Abstract
Background:
Tumor necrosis factor alpha-induced protein 3 (TNFAIP3) is a multifunctional ubiquitin binding and editing enzyme that regulates inflammation. Genetic studies have implicated polymorphisms within the TNFAIP3 locus to the development of numerous immune-related diseases. This study evaluated the frequencies of single-nucleotide polymorphism (SNPs) within the exonic regions of the TNFAIP3 gene and an associated point mutation from the Illumina array among a predominantly Hispanic cohort.
Methods:
Genomic DNA was obtained from 721 participants and sequencing of all TNFAIP3 exons and an intergenic point mutation (rs6920220) was performed. In-vitro functional assessment was performed by transfecting mutated TNFAIP3 constructs into TNFAIP3 knockout cells containing the NF-kB luciferase reporter and stimulating with TNFα. Comparative statistics were performed with Student’s t-test for continuous variables and chi-squared test for categorical variables.
Results:
Sequencing revealed two missense SNPs, rs146534657:A>G and rs2230926:T>G, both within exon 3 of TNFAIP3, which encodes the protein’s deubiquitinating enzymatic domain. Frequencies of all three point mutations differed significantly across racial groups (χ2-test, P = 0.014 to P < 0.001). Compared to Caucasians, rs146534657:A>G was overrepresented among Hispanics (odds ratio (OR) [95% CI] 4.05 [1.24−13.18]), and rs2230926:T>G was more prevalent among African-Americans (OR [95% CI] 3.65 [1.58−8.43]). In-vitro assays confirm rs146534657:A>G and rs2230926:T>G decrease the ability of TNFAIP3 to abrogate NF-κB activation by 2-fold (P < 0.01) and 1.7-fold (P < 0.01), respectively.
Conclusions:
This study reports the frequency of rs146534657:A>G among Hispanics and is the first to evaluate its potential physiologic impact, establishing a basis for future research as a potential biomarker among this population.
Keywords: TNFAIP3, A20, Hispanic, SNP, autoimmune, NF-kB, RA, IBD
Introduction
The protein TNF-alpha-induced protein 3 (TNFAIP3, also known as A20) is a potent suppressor of inflammatory responses [1]. Structurally, it contains an amino-terminal ovarian tumor-like unit (OTU) domain capable of deubiquitinating (DUB) activity, while the fourth carboxyl-terminal zinc finger (ZnF) domain contains its E3 ubiquitin ligase activity [2]. The seventh ZnF has recently been shown to bind to linear ubiquitin motifs, which is important for its regulatory function downstream of TNF-receptor signaling (Fig. 1) [3,4]. These functions allow A20 to abrogate inflammation through modifying the ubiquitination of specific proteins in the signaling pathways of TNFα [5], toll-like receptor (TLR) [6], nucleotide-binding oligomerization domain-containing protein 2 (NOD2) [7], and interleukin 1 receptor (IL1R) [8]. A20-deficient mice demonstrate hypersensitivity to TNFα and die perinatally from spontaneous multi-organ inflammation [5], whereas A20ZF4 or A20°TU knock-in mice develop pronounced response to TNFα injection and dextran sodium sulfate (DSS)-induced colitis [9]. Finally, mice harboring tissue-specific A20 deficiencies develop distinct abnormal pathologies including polyarthritis [10], colitis [11,12], lupus-like systemic condition [13], keratinocyte hyperproliferation [14], and intestinal polyposis [15].
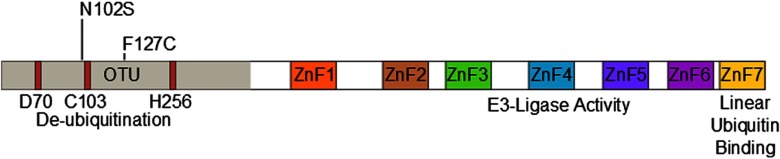
Fig. 1.
Domain structure of A20 and SNP locations.
