Abstract
We have successfully integrated techniques for controlling cell adhesion and performing electrochemical differential pulse voltammetry (DPV) through the use of digitally controlled microfluidics and patterned transparent indium tin oxide electrode arrays to enable rapid and sensitive enumeration of cancer cells in a scalable microscale format. This integrated approach leverages a dual-working electrode (WE) surface to improve the specificity of the detection system. Here, one of the WE surfaces is functionalized with anti-Melanocortin 1 Receptor antibodies specific to melanoma cancer cells, while the other WE acts as a control (i.e., without antibody), for detecting non-specific interactions between cells and the electrode. The method is described and shown to provide effective detection of melanoma cells at concentrations ranging between 25 to 300 cells per 20 μL sample volume after a 5 min incubation and 15 s of DPV measurements. The estimated limit of detection was ~17 cells. The sensitivity and specificity of the assay were quantified using addition of large fractions of non-target cells and resulted in a detection reproducibility of ~97%. The proposed approach demonstrates a unique integration of electrochemical sensing and microfluidic cell adhesion technologies with multiple advantages such as label-free detection, short detection times, and low sample volumes. Next steps for this platform include testing with patient samples and use of other cell-surface biomarkers for detection and enumeration of circulating tumor cells in prostate, breast, and colon cancer.
Keywords: differential pulse voltammetry, cancer cells, microfluidics, point-of-care, label-free detection
Graphical Abstract
Integrated technique for controlling cell adhesion and performing electrochemical differential pulse voltammetry (DPV) through the use of digitally controlled microfluidics and patterned transparent indium tin oxide (ITO) electrode arrays to enable rapid and sensitive enumeration of melanoma cells in a scalable microscale format.
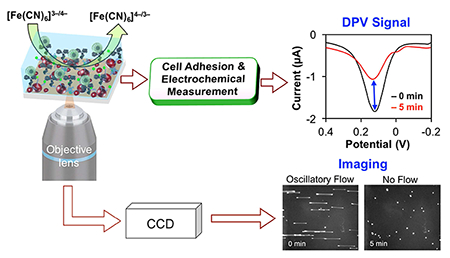
1. Introduction
Electrochemical sensing technologies (ESTs) have many strengths, which include label-free sensing, high sensitivity, high specificity in complex backgrounds, rapid and simple digital quantification, and low cost [1–4]. Given these strengths, ESTs have been applied in many fields, including in recent efforts to detect and enumerate circulating tumor cells (CTCs) in patient blood samples. These efforts are driven by significant interest in using CTCs to develop new prognostic, diagnostic, and pharmacodynamic biomarkers [5–9]. For example, CTCs have been found to correlate significantly with tumor stage and patient survival [10], and can serve as a biomarker for predicting anti-tumor responses in patients undergoing systemic therapies [11]. At present, CTC detection / enumeration are typically separated into two steps, CTC isolation (e.g., size-based filtration) and quantification (e.g., antibody staining, microscopy, and image analysis), each requiring specialized technologies and/or methodologies [8, 12–19]. Here, we focus on CTC quantification, where increasingly reliable and simple technologies are needed to help advance clinical application.
Integration of electrochemical sensing holds promise for significantly advancing efforts in this area of research. Recently, Safaei et al.[20] developed a microfluidic device utilizing in situ electrochemical ELISA for on-chip identification of captured cancer cells. However, such an approach requires labeled antibodies and various incubation steps for effective CTC detection and enumeration, resulting in lower reproducibility. Weng et al.[21] modified the surface of different types of electrodes (boron-doped diamond or indium tin oxide (ITO)-coated glass) with gold nanoparticles (AuNPs) functionalized with folic acid to enable the detection of HeLa cells in ~20 min via electrochemical impedance. Likewise, electrochemistry is being used alongside fluorescence imaging to enable detection followed by endpoint quantification [22–24]. Indeed, integration of electrochemical and imaging techniques is a rapidly advancing area [25] and holds promise for the development of cost-effective and reliable cell detection strategies for a variety of applications in cell research and clinical assessment. A dual quantification strategy (e.g., EST + microscopy) helps to guard against false-positive and negative results by providing secondary confirmation/validation. There also exist opportunities to leverage microfluidics for manipulating and interfacing test samples with ESTs for simpler and more reliable use in point-of-care applications. Thus, continued integration holds significant promise for further streamlining and improving CTC detection and quantification.
Previously, we demonstrated use of differential pulse voltammetry (DPV) for highly selective and sensitive label-free detection of multiple cancer cell types within complex samples in ~15 min [26, 27]. Herein, we describe the development of a microscale device that integrates highly sensitive DPV-based electrochemical sensing along with digitally controlled microfluidic flow and microscopy to enable label-free cancer cell enumeration and sensor validation in roughly one third of the previous detection time (~5 min vs ~15 min) [26, 27]. The sensor consists of a microfluidic channel placed over an optically transparent ITO-patterned electrode array where the detection electrode is coated with Melanocortin 1 Receptor (MC1R) antibodies (Ab)-functionalized AuNPs via carbamate chemistry. The AuNPs were functionalized with anti-MC1R-Ab and used for label-free detection of MC1R antigen expressed on melanoma cells [28, 29]. Digital control of oscillatory microfluidic flow [30] is used to tightly control interaction of the cells with the electrode (i.e., on or off) while DPV is used to quantify the extent and specificity of those interactions [27]. The use of the optically transparent ITO allows imaging through the device during sensing to enable direct validation of the sensor. This integrated approach provides a label-free method of target cell detection in a microscale format for scalable, precise, sensitive, and specific detection of cancer cells in complex backgrounds. In the future, we plan to test this new embodiment with patient samples and other cancer types to demonstrate its utility for clinical application.
