Abstract
The outcome of microbial infections in mammals, including humans, is affected by the age, sex, and reproductive status of the host suggesting a role for sex steroid hormones. Testosterone, estradiol, and progesterone, signaling through their respective steroid receptors, affect the functioning of immune cells to cause differential susceptibility to parasitic, bacterial, and viral infections. Microbes, including fungi, bacteria, parasites, and viruses, can also use sex steroid hormones and manipulate sex steroid receptor signaling mechanisms to increase their own survival and replication rate. The multifaceted use of sex steroid hormones by both microbes and hosts during infection forms the basis of this review. In the arms race between microbes and hosts, both hosts and microbes have evolved to utilize sex steroid hormone signaling mechanisms for survival.
Keywords: estrogen, influenza, malaria, parasites, progesterone, testosterone, toxoplasma
Graphic abstract

Differential susceptibility to microbial infection depends on the age, sex, and reproductive status of the host, among diverse species, including humans. For example, although younger people are more likely to be infected with microbes, older individuals tend to suffer a more severe outcome from several infectious diseases, including influenza (Fink and Klein, 2015). Sex differences in the outcome of infectious diseases are also commonly reported in humans, in which the prevalence (i.e., the number of infected individuals in a population) and intensity (i.e., microbial load within an individual) of infections tend to be higher in males than females (vom Steeg and Klein, 2016). The reproductive status of an individual, including puberty, pregnancy, and reproductive senescence, alter immune responses and the outcome of many infectious diseases, including Zika, which may involve changes in the concentrations of sex steroid hormones (Klein and Flanagan, 2016).
We and others hypothesize that differences in sex steroid hormone concentrations that occur with age, reproductive status, sex, and even gender-associated factors contribute significantly to infectious disease susceptibility (Klein and Roberts, 2010, 2015). Sex steroids, specifically testosterone, estrogens, and progesterone, occur in different concentrations between the sexes, with males typically having greater levels of testosterone and females often having greater levels of estrogen and progesterone at reproductive ages. Concentrations of sex steroids also differ between the sexes during perinatal development and during reproductive senescence, but not to the same extent as during the years between puberty and reproductive senescence. During reproductive senescence, concentrations of estradiol and progesterone fluctuate and then decline rapidly in women, whereas concentrations of testosterone decline gradually in men throughout adulthood. Finally, concentrations of estrogens and progesterone are at their highest during pregnancy, with third trimester concentrations being several fold higher in pregnant compared with non-pregnant females. From the perspective of the host, differences in the concentrations of sex steroid hormones over the life course and between the sexes can alter physiology, including immune function, to affect susceptibility to and the outcome of infectious diseases. Often overlooked is the fact that microbes can also utilize sex steroid hormones for their own survival and reproductive success.
The goal of this review will be to illustrate the complex interactions that occur between microbial infection and host sex steroid hormones and show that this is bidirectional, in which microbes can alter and utilize host hormones to facilitate growth and survival. Further, host immune responses that control the ability to contain and clear an infection can be affected by changes in the concentrations of sex steroid hormones.
Hormones affect host immune responses to microbes
Immune cells have sex steroid receptors.
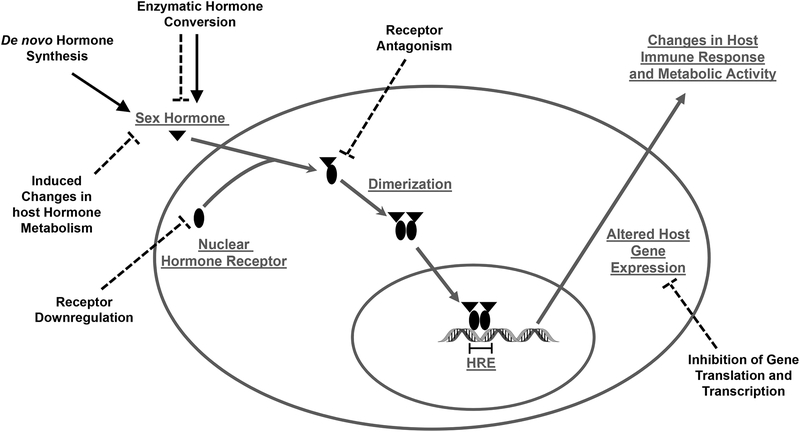
Sex steroids can influence the functioning of host immune cells by binding to specific receptors that are expressed in most immune cells, including lymphocytes, macrophages, and dendritic cells (Kovats et al., 2010). Sex steroids have a majority of their cellular effects by binding to receptors located in the cytoplasm. Once bound, the hormone-receptor complex translocates to the nucleus of the cell and binds to segments of DNA that contain specific hormone response elements (HREs) (Figure 1). The binding of sex steroids to their respective steroid receptors directly influences signaling pathways associated with the production of cytokines and chemokines (Kovats et al., 2010). Genes that encode for immunological proteins (e.g., interferon [IFN]-γ) can have HREs in their promoters allowing for sex hormone receptors to act as transcriptional factors directly altering gene expression (Fox et al., 1991). Non-classical sex steroid receptor signaling also occurs in immune cells, enabling protein-protein interactions between sex steroid receptors and HRE-independent transcription factors, including NF-κB, specific protein 1 (Sp1), and activator protein 1 (AP-1) (Kovats, 2015). The effects of sex steroids on immune responses to microbial infection have been most well characterized in the context of sex differences and the effects of pregnancy on the outcome of infection in mouse models. There are few studies that have considered how hormonal changes during puberty or reproductive senescence affect immune responses during microbial infection in mice, humans, or other animal models.
Figure 1.
