Supplemental Digital Content is available in the text.
Keywords: brain natriuretic peptide; hypertension, pulmonary; prostacyclin receptor; risk assessment
Abstract
Background:
NT-proBNP (N-terminal pro brain natriuretic peptide) levels are included in the multiparametric risk assessment approach for pulmonary arterial hypertension (PAH) outlined in PAH guidelines. However, data supporting the use of NT-proBNP risk thresholds in assessing prognosis in PAH are limited. The GRIPHON trial (Prostacyclin [PGI2] Receptor Agonist In Pulmonary Arterial Hypertension) provides an opportunity to assess the prognostic value of NT-proBNP thresholds in a controlled clinical trial and to evaluate the response to selexipag according to these thresholds.
Methods:
The event-driven GRIPHON trial randomly assigned patients to selexipag or placebo. NT-proBNP was measured at regular intervals in GRIPHON. Here, patients were categorized post hoc into low, medium, and high NT-proBNP subgroups according to 2 independent sets of thresholds: (1) baseline tertiles: <271 ng/L; 271 to 1165 ng/L; >1165 ng/L; and (2) 2015 European Society of Cardiology/European Respiratory Society guidelines cutoffs: <300 ng/L; 300 to 1400 ng/L; >1400 ng/L. Hazard ratios (selexipag versus placebo) with 95% CIs were calculated for the primary end point (composite morbidity/mortality events) by NT-proBNP category at baseline using Cox proportional-hazards models, and at any time during the exposure period using a time-dependent Cox model.
Results:
With both thresholds, baseline and follow-up NT-proBNP categories were highly prognostic for future morbidity/mortality events during the study (P<0.0001). In the time-dependent analysis, the risk of experiencing a morbidity/mortality event was 92% and 83% lower in selexipag-treated patients with a low and medium NT-proBNP level, and 90% and 56% lower in placebo-treated patients with a low and medium NT-proBNP level, in comparison with patients with a high NT-proBNP level. Selexipag reduced the risk of morbidity/mortality events across all 3 NT-proBNP categories in both the baseline and time-dependent analyses, with a more pronounced treatment benefit of selexipag seen in the medium and low NT-proBNP subgroups (interaction P values 0.20 and 0.007 in the baseline and time-dependent analyses).
Conclusions:
These analyses further establish the prognostic relevance of NT-proBNP levels in PAH and provide first evidence for the association of NT-proBNP level and treatment response. Using 2 similar sets of thresholds, these analyses support the relevance of the low, medium, and high NT-proBNP categories as part of the multiparametric risk assessment approach outlined in the European Society of Cardiology/European Respiratory Society guidelines for the management of PAH patients.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01106014.
Clinical Perspective.
What Is New?
These post hoc analyses from the GRIPHON study (Prostacyclin [PGI2] Receptor Agonist In Pulmonary Arterial Hypertension) provide the first assessment of response to pulmonary arterial hypertension therapy in patients categorized according to NT-proBNP (N-terminal pro brain natriuretic peptide) levels.
Although a positive treatment response was seen across all 3 NT-proBNP categories, some analyses suggested a more pronounced treatment response for patients with levels of NT-proBNP in the low and medium ranges.
Our findings also highlight the prognostic value of NT-proBNP levels.
What Are the Clinical Implications?
NT-proBNP levels are highly prognostic of pulmonary arterial hypertension progression, and patients with persistently high NT-proBNP have the highest risk of disease progression.
This indicates that having NT-proBNP in the low range, by improving to or maintaining low NT-proBNP levels, is a clinically relevant treatment goal for patients with pulmonary arterial hypertension.
Although selexipag was beneficial in all NT-proBNP categories, the treatment effect may be greater in the low and medium categories (versus high), suggesting that earlier selexipag treatment may be of greater benefit.
Treatment escalation should be considered for patients with persistently high NT-proBNP or who experience deterioration to the high NT-proBNP subgroup while receiving selexipag.
Multiparameter risk assessment has an essential role in determining prognosis and guiding treatment decisions for patients with pulmonary arterial hypertension (PAH). NT-proBNP (N-terminal pro brain natriuretic peptide) is an integral component of risk assessment in PAH and is included in both the risk score developed from the REVEAL registry (Registry to Evaluate Early And Long-term PAH Disease Management)1–3 and the risk stratification approach outlined in the 2015 European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines.3–5 NT-proBNP is secreted by cardiomyocytes in response to ventricular stretch and is an established noninvasive marker of right ventricular dysfunction.6 In pulmonary hypertension, including PAH, plasma NT-proBNP levels correlate with functional capacity, right ventricular function, and echocardiographic and hemodynamic variables7–9 and have been shown to be an independent predictor of survival in a number of small observational studies.1,9–15 Recent data from the REVEAL registry indicate that a baseline NT-proBNP level of ≤340 ng/L strongly predicted improved survival up to 5 years in patients with PAH.16 The ESC/ERS guidelines recommend that NT-proBNP levels are categorized as low (<5%), intermediate (5%–10%), or high (>10%) risk of 1-year mortality by using thresholds of 300 ng/L and 1400 ng/L. However, the data supporting these thresholds are limited, because they were selected based on data from only a few observational studies in PAH1,9 and expert consensus. To date these thresholds have not been validated in a clinical trial setting or in a population that included prevalent patients. Furthermore, there are no data investigating the association of NT-proBNP category with response to treatment in PAH.
Selexipag is an oral, selective IP prostacyclin receptor agonist approved for the long-term treatment of PAH in adult patients with World Health Organization functional class (WHO FC) II to III. In the phase III GRIPHON study (Prostacyclin [PGI2] Receptor Agonist In Pulmonary Arterial Hypertension), the largest PAH randomized, controlled trial to date, selexipag reduced the risk of the primary composite outcome of morbidity/mortality by 40% (P<0.001) in comparison with placebo in a largely pretreated population.17 Change in NT-proBNP was included as an exploratory end point in GRIPHON, which showed that the median NT-proBNP level at 26 weeks decreased in the selexipag group and increased in the placebo group (median treatment effect of –123 ng/L [P<0.001] for selexipag versus placebo).17
The objective of these analyses was to evaluate the prognostic and predictive value of low, medium, and high NT-proBNP categories in the large GRIPHON population of patients with PAH to validate their use in a multiparameter risk assessment approach in PAH.
Methods
The data sharing policy of the Sponsor is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.18.
