Summary
Mantle cell lymphomas have generally a dismal prognosis. Intensified induction treatment with rituximab and high dose cytarabine, and consolidation with high-dose therapy with autologous stem cell support has resulted in 10 year overall survival (OS) higher than 60%. However, the clinical course varies. Diagnostic tools capable of stratifying patients include the Mantle Cell Lymphoma International Prognostic Index (MIPI), gene expression-based proliferation signature, Ki-67 proliferation index or tumor cell morphology. Here, we tested the performance of a newly developed Nanostring-based RNA expression -based proliferation assay (MCL35) on formalin-fixed paraffin-embedded tumor tissue from younger patients recruited in or treated according to Nordic MCL protocols compared to the prognosticators listed above. Seventy-four patients were included and the assay performed well in all cases except four with inadequate RNA quality. The patients were evenly distributed in the MCL35 low-, intermediate- and high risk categories. MCL35 low- and intermediate- risk groups had overlapping progression-free survival, while patients in the high-risk category had significantly inferior progression-free survival. Combining MCL35 with MIPI or MIPI-C (MIPI with the additional score for Ki67 score if ≥30%) showed a better discrimination than either assessment alone. In conclusion, the MCL35 assay alone or combined with MIPI or MIPI – C scores can identify patients who still have a dismal outcome despite intensified treatment.
Keywords: Mantle cell lymphoma, gene expression, prediction, therapy, FFPE
Introduction
Mantle cell lymphoma (MCL) is an aggressive disease with poor overall survival (OS). Although new intensive treatment regimens have improved survival, there is no indication that the disease can be cured with standard treatments (Dreyling et al, 2014, Geisler et al, 2012, Kolstad et al, 2014, Abrahamsson et al, 2014, Kluin-Nelemans et al, 2012). The outcome is variable, but clinical factors can discern between prognostic groups based on age, performance status, lactate dehydrogenase (LDH), and leukocyte count, when combined as the Mantle Cell lymphoma International Prognostic Index (MIPI) (Hoster et al, 2008).
Microarray RNA expression analyses have expanded the diagnostic accuracy in malignant lymphomas and have identified a proliferation signature as a strong predictor of survival in MCL (Rosenwald et al, 2003). These analyses were pioneered from the National Cancer Institute and the Leukemia and Lymphoma Molecular Profiling Project (LLMPP). However, gene expression analyses were performed on fresh frozen tissue which is not readily available from the majority of patients. To translate these findings to clinical practice, tumor cell proliferation index, based on Ki-67 staining by immunohistochemistry (IHC) has been tested and recognized as a strong adverse prognostic factor (Hoster et al, 2014, Hoster et al, 2016, Katzenberger et al, 2006, Raty et al, 2002, Tiemann et al, 2005, Determann et al, 2008), and was the only biological factor at diagnosis with predictive power in multivariate analysis in a Nordic prospective phase II study (Geisler et al, 2012). However, several studies on IHC analysis in lymphoma, including MCL, have shown large inter-observer variability, which poses a challenge for comparison of results between labs (de Jong et al, 2007, Klapper et al, 2009, Sander et al, 2014). Therefore, a standardization of IHC evaluation of Ki-67 index in MCL is recommended (Klapper et al, 2009, Dreyling et al, 2013).
An assay using Nanostring® technology to measure RNA expression of a selected number of genes extracted from formalin-fixed, paraffin-embedded tissue (FFPE) has been developed (Roberts et al, 2007). This technology has been applied to discern between the two major subtypes of diffuse large B-cell lymphomas, Activated B-cell like and Germinal Centre B-cell like (Scott et al, 2014). An assay which applies the same technology has been developed for MCL by selecting 35 different genes, of which 17 were associated with proliferation and 18 were housekeeping genes. The MCL35 assay stratified patients into high-, standard- and low risk in a training set of 47 biopsies and was validated in a separate cohort of 110 patients (Scott et al, 2017). The validation cohort originated from British Columbia and consisted of patients who received rituximab-CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) chemoimmunotherapy. Of note, in approximately half the patients, there was an intention to consolidate with high dose chemotherapy and autologous stem cell support (HD-ASCT). In this study, the pre-treatment biopsies contained at least 60% tumor cells and were excisional nodal biopsies.
For the above assay to be recommended in routine clinical practice, it should also be validated in a separate cohort of patients who have been treated with a regimen that now is considered to be standard for younger patients: Alternating rituximab-CHOP with a rituximab-high dose cytarabine – containing regimen (total of 6 courses) followed by HD-ASCT. We have, therefore, tested the MCL35 assay on a cohort of Norwegian patients, recruited in two Nordic MCL2 and MCL3 clinical trials (Geisler et al, 2012, Kolstad et al, 2014) or treated accordingly after closure of the trials.
Methods
Patients and Material
All Norwegian patients diagnosed with MCL with available FFPE diagnostic material and who had been treated in two consecutive Nordic phase II trials (MCL2 and MCL3) or according to the MCL2 protocol were eligible for this study - in total 153 cases. Danish and Swedish patients were not included. Further selection of patients was performed according to the Consort diagram (Supplementary Figure 1). Only surgical material from lymph nodes or Waldeyer’s ring and with at least 60% tumor infiltration was considered suitable. Thus, out of 153 cases, 78 were deemed suitable for the MCL35 assay.
The study was approved by Regional Committee for Medical and Health Research Ethics South East (reg. no. 2016/1459) and by the Protection Officer at Oslo University Hospital.
