Abstract
Purpose of Review
This review discusses global trends in cancer mortality and survival, the socioeconomic drivers of those trends, and recent innovations in cancer surgery.
Recent Findings
Cancer is a leading cause of death worldwide. Cancer, previously a disease primarily of wealthy countries, is rapidly becoming a leading cause of death in low- and middle-income countries. Major economic forces driving global cancer trends include aging, frailty, and obesity. Alcohol consumption, poor diet, and lack of exercise also contribute to cancer types associated with modifiable causes. Surgery is responsible for 65% of cancer care globally, providing an opportunity for anesthesiologists to improve that care. Anesthesiologists can contribute to cancer remission through perioperative interventions that reduce risk of metastasis and speed return to intended oncologic therapy.
Summary
Cancer surgery comprises a large proportion of anesthetic caseload. Good outcomes come from high volume cancer centers using a multidisciplinary approach.
Keywords: cancer, anesthesia, perioperative medicine, return to intended oncologic therapy, postoperative complications, global surgery
Introduction
Despite enormous effort, cancer remains a major cause of death worldwide. However, more and more people around the world are surviving cancer, particularly in countries with a higher level of development. Anesthesiologists and other leaders in perioperative medicine can better predict the future surgical population by understanding current trends in global cancer diagnosis and mortality.
Economic factors are driving these global trends. Improvements in the standard of living mean that more people can live long enough to be diagnosed with cancer. Modifiable risk factors such as obesity and smoking are becoming more common. These drivers will increase the prevalence of associated cancers, which will be discussed in this review.
Surgery is responsible for 65% of cancer care globally, so the perioperative period presents a rich opportunity for improvement in cancer care.[1] Quality and results vary depending on the type of institution where cancer treatment is performed. The leading cause of mortality after any kind of surgery is cancer, and there is evidence to believe that the surgery creates a favorable environment for metastasis. Hospitals around the world are experimenting with pathways that combine several “best practices” for surgery and anesthesia to improve patient outcomes. Both surgical and cancer outcomes, specifically disease-free survival, must be evaluated.
This manuscript reviews the current trends in the worldwide cancer mortality and survivorship, the economic burden of cancer and cancer-related diseases, and the trends in cancer surgery.
Worldwide Cancer Mortality and Survivorship
Cancer is the second most common cause of death after ischemic heart disease, according to World Health Organization (WHO) estimates. Among women aged 30–59, cancer is the leading cause of death. In 2016, 9.2 million people died of cancer worldwide, comprising 16.1% of all deaths.[2] This corresponds to over 25,000 deaths per day. Only ischemic heart disease was more prevalent at 16.6% of all deaths. Since the year 2000, other common causes of death have a neutral or slightly downward trend. However, cancer and heart disease continue to rise both in absolute terms and as a percentage of all deaths. From 2010 to 2016, the percentage of all deaths due to cancer rose by 0.8%. The Global Burden of Disease (GBD) study, which uses a different methodology, has a lower estimate of 8.9 million people for cancer deaths in 2016.[3]
Projections made by WHO in 2013 predict that by the year 2030 cancer will surpass ischemic heart disease and become the most common cause of death, accounting for 18.5% of all deaths.[4] People who in previous generations would have died of tuberculosis, HIV/AIDS, or complications of birth are now living long enough to die of cancer.
Of global cancer deaths in the year 2016, 57% were in men. The types of cancer responsible for deaths in men are predominately lung (23%), liver (11%), stomach (9%), colon (8%), and prostate (7%). In women, breast cancer predominates (15%), followed by lung (13%), colon (9%), cervical (7%), and stomach (also 7%). These data are summarized in Table 1.
