Abstract
The objective of this study was to quantitatively analyze the effect of diurnal loading on the transport of various charged antibiotics into negatively charged human IVDs. Transport of charged antibiotics into a human lumbar disc was analyzed using a 3D finite element model. The valence (z) of the electrical charge of antibiotics varied from z=+2 (positively charged) to z=−2 (negatively charged). An uncharged antibiotic (z=0) was used as a control. Cases with transient antibiotic concentration at disc boundaries [to mimic intravenous (IV) infusion] were simulated. Our results showed that diurnal compression increased the concentrations in the NP region, but degreased the concentrations in the AF region for all charged or non-charged drugs. The overall concentration (averaged over disc) increased with diurnal compression. The diurnal compression had more effects on negatively charged antibiotics than positively charged ones. For example, at day 5 with diurnal compression, the diurnal compression increased the concentration of negatively drug (z = −1) in NP by 18.3%, but only by 6.6% for positively charged one (z = +1). In AF, diurnal compression decreased the concentration by 13.2% for negatively charged drug (z = −1) versus 1.2% for positively charged one (z=+1). Note these percentages are the averaged values over day 5. This study provides quantitative information on understanding the mechanisms of charged drug transport in human IVDs.
Keywords: intervertebral disc, charged antibiotics, diurnal compression, drug delivery, finite element method
1. Introduction
Intervertebral disc (IVD) infection is caused by the bacteria invasion into the disc space. The incidence of disc infection is growing due to many factors such as the aging population, the rise of intravenous (IV) drug abuse, an increase in spinal surgeries, particularly in immunosuppressed patients (e.g., HIV, cancer, rheumatic disease patients) (Acosta et al., 2006). Currently, treatment of disc infections are difficult and costly, often requiring weeks of inpatient care and IV administered antibiotics.
Many commonly used antibiotics are small molecules, and electrically charged. Due to the avascular nature of the IVD tissue, transport of antibiotics into the disc is mainly through diffusion from vasculatures surrounding the disc. The rate of antibiotics transport into the IVD depends on several factors, including antibiotic molecular size, electric charge (positive or negative), as well as disc tissue properties such as porosity (Currier et al., 1994; Eismont et al., 1987; Gibson et al., 1987; Gu et al., 2003; Gu et al., 2004; Maroudas, 1976; Riley et al., 1994; Thomas et al., 1995; Urban et al., 1977; Zhu et al., 2016). In general, drugs with smaller size, and/or with positive charge have easier access to, or higher uptake in the discs than the ones with larger sizes and/or negative charge (Currier et al., 1994; Eismont et al., 1987; Gibson et al., 1987; Riley et al., 1994; Thomas Rde et al., 1995; Urban et al., 1977; Zhu et al., 2016) because IVDs are negatively charged due to the charged groups (e.g., SO3−, COO−) on the side chain of proteoglycan, one of the major component of the IVD matrix (Urban and Maroudas, 1979). The concentration of charged groups is called fixed charge density.
Solute transport in the disc is also affected by mechanical loading (Arun et al., 2009; Ferguson et al., 2004; Gullbrand et al., 2015; Huang and Gu, 2008; Jackson et al., 2011; Urban et al., 1982; Yao and Gu, 2004, 2006). It is generally found that static loading decreased the transport rate of solutes in human IVDs (Arun et al., 2009; Huang and Gu, 2008; Jackson et al., 2011), while dynamic loading enhanced the transport rates of solutes (Ferguson et al., 2004; Gullbrand et al., 2015; Huang and Gu, 2007; Urban et al., 1982; Yao and Gu, 2006). However, most of the studies are focused on non-charged solutes, and to our knowledge, there is limited information on how mechanical loading affects the transport of charged solutes in the highly negatively charged human disc. Due to the charged nature of the IVD and some antibiotics, electric interaction between charged tissue and antibiotics plays an important role in governing antibiotic penetration rate, antibiotic concentration profile, and duration for antibiotic maintaining its concentration at the level above minimal inhibitory concentration within the IVD.