RNA extraction and gene expression profiling
Unstained sections from FFPE tissue (1–2 slides if the surface area was > 1 cm2, 5 slides for 0.1–1 cm2 and 10 slides for < 0.1 cm2) were sent to Vancouver for deparaffinization with the QIAGEN Deparaffinization Solution (catalogue number 19093) and RNA extraction with the QIAGEN AllPrep DNA/RNA FFPE Kit (catalogue number 80234, QIAGEN GmbH, Hilden, Germany). The RNA was quantified with spectrophotometry (NanoDrop, Thermo Scientific, DE). The MCL35 assay was performed on a minimum of 200 ng of RNA on NanoString technology (NanoString Technologies, Seattle, WA) as previously described (Scott et al, 2014).
Pathology
Biopsies from patients included in the Nordic MCL2 and MCL3 studies or treated after closure of the Nordic MCL studies according to the MCL2 protocol were reviewed. Only biopsy samples originating from a lymph node or Waldeyer’s ring, fulfilling the histological and immunohistochemical criteria established by the WHO (2017) for the diagnosis of MCL and containing ≥ 60% tumor cells were selected for this study. Following WHO criteria, tumors were subclassified into those with blastoid/pleomorphic (referred to as blastic) and those with clasical histology. Ki-67 score was calculated as an average percentage of positive cells after counting immunohistochemically positive cells in representative tumor areas by an experienced pathologist.
Statistics
The chi square or Fisher tests were applied to examine differences in patient characteristics between MCL35 risk categories. The primary endpoint was OS, which was calculated from date of diagnosis or inclusion in prospective studies until date of death from any cause. Secondary end point was progression free survival (PFS), calculated from date of diagnosis or study until date of progression or death from any cause (Cheson et al, 2007). OS and PFS were estimated using the Kaplan-Meier method. Log-rank test was used to examine the relationship between categorical variables and OS or PFS. Univariable analyses using Cox models were used to examine the relationship between defined variables and OS, including continuous variables. Cox proportional hazards regression tests were used to test the association of variables with OS in combination with other variables (adjusted analysis in Table 2). Two-sided p values < .05 were considered significant.
Table 2.
Prognostic value of clinical and tumor proliferation associated variables including MCL35 raw score for overall and progression free survival
| OS | PFS | ||||
|---|---|---|---|---|---|
| type | variable | Pval | HR | Pval | HR |
| Unadjusted | Age (>60 vs <=60) | 0.20 | 1.7496 | 0.62 | 1.2171 |
| Unadjusted | Gender (Female vs Male) | 0.60 | 1.3595 | 0. | 1.6261 |
| Unadjusted | LDH (High vs Normal) | 0.0041 | 4.9138 | 0.0043 | 3.2600 |
| Unadjusted | WHO (2–4 vs 0–1) | 0.0248 | 3.5401 | 0.0025 | 4.4789 |
| Unadjusted | Ki67 index (+5%) | 0.0001 | 1.4268 | 0.0002 | 1.3502 |
| Unadjusted | Ki67 index (>=30%) | 0.0129 | 3.1364 | 0.0315 | 2.2314 |
| Unadjusted | Histology (Blastic vs Classic) | 0.0339 | 2.5004 | 0.1073 | 1.8436 |
| Unadjusted | MIPI (+1) | 0.0009 | 2.3200 | 0.0059 | 1.9666 |
| Unadjusted | MIPI (3 cat) | 0.0002 | 2.9070 | 0.0005 | 2.2900 |
| Unadjusted | MIPI-C (4 cat) | 0.0001 | 2.7082 | 0.0002 | 2.0987 |
| Unadjusted | MCL35 score (+100) | 0.0101 | 1.7152 | 0.0441 | 1.4297 |
| Adjusted | MCL35 score (+100) | 0.0316 | 1.6252 | 0.0710 | 1.4068 |
| MIPI score (+1) | 0.0044 | 2.0499 | 0.0111 | 1.8536 | |
| Adjusted | MCL35 score (+100) | 0.94 | 1.0234 | 0.48 | 0.8329 |
| Ki67 index (+5%) | 0.0064 | 1.1903 | 0.0025 | 1.1982 | |
| Adjusted | MCL35 score (+100) | 0.96 | 0.9839 | 0.50 | 0.8350 |
| Ki67 index (+5%) | 0.0101 | 1.1727 | 0.0033 | 1.1833 | |
| MIPI score (+1) | 0.0088 | 2.1322 | 0.0178 | 1.9553 | |
| Adjusted | MCL35 score (+100) | 0.14 | 1.4243 | 0.35 | 1.2223 |
| Ki67 index (>=30%) | 0.13 | 2.2188 | 0.20 | 1.7778 | |
| Adjusted | MCL35 score (+100) | 0.36 | 1.2734 | 0.50 | 1.1642 |
| Ki67 index (>=30%) | 0.0710 | 2.6588 | 0.12 | 1.9893 | |
| MIPI score (+1) | 0.0029 | 2.3238 | 0.0074 | 2.0288 | |
Results
Description of patient material
The MCL35 assay was applied to the biopsies of 78 patients, with 74 (95%) passing quality control. The assay assigned 27 (36%), 29 (39%) and 18 (24%) of the patients to low-, standard- and high-risk MCL35 categories, (thresholds −143 and −28) respectively. The median MCL35 value was −113.05 (range −303.6 – +129.9). The demographic data, disease characteristics and treatments of all 74 MCL patients with MCL35 score (Scott et al, 2017) are shown in Table 1. There was a male predominance; the median age was 59 years, most patients had ECOG performance status 0–1 and bone marrow infiltration, and the distribution of MIPI and MIPI-C was as expected. There was an even distribution of Ki-67 score less than or greater than 30%. A quarter of the biopsies were of blastic (blastoid or pleomorphic) morphology. Of note, the majority of patients completed the intended treatment with alternating R-Maxi CHOP/R-HD cytarabine and consolidation with HD-ASCT. The median observation time was 5.3 years, there were no missing data in the final cohort and no patients were lost to follow up.