Table 1:
Global Top 15 Cancer Types by Estimated Deaths and Gender, 2016
| Male Cancer Type |
Male Deaths |
Female Cancer Type |
Female Deaths |
|||
|---|---|---|---|---|---|---|
| Trachea, bronchus, lung cancers |
1,176,732 |
23% | Breast cancer |
582,444 |
15% | |
| Liver cancer |
585,705 |
11% | Trachea, bronchus, lung cancers |
531,008 |
13% | |
| Stomach cancer |
495,908 |
9% | Colon and rectum cancers |
362,778 |
9% | |
| Colon and rectum cancers |
431,717 |
8% | Cervix uteri cancer |
283,439 |
7% | |
| Prostate cancer |
355,506 |
7% | Stomach cancer |
264,264 |
7% | |
| Esophagus cancer |
308,688 |
6% | Liver cancer |
243,894 |
6% | |
| Mouth and oropharynx cancers |
240,739 |
5% | Pancreas cancer |
176,291 |
4% | |
| Lymphomas, multiple myeloma |
202,357 |
4% | Ovary cancer |
164,568 |
4% | |
| Pancreas cancer |
195,523 |
4% | Lymphomas, multiple myeloma |
152,369 |
4% | |
| Leukemia |
167,551 |
3% | Leukemia |
124,791 |
3% | |
| Bladder cancer |
143,034 |
3% | Esophagus cancer |
120,456 |
3% | |
| Brain and nervous system cancers |
123,324 |
2% | Gallbladder and biliary tract cancer |
108,058 |
3% | |
| Kidney cancer |
101,559 |
2% | Brain and nervous system cancers |
95,319 |
2% | |
| Larynx cancer |
80,777 |
2% | Mouth and oropharynx cancers |
91,821 |
2% | |
| Gallbladder and biliary tract cancer |
70,618 |
1% | Corpus uteri cancer |
84,161 |
2% | |
| All Other |
547,531 |
10% | All Other |
568,557 |
14% | |
| All Sites |
5,227,269 |
100% | All Sites |
3,954,218 |
100% | |
Data from World Health Organization Global Health Estimates 2016.[2]
The level of economic development has a significant impact on cancer mortality. Development can be measured with the Human Development Index (HDI), which ranks countries based on life expectancy, education, and income. In very high HDI countries, the leading types of cancer responsible for death are breast, prostate, lung, colorectal, and stomach.[5] Liver cancer is not as common in first world countries as it is in poorer regions. Infectious disease is more common in poorer regions, especially hepatitis B virus and hepatitis C virus, both of which are associated with increased risk for liver cancer. Prostate cancer is an infrequent cause of death in low HDI areas. The reasons for this are not entirely clear, but are likely some combination of improved screening and data collection and greater exposure to risk factors such as obesity, low exercise, and excess dietary fat.[6]
Improved cancer treatment has increased the number of people living after a cancer diagnosis. The prevalence of cancer five years after diagnosis reflects the number of survivors. Using data from GLOBOCAN 2012,(reference) there were an estimated 32.4 million people living with cancer worldwide in 2012, 53% of which are women. The top five cancer types in cancer survivors, includes: breast cancer (19%), prostate cancer (12%), colorectal cancer (11%), lung cancer (6%), and cervical cancer (5%).
Cancer survivorship is highly dependent on HDI level. Low HDI countries have 18% of the population but only 6% of all survivors. For all cancers except for cervical cancer, there are fewer survivors per capita as the HDI level decreases.[7] In fact, very high HDI countries only comprise 16% of the world population yet comprise 50% of all cancer survivors.
The CONCORD-3 study of cancer survival for 18 different cancers found that global 5-year net survival varies widely between countries.[8] For example, breast cancer survival varies from 90.2% in the United States to 66.1% in India. Survival trends are improving worldwide, even for cancers of the liver, pancreas, and lung.
Within the United States, the five-year relative survival rate for patients diagnosed with any cancer is 69%.[9] This is similar to recent five-year relative survival rates in Australia (68%) and Norway (72.2% for males, 71.6% for females), but higher than China (40.5%).[10–12]
Economic Burden of Cancer and Cancer-related Disease
The worldwide annual economic cost of cancer in 2010 was estimated at $1.16 trillion (USD).[13] This estimate does not include the indirect costs to the families of people with cancer.
Within the United States, as of 2015, cancer is the 6th most expensive health condition at $80.2 billion per year after mental disorders, heart conditions, trauma, diabetes, and osteoarthritis.[14] Of that expenditure, most (55%) is spent on office and hospital outpatient visits.
These costs are one way of measuring the global burden of cancer. This burden is expected to increase for several reasons, of which we will focus on two major contributors. First, the world population is becoming older. Second, rates of obesity and other modifiable risk factors for cancer are increasing, particularly in low- and middle-income countries.