Microbes can increase or inhibit sex steroid hormone signaling in mammalian hosts. Several pathogens, including Toxoplasma gondii and Clostridium scindens, can increase the synthesis of sex steroid hormones and conversion of sex steroids to metabolites (solid lines). In contrast, other microbes, including Schistosoma haematobium, can inhibit metabolic conversion of sex steroids, downregulate cytoplasmic steroid receptors, antagonize steroid receptors, and inhibit transcription and translation of host genes that contain hormone response elements (HRE) (stippled lines).
Sex steroids cause sex differences in host immune responses during microbial infections.
Sex steroids can have profound effects on immune responses during microbial infection. Studies of rodent models of infectious diseases reveal that males suffer a worse outcome following infection with Plasmodium spp.(Cernetich et al., 2006; Wunderlich et al., 1991), Leishmania spp. (Mock and Nacy, 1988; Satoskar et al., 1998), Brugia malayi (Rajan et al., 1994), Trichinella spiralis (Klein et al., 1999), and hepatitis B virus (HBV) (Tian et al., 2012), to name a few that have been studied extensively in the context of hormonal modulation of sex differences. In many of these rodent host-pathogen systems with a male-bias in the outcome of infection, castration of males improves, whereas exogenous administration of testosterone worsens, the outcome of infection (Cernetich et al., 2006; DeLoia et al., 1989; Farza et al., 1987; Mock and Nacy, 1988; Tian et al., 2012; Wunderlich et al., 1991).
Testosterone has immunomodulatory effects during infection. For example, during Plasmodium chabaudi infection, exposure of adult female mice to testosterone reduces antibody production, decreases major histocompatibility complex (MHC) class II cells in the spleen, and increases CD8+ T cells in the spleen making females more susceptible to an adverse outcome following infection (Benten et al., 1997). In other host-pathogen systems with a male-bias, estrogens are protective against development of severe disease in females. For example, female protection against Leishmania mexicana appears to be mediated by the effects of estrogens on increased synthesis of interferon (IFN)-γ and production of helper T cell type 1 (Th1) responses (Satoskar et al., 1998; Satoskar and Alexander, 1995).
Sex steroids mediate sex differences in susceptibility to chronic diseases caused by viral infection. For example, among men who test positive for the surface antigen of the HBV (HBsAg), elevated concentrations of testosterone and expression of certain androgen receptor (AR) gene alleles (SRD5A2 and V89L) correlate with increased risk of hepatocellular carcinoma (Yu et al., 2000; Yu et al., 2001). The development of chemically-induced hepatocellular carcinoma is delayed in androgen receptor (AR) male knockout mice as compared with wild-type male mice (Ma et al., 2008). In HBV transgenic mice, castration of males reduces, whereas replacement of testosterone or treatment with a testosterone agonist (i.e., R1881) in castrated males increases, serum HBsAg concentrations (DeLoia et al., 1989; Farza et al., 1987; Tian et al., 2012). Male HBV transgenic mice have higher concentrations of HBsAg than females after, but not before, puberty (Tian et al., 2012). The effect of androgens on HBV is mediated by the AR because male Tfm mice (i.e., mice with a mutation in the AR) do not show elevated concentrations of HBsAg as do wild-type males following HBV infection (Breidbart et al., 1993). The effects of sex steroids on immune responses are hypothesized to underlie sex and age associated differences in the outcome of HBV. For example, chemically-induced hepatocellular carcinoma is more severe in male than female mice, which is mediated by increased IL-6 production by Kupffer cells in the livers of male mice (Naugler et al., 2007). Estradiol reduces the synthesis of IL-6 by Kupffer cells through inhibition of Myd88-dependent induction of NF-κB (Naugler et al., 2007). Thus, sex steroids modulate sex differences in the prevalence of HBV and development of liver cancer partly through effects on host immune responses.
For other microbial infections, including Schistosoma mansoni (Eloi-Santos et al., 1992), Toxoplasma gondii (Walker et al., 1997), Taenia crassiceps (Larralde et al., 1995), and influenza A viruses (Lorenzo et al., 2011; Robinson et al., 2011), adult females suffer a worse outcome than males. For some of these microbes, including S. mansoni, administration of testosterone protects, whereas castration exacerbates worm burden and death in males following inoculation with S. mansoni parasites (Nakazawa et al., 1997). In mouse models of T. gondii, females develop more severe brain inflammation and are more likely to die following infection than males (Walker et al., 1997). Ovariectomy of female mice reduces, whereas administration of estradiol exacerbates, the development of tissue cysts caused by T. gondii infection (Liesenfeld et al., 2001; Pung and Luster, 1986). Male mice produce higher concentrations of tumor necrosis factor (TNF)-α, IL-12, and IFN-γ than females early during infection (Walker et al., 1997). Studies of mice infected with T. crassiceps reveal that females develop more cysticerci than males (Larralde et al., 1995). Estrogens favor, whereas androgens inhibit, T. crassiceps growth and development (Morales-Montor et al., 2002; Terrazas et al., 1998). Males develop higher Th1 responses, including elevated IFN-γ synthesis, whereas females exhibit heightened IL-10 production, during the early phase of infection (Terrazas et al., 1998). Because Th1 responses inhibit parasite growth, this is hypothesized to be the mechanism mediating reduced susceptibility to infection in males (Terrazas et al., 1998).
