Summary
Calories from any food have the potential to increase risk for obesity and cardiometabolic disease because all calories can directly contribute to positive energy balance and fat gain. However, various dietary components or patterns may promote obesity and cardiometabolic disease by additional mechanisms that are not mediated solely by caloric content. Researchers explored this topic at the 2017 CrossFit Foundation Academic Conference ‘Diet and Cardiometabolic Health – Beyond Calories’, and this paper summarizes the presentations and follow-up discussions. Regarding the health effects of dietary fat, sugar and non-nutritive sweeteners, it is concluded that food-specific saturated fatty acids and sugar-sweetened beverages promote cardiometabolic diseases by mechanisms that are additional to their contribution of calories to positive energy balance and that aspartame does not promote weight gain. The challenges involved in conducting and interpreting clinical nutritional research, which preclude more extensive conclusions, are detailed. Emerging research is presented exploring the possibility that responses to certain dietary components/patterns are influenced by the metabolic status, developmental period or genotype of the individual; by the responsiveness of brain regions associated with reward to food cues; or by the microbiome. More research regarding these potential ‘beyond calories’ mechanisms may lead to new strategies for attenuating the obesity crisis.
Keywords: Cardiometabolic disease, dietary fat, dietary sugar, obesity
Introduction: Janet King, Laura Schmidt
With the emergence and global spread of the highly palatable, processed foods that are major components of the Western diet and the rising rates of obesity and cardio-metabolic diseases (e.g. cardiovascular disease [CVD] and type 2 diabetes [T2D]), the focus of nutrition science has shifted from concerns about dietary deficiencies to concerns about dietary excesses. Given the well-documented relationship between Western diet and obesity (1–4) and between obesity and cardiometabolic disease (5) (Fig. 1), the dietary excess of greatest concern is caloric consumption. Unquestionably, most Americans are consuming too many calories; 69% of US adults are overweight (6), and new estimates, just released, indicate 39.8% of US adults are obese (7).
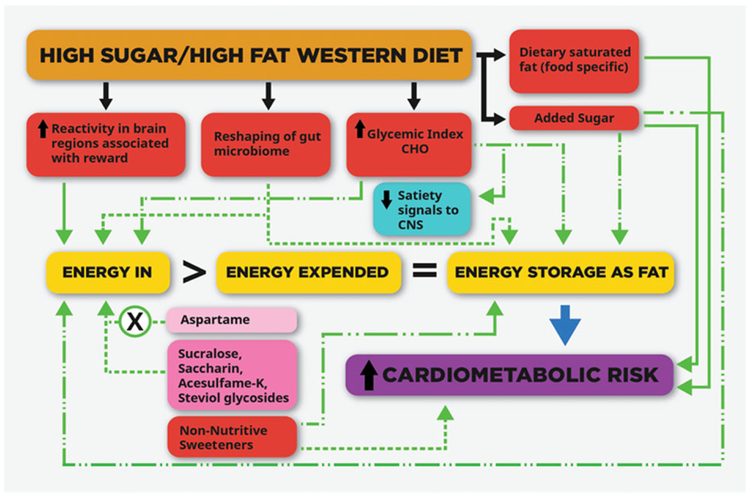
Figure 1.
The potential links between dietary patterns and components and cardiometabolic risk. The totality of the evidence suggests that added sugar and certain saturated-fat-containing foods increase risk for cardiometabolic disease by metabolic mechanisms that are not mediated solely by positive energy balance and fat gain. There is also evidence that certain dietary patterns or components can increase ‘energy in’ and/or ‘energy storage as fat’ via mechanisms that are not explained solely by their specific contribution of calories to the ‘energy in’ side of the energy balance equation. The strength of the links is indicated by the green lines as follows. Solid green line: supported by evidence from animal studies and clinical observational and dietary intervention studies. Dashed line: evidence from prospective cohort studies and/or clinical dietary intervention studies suggests heightened risk during critical developmental periods and in persons with compromised glucose tolerance or insulin sensitivity. Dotted line: supported mainly by evidence from observational and/or animal studies only. Dotted line w/X: evidence from 100% of the clinical dietary intervention studies do not support the evidence from the observational and animal studies.
Yet a major question remains: are all calories equal with regard to effects on cardiometabolic disease and obesity? This was the question that was deliberated by national and international researchers at the 2017 CrossFit Foundation Academic Conference ‘Diet and Cardiometabolic Health – Beyond Calories’ in San Francisco, California.
In one important aspect, the answer to this question is clearly ‘yes’. The first law of thermodynamics states that energy can be neither created nor destroyed: energy in = energy out. Nutritionally, this means that once full growth is reached, if the energy consumed from foods and beverages is greater than the energy expended through metabolism, thermogenesis and physical activity, the surfeit will be stored mostly as body fat. Thus, our traditional energy balance paradigm dictates that excess consumption of calories from any food will drive energy storage and increase the risk of obesity and cardiometabolic disease as defined by
There is no doubt that positive energy balance, due to excessive caloric consumption and/or inadequate physical activity, is the main driver of the obesity and cardiometabolic epidemics. However, it was the first objective of the Conference to consider whether certain dietary components increase risk for cardiometabolic disease by metabolic effects that are not driven solely by positive energy balance and fat gain. The evidence specific for saturated fat and added sugar, and the challenges pertaining to obtaining such evidence, were presented and discussed. It was the second objective of the conference to consider whether certain dietary patterns or components have the potential to promote fat gain via mechanisms that are in addition to their specific contribution of calories to the ‘energy in’ side of the energy balance equation. These mechanisms could include promoting excess energy consumption, more efficient extraction of energy from food within the intestine or preferential partitioning of energy towards fat storage. Evidence addressing this question was presented and discussed for long-debated topics such as high carbohydrate (CHO) diets and non-nutritive sweeteners (NNSs). Also, newer, emerging evidence was presented for the effects of caloric and NNSs during critical periods of development and for effects of the Western diet on reactivity in brain regions associated with reward and on reshaping gut microbiota. This paper summarizes the evidence presented for each topic, the areas of agreement, the topics that require more research and the nutritional approaches that should be emphasized for improving public health.
Objective 1: do certain dietary components increase risk for cardiometabolic disease by metabolic effects that are not driven solely by positive energy balance and fat gain?
Dietary fats: Ronald Krauss
A review, published in 1958, on the relationship of dietary fat to atherosclerotic disease stated:
The evidence now appears to be conclusive that sufficient quantities of polyunsaturated fat in the diet, with proportional decrease in saturated fat, will result in major decrease in blood lipid. Some evidence indicates that such blood lipid lowering produces a desirable effect upon existing atherosclerosis (8).