Older people represent a significant proportion of the world population. As of 2017, 13% of the global population is age 60 or above, a proportion projected to grow by 3% each year.[15] By 2050, all regions of the world except for Africa will see that proportion grow to nearly 25% or more. Older patients are more likely to receive a new diagnosis of cancer, particularly the very old. In 2016 it is estimated that of new cases of cancer, 15% were in patients age 80 or older, 22% in patients aged 70 to 79, and 26% in patients aged 60 to 69.[16] That is a total of 63% of all new cancer diagnoses were in patients aged 60 or older. Figure 1 demonstrates the increasing prevalence, incidence, and mortality as age increases. In the United States, the five-year relative survival for patients at or over 65 years old is 59.3% versus 73.9% for younger patients.[9]
Figure 1:
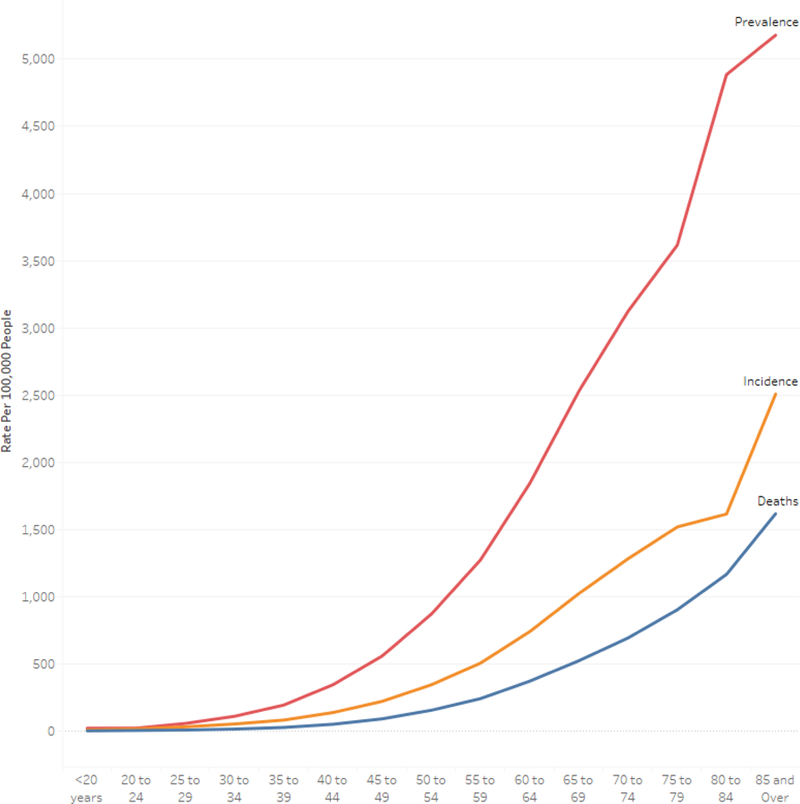
Global Prevalence, Incidence, and Mortality by Age for All Cancers, 2016 Data from Global Burden of Disease Study.[16]
Older cancer patients are both undertreated due to an often erroneous sense that they are too old to benefit from anti-cancer therapies, and overtreated when patients who have multiple comorbidities that warrant better shared decision making and tailored therapy rather than aggressive treatment for all. “Frailty” is a geriatric syndrome that describes patients who are vulnerable to minor stressors.[17] Frail patients are at higher risk for disability, falls, institutional admission, and death. While there is a high prevalence of frailty in elderly cancer patients, it is not clear whether frailty is a risk factor for cancer or vice versa. Cancer centers have improved the care of elderly patients through geriatric assessments and data-driven risk calculators that provide a stronger foundation for treatment decision-making.[18]
Increases in obesity are another reason for the upward trend in cancer diagnoses and mortality. Obesity raises the risk of 13 cancers, including colorectal, breast (post-menopausal), ovarian, esophageal, endometrial, kidney, pancreatic, gallbladder, and aggressive prostate cancer.[19] Worldwide, the WHO estimated that from 2000 to 2016, the prevalence of obesity in people over 18 rose from 8.7% to 13.1%.[20]
In the United States, the incidence of obesity-related cancers excluding colorectal cancer have increased by 7% among people aged 20–74 from 2005 to 2014, a statistically significant increase.[21] A study of United States adults found that moderate weight gain from early to middle adulthood was associated with significantly increased risk of major chronic diseases.[22] In the Nurses' Health Study cohort, the incident risk ratio (IRR) for obesity-related cancer in those who gained between 2.5 and 10 kg was 1.09 (95% CI 1.01–1.17), and in those who gained over 20 kg the IRR was 1.52 (95% CI 1.40–1.64).