Note: The N-terminal ovarian tumor-like unit (OTU) domain contains the catalytic triad of aspartate (D70), cysteine (C103), and histidine (H256). Seven zinc-fingers (ZnF1-ZnF7) are located in the C-terminal portion of the protein. The E3 ligase function of A20 is contained within ZnF4, while the linear ubiquitin binding function is dependent upon ZnF7. Both rs146534657 (N102S) and rs2230926 (F127C) are located with the N-terminal OTU domain.
Given A20’s regulatory role on inflammation and the NF-κB pathway, its implications in immune-related disorders are not unexpected. Genetic studies have linked A20 polymorphisms to the development of multiple chronic inflammatory conditions, including rheumatoid arthritis (RA) [16,17], celiac disease [18–20], inflammatory bowel disease (IBD) [21–23], systemic lupus erythematosus (SLE) [24–26], systemic sclerosis [27], Sjogren’s syndrome [28], psoriasis [29], and Behcet’s disease [30]. Many point mutations cause detrimental alterations to protein function or expression, diminishing A20’s capacity to abolish inflammation [31–33].
The Los Angeles County Health System and the Keck Hospital of the University of Southern California (USC) serve the region’s diverse racial and ethnic population – a significant percentage consisting of first- or second-generation immigrants. Over 60% of the patients self-report a Hispanic background, providing the unique opportunity to study genetic polymorphisms in this understudied population. In this study, we sequenced the exons encoding A20 of over 700 patients who received care in our clinics. We also evaluated the minor allele frequency (MAF) of a well-studied single-nucleotide polymorphism (SNP). Among the Hispanic patients, we identified two SNPs with significantly different MAF than reported in the literature among Caucasians. Subsequent in-vitro studies confirm that these SNPs inhibit the ability of A20 to regulate inflammation, suggesting that they may carry critical clinical implications.
Methods
Study Population
This study was approved by the institutional review board of the USC (Protocol no. IRB #HS-09-00543). Patients who received care at the Gastroenterology clinics of the Los Angeles County hospital (LAC+USC) were recruited randomly. All patients were recruited when they presented for routine medical care and had a variety of reasons for being in clinic. Buccal swabs containing DNA were obtained from those who agreed to participate and provided written informed consent, along with their self-identified gender (male or female), race/ethnicity (Caucasian, Hispanic, Middle Eastern, African-American, Asian, or other), and whether they were born abroad (yes or no).
Sequencing and Genotyping
Genomic DNA was extracted from buccal swabs using QuickExtract DNA Extraction Solution (Epicentre, Madison, WI, USA). Briefly, swabs were immersed in 500 µL of extraction solution. Tubes were vortexed for 10 s and then incubated at 65°C for 1 min, then mixed again for 15 s, and finally incubated at 98C for 2 min. DNA samples were stored at −70°C. DNA concentrations were measured by Nanodrop (Thermofisher, Waltham, MA, USA). For rs146534657 (NM_001270507.1) and rs2230936 (NM_001270507.1), genomic DNA samples (∼1 ug) were used to amplify exon 3 of TNFAIP3 using the following primers: exon 3 forward 5’-TTGCTGGGTCTTACATGCA G-3’ and exon 3 reverse 5’-CCCACCATGGAGCTCTGTTA -3’. PCR primers used to sequence the remaining exons of A20 were published previously [34]. PCR products were then enzymatically digested with exonuclease I and shrimp alkaline phosphatase (both from NEB, Ipswich, MA, USA) to prepare samples for direct Sanger sequencing using the exon 3 reverse primer. For rs6920220 (NC_000006.11), genomic DNA samples were subjected to qPCR using specific Taqman primers and probes (Applied Biosystems, Foster City, CA, USA).