Table 1:
Demographic data and disease characteristics distributed among the MCL35 risk groups
| Patient Demographic Data and Disease Characteristics | |||||
|---|---|---|---|---|---|
| Variable | Total Cohort, n (%) | Low Risk Group n (%) | Standard-Risk Group n (%) | High-Risk Group n (%) | P |
| Assessable patients | 74 | 27 (36) | 29 (39) | 18 (24) | 1 |
| Male | 64 (86) | 23 (85) | 25 (86) | 16 (89) | |
| Female | 10 (14) | 4 (15) | 4 –(14) | –2 (11) | |
| Median age, years (range) | 59 (41–67) | 60 (45–67) | 58 (41–67) | 60.5 (43–65) | |
| > 65 | 2 | 1 | 1 | 0 | |
| Clinical features | |||||
| ECOG performance stat | |||||
| 0–1 | 68 (92) | 26 (96) | 27 (93) | 15 (83) | 0.32 |
| 2–4 | 6 (8) | 1 (4) | 2 (7) | 3 (7) | |
| Missing | |||||
| White cell count, median (range) | 6.8 (3.4–56.2) | 6,3 (4.2–20.5) | 7,2 (3.4–56.2) | 8,4 (4.4–18.2) | 0.31 |
| Bone marrow infiltration | 59 (80) | 24 (89) | 19 (66) | 16 (89) | 0.051 |
| LDH | |||||
| Normal | 35 (47) | 16 (59) | 15 (52) | 4 (25) | 0.043 |
| >ULN | 39 (53) | 11 (41) | 14 (48) | 14 (75) | |
| MIPI | |||||
| Low < 5.7 | 36 (49) | 15 (56) | 14 (48) | 7 (39) | 0.81 |
| Intermediate (5.7–6.2 | 26 (35) | 9 (33) | 10 (35) | 7 (39) | |
| High (>= 6.2) | 12 (16) | 3 (11) | 5 (17) | 4 (27) | |
| MIPI – c | |||||
| Low | 25 (34) | 13 (48) | 10 (35) | 2 (11) | 0.16 |
| Low-intermediate | 22 (30) | 8 (30) | 8 (28) | 6 (33) | |
| High-intermediate | 19 (26) | 5 (19) | 8 (28) | 6 (33) | |
| High | 8 (11) | 1 (4) | 3 (10) | 4 (22) | |
| Ki67 proliferation index | |||||
| < 30 | 40 (54) | 21 (78) | 16 (55) | 3 (17) | <0.001 |
| >=30 | 34 (46) | 6 (12) | 13 (45) | 15 (83) | |
| MIPI-B | |||||
| Low < 5.7 | 9 (12) | 5 (19) | 3 (10) | 1 (6) | 0.064 |
| Intermediate 5.7–6.5 | 37 (50) | 15 (56) | 17 (59) | 5 (28) | |
| High >= 6.5 | 28 (38) | 7 (26) | 9 (31) | 12 (67) | |
| Pathology | |||||
| Classic | 54 (73) | 25 (93) | 23 (80) | 6 (33) | <0.001 |
| Blastic (pleomorphic, blastoid) | 20 (27) | 2 (7) | 6 (21) | 12 (67) | |
| Given HDT with BEAM | 68 (92) | 24 (89) | 27 (93) | 17 (94) | |
| Reason not given HDT | |||||
| Toxicity | 1 | 1 | |||
| Harvest failure | 2 | 1 | 1 | ||
| Stage I | 1 | 1 | |||
| Age | 1 | 1 | |||
| No response induction | 1 | 1 | |||
| CR | 66 (90) | 24 (90) | 26 (90) | 16 (90) | 0.92 |
| CR / PR | 72 (98) | 26 (97) | 28 (97) | 18 (100) | |
Distribution of patient and tumor characteristics across MCL35 risk groups
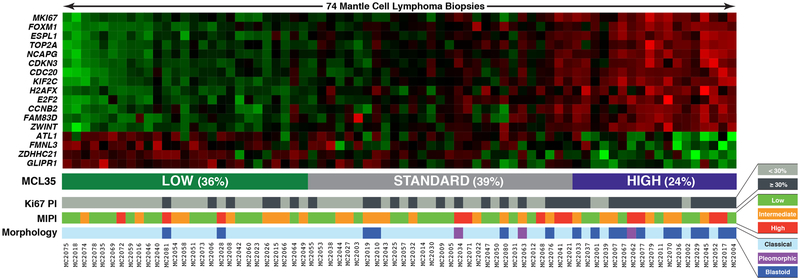
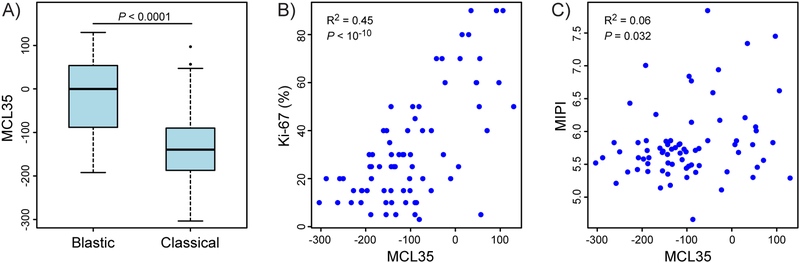
Figure 1 shows the heat map of RNA expression of the 35 genes (rows) for the 74 cases included in the analyses (columns). Underneath is depicted the corresponding Ki-67dichotomized score, MIPI score and histology (blastic versus classical). Table 1 shows the baseline distribution of disease characteristics across the three MCL35 risk groups. There were significant correlations between MCL35 high-risk and elevated LDH (p = 0.043), blastic histology (p < 0.001) and Ki-67 score > 30% (p < 0.001) (Table 1 and Figure 2A and 2B), but no significant correlations to MIPI (Table 1, Figure 2C) MIPI-B (MIPI with the addition of Ki67 score <10%, ≥10%−29% or ≥ 30%, Hoster et al, 2014) or MIPI-C (MIPI with the addition of binary Ki67 score +/−30%, Hoster et al, 2016) (Table 1).