Other potentially modifiable behaviors that are linked to cancer include smoking cigarettes, drinking alcohol, poor diet, and lack of exercise. The proportion of the population who smoke has declined at a rate of 0.9% from 2006 to 2012.[23] Whilst the global prevalence of smoking in 2012 dropped to 31.1% for men and 6.2% for women, the global number of smokers increased to 967 million. According to a recent study, in the population aged 50 years and older 27.1% (95% CI 21.2–33.3) of alcohol-attributable deaths in 2016 were due to cancer.[24] A diet with excess red meat, low fiber, and low calcium raises colorectal cancer risk.[25] Other potentially modifiable exposures include ultraviolet radiation and six infectious diseases linked to cancer. These diseases are H. pylori, hepatitis B virus, hepatitis C virus, human herpes virus type 8, human immunodeficiency virus (HIV), and human papillomavirus.[26] Colorectal cancer is increasing in developing countries as infection-related cancers decline.[27] A group of epidemiologists estimated that 5.7% of all new cancers in 2012 were attributable to the combination of high BMI and diabetes when treated as independent risk factors, or 4.5% of all new cancers using a more conservative model.[28] The American Cancer Society found that the modifiable risk factors that accounted for the highest proportion of cancer cases were cigarette smoking (19%) and excess body weight (7.8%).[29] The types of cancer most associated with modifiable risk factors were lung cancer and colorectal cancer.
Trends in Cancer Surgery
Globally, the number of surgical operations performed is estimated to be between 266 to 360 million, an increase of 38% over the prior eight years.[30] While there was a large increase of surgical operations seen in very low health expenditure WHO member states, this comprised only 5% of the worldwide surgical total.
As countries increase the volume of surgical operations, a significant number of these operations will be for cancer, estimated at approximately 65% of surgeries.[1] Combining data from the Swedish Cancer registry and GLOBOCAN 2012, Sullivan and colleagues estimate that the - global need for cancer surgery will be at least 45 million operations in 2030.[31] In that analysis the need for surgery for a given cancer admission varied from 25% for bone cancer to over 70% for bladder and breast cancer. The largest increase in cancer surgery need will be in low Human Development Index countries (estimated 59% increase from 2015 to 2030.) It is likely that the cancer types with the greatest need will be prostate cancer, bladder cancer, and breast cancer. A survey of 173 global health providers asked what proportion of patients would, in an ideal setting, benefit from surgical involvement in disease management. Respondents felt that 62.5% of cancer patients would benefit from surgical input.[32] These results show that the burden of surgical disease is significant among cancer patients.
United States data also reflects the frequent need for surgery during cancer admissions. A study of United States inpatient data found that of patients admitted for cancer, 61.4% had an operation during that admission.[33] Out of all the inpatient admissions, cancer was the second most frequent admission category to receive an operation after musculoskeletal admissions (84%.) Of all operations in that study, cancer surgery comprised 6.8% of the total.
There is evidence that cancer patients at dedicated cancer centers have lower mortality than those who present to community hospitals. Within the United States, risk-adjusted mortality overall among cancer patients is lowest at cancer specialty hospitals and highest at community hospitals.[34] Among National Cancer Institute (NCI) designated cancer centers, a subset of the oldest cancer hospitals had the lowest risk of death.
The United States model of comprehensive cancer centers has been difficult to replicate in other countries for a variety of reasons. The United States has benefited from a strong private sector and a robust research program which drove the development of centers devoted to high-quality cancer care[35] Low- and middle-income countries (LMICs) have seen a huge rise in private cancer centers, frequently in response to low public funding and unclear national cancer strategy. These facilities vary wildly due to a lack of standardization on what “comprehensive cancer center” means in LMICs. Many of these cancer centers emphasize advanced technology and novel medications over outcomes.