Study Population
GRIPHON (NCT01106014) was a global, double-blind, randomized, placebo-controlled event-driven phase III study that assessed the safety and efficacy of selexipag in patients with PAH and has previously been described in detail.17 Patients (18–75 years old) with a diagnosis of idiopathic PAH, heritable PAH, or PAH associated with connective tissue disease, repaired congenital systemic-to-pulmonary shunts, HIV infection, drug use, or toxin exposure were eligible. The diagnosis of PAH had to be confirmed by right heart catheterization at any time before screening with a pulmonary vascular resistance ≥400 dyn·sec·cm−5, and patients were required to have a 6-minute walk distance (6MWD) of 50 to 450 m at screening. Eligible patients were permitted to take stable PAH background therapy including an endothelin receptor antagonist, a phosphodiesterase type-5 inhibitor, or both.
Study Design and Outcomes
The GRIPHON trial was conducted in accordance with the amended Declaration of Helsinki and the protocol was reviewed by local institutional review boards with written informed consent obtained from all patients. Patients were randomly assigned 1:1 to receive selexipag or placebo twice daily, and study drug was titrated to the highest tolerated dose or the maximum dose of 1600 µg twice daily. Double-blind treatment continued until a patient experienced a primary end point event, or until premature discontinuation of double-blind treatment (eg, because of an adverse event [AE]) or until the end of the study. End of treatment was defined as 7 days after the last intake of selexipag or placebo. GRIPHON used a composite primary end point of time from randomization to first morbidity/mortality event up to end of treatment. Morbidity events were defined as disease progression, or worsening of PAH that resulted in hospitalization, initiation of parenteral prostanoid therapy or long-term oxygen therapy, or need for lung transplantation or balloon atrial septostomy. Disease progression was defined as a decrease from baseline of at least 15% in 6MWD, and worsening in WHO FC (for patients in WHO FC II or III at baseline) or the need for additional PAH therapy (for patients in WHO FC III or IV at baseline). All primary end point events were adjudicated by a blinded independent critical event committee.
NT-proBNP Measurement
Plasma concentrations of NT-proBNP were determined at a central laboratory using a validated electrochemiluminescence immunoassay (Roche Elecsys proBNP II) at baseline, weeks 4, 8, 16, and 26, and at 6-month intervals thereafter.
NT-proBNP Subgroups
For each NT-proBNP assessment time point, patients were categorized into low, medium, and high NT-proBNP subgroups according to 2 sets of thresholds: (1) thresholds defined as the tertiles of the baseline distribution of NT-proBNP values in GRIPHON (low: <271 ng/L, medium: 271−1165 ng/L, high: >1165 ng/L) and (2) the thresholds outlined in the ESC/ERS guidelines4,5 (low: <300ng/L, medium: 300−1400 ng/L, high: >1400 ng/L). The main analyses were based on baseline tertile thresholds, and results from the guidelines thresholds are included in the online-only Data Supplement. Patients were also categorized by change in NT-proBNP category over time as follows: stable low (low NT-proBNP at baseline and at week 26), stable medium (medium NT-proBNP at baseline and at week 26), stable high (high NT-proBNP at baseline and at week 26), improved (high at baseline and medium or low at week 26, or medium at baseline and low at week 26), and deteriorated (low at baseline and medium or high at week 26, or medium at baseline and high at week 26).
Statistical Analyses
Post hoc analyses on the primary end point (composite morbidity/mortality events) were performed in patient subgroups determined by NT-proBNP category and by the change in NT-proBNP category from baseline to week 26. The analysis by NT-proBNP category at baseline included all randomly assigned patients with an NT-proBNP level measured at baseline. For the analysis by change in NT-proBNP category that follows patients for the occurrence of a primary end point from the week 26 time point, the landmark methodology19 was used. This analysis included data from patients who were still receiving double-blind treatment at week 26 (ie, excluding patients who had a primary end point or had prematurely discontinued treatment before the week 26 time point) and for whom NT-proBNP levels were available at baseline and week 26. The association between NT-proBNP category at baseline, or change in NT-proBNP category, and the risk of a primary end point was assessed using Kaplan-Meier estimates by treatment arm and subgroup, and using Cox proportional-hazards models to calculate hazard ratios (HRs) with 95% CIs. A time-dependent Cox proportional model was used to evaluate the association of the NT-proBNP category at any time during the study and the primary end point. This model evaluates the risk of an event for the low, medium, and high NT-proBNP categories at any time during the study.
Sensitivity analyses were performed that adjusted for the following prognostic baseline covariates: treatment, background PAH therapy, WHO FC, sex, race, age group (<65 years versus ≥65 years), PAH classification, geographical region, creatinine, and 6MWD. Consistency of treatment effects across subgroups were assessed using interaction tests.
A subpopulation treatment effect pattern plot analysis20 was performed to descriptively explore whether the hazard ratio (ie, treatment effect) of selexipag versus placebo on the primary end point changed as a function of NT-proBNP level at baseline. Patients were ranked according to their NT-proBNP level at baseline (from lowest to highest) and divided into multiple overlapping subpopulations. A Cox model was fitted separately to each subpopulation to obtain a hazard ratio for selexipag versus placebo. The hazard ratios were plotted against the median values for NT-proBNP level at baseline for each of the subpopulations.