2. Experimental
2.1. Materials
Anti-MC1R-Ab (200 μg/mL) was obtained from Santa Cruz Bio-technology Inc. (Santa Cruz, CA, USA, # SC-28990). 1.1-mm thick ITO-coated glass slides (25.4 × 76.2 mm) with a surface resistance of 100 Ω/sq and optical transmittance over 85 % were obtained from Nanocs Inc. (New York, NY, USA, # IT100-111-25). Gold (III) chloride trihydrate (99.9+%), 28–30% ammonium hydroxide (NH4OH), and cysteamine were purchased from Acros Organics, USA. MICROPOSIT positive photoresist S1813 and developer MF-321 were purchased from Rohm and Haas (Marlborough, MA, USA).
2.2. Cell culture and cell suspension preparation
Model cell lines of melanoma (SK-MEL-2) and prostate (PC 3) cancer were cultured as previously described [27, 31]. Human embryonic kidney cells HEK-293 were obtained from American Type Culture Collection (ATCC) and cultured according the supplier’s protocols. Subconfluent cells were trypsinized and washed with phosphate buffered saline (PBS) and suspended in cell culture media for further use. Viable cells were counted on a hemocytometer using trypan blue exclusion.
2.3. Patterned ITO electrode fabrication
Photolithographic patterning of two working electrodes (WEs), one counter electrode (CE), one reference electrode (RE), and four contact pads on an ITO-coated glass using MASK 1 and the detailed pattering scheme is depicted in Fig. S1. The dimensions of each feature are as follows: each WE = 1.2 mm × 1.5 mm, CE = 0.5 mm × 1.5 mm, RE = 0.5 mm × 0.5 mm, and each contact pad = 3 mm × 3 mm. The RE was coated with pseudo silver paint as we described previously [27].
2.4. Fabrication of electrochemical immunosensor
The anti-MC1R-Ab-modified AuNPs and AuNPs-coated WEs were prepared following our previously published method with slight modifications [27]. First, the ITO is masked by placing PDMS (PDMS Sylgard 184 Silicon Elastomer Kit, Dow Corning 100 Corporation, Midland, MI), leaving only the areas corresponding to each WE exposed. The hydroxyl (–OH) group is generated on each WE surface by immersing the PDMS-masked ITO electrode in 2% NH4OH solution at 80 C for 1 h followed by washing with deionized (DI) water. Next, a 0.3 M carbonyldiimidazole (CDI) solution is placed on the WE surface at 37 °C for 3 h to activate the –OH group and washed several times with DI water. Then, the CDI-activated WEs are reacted with cysteamine functionalized AuNPs in solution for 2 h at room temperature to effectively load the WEs with AuNPs. This results in covalent binding of the AuNPs to the WE surface via carbamate linkage. Subsequently, 0.3 M CDI solution is used to initiate a reaction between the free terminal amino groups on the AuNPs and CDI for 3 h at room temperature. The WE is then rinsed with cold-1× PBS (pH 7.4) and incubated with 3 μL of anti-MC1R-Ab solution (1:10 v/v with cold 1× PBS, pH 7.4) overnight at 4 °C to immunofunctionalize the AuNPs against MCIR, the target protein. Lastly, after thorough washing with cold 1× PBS (pH 7.4), the WE is blocked with 1% BSA for 30 min at room temperature (Scheme S1). The PDMS mask is then removed and a microfluidic channel is placed to enable the EST- and cell-adhesion-based detection and enumeration of MC1R-expressing SK-MEL-2 melanoma cells (Scheme 1A & B).
SCHEME 1.
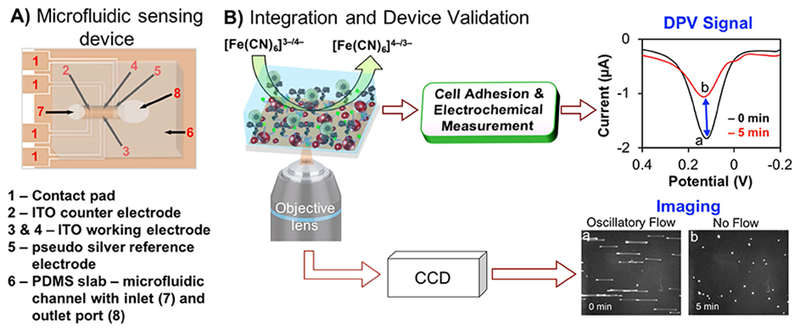
A) Schematic drawing of a fully assembled microfluidic sensing device. B) Schematic of electron transfer reaction at the working electrode surface in the absence (curve a) and in the presence of melanoma cells (curve b) and simultaneous microscopic image acquisition of cell adhesion during oscillatory flow (image a) and after stopping flow (image b). [Note: color in figures and images are for illustration only].
2.5. Microfluidic integration
The electrochemical immunosensor was integrated with a modified version of a digitally controlled high-content microfluidic cell adhesion platform that we have previously described [30]. Key advantages of the assay for this application include microfluidic channels that can be loaded with a simple pipette, the ability to work with small sample volumes, and digital flow control that is simple, flexible, and affordable. A further advantage of the approach is that it uses oscillatory fluid flow. Oscillatory flow enables control of cell interaction with the surface (i.e., adhesion can be allowed or prevented) while keeping cells positioned over the sensor for highly controlled measurements before and after cell-sensor interaction. Thus, no fluid exchange needs to take place before and after measurement.
