 patients (42%) in the initial observation group received sRT at a median (IQR) preradiotherapy PSA level of 0.55 ng/mL (0.33 to 1.40 ng/mL). The median (IQR) time from RP to sRT was 11.1 months (4.9 to 30.8 months).
Table 1.
Descriptive Statistics of Patients With Prostate Cancer Treated With Radical Prostatectomy at One of Four Academic Centers in the United States Between 1990 and 2010 (N = 512)*
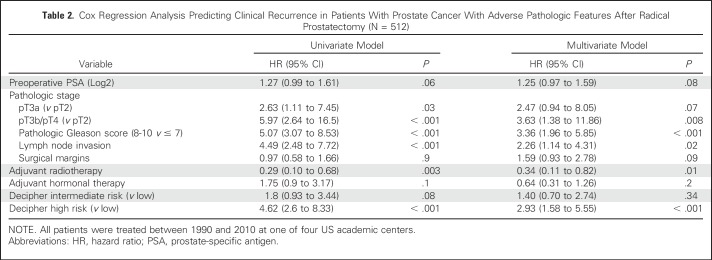
Overall, 62 patients (12.1%) had documented CR through the study period. Median (IQR) follow-up among those who did not experience CR during the study period was 8.3 years (5.4 to 11.3 years). The 10-year cumulative incidence of CR was 4.9% (95% CI, 0.0% to 9.6%) in aRT patients versus 17.4% (95% CI, 12.7% to 21.9%) in initial observation patients (P < .001). In multivariable analysis, pathologic pT3b/T4 disease (hazard ratio [HR], 3.63), pathologic Gleason score 8 to 10 (HR, 3.36), presence of LNI (HR, 2.26), and high (v low) Decipher score (HR, 2.93) were all independent predictors of higher risk of CR (all P ≤ .02; Table 2). Of note, patients treated with aRT had a 66% lower CR risk than their counterparts treated with initial observation (HR, 0.34; 95% CI, 0.11 to 0.82; P = .01). The coefficients of these independent predictors were used to develop a novel nomogram stratified by aRT status (Fig 2). Noteworthy, the Decipher score was used as a continuous variable in the nomogram (HR, 1.25 [95% CI, 1.09 to 1.44]; P = .002; Appendix Table A2, online only). The discrimination accuracy of the novel nomogram for predicting 5-year CR risk was 85% (v 79% for the clinicopathologic model only, although the 95% CI of the C indices for the two models overlapped) and its calibration characteristics were favorable (Hosmer-Lemeshow P = .1; Appendix Fig A1, online only).
Table 2.
Cox Regression Analysis Predicting Clinical Recurrence in Patients With Prostate Cancer With Adverse Pathologic Features After Radical Prostatectomy (N = 512)
Fig 2.
Nomogram predicting the probability of clinical recurrence-free survival (CRFS) in patients with prostate cancer with adverse pathologic features at radical prostatectomy on the basis of pathologic stage, pathologic Gleason score, lymph node invasion, and Decipher score. Nomogram outcomes were stratified based on adjuvant radiotherapy (aRT) status. For example, locate the patient’s Decipher score on the Decipher axis. Draw a line straight up to the point axis to determine how many points toward the probability of CRFS the patient receives for his Decipher score. Repeat the process for each additional variable. Sum the points for each predictor. Locate the final sum on the total-point axis. Draw a line straight down to find the patient’s 5- and 10-year probability of CRFS. CR, clinical recurrence.
In addition to the nomogram, a simple risk score was calculated for all patients in our cohort on the basis of multivariable analysis results. Specifically, the novel risk score represented the cumulative number of independent predictors of CR found in each patient (ie, pT3b/T4, pathologic Gleason score 8 to 10, LNI, and high Decipher score). The risk score was 0, 1, 2, and 3 to 4 in 238 (46.5%), 148 (28.9%), 88 (17.2%), and 38 (7.4%) of patients, respectively. Figure 3 shows the cumulative incidence of CR in our cohort after stratifying patients according to the novel risk score. At 10 years, the CR rate was 5.6%, 12.4%, 27.3%, and 57.4% in patients with risk scores of 0, 1, 2, and 3 to 4, respectively (P < .001). In patients treated with aRT versus initial observation, the 10-year CR rate was 3.5% versus 9.3% in patients with a risk score < 2 (P = .18; Fig 4A), and 10.1% versus 42.1% in patients with a risk score ≥ 2 (P = .012; Fig 4B). At 10 years, NNT to prevent one CR was 3.1 (95% CI, 2.9 to 3.3) in patients with risk score ≥ 2.