Fast forward to 2014 and there are at least two reviews on the same topic. One of them concludes that ‘data provide support for current recommendations to replace saturated fat with polyunsaturated fat for primary prevention of coronary heart disease (CHD)’ (9). The other concludes ‘current evidence does not clearly support CVD guidelines that encourage high consumption of polyunsaturated fatty acids and low consumption of total saturated fats’ (10). Clearly, both cannot be correct. Therefore, in addition to reviewing the evidence that supports the conclusion that dietary fats differ in their weight-independent effects on cardiometabolic risk, it is also important to consider the challenges involved in conducting and interpreting nutritional research on the topic of dietary macronutrients. These challenges have contributed to 60 years of sometimes inconsistent and conflicting conclusions regarding the role of dietary fat and other macronutrients in the development and prevention of cardiometabolic disease.
Macronutrient subtypes
The major macronutrients, particularly fat and CHO, include subtypes that need to be carefully controlled/monitored and reported when conducting nutrition studies. This may seem obvious, but the results of many nutrition studies are challenging to interpret because the amounts and types of fat or CHO were not monitored and/or reported. One of the many examples is a study designed to compare the cholesterol-lowering and triglyceride (TG)-lowering effects of four fat-restricted diets (30%, 26%, 22% and 18% of energy as fat), achieved via CHO replacement. The results were reported in the Journal of the American Medical Association in 1997 without any description of the type of CHO prescribed or consumed. The words sugar, simple CHO, sucrose, high-fructose corn syrup (HFCS) or other key CHO descriptors do not appear in the article (11). In the case of fats, one must also consider that beyond the major categories of saturated, monounsatu-rated, n-3 and n-6 polyunsaturated, and trans fatty acids, there is heterogeneity within each group that may contribute to differing biologic and clinically relevant effects. Failure to distinguish between macronutrient subtypes, which has occurred even in recent reports (12), has likely contributed to conflicting dietary conclusions and to the ‘high fat versus high CHO diet’ debate that still rages today.
Food context
The food context within which macronutrients are consumed can also have a significant impact on CVD risk. For example, a 10-year cohort study provided evidence that the consumption of saturated fatty acids (SFA) from dairy foods was associated with decreased risk of CVD, while consumption of the same amount of SFA from meat (including red and processed meat, fish and poultry) was associated with increased CVD risk (13). Yet, even within the same food category, differences in formulations or processing can impact health effects. The fermentation of dairy products provides a notable example. While current evidence does not support an association between intake of dairy products and risk of cardiometabolic disease, fermented dairy products, such as cheese and yogurt, generally show inverse associations (14). Indeed, results from randomized crossover trials show that consumption of SFA in cheese lowers total and/or low-density lipoprotein cholesterol (LDL-C) compared with consumption of SFA in butter (15–18). As another example, a meta-analysis of 17 prospective cohorts and three case–control studies indicated that consumption of processed meat was associated with increased risk of CHD, but consumption of red meat was not (19).
Meta-analyses
Meta-analyses that combine studies that compared macro-nutrient A with B and studies that compared A with C or D or E can lead to misleading conclusions (20). Hooper et al. (21) illustrated this by pooling randomized controlled trials (RCTs) comparing SFA with polyunsaturated fatty acids (PUFAs; n-6 fatty acids), monounsaturated fat, CHO or protein and reporting a 17% reduction in CVD events with replacement of SFA. However, in separate analyses with each of the replacement nutrients, replacement of SFA with n-6 fatty acids yielded a significant 27% reduction in CVD events. The separate analyses for replacement of SFA with monounsaturated fat, CHO or protein all showed non-significant effects on CVD events (21). Differences in the effects of the replacement nutrients in both RCTs and observational cohort studies, and the pooling of these effects, likely help to explain the discrepant results from studies and meta-analyses investigating the effects of dietary SFA and other macronutrients on CVD risk (9,10,22–25).
Conclusions drawn from meta-analyses that include inappropriate studies can also be misleading. For example, the 2014 meta-analysis (10) that challenged the CVD benefit of replacing SFA with n-6 fatty acids has been criticized (26) for including potentially confounded data from the Sydney Diet Heart Study (27). The Sydney Diet Heart Study had included margarine with high trans fats in the n-6 fatty acids supplementation arm. Exclusion of the Sydney Diet Heart Study resulted in a seven-study meta-analysis with a relative risk estimate of 0.81 (0.68–0.98), thus supporting a CVD benefit of replacing SFA with n-6 fatty acids (28). However, concerns have been raised regarding the rationale for exclusion of this trial (29), as well as the inclusion of other trials (the Finnish Mental Hospital (30) and Oslo Diet-Heart (31) studies) that allowed for the continued consumption of trans fats from margarines in the control arms of the studies, thus confounding comparisons with the n-6 fatty acid diets (28). Furthermore, during the Oslo Heart Study, the experimental patients and spouses were taught in their homes how to select and prepare study foods, while the control group was not (31). In short, decisions regarding what studies are appropriate to include or exclude from a meta-analysis can vary among investigators and contribute to conflicting conclusions.
Randomized controlled trials with cardiovascular disease outcomes
Despite the limitations of the Finnish Mental Hospital (30) and Oslo Diet-Heart (31) studies, both of these RCTs, along with two others conducted at Wadsworth Hospital and Veterans Administration Center in Los Angeles (32) and by the British Medical Research Council (33), have been described as providing the highest-quality evidence available regarding the effects of dietary SFA compared with n-6 fatty acids (24). The population sizes in these four trials ranged from 400 to 1,200, and maximum diet exposure was 4–8 years, long enough to obtain CVD outcomes. All four trials monitored blood lipid levels and compliance biomarkers, and the two longest and largest trials also provided standardized diets (30,32). These studies were conducted more than 50 years ago, yet they compose the core evidence upon which a 2017 Presidential Advisory from the American Heart Association based the following summary: ‘randomized controlled trials that lowered intake of dietary saturated fat and replaced it with polyunsaturated vegetable oil reduced CVD by approximately 30%’ (24). Given the challenges and escalating costs associated with conducting nutritional RCTs with CVD outcomes, these four trials are likely to remain the highest-quality evidence available regarding the effects of dietary SFA compared with n-6 fatty acids (24).