Thus, the objective of this study was to quantitatively analyze the effect of dynamic loading (e.g., diurnal loading) on the transport of charged antibiotics into charged IVDs using a finite element method. Such knowledge is important for treating disc infections with the proper dosage and timing of antibiotics.
2. Methods
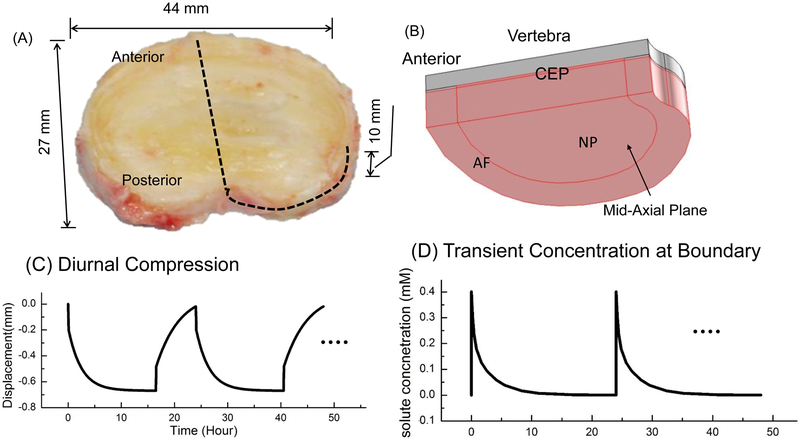
A previously developed finite element model (Zhu et al., 2016) was used in this study, in which the transport of various charged antibiotics into human lumbar discs without mechanical loading was analyzed. In the current study, the method was similar to previously published paper (Zhu et al., 2016), except the mechanical loading condition. In the current study, mechanical compression with the profile shown in Figure 1C was applied on the top of the vertebra to mimic the diurnal loading on the IVD. This compression profile (Fig. 1C) was determined based on experimental data that the disc height decreased by a maximum of 1.3 mm at the end of the 16.5-hour day activity, and it recovered back to its original thickness at the end of the 7.5-hour sleep (Broberg, 1993), assuming the disc has a height of 10 mm. The material properties (including the mechanical properties, transport properties, and electrochemical properties) used in this study can be found in Table 1 of our previous study (Zhu et al., 2016). In real situation, there is no discontinuity in permeability or diffusivity at the AF-NP boundary or interface. However, information on these properties at AF-NP boundary is not available in the literature. In order to avoid possible discontinuity in transport properties at the AF-NP interface, in the current study, the constitutive relationship for hydraulic permeability and diffusivities for nucleus pulposus (NP) were used for those for annulus fibrosus (AF).
Figure 1:
(A) Picture of a human lumbar intervertebral disc (IVD, L2–3, non-degenerated). (B) Schematic of a quarter of the IVD-vertebra segment used in the FEM analysis (due to symmetry). (C) Schematic of the diurnal compression (on a quarter of the disc). (D) Schematic of the transient antibiotic concentration at the disc boundary. NP: Nucleus pulposus. AF: Annulus fibrosus. CEP: Cartilaginous endplate.
Three groups of antibiotics were investigated: a positively charged group, a negatively charged group, and a neutral group. To eliminate the effects of differences in molecular characteristics on the transport of antibiotics into discs, all the antibiotics were assumed to have the same molecular properties [e.g., molecular weight (400 g/mol), diffusivity in water at 37 °c (6.2×10−10 m2/s), and hydraulic radius (0.53 nm)], except electrical charge. The reason for choosing this molecule weight of 400 g/mol in our simulation is that many commonly used antibiotics have a similar molecular weight (around 300~500 g/mole).