Figure 1.
Heatmap of RNA expression for the 35 genes included (rows) for the 74 individual biopsies included (columns). The total score indicate the three categories (low, standard, high). Underneath is depicted the corresponding Ki-67 score dichotomized into +/−30%, the MIPI score and whether the biopsies had a blastic or classical morphology type.
Figure 2.
A. Box plot of MCL35 score in blastic versus classical MCL type. Statistics: Welch’s unequal variances t-test. B. MCL35 score versus Ki-67 score. Correlation by Spearman rank test. C. MCL35 score versus MIPI score. Correlation by Spearman rank test.
Survival prediction for risk factors
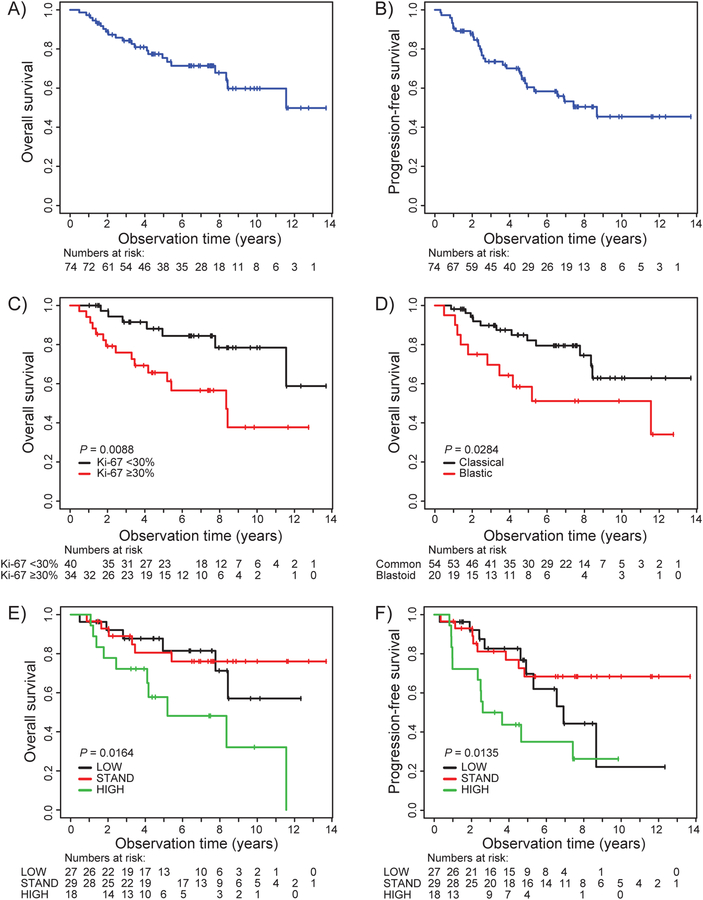
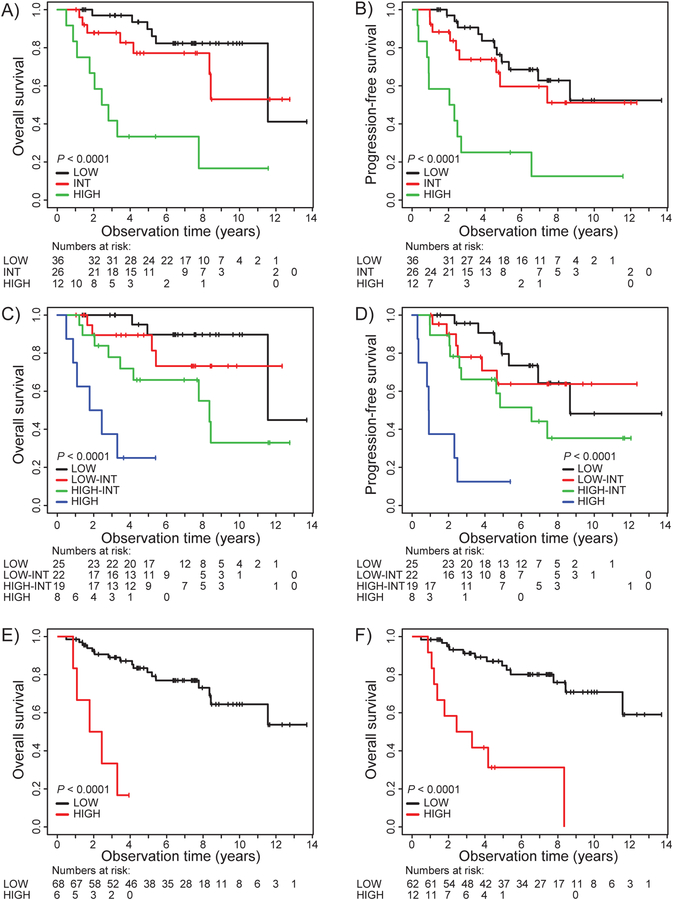
The median observation time for all patients was 5.2 years (range 0.5–13.7). The estimated 5-year OS and PFS was 77% and 60%, respectively, and at 10 years 60% and 48% (Figure 3A and 3B). Patients with Ki-67 ≥30% and blastic morphology (Figures 3C and 3D) had an inferior survival. Compared with data in the original manuscript16, low- and standard-risk groups did not have significantly different outcomes, while the high-risk group had inferior outcome (Figure 3E and 3F). MIPI and MIPI-C discriminated well between the different risk categories (Figures 4A – 4D). In Cox unadjusted analysis, LDH (normal versus elevated), WHO performance status (0–1 versus 2–4), Ki-67 5% incremental up to 30%, Ki-67 +/− 30%, MIPI Score, MIPI-C score and MCL35 score predicted OS and PFS (albeit MCL35 score only of borderline significance for PFS (Table 2). In Cox adjusted multivariate analysis including Ki-67% with 5% increments and MCL35, only Ki-67 was significant for OS and PFS. However, when Ki-67 was included as a dichotomized variable (≥ versus < 30%), neither were associated with survival (Table 2). When MIPI and MCL35 were included in multivariable analysis, both variables were associated with OS, but with the MIPI score showing a better survival prediction (Table 2). Lastly, in multivariable analysis including MCL35, Ki-67 and MIPI, the two latter variables were significantly associated with OS and PFS. Unadjusted analyses of MCL35 score (standard versus low and high versus low) and adjusted multivariate analysis of the same variables, including Ki-67 and MIPI score, is shown in Supplementary Table 1. As MCL35 score did not correlate with the MIPI scores, there is a rationale for combining the MCL35 scores with MIPI scores in an exploratory analysis. When combining high risk MCL35 with high risk MIPI, we identify six patients with a very high risk (Figure 4E), and when combining with high or high intermediate MIPI-C, we identify 12 patients with substantially inferior survival (Figure 4F). Unadjusted and adjusted analyses of MCL35 and MIPI for Ki67 are shown in Supplementary Table 2.
Figure 3.
Kaplan Meier survival plots. A. OS, all patients. B. PFS, all patients. C. OS for Ki-67 score categorized as ≥ or < 30%. D. OS for blastic (blastoid or pleomorphic) versus classical / small cell MCL histology. E. OS for MCL35 risk groups. F. PFS for MCL35 risk groups.
Figure 4.