Exemplifying the challenge and the expense of RCTs with CVD outcomes is the more recent Women’s Health Initiative Dietary Modification Study of ~48,000 women with an estimated cost of over $400m (34). Women were randomized to receive intensive behaviour modification in group and individual sessions aimed at reducing total fat intake to 20% of calories and increasing intake of vegetables/fruits to five servings per day and grains to at least six servings per day or to the no-diet-modification group that received only diet-related education material. By year 6, the diet-modification group reported a mean increase of 1.1 and 0.5 servings per day of fruit/vegetable and grain, respectively, and a mean 8% reduction in total fat energy. This resulted in a group reduction in fat consumption that was less than anticipated (37% of daily energy down to 28.8%), no significant reduction in CVD outcomes and only a modest reduction in LDL-C of 3.6% during the ~8-year intervention (35). It is not possible to know if the intervention failed to achieve reduction in CVD outcomes because the fat restriction was focused on total fat rather than subtype or because the dietary modifications reported by the participants were too modest or were overestimations of the dietary changes that actually occurred. The two latter limitations, potential non-compliance (36) and inaccurate reporting of food consumption (37) by research participants, are formidable challenges faced by all nutritional research studies, with the exception of those conducted in well-monitored inpatient facilities.
In contrast to the Women’s Health Initiative, a recent and smaller multicentre trial conducted in Spain focused on sub-types of fat in specific foods (38). Approximately 7,000 participants with T2D or three CVD risk factors were randomized to ad libitum Mediterranean diets supplemented either with extra-virgin olive oil (50 mL d−1 prescribed for participants, 1 L week−1 provided for family needs) or with mixed nuts (30 g d−1 provided for participants, 1000 g/3 months provided for family needs) or to a control diet (non-food gifts provided) that was centred around a Mediterranean diet with advice to reduce dietary fat. After 4 years, the Mediterranean diets supplemented with either extra-virgin olive oil or nuts resulted in a relative risk reduction of approximately 30% for major CVD events (primarily stroke) compared with the control diet (38). These beneficial results were mainly mediated by consumption of the supplemental extra-virgin olive oil and the nuts, as there were few diet differences among the groups that were not due to these foods (38). It is worth noting that despite receiving supplemental calories in the olive oil (~9,000 kcal week−1) or nuts (~1,400 kcal week−1), neither of the experimental groups nor the control group gained body weight during the trial (group means for Δbody weight at 4.8 years ranged from −0.9 to −0.4 kg) (39). The authors suggest that results provide evidence that restricting intake of healthy fats is not required for maintenance of body weight (39).
Biomarkers that can demonstrate causality
Given the high costs and challenges associated with RCTs with CVD outcomes, the next strongest line of research to draw on is prospective cohort studies. However, while these studies increase the feasibility of studying CVD outcomes, they cannot prove causality and are limited by the inaccuracies of self-reported food intake (37) and the challenges related to identifying and adjusting for relevant covariates. Evidence of causality must often rely on RCTs in which outcomes are biomarkers of CVD rather than CVD events. Among modifiable CVD risk biomarkers, LDL-C and blood pressure have been the most strongly validated (40), and they provide the major rationale for therapies aimed at reducing disease risk (28). However, reliance on identifying dietary macronutrient effects on LDL-C may obscure effects on LDL particles, especially small dense LDL (sdLDL). sdLDLs may have more direct and specific effects on the development and progression of CVD than are predicted by the LDL-C measurement (41). Increased atherogenicity of sdLDL may be due in part to a longer residence time in plasma, which exposes the arterial endothelium to proinflammatory and proatherogenic particle components such as apolipoprotein (apo)CIII (28,41,42). sdLDLs have been shown to be reduced with lower CHO intake (43). Further, the prevalence of LDL subclass pattern B, a categorical marker for atherogenic dyslipidaemia defined by the predominance of sdLDL, has been linearly and positively associated with increasing concentrations of dietary CHO (consisting of 50% starch and 50% sugar) in RCTs owing to effects of CHO that can occur in as few as 3 d (43). In contrast to CHO, dietary SFA in the ranges generally consumed appears to mainly increase larger LDL particles, which are less strongly associated with risk of CVD (41). Thus, SFA-induced increases in LDL-C may not signify an increase in CVD risk commensurate with that predicted from the relationship of LDL-C to CVD risk in the population (41). For example, recent results from the large multinational Prospective Urban Rural Epidemiology study found a correlation between SFA intake and LDL-C. However, simulation models indicated that LDL-C provided a poor measure of risk of CVD events and mortality as opposed to the ratio of serum apolipoprotein (apo)B (a measure of the total number of atherogenic lipoprotein particles) to apoAI (the principal high-density lipoprotein [HDL] protein), a ratio that in turn is associated with sdLDL levels (44). More studies are needed regarding whether there are specific pathophysiological properties of particles within the spectrum of sdLDL that merit the use of standardized assays for their measurement as a more informative biomarker of CVD risk than LDL-C (41). Another candidate CVD biomarker is apoCIII (28). apoCIII has been found consistently to be positively associated with the risk of CVD, likely because of its capacity to retard plasma clearance of atherogenic remnant lipoproteins (45) as well as its direct proinflammatory activity (46). Further, apoCIII in apoB-containing particles is increased with high-CHO diets (47,48). Given our reliance on the totality of the scientific evidence (prospective cohort studies: association between nutrient and disease outcome + RCTs: direct effects of nutrient on disease biomarker), valid biomarkers are essential.
Nutrient substitutions
Any isocaloric change in one macronutrient requires changes in others, and hence, it is difficult to determine if effects are caused by the increase in macronutrient A or the decrease in macronutrient B. Thus, rather than focus on effects of high versus low consumption of single nutrients on CVD risk, it is more appropriate to statistically evaluate the effects of nutrient or, better yet, food substitutions. The effects of nutrient substitutions for SFA were evaluated in over 127,500 men and women who were followed for 24–30 years (49). Replacing 5% of energy intake from SFA with equivalent energy intake from PUFAs, monounsaturated fatty acids or CHOs from whole grains was associated with a 25%, 15%, and 9% lower risk of CHD, respectively. Replacing SFA with trans fats or CHOs from refined starches/added sugars was not significantly associated with CHD risk (49). These results are consistent with the findings from recent meta-analyses that are not confounded by the challenges discussed above (9,21,24), although the effects of macronutrient heterogeneity and food context, as discussed above, also require consideration.