Results
Patient Characteristics
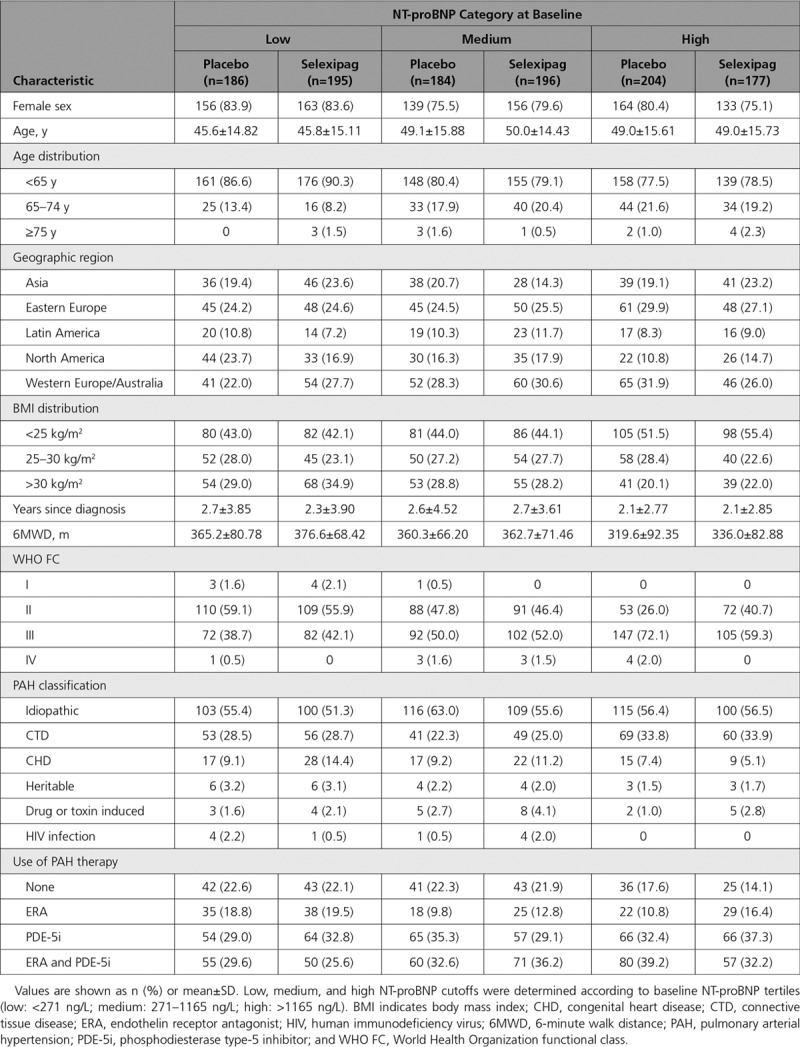
In the 1142 GRIPHON patients with an NT-proBNP value at baseline, median NT-proBNP levels at baseline were 546.5 ng/L in the selexipag group and 562.5 ng/L in the placebo group. Using NT-proBNP tertiles defined at baseline, 381, 380, and 381 patients were classified in the low, medium, and high NT-proBNP subgroups, respectively (Figure I in the online-only Data Supplement). Baseline characteristics by NT-proBNP subgroup are shown in Table 1. Patients in the low NT-proBNP subgroup tended to be younger, to have higher 6MWD, were less likely to be in WHO FC III/IV, and were less likely to be on combination therapy in comparison with patients in the high NT-proBNP group (Table 1).
Table 1.
Baseline Characteristics for Patients Grouped According to NT-proBNP (N-Terminal Pro Brain Natriuretic Peptide) Level
For the 867 patients with an NT-proBNP value at baseline and at week 26, the number and percentage of patients with NT-proBNP levels classified as stable low, stable medium, stable high, improved, or deteriorated are shown in Figure 1. An improvement in NT-proBNP category was observed more frequently for the selexipag-treated patients than for the placebo-treated patients (17.4% versus 8.3%). Conversely, fewer selexipag-treated patients experienced a deterioration in NT-proBNP category than placebo-treated patients (9.3% versus 15.8%). Similar results were obtained using subgroups defined according to the guidelines thresholds (Figure II in the online-only Data Supplement).
Figure 1.
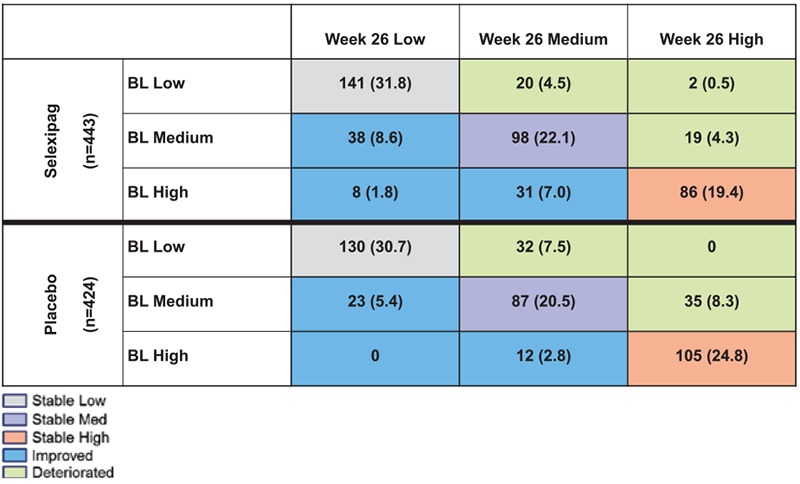
Shift table for change in NT-proBNP (N-terminal pro brain natriuretic peptide) category from baseline to week 26. Values are shown as n (%). Low, medium, and high NT-proBNP cutoffs were determined according to baseline NT-proBNP tertiles (low: <271 ng/L; medium: 271–1165 ng/L; high: >1165 ng/L). Analyses were performed only in patients who were still in the double-blind treatment at week 26 and who had an NT-proBNP value at baseline and week 26; 289 patients randomly assigned at baseline were excluded from these analyses for the following reasons (categories are not mutually exclusive): primary end point event before week 26 (n=147), premature discontinuation of double-blind treatment before week 26 (n=121), missing NT-proBNP value at baseline (n=14) or at week 26 (n=239). BL indicates baseline.
NT-proBNP Category and Long-Term Outcome
When placebo-treated patients were categorized based on their NT-proBNP level at baseline, the percentages of patients who experienced a morbidity/mortality event increased from 21.5% to 43.5% to 57.8% in the low, medium, and high NT-proBNP subgroups, respectively (Table 2). A similar pattern was observed for selexipag-treated patients (Figure 2, Table 2). NT-proBNP category at baseline was highly prognostic for the occurrence of morbidity/mortality events (P<0.0001), both with and without adjustment for baseline covariates (P<0.0001). Similar results were observed in the subgroups defined using the thresholds in the ESC/ERS guidelines (Figure III in the online-only Data Supplement).
Table 2.
Proportion of Patients With Morbidity/Mortality Events by NT-proBNP (N-Terminal Pro Brain Natriuretic Peptide) Category at Baseline
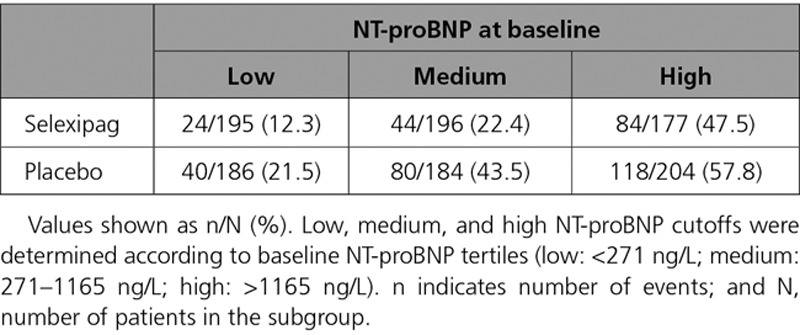
Figure 2.