Females develop higher pulmonary inflammatory responses and experience a more severe outcome from influenza A virus infection than males (Hoffmann et al., 2015; Larcombe et al., 2011; Lorenzo et al., 2011; Robinson et al., 2011). Acute infection with influenza A viruses causes sickness in male and female mice, leading to a transient reduction in testosterone during the acute phase of infection and a more persistent reduction in circulating estradiol and progesterone and a loss of reproductive function in females (Robinson et al., 2011). In males, testosterone protects and castration exacerbates pulmonary inflammation during influenza A virus infection (Vom Steeg et al., in press). Treatment with either estradiol or progesterone protects females against infection-induced morbidity and mortality (Hall et al., in press; Nguyen et al., 2011; Pazos et al., 2012; Robinson et al., 2014; Robinson et al., 2011). Treatment of female mice with estradiol appears to protect against influenza A virus infection by dampening the inflammatory responses associated with tissue damage, including excessive production of IFN-γ, TNF-α, and CCL2, and by promoting higher antibody responses to influenza vaccination (Nguyen et al., 2011; Pazos et al., 2012; Robinson et al., 2014; Robinson et al., 2011). Some (Pazos et al., 2012), but not all (Robinson et al., 2014; Robinson et al., 2011), studies suggest that treatment of females with estradiol affects type I IFN responses and virus replication in the lungs. Progesterone, on the other hand, dampens pulmonary inflammation by upregulating regulatory and repair responses to infection, including production of amphiregulin, a growth factor produced by immune cells and epithelial cells (Hall et al., in press). Collectively, these data illustrate that sex differences in the outcome of microbial infection are at least partly mediated by the effects of sex steroid hormones on immune responses during infection. Furthermore, the anti-inflammatory effects of sex steroids protect both males and females from severe influenza outcome, highlighting that host inflammatory responses can be the cause of severe outcome from infectious diseases.
Sex steroids affect host immune responses to microbial infections during pregnancy.
The hormonal conditions associated with pregnancy also alter immune responses to microbial infection by reducing the activity of natural killer (NK) cells, inflammatory macrophages, and Th1 cells and production of inflammatory cytokines, while increasing the activity of regulatory T cells and production of anti-inflammatory cytokines (Robinson and Klein, 2012). For example, pregnant female mice are more susceptible to infection with T. gondii and experience worse disease outcome than non-pregnant females (Luft and Remington, 1982; Shirahata et al., 1992). The activity of NK and T cells as well as the production of IL-12, IFN-γ, and TNF-α during the early stages of infection are necessary for induction of adaptive immune responses and clearance of parasites (Roberts et al., 2001). Pregnant females produce significantly less IFN-γ than non-pregnant females during T. gondii infection (Luft and Remington, 1982; Shirahata et al., 1992). Administration of recombinant IFN-γ to pregnant female mice improves the outcome of T. gondii infection and can reduce congenital transmission of parasites (Abou-Bacar et al., 2004a; Abou-Bacar et al., 2004b; Shirahata et al., 1992) but can also directly harm the developing fetus (Pfaff et al., 2007). There is growing evidence that hormones underlie increased susceptibility of pregnant females to toxoplasmosis. In female mice, estradiol exacerbates, whereas gonadectomy reduces, parasite burden and disease pathogenesis (Kittas and Henry, 1979, 1980). High concentrations of progesterone also increase susceptibility to T. gondii during pregnancy by suppressing production of IL-12 and IFN-γ (Jones et al., 2008).
Pregnancy-associated changes in cell-mediated immune responses and increased susceptibility to Plasmodium infections have been attributed to hormonal changes that occur during pregnancy (Rogerson et al., 2007). Studies of women in malaria endemic regions, as well as mouse models of Plasmodium berghei, reveal that concentrations of glucocorticoids (i.e., cortisol in human and nonhuman primates and corticosterone in rodents) are higher in pregnant females infected with malaria parasites than in uninfected pregnant females (Bayoumi et al., 2009; Van Zon et al., 1983; Van Zon et al., 1986; Vleugels et al., 1989; Vleugels et al., 1987). Elevated glucocorticoids increase, while adrenalectomy decreases, parasitemia in pregnant female mice which may be caused by glucocorticoid-induced suppression of inflammatory responses (Van Zon et al., 1982). Prolactin concentrations are either reported to not change with malaria infection during pregnancy (Bouyou-Akotet et al., 2005) or to be lower in P. falciparum infected than uninfected pregnant females (Bayoumi et al., 2009). Malaria infection also reduces estradiol concentrations in late pregnancy (Watkinson et al., 1985). More research is required to fully understand how changes in pregnancy-associated hormones modulate immune responses and the outcome of infection, but these data provide proof-of-principle examples of how sex steroid hormone changes during pregnancy alter the outcome of microbial infections, at least in mice.
Microbes manipulate host sex steroids for growth, survival, and transmission
In the biomedical sciences, we rarely consider that microbes, both commensal and pathogenic, have evolved multiple mechanisms to manipulate host hormone production and receptor signaling to promote their growth, survival, and transmission (Figure 1). In addition to effects on host immune responses during infection, sex steroids can directly affect and even be utilized by microbes.
Commensal microbes alter sex steroids.
The microbiome consists of the microbial communities that exist within the skin, gut, lungs, oral cavity, and genitals of vertebrate species. Significant interplay exists between the mammalian microbiome and the hormonal milieu, and this interaction influences age and sex differences in immune function and the pathogenesis of diseases (Belkaid and Hand, 2014; Markle et al., 2013; Yurkovetskiy et al., 2013). Sex steroid hormones can influence the composition of the microbiome outside of the reproductive tract. Indirect evidence comes from the observation that sex differences in the composition of the gut microbiome are only observed after puberty, at least in mice (Markle et al., 2013; Steegenga et al., 2014). Direct evidence comes from studies illustrating that adoptive transfer of gut commensals from male to female mice causes systemic changes in hormone levels (i.e., increased concentrations of androgens in females) and changes in the pathogenesis of autoimmune diseases (e.g., type 1 diabetes) (Markle et al., 2013).