After the disc reaches steady state upon the application of this diurnal compression, a transient concentration was applied at the disc boundary with a function of <MI> (mM, where t0=30s), see figure 1D, to mimic drug level changes in the serum post intravenous (IV) administration as reported in the literature (Currier et al., 1994; Pinto et al., 2011; Shi et al., 2010; Walters et al., 2006). This boundary condition was repeated every 24 hours to simulate multiple IV infusions. For the convenience (without losing generosity), we assumed that the blood-disc partition coefficient is unity at both the CEP boundary and the AF periphery for the antibiotics in the simulation. The uptake of the antibiotics by the cells was not considered in the current study due to lack of information in the literature. Transport of charged antibiotics in IVD under diurnal compression (Fig. 1C) was studied and compared to those without compression.
Results
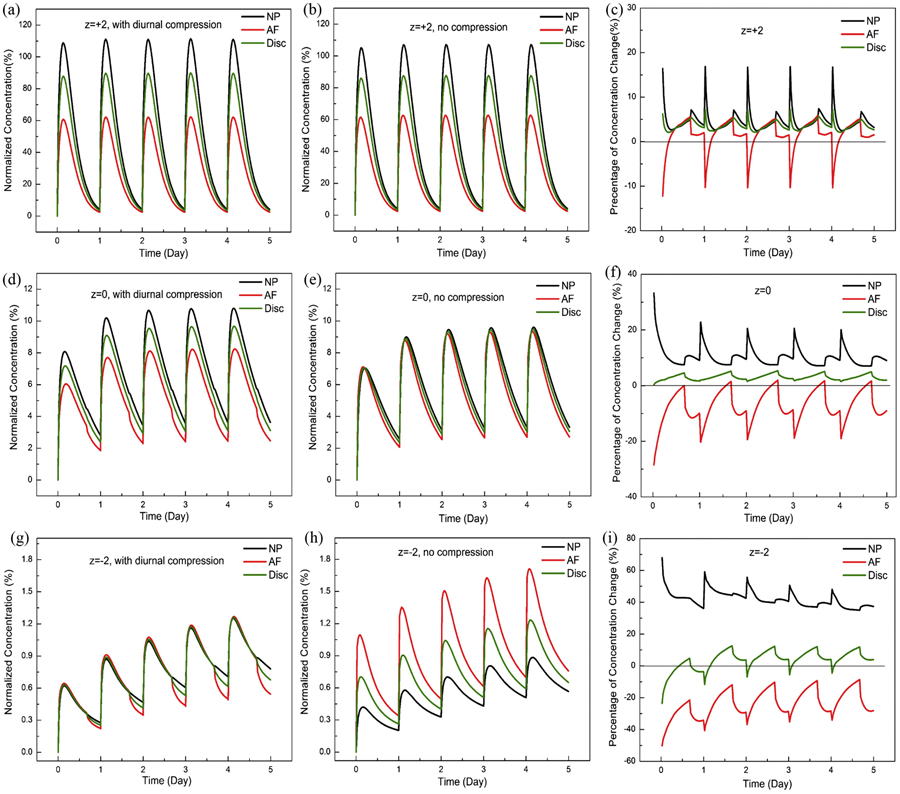
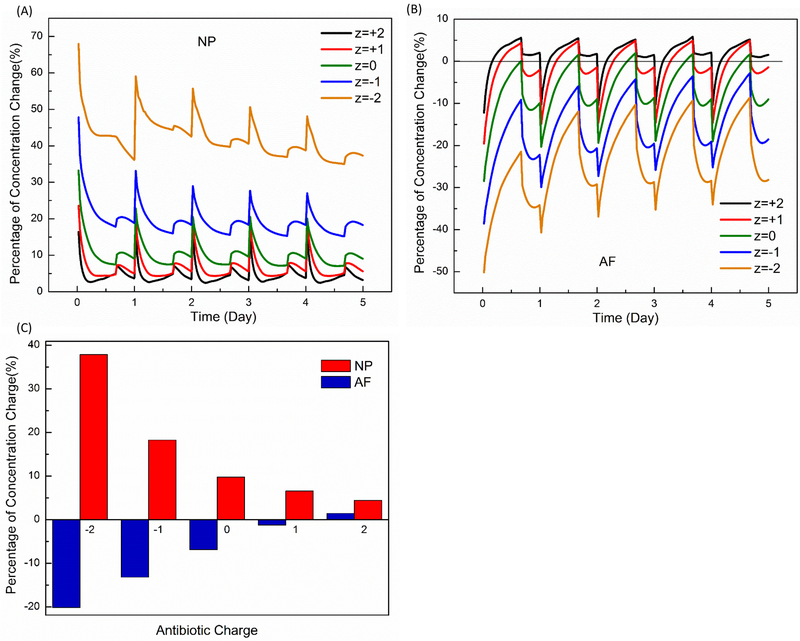
Diurnal compression increased the concentrations in the NP region for all charged or non-charged drugs, but generally decreased the concentrations in the AF region (Figs. 2 and 3). The overall concentration (averaged over disc) was increased with diurnal compression (Fig. 2). The diurnal compression had more effects on negatively charged antibiotics than positively charged ones (Fig. 3). For example, at day 5 with diurnal compression, the diurnal compression increased the concentration of negatively drug (z = −1) in NP by 18.3%, but only by 6.6% for positively charged one (z = +1). In AF, diurnal compression decreased the concentration by 13.2% for negatively charged drug (z = −1) versus 1.2% for positively charged one (z=+1), see Figure 3C. Note these percentages in this example are averaged values over the day 5. The effect of diurnal compression on the concentration in the disc monotonically and nonlinearly decreased when the charge number (or valence) increased from −2 (negatively charged) to +2 (positively charged), see Figure 3C.
Figure 2:
Normalized antibiotic concentrations in the disc, with diurnal compression (A, D, G), without diurnal compression (B, E, H), and their relative changes due to diurnal compression (C, F, I). In (A, C, D, F, G, I), the concentrations were normalized to the peak value of the concentration in serum (i.e., 0.4 mM)], while in (C, F, I), relative changes in concentrations (due to diurnal compression) to those without diurnal compression. z=valence of the antibiotic. NP: Nucleus Pulposus; AF: Annulus Fibrosus.