Fig 3.
Cumulative incidence plot depicting clinical recurrence (CR) curves of 512 patients with prostate cancer with adverse pathologic features at radical prostatectomy (RP). Patients were stratified based on the risk score described in this study. All patients were treated at one of four academic centers in the United States between 1990 and 2010. P value was calculated using Gray test.
Fig 4.
Cumulative incidence plot depicting clinical recurrence (CR) curves in patients with prostate cancer with adverse pathologic features at radical prostatectomy (RP). Patients were stratified based on adjuvant radiotherapy (aRT) status and the risk score described in this study: (A) risk score < 2 and (B) risk score ≥ 2. All patients were treated at one of four academic centers in the United States between 1990 and 2010. P value was calculated using Gray test.
Last, given that only one patient with LNI received aRT in our cohort, we conducted post hoc sensitivity analyses after excluding all men with LNI (Appendix). Independent risk factors for CR were pathologic stage pT3b/T4 (HR, 2.65,), pathologic Gleason score 8 to 10 (HR, 3.85), and high Decipher score (HR, 2.90; all P ≤ .04). For patients treated with aRT versus initial observation, the 10-year CR rate was 3.5% versus 9.6% in patients with a risk score < 2 (P = .21), and 10.6% versus 37.2% in patients with a risk score ≥ 2 (P = .04).
DISCUSSION
Despite level I evidence data demonstrating the favorable impact of aRT on cancer control outcomes in patients with PCa with adverse pathologic characteristics at surgery,5-7 the use of this treatment modality is still very limited across the United States (approximately 10% to 12%).8,9 This might be attributed to many reasons, such as concerns about overtreatment, radiation toxicity,5-7 adverse impact of radiation on post-RP functional outcomes,10 and patient preferences.31 As a result, many physicians prefer an approach of initial observation with or without sRT at biochemical recurrence. However, the efficacy of the latter treatment modality has not been demonstrated. One approach to overcome this clinical dilemma might be a better definition of patients who actually need aRT. In this study, we combined genomic data (ie, the Decipher risk score) and histopathology data to identify the optimal candidate for aRT after surgery among patients with PCa with adverse pathologic characteristics.8,9
Our analyses showed several important findings. First, we demonstrate that incorporating Decipher added incremental prognostic value in identifying patients with adverse pathologic features (ie, extraprostatic disease, pathologic Gleason score 8 to 10, or presence of LNI) at a higher risk of CR after RP, with a predictive accuracy of 85%. Based on the combined clinicopathologic and genomic parameters, we created a novel nomogram to quantify the likelihood of CR-free survival 5 and 10 years after RP (stratified by receipt of aRT), which showed optimal discrimination and calibration characteristics. Whereas prior studies have combined Decipher with pre-existing validated models (such as the postsurgical Cancer of the Prostate Risk Assessment [CAPRA-S] or the Stephenson nomogram),11,13 our study attempted to incorporate genomic features with routinely available clinicopathologic parameters to create a de novo prediction model. This was then used to derive an easily used risk score. Importantly, every one-point increment in the risk score was associated with a doubling of CR risk at 10 years post-RP. Of note, this study is based on genomic parameters that adhere to the recently published TRIPOD guidelines for developing and reporting prognostic models.20 Adherence to the guidelines increases the methodological strengths of our study and allows readers to transparently assess the risk of bias and potential usefulness in a given clinical setting. Our work represents a type 1b study per the TRIPOD statement (corresponding to development and internal validation) and lays the foundation for future external validation studies.