The previous embodiment of the microfluidic system was improved in multiple ways. The old system employed PDMS for all channel components, which proved difficult to keep adhered and sealed to the ITO chip during fluid manipulations. The new channel construction (Fig. 1) is made by razor-printing the channel from biocompatible double-sided adhesive tape [32] and micromilling of port holes into sheet polystyrene (#ST313300, Goodfellow Cambridge Ltd., Huntingdon, England) [33] to create a top layer with excellent clarity for imaging. The solid polystyrene channel and double-sided adhesive not only made the sensor more robust, but also allowed the sensor to be cleaned and reused. After filling the channel with detection fluid (see Characterization), a flexible PDMS membrane is used to cover the larger port and held in place using razor-printed double-sided adhesive to create a sealed air chamber. Thus, like a drum, the membrane can be pressed downward to induce fluid flow in the channel. The membrane is perturbed via digital control of a piezoelectric actuator. The old system used an expensive signal generator (>$1000) while the new system uses a highly flexible and affordable (~$30) electronic prototyping platform, Arduino Due (SparkFun, DEV-11589). The Arduino is used to create a sawtooth electrical signal that generates oscillatory flow with near-constant shear stress and can be controlled with a single button press.
FIG. 1.
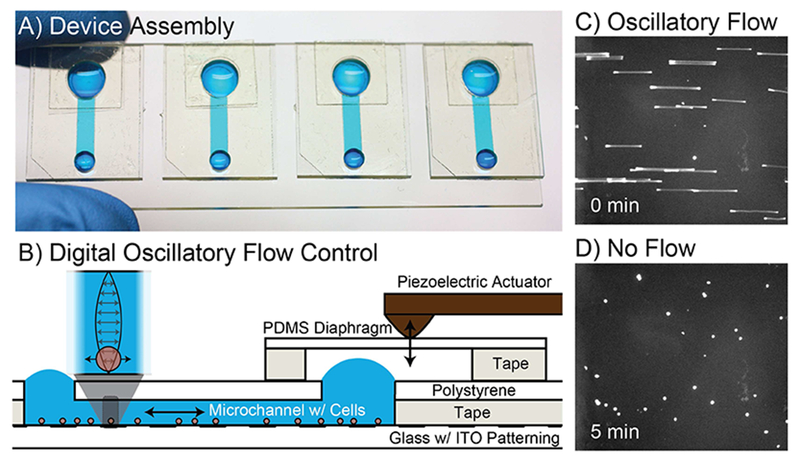
Microfluidic interface. A) Each ITO-pattemed glass slide contains four microfluidic devices to facilitate sample loading and control of adhesion for sensing. Blue food coloring is used for illustration. B) Devices are constructed on the ITO-patterned glass using razor-printed double-sided adhesive, polystyrene, and PDMS. C) unlabeled cells are imaged in bright-field mode with an extended exposure to show the oscillatory motion of each cell as a streak. D) The same unlabeled cells are imaged in bright-field mode after stopping flow to allow the cells to interact with the antibody functionalized surface.
2.6. Characterization
To perform measurements, channels are first filled with 20 μL of cell culture media containing redox probe of 0.25 mM of potassium ferricyanide (K3[Fe(CN)6]) / potassium ferrocyanide (K4[Fe(CN)6]) i.e., [Fe(CN)6]3−/[Fe(CN)6]4− (from Fisher Scientific) and 0.1 M potassium chloride, KCl (from Fisher Scientific). The PDMS diaphragm is then adhered over the larger microchannel port. The piezoelectric actuator is placed in contact with the membrane to start the oscillatory flow, during which cells are introduced via the open channel port. The membrane is tapped gently to distribute cells throughout the channel. Initially, the fluid flow is sufficient to prevent cell adhesion. Cells oscillate near the substrate with an amplitude of ~100 μm and a frequency of 2 Hz. At this time, a DPV baseline signal is obtained (image a and curve a in Scheme 1B); then the flow is stopped, allowing the cells to interact with the surface. The final DPV signal is acquired at 5 min (image b and curve b in Scheme 1B). Data were acquired for each Ab-modified and unmodified electrode during flow and after stopping flow. All these steps are performed while on a microscope (Nikon Ti-E equipped with Hamamatsu Flash4.0 camera and SpectraX light engine) to visually verify the operation and image the number of cells on each electrode. All DPV, cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) measurements were performed on an electrochemical workstation (CHI660D, CH Instruments Inc., Austin, TX, USA) connected to a laptop computer.
3. Results and discussion
We previously presented a DPV-based EST for detecting cancer cells in complex samples [27]. Here, we provide multiple significant advancements. Patterning of ITO is used to generate the sensor electrodes (2 WEs, 1 RE, 1 CE, contacts), eliminating bulky electrochemical cells and electrodes (see Fig. S1). By patterning 2 WEs, we were able to add an internal control into the sensor for increased sensitivity and specificity. The thin and transparent ITO also allowed us to integrate the sensor with microfluidics (Scheme 1). The microfluidic components facilitated loading of small samples using a simple micropipette while also providing digital flow-based control over cell adhesion during testing to improve sensor reproducibility. Lastly, the optically transparent device allowed use of microscopy for validation of sensor performance. Together, these advancements enable sensitive and specific, label-free cancer cell enumeration in ~5 min and represent a significant step towards clinical application of the EST for CTC detection and enumeration.