In-Vitro Experiments
Human A20 cDNA was obtained from Dharmacon (Lafayette, CO, USA) and cloned into a mammalian expression vector (pCMV, Agilent Santa, Clara, CA, USA). A20 with the rs146534657:A>G (p.N102S) SNP, or A20 with the rs2230926:T>G (p.F127C) SNP were constructed from the wild-type A20 plasmid by QuikChange Lightning site-directed mutagenesis (Agilent, Santa Clara, CA, USA). Wild-type, p.N102S, or p.F127C A20 constructs were transfected into A20 knockout 293 cells, generated previously [35] containing the NF-κB Firefly luciferase reporter gene at concentrations of 10, 50, 100, or 500 ng. Total plasmid concentrations were normalized using empty pCMV vector. Cells were subsequently stimulated with TNFα at a concentration of 10 ng/mL for 8 h, and luciferase activity was assayed and normalized to Renilla luciferase. Protein levels of wild-type A20, and the A20 mutant-types p.N102S and p.F127C obtained from the transfected A20 knockout 293 cells, were quantified by Western blot using antibodies toward A20 (Cell Signaling, Danvers, MA, USA) and normalized to GAPDH (Merck Millipore, Darmstadt, Germany).
Statistical Analysis
Comparative statistics was performed with Student’s t-test for continuous variables, and chi-squared test for categorical variables. All analyses were executed on IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA).
Results
Participant Demographics
In total, 721 participants were recruited over a 10-year period. Approximately half were male, and 64.9% were born abroad (Table 1). The majority self-identified as Hispanic or non-Hispanic, followed by Caucasian, Asian, African-American, Middle Eastern, and others.
Table 1.
Demographics of study participants
| Demographics | Total (n = 721) | % |
|---|---|---|
| Male | 362 | 50.2 |
| Born abroad | 468 | 64.9 |
| Race/ethnicity | ||
| Caucasian | 139 | 19.3 |
| Hispanic | 469 | 65.0 |
| Asian | 50 | 6.9 |
| African-American | 45 | 6.2 |
| Middle Eastern | 16 | 2.2 |
| Others | 2 | 0.3 |
| Race/ethnicity (other than Caucasian) | 582 | 80.7 |
Demographic data of the participants are presented, along with the numbers of participants with each characteristic and the respective percentage (of the total 721 participants).
Single-Nucleotide Polymorphisms
Twenty-eight samples from Hispanic individuals were used in a pilot study in which all exons within the A20 gene were sequenced in their entirety. This subset analysis revealed two SNPs, both within exon 3 which contains the OTU enzymatic domain responsible for DUB activity (Fig. 1). The rs146534657 locus is located directly upstream to the catalytic cysteine of the OTU, where the missense mutation 305A>G converts the amino-acid N102S (NP_001257436.1:p. Asn102Ser). This SNP has a reported MAF of 0.11–1.3% [36], but is significantly overrepresented within this cohort at an MAF of 3.12% (45/1442) (Table 2). The significant majority of individuals with this SNP (40/44) self-reported as Hispanic (χ2 = 14.229, P = 0.014), including one with alleles GG. As there are 40 Hispanic participants with this SNP with 41 alleles present, it can be determined that there are 39 with the heterozygote genotype (AG) and one homozygote (GG). The MAF of this polymorphism among the Caucasian participants was 1.08%, conforming to literature and the MAF seen in the 1000 Genomes database [37], whereas it was 4.37% among Hispanics. Compared to Caucasians, the odds of acquiring this SNP as a Hispanic is 4.05 (95% CI 1.24–13.18).
Table 2.
Distribution of the SNP rs146534657:A>G
| Race/ethnicity | No. of participants |
Single-nucleotide polymorphism | Minor allele frequency (%) |
Odds ratio vs. Caucasian (95% CI) |
|---|---|---|---|---|
| Caucasian | 3 | 3 | 1.08 | – |
| Hispanic | 40 | 41 | 4.37 | 4.05 (1.24−13.18) |
| Asian | 1 | 1 | 1 | 0.93 (0.10−9.01) |
| African-American | 0 | 0 | 0 | 0 |
| Middle Eastern | 0 | 0 | 0 | 0 |
| Others | 0 | 0 | 0 | 0 |
Distribution of rs146534657:A>G among the ethnic/racial groups. The MAF of this SNP (0.11–1.3%) per NCBI dbSNP is similar to the Caucasian participants of our cohort. The MAF of this SNP among the Hispanic participants of our cohort is significantly different.