Second, our results provide vital information to guide decision making regarding aRT in patients with aggressive pathology. aRT use did not substantially alter the nomogram-predicted CR-free survival in patients with few pathologic risk factors and low Decipher score (ie, risk score < 2), allowing a substantial proportion of patients to safely omit unnecessary aRT. Conversely, those with more aggressive disease and higher Decipher scores (ie, risk score ≥ 2) demonstrated greater benefit with upfront aRT versus initial observation. Indeed, for patients in the latter group at 5 and 10 years post-RP, the absolute reduction in risk of CR was 17.3% and 32.0%, respectively (corresponding to a relative risk reduction of 63% and 76%, respectively). At 10 years, the NNT to prevent one CR in patients with risk score ≥ 2 was 3.1; the corresponding NNT on the basis of current recommendations (derived from the European Organization for Research and Treatment of Cancer trial 22911 and the Southwest Oncology Group 8794 trials) would be approximately 10. Although the risk-scoring system has practical use in a busy clinical setting for patient counseling regarding the need for aRT, our nomogram can provide individualized estimation of CR risk (both at 5 and 10 years post-RP) in men who opt for aRT and those preferring an initial observation approach. Given the fairly protracted natural history of PCa, 10-year CR estimates may arguably provide greater clinical use to Decipher-based models. This, in turn, may enhance shared decision making between urologists and their patients. Pending the results of the RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery),32 GETUG-17/070233 and RAVES (Radiotherapy–Adjuvant Versus Early Salvage) 34 trials comparing aRT with initial observation with or without salvage RT, our results suggest it might not be prudent to withhold aRT in favor of an initial observation approach patients with risk score ≥ 2.
Last, our results corroborate the findings reported by Den et al,13 who suggested that aRT may offer significantly greater CR-free survival than salvage RT in men with high Decipher score (≥ 0.4), but not in men with lower Decipher scores. Nonetheless, there are key differences as well. Most importantly, the control group (ie, sRT) in the Den et al13 study excluded men treated with initial observation: Given that 35% to 40% of patients treated with initial observation may not experience a biochemical recurrence5,7 within 10 years of RP (thereby obviating the need for salvage therapy), inclusion of such patients is necessary in deriving conclusions regarding the oncologic efficacy for aRT.35 Of note, the median time to sRT was 11.1 months in our cohort (compared with 5 months in the Den et al13 study), more closely reflecting the natural history of an initial observation versus an upfront aRT approach in the post-RP setting. Next, the C index of the Decipher-only model in our study was somewhat lower compared with that reported by Den et al.13 As alluded to earlier, this could be due to different patient populations in the initial observation arm (with or without sRT), and although direct comparison of aRT with only salvage RT may improve the discrimination power from a statistical standpoint, it may not be truly reflective of a clinical setting. Finally, risk stratification of patients into those likely to benefit from upfront aRT versus those who may not was based solely on low versus intermediate/high Decipher score in the Den et al study.13 We have previously shown the prognostic role of histopathologic features (ie, pathologic stage, Gleason score, and presence of LNI) in predicting cancer-specific mortality in patients undergoing aRT.19 As such, our current study shows that incorporating both Decipher and clinicopathologic feature results in a better accuracy when predicting CR after surgery.
Our study must be interpreted in light of its limitations. First, this retrospective study spanned patients diagnosed and treated over two decades (1990 to 2010); the widespread dissemination of PSA screening leading to downstaging of PCa, evolution of aRT or adjuvant ADT protocols, and modifications in the Gleason grading system over the study period may be meaningful confounders of results. These data originated at four high-volume academic centers and, as such, would require validation at the community level with both low- and high-volume facilities. The median RT dose was 66.6 Gy, which, although somewhat lower by contemporary standards, is comparable to what has been reported in the three large-scale randomized controlled trials on aRT (60 to 64 Gy).5-7 Second, although we accounted for the use of adjuvant ADT, its use was largely driven by surgeon and/or institutional preferences. There continues to be a lack of consensus regarding the role of ADT in the adjuvant setting,36-38 and the role of aRT with or without ADT, especially in patients with pN1 disease, is currently a matter of investigation.39 Third, a central pathologic review was not undertaken; however, dedicated genitourinary pathologists at each center examined the histopathology specimens, which may reduce but not entirely eliminate the possibility of interobserver and/or misclassification bias. Fourth, we did not see a statistically significant association between PSM and CR rate: Although it is conceivable that relatively fewer patients precluded this finding, evidence regarding association of PSM with metastases-free survival is rather contradictory, even in level I studies.5-7
Appendix
Given the retrospective nature of the study, a formal sample-size calculation was not performed. A complete-case analytic approach was used; 11 patients were excluded from the study because of missing clinical or outcomes data.
Five-Year Cumulative Incidence of Patients With Clinical Recurrence Undergoing Initial Observation Versus Upfront Adjuvant Radiotherapy
Primary analyses.