The evidence concerning omega-3 (n-3) PUFAs and cardiometabolic risk remains unclear. The consumption/supplementation of n-3 fatty acid is associated with reduced CVD risk in prospective cohort studies (10,50), but randomized controlled supplementation trials have not shown significant beneficial effects (10). Nevertheless, a recent Science Advisory from the American Heart Association (51) stated that treatment with omega-3 PUFA supplements of patients with prevalent CHD such as a recent myocardial infarction is reasonable as even a potential modest reduction in CHD mortality (10%) in this clinical population would justify treatment with a relatively safe therapy.
Conclusions
Focusing on effects of nutrient substitution rather than high versus low SFA indicates that replacement of SFA with n-6 fatty acids is associated with lower CVD risk, while replacement of SFA with refined CHOs (starches and sugars) is associated with a neutral or adverse effect. However, more research is needed to consider the food and dietary context in which specific fats are consumed and to develop better tools to assess dietary patterns. Given the challenges of large RCTs with CVD disease endpoints, and the limitations of observational studies, identification and validation of additional surrogate biomarkers for RCTs would be of great value.
Dietary sugars: Kimber Stanhope and Jean-Marc Schwarz
All the challenges involved with conducting and interpreting research on dietary fat are also pertinent to the topic of dietary CHO/sugar. Therefore, similar to dietary fat, recent reviews (52–58) and meta-analyses (59–65) offer very conflicting conclusions concerning the effects of added sugar on cardiometabolic risk.
Definitions of added sugars and free sugars
As defined by the US Food and Drug Administrations, added sugars include sugars that are either added during the processing of foods or are packaged as such and include sugars (free, monosaccharides and disaccharides), sugars from syrups and honey and sugars from concentrated fruit or vegetable juices that are in excess of what would be expected from the same volume of 100% fruit or vegetable juice of the same type (66). The World Health Organization’s definition of free sugars is similar except that it also includes the sugar naturally present in 100% fruit juices and fruit juice concentrates (67).
Natural sugars
The sugars naturally present in whole fruit are exempt from both definitions, and dietary guidelines emphasize the importance of consuming whole fruits and vegetables. Prospective cohort studies consistently support this with evidence that fruit consumption (68–75) or fruit-plus-vegetable consumption (76–80) is inversely associated with incidence of CVD and T2D (81). The evidence from RCTs is inconclusive (82,83), and this is possibly due to the generally modest changes in fruit and vegetable intake that have been achieved in these studies (84). While dietary intervention studies comparing the consumption of added sugar to isocaloric amounts of sugar in whole fruit are lacking, the results from three RCTs suggest that consumption of naturally sweetened orange juice (85,86) or grape juice (87) decreases risk factors compared with sugar-sweetened beverages (SSB). In support of this, there are population studies that report that incidence/prevalence of metabolic syndrome (88,89), CVD (90) and T2D (91–93) and their risk factors (94–96) are associated with consumption of SSB or fruit juice with added sugar (93), but not with consumption of 100% fruit juice. However, the findings from several prospective cohort studies suggest that both 100% fruit juice and SSB consumption are positively and comparably associated with metabolic syndrome (97) and T2D (98,99). Therefore, more RCTs comparing SSB and naturally sweetened fruit juice are warranted, as well as RCTs comparing added sugar with the sugar in whole fruit (82).
Added sugar
Regarding disagreements among meta-analyses concerning the health effects of added sugar, conclusions differ between those that compare fructose with any CHO, including sucrose and HFCS (60,100), and those that compare high added sugar diets with lower added sugar diets (62). Meta-analyses that include inappropriate studies also provide conflicting conclusions. An example of an inappropriate study, included in at least four meta-analyses reporting little or no detrimental effects of fructose consumption (61,100–102), is one in which the fructose in the high-fructose diet (60 g of fructose per day) was not added sugar but was rather provided by whole fruit (103). Furthermore, both the high-fructose and low-fructose (20 g of fructose per day) diets were weight loss diets; thus, subjects in both interventions lost significant amounts of weight (diet with fruit: −4.2 ± 0.3 kg; diet without fruit: −2.8 ± 0.3 kg) and, not unexpectedly, exhibited improvements in cardiometabolic risk factors (103).
Prospective cohort studies
The recent meta-analyses of prospective cohort studies investigating consumption of SSB have consistently shown positive relationships with CVD (59,104,105), T2D (106–110) and hypertension (59,111). The Nutrition and Chronic Diseases Expert Group systematically reviewed the evidence for effects of dietary factors on cardiometabolic diseases, including comprehensively assessing evidence for causality (112). They concluded that evidence from prospective studies suggests a body mass index (BMI)-independent effect of SSB on incidence of T2D and CHD and an additional effect on adiposity (112).
The consumption of added sugar has been less extensively studied than SSB. In 2014, two large prospective cohort studies came to differing conclusions concerning the association between added sugar consumption and CVD mortality. Consumption of added sugar was positively associated with CVD mortality over 15 years in 11,733 National Health and Nutrition Examination Survey (NHANES) participants (age 20 and above) (113), but not over 13 years in 353,751 National Institutes of Health–American Association of Retired Persons (NIH-AARP) Diet and Health Study participants (age 50–71) (114). In the latter study (114), fructose in beverage was positively associated with CVD mortality. Study differences that may have influenced the results include sugar intake: the NHANES participants reported a mean baseline level of added sugar consumption of ~15.7% of daily energy (113), while the NIH-AARP Diet and Health Study participants reported a mean baseline level of free sugar consumption of ~9.6% of daily energy (114). The definition of free sugars for this study included sugar from dried fruit and applesauce, as well as from 100% fruit juice (114). A 10-year prospective study in 2,379 girls (9–10 years at baseline) showed that consuming <10% of energy as added sugar resulted in increasing concentrations of HDL-C compared with consuming ≥10% of energy as added sugar (115). However, a study that divided added sugar into sugar added to beverage (mean intake: 2.6% of daily energy) and sugar added to solid food (mean intake: 9.4% of daily energy) found that the development of impaired glucose homeostasis and insulin resistance over 2 years in 8- to 10-year-old children (n = 564) at risk for obesity was only associated with added sugar in beverages (116).