The second SNP rs2230926 identifies a missense mutation 380T>G which converts the amino-acid F127C (NP_001257436.1:p. Phe127Cys). The MAF of this SNP among our cohort is 4.30% (62/1442) (Table 3), slightly lower than the 6.13–17.85% reported in the literature [36]. A significant difference in the distribution of this SNP among the racial/ethnic groups was found (χ2 = 24.886, P < 0.001), with the highest MAF of 14.44% among African-Americans. Notably, the 1000 Genomes database demonstrates a total MAF of 14% with only 2.3% of Mexicans living in Los Angeles carrying the SNP [37]. However, differences in the racial and ethnic characterization between our cohort and the 1000 Genomes database preclude a direct statistical comparison between the two. Twenty-eight Hispanic participants with this SNP carried the heterozygote genotype and four carried the homozygote genotype. Eleven African-American participants carried the heterozygote genotype and one had the homozygote genotype of this SNP.
Table 3.
Distribution of the SNP rs2230926:T>G
| Race/ethnicity | No. of participants |
Single- nucleotide polymorphism |
Minor allele frequency (%) |
Odds ratio vs. Caucasian (95% CI) |
|---|---|---|---|---|
| Caucasian | 11 | 11 | 3.96 | – |
| Hispanic | 30 | 32 | 3.41 | 0.86 (0.43−1.73) |
| Asian | 4 | 4 | 4 | 1.01 (0.31−3.25) |
| African-American | 12 | 13 | 14.44 | 3.65 (1.58−8.43) |
| Middle Eastern | 2 | 2 | 6.25 | 1.58 (0.34−7.45) |
| Others | 0 | 0 | 0 | 0 |
Distribution of rs2230926:T>G among the ethnic/racial groups. The MAF of this SNP (6.13–17.85%) per NCBI dbSNP is lower among the Caucasian participants of our cohort. The MAF of this SNP among African-American participants is significantly higher.
Many genome-wide association studies utilize the Illumina array chips to locate SNPs associated with autoimmune diseases [34,38,39]. We explored the MAF of the point mutation at rs6920220, one of the sites targeted by the Illumina chipset located within the intergenic gene region of A20, among our participants. 714 participants were screened for this SNP with using specific Taqman primers and probes. We again discovered heterogeneous distribution among the races/ethnicity within our cohort (χ2 = 24.8235, P < 0.001) (Table 4). Compared to Caucasian participants, this SNP is less prevalent among Hispanics and Asians. According to the 1000 Genomes database, this SNP has an MAF of 9% [37]; our population had an MAF of 12.5%.
Table 4.
Distribution of the SNP rs6920220:G>A
| Race/ethnicity | No. of participants |
Single- nucleotide polymorphism |
Minor allele frequency (%) |
Odds ratio vs. Caucasian (95% CI) |
|---|---|---|---|---|
| Caucasian | 43 | 51 | 19.03 | – |
| Hispanic | 95 | 101 | 10.79 | 0.57 (0.39−0.82) |
| Asian | 5 | 5 | 5.10 | 0.27 (0.10−0.69) |
| African-American | 14 | 15 | 16.67 | 0.88 (0.47−1.63) |
| Middle Eastern | 6 | 7 | 21.88 | 1.15 (0.48−2.75) |
| Others | 0 | 0 | 0 | 0 |
Distribution of rs6920220:G>A among the ethnic/racial groups. Compared to the Caucasian participants of our cohort, the MAF of this SNP is less prevalent among the Hispanic and Asian participants.