3.1. Surface characterization of working electrodes
FE-SEM images of bare ITO-coated glass, –OH functionalized on ITO, and covalently modified AuNPs on ITO are shown in Fig. 2. A slightly rough texture was observed for both bare ITO (Fig. 2a) and –OH functionalized ITO (Fig. 2b). The spherical and uniformly sized AuNPs display strong adhesion to the ITO substrate despite extensive washing (Figs. 2c, d), suggesting covalent linkage between the amino groups of the AuNPs with the hydroxyl groups on the ITO substrate via carbamate linkage. The AuNPs are slightly more aggregated on the ITO surface than in our previous report where AuNPs were electrostatically coated on the ITO surface and pre-functionalized with anti-MC1R-Ab in solution [27, 34]. However, aggregation seen here is similar to that seen with electrodeposited AuNPs on ITO [35], AuNPs loaded on (3-aminopropyl) triethoxysilane (APTES)-modified ITO [36, 37], and AuNP-modified SiNWs/ITO via Au-S bonds [38]. The AuNPs increase the active surface area which improves the conductivity of the electrode and increases the functionalization capacity for anti-MC1R-Ab, which thereby improves the immunosensor sensitivity. The distribution of covalently linked AuNPs on the ITO surface was higher and more uniform than previously reported for AuNPs electrostatically modified on APTES-ITO [36]. In addition, AuNPs-modified ITO did not interfere with imaging, making this approach useful for integrating imaging endpoints with electrochemical sensing.
FIG. 2.
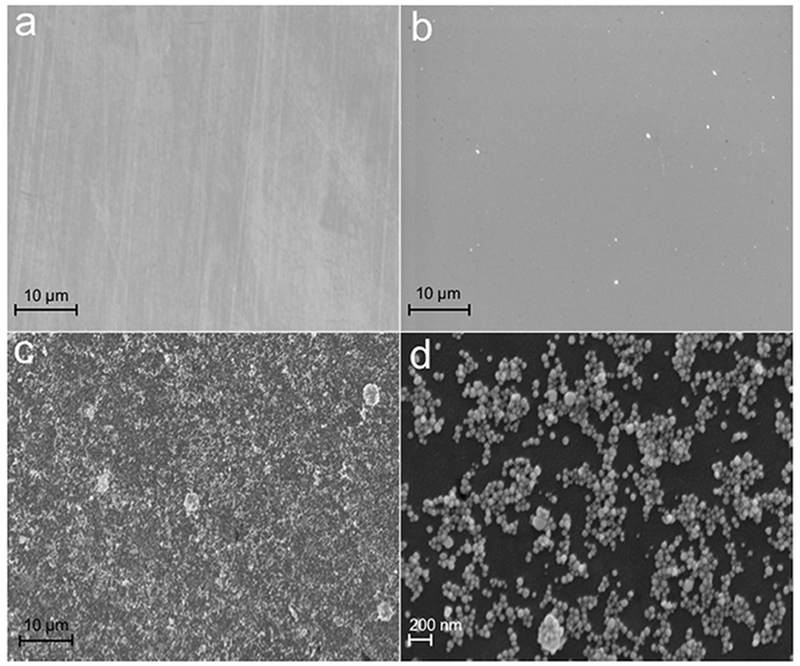
FE-SEM images of a) patterned working electrode surface of bare ITO–coated glass, b) –OH group functionalized ITO, and (c) covalently modified cysteamine functionalized AuNPs on the WE. (d) Magnified image of (c).
The electron transfer properties of anti-MC1R-Ab-modified and unmodified ITO electrodes were investigated with the help of electrochemical redox signal probe Fe(CN)63− /Fe(CN)64− with cell culture media. This redox signal probe is chosen both for sensitivity and relative biocompatibility for cell-based applications [26, 27]. Fig. S2 demonstrates CV response results of 0.25 mM of Fe(CN)63−/Fe(CN)64− and 0.1 M KCl at ITO, AuNPs/ITO, and anti-MC1R-Ab/AuNPs/ITO electrodes. After successive CV scanning from −0.1 V to +0.4 V with a scan rate of 50 mV/s, a noticeable broad and irreversible CV redox peak was observed for bare ITO electrode (Fig. S2a). The irreversible peak is due to the poor electron transfer properties of ITO via oxide groups [39]. However, a well-defined reversible CV redox peak with significantly enhanced electrocatalytic response is observed after covalently introducing cysteamine-capped AuNPs on the ITO surface (Fig. S2b). The AuNPs greatly increase the active surface area of the working electrode and enhance the conductivity of the electrode, resulting in improved performance relative to that reported for electrodeposited AuNPs/ITO electrodes [34]. Further, the addition of anti-MC1R-Ab on the surface of the AuNPs-modified ITO altered both the anodic and cathodic CV peak currents (Fig. S2c). Changes indicate that the electron transfer rate is hindered by the presence of the anti-MC1R-Ab. The surface coverage of AuNPs and anti-MC1R-Ab on the ITO is calculated using the Laviron equation (1),
| (1) |
where Γ is the surface density of AuNPs/anti-MC1R-Ab on the working electrode surface (mol/cm2), Q is the charge density charge (C) obtained by integrating the anodic peak obtained at 50 mVs−1, n is the number of electrons involved in the reaction, F is Faraday’s constant (C mol−1), and A is the surface area of the working electrode (cm2). The calculated surface coverage of AuNPs on the ITO surface is 0.86 × 10−9 mol/cm2. After anti-MC1R-Abs are functionalized on the AuNPs/ITO, surface coverage is 0.57 × 10−9 mol/cm2.