Kaplan-Meier survival plots demonstrate stratification of MCL35 assay alone or in combination with MIPI or MIPI-C score. A. OS for MIPI risk groups. B. PFS for MIPI risk groups. C. OS for MIPI-C risk groups. D. PFS for MIPI-C risk groups. E. OS for MCL35 high risk combined with MIPI high risk versus others.. F. OS for MCL35 high risk combined with MIPI-C high/high-intermediate versus others.
Discussion
The intention of the current analysis was to test the ability of the MCL35 assay to predict survival in a cohort of MCL patients younger than 70 years treated with alternate R-MaxiCHOP/R-HD Cytarabine and HD-ASCT (BEAM). The estimated 5-year OS of 77% was comparable to what has been reported from two Nordic MCL studies where the patients received a similar regimen (Geisler et al, 2012, Kolstad et al, 2014) and with no plateaus in the survival curves. The overall outcome was superior to that shown in the original report on the MCL35 assay (Scott et al, 2017), where the patients received R-CHOP and only around half of them were consolidated with HD-ASCT. Outcome for our total cohort was comparable to that of the MCL35 low risk group in the original report. This justifies our evaluation of the performance of the MCL35 assay in a patient cohort of younger patients treated with, what is now considered to be, standard of care. While in the original publication there was a very clear discrimination among three risk groups, the MCL35 assay did not separate the standard-risk group from the low-risk group in our cohort. However, the MCL35 assay was able to identify a subgroup with inferior outcome despite treatment with intensified chemo-immunotherapy and HD-ASCT therapy. This indicates that survival was improved, especially for patients in the MCL35 standard-risk upon introduction of current intensified treatment regimens.
When comparing the predictive value of the assay relative to MIPI and MIPI-C in our cohort, the MIPI scores provided improved stratification compared to the MCL35 assay. Similarly, Ki-67 score (≥ versus < 30%) alone and histology (blastic versus classical) stratified patient outcome better than the MCL35 assay. However, there is still a rationale to use the MCL35 assay in risk stratification: the MIPI score identifies clinical risk factors only, and was not correlated to MCL35 scores. In addition, the Ki-67 score included in the MIPI-C prognostic index is difficult to assess in routine clinical practice and has shown considerable inter-observer variability (de Jong et al. 2007, Sander et al 2014 Klapper et al, 2009), although the reproducibility can be improved by standardizing the IHC evaluation (Klapper et al 2009, Dreyling et al 2013). In comparison, the MCL35 assay has been shown to have a good reproducibility across different centers (Scott et al, 2017). The data suggest that the prognostic effect of the MCL35 assay is independent of clinical factors, but it remains unclear whether its prognostic value is comparable to the Ki-67 index. A future comparative analysis of MCL35 and Ki-67 index using a larger patient cohort is warranted to clarify whether the presumed higher reproducibility of the MCL35 score is at the expense of lower prognostic value. Further, there is a need for validating our findings in a separate and larger, but similarly treated patient cohort.