Functional Significance of SNPs
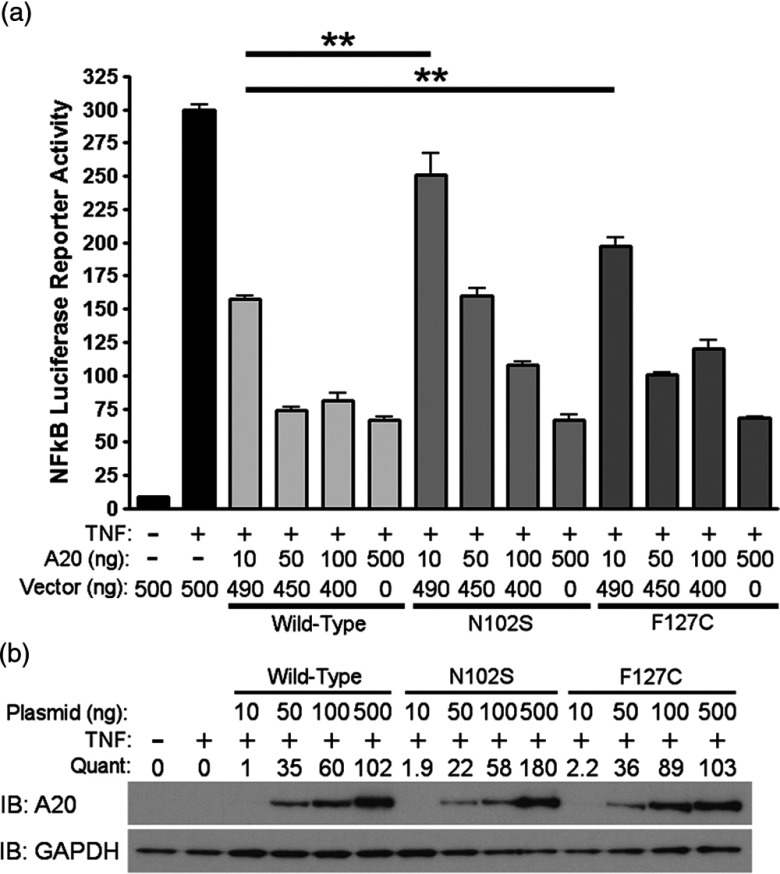
Since A20 is a well-known negative regulator of TNFα-induced NF-κB activity, we evaluated the functional significance of rs146534657:A>G and rs2230926:T>G with an NF-κB luciferase reporter assay. After stimulating with TNFα, cells pre-transfected with 10 ng of plasmid containing rs146534657:A>G (N102S) demonstrated a 2-fold increase in NF-κB luciferase activity (P < 0.01) compared to cells transfected with 10 ng of wild-type A20 plasmid. Similarly, cells transfected with 10 ng of plasmid containing rs2230926:T>G (F127C) A20 showed a 1.7-fold increase in NF-κB luciferase activity (P < 0.01) compared to cells transfected with the same concentration of wild-type A20 plasmid after TNFα stimulation (Fig. 2A). These deficits introduced by the SNPs gradually diminished with a higher quantity of transfected plasmids (i.e., 50, 100, and 500 ng) correlating to increasing protein concentrations (Fig. 2B), suggesting a baseline activation of NF-κB after TNFα stimulation regardless of A20 level or activity.
Fig. 2.
Functional consequences of rs146534657:A>G and rs2230926:T>G. (A) Wild-type A20 plasmid or plasmids containing the rs146534657:A>G (N102S) and rs2230926:T>G (F127C) were transfected into A20 knockout 293 cells at the indicated concentrations along with an NF-kB firefly luciferase reporter. Cells were stimulated with 10 ng/ml of TNFα (TNF) for 8 h then harvested for luciferase assay. Firefly luciferase activity was normalized to a co-transfected Renilla luciferase reporter. Total plasmid concentrations were normalized using empty pCMV vector. (B) Protein from these cells was also used in Western blot. Quantifications of transfected A20 band intensities shown as immuno-blots (IB) are normalized to the housekeeping protein GAPDH.
Note: ** P < 0.01. Figures are representative of three independent experiments.
Discussion
A majority of genetic studies to date have focused on populations of European descent [40,41], while few feature the comparison of genetic and clinical differences among Caucasians and other races [38,42]. Risk alleles may differ significantly across races and ethnicity, as evidenced by the unique genetic signature of African-American patients with RA and the near absence of mutations in the bacterial sensor NOD2 in Asian populations, which was a major advancement in the genetics of IBD among Europeans and Americans [38,43–47]. Taken together, the inclusion of participants representing diverse racial and ethnic background in genetic studies to identify population-specific mutations is crucial.