While so far we have focused on cancer surgery, it is important to note that patients having any kind of surgery may ultimately die from cancer., Across all types of surgical procedures, cancer was identified as a major cause of long-term postoperative mortality. In a prospective trial of patients undergoing major non-cardiac surgery, cancer was the leading cause of post surgery mortality at one year.[36] Of the 1,064 patients in this study, cancer was the cause of death 51.7% of patients who died at one-year post surgery, followed by cardiovascular causes (17.2%.) In a later study of mortality at two years after surgery, cancer was again the leading cause of death (75% in year 1, 65% in year 2.)[37] A powerful predictor of 2-year mortality in the survival model was preexisting malignancy with poor prognosis (hazard ratio 9.3, 95% CI 6.60–13.1.)
Surgery is often intended as a curative treatment for cancer. However, there is evidence that the anesthesia and surgery creates conditions that encourage metastasis. Anecdotal reports at the turn of the century suggested that tumor removal may promote tumor recurrence. Since then, a fragmentary body of evidence has accumulated to support the idea that adrenergic and inflammatory changes caused by surgery provide a fertile environment for metastasis.[38] However, it is unclear how much real world impact this phenomenon has on cancer patients. The theory that reduced adrenergic and inflammatory stimulus can lead to reduced cancer recurrence has led to several proposals to adapt perioperative management for the cancer surgery patient accordingly. Suggested perioperative interventions include using selective non-selective β-adrenergic blockers and COX-2 inhibitors (in an attempt to also reduce opiate requirements), regional anesthesia, and reducing of psychological stress.[39]
Sustained change in cancer outcomes requires an organized and consistent application of perioperative interventions. Enhanced Recovery Pathways (ERPs) have been developed for a variety of cancer types, including gynecologic cancers, breast cancer, colorectal cancer, and prostate cancer.[40–43] Enhanced recovery protocols have shown reduced length of stay, reduced postoperative opioid requirement, and improved patient satisfaction. ERPs extend beyond the operating suite to include preoperative care, postoperative recovery, and postdischarge follow up.
The metrics discussed above to demonstrate ERP performance describe good surgical outcomes, but do not address cancer outcomes. Metrics that connect anesthetic practice with cancer treatment and remission are more useful. One example of a novel metric that does this is “return to intended oncologic treatment” (RIOT), that is have postoperative adjuvant therapy. This metric was initially described in a study of liver cancer patients, for whom adjuvant chemotherapy is indicated after resection of hepatic metastases. Of the open approach patients followed in the study, 75% were able to RIOT, while 100% of the minimally invasive approach group were able to RIOT.[44] Hypertension, multiple preoperative chemotherapy regimens, and postoperative complications were all associated with the inability to RIOT. It is difficult to determine ability to RIOT in circumstances where the intended therapy is not clear prior to surgery. Surgeons, oncologists, and anesthesiologists need to work together to determine the treatment pathway and then measure how many patients stay on it.
In addition to inability to RIOT, postoperative complications have been shown to reduce disease-free survival. Postoperative infection was associated with decreased overall and disease-free survival in a study of patients who had surgical resection of colorectal liver metastases.[45] Postoperative complications have been associated with worse disease-free and overall survival in rectal cancer and esophageal cancer.[46, 47] Efforts in the perioperative period to reduce preventable postoperative complications may lead to improved disease-free and overall survival for patients.
Conclusions
The global death rate of cancer has continued to rise in the last 25 years, although there is a reduction in some countries. Within the United States, the combined cancer death rate has dropped by 26% from 1991 to 2015.[48] Improvements in cancer therapy have resulted in a large number of cancer survivors, concentrated in high-income countries. Global projections demonstrate that more cancer surgery patients in the future will be elderly, and likely more frail, requiring additional care in anesthetic technique. Worldwide efforts to prevent cancer are confounded by increasing rates of obesity, an increasing population of smokers, and other modifiable lifestyle factors that are connected to specific cancer types. Infectious disease remains a leading cause of liver and related cancers.