The 5-year cumulative incidence of clinical recurrence (CR) was 2.0% (95% CI, 0.0% to 4.7%) in patients who received adjuvant radiotherapy (aRT) versus 9.1% (95% CI, 6.2% to 12.0%) in initial observation patients. At 5 years, the CR rates were 0.9%, 4.3%, 14.9%, and 45.4% in patients with risk scores of 0, 1, 2, and 3-4, respectively. The 5-year CR rate was 0.0% versus 2.8% in patients with a risk score < 2 (P = .18; Fig 4A), whereas in patients with a risk score ≥ 2, the 5-year CR rate was 10.1% v 27.4% (P = .012; Fig 4B).
Post hoc analyses (excluding all men with lymph node invasion).
At 5 years, the CR rates were 0.9%, 4.5%, 15.6%, and 42.9% in patients with risk scores of 0, 1, 2, and 3, respectively. The 5-year CR rate was 0.0% v 2.9% in patients with a risk score < 2 (P = .2), and 10.6% v 21.9% in patients with a risk score ≥ 2 (P = .04).
Results of Sensitivity Analyses After Excluding All Men With Lymph Node Invasion on Pathology
Independent risk factors for CR were pathologic stage pT3b/T4 (hazard ratio [HR], 2.65), pathologic Gleason score 8 to 10 (HR, 3.85) and high Decipher score (HR, 2.90; all P < .04). Patients treated with aRT had a 68% lower CR risk than their counterparts treated with initial observation (P = .009). The predictive accuracy of the model was 82% (v 77% and 74% for the clinicopathologic and Decipher-only models, respectively) and showed favorable calibration characteristics (data not shown). The proportion of patients with risk scores of 0, 1, 2, and 3 was 238 (50.9%), 141 (30.1%), 77 (16.5%), and 12 (2.6%). At 10 years, the CR rates were 5.6%, 13.1%, 29.6%, and 42.9% in patients with risk scores of 0, 1, 2, and 3, respectively (P < .001). In patients treated with aRT versus initial observation, the 10-year CR rate was 3.5% v 9.6% in patients with a risk score < 2 (P = .2), and 10.6 v 37.2% in patients with a risk score ≥ 2 (P = .04).
Fig A1.
(A) Discrimination curve for the nomogram predicting clinical recurrence (CR) in patients with adverse pathologic features after radical prostatectomy, compared with the predictive models based on clinicopathologic parameters and Decipher score only. (B) Calibration characteristics of the nomogram. All data originated from 512 patients with prostate cancer who were treated with radical prostatectomy at one of four academic centers in the United States, between 1990 and 2010. Blue bars represent the frequency of the 5-year predicted probability of clinical recurrence. Both graphs were generated based on the 5-year end point.
Table A1.
Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis Checklist20
Table A2.
Multivariate Cox Regression Model of the Association Between Routine Clinicopathologic Parameters, Decipher Genomic Classifier Risk Score, Receipt of Adjuvant Radiotherapy and Clinical Recurrence in Patients With Adverse Pathologic Features After Radical Prostatectomy
Footnotes
A.E.R. is supported by a Prostate Cancer Foundation Young Investigator Award and a Department of Defense Physician Research Training Award.
See accompanying Editorial on page 1973
AUTHOR CONTRIBUTIONS
Conception and design: Kasra Yousefi, R. Jeffrey Karnes, Elai Davicioni, Firas Abdollah
Administrative support: R. Jeffrey Karnes, Mani Menon
Provision of study materials or patients: María Santiago-Jiménez, Stephen J. Freedland, Mani Menon, Elai Davicioni
Collection and assembly of data: María Santiago-Jiménez, Kasra Yousefi, R. Jeffrey Karnes, Stephen J. Freedland
Data analysis and interpretation: Deepansh Dalela, Kasra Yousefi, R. Jeffrey Karnes, Ashley E. Ross, Robert B. Den, Stephen J. Freedland, Edward M. Schaeffer, Adam P. Dicker, Mani Menon, Alberto Briganti, Firas Abdollah
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Genomic Classifier Augments the Role of Pathological Features in Identifying Optimal Candidates for Adjuvant Radiation Therapy in Patients With Prostate Cancer: Development and Internal Validation of a Multivariable Prognostic Model
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Deepansh Dalela
No relationship to disclose
María Santiago-Jiménez
Employment: GenomeDx Biosciences
Kasra Yousefi
Employment: GenomeDx Biosciences
Travel, Accommodations, Expenses: GenomeDx Biosciences
R. Jeffrey Karnes
Research Funding: GenomeDx Biosciences
Travel, Accommodations, Expenses: GenomeDx Biosciences
Ashley E. Ross
Consulting or Advisory Role: GenomeDx Biosciences
Stock or Other Ownership: GenomeDx Biosciences
Robert B. Den
Consulting or Advisory Role: GenomeDx Biosciences