We selected A20 as a candidate gene to investigate because of its regulatory role on NF-κB activation and multiple other inflammatory signaling pathways. We identified two potential SNPs in the A20 gene which may have critical implications on chronic inflammatory conditions. Based on findings from our in-vitro assays, the locations of these SNPs within the exon 3 and their proximities to the OTU where deubiquitination occurs, these mutations may severely compromise the ability of A20 to control inflammation. These SNPs within exon 3 were identified after screening 28 Hispanic patients for all A20 exons; therefore, we focused specifically on these exon 3 SNPs within the entire cohort. Furthermore, we evaluated the SNP included in the Illumina array located at the intergenic region close to A20. We found this mutation to be significantly underrepresented among the Hispanics and Asians in our cohort, possibly limiting its potential as a susceptibility variant among these racial and ethnic groups.
The rs6920220 SNP has been associated with autoimmune and inflammatory conditions such as IBD and RA in the literature [48]. This SNP was included in the study to determine if the frequency of rs6920220 in Hispanics is as common as the other two SNPs being studied. The MAF of this SNP was found to be less prevalent among Hispanic and Asian patients in our cohort compared to Caucasian patients.
This study expands upon current genetics literature in multiple aspects. Access to a diverse population enabled us to validate the existence of racial differences in the distribution and frequencies of SNPs. Sequencing the exon regions of TNFAIP3 revealed two SNPs in close proximity to the OTU catalytic site. While rs2230926:T>G has been well-studied in the context of autoimmune diseases [39,49,50], rs146534657:A>G is poorly-characterized with only one report linking it to poor outcome among Chinese RA patients [34]. The function of this SNP in altering the amino acid directly adjacent to the catalytic cysteine within the OTU and its prevalence among Hispanics highlight clinical implications among this population. Consistent with a prior report on rs2230926:T>G in African-Americans with IBD, we found that this missense mutation significantly impaired the ability of A20 to inhibit inflammatory signaling [31]. We also demonstrated for the first time that the polymorphism associated with rs146534657:A>G present at high frequency in the Hispanic population is similarly defective.
The SNP allele frequencies in our cohort were unable to be directly compared statistically to the 1000 Genomes database due to differences in the categorization of cohorts; however, the general frequencies appeared similar. Specific challenges also merit consideration, the most pronounced being the lack of clinical information to correlate the three SNPs with development or progression of autoimmune conditions. Although our study demonstrates the prevalence of these SNPs among different ethnicities, it is possible that these differences in prevalence have no relation to chronic inflammatory conditions such as IBD. Participants’ races and ethnicity were based on self-report, which may lack specificity compared to the genetic determination [50]. Self-identification of ethnicity poses a weakness in our study as some participants may misreport their ethnicity, knowingly or unknowingly. Participants identified as Hispanic originate from multiple diverse ethnic groups which may carry significant genetic differences. Future studies should use genetic sequencing to definitively determine the ethnicity of the study participants. The cohort evaluated in this study was patients presenting to the LAC+USC Gastroenterology Clinic; therefore, a weakness is the suspected higher prevalence of GI pathology compared to a healthy control population. Furthermore, the intronic regions of A20 were omitted from sequencing, potentially excluding some relevant SNPs.
Study Highlights
Genetic studies have identified associations between certain SNPs, such as rs2230926 and s6920220, with inflammatory conditions; however, most studies are focused on populations of European descent. Our study aimed to sequence the A20 gene in a predominantly Hispanic population to identify new SNPs associated with inflammatory conditions, and to compare the frequency of SNPs associated with such conditions in Caucasian patients, such as rs2230926 and rs6920220, with the prevalence in Hispanic patients. We demonstrated for the first time that the polymorphism associated with rs146534657 is present at higher frequency in the Hispanic population and impairs the ability of A20 to inhibit TNFα activity and inflammatory signaling. As previously demonstrated, we showed that the polymorphism at rs2230926, present in higher frequency in African-Americans is similarly defective. Further studies should determine the clinical significance of the rs146534657 mutation and its clinical role in assessing susceptibility to inflammatory conditions such as IBD.