A combination of MIPI score and MCL35 score is plausible for taking into account both clinical and biological risk factors. When combining high-risk MCL35 with high-risk MIPI score, we identified a smaller subgroup with dismal prognosis. Such patients should probably not receive standard treatment but rather be recruited to up-front clinical trials with novel investigational drugs like ibrutinib, lenalidomide and venetoclax as single agents or in combination with other drugs (Wang et al, 2013, Wang et al, 2016, Davids et al, 2017, Trneny et al, 2016, Dreyling et al, 2016). Indeed, both clinical and biological risk factors have to be assessed when new therapeutic approaches are introduced.
Novel biological risk factors have been identified during recent years like non-functional TP53 through genetic loss of p17 or mutations (Eskelund et al, 2017, Aukema et al, 2018) for identifying patients with inferior outcome. Hence, a combination of MIPI and TP53 alterations has been proposed as a new predictive marker.
Strengths of this study include that patients were uniformly treated and the fact that there were no missing values of any of the variables. Furthermore, the MCL35 analysis was performed by the group who developed the assay. For two patients, ultimately excluded from the study, results of the MCL35 assay did not fit with what was expected for a MCL biopsy. A second review of the diagnosis in these cases revealed that MCL could not be confirmed in one patient and for the other patient the sample was massively necrotic (data not shown) resulting in a viable tumor content of < 60%. Thus, the assay, although developed for predictive purposes, might also be of diagnostic value.
A weakness of this study is the limited sample size which reduces the power of the analyses. Additionally, the assay was developed for surgical biopsies from lymph nodes with tumor content of at least 60%, excluding samples from bone marrow and non-lymphoid tissue. However, OS and PFS at 5 and 10 years are nearly identical to those published for the whole cohort of Nordic patients included in the MCL2 and MCL3 studies (Kolstad A et al. 2014). Nevertheless, the MCL35 assay remains to be validated in samples that do not meet these criteria and, this so far reduces the applicability of the assay.
In conclusion, the MCL35 assay was able to identify a group of MCL patients with inferior outcome after intensive first-line treatment, including HD-ASCT, but standard- and low-risk groups had similar outcomes. By combining MIPI or MIPI-C and MCL35, patients with dismal prognosis were identified, and they should be considered for novel strategies.
Supplementary Material
Supplementary Figure 1. Consort diagram. Selection of Norwegian MCL patients for the MCL35 assay
Footnotes
Conflict of interest statement: Andreas Rosenwald, Lisa M. Rimsza, Erlend B Smeland and David W. Scott are named inventors on two patents filed by the National Cancer Institute: “Methods for selecting and treating lymphoma types” licensed to NanoString Technologies, and “Evaluation of mantle cell lymphoma and methods related thereof.” Harald Holte is named inventor on one patent filed by the National Cancer Institute: “Methods for selecting and treating lymphoma types” licensed to NanoString Technologies.
References
- Abrahamsson A, Albertsson-Lindblad A, Brown PN, Baumgartner-Wennerholm S, Pedersen LM, D’amore F, Nilsson-Ehle H, Jensen P, Pedersen M, Geisler CH & Jerkeman M 2014. Real world data on primary treatment for mantle cell lymphoma: a Nordic Lymphoma Group observational study. Blood, 124, 1288–95. [DOI] [PubMed] [Google Scholar]
- Aukema SM, Hoster E, Rosenwald A, Canoni D, Delfau-Larue MH, Rymkiewicz G, Thorns C, Hartmann S, Kluin-Nelemans H, Hermine O, Dreyling M & Klapper W 2018. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood, 131, 417–420. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V & International Harmonization Project On, L. 2007. Revised response criteria for malignant lymphoma. J Clin Oncol, 25, 579–86. [DOI] [PubMed] [Google Scholar]
- Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, Puvvada S, Kipps TJ, Anderson MA, Salem AH, Dunbar M, Zhu M, Peale F, Ross JA, Gressick L, Desai M, Kim SY, Verdugo M, Humerickhouse RA, Gordon GB & Gerecitano JF 2017. Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. J Clin Oncol, 35, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong D, Rosenwald A, Chhanabhai M, Gaulard P, Klapper W, Lee A, Sander B, Thorns C, Campo E, Molina T, Norton A, Hagenbeek A, Horning S, Lister A, Raemaekers J, Gascoyne RD, Salles G, Weller E & Lunenburg Lymphoma Biomarker C 2007. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications--a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol, 25, 805–12. [DOI] [PubMed] [Google Scholar]