Figure 3:
Comparisons of the relative change in concentration due to diurnal compression for various electrically charged antibiotics in NP (A) and AF (B). (C) Correlation between antibiotic charge and percentage of concentration change (averaged over day 5). NP: Nucleus Pulposus. AF: Annulus Fibrosis.
Discussion
The objective of this study was to investigate the effect of diurnal loading on antibiotic penetration into IVD with IV infusions. Due to transient drug concentration at the disc boundary (Fig. 1D), the concentration gradient in the disc varies with time. During the penetration process, IVD also deforms under diurnal loading (Fig. 1C), causing changes in tissue hydration and its related transport properties (Gu et al., 2003; Gu et al., 2004; Johnson and Deen, 1996; Mackie and Meares, 1955; Masaro and Zhu, 1999; Mow et al., 1980). The strain field in the IVD is complex, mainly due to its 3D geometry (Fig. 1A). The combination of concentration gradient and mechanical effects governs the transport of non-charged drug (i.e., z=0) in the IVD. In some regions at a certain time, the concentration gradient and mechanical effects might work together to enhance the transport of non-charged antibiotic; or they might work against each other to impede its transport, resulting in a higher (or lower) antibiotic concentration in some regions relative to those without mechanical compression. This might explain why dynamic compression enhances the concentration of non-charged antibiotic in the NP, but not in the AF (Figs. 2 and 3).
For charged drugs, the driving force is the gradient of its electrochemical potential which is explicitly related to the valence of the drug (Gu et al., 1998; Lai et al., 1991). The electric interaction of charged drug with charged tissue further enhances the effect of dynamic compression on drug transport. Since the concentration is lower in IVD with higher negatively charged drugs (even with dynamic compression), compared to that of positively charged ones, the effect of dynamic compression on the change in relative concentration is more pronounced for higher negatively charged drugs (Fig. 3).