Anesthesiologists are already heavily involved in cancer care as most cancer admissions require surgery. When performing anesthesia for non-cancer surgery, our specialty needs to be cognizant that these patients will often develop cancer later in life. Evidence that the physiologic and psychological stress of surgery may lead to metastasis leads to the tantalizing suggestion that reducing this stress may improve recurrence-free survival. However, significant research is required to prove that theory. Anesthesiologists can lead the way in measuring both surgical and cancer survival outcomes for patients undergoing surgical procedures. These outcomes include a patient’s return to intended oncologic therapy and the incidence of preventable postoperative complications. The hope is that future research will show that perioperative interventions can lead to fewer cancer deaths and more cancer survivors.
Acknowledgments
Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Patrick J. McCormick declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Meara JG, Leather AJM, Hagander L, et al. (2015) Global Surgery 2030: Evidence and solutions for achieving health, welfare, and economic development. Lancet 386:569–624 [DOI] [PubMed] [Google Scholar]
- 2.•World Health Organization (2018) Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016 http://www.who.int/healthinfo/global_burden_disease/estimates/en/. Accessed 24 Aug 2018. [Google Scholar]; Statistical estimates for 176 causes of death worldwide, grouped by gender, location, and year.
- 3.•Naghavi M, Abajobir AA, Abbafati C, et al. (2017) Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England) 390:1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides annual estimates for more causes of death than the WHO GHE grouped by age, gender, location, and year.
- 4.World Health Organization (2013) Projections of mortality and causes of death, 2015 and 2030: global summary projections - top 20 causes http://www.who.int/healthinfo/global_burden_disease/GHE_DthGlobal_Proj_2015_2030.xls. Accessed 24 Aug 2018 [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 http://globocan.iarc.fr. Accessed 24 Aug 2018 [DOI] [PubMed] [Google Scholar]
- 6.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F (2012) International variation in prostate cancer incidence and mortality rates. Eur Urol 61:1079–1092 [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society (2014) The Burden: Survivorship http://canceratlas.cancer.org/the-burden/cancer-survivorship/. Accessed 24 Aug 2018 [Google Scholar]
- 8.••Allemani C, Matsuda T, Di Carlo V, et al. (2018) Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of the third cycle of the CONCORD program to estimate worldwide cancer survival metrics. Survival is generally increasing in most countries, with increases of up to 5% for liver, pancreatic, and lung cancer.
- 9.•Noone A, Howlader N, Krapcho M, et al. (2018) SEER Cancer Statistics Review, 19752015, National Cancer Institute https://seer.cancer.gov/csr/1975_2015/. Accessed 24 Aug 2018. [Google Scholar]; Comprehensive statistics for United States cancer patients, including mortality and survivorship.
- 10.Cancer Australia (2018) 5-year relative survival https://ncci.canceraustralia.gov.au/outcomes/relative-survival-rate/5-year-relativesurvival. Accessed 2 Oct 2018 [Google Scholar]
- 11.Cancer Registry of Norway (2017) Cancer in Norway 2016 - Cancer incidence, mortality, survival and prevalence in Norway. Cancer Registry of Norway, Oslo [Google Scholar]
- 12.Zeng H, Chen W, Zheng R, et al. (2018) Changing cancer survival in China during 2003– 15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Heal 6:e555– e567 [DOI] [PubMed] [Google Scholar]
- 13.Stewart BW, Wild CP (eds) (2014) World Cancer Report 2014. WHO Press, Lyon [Google Scholar]
- 14.Agency for Healthcare Research & Quality (2018) Total expenditures in millions by condition, United States, 1996–2015. Medical Expenditure Panel Survey https://meps.ahrq.gov/mepstrends/hc_cond/. Accessed 24 Aug 2018 [Google Scholar]
- 15.United Nations (2017) World Population Prospects The 2017 Revision Key Findings and Advance Tables. World Popul Prospect 2017 1–46 [Google Scholar]
- 16.Institute for Health Metrics and Evaluation (2018) Global Burden of Disease Results Tool http://ghdx.healthdata.org/gbd-results-tool. Accessed 24 Aug 2018 [Google Scholar]
- 17.Baijal P, Periyakoil V (2014) Understanding frailty in cancer patients. Cancer J (United States) 20:358–366 [DOI] [PubMed] [Google Scholar]
- 18.••Shahrokni A, Kim SJ, Bosl GJ, Korc-Grodzicki B (2017) How We Care for an Older Patient With Cancer. J Oncol Pract 13:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes the importance of the geriatric assessment in the colorectal cancer population. Includes a list of interventions by the authors’ geriatric service at each stage of perioperative care.