Disruption of the host microbiota-hormonal balance, can be advantageous for infectious pathogens. In mice, infection with Salmonella enterica serovar Typhimurium, but not other enteric pathogens including Escherichia coli or Vibrio cholera (Huang et al., 1980 & Huang et al., 1982), disrupts host steroid metabolism (Antunes et al., 2011) and reduces inflammatory responses, allowing S. enterica to overcome the commensal microbiome and establish infection (Stecher et al., 2007). More studies of microbiome-sex steroid interactions are required to fully evaluate how these interactions alter and are altered by pathogenic infections and inflammatory diseases, including autoimmunity.
Microbes synthesize and metabolize sex steroids.
Several members of the parasite genus Schistosoma can synthesize estrogenic compounds and also express estrogen receptors suggesting that these parasites can directly respond to circulating estrogens (Botelho et al., 2009; Escobedo et al., 2010). Estrogenic signaling in the host has deleterious consequences on Schistosoma haematobium by limiting parasite replication through effects on host immune responses (see above). To counteract this, S. haematobium is capable of producing an estrogenic compound that antagonizes both estrogen receptor signaling and expression (Botelho et al., 2010).
Microbes are also capable of enzymatically altering host sex steroid concentrations. Female mice are more susceptible to infection with Taenia crassiceps than males in part because estradiol enhances parasite reproduction (Larralde et al., 1995). In male rodents, T. crassiceps can enzymatically reduce both serum and testicular testosterone concentrations while increasing estradiol concentrations to promote its own reproduction (Escobedo et al., 2010). In humans, pathogenic periodontal bacteria can enzymatically convert testosterone to dihydrotestosterone (DHT) and 4-androstenedione (Soory, 1995), while the Clostridium scindens, a component of the human microbiome is capable of converting glucocorticoids to androgens (Ridlon et al., 2013). Whether enzymatic conversion of steroid hormones by microbes is conserved across several classes of pathogens has not been adequately addressed.
In men, HIV infection causes hypogonadism (i.e., reduced androgen concentrations), which is associated with wasting syndrome, loss of bone mass, and depression (Grinspoon, 2005). In parallel with reduced androgen concentrations, estrone and estradiol concentrations increase with the progression of HIV (Christeff et al., 1996; Teichmann et al., 2003). Consequently, estradiol augments transcription of HIV in vitro and this effect can be reversed by exposure to the estrogen receptor antagonist ICI 182,780 (Katagiri et al., 2006).
Microbes use sex steroids for growth and survival.
Microbes can respond to host sex steroids and their metabolites to regulate their growth and survival. The genome of human papillomavirus (HPV) high-risk type 16 and 18 contains a progesterone response element (PRE). When progesterone activates the PRE, this regulates part of the HPV life cycle and transformation process, which may explain the higher frequency of malignant HPV lesions in females compared with males (Chan et al., 1989). One mechanism by which androgens affect HBV replication is through direct binding to androgen response elements that have been identified in the enhancer I of HBV (Wang et al., 2009).
Host sex hormones can also regulate microbial lifecycle progression. Candida albicans contains an estrogen binding protein that has a high affinity for estradiol, which can stimulate transition of the yeast into a hyphal form that may increase fungal virulence (Madani et al., 1994). The opposite has been shown for the pathogenic dimorphic fungi Paracoccidioides, which expresses a cytosolic protein capable of binding estrogen (Loose et al., 1983). The binding of estradiol to this protein receptor blocks the transition to the parasitic yeast form and may in part explain the male bias of disease (Shankar et al., 2011a; Shankar et al., 2011b). T. crassiceps expresses both estrogen and androgen receptors, with testosterone treatment inhibiting, and estrogen treatment enhancing, reproduction (Escobedo et al., 2004).
Bacteria are capable of sensing host sex hormones and subsequently expressing enzymes allowing their use as substrates to fulfill growth and metabolic requirements. The soil bacterium Comamonas testosteroni is an extreme example of this, and in vitro, the bacterium is capable of growth on media containing only testosterone as a carbon source (Gohler et al., 2008; Horinouchi et al., 2012). Bacterial species capable of estrogen metabolism have also been identified in both the environment and as components of the human microbiome (Yu et al., 2013). Given the potential for these bacteria to respond to and modify host sex steroids levels, their role in mediating age and sex related changes in sex steroid levels as a component of the microbiome, warrants further study.
In vitro treatment of P. faliciparum with testosterone, progesterone, or estradiol results in an increased number of gametocytes (i.e., the sexual stage of the parasite life cycle) in human blood, without changing overall levels of parasitemia (i.e., numbers of parasites in blood samples) (Lingnau et al., 1993; Remoue et al., 2002). Sex hormones can also act directly on parasites to inhibit parasite reproduction. Testosterone can bind to the glutathione S-transferase of S. haematobium, inhibiting its metabolism and reproductive success. This may in part explain why male mice have lower levels of parasitemia than females.
Microbial tissue-tropisms involve sex steroids.
Sex steroid hormones can influence microbial tissue specificity. Synthetic progesterone analogs (i.e., progestins) differentially influence the germination of spores from Clostridium difficile and Clostridium sordellii (Liggins et al., 2011). For C. sordellii, a bacterium commonly associated with soft tissue infections of the female reproductive tract following pregnancy, treatment of spores with progestins significantly increased the rate of germination. The opposite is observed for C. difficile, an intestinal microbe capable of causing severe diarrheal and intestinal disease, were sporulation is inhibited by the presence of progestins (Liggins et al., 2011). Sporulation of C. difficile is caused by the presence of bile salts, such as taurocholate (Paredes-Sabja et al., 2014), with progestins likely competing with taurocholate for the purported germination receptor (Liggins et al., 2011).
Microbes use sex steroids for transmission.