Speakers' Bureau: Bayer
Research Funding: Medivation/Astellas, GenomeDx Biosciences
Travel, Accommodations, Expenses: GenomeDx Biosciences
Stephen J. Freedland
Stock or Other Ownership: Armune Bioscience, Parallel6
Consulting or Advisory Role: Astellas Pharma, Medivation, Janssen Biotech, Dendreon, MDxHealth, Sanofi, Bayer, GenomeDx Biosciences, Armune Bioscience, Parallel6
Research Funding: Dendreon (Inst), Bayer (Inst), Amgen (Inst), Janssen Biotech (Inst), Myriad Genetics (Inst), GenomeDx Biosciences (Inst), Mitomics (Inst), Metabolon (Inst), POM Wonderful (Inst), Progenika (Inst)
Travel, Accommodations, Expenses: Sanofi, Myriad Genetics, GenomeDx Biosciences
Edward M. Schaeffer
Honoraria: GenomeDx Biosciences
Adam P. Dicker
Consulting or Advisory Role: Merck, Glenview Consulting, Johnson & Johnson
Travel, Accommodations, Expenses: Merck, Agios
Other Relationship: NRG Oncology, Department of Defense-Prostate Cancer Research Program
Mani Menon
No relationship to disclose
Alberto Briganti
Honoraria: Astellas Pharma, Ferring, Janssen-Cilag
Consulting or Advisory Role: Astellas Pharma, OPKO Health, MDxHealth, Bayer
Research Funding: Sandoz
Elai Davicioni
Employment: GenomeDx Biosciences
Leadership: GenomeDx Biosciences
Stock or Other Ownership: GenomeDx Biosciences
Patents, Royalties, Other Intellectual Property: Cancer diagnostics using biomarkers 20140066323
Travel, Accommodations, Expenses: GenomeDx Biosciences
Firas Abdollah
Consulting or Advisory Role: GenomeDx Biosciences
Travel, Accommodations, Expenses: GenomeDx Biosciences
REFERENCES
- 1.Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–1283. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalbasi A, Swisher-McClure S, Mitra N, et al. Low rates of adjuvant radiation in patients with nonmetastatic prostate cancer with high-risk pathologic features. Cancer. 2014;120:3089–3096. doi: 10.1002/cncr.28856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silberstein JL, Vickers AJ, Power NE, et al. Reverse stage shift at a tertiary care center: Escalating risk in men undergoing radical prostatectomy. Cancer. 2011;117:4855–4860. doi: 10.1002/cncr.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 5.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–2027. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 6.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J Urol. 2009;181:956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66:243–250. doi: 10.1016/j.eururo.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Mahal BA, Hoffman KE, Efstathiou JA, et al. National trends in the recommendation of radiotherapy after prostatectomy for prostate cancer before and after the reporting of a survival benefit in March 2009. Clin Genitourin Cancer. 2015;13:e167–e172. doi: 10.1016/j.clgc.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Sineshaw HM, Gray PJ, Efstathiou JA, et al. Declining use of radiotherapy for adverse features after radical prostatectomy: Results from the National Cancer Data Base. Eur Urol. 2015;68:768–774. doi: 10.1016/j.eururo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Suardi N, Gallina A, Lista G, et al. Impact of adjuvant radiation therapy on urinary continence recovery after radical prostatectomy. Eur Urol. 2014;65:546–551. doi: 10.1016/j.eururo.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Cooperberg MR, Davicioni E, Crisan A, et al. Combined value of validated clinical and genomic risk stratification tools for predicting prostate cancer mortality in a high-risk prostatectomy cohort. Eur Urol. 2015;67:326–333. doi: 10.1016/j.eururo.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Den RB, Feng FY, Showalter TN, et al. Genomic prostate cancer classifier predicts biochemical failure and metastases in patients after postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:1038–1046. doi: 10.1016/j.ijrobp.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Den RB, Yousefi K, Trabulsi EJ, et al. Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. J Clin Oncol. 2015;33:944–951. doi: 10.1200/JCO.2014.59.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erho N, Crisan A, Vergara IA, et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS One. 2013;8:e66855. doi: 10.1371/journal.pone.0066855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karnes RJ, Bergstralh EJ, Davicioni E, et al. Validation of a genomic classifier that predicts metastasis following radical prostatectomy in an at risk patient population. J Urol. 2013;190:2047–2053. doi: 10.1016/j.juro.