Conclusions
In conclusion, we identified two SNPs within the third exon of A20 affecting protein function with significantly higher MAF among self-reported Hispanics and African-Americans as compared to Caucasians. The SNP rs146534657:A>G is not well described in literature and is significantly overrepresented in Hispanics compared to Caucasians. Further research, with genetically confirmed analysis of ethnicity, is warranted to validate the clinical significance of this mutation to establish it as a susceptibility locus for autoimmune diseases among this population.
Acknowledgments
We thank the USC Libraries Bioinformatics Service for assisting with data analysis. The bioinformatics software and computing resources used in the analysis are funded by the USC Office of Research and Norris Medical Library. AP would like to thank the IBD Support Foundation.
Footnotes
Financial Support
This research was supported by a K-08 award from the NIDDK (DK100462), the Wright Foundation (LS), and the Margaret E. Early Foundation (LS). Additional core facility support was provided by USC Research Center for Liver Diseases, NIH grants Nos. P30 DK048522 and S10 RR022508. This research was also supported by the National Center for Advancing Translational Sciences (NCATS) of the U.S. National Institutes of Health [grant Nos. UL1TR001855 and UL1TR000130].
Disclosures
All authors have no financial and non-financial competing interests to declare.
Ethical Approval
This study was approved by the institutional review board (IRB) of the University of Southern California (Protocol no. IRB #HS-09-00543). Verbal and written consents were obtained from all participants of the study prior to their enrollment.
Availability of Data and Material
Raw trace files are available through the NCBI Trace Archive (link to be supplied upon manuscript acceptance). Cell lines and constructs are available upon request.
References
- 1.Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nature Reviews Immunology 2012; 12(11): 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosanac I, et al. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-kappaB signaling. Molecular Cell 2010; 40(4): 548–557. [DOI] [PubMed] [Google Scholar]
- 3.Tokunaga F, et al. Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-kappaB regulation. EMBO Journal 2012; 31(19): 3856–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verhelst K, et al. A20 inhibits LUBAC-mediated NF-kappaB activation by binding linear polyubiquitin chains via its zinc finger 7. EMBO Journal 2012; 31(19): 3845–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee EG, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 2000; 289(5488): 2350–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boone DL, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nature Immunology 2004; 5(10): 1052–1060. [DOI] [PubMed] [Google Scholar]
- 7.Hitotsumatsu O, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity 2008; 28(3): 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Letters 1999; 442(2–3): 147–150. [DOI] [PubMed] [Google Scholar]
- 9.Lu TT, et al. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity 2013; 38(5): 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matmati M, et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nature Genetics 2011; 43(9): 908–912. [DOI] [PubMed] [Google Scholar]
- 11.Hammer GE, et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nature Immunology 2011; 12(12): 1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vereecke L, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. Journal of Experimental Medicine 2010; 207(7): 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kool M, et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 2011; 35(1): 82–96. [DOI] [PubMed] [Google Scholar]
- 14.Lippens S, et al. Keratinocyte-specific ablation of the NF-kappaB regulatory protein A20 (TNFAIP3) reveals a role in the control of epidermal homeostasis. Cell Death and Differentiation 2011; 18(12): 1845–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao L, et al. A20 restricts WNT signaling in intestinal epithelial cells and suppresses colon carcinogenesis. PLoS One 2013; 8(5): e62223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plenge RM, et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nature Genetics 2007; 39(12): 1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson W, et al. Rheumatoid arthritis association at 6q23. Nature Genetics 2007; 39(12): 1431–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trynka G, et al. Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-kappaB signalling. Gut 2009; 58(8): 1078–1083. [DOI] [PubMed] [Google Scholar]