Microbial manipulation of host hormones can promote pathogen transmission. Infection of male rats with Toxoplasma gondii can elevate testosterone through the upregulation of luteinizing hormone receptor and other genes involved in testosterone synthesis (Lim et al., 2013). The resulting increase in testosterone leads to enhanced expression of sexually selected traits and attractiveness of infected males (Dass et al., 2011), while potentiating sexual transmission (Vyas, 2013). Upregulation of testosterone production following T. gondii infection may also enhance trophic transmission through decreased predator avoidance behavior (Hari Dass and Vyas, 2014). These effects, however, may be species specific as T. gondii infection in mice resulted in decreased testosterone levels (Kankova et al., 2011) and fails to alter predator avoidance or reproductive potential (Soh et al., 2013). How pathogens utilize and even produce sex steroids to promote transmission differentially between the sexes or during different reproductive states requires consideration.
Conclusions
The significance of sex steroid hormones for the survival and reproductive success of vertebrate host species is well established. These hormones modulate diverse physiological functions ranging from reproduction to metabolism and immune function. Their role in immune function, specifically, directly affects the outcome of infectious diseases (reviewed here) as well autoimmune diseases and cancers (Klein and Flanagan, 2016). Sex steroid hormones are derived from cholesterol, which is also commonly used for survival by microbes. In this review, we have consolidated diverse evidence that sex steroid hormones can be altered, metabolized, and utilized by microbes for survival and reproduction. This point has been overlooked in the biomedical sciences, but deserves greater consideration.
The contents of this review should provide the impetus for future studies to consider that microbial infections alter the host hormonal milieu which impacts both host recovery from infection as well as transmission and reproduction of the microbes. Future studies should continue to explore whether microbial utilization of sex steroids is conserved across diverse microbial species, beyond the ones discussed in this review. The observation that sex steroid hormones are utilized by microbes for fundamental processes required for survival (e.g., metabolism, reproduction, and transmission), suggests that these processes are evolutionarily well conserved. How the sex, age, and reproductive status of the host affects microbial utilization of sex steroid hormones represents a novel area of investigation. The co-evolutionary arms race between hosts and microbes results in the evolution of counter-adaptations as each seeks to survive the other’s defenses. The concept that sex steroids, being utilized by both hosts and microbes, have evolved to serve dual roles is intriguing and reminds us that there is still much we need to learn about how evolution shapes our interactions with the microbial environment.
References
- Abou-Bacar A, Pfaff AW, Georges S, Letscher-Bru V, Filisetti D, Villard O, Antoni E, Klein JP, Candolfi E, 2004a. Role of NK cells and gamma interferon in transplacental passage of Toxoplasma gondii in a mouse model of primary infection. Infect Immun 72, 1397–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Bacar A, Pfaff AW, Letscher-Bru V, Filisetti D, Rajapakse R, Antoni E, Villard O, Klein JP, Candolfi E, 2004b. Role of gamma interferon and T cells in congenital Toxoplasma transmission. Parasite Immunol 26, 315–318. [DOI] [PubMed] [Google Scholar]
- Antunes LC, Andersen SK, Menendez A, Arena ET, Han J, Ferreira RB, Borchers CH, Finlay BB, 2011. Metabolomics reveals phospholipids as important nutrient sources during Salmonella growth in bile in vitro and in vivo. J Bacteriol 193, 4719–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoumi NK, Elhassan EM, Elbashir MI, Adam I, 2009. Cortisol, prolactin, cytokines and the susceptibility of pregnant Sudanese women to Plasmodium falciparum malaria. Ann Trop Med Parasitol 103, 111–117. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW, 2014. Role of the microbiota in immunity and inflammation. Cell 157, 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benten WP, Ulrich P, Kuhn-Velten WN, Vohr HW, Wunderlich F, 1997. Testosterone-induced susceptibility to Plasmodium chabaudi malaria: persistence after withdrawal of testosterone. J Endocrinol 153, 275–281. [DOI] [PubMed] [Google Scholar]
- Botelho MC, Crespo M, Almeida A, Vieira P, Delgado ML, Araujo L, Machado JC, Correia da Costa JM, 2009. Schistosoma haematobium and Schistosomiasis mansoni: production of an estradiol-related compound detected by ELISA. Exp Parasitol 122, 250–253. [DOI] [PubMed] [Google Scholar]
- Botelho MC, Soares R, Vale N, Ribeiro R, Camilo V, Almeida R, Medeiros R, Gomes P, Machado JC, Correia da Costa JM, 2010. Schistosoma haematobium: identification of new estrogenic molecules with estradiol antagonistic activity and ability to inactivate estrogen receptor in mammalian cells. Exp Parasitol 126, 526–535. [DOI] [PubMed] [Google Scholar]
- Bouyou-Akotet MK, Adegnika AA, Agnandji ST, Ngou-Milama E, Kombila M, Kremsner PG, Mavoungou E, 2005. Cortisol and susceptibility to malaria during pregnancy. Microbes Infect 7, 1217–1223. [DOI] [PubMed] [Google Scholar]
- Breidbart S, Burk RD, Saenger P, 1993. Hormonal regulation of hepatitis B virus gene expression: influence of androgen receptor. Pediatr Res 34, 300–302. [DOI] [PubMed] [Google Scholar]