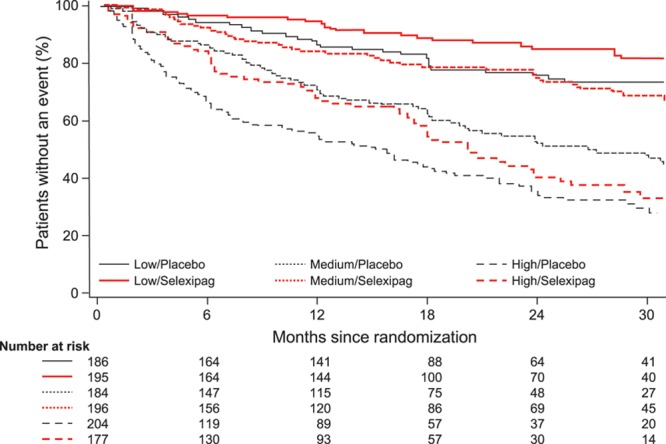
Time from randomization to first morbidity or mortality event in subgroups defined by NT-proBNP (N-terminal pro brain natriuretic peptide) level at baseline. Low, medium, and high NT-proBNP cutoffs were determined according to baseline NT-proBNP tertiles (low: <271 ng/L; medium: 271–1165 ng/L; high: >1165 ng/L).
Differences in the risk of a morbidity/mortality event between NT-proBNP categories determined at baseline were also assessed using a time-dependent Cox model (Table 3). Selexipag-treated patients with a low or medium NT-proBNP level had a 92% and 83% reduced risk of experiencing a morbidity/mortality event in comparison with patients with a high NT-proBNP level, irrespective of when NT-proBNP was assessed (Table 3). Similar, albeit less pronounced, differences in risk were observed for placebo-treated patients. Consistent results were observed after adjusting for baseline covariates (Table 3). Sensitivity analyses restricted to 36 months follow-up provided comparable results. Similar results were observed using the thresholds in the ESC/ERS guidelines (data not shown).
Table 3.
Risk of Morbidity/Mortality Adjusted by Time-Dependent NT-proBNP (N-Terminal Pro Brain Natriuretic Peptide) Category
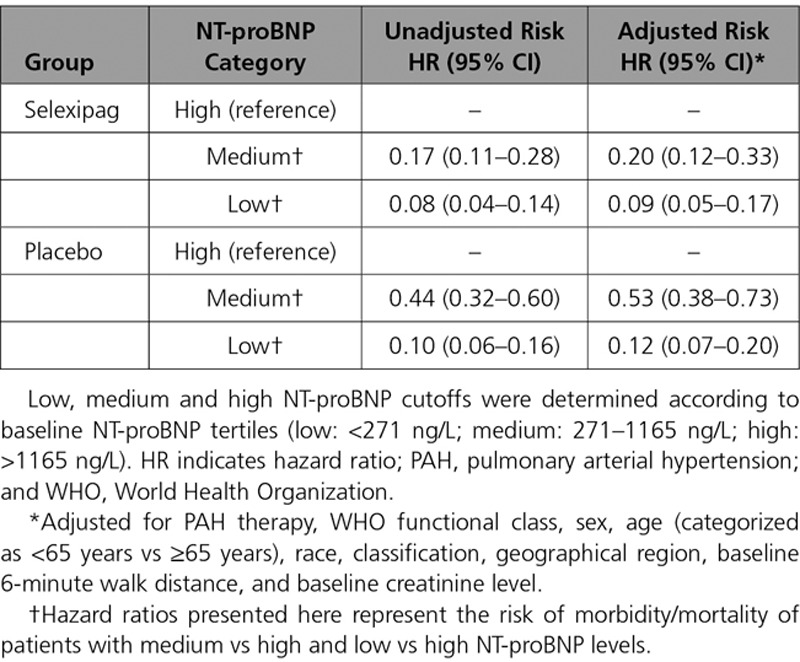
The incidence of morbidity/mortality events for placebo- and selexipag-treated patients in the stable low, stable medium, stable high, improved, and deteriorated NT-proBNP subgroups are shown in Figure 3. Irrespective of treatment, the risk of morbidity/mortality was lowest for patients with a stable low or improved NT-proBNP level. The highest risk was observed for patients receiving selexipag with a stable high NT-proBNP.
Figure 3.