With transient antibiotic concentration at tissue boundaries, our simulation predicts that diurnal compression is beneficial for enhancing transport of charged and non-charged antibiotics into the NP region of the disc (Figs. 2 and 3). Our result is consistent with an in-vivo study on the transport of gadodiamide (small neutral molecule, Mw=573 g/mol) into the NP of rabbits with the IV injection (Gullbrand et al., 2015). In this in-vivo study, the authors found that the transport was enhanced in the NP of rabbits under dynamic compression. In our study, our model predicted that the concentration of non-charged antibiotic in the NP increased with diurnal compression.
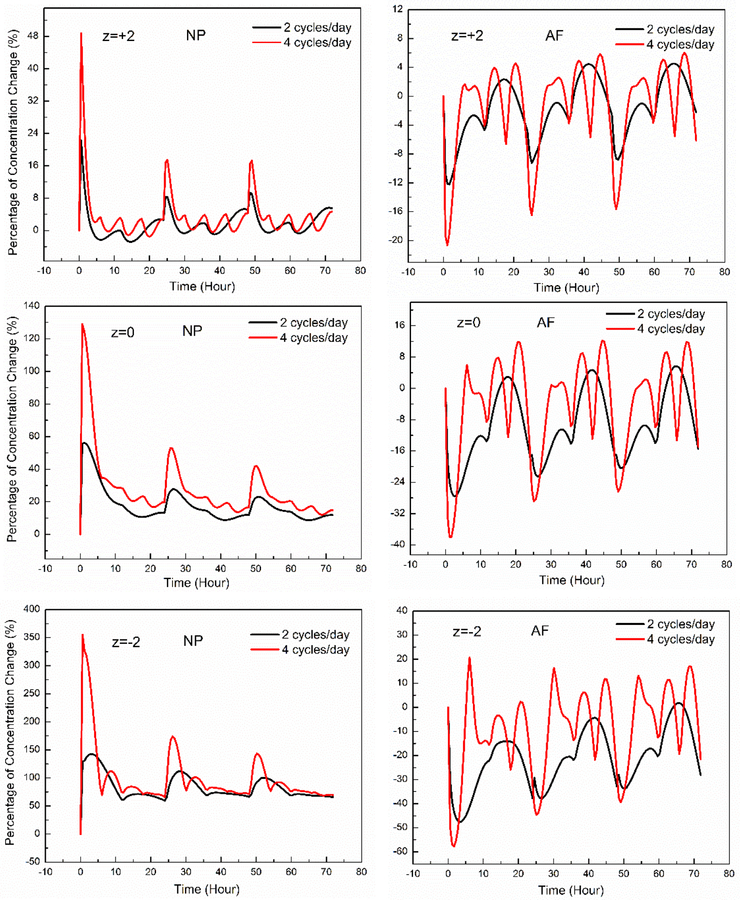
In order to understand drug transport in the disc with higher loading frequency, we also simulated the cases with 2 and 4 cycles of compression per day. The compression profile was in a half-sinusoidal fashion with the peak strain of −13%. Our results show that dynamic loading with higher frequency enhances the concentration of both charged and non-charged antibiotics in the NP, and decreases the concentrations in the AF region (Fig. 4), which is consistent with our findings with diurnal loading.
Figure 4:
The effect of loading frequency on percentage change in drug concentration (relative to those without dynamic compression). NP: Nucleus Pulposus. AF: Annulus Fibrosis.
Some limitations in the current study include that the anisotropy of the transport properties in AF is not considered in this study, this may affect the concentration distribution of the antibiotic in the AF. However in this study, we used the average concentration in the AF region in our analysis, thus the findings related to AF in our current study may not be significantly influenced by this limitation. Another limitation is that the repeated IV infusions have the same frequency as the diurnal loading in this study. In the future studies, we will investigate cases with different infusion frequencies, to further understand the interaction between mechanical and chemical loading.