- Cernetich A, Garver LS, Jedlicka AE, Klein PW, Kumar N, Scott AL, Klein SL, 2006. Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect Immun 74, 3190–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WK, Klock G, Bernard HU, 1989. Progesterone and glucocorticoid response elements occur in the long control regions of several human papillomaviruses involved in anogenital neoplasia. J Virol 63, 3261–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christeff N, Lortholary O, Casassus P, Thobie N, Veyssier P, Torri O, Guillevin L, Nunez EA, 1996. Relationship between sex steroid hormone levels and CD4 lymphocytes in HIV infected men. Exp Clin Endocrinol Diabetes 104, 130–136. [DOI] [PubMed] [Google Scholar]
- Dass SA, Vasudevan A, Dutta D, Soh LJ, Sapolsky RM, Vyas A, 2011. Protozoan parasite Toxoplasma gondii manipulates mate choice in rats by enhancing attractiveness of males. PLoS One 6, e27229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoia JA, Burk RD, Gearhart JD, 1989. Developmental regulation of hepatitis B surface antigen expression in two lines of hepatitis B virus transgenic mice. J Virol 63, 4069–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloi-Santos S, Olsen NJ, Correa-Oliveira R, Colley DG, 1992. Schistosoma mansoni: mortality, pathophysiology, and susceptibility differences in male and female mice. Exp Parasitol 75, 168–175. [DOI] [PubMed] [Google Scholar]
- Escobedo G, De Leon MA, Morales-Montor J, 2010. Sex differences in parasitic infections: Beyond the dogma of female-biased resistance, in: Roberts, S.L.K.a.C.W. (Ed.), Sex Hormones and Immunity to Infection. Springer-Verlag, Berlin. [Google Scholar]
- Escobedo G, Larralde C, Chavarria A, Cerbon MA, Morales-Montor J, 2004. Molecular mechanisms involved in the differential effects of sex steroids on the reproduction and infectivity of Taenia crassiceps. J Parasitol 90, 1235–1244. [DOI] [PubMed] [Google Scholar]
- Farza H, Salmon AM, Hadchouel M, Moreau JL, Babinet C, Tiollais P, Pourcel C, 1987. Hepatitis B surface antigen gene expression is regulated by sex steroids and glucocorticoids in transgenic mice. Proc Natl Acad Sci U S A 84, 1187–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AL, Klein SL, 2015. Sex and Gender Impact Immune Responses to Vaccines Among the Elderly. Physiology (Bethesda) 30, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HS, Bond BL, Parslow TG, 1991. Estrogen regulates the IFN-gamma promoter. J Immunol 146, 4362–4367. [PubMed] [Google Scholar]
- Gohler A, Xiong G, Paulsen S, Trentmann G, Maser E, 2008. Testosterone-inducible regulator is a kinase that drives steroid sensing and metabolism in Comamonas testosteroni. J Biol Chem 283, 17380–17390. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, 2005. Androgen deficiency and HIV infection. Clin Infect Dis 41, 1804–1805. [DOI] [PubMed] [Google Scholar]
- Hall OJ, Limjunyawong N, Vermillion MS, Wohlgemuth N, Robinson DP, Pekosz A, Mitzner W, Klein SL, in press. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari Dass SA, Vyas A, 2014. Toxoplasma gondii infection reduces predator aversion in rats through epigenetic modulation in the host medial amygdala. Mol Ecol 23, 6114–6122. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Otte A, Thiele S, Lotter H, Shu Y, Gabriel G, 2015. Sex differences in H7N9 influenza A virus pathogenesis. Vaccine. [DOI] [PubMed] [Google Scholar]
- Horinouchi M, Hayashi T, Kudo T, 2012. Steroid degradation in Comamonas testosteroni. J Steroid Biochem Mol Biol 129, 4–14. [DOI] [PubMed] [Google Scholar]
- Jones LA, Anthony JP, Henriquez FL, Lyons RE, Nickdel MB, Carter KC, Alexander J, Roberts CW, 2008. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology 125, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankova S, Kodym P, Flegr J, 2011. Direct evidence of Toxoplasma-induced changes in serum testosterone in mice. Exp Parasitol 128, 181–183. [DOI] [PubMed] [Google Scholar]
- Katagiri D, Hayashi H, Victoriano AF, Okamoto T, Onozaki K, 2006. Estrogen stimulates transcription of human immunodeficiency virus type 1 (HIV-1). Int Immunopharmacol 6, 170–181. [DOI] [PubMed] [Google Scholar]
- Kittas C, Henry L, 1979. Effect of gonadectomy and oestrogen administration on the response of lymph-node post-capillary venules to infection with Toxoplasma gondii. J Pathol 127, 129–136. [DOI] [PubMed] [Google Scholar]
- Kittas C, Henry L, 1980. Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. Br J Exp Pathol 61, 590–600. [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL, 2016. Sex differences in immune responses. Nat Rev Immunol. [DOI] [PubMed] [Google Scholar]
- Klein SL, Gamble HR, Nelson RJ, 1999. Role of steroid hormones in Trichinella spiralis infection among voles. Am J Physiol 277, R1362–1367. [DOI] [PubMed] [Google Scholar]
- Klein SL, Roberts CW, 2010. Sex hormones and immunity to infection. Springer; Verlag, Berlin. [Google Scholar]
- Klein SL, Roberts CW, 2015. Sex and gender differences in infection and treatments for infectious diseases. Springer International Publishing, Switzerland. [Google Scholar]
- Kovats S, 2015. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats S, Carreras E, Agrawal H, 2010. Sex steroid receptors in immune cells, in: Klein SL, Roberts CW (Eds.), Sex hormones and immunity to infection. Springer-Verlag, Berlin, pp. 53–92. [Google Scholar]
- Larcombe AN, Foong RE, Bozanich EM, Berry LJ, Garratt LW, Gualano RC, Jones JE, Dousha LF, Zosky GR, Sly PD, 2011. Sexual dimorphism in lung function responses to acute influenza A infection. Influenza Other Respir Viruses 5, 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larralde C, Morales J, Terrazas I, Govezensky T, Romano MC, 1995. Sex hormone changes induced by the parasite lead to feminization of the male host in murine Taenia crassiceps cysticercosis. J Steroid Biochem Mol Biol 52, 575–580. [DOI] [PubMed] [Google Scholar]