3.2. Detection and Enumeration of Melanoma Cells
To test the feasibility of developing a scalable microscale device for rapid detection and enumeration of cells in a complex sample, we integrated our DPV-based EST with pipette-friendly digitally controlled microfluidics (Scheme 1B). Figure 3 shows the DPV responsive results of anti-MC1R-Ab-modified and unmodified devices in the presence and absence of various amounts of SK-MEL-2 cells in 20 μL total working volume. For detection, the suspension contains 0.25 mM of Fe(CN)63−/Fe(CN)64− and 0.1 M KCl. Validation images show that cells attach when the AuNPs/ITO are modified with anti-MC1R-Ab but not when left unmodified (Fig. S3). The images were used to count the total number of SK-MEL-2 cells on the anti-MC1R-Ab-coated WE surface (Fig. S3A). The corresponding redox probe DPV results from before and after oscillatory flow (Fig. 3A) clearly show that the anti-MC1R-Abs are effectively immobilized on the AuNPs/ITO and show good affinity to the MC1R antigens. When target cells are present, the high affinity interaction between the MC1R cell surface proteins and anti-MC1R-Ab immobilized on the nanoparticles hinder the electron transfer reaction of Fe(CN)6]3− /[Fe(CN)6]4− and reduce the size of the voltammetric peak. The validation images show that cells do not attach in the absence of anti-MC1R-Ab during oscillation and after flow is stopped (Fig. S3B). These results also show that the SK-MEL-2 cells produced a significant change in signal only when on the anti-MC1R-Ab-modified AuNPs/ITO electrodes and not on the unmodified AuNPs/ITO (Fig. 3B). After 5 min of incubation and oscillatory flow, the number of cells in the validation images were counted used to generate a calibration curve for subsequent electrochemical enumeration of CTCs using the ITO-based immunosensor.
FIG. 3.
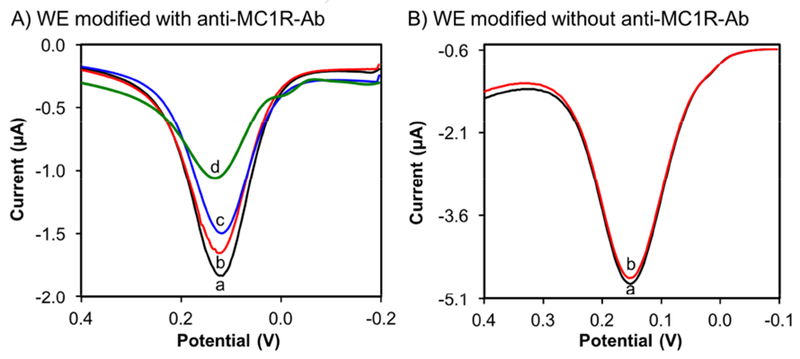
A) Data obtained via DPV signals in microfluidic channel (0 and 5 min oscillatory flow incubation) before (a) and after (b) 55 (c) 120 and (d) 300 SK-MEL-2 cells attach to the anti-MC1R-Ab-modified on AuNPs/ITO, and B) DPV results (a) 0 min (b) 5 min response after loading ~300 SK-MEL-2 cells without anti-MC1R-Ab-modified AuNPs/ITO electrode (background control).
3.3. Sensitivity and selectivity
Figure 4 shows the calibration results in the range from 25 to 300 SK-MEL-2 cells (0 = No-cell control). The DPV current signal has a highly linear relationship with the amounts of cells and required less time (5 min for incubation, plus 15 s for detection) than other reported microarray and immunosensors to detect the cancer cells [21, 26, 27, 40]. Overall the approach demonstrates multiple strengths including simple fabrication, label-free detection, pipette-friendly microfluidics and digital fluid control, and a highly-sensitive DPV method for more sensitive detection and enumeration compared to other electrochemical approaches [26, 41]. However, if this approach is to be feasible in future clinical applications, we must also evaluate specificity.
FIG. 4.
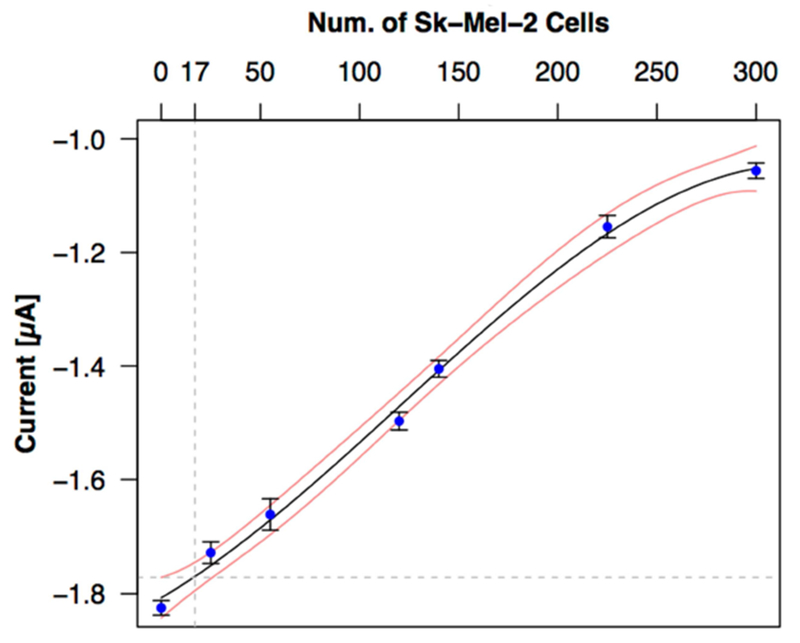
Detection sensitivity. Although a linear fit (y = −1.802 + 2.6e−03x, R2=0.984) can explain the majority of the response, a cubic fit is both superior (R2 = 0.997) and justified using ANOVA comparison of 1st, 2nd, 3rd and 4th order fits and a criterion of p< 0.01 (R statistical software). The cubic fit and corresponding 99% confidence band suggest a LOD of ~17 cells.