- 19.••Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group (2016) Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authoritative review of evidence for the association of obesity with site-specific cancer. The review found that 13 different cancers had sufficient evidence to say they are associated with obesity.
- 20.World Health Organization (2017) Global Health Observatory Data Repository: Prevalence of obesity among adults http://apps.who.int/gho/data/view.main.REGION2480A. Accessed 24 Aug 2018 [Google Scholar]
- 21.Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, Puckett M, Richardson LC (2017) Vital Signs : Trends in Incidence of Cancers Associated with Overweight and Obesity — United States, 2005–2014. MMWR Morb Mortal Wkly Rep 66:1052–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.•Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, Willett WC, Hu FB (2017) Associations of Weight Gain From Early to Middle Adulthood With Major Health Outcomes Later in Life. JAMA - J Am Med Assoc 318:255. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important analysis of two large cohort studies showing that weight gain during adulthood was associated with significantly increased risk of major chronic diseases, including cancer. Higher amounts of weight gain were associated with greater risks of major chronic diseases and a lower chance of healthy aging.
- 23.Ng M, Freeman MK, Fleming TD, et al. (2014) Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA - J Am Med Assoc 311:183–192 [DOI] [PubMed] [Google Scholar]
- 24.•GBD 2016 Alcohol Collaborators (2018) Alcohol use and burden: a systematic analysis from the Global Burden of Disease Study 2016 for 195 countries and territories, 19902016. Lancet 6736:1–21. [Google Scholar]; Controversial study calling for global reduction in alcohol consumption due to the number of annual global deaths that can be attributed to alcohol. Alcohol use was the leading risk factor in deaths among those aged 15–49. In the over 50 year old population, cancers constituted a large proportion of alcoholattributable deaths. The level of alcohol consumption that minimized harm was zero (95% CI 0.0–0.8) standard drinks per week.
- 25.World Cancer Research Fund/American Institute for Cancer Research (2018) Continuous Update Project Report: Diet, Nutrition, Physical Activity and Colorectal Cancer https://www.wcrf.org/sites/default/files/Colorectal-cancer-report.pdf. Accessed 24 Aug 2018 [Google Scholar]
- 26.Bouvard V, Baan R, Straif K, et al. (2009) A review of human carcinogens–Part B: biological agents. Lancet Oncol 10:321–2 [DOI] [PubMed] [Google Scholar]
- 27.Bray F, Jemal A, Grey N, Ferlay J, Forman D (2012) Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol 13:790–801 [DOI] [PubMed] [Google Scholar]
- 28.•Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M (2018) Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 6:e6–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using GLOBOCAN and a review of articles associating high BMI and diabetes with site specific cancer, these researchers estimated the number of new cancer cases attributable to obesity and diabetes.
- 29.Islami F, Goding Sauer A, Miller KD, et al. (2018) Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 68:31–54 [DOI] [PubMed] [Google Scholar]
- 30.Weiser TG, Haynes AB, Molina G, et al. (2016) Size and distribution of the global volume of surgery in 2012. Bull World Health Organ 94:201–209F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.••Sullivan R, Alatise OI, Anderson BO, et al. (2015) Global cancer surgery: Delivering safe, affordable, and timely cancer surgery. Lancet Oncol 16:1193–1224. [DOI] [PubMed] [Google Scholar]; Comprehensive look at the need for cancer surgery worldwide, presented by the Lancet Oncology Commission. Describes gaps in access to surgery, need for surgical personnel, and lack of key adjunct treatment modalities.
- 32.Shrime MG, Bickler SW, Alkire BC, Mock C (2015) Global burden of surgical disease: an estimation from the provider perspective. Lancet Glob Heal 3 Suppl 2:S8–9 [DOI] [PubMed] [Google Scholar]
- 33.Rose J, Chang DC, Weiser TG, Kassebaum NJ, Bickler SW (2014) The role of surgery in global health: Analysis of United States inpatient procedure frequency by condition using the global burden of disease 2010 framework. PLoS One doi: 10.1371/journal.pone.0089693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.•Pfister DG, Rubin DM, Elkin EB, Neill US, Duck E, Radzyner M, Bach PB (2015) Risk adjusting survival outcomes in hospitals that treat patients with cancer without information on cancer stage. JAMA Oncol 1:1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large differences in cancer survival exist for patients over 65 between different classes of hospitals within the United States, even after risk adjustment.