Fructose versus glucose
There is evidence and plausible mechanisms to suggest that there are differences between CHOs with regard to their effects on cardiometabolic risk factors that mainly involve the differential metabolism of fructose and glucose. Even though both pure fructose and pure glucose are generally not used as added sugars, investigations of their specific metabolic effects have provided important mechanistic insights into the effects of sucrose and HFCS, the most commonly used added sugars that contain both fructose and glucose. The effects of fructose and glucose were compared in adults (mean age: 54 years, mean BMI: 29 kg m−2) who consumed 25% of their energy requirement (Ereq) as fructose-sweetened or glucose-sweetened beverages for 10 weeks (117). These subjects resided at the clinical research centre and consumed eucaloric diets consisting of 55% Ereq as complex CHO for 2 weeks while baseline procedures were conducted. This was followed by an 8-week outpatient period during which subjects consumed the fructose-sweetened or glucose-sweetened beverages along with their usual ad libitum diets. Intervention procedures were conducted during the last 2 weeks of the study while subjects resided at the clinical research centre and consumed eucaloric diets consisting of 30% Ereq complex CHO and 25% Ereq as the assigned beverage. Although both groups gained comparable amounts of body weight (~1.5%) and body fat (~3%), there were marked differences between the effects of the two sugars. Subjects consuming glucose exhibited markedly higher post-meal glucose and insulin responses than those consuming fructose (118). Also, in keeping with the established paradigm by which positive energy balance promotes the development of metabolic syndrome through the increased insulin resistance and lipolytic activity of enlarged adipocytes (119) (Fig. 2), subjects consuming glucose exhibited higher 24-h circulating free fatty acids (FFA) (117). In contrast, fructose consumption did not affect FFA levels. Yet it was the subjects consuming fructose who exhibited increased de novo lipogenesis (DNL), reduced fat oxidation, increased circulating TG (postprandial only), LDL-C, sdLDL-C, oxidized LDL, apoB, apoCIII and uric acid and decreased insulin sensitivity (117,120,121). The essential mechanistic feature that explains these results is that the hepatic uptake and metabolism of glucose is regulated by hepatic energy status, which allows glucose to bypass the energy-replete liver and raise post-meal blood glucose and insulin levels. In contrast, the hepatic uptake and metabolism of fructose is unregulated (122,123); thus, excessive fructose consumption results in a hepatic substrate overload that increases uric acid production (121,123–125) and up-regulates DNL (117,126,127). As illustrated in Fig. 3, the major downstream effects include inhibition of fat oxidation (120,127), increased liver lipid content (127–129), up-regulated secretion of large very-low-density lipoproteins 1 (130), dyslipidaemia (131) and hepatic insulin resistance (132,133). Increased inflammation induced by increases in visceral fat (117,128) or fructose exposure in the intestine (134,135) or liver (136) may also mediate or enhance metabolic dys-regulation.
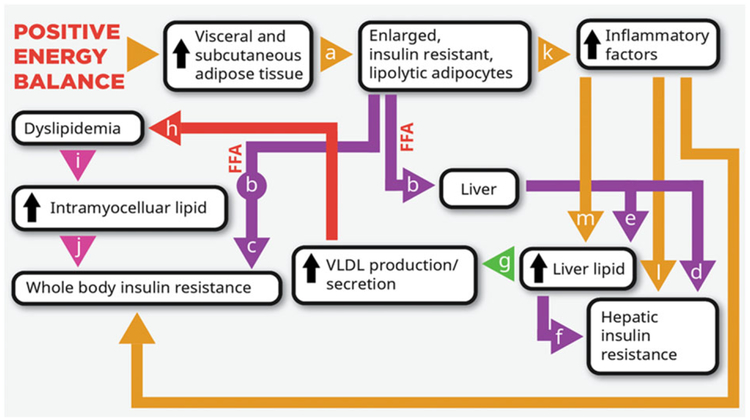
Figure 2.
Established paradigm by which positive energy balance promotes the development of the metabolic syndrome. Positive energy balance leads to fat accumulation and larger and more insulin-resistant adipocytes (a). The insulin resistance increases lipolytic activity and circulating free fatty acids (FFA) (b). While lipolytic activity is higher in visceral adipose tissue than subcutaneous adipose, upper body subcutaneous adipose is a major contributor to the increased levels of FFA (328,329). High levels of FFA can mediate muscle (c) (330) and liver (d) (331) insulin resistance. Hepatic uptake of FFA leads to increased liver lipid (e), which is associated with liver insulin resistance (f) (332) and promotes very-low-density lipoprotein (VLDL) production/secretion (g) and dyslipidaemia (h) (328,333). Increased exposure to circulating triglyceride promotes intramyocellular lipid accumulation (i) (334), which is associated with insulin resistance (j) and type 2 diabetes (332). Inflammatory factors released by insulin-resistant visceral adipose tissue (k) may also promote hepatic insulin resistance (l) and lipid accumulation (m) (119).
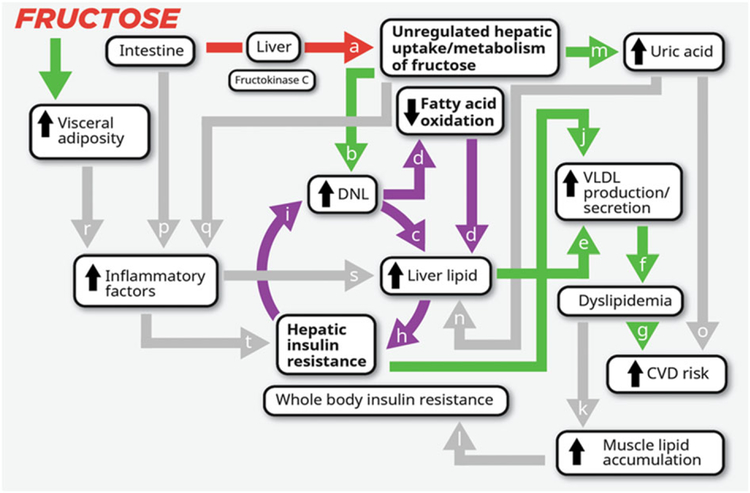
Figure 3.