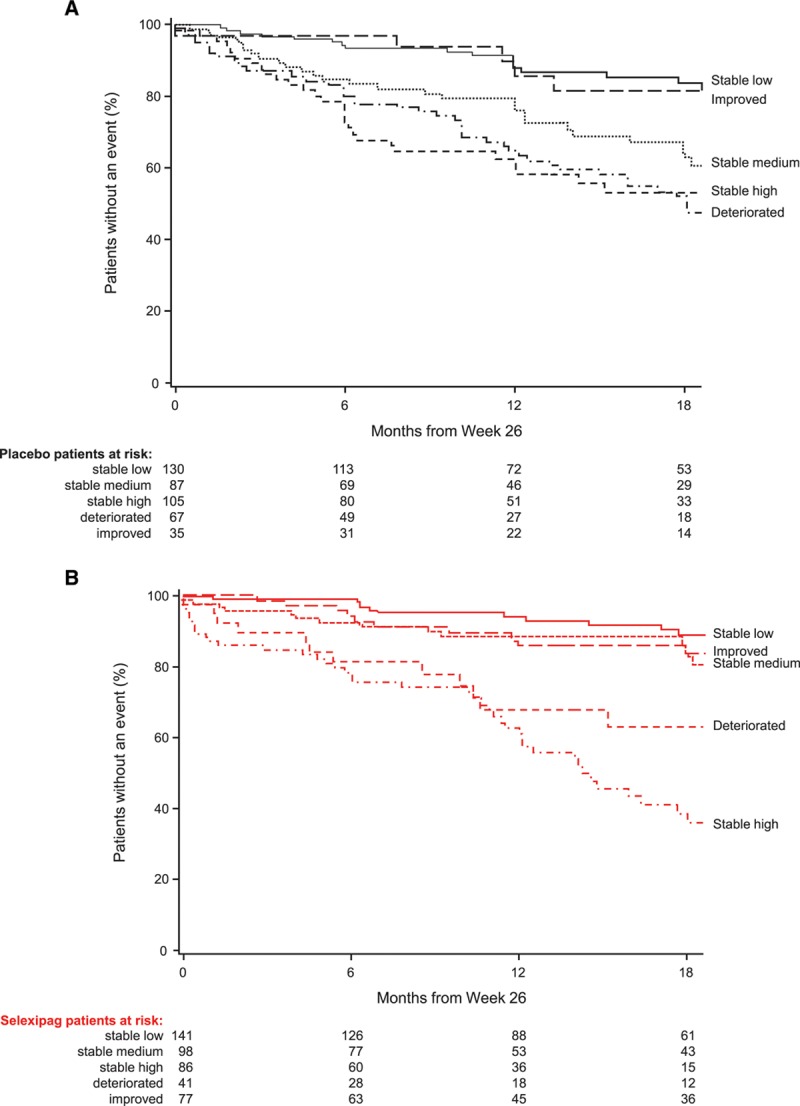
Time from week 26 to first morbidity or mortality event based on change in NT-proBNP (N-terminal pro brain natriuretic peptide) category from baseline to week 26 for placebo and selexipag patients. Data shown for (A) patients receiving placebo and (B) patients receiving selexipag. Low, medium, and high NT-proBNP cutoffs determined according to baseline NT-proBNP tertiles (low: <271 ng/L; medium: 271–1165 ng/L; high: >1165 ng/L). Subgroups are defined as: stable low (low NT-proBNP at baseline and at week 26), stable medium (medium NT-proBNP at baseline and at week 26), stable high (high NT-proBNP at baseline and at week 26), improved (high at baseline and medium or low at week 26, or medium at baseline and low at week 26), and deteriorated (low at baseline and medium or high at week 26, or medium at baseline and high at week 26).
NT-proBNP Category and Response to Treatment
In the overall GRIPHON population, the hazard ratio for the primary morbidity/mortality end point was 0.60 (99% CI, 0.46–0.78) for selexipag versus placebo.17 Hazard ratios for the primary end point indicated a benefit from selexipag in all baseline NT-proBNP subgroups: low subgroup HR 0.57 (95% CI, 0.34–0.94), medium subgroup 0.48 (95% CI, 0.33–0.70), and high subgroup 0.73 (95% CI, 0.55–0.96; Figure 4A). Although the point estimates of the HRs were greater in the low and medium NT-proBNP subgroups, the interaction test showed no evidence for heterogeneity in the treatment effect of selexipag between the low, medium, and high NT-proBNP subgroups (nonsignificant interaction P value 0.20). Similar results were obtained in sensitivity analyses, which adjusted for baseline covariates (Table I in the online-only Data Supplement) and in subgroups defined according to the ESC/ERS guidelines thresholds (Table I in the online-only Data Supplement and Figure III in the online-only Data Supplement).
Figure 4.
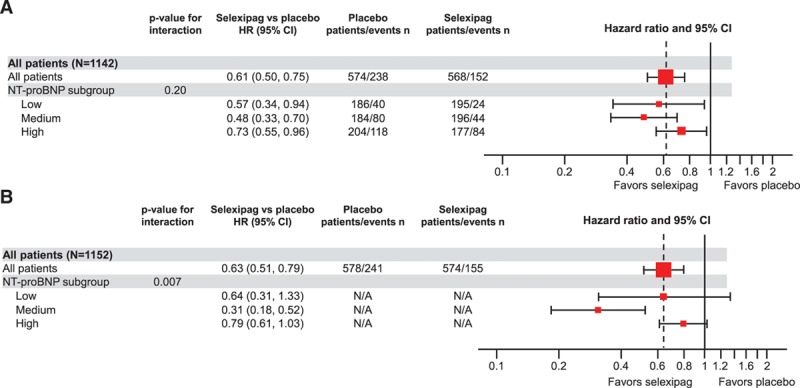
Treatment effect of selexipag on time from randomization to first morbidity or mortality event. A, Treatment effect in subgroups defined by NT-proBNP (N-terminal pro brain natriuretic peptide) level at baseline. B, Treatment effect by time-dependent NT-proBNP category. Low, medium, and high NT-proBNP cutoffs were determined according to baseline NT-proBNP tertiles (low: <271 ng/L; medium: 271–1165 ng/L; high: >1165 ng/L). HR indicates hazard ratio; and N/A, not available.
To further explore the relationship between NT-proBNP levels at baseline and the treatment effect of selexipag on the primary end point of morbidity/mortality, a subpopulation treatment effect pattern plot analysis was performed (Figure 5). Results of this analysis suggest that the treatment effect of selexipag versus placebo may be greater in magnitude in subpopulations with NT-proBNP levels in the middle and low range.
Figure 5.
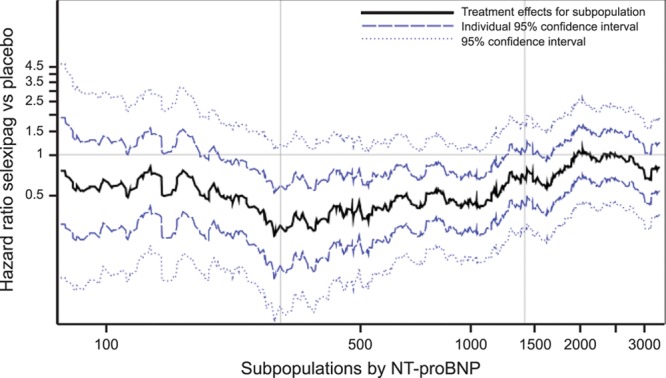
Subpopulation treatment effect pattern plot showing the treatment effect of selexipag vs placebo on morbidity/mortality (hazard ratio plus 95% CIs and 95% confidence bands) by NT-proBNP (N-terminal pro brain natriuretic peptide) level at baseline. The black line indicates the hazard ratio of each subpopulation, the dark blue dashed line indicates the 95% CI for each individual subpopulation. The light blue dashed line shows the 95% confidence bands, which define the confidence interval of the best-fit line and indicate with 95% certainty the bounds within which the true curve will fall. Vertical lines indicate the 300 ng/L and 1400 ng/L NT-proBNP thresholds. HR indicates hazard ratio.