- 19.Zhernakova A, et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genetics 2011; 7(2): e1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois PC, et al. Multiple common variants for celiac disease influencing immune gene expression. Nature Genetics 2010; 42(4): 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majumdar I, Ahuja V, Paul J. Altered expression of Tumor Necrosis Factor Alpha-Induced Protein 3 correlates with disease severity in Ulcerative Colitis. Scientific Reports 2017; 7(1): 9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang K, et al. Comparative genetic analysis of inflammatory bowel disease and type 1 diabetes implicates multiple loci with opposite effects. Human Molecular Genetics 2010; 19(10): 2059–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wellcome Trust Case Control C. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007; 447(7145): 661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adrianto I, et al. Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nature Genetics 2011; 43(3): 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham RR, et al. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nature Genetics 2008; 40(9): 1059–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SK, et al. TNFAIP3 gene polymorphisms associated with differential susceptibility to rheumatoid arthritis and systemic lupus erythematosus in the Korean population. Rheumatology (Oxford) 2014; 53(6): 1009–1013. [DOI] [PubMed] [Google Scholar]
- 27.Dieude P, et al. Association of the TNFAIP3 rs5029939 variant with systemic sclerosis in the European Caucasian population. Annals of the Rheumatic Diseases 2010; 69(11): 1958–1964. [DOI] [PubMed] [Google Scholar]
- 28.Musone SL, et al. Sequencing of TNFAIP3 and association of variants with multiple autoimmune diseases. Genes and Immunity 2011; 12(3): 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair RP, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nature Genetics 2009; 41(2): 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, et al. TNFAIP3 gene polymorphisms confer risk for Behcet’s disease in a Chinese Han population. Human Genetics 2013; 132(3): 293–300. [DOI] [PubMed] [Google Scholar]
- 31.Lodolce JP, et al. African-derived genetic polymorphisms in TNFAIP3 mediate risk for autoimmunity. The Journal of Immunology 2010; 184(12): 7001–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, et al. Alternative expression pattern of MALT1-A20-NF-kappaB in patients with rheumatoid arthritis. The Journal of Immunology Research 2014; 2014: 492872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nature Genetics 2016; 48(1): 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, et al. Characteristics of A20 gene polymorphisms and clinical significance in patients with rheumatoid arthritis. Journal of Translational Medicine 2015; 13: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura BN, et al. A20 regulates canonical WNT-signaling through an interaction with RIPK4. PLoS One 2018; 13(5): e0195893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherry ST, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Research 2001; 29(1): 308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auton A, et al. A global reference for human genetic variation. Nature 2015; 526(7571): 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu JZ, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nature Genetics 2015; 47(9): 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, et al. Associations between TNFAIP3 gene polymorphisms and rheumatoid arthritis risk: a meta-analysis. Archives of Medical Research 2017; 48(4): 386–392. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg NA, et al. Genome-wide association studies in diverse populations. Nature Reviews Genetics 2010; 11(5): 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends in Genetics 2009; 25(11): 489–494. [DOI] [PubMed] [Google Scholar]
- 42.Afzali A, Cross RK. Racial and ethnic minorities with inflammatory bowel disease in the United States: a systematic review of disease characteristics and differences. Inflammatory Bowel Diseases 2016; 22(8): 2023–2040. [DOI] [PubMed] [Google Scholar]
- 43.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001; 411(6837): 599–603. [DOI] [PubMed] [Google Scholar]
- 44.Danila MI, et al. Dense genotyping of immune-related regions identifies loci for rheumatoid arthritis risk and damage in African Americans. Molecular Medicine 2017; 23: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001; 411(6837): 603–606. [DOI] [PubMed] [Google Scholar]
- 46.Inoue N, et al. Lack of common NOD2 variants in Japanese patients with Crohn’s disease. Gastroenterology 2002; 123(1): 86–91. [DOI] [PubMed] [Google Scholar]
- 47.Leong RW, et al. NOD2/CARD15 gene polymorphisms and Crohn’s disease in the Chinese population. Alimentary Pharmacology & Therapeutics 2003; 17(12): 1465–1470. [DOI] [PubMed] [Google Scholar]
- 48.Stahl EA, et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nature Genetics 2010; 42(6): 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang MY, et al. Associations between TNFAIP3 gene polymorphisms and systemic lupus erythematosus risk: an updated meta-analysis. HLA 2016; 88(5): 245–252. [DOI] [PubMed] [Google Scholar]
- 50.Damas OM, et al. Genetic characterization and influence on inflammatory bowel disease expression in a diverse Hispanic South Florida Cohort. Clinical and Translational Gastroenterology 2017; 8(4): e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw trace files are available through the NCBI Trace Archive (link to be supplied upon manuscript acceptance). Cell lines and constructs are available upon request.