- Determann O, Hoster E, Ott G, Wolfram Bernd H, Loddenkemper C, Leo Hansmann M, Barth TE, Unterhalt M, Hiddemann W, Dreyling M, Klapper W, European Mantle Cell Lymphoma, N. & The German Low Grade Lymphoma Study, G. 2008. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood, 111, 2385–7. [DOI] [PubMed] [Google Scholar]
- Dreyling M, Geisler C, Hermine O, Kluin-Nelemans HC, Le Gouill S, Rule S, Shpilberg O, Walewski J, Ladetto M & Group EGW 2014. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 25 Suppl 3, iii83–92.25210087 [Google Scholar]
- Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, Offner F, Caballero D, Joao C, Witzens-Harig M, Hess G, Bence-Bruckler I, Cho SG, Bothos J, Goldberg JD, Enny C, Traina S, Balasubramanian S, Bandyopadhyay N, Sun S, Vermeulen J, Rizo A & Rule S 2016. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet, 387, 770–8. [DOI] [PubMed] [Google Scholar]
- Dreyling M, Thieblemont C, Gallamini A, Arcaini L, Campo E, Hermine O, Kluin-Nelemans JC, Ladetto M, Le Gouill S, Iannitto E, Pileri S, Rodriguez J, Schmitz N, Wotherspoon A, Zinzani P & Zucca E 2013. ESMO Consensus conferences: guidelines on malignant lymphoma. part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol, 24, 857–77. [DOI] [PubMed] [Google Scholar]
- Eskelund CW, Dahl C, Hansen JW, Westman M, Kolstad A, Pedersen LB, Montano-Almendras CP, Husby S, Freiburghaus C, Ek S, Pedersen A, Niemann C, Raty R, Brown P, Geisler CH, Andersen MK, Guldberg P, Jerkeman M & Gronbaek K 2017. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood, 130, 1903–1910. [DOI] [PubMed] [Google Scholar]
- Geisler CH, Kolstad A, Laurell A, Jerkeman M, Raty R, Andersen NS, Pedersen LB, Eriksson M, Nordstrom M, Kimby E, Bentzen H, Kuittinen O, Lauritzsen GF, Nilsson-Ehle H, Ralfkiaer E, Ehinger M, Sundstrom C, Delabie J, Karjalainen-Lindsberg ML, Brown P, Elonen E & Nordic Lymphoma G 2012. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol, 158, 355–62. [DOI] [PubMed] [Google Scholar]
- Hoster E, Dreyling M, Klapper W, Gisselbrecht C, Van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, Jung W, Wormann B, Ludwig WD, Duhrsen U, Eimermacher H, Wandt H, Hasford J, Hiddemann W, Unterhalt M, German Low Grade Lymphoma Study, G. & European Mantle Cell Lymphoma, N. 2008. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood, 111, 558–65. [DOI] [PubMed] [Google Scholar]
- Hoster E, Klapper W, Hermine O, Kluin-Nelemans HC, Walewski J, Van Hoof A, Trneny M, Geisler CH, Di Raimondo F, Szymczyk M, Stilgenbauer S, Thieblemont C, Hallek M, Forstpointner R, Pott C, Ribrag V, Doorduijn J, Hiddemann W, Dreyling MH & Unterhalt M 2014. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol, 32, 1338–46. [DOI] [PubMed] [Google Scholar]
- Hoster E, Rosenwald A, Berger F, Bernd HW, Hartmann S, Loddenkemper C, Barth TF, Brousse N, Pileri S, Rymkiewicz G, Kodet R, Stilgenbauer S, Forstpointner R, Thieblemont C, Hallek M, Coiffier B, Vehling-Kaiser U, Bouabdallah R, Kanz L, Pfreundschuh M, Schmidt C, Ribrag V, Hiddemann W, Unterhalt M, Kluin-Nelemans JC, Hermine O, Dreyling MH & Klapper W 2016. Prognostic Value of Ki-67 Index, Cytology, and Growth Pattern in Mantle-Cell Lymphoma: Results From Randomized Trials of the European Mantle Cell Lymphoma Network. J Clin Oncol, 34, 1386–94. [DOI] [PubMed] [Google Scholar]
- Katzenberger T, Petzoldt C, Holler S, Mader U, Kalla J, Adam P, Ott MM, Muller-Hermelink HK, Rosenwald A & Ott G 2006. The Ki67 proliferation index is a quantitative indicator of clinical risk in mantle cell lymphoma. Blood, 107, 3407. [DOI] [PubMed] [Google Scholar]
- Klapper W, Hoster E, Determann O, Oschlies I, Van Der Laak J, Berger F, Bernd HW, Cabecadas J, Campo E, Cogliatti S, Hansmann ML, Kluin PM, Kodet R, Krivolapov YA, Loddenkemper C, Stein H, Moller P, Barth TE, Muller-Hermelink K, Rosenwald A, Ott G, Pileri S, Ralfkiaer E, Rymkiewicz G, Van Krieken JH, Wacker HH, Unterhalt M, Hiddemann W, Dreyling M & European MCLN 2009. Ki-67 as a prognostic marker in mantle cell lymphoma-consensus guidelines of the pathology panel of the European MCL Network. J Hematop, 2, 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluin-Nelemans HC, Hoster E, Hermine O, Walewski J, Trneny M, Geisler CH, Stilgenbauer S, Thieblemont C, Vehling-Kaiser U, Doorduijn JK, Coiffier B, Forstpointner R, Tilly H, Kanz L, Feugier P, Szymczyk M, Hallek M, Kremers S, Lepeu G, Sanhes L, Zijlstra JM, Bouabdallah R, Lugtenburg PJ, Macro M, Pfreundschuh M, Prochazka V, Di Raimondo F, Ribrag V, Uppenkamp M, Andre M, Klapper W, Hiddemann W, Unterhalt M & Dreyling MH 2012. Treatment of older patients with mantle-cell lymphoma. N Engl J Med, 367, 520–31. [DOI] [PubMed] [Google Scholar]