Potential mechanisms by which consumption of fructose promotes the development of metabolic syndrome The initial phosphorylation of dietary fructose in the liver is largely catalysed by fructokinase C (a), which is not regulated by hepatic energy status (122,123). This results in unregulated fructose uptake and metabolism by the liver. The excess substrate leads to increased de novo lipogenesis (DNL) (b) (117,127). DNL increases the intra-hepatic lipid supply directly (127–129), via synthesis of fatty acids (c), and indirectly by inhibiting fatty acid oxidation (d) (120,127). Increased intra-hepatic lipid content promotes very-low-density lipoprotein (VLDL) production and secretion (e) (130). This leads to increased levels of circulating triglyceride (TG) and low-density lipoprotein particles (dyslipidaemia) (f) (131), risk factors for cardiovascular disease (CVD) (g). Increased levels of hepatic lipid may also promote hepatic insulin resistance (132) by increasing levels of diacylglycerol, which may activate novel protein kinase C and lead to serine phosphorylation (serine P) of the insulin receptor and insulin receptor substrate 1 and impaired insulin action (h) (335). Because of selective insulin resistance, DNL is even more strongly activated in the insulin resistant liver (i) (336), which has the potential to generate a vicious cycle (circular arrows) that would be perpetuated by sustained fructose consumption. This cycle would be expected to further exacerbate VLDL production and secretion via increased intrahepatic lipid supply (130). Hepatic insulin resistance also promotes VLDL production/secretion (j) by increasing apolipoprotein B availability (337,338) and apolipoprotein CIII synthesis (339) and by up-regulating microsomal TG transfer protein expression (MTP) (336). This exacerbates and sustains exposure to circulating TG, leading to intramyocellular lipid accumulation (k) (334), impaired insulin signalling and whole-body insulin resistance (l) (332). The fructokinase-catalysed phosphorylation of fructose to fructose-1-phosphate, which results in conversion of adenosine triphosphate to adenosine monophosphate and a depletion of inorganic phosphate, leads to uric acid production via the purine degradation pathway (m) (121,123–125). High levels of uric acid are associated and may contribute to increased risk for development of fatty liver (n) and CVD (o) (340–342). Fructose exposure in the intestine (p) (134,135) and liver (q) (136) and fructose-induced increases of visceral adipose (r) may promote inflammatory responses (117,343) that further promote liver lipid accumulation (s) and/or impair hepatic insulin signalling (t) (119).
High fructose corn syrup versus fructose and glucose
The results from the 10-week intervention comparing fructose and glucose (117) suggest that the hepatic substrate overload induced by excessive consumption of fructose (Fig. 3) is a more rapid pathway to metabolic dys-regulation than the increased FFA (Fig. 2) and post-meal hyperglycaemia/hyperinsulinaemia induced by excessive consumption of glucose. These studies also suggest that because the commonly consumed sugars HFCS and sucrose contain 50–55% fructose, their overconsumption would have less detrimental effects on risk factors than isocaloric amounts of pure fructose. Data from a 2-week dietary intervention study (125,137), in which adults (mean age: 26 years, mean BMI: 25 kg m−2) consumed beverages containing 25% Ereq as fructose, glucose or HFCS along with their usual ad libitum diet, showed that circulating post-prandial TG, apoCIII and uric acid concentrations were highest during fructose consumption (Δbody weight: −0.1 kg), lowest during glucose consumption (Δbody weight: 0.4 kg) and intermediate during HFCS consumption (Δbody weight: 0.4 kg) (137). However, surprisingly, this was not the pattern for fasting and postprandial concentrations of LDL-C, non-HDL-C and apoB. The levels of these CVD risk factors were highest after HFCS consumption, lowest after glucose consumption and intermediate after fructose consumption (137), suggesting potentially synergistic effects of fructose and glucose consumption on these measures when the two sugars are consumed concurrently.
Utilizing the same 2-week dietary intervention protocol (125,137), adults (mean age: 25 years, mean BMI: 26 kg m−2) consuming beverages containing 0% (aspar-tame-sweetened beverage), 10%, 17.5% or 25% Ereq from HFCS with their usual ad libitum diets exhibited dose-dependent increases of fasting and postprandial non-HDL C, LDL-C, apoB and uric acid and postprandial apoCIII and TG concentrations (all P < 0.001) (125). HFCS consumption also resulted in a dose-dependent increase in body weight (Δbody weight: 0%, 0.1 kg; 10%, 0.0 kg; 17.5%, 0.3 kg; 25%, 0.8; P < 0.05); however, in adjusted statistical models, the variance attributed to Δbody weight was 1–3% and the variance attributed to HFCS dose was 9–29% (125). These results are in contrast to those from a study in which adults consuming beverages containing 8%, 18% or 30% Ereq as sucrose or HFCS with usual ad libitum diets for 10 weeks exhibited no differences in cholesterol and LDL-C (138) or 24-h uric acid and TG area under the curve (139) between doses. As previously discussed in detail (58,125), possible explanations for the conflicting results include differences in the statistical analyses employed, use of three cups per day of low-fat milk as a vehicle for the sugars, lack of a vehicle control group and lack of an objective measure of compliance in the 10-week study (138,139).
Sucrose
Data from several older studies suggest that consumption of sucrose also increases total and/or LDL-C (140–144) or postprandial TG (142,145). More recently, men (mean age: 26 years, mean BMI: 22 kg m−2) consuming beverages containing either 80 g d−1 sucrose or fructose with their usual ad libitum diets for 3 weeks had higher concentrations of total and LDL-C and reduced LDL particle size than when they consumed beverages containing 80 g of glucose (146,147). This study also showed that the fructose-sweetened beverages decreased hepatic insulin sensitivity compared with the glucose-sweetened beverages, even though body weight, body fat and waist circumference were reduced after fructose consumption compared with glucose consumption (146). In the longest of the recent intervention trials, liver fat, and fasting TG, total cholesterol and uric acid concentrations were increased in adults (mean age: 39 years, mean BMI: 32 kg m−2) consuming 1 L of sucrose-sweetened cola per day with their usual ad libitum diets for 6 months compared with subjects consuming isocaloric amounts of low-fat milk or 1 L of aspartame-sweetened cola or water per day (124,128). The changes in body weight ranged from +0.1 (aspartame) to +1.4% (low-fat milk) and did not differ among the four diet groups (P = 0.8) (128). A 2-week study that concurrently investigated consumption of 25% Ereq as sucrose-sweetened (Δbody weight: 0.5 kg) or HFCS-sweetened beverages (Δbody weight: 0.8 kg) with ad libitum diets in adults (mean age: 26 years, mean BMI: 25 kg m−2) showed that the effects of the two added sugars on LDL-C, apoB, apoCIII, uric acid and postprandial TG were comparable (148).