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein EA, Yousefi K, Haddad Z, et al. A genomic classifier improves prediction of metastatic disease within 5 years after surgery in node-negative high-risk prostate cancer patients managed by radical prostatectomy without adjuvant therapy. Eur Urol. 2015;67:778–786. doi: 10.1016/j.eururo.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Ross AE, Johnson MH, Yousefi K, et al. Tissue-based genomics augments post-prostatectomy risk stratification in a natural history cohort of intermediate- and high-risk men. Eur Urol. 2016;69:157–165. doi: 10.1016/j.eururo.2015.05.042. [DOI] [PubMed] [Google Scholar]
- 18.Yamoah K, Johnson MH, Choeurng V, et al. Novel biomarker signature that may predict aggressive disease in African American men with prostate cancer. J Clin Oncol. 2015;33:2789–2796. doi: 10.1200/JCO.2014.59.8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdollah F, Suardi N, Cozzarini C, et al. Selecting the optimal candidate for adjuvant radiotherapy after radical prostatectomy for prostate cancer: A long-term survival analysis. Eur Urol. 2013;63:998–1008. doi: 10.1016/j.eururo.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Collins GS, Reitsma JB, Altman DG, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med. 2015;162:55–63. doi: 10.7326/M14-0697. [DOI] [PubMed] [Google Scholar]
- 21. doi: 10.1016/j.eururo.2016.01.008. Freedland SJ, Choeurng V, Howard L, et al: Utilization of a genomic classifier for prediction of metastasis following salvage radiation therapy after radical prostatectomy. Eur Urol 70:588-596, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Lockstone HE. Exon array data analysis using Affymetrix power tools and R statistical software. Brief Bioinform. 2011;12:634–644. doi: 10.1093/bib/bbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccolo SR, Sun Y, Campbell JD, et al. A single-sample microarray normalization method to facilitate personalized-medicine workflows. Genomics. 2012;100:337–344. doi: 10.1016/j.ygeno.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 25.Vickers AJ, Sjoberg DD. Guidelines for reporting of statistics in European Urology. Eur Urol. 2015;67:181–187. doi: 10.1016/j.eururo.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 27.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 28.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 29. D'Agostino RB, Nam B-H: Evaluation of the performance of survival analyses models: Discrimination and calibration measures, Balakrishnan N, Rao C, (eds): Handbook of Statistics. Amsterdam, the Netherlands, Elsevier BV, 2004, pp 1-25. [Google Scholar]
- 30.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319:1492–1495. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayman JA, Fairclough DL, Harris JR, et al. Patient preferences concerning the trade-off between the risks and benefits of routine radiation therapy after conservative surgery for early-stage breast cancer. J Clin Oncol. 1997;15:1252–1260. doi: 10.1200/JCO.1997.15.3.1252. [DOI] [PubMed] [Google Scholar]
- 32.Parker C, Clarke N, Logue J, et al. RADICALS (Radiotherapy and Androgen Deprivation in Combination after Local Surgery) Clin Oncol (R Coll Radiol) 2007;19:167–171. doi: 10.1016/j.clon.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Richaud P, Sargos P, Henriques de Figueiredo B, et al. Postoperative radiotherapy of prostate cancer [in French] Cancer Radiother. 2010;14:500–503. doi: 10.1016/j.canrad.2010.07.224. [DOI] [PubMed] [Google Scholar]
- 34. doi: 10.1111/bju.12623. Pearse M, Fraser-Browne C, Davis ID, et al: A phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: Background and rationale of the Radiotherapy -- Adjuvant Versus Early Salvage (RAVES) trial. BJU Int 113:7-12, 2014, (suppl 2) [DOI] [PubMed] [Google Scholar]
- 35.Isbarn H, Giannarini G. Re: Genomic classifier identifies men with adverse pathology after radical prostatectomy who benefit from adjuvant radiation therapy. Eur Urol. 2015;68:337–338. doi: 10.1016/j.eururo.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 37.Wong YN, Freedland S, Egleston B, et al. Role of androgen deprivation therapy for node-positive prostate cancer. J Clin Oncol. 2009;27:100–105. doi: 10.1200/JCO.2007.14.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 39.Abdollah F, Karnes RJ, Suardi N, et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. 2014;32:3939–3947. doi: 10.1200/JCO.2013.54.7893. [DOI] [PubMed] [Google Scholar]