In conclusion, in this study, we numerically investigated the effect of diurnal loading on transport of charged antibiotics in the negatively charged human IVDs under transient antibiotic concentration at disc boundary. Our results indicate that diurnal compression enhances (reduces) drug concentration in NP (in AF), but overall concentration in disc increases with diurnal loading. Our predict results are consistent with the findings reported in the literature. This study provides a new insight into the mechanism of transport of charged antibiotics into the disc under dynamic loading conditions.
Acknowledgement
This study was supported in part by a research grant from the NIH (AR066240) and Miami Center for Orthopedic Research and Education (Miami CORE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
No financial support or benefit has been obtained from any commercial source related directly or indirectly to the scientific work reported in this manuscript.
References
- Acosta FL Jr., Galvez LF, Aryan HE, Ames CP, 2006. Recent advances: infections of the spine. Curr Infect Dis Rep 8, 390–393. [DOI] [PubMed] [Google Scholar]
- Arun R, Freeman BJC, Scammell BE, McNally DS, Cox E, Gowland P, 2009. 2009 ISSLS Prize Winner: What Influence Does Sustained Mechanical Load Have on Diffusion in the Human Intervertebral Disc? An In Vivo Study Using Serial Postcontrast Magnetic Resonance Imaging. Spine 34, 2324–2337. [DOI] [PubMed] [Google Scholar]
- Broberg KB, 1993. Slow deformation of intervertebral discs. J Biomech 26, 501–512. [DOI] [PubMed] [Google Scholar]
- Currier BL, Banovac K, Eismont FJ, 1994. Gentamicin penetration into normal rabbit nucleus pulposus. Spine 19, 2614–2618. [PubMed] [Google Scholar]
- Eismont FJ, Wiesel SW, Brighton CT, Rothman RH, 1987. Antibiotic penetration into rabbit nucleus pulposus. Spine 12, 254–256. [DOI] [PubMed] [Google Scholar]
- Ferguson SJ, Ito K, Nolte LP, 2004. Fluid flow and convective transport of solutes within the intervertebral disc. J Biomech 37, 213–221. [DOI] [PubMed] [Google Scholar]
- Gibson MJ, Karpinski MR, Slack RC, Cowlishaw WA, Webb JK, 1987. The penetration of antibiotics into the normal intervertebral disc. J Bone Joint Surg Br. 69, 6. [DOI] [PubMed] [Google Scholar]
- Gu WY, Lai WM, Mow VC, 1998. A mixture theory for charged-hydrated soft tissues containing multi-electrolytes: passive transport and swelling behaviors. Journal of biomechanical engineering 120, 169–180. [DOI] [PubMed] [Google Scholar]
- Gu WY, Yao H, Huang CY, Cheung HS, 2003. New insight into deformation-dependent hydraulic permeability of gels and cartilage, and dynamic behavior of agarose gels in confined compression. J. Biomech 36, 593–598. [DOI] [PubMed] [Google Scholar]
- Gu WY, Yao H, Vega AL, Flagler D, 2004. Diffusivity of ions in agarose gels and intervertebral disc: Effect of porosity. Annals of Biomedical Engineering 32, 1710–1717. [DOI] [PubMed] [Google Scholar]
- Gullbrand SE, Peterson J, Ahlborn J, Mastropolo R, Fricker A, Roberts TT, Abousayed M, Lawrence JP, Glennon JC, Ledet EH, 2015. ISSLS Prize Winner: Dynamic Loading-Induced Convective Transport Enhances Intervertebral Disc Nutrition. Spine 40, 1158–1164. [DOI] [PubMed] [Google Scholar]
- Huang CY, Gu WY, 2007. Effects of tension-compression nonlinearity on solute transport in charged hydrated fibrous tissues under dynamic unconfined compression. Journal of biomechanical engineering 129, 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Gu WY, 2008. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J Biomech 41, 1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AR, Huang CY, Brown MD, Gu WY, 2011. 3D finite element analysis of nutrient distributions and cell viability in the intervertebral disc: effects of deformation and degeneration. Journal of biomechanical engineering 133, 091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EM, Deen WM, 1996. Hydraulic permeability of agarose gels. Aiche J 42, 1220–1224. [Google Scholar]