In efforts to adapt this system for eventual use with blood samples, we performed detection of melanoma cells in the presence of an overwhelming number of non-melanoma cells. This was done by mixing of different numbers of SK-MEL-2 cells with media, or media containing an abundance of non-target prostate cancer (PC 3) and human embryonic kidney (HEK-293) cells. The selectivity results are shown in Fig. 5. The signal obtained in the presence of 10,000 PC 3 cells (Fig. 5b vs 5a) or 10,000 HEK-293 (Fig. 5c) was largely unperturbed compared to the no-cell control (Fig. 5a). In contrast, we see the expected drop in current amplitude with addition of more SK-MEL-2 cells (Fig. 5d vs. 5f) compared to the no-cell control (Fig 5a). In agreement with both of these results, the addition of 10,000 HEK-293 cells to SK-MEL-2 samples did not significantly alter the signal amplitudes relative to the corresponding SK-MEL-2-only samples (Fig. 5d vs. 5e and Fig. 5f vs. 5g). There is potentially a slight increase in the signal noise in the presence of non-target cells. However, our integrated system shows better reproducibility (over 97 %) in the presence of large fractions of non-target cells than what was reported for an electrochemical linked ELISA approach that was tested using larger numbers of cancer cells [20].
FIG. 5.
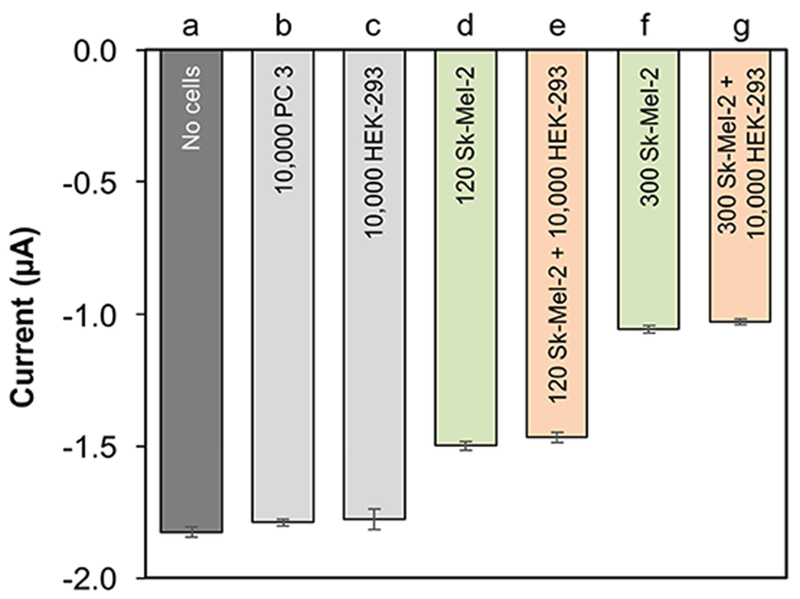
Specificity of responses obtained from DPV signal in the presence of (a) no cells, (b) 10,000 PC 3, (c) 10, 000 HEK-293, (d) 120 SK-MEL-2, e) 120 SK-MEL-2 + 10,000 HEK-293, (f) 300 SK-MEL-2, and (g) 300 SK-MEL-2 + 10,000 HEK-293 cells.
Taken together, these results demonstrate sufficient sensitivity and specificity to warrant follow-on pilot studies with patient samples. Given the microscale nature of the assay, upstream processing of samples will still be required to reduce sample volumes and eliminate a large majority of contaminating cells. Common isolation methods such as filter- and bead-based isolation can readily achieve adequate purities (i.e., 100% × number of CTCs / Total Cells) of ≳ 0.1-10% [17, 19]. Following isolation, the most common strategy for enumeration and quantification is immunostaining. However, immunostaining involves multiple steps that can result in the damage or loss of these already exceedingly rare cells [16–18]. Further, separate staining protocols performed on each patient sample introduce significant potential for sample-to-sample variation. Our proposed methodology minimizes sample processing and the potential for additional cell loss or damage by using label-free detection. Likewise, and sensor can be cleaned and reused to help minimize sample-to-sample variation. However, in such future studies, we expect additional modifications will be needed to fully realize this potential. For example, we expect to expand the overall foot-print of the arrays to accommodate higher sample volumes while maintaining or improving sensitivity. Moving forward, an important advantage of our approach will be the ability to incorporate imaging endpoints for validation of the approach against current immunostaining-based methods and for sensor validation to ensure accuracy and reproducibility.
4. Conclusions
We integrated easy-to-use microfluidics and highly sensitive label-free electrochemical sensing into a microscale assay for detection/enumeration of melanoma cells. The assay is rapid, requiring only 5 min for sample incubation, plus 15 s for detection. The cost of fabricating the electrode arrays is estimated to be around $2.50. The assay is affordable and also incorporates multiple features that enhance its performance. The microfluidic components offer easy pipette-based sample loading and tight control over cell interaction with the surface via digitally controlled oscillatory fluid flow. The sensitivity and specificity of the design is enhanced by the use of Ab-functionalized AuNPs, which increase detection Ab capacity and electrode conductivity, and by the dual WE design, which provides an internal control for non-specific cell adhesion. Integration with microscopy enabled on chip validation of cell numbers while also opening future opportunities to incorporate other imaging endpoints. Taken together, the integration of these components enables sensitive and specific detection of target cells amidst an abundance of non-target cells and provides multiple significant advancements towards the development EST-based point-of-care devices for detection/enumeration of CTCs. In the near future, we plan to use the new miniaturized device to validate the selective detection/enumeration of less than 10 melanoma cells in untreated blood samples. We also plan to expand application of the technology to other cancer types such as breast, prostate, and colon, where CTC enumeration has been used as a prognostic predictor of survival [42].
Supplementary Material
Highlights.