The treatment effect of selexipag by NT-proBNP category at any time during the study was assessed using a time-dependent Cox model analysis. The HRs for the primary end point favored selexipag in all NT-proBNP subgroups: low subgroup HR 0.64 (95% CI, 0.31–1.33), medium subgroup 0.31 (95% CI, 0.18–0.52), and high subgroup 0.79 (95% CI, 0.61–1.03). A significant interaction P value was seen, suggesting a greater treatment effect for the medium and low NT-proBNP subgroups in comparison with the high NT-proBNP subgroup (interaction P value 0.007; Figure 4B). Similar HRs were observed using the thresholds in the ESC/ERS guidelines (data not shown).
Safety
Overall, 11.3%, 15.7%, and 16.4% of selexipag-treated patients and 6.5%, 6.0%, and 8.8% of placebo-treated patients in the low, medium, and high NT-proBNP subgroups, respectively, discontinued treatment prematurely because of an AE. The most frequent AE associated with therapies targeting the prostacyclin pathway was headache, which affected 33.1%, 32.8%, and 29.2% of the selexipag-treated patients (placebo-corrected) in the low, medium, and high-NT-proBNP subgroups, respectively (Table II in the online-only Data Supplement). Irrespective of treatment, patients in the medium and high NT-proBNP subgroups experienced more serious AEs than patients in the low NT-proBNP subgroup (Table II in the online-only Data Supplement). This result was driven by serious AEs related to the underlying disease (eg, PAH and right ventricular failure). In all 3 NT-proBNP subgroups, the frequency of a serious AE of PAH was higher in the placebo group than in the selexipag group (low: 10.8% versus 5.6%; medium: 19.1% versus 13.2%; high: 34.3% versus 24.9%).
Discussion
The GRIPHON trial included the largest number of patients with PAH evaluated to date in a randomized, controlled trial, of which >98% patients had an NT-proBNP value measured at baseline. This provided an opportunity to assess the prognostic and predictive value of NT-proBNP thresholds in a large population using both the ESC/ERS guidelines thresholds and newly derived baseline tertiles thresholds. In this GRIPHON post hoc analysis, NT-proBNP category was highly prognostic for long-term outcomes using both NT-proBNP baseline tertiles and the ESC/ERS guidelines thresholds. These analyses also suggest that NT-proBNP category during follow-up can be used to predict long-term treatment response.
The ESC/ERS guidelines recommend the use of NT-proBNP as part of a multiparametric assessment for prognosis and as treatment goals for patients with PAH, and provide thresholds to define low (<300 ng/L), intermediate (300–1400 ng/L), and high risk (>1400 ng/L) levels of NT-proBNP.5 Although recent registry analyses have demonstrated that these thresholds have strong prognostic relevance in patients with incident PAH,21,22 data supporting the specific thresholds currently recommended and data in prevalent patients are more limited. Four studies in PAH or PAH plus chronic thromboembolic pulmonary hypertension used receiver operating characteristic curve analysis to identify threshold values to predict survival in treatment naïve patients.9,10,13,14 Cutoff values of 705 ng/L (n=95, Zelniker et al14), 1256 ng/L (n=161, Mauritz et al13), 1400 ng/L (n=55, Fijalkowska et al9), and 1800 ng/L (n=109, Nickel et al10) were identified. Although generally in the range of the high-risk cutoff used in the current guidelines (>1400 ng/L),5 considerable variability was seen. In addition, the lowest-risk tier in the current guidelines, an NT-proBNP level <300 ng/L,5 was not initially identified in PAH receiver operating characteristic curve analysis (although it is used in excluding acute heart failure in other settings23). Limitations of the current thresholds therefore include the small samples sizes, the lack of separate validation cohorts, the inclusion of only treatment-naïve patients, the variability in the thresholds identified in receiver operating characteristic analyses9,10,13,14 and the lack of studies specifically designed to identify cutoffs for 3 tiers of risk.
Given these limitations, both baseline tertile values, and the NT-proBNP threshold values from the 2015 ESC/ERS guidelines were analyzed in the current study. Our results support the relevance of using low, medium, and high NT-proBNP categories in PAH risk assessment. The differences in cutoff values used in the ESC/ERS guidelines and the baseline NT-proBNP tertiles (300 ng/L versus 271 ng/L for the low/medium threshold, and 1165 ng/L versus 1400 ng/L for the medium/high threshold) also suggest that these thresholds should be considered as guidance to clinicians and not as absolute boundaries between risk categories.
Our analyses by change in NT-proBNP category provide evidence that highlights the importance of maintaining patients in the low NT-proBNP category or improving their NT-proBNP category as potential treatment goals.
This study also presents the first data showing an association between NT-proBNP category and response to long-term treatment in PAH. Selexipag reduced the risk of morbidity/mortality across all baseline NT-proBNP categories. The treatment effect appeared numerically higher in the low and medium subgroups, but the interaction P value was not statistically significant. In the time-dependent analysis, a benefit was also observed in all subgroups regardless of when the NT-proBNP categorization was performed. However, more pronounced treatment effects were seen in the low and medium NT-proBNP subgroups, and the interaction P value in this case was statistically significant. These results combined with the poor long-term outcomes and higher discontinuation rates for patients with NT-proBNP in the persistently high range suggest that treatment escalation should be considered for individual patients who remain in or experience deterioration to the high NT-proBNP subgroup while receiving selexipag. These findings also support close monitoring of patients at any time during follow-up to ensure that changes in treatment are made at the appropriate time.
The results presented here provide an in-depth analysis of NT-proBNP threshold values in the context of a randomized, controlled trial and further support the use of the NT-proBNP categories outlined in the ESC/ERS guidelines in the assessment and follow-up of patients with PAH. It is not surprising that patients with high NT-proBNP at baseline also tended to be in a higher WHO FC and have low 6MWD values. However, it is interesting to note that 32.8% of patients in the high NT-proBNP subgroup were also in WHO FC II, demonstrating that a single parameter cannot provide sufficient information to fully assess risk in patients with PAH, and multiple parameters should be considered when determining prognosis and making treatment decisions. Using such a multiparametric approach to risk assessment is recommended in the ESC/ERS guidelines4,5 and supported by several analyses of PAH registry data, which have highlighted the strong prognostic relevance of WHO FC, 6MWD, and NT-proBNP levels at baseline and follow-up.21,22,24
Selexipag was generally well-tolerated, irrespective of NT-proBNP category at baseline. The rates of discontinuations and number of serious AEs related to PAH were higher in patients in the medium and high NT-proBNP subgroups than in those in the low NT-proBNP subgroup. These results are not unexpected given that NT-proBNP is a marker for ventricular dysfunction and patients with high NT-proBNP are likely to have more advanced disease.