- Lai WM, Hou JS, Mow VC, 1991. A triphasic theory for the swelling and deformation behaviors of articular cartilage. Journal of biomechanical engineering 113, 245–258. [DOI] [PubMed] [Google Scholar]
- Mackie JS, Meares P, 1955. The Diffusion of Electrolytes in a Cation-Exchange Resin Membrane .2. Experimental. Proc R Soc Lon Ser-A 232, 510–518. [Google Scholar]
- Maroudas A, 1976. Transport of Solutes through Cartilage - Permeability to Large Molecules. J Anat 122, 335–347. [PMC free article] [PubMed] [Google Scholar]
- Masaro L, Zhu XX, 1999. Physical models of diffusion for polymer solutions, gels and solids. Prog Polym Sci 24, 731–775. [Google Scholar]
- Mow VC, Kuei SC, Lai WM, Armstrong CG, 1980. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. Journal of biomechanical engineering 102, 73–84. [DOI] [PubMed] [Google Scholar]
- Pinto N, Schumacher J, Taintor J, Degraves F, Duran S, Boothe D, 2011. Pharmacokinetics of amikacin in plasma and selected body fluids of healthy horses after a single intravenous dose. Equine Vet J 43, 112–116. [DOI] [PubMed] [Google Scholar]
- Riley LH 3rd, Banovac K, Martinez OV, Eismont FJ, 1994. Tissue distribution of antibiotics in the intervertebral disc. Spine 19, 2619–2625. [PubMed] [Google Scholar]
- Shi S, Liu Y, Li Z, Zheng H, Lv Y, Chen H, 2010. Pharmacokinetics and tolerability of intravenous cefotetan disodium for injection in healthy Chinese volunteers: A randomized, open-label, single- and multiple-dose study. Clin Ther 32, 1832–1841. [DOI] [PubMed] [Google Scholar]
- Thomas Rde W, Batten JJ, Want S, McCarthy ID, Brown M, Hughes SP, 1995. A new in-vitro model to investigate antibiotic penetration of the intervertebral disc. The Journal of bone and joint surgery. British volume 77, 967–970. [PubMed] [Google Scholar]
- Thomas RW, Batten JJ, Want S, McCarthy ID, Brown M, Hughes SP, 1995. A new in-vitro model to investigate antibiotic penetration of the intervertebral disc. The Journal of bone and joint surgery. British volume 77, 967–970. [PubMed] [Google Scholar]
- Urban JP, Holm S, Maroudas A, Nachemson A, 1977. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clinical orthopaedics and related research, 101–114. [PubMed] [Google Scholar]
- Urban JP, Holm S, Maroudas A, Nachemson A, 1982. Nutrition of the intervertebral disc: effect of fluid flow on solute transport. Clinical orthopaedics and related research, 296–302. [PubMed] [Google Scholar]
- Urban JPG, Maroudas A, 1979. The measurement of fixed charged density in the intervertebral disc. Biochimica et Biophysica Acta (BBA)-General Subjects 586. [Google Scholar]
- Walters R, Moore R, Fraser R, 2006. Penetration of cephazolin in human lumbar intervertebral disc. Spine 31, 567–570. [DOI] [PubMed] [Google Scholar]
- Yao H, Gu WY, 2004. Physical signals and solute transport in cartilage under dynamic unconfined compression: Finite element analysis. Annals of Biomedical Engineering 32, 380–390. [DOI] [PubMed] [Google Scholar]
- Yao H, Gu WY, 2006. Physical signals and solute transport in human intervertebral disc during compressive stress relaxation: 3D finite element analysis. Biorheology 43, 323–335. [PubMed] [Google Scholar]
- Zhu QQ, Gao X, Li N, Gu WY, Eismont F, Brown MD, 2016. Kinetics of charged antibiotic penetration into human intervertebral discs: A numerical study. J Biomech 49, 3079–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]