Integration of microfluidics to enhance label-free electrochemical sensing
Label-free detection and enumeration of melanoma cells in a scalable microscale format
Rapid detection of as few as 25 cells in ~5 min with an LOD of ~17 cells
Potential portable point-of-care system for routine monitoring of rare cell types
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH) under Award Number P30 AR066524, Skin Diseases Research Center at the University of Wisconsin and the University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cosnier S, Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films. A review, Biosens. Bioelectron, 14 (1999) 443–456. [DOI] [PubMed] [Google Scholar]
- [2].Wang J, Analytical electrochemistry, John Wiley & Sons VCH, Hoboken, New Jersey, USA: 2006. [Google Scholar]
- [3].Ronkainen NJ, Halsall HB, Heineman WR, Electrochemical biosensors, Chem. Soc. Rev, 39 (2010) 1747–1763. [DOI] [PubMed] [Google Scholar]
- [4].Ranjan R, Esimbekova EN, Kratasyuk VA, Rapid biosensing tools for cancer biomarkers, Biosens. Bioelectron, 87 (2017) 918–930. [DOI] [PubMed] [Google Scholar]
- [5].Atkinson AJ, Colburn WA, DeGruttola VG, DeMets DL, Downing DO GJ, Hoth DF, Oates JA, Peck CC, Schooley RT, Spilker BA, Woodcock J, Zeger SL, Biomarkers definitions working group: Biomarkers and surrogate endpoints: preferred definitions and conceptual framework, Clin. Pharmacol. Ther, 69 (2001) 89–95. [DOI] [PubMed] [Google Scholar]
- [6].Pantel K, Brakenhoff RH, Brandt B, Detection, clinical relevance and specific biological properties of disseminating tumour cells, Nat. Rev. Cancer, 8 (2008) 329–340. [DOI] [PubMed] [Google Scholar]
- [7].Danila DC, Fleisher M, Scher HI, Circulating tumor cells as biomarkers in prostate cancer, Clin. Cancer Res, 17 (2011) 3903–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alix-Panabieres C, Pantel K, Circulating tumor cells: liquid biopsy of cancer, Clin. Chem, 59 (2013) 110–118. [DOI] [PubMed] [Google Scholar]
- [9].Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C, Molecular analysis of circulating tumour cells-biology and biomarkers, Nat. Rev. Clin. Oncol, 11 (2014) 129–144. [DOI] [PubMed] [Google Scholar]
- [10].Mocellin S, Hoon D, Ambrosi A, Nitti D, Rossi CR, The prognostic value of circulating tumor cells in patients with melanoma: a systematic review and meta-analysis, Clin. Cancer Res, 12 (2006)4605–4613. [DOI] [PubMed] [Google Scholar]
- [11].Koyanagi K, O’Day SJ, Boasberg P, Atkins MB, Wang HJ, Gonzalez R, Lewis K, Thompson JA, Anderson CM, Lutzky J, Amatruda TT, Hersh E, Richards J, Weber JS, Hoon DSB, Serial monitoring of circulating tumor cells predicts outcome of induction biochemotherapy plus maintenance biotherapy for metastatic melanoma, Clin. Cancer Res, 16 (2010) 2402–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hong B, Zu Y, Detecting circulating tumor cells: current challenges and new trends, Theranostics, 3 (2013) 377–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Karabacak NM, Spuhler PS, Fachin F, Lim EJ, Pai V, Ozkumur E, Martel JM, Kojic N, Smith K, Chen PI, Yang J, Hwang H, Morgan B, Trautwein J, Barber TA, Stott SL, Maheswaran S, Kapur R, Haber DA, Toner M, Microfluidic, marker-free isolation of circulating tumor cells from blood samples, Nat. Protoc, 9 (2014) 694–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS, Circulating tumor cells: a multifunctional biomarker, Clin. Cancer Res, 20 (2014) 2553–2568. [DOI] [PubMed] [Google Scholar]
- [15].Ferreira MM, Ramani VC, Jeffrey SS, Circulating tumor cell technologies, Mol. Oncol, 10 (2016) 374–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M, Isolation of rare circulating tumour cells in cancer patients by microchip technology, Nature, 450 (2007) 1235–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sperger JM, Strotman LN, Welsh A, Casavant BP, Chalmers Z, Horn S, Heninger E, Thiede SM, Tokar J, Gibbs BK, Guckenberger DJ, Carmichael L, Dehm SM, Stephens PJ, Beebe DJ, Berry SM, Lang JM, Integrated analysis of multiple biomarkers from circulating tumor cells enabled by exclusion-based analyte isolation, Clin. Cancer Res, 23 (2017) 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu CL, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S, Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer, Sci. Transl. Med, 2 (2010) 25ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yusa A, Toneri M, Masuda T, Ito S, Yamamoto S, Okochi M, Kondo N, Iwata H, Yatabe Y, Ichinosawa Y, Kinuta S, Kondo E, Honda H, Arai F, Nakanishi H, Development of a new rapid isolation device for circulating tumor cells (ctcs) using 3d palladium filter and its application for genetic analysis, Plos One, 9 (2014) e88821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Safaei TS, Mohamadi RM, Sargent EH, Kelley SO, In situ electrochemical ELISA for specific identification of captured cancer cells, ACS Appl. Mater. Inter, 7 (2015) 14165–14169. [DOI] [PubMed] [Google Scholar]
- [21].Weng J, Zhang Z, Sun L, Wang JA, High sensitive detection of cancer cell with a folic acid-based boron-doped diamond electrode using an AC impedimetric approach, Biosens. Bioelectron, 26 (2011) 1847–1852. [DOI] [PubMed] [Google Scholar]