These analyses are subject to a number of limitations. Although GRIPHON provided a large data set appropriate for the analysis of NT-proBNP levels, analyses presented here are post hoc and exploratory in nature. The landmark analysis used to assess the effect of change in NT-proBNP category uses a single time point at week 26 and therefore excludes patients who are no longer receiving double-blind treatment at week 26. Because these analyses do not provide an overall effect of change in NT-proBNP category throughout the study, analyses using a time-dependent covariate model were performed to address these limitations. Finally, the NT-proBNP baseline tertile thresholds used in this study were derived from and applied to the same patient population.
Conclusions
The GRIPHON trial provided an opportunity to evaluate the prognostic and predictive value of NT-proBNP in patients with PAH in detail in the context of an randomized, controlled trial. In our analyses, NT-proBNP category at baseline and at any time during follow-up, irrespective of whether the categories were based on the ESC/ERS guidelines thresholds or baseline tertiles, was shown to be highly prognostic for long-term outcomes and can be used to predict response to selexipag treatment. In addition, we show that change in NT-proBNP category has some prognostic value. The data presented in this study therefore support the inclusion of NT-proBNP categories as part of the multiparametric risk assessment of patients with PAH at baseline and follow-up, as outlined in the ESC/ERS guidelines.
Acknowledgments
Medical writing assistance was provided by S. Carter and J. Glasper (nspm ltd, Meggen, Switzerland).
Sources of Funding
GRIPHON was funded by Actelion Pharmaceuticals Ltd, Allschwil, Switzerland.
Disclosures
Dr Chin has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received research grants from Actelion Pharmaceuticals Ltd, National Institutes of Health, Ironwood Pharmaceuticals, National Institutes of Health and SoniVie; has served on an advisory board for Bayer Healthcare (through UCSD) and Flowonix; has served as an adjudication committee member for Arena Pharmaceuticals; is Circulation Associate Editor for American Heart Association; and has received consultancy fees from Actelion Pharmaceuticals Ltd. Dr Rubin has served as a steering committee member for Actelion Pharmaceuticals Ltd; and has received consultancy fees from Actelion Pharmaceuticals Ltd, Arena Pharmaceuticals, GeNO Pharmaceuticals, Gilead, Karos Pharmaceuticals, Pfizer, and SoniVie Ltd. Dr Channick has served as a steering committee member for Actelion Pharmaceuticals Ltd; has served on an advisory board for Actelion Pharmaceuticals Ltd and Bayer; has received consultancy fees from Bayer and Arena Pharmaceuticals; and has received research grants from Actelion Pharmaceuticals Ltd and United Therapeutics. Dr Di Scala is an employee of Actelion Pharmaceuticals Ltd and in the past held stock/stock options for Actelion Pharmaceuticals Ltd and currently holds stock/stock options in the parent company Johnson&Johnson. Dr Gaine has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received speaker fees from Actelion Pharmaceuticals Ltd; has received advisory board fees from Actelion Pharmaceuticals Ltd, and Daiichi-Sankyo; and has served on a data and safety monitoring board for United Therapeutics. Dr Galiè is a steering committee member for Actelion Pharmaceuticals Ltd; has received grant support, personal fees, and nonfinancial support from Actelion Pharmaceuticals Ltd; and has received grant support and personal fees from Bayer Healthcare, Pfizer, and GlaxoSmithKline. Dr Ghofrani has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received advisory board and speaker fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, Novartis, and Pfizer; has received consultancy fees from Actelion Pharmaceuticals Ltd, Bayer, Bellerophon Pulse Technologies, GlaxoSmithKline, Merck Sharp & Dohme, Novartis, and Pfizer; and has received research grants from Actelion Pharmaceuticals Ltd and Deutsche Forschungsgemeinschaft. Dr Hoeper has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received speaker and consultancy fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, Merck Sharp & Dohme, and Pfizer; and has received research grants from Actelion Pharmaceuticals Ltd. Dr Lang has served as a steering committee member for Actelion Pharmaceuticals Ltd; has received speaker fees from Actelion Pharmaceuticals Ltd, Merck Sharp & Dohme, and AOP Orphan Pharmaceuticals; and has received research grants from Actelion Pharmaceuticals Ltd and AOP Orphan Pharmaceuticals. Dr McLaughlin reports grants, personal fees and nonfinancial support from Actelion Pharmaceuticals Ltd and Bayer; grants from Eiger and SoniVie; and personal fees from United Therapeutics, Arena, Caremark, Medtronic, and Merck Sharp & Dohme. Dr Preiss is an employee of Actelion Pharmaceuticals Ltd and in the past held stock/stock options for Actelion Pharmaceuticals Ltd and currently holds stock/stock options in the parent company Johnson&Johnson. Dr Simonneau has served as a steering committee member for and received research grants from Actelion Pharmaceuticals Ltd and Bayer; and has received speaker and consultancy fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, Merck Sharp & Dohme, and Pfizer. Dr Sitbon has served as a steering committee member for Actelion Pharmaceuticals Ltd; has served as an advisory board member for and received research grants from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, and Merck Sharp & Dohme; has received consultancy fees from Actelion Pharmaceuticals Ltd, Arena, Bayer, GlaxoSmithKline, and Merck Sharp & Dohme; has received speaker fees from Actelion Pharmaceuticals Ltd, Bayer, GlaxoSmithKline, and Merck Sharp & Dohme; has served on a scientific advisory board for Arena Pharmaceuticals and Gossamer Bio; and has received writing assistance from Actelion Pharmaceuticals Ltd and GlaxoSmithKline. Dr Tapson has served as a steering committee member for Actelion Pharmaceuticals Ltd, Bayer, and United Therapeutics; has received consultancy fees from Actelion Pharmaceuticals Ltd, Arena Pharmaceuticals, Bayer, Daiichi-Sankyo, EKOS/BTG, Gilead Sciences, Janssen, Reata, and United Therapeutics; has received research grants from Arena Pharmaceuticals, Arena, Bayer, EKOS/BTG, and Riata; has received speaker fees from Bayer, Gilead Sciences, and Janssen.