- Liesenfeld O, Nguyen TA, Pharke C, Suzuki Y, 2001. Importance of gender and sex hormones in regulation of susceptibility of the small intestine to peroral infection with Toxoplasma gondii tissue cysts. J Parasitol 87, 1491–1493. [DOI] [PubMed] [Google Scholar]
- Liggins M, Ramirez N, Magnuson N, Abel-Santos E, 2011. Progesterone analogs influence germination of Clostridium sordellii and Clostridium difficile spores in vitro. J Bacteriol 193, 2776–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim A, Kumar V, Hari Dass SA, Vyas A, 2013. Toxoplasma gondii infection enhances testicular steroidogenesis in rats. Mol Ecol 22, 102–110. [DOI] [PubMed] [Google Scholar]
- Lingnau A, Margos G, Maier WA, Seitz HM, 1993. The effects of hormones on the gametocytogenesis of Plasmodium falciparum in vitro. Appl Parasitol 34, 153–160. [PubMed] [Google Scholar]
- Loose DS, Stover EP, Restrepo A, Stevens DA, Feldman D, 1983. Estradiol binds to a receptor-like cytosol binding protein and initiates a biological response in Paracoccidioides brasiliensis. Proc Natl Acad Sci U S A 80, 7659–7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo ME, Hodgson A, Robinson DP, Kaplan JB, Pekosz A, Klein SL, 2011. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine 29, 9246–9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft BJ, Remington JS, 1982. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect Immun 38, 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai JJ, Jou YS, Chen CW, Yeh S, Chang C, 2008. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology 135, 947–955, 955 e941–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani ND, Malloy PJ, Rodriguez-Pombo P, Krishnan AV, Feldman D, 1994. Candida albicans estrogen-binding protein gene encodes an oxidoreductase that is inhibited by estradiol. Proc Natl Acad Sci U S A 91, 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS, 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088. [DOI] [PubMed] [Google Scholar]
- Mock BA, Nacy CA, 1988. Hormonal modulation of sex differences in resistance to Leishmania major systemic infections. Infect Immun 56, 3316–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Montor J, Baig S, Hallal-Calleros C, Damian RT, 2002. Taenia crassiceps: androgen reconstitution of the host leads to protection during cysticercosis. Exp Parasitol 100, 209–216. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Fantappie MR, Freeman GL Jr., Eloi-Santos S, Olsen NJ, Kovacs WJ, Secor WE, Colley DG, 1997. Schistosoma mansoni: susceptibility differences between male and female mice can be mediated by testosterone during early infection. Exp Parasitol 85, 233–240. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M, 2007. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317, 121–124. [DOI] [PubMed] [Google Scholar]
- Nguyen DC, Masseoud F, Lu X, Scinicariello F, Sambhara S, Attanasio R, 2011. 17beta-Estradiol restores antibody responses to an influenza vaccine in a postmenopausal mouse model. Vaccine 29, 2515–2518. [DOI] [PubMed] [Google Scholar]
- Paredes-Sabja D, Shen A, Sorg JA, 2014. Clostridium difficile spore biology: sporulation, germination, and spore structural proteins. Trends Microbiol 22, 406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos MA, Kraus TA, Munoz-Fontela C, Moran TM, 2012. Estrogen mediates innate and adaptive immune alterations to influenza infection in pregnant mice. PLoS ONE 7, e40502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff AW, Abou-Bacar A, Letscher-Bru V, Villard O, Senegas A, Mousli M, Candolfi E, 2007. Cellular and molecular physiopathology of congenital toxoplasmosis: the dual role of IFN-gamma. Parasitology 134, 1895–1902. [DOI] [PubMed] [Google Scholar]
- Pung OJ, Luster MI, 1986. Toxoplasma gondii: decreased resistance to infection in mice due to estrogen. Exp Parasitol 61, 48–56. [DOI] [PubMed] [Google Scholar]
- Rajan TV, Nelson FK, Shultz LD, Shultz KL, Beamer WG, Yates J, Greiner DL, 1994. Influence of gonadal steroids on susceptibility to Brugia malayi in scid mice. Acta Trop 56, 307–314. [DOI] [PubMed] [Google Scholar]
- Remoue F, Mani JC, Pugniere M, Schacht AM, Capron A, Riveau G, 2002. Functional specific binding of testosterone to Schistosoma haematobium 28-kilodalton glutathione S-transferase. Infect Immun 70, 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridlon JM, Ikegawa S, Alves JM, Zhou B, Kobayashi A, Iida T, Mitamura K, Tanabe G, Serrano M, De Guzman A, Cooper P, Buck GA, Hylemon PB, 2013. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res 54, 2437–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Walker W, Alexander J, 2001. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev 14, 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL, 2014. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DP, Klein SL, 2012. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 62, 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DP, Lorenzo ME, Jian W, Klein SL, 2011. Elevated 17beta-estradiol protects females from influenza a virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7, e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW, 2007. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis 7, 105–117. [DOI] [PubMed] [Google Scholar]
- Satoskar A, Al-Quassi HH, Alexander J, 1998. Sex-determined resistance against Leishmania mexicana is associated with the preferential induction of a Th1-like response and IFN-gamma production by female but not male DBA/2 mice. Immunol Cell Biol 76, 159–166. [DOI] [PubMed] [Google Scholar]