- Kolstad A, Laurell A, Jerkeman M, Gronbaek K, Elonen E, Raty R, Pedersen LB, Loft A, Bogsrud TV, Kimby E, Hansen PB, Fagerli UM, Nilsson-Ehle H, Lauritzsen GF, Lehmann AK, Sundstrom C, Karjalainen-Lindsberg ML, Ralfkiaer E, Ehinger M, Delabie J, Bentzen H, Schildt J, Kostova-Aherdan K, Frederiksen H, Brown Pde N, Geisler CH & Nordic Lymphoma G 2014. Nordic MCL3 study: 90Y-ibritumomab-tiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood, 123, 2953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raty R, Franssila K, Joensuu H, Teerenhovi L & Elonen E 2002. Ki-67 expression level, histological subtype, and the International Prognostic Index as outcome predictors in mantle cell lymphoma. Eur J Haematol, 69, 11–20. [DOI] [PubMed] [Google Scholar]
- Roberts RA, Sabalos CM, Leblanc ML, Martel RR, Frutiger YM, Unger JM, Botros IW, Rounseville MP, Seligmann BE, Miller TP, Grogan TM & Rimsza LM 2007. Quantitative nuclease protection assay in paraffin-embedded tissue replicates prognostic microarray gene expression in diffuse large-B-cell lymphoma. Lab Invest, 87, 979–97. [DOI] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Wiestner A, Chan WC, Connors JM, Campo E, Gascoyne RD, Grogan TM, Muller-Hermelink HK, Smeland EB, Chiorazzi M, Giltnane JM, Hurt EM, Zhao H, Averett L, Henrickson S, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Montserrat E, Bosch F, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Fisher RI, Miller TP, Leblanc M, Ott G, Kvaloy S, Holte H, Delabie J & Staudt LM 2003. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell, 3, 185–97. [DOI] [PubMed] [Google Scholar]
- Sander B, De Jong D, Rosenwald A, Xie W, Balague O, Calaminici M, Carreras J, Gaulard P, Gribben J, Hagenbeek A, Kersten MJ, Molina TJ, Lee A, Montes-Moreno S, Ott G, Raemaekers J, Salles G, Sehn L, Thorns C, Wahlin BE, Gascoyne RD & Weller E 2014. The reliability of immunohistochemical analysis of the tumor microenvironment in follicular lymphoma: a validation study from the Lunenburg Lymphoma Biomarker Consortium. Haematologica, 99, 715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DW, Abrisqueta P, Wright GW, Slack GW, Mottok A, Villa D, Jares P, Rauert-Wunderlich H, Royo C, Clot G, Pinyol M, Boyle M, Chan FC, Braziel RM, Chan WC, Weisenburger DD, Cook JR, Greiner TC, Fu K, Ott G, Delabie J, Smeland EB, Holte H, Jaffe ES, Steidl C, Connors JM, Gascoyne RD, Rosenwald A, Staudt LM, Campo E, Rimsza LM & Lymphoma/Leukemia Molecular Profiling, P. 2017. New Molecular Assay for the Proliferation Signature in Mantle Cell Lymphoma Applicable to Formalin-Fixed Paraffin-Embedded Biopsies. J Clin Oncol, 35, 1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DW, Wright GW, Williams PM, Lih CJ, Walsh W, Jaffe ES, Rosenwald A, Campo E, Chan WC, Connors JM, Smeland EB, Mottok A, Braziel RM, Ott G, Delabie J, Tubbs RR, Cook JR, Weisenburger DD, Greiner TC, Glinsmann-Gibson BJ, Fu K, Staudt LM, Gascoyne RD & Rimsza LM 2014. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood, 123, 1214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann M, Schrader C, Klapper W, Dreyling MH, Campo E, Norton A, Berger F, Kluin P, Ott G, Pileri S, Pedrinis E, Feller AC, Merz H, Janssen D, Hansmann ML, Krieken H, Moller P, Stein H, Unterhalt M, Hiddemann W, Parwaresch R & European MCLN 2005. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL Network. Br J Haematol, 131, 29–38. [DOI] [PubMed] [Google Scholar]
- Trneny M, Lamy T, Walewski J, Belada D, Mayer J, Radford J, Jurczak W, Morschhauser F, Alexeeva J, Rule S, Afanasyev B, Kaplanov K, Thyss A, Kuzmin A, Voloshin S, Kuliczkowski K, Giza A, Milpied N, Stelitano C, Marks R, Trumper L, Biyukov T, Patturajan M, Bravo ML, Arcaini L, Investigators ST & In Collaboration with the European Mantle Cell Lymphoma, N. 2016. Lenalidomide versus investigator’s choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol, 17, 319–31. [DOI] [PubMed] [Google Scholar]
- Wang ML, Lee H, Chuang H, Wagner-Bartak N, Hagemeister F, Westin J, Fayad L, Samaniego F, Turturro F, Oki Y, Chen W, Badillo M, Nomie K, Dela Rosa M, Zhao D, Lam L, Addison A, Zhang H, Young KH, Li S, Santos D, Medeiros LJ, Champlin R, Romaguera J & Zhang L 2016. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol, 17, 48–56. [DOI] [PubMed] [Google Scholar]
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Li L, Zhang L, Newberry K, Ou Z, Cheng N, Fang B, Mcgreivy J, Clow F, Buggy JJ, Chang BY, Beaupre DM, Kunkel LA & Blum KA 2013. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med, 369, 507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Consort diagram. Selection of Norwegian MCL patients for the MCL35 assay