Sucrose versus starch
There appears to be only one study that has investigated the effects of replacing starch with sucrose in solid food, as opposed to SSB, at levels less than 30% Ereq. Twenty-four women and men with hyperinsulinaemia (mean age: 36 years, mean BMI: 25 kg m−2) consumed three eucaloric (42% Ereq fat, 14% protein and 44% CHO), crossover diets in which the 44% CHO component was provided as 39% starch + 5% sucrose, 26% starch + 18% sucrose or 11% starch + 33% sucrose in solid form for 6 weeks each. Fasting lipids (143), glucose and insulin concentrations, and the glucose and insulin responses to an oral sucrose tolerance test (149) were increased when subjects consumed the 18% and 33% Ereq sucrose diets compared with the higher starch diet containing only 5% Ereq sucrose (143,149). More studies are needed to compare the metabolic effects of sugar consumed in solid and liquid forms with both refined and whole-grain CHO in normoinsulinaemic individuals consuming isocaloric diets.
Weight-independent effect of fructose/sugar
All of the studies detailed above, with the exception of the investigation on solid-form sucrose versus starch (143,149), provided the intervention sugar as supplements to the participants’ usual ad libitum diets. While the modest weight gains suggest that most of the subjects partially compensated for the sugar supplements by consuming less of their usual diet, there is still the potential for confounding by excess energy consumption and weight gain. However, studies with dietary protocols that included the provision of eucaloric diets with matched macronutrient distribution between the high-sugar and low-sugar diets have demonstrated that fructose and sucrose consumption can increase risk factors in the absence of positive energy balance and weight gain (127,140,143–145,149–158). These studies include a recent crossover study that provided evidence that all the major effects of fructose overload, detailed in Fig. 3, can occur in the absence of positive energy balance and weight gain. Eight men (mean age: 42 years, mean BMI: 24 kg m−2) resided at a clinical research centre and consumed eucaloric diets in which the CHO component consisted of 25% Ereq fructose-sweetened beverage + 25% complex CHO or 50% Ereq complex CHO for 9 d each (127). Despite weight maintenance, this short-term exposure to fructose-sweetened beverage increased DNL, inhibited fat oxidation, increased liver fat and postprandial plasma TG concentrations and decreased hepatic insulin sensitivity compared with the complex CHO diet. Each of these effects was observed in all eight participants, with the exception that postprandial TG was not increased in one individual (127). It is important to note, however, that these results could have been affected by both the differences between fructose and complex CHO and the differences between beverage and solid food. However, in a much older study, Hallfrisch et al. compared the isocaloric substitution of starch with 0%, 7.5% or 15% Ereq as fructose in solid food, utilizing eucaloric crossover diets that were provided for 5 weeks each to 12 hyperinsulinaemic and 12 normoinsulinaemic men (mean age: 40 years, mean BMI: 26 kg m−2). Compared with the 0% fructose diet, the 7.5% and 15% fructose diets increased total cholesterol and LDL-C (154) and the 15% fructose diet increased glucose and insulin responses to a 3-h oral sucrose tolerance test in both groups of subjects (153). More recently, a study that provided eucaloric diets including 25% Ereq as fructose-sweetened (n = 15) or glucose-sweetened beverages (n = 17) for 2 weeks to men (mean age: 34 years, mean BMI: 29 kg m−2) did not reveal differences in liver fat, but fasting uric acid concentrations and homeostatic model assessment insulin resistance (HOMA-IR) were increased by fructose compared with glucose consumption (155). Similar results were reported from a crossover study in which men (mean age: 46 years, mean BMI: 32 kg m−2) were provided eucaloric low-sucrose (sucrose 5.2%, total sugar 17.1% of daily calories) or high-sucrose (sucrose 14.9%, total sugar 30.2% of daily calories – details about non-sucrose sugar are not provided) diets for 6 weeks (156). While there were no differences in peripheral glucose utilization and suppression of endogenous glucose production during a two-step hyperinsulinaemic euglycaemic clamp, fasting and oral glucose tolerance test areas under the curve were higher for both glucose and insulin after the high-sucrose versus lowsucrose diet (156).
Sugar restriction
In a study designed to demonstrate weight-independent effects of fructose restriction to reverse the adverse metabolic effects of excessive fructose consumption, eucaloric diets were provided for home consumption to 43 Latino and African–American adolescents (mean age: 13 years) with metabolic syndrome for 9 d (159). The diets contained 10% Ereq as added sugar, considerably less than the average of 27% Ereq recorded for the participants’ usual diets (159). In response to the lower-sugar diets, the adolescents exhibited numerous benefits including reductions of liver fat, visceral adipose tissue and DNL (160), plasma lipids and lipoproteins (161), glucose and insulin excursions during oral glucose tolerance test and diastolic blood pressure (159). Body fat was not affected (−0.3 kg, P = 0.17), but body weight was significantly decreased (−0.9 kg, P < 0.01). Despite efforts by the investigators to promote body weight maintenance by providing more food, body scales for daily weight monitoring at home, and individualized weight maintenance counselling, 34 of the 43 participants exhibited a decrease in body weight (159). This confounds assessment of the direct metabolic effects of fructose restriction as opposed to those mediated by negative energy balance and weight loss. However, the subgroup of nine subjects who did not lose weight also had statistically significant reductions in most of the outcomes (DNL, liver fat, visceral adipose tissue, fasting glucose, insulin and HOMA-IR) (159–161).
Two more recent studies demonstrate benefits of sugar restriction in children with obesity (162,163). In response to a 6-week dietary intervention consisting of advice to reduce fructose consumption from a usual intake >70 to <20 g d−1, 54 children (age range: 6–11 years) exhibited significant decreases in liver fat and fasting TG, but no changes in body weight or BMI (162). A 6-month dietary intervention consisting of advice to reduce consumption of fructose and high glycaemic index foods resulted in lowered systolic blood pressure, alanine aminotransferase, apoB and HOMA-IR in 12 children (age range: 7–18 years) with non-alcoholic fatty liver disease (NAFLD) (163). An important limitation of all three of these studies (159,162,163) is they did not include control groups. However, in a recent RCT, adults (mean BMI: 31 kg m−2), whose normal diets contained ~94 g of sugar per day from SSB, were randomized to receive and consume their usual quantity of beverage sweetened with sucrose (n = 14) or with an NNS (n = 13) for 12 weeks (164). The group consuming NNS exhibited significant decreases in liver fat compared with the group who continued to consume SSB (87.9 g sugar from SSB consumed per day at 12 weeks) (164). However, while the changes in body weight were not significant, the NNS group lost ~1.4 kg and the SSB group gained ~1 kg. More adequately powered RCTs are required to delineate the weight-independent and weight-dependent effects of sugar restriction.