- [22].Amatore C, Arbault S, Chen Y, Crozatier C, Lemaitre F, Verchier Y, Coupling of electrochemistry and fluorescence microscopy at indium tin oxide microelectrodes for the analysis of single exocytotic events, Angew. Chem. Int. Ed, 45 (2006) 4000–4003. [DOI] [PubMed] [Google Scholar]
- [23].Meunier A, Jouannot O, Fulcrand R, Fanget I, Bretou M, Karatekin E, Arbault S, Guille M, Darchen F, Lemaitre F, Amatore C, Coupling amperometry and total internal reflection fluorescence microscopy at ito surfaces for monitoring exocytosis of single vesicles, Angew. Chem. Int. Ed, 50 (2011) 5081–5084. [DOI] [PubMed] [Google Scholar]
- [24].Kisler K, Kim BN, Liu X, Berberian K, Fang Q, Mathai CJ, Gangopadhyay S, Gillis KD, Lindau M, Transparent electrode materials for simultaneous amperometric detection of exocytosis and fluorescence microscopy, J. Biomater. Nanobiotechnol, 3 (2012) 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang H, Molino PJ, Wallace GG, Higgins MJ, Quantifying molecular-level cell adhesion on electroactive conducting polymers using electrochemical-single cell force spectroscopy, Sci. Rep, 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moscovici M, Bhimji A, Kelley SO, Rapid and specific electrochemical detection of prostate cancer cells using an aperture sensor array, Lab Chip, 13 (2013) 940–946. [DOI] [PubMed] [Google Scholar]
- [27].Seenivasan R, Singh CK, Warrick JW, Ahmad N, Gunasekaran S, Microfluidic-integrated patterned ITO immunosensor for rapid detection of prostate-specific membrane antigen biomarker in prostate cancer, Biosens. Bioelectron, 95 (2017) 160–167. [DOI] [PubMed] [Google Scholar]
- [28].Robinson SJ, Healy E, Human melanocortin 1 receptor (MC1R) gene variants alter melanoma cell growth and adhesion to extracellular matrix, Oncogene, 21 (2002) 8037–8046. [DOI] [PubMed] [Google Scholar]
- [29].Salazar-Onfray F, Lopez M, Lundqvist A, Aguirre A, Escobar A, Serrano A, Korenblit C, Petersson M, Chhajlani V, Larsson O, Kiessling R, Tissue distribution and differential expression of melanocortin 1 receptor, a malignant melanoma marker, Br. J. Cancer, 87 (2002) 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Warrick JW, Young EWK, Schmuck EG, Saupe KW, Beebe DJ, High-content adhesion assay to address limited cell samples, Integr. Biol, 5 (2013) 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Seenivasan R, Maddodi N, Setaluri V, Gunasekaran S, An electrochemical immunosensing method for detecting melanoma cells, Biosens. Bioelectron, 68 (2015) 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stallcop LE, Alvarez-Garcia YR, Reyes-Ramos AM, Ramos-Cruz KP, Morgan MM, Shi Y, Li L, Beebe DJ, Domenech M, Warrick JW, Razor-printed sticker microdevices for cell-based applications, Lab Chip, (2018) DOI: 10.1039/C1037LC00724H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guckenberger DJ, de Groot TE, Wan AMD, Beebe DJ, Young EWK, Micromilling: a method for ultra-rapid prototyping of plastic microfluidic devices, Lab Chip, 15 (2015) 2364–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang J, Wang L, Di J, Tu Y, Electrodeposition of gold nanoparticles on indium/tin oxide electrode for fabrication of a disposable hydrogen peroxide biosensor, Talanta, 77 (2009) 1454–1459. [DOI] [PubMed] [Google Scholar]
- [35].Zhang J, Kambayashi M, Oyama M, A novel electrode surface fabricated by directly attaching gold nanospheres and nanorods onto indium tin oxide substrate with a seed mediated growth process, Electrochem. Commun, 6 (2004) 683–688. [Google Scholar]
- [36].Aziz MA, Patra S, Yang H, A facile method of achieving low surface coverage of Au nanoparticles on an indium tin oxide electrode and its application to protein detection, Chem. Commun, (2008) 4607–4609. [DOI] [PubMed] [Google Scholar]
- [37].Ballarin B, Cassani MC, Scavetta E, Tonelli D, Self-assembled gold nanoparticles modified ITO electrodes: The monolayer binder molecule effect, Electrochim. Acta, 53 (2008) 8034–8044. [Google Scholar]
- [38].Rashid JIA, Yusof NA, Abdullah J, Hashim U, Hajian R, The utilization of SiNWs/AuNPs-modified indium tin oxide (ITO) in fabrication of electrochemical DNA sensor, Mat. Sci. Eng. C-Mater, 45 (2014) 270–276. [DOI] [PubMed] [Google Scholar]
- [39].Aziz MA, Park S, Jon S, Yang H, Amperometric immunosensing using an indium tin oxide electrode modified with multi-walled carbon nanotube and poly(ethylene glycol)-silane copolymer, Chem. Commun, (2007) 2610–2612. [DOI] [PubMed] [Google Scholar]
- [40].An L, Wang G, Han Y, Li T, Jin P, Liu S, Electrochemical biosensor for cancer cell detection based on a surface 3D micro-array, Lab Chip, DOI: 10.1039/C7LC01117B (2018). [DOI] [PubMed] [Google Scholar]
- [41].Kavosi B, Salimi A, Hallaj R, Moradi F, Ultrasensitive electrochemical immunosensor for PSA biomarker detection in prostate cancer cells using gold nanoparticles/PAMAM dendrimer loaded with enzyme linked aptamer as integrated triple signal amplification strategy, Biosens. Bioelectron, 74 (2015) 915–923. [DOI] [PubMed] [Google Scholar]
- [42].Toss A, Mu Z, Fernandez S, Cristofanilli M, CTC enumeration and characterization: moving toward personalized medicine, Ann. Transl. Med, 2 (2014) 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