Supplementary Material
Footnotes
Sources of Funding, see page 2449
Guest editor for this article was Werner Budts, MD, PhD.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.118.039360.
References
- 1.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 2.Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, Badesch DB, McGoon MD. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141:354–362. doi: 10.1378/chest.11-0676. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2018;53:1801889. doi: 10.1183/13993003.01889-2018. doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 6.Lador F, Soccal PM, Sitbon O. Biomarkers for the prognosis of pulmonary arterial hypertension: Holy Grail or flying circus? J Heart Lung Transplant. 2014;33:341–343. doi: 10.1016/j.healun.2013.12.012. doi: 10.1016/j.healun.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Souza R, Bogossian HB, Humbert M, Jardim C, Rabelo R, Amato MB, Carvalho CR. N-terminal-pro-brain natriuretic peptide as a haemodynamic marker in idiopathic pulmonary arterial hypertension. Eur Respir J. 2005;25:509–513. doi: 10.1183/09031936.05.00100504. doi: 10.1183/09031936.05.00100504. [DOI] [PubMed] [Google Scholar]
- 8.Souza R, Jardim C, Julio Cesar Fernandes C, Silveira Lapa M, Rabelo R, Humbert M. NT-proBNP as a tool to stratify disease severity in pulmonary arterial hypertension. Respir Med. 2007;101:69–75. doi: 10.1016/j.rmed.2006.04.014. doi: 10.1016/j.rmed.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Fijalkowska A, Kurzyna M, Torbicki A, Szewczyk G, Florczyk M, Pruszczyk P, Szturmowicz M. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129:1313–1321. doi: 10.1378/chest.129.5.1313. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 10.Nickel N, Golpon H, Greer M, Knudsen L, Olsson K, Westerkamp V, Welte T, Hoeper MM. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2012;39:589–596. doi: 10.1183/09031936.00092311. doi: 10.1183/09031936.00092311. [DOI] [PubMed] [Google Scholar]
- 11.Nagaya N, Nishikimi T, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Kakishita M, Fukushima K, Okano Y, Nakanishi N, Miyatake K, Kangawa K. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102:865–870. doi: 10.1161/01.cir.102.8.865. doi: 10.1161/01.CIR.102.8.865. [DOI] [PubMed] [Google Scholar]
- 12.Leuchte HH, El Nounou M, Tuerpe JC, Hartmann B, Baumgartner RA, Vogeser M, Muehling O, Behr J. N-terminal pro-brain natriuretic peptide and renal insufficiency as predictors of mortality in pulmonary hypertension. Chest. 2007;131:402–409. doi: 10.1378/chest.06-1758. doi: 10.1378/chest.06-1758. [DOI] [PubMed] [Google Scholar]
- 13.Mauritz GJ, Rizopoulos D, Groepenhoff H, Tiede H, Felix J, Eilers P, Bosboom J, Postmus PE, Westerhof N, Vonk-Noordegraaf A. Usefulness of serial N-terminal pro-B-type natriuretic peptide measurements for determining prognosis in patients with pulmonary arterial hypertension. Am J Cardiol. 2011;108:1645–1650. doi: 10.1016/j.amjcard.2011.07.025. doi: 10.1016/j.amjcard.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Zelniker T, Uhlmann L, Spaich S, Friedrich J, Preusch MR, Meyer FJ, Katus HA, Giannitsis E. Novel biomarkers for risk stratification in pulmonary arterial hypertension. ERJ Open Res. 2015;1:000082015. doi: 10.1183/23120541.00008-2015. doi: 10.1183/23120541.00008-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghofrani HA, Grimminger F, Grünig E, Huang Y, Jansa P, Jing ZC, Kilpatrick D, Langleben D, Rosenkranz S, Menezes F, Fritsch A, Nikkho S, Humbert M. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4:361–371. doi: 10.1016/S2213-2600(16)30019-4. doi: 10.1016/S2213-2600(16)30019-4. [DOI] [PubMed] [Google Scholar]
- 16.Frantz RP, Farber HW, Badesch DB, Elliott CG, Frost AE, McGoon MD, Zhao C, Mink DR, Selej M, Benza RL. Baseline and serial brain natriuretic peptide level predicts 5-year overall survival in patients with pulmonary arterial hypertension: data from the REVEAL Registry. Chest. 2018;154:126–135. doi: 10.1016/j.chest.2018.01.009. doi: 10.1016/j.chest.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galiè N, Ghofrani HA, Hoeper MM, Lang IM, Preiss R, Rubin LJ, Di Scala L, Tapson V, Adzerikho I, Liu J, Moiseeva O, Zeng X, Simonneau G, McLaughlin VV GRIPHON Investigators. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–2533. doi: 10.1056/NEJMoa1503184. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 18.Yale University. The Yale University Open Data Access (YODA) Project. Center for Outcomes Research and Evaluation (CORE). 2018. https://yoda.yale.edu/welcome-yoda-project. Accessed March 19, 2019.
- 19.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4:363–371. doi: 10.1161/CIRCOUTCOMES.110.957951. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 20.Bonetti M, Gelber RD. A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data. Stat Med. 2000;19:2595–2609. doi: 10.1002/1097-0258(20001015)19:19<2595::aid-sim562>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Boucly A, Weatherald J, Savale L, Jais X, Cottin V, Prevot G, Picard F, de Groote P, Jevnikar M, Bergot E, Chaouat A, Chabanne C, Bourdin A, Parent F, Montani D, Simonneau G, Humbert M, Sitbon O. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50:1700889. doi: 10.1183/13993003.00889-2017. doi: 10.1183/13993003.00889-2017. [DOI] [PubMed] [Google Scholar]
- 22.Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, Olsson KM, Meyer K, Vizza CD, Vonk-Noordegraaf A, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Huscher D, Pittrow D, Rosenkranz S, Grunig E. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50:1700740. doi: 10.1183/13993003.00740-2017. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]
- 23.NICE. Acute heart failure: diagnosis and management. 2014. https://www.nice.org.uk/guidance/cg187. Accessed March 20, 2019.
- 24.Kylhammar D, Kjellström B, Hjalmarsson C, Jansson K, Nisell M, Söderberg S, Wikström G, Rådegran G. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39:4175–4181. doi: 10.1093/eurheartj/ehx257. doi: 10.1093/eurheartj/ehx257. [DOI] [PubMed] [Google Scholar]