- 35.•Sirohi B, Chalkidou K, Pramesh CS, et al. (2018) Developing institutions for cancer care in low-income and middle-income countries: from cancer units to comprehensive cancer centres. Lancet Oncol 19:e395–e406. [DOI] [PubMed] [Google Scholar]; Overview of cancer center development history and the current development of cancer centers in low and middle income countries.
- 36.Monk TG, Saini V, Weldon BC, Sigl JC (2005) Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg 100:4–10 [DOI] [PubMed] [Google Scholar]
- 37.Lindholm M- LL, Träff S, Granath F, Greenwald SD, Ekbom A, Lennmarken C, Sandin RH (2009) Mortality within 2 years after surgery in relation to low intraoperative bispectral index values and preexisting malignant disease. Anesth Analg 108:508–12 [DOI] [PubMed] [Google Scholar]
- 38.••Tohme S, Simmons RL, Tsung A (2017) Surgery for cancer: A trigger for metastases. Cancer Res 77:1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review summarizes current research linking surgery, anesthesia, and inflammation to cancer recurrence.
- 39.Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S (2015) Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol 12:213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musser JE, Assel MJ, Meeks JJ, et al. (2015) Ambulatory Extended Recovery: Safely Transitioning to Overnight Observation for Minimally Invasive Prostatectomy. Urol Pract 2:121–125 [DOI] [PubMed] [Google Scholar]
- 41.•Chiu C, Aleshi P, Esserman LJ, Inglis-Arkell C, Yap E, Whitlock EL, Harbell MW (2018) Improved analgesia and reduced post-operative nausea and vomiting after implementation of an enhanced recovery after surgery (ERAS) pathway for total mastectomy. BMC Anesthesiol 18:41. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study comparing mastectomy outcomes before and after implementation of an Enhanced Recovery After Surgery (ERAS) pathway. The ERAS group had lower perioperative opioid consumption, lower incidence of postoperative nausea and vomiting, but no difference in length of stay.
- 42.McEvoy MD, Wanderer JP, King AB, Geiger TM, Tiwari V, Terekhov M, Ehrenfeld JM, Furman WR, Lee LA, Sandberg WS (2016) A perioperative consult service results in reduction in cost and length of stay for colorectal surgical patients : evidence from a healthcare redesign project. Perioper Med 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman JS, Roddy E, Ueda S, Brooks R, Chen L- L, Chen L- M (2016) Enhanced Recovery Pathways for Improving Outcomes After Minimally Invasive Gynecologic Oncology Surgery. Obstet Gynecol 128:138–44 [DOI] [PubMed] [Google Scholar]
- 44.••Aloia TA, Zimmitti G, Conrad C, Gottumukalla V, Kopetz S, Vauthey JN (2014) Return to intended oncologic treatment (RIOT): A novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol 110:107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]; Patients who failed to return to intended oncologic therapy had lower disease-free and overall survival. All patients who had minimally invasive surgery completed the intended oncologic therapy, versus only 75% of patients who had surgery via open approach.
- 45.Memeo R, de Blasi V, Adam R, et al. (2018) Postoperative Infectious Complications Impact Long-Term Survival in Patients Who Underwent Hepatectomies for Colorectal Liver Metastases: a Propensity Score Matching Analysis. J Gastrointest Surg doi: 10.1007/s11605-018-3854-2 [DOI] [PubMed] [Google Scholar]
- 46.Tevis SE, Kohlnhofer BM, Stringfield S, Foley EF, Harms BA, Heise CP, Kennedy GD (2013) Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum 56:1339–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luc G, Durand M, Chiche L, Collet D (2015) Major post-operative complications predict long-term survival after esophagectomy in patients with adenocarcinoma of the esophagus. World J Surg 39:216–22 [DOI] [PubMed] [Google Scholar]
- 48.•Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68:7– 30. [DOI] [PubMed] [Google Scholar]; Annual statement of trends in United States cancer mortality and incidence, focusing on regional and racial disparities.