- Satoskar A, Alexander J, 1995. Sex-determined susceptibility and differential IFN-gamma and TNF-alpha mRNA expression in DBA/2 mice infected with Leishmania mexicana. Immunology 84, 1–4. [PMC free article] [PubMed] [Google Scholar]
- Shankar J, Restrepo A, Clemons KV, Stevens DA, 2011a. Hormones and the resistance of women to paracoccidioidomycosis. Clin Microbiol Rev 24, 296–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar J, Wu TD, Clemons KV, Monteiro JP, Mirels LF, Stevens DA, 2011b. Influence of 17beta-estradiol on gene expression of Paracoccidioides during mycelia-to-yeast transition. PLoS One 6, e28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata T, Muroya N, Ohta C, Goto H, Nakane A, 1992. Correlation between increased susceptibility to primary Toxoplasma gondii infection and depressed production of gamma interferon in pregnant mice. Microbiol Immunol 36, 81–91. [DOI] [PubMed] [Google Scholar]
- Soh LJ, Vasudevan A, Vyas A, 2013. Infection with Toxoplasma gondii does not elicit predator aversion in male mice nor increase their attractiveness in terms of mate choice. Parasitol Res 112, 3373–3378. [DOI] [PubMed] [Google Scholar]
- Soory M, 1995. Bacterial steroidogenesis by periodontal pathogens and the effect of bacterial enzymes on steroid conversions by human gingival fibroblasts in culture. J Periodontal Res 30, 124–131. [DOI] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD, 2007. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5, 2177–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegenga WT, Mischke M, Lute C, Boekschoten MV, Pruis MG, Lendvai A, Verkade HJ, Boekhorst J, Timmerman HM, Plosch T, Muller M, 2014. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol Sex Differ 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann J, Schmidt A, Lange U, Stracke H, Discher T, Friese G, Lohmeyer J, Bretzel RG, 2003. Longitudinal evaluation of serum estradiol and estrone in male patients infected with the human immunodeficiency virus. Eur J Med Res 8, 77–80. [PubMed] [Google Scholar]
- Terrazas LI, Bojalil R, Govezensky T, Larralde C, 1998. Shift from an early protective Th1-type immune response to a late permissive Th2-type response in murine cysticercosis (Taenia crassiceps). J Parasitol 84, 74–81. [PubMed] [Google Scholar]
- Tian Y, Kuo CF, Chen WL, Ou JH, 2012. Enhancement of hepatitis B virus replication by androgen and its receptor in mice. J Virol 86, 1904–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zon AA, Eling WM, Hermsen CC, Koekkoek AA, 1982. Corticosterone regulation of the effector function of malarial immunity during pregnancy. Infect Immun 36, 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zon AA, Eling WM, Hermsen CC, Van de Wiel TJ, Duives ME, 1983. Malarial immunity in pregnant mice, in relation to total and unbound plasma corticosterone. Bull Soc Pathol Exot Filiales 76, 493–502. [PubMed] [Google Scholar]
- Van Zon AA, Termaat RM, Schetters TP, Eling WM, 1986. Plasmodium berghei: reduction of the mouse’s specific lymphoproliferative response in relation to corticosterone and pregnancy. Exp Parasitol 62, 71–78. [DOI] [PubMed] [Google Scholar]
- Vleugels MP, Brabin B, Eling WM, de Graaf R, 1989. Cortisol and Plasmodium falciparum infection in pregnant women in Kenya. Trans R Soc Trop Med Hyg 83, 173–177. [DOI] [PubMed] [Google Scholar]
- Vleugels MP, Eling WM, Rolland R, de Graaf R, 1987. Cortisol and loss of malaria immunity in human pregnancy. Br J Obstet Gynaecol 94, 758–764. [DOI] [PubMed] [Google Scholar]
- vom Steeg LG, Klein SL, 2016. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog 12, e1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, Zirkin BR, Klein SL, in press. Age and testisterone mediate influenza pathogenesis in male mice. American Journal of Physiology Lung Cellular and Molecular Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, 2013. Parasite-augmented mate choice and reduction in innate fear in rats infected by Toxoplasma gondii. J Exp Biol 216, 120–126. [DOI] [PubMed] [Google Scholar]
- Walker W, Roberts CW, Ferguson DJ, Jebbari H, Alexander J, 1997. Innate immunity to Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production. Infect Immun 65, 1119–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Yeh SH, Lin WH, Wang HY, Chen DS, Chen PJ, 2009. Identification of androgen response elements in the enhancer I of hepatitis B virus: a mechanism for sex disparity in chronic hepatitis B. Hepatology 50, 1392–1402. [DOI] [PubMed] [Google Scholar]
- Watkinson M, Rushton DI, Lunn PG, 1985. Placental malaria and foetoplacental function: low plasma oestradiols associated with malarial pigmentation of the placenta. Trans R Soc Trop Med Hyg 79, 448–450. [DOI] [PubMed] [Google Scholar]
- Wunderlich F, Marinovski P, Benten WP, Schmitt-Wrede HP, Mossmann H, 1991. Testosterone and other gonadal factor(s) restrict the efficacy of genes controlling resistance to Plasmodium chabaudi malaria. Parasite Immunol 13, 357–367. [DOI] [PubMed] [Google Scholar]
- Yu CP, Deeb RA, Chu KH, 2013. Microbial degradation of steroidal estrogens. Chemosphere 91, 1225–1235. [DOI] [PubMed] [Google Scholar]
- Yu MW, Cheng SW, Lin MW, Yang SY, Liaw YF, Chang HC, Hsiao TJ, Lin SM, Lee SD, Chen PJ, Liu CJ, Chen CJ, 2000. Androgen-receptor gene CAG repeats, plasma testosterone levels, and risk of hepatitis B-related hepatocellular carcinoma. J Natl Cancer Inst 92, 2023–2028. [DOI] [PubMed] [Google Scholar]
- Yu MW, Yang YC, Yang SY, Cheng SW, Liaw YF, Lin SM, Chen CJ, 2001. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst 93, 1644–1651. [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV, 2013. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]