Conclusions
Consumption of fructose-sweetened, HFCS-sweetened or sucrose-sweetened beverages leads to greater increases in risk factors for cardiometabolic disease than isocaloric amounts of starch. More studies comparing the metabolic effects of SSB with those of added sugar in solid food and comparing added sugar in solid foods with both refined and whole-grain complex CHO are required. The metabolic dys-regulation induced by excessive consumption of fructose-containing sugar is mainly caused by hepatic fructose overload increasing DNL, which leads to inhibited fat oxidation, increased liver fat and increased very-low-density lipoprotein production/secretion. Risk factors associated with metabolic dys-regulation increase even when fructose-containing sugars are consumed with diets that do not result in positive energy intake and weight gain.
Objective 2: do certain dietary patterns or components have the potential to promote fat gain via mechanisms that are in addition to their specific contribution of calories to the ‘energy in’ side of the energy balance equation?
Older and emerging evidence on the high-carbohydrate/high-glycaemic-index diet: Anja Bosy-Westphal
The high-carbohydrate versus high-fat diet debate
The potential to promote fat gain via mechanisms that are in addition to caloric content has been attributed to both high-CHO and high-fat diets. Thirty years ago, the consensus was that a high-fat diet promotes greater fat gain than a high-CHO diet because dietary fat is converted to body fat much more efficiently than dietary CHO (165). At that time, clinical studies showed that the body does not handle fat ingestion in the same way as CHO or protein ingestion. More specifically, (a) unlike CHO ingestion, which stimulates CHO oxidation and even DNL in extreme cases (166), the ingestion of dietary fat is not reciprocated by an increase in fat oxidation (167–169); (b) similarly, under eucaloric conditions, replacement of CHO by fat in the diet takes several days or weeks to stimulate fat oxidation, even in the presence of increased physical activity (169–172); (c) the thermic effect of dietary fat is lower than that from CHO or protein (173–175); (d) because of the higher energy density of fat and its low satiating effect, fat ingestion leads to food overconsumption (176,177). Together, these results strongly suggested that dietary fat was a key culprit in the western diet leading to enhanced storage of body fat (178). All these studies lead to the development of the ‘Flatt hypothesis’, which proposes that unlike CHO and protein, excess fat intake is not rapidly buffered by increased fat oxidation but requires a significant amount of weight gain to re-establish a new fat balance (179). At this new weight and body composition, the amount of CHO, fat and protein oxidized will match the amount of CHO, fat and protein consumed (i.e. respiratory quotient = food quotient) and weight can be maintained (179).
The consensus that dietary fat is the specific culprit in the obesity crises has waned with the perception that while Americans decreased their intake of dietary fat, the rates of overweight and obesity have risen (180). Whether this occurred because dietary fat does not promote fat gain more efficiently than CHO or because Americans did not achieve substantial reductions in fat intake or because their fat calories were replaced by even more calories of low-fat/high-CHO/high-sugar foods is not known. However, it increased focus on the possibility that a high-CHO diet has the potential to increase fat gain by affecting both sides of the energy balance equation. Specifically, when meals with a high proportion of glucose-containing CHOs are consumed, higher post-meal glucose excursions lead to larger meal-associated insulin excursions than when isocaloric high-fat/low-CHO meals are consumed (181). This physiologic hyperinsulinaemia has the potential to promote fat gain by driving glucose and FFA into storage forms, which also decreases the availability of circulating metabolic fuels and promotes hunger (182).
Energy-restricted weight loss diets
This mechanism serves as the rationale for many popular high-fat weight loss diets. However, it is not supported by a recent 14-day inpatient metabolic balance study examining the effect of selective isocaloric reduction of dietary CHO versus fat on body weight, energy expenditure and fat balance in 19 volunteers with obesity. The results showed that calorie for calorie, the high-CHO weight loss diet led to greater body fat loss than the high-fat diet, despite the fact it was the high-fat diet that led to decreased insulin secretion (183). A 2014 meta-analysis of 19 RCTs lasting 3 months to 2 years with ~3,200 participants showed no significant differences in loss of body weight between participants assigned to consume low-CHO weight loss diets compared with those assigned to consume isocaloric higher-CHO (45–65% of energy) weight loss diets (184). A second meta-analysis also indicated there was no significant difference in weight loss between low-fat and high-fat weight loss interventions when the interventions were concordant for caloric restriction (185). Furthermore, an RCT with a 2 × 2 factorial design comparing four energy-restricted diets with low or high amounts of protein (10% or 20% of energy) and low or normal amounts of CHO (25% or 50% of energy) showed that weight loss after the 12-month intervention was unaffected by CHO content but significantly greater on the high protein diets (186). A recent 12-month weight loss diet study showed comparable weight reduction in subjects consuming a healthy low-fat diet (−5.3 kg) vs. a healthy low-CHO diet (−6.0 kg). Notably, the dietary instructions for both diet groups included the following: (1) maximize vegetable intake; (2) minimize intake of added sugars, refined flours and trans fats; and (3) focus on whole foods that were minimally processed, nutrient dense and prepared at home whenever possible (187). Collectively, these results suggest that hypocaloric high-CHO diets do not impede fat loss compared with hypocaloric low-CHO diets in the majority of the population.
Ad libitum diets
However, because the majority of people do not spend the majority of their time consuming hypocaloric diets, far more important to the obesity epidemic is the question of whether high-CHO/lower-fat diets promote fat gain compared with low-CHO/higher-fat diets when consumed ad libitum (188). In the ad libitum condition, a differential effect between the two diets to promote weight gain could be mediated by mechanisms related to satiety and energy in-take, as well as by differences in their potential to promote fat storage versus fat oxidation. Results from a meta-analysis of 16 studies suggest high-CHO/lower-fat diets do not cause more weight gain than low-CHO/high-fat diets. Instead it was concluded that a reduction in dietary fat to 27% of energy without intentional restriction of energy intake caused weight loss compared with control diets containing 37% of energy as fat (and less CHO) in subjects without obesity (189). A more recent meta-analysis of 30 RCTs comparing participants consuming ad libitum lower-fat versus usual or moderate-fat diets also showed a consistent but small effect of low-fat intake to reduce body fat and/or weight (190). However, the majority of the studies included in both of these meta-analyses provided the participants with dietary guidelines rather than diets, and the dietary instructions and counselling time spent with the groups consuming the fat-restricted diets were considerably more extensive than those provided to the groups consuming the higher-fat control diets. A separate analysis with five trials that did equalize attention between both diets groups showed there was still significantly more weight loss in the groups consuming the lower-fat diets (190).
Another recent meta-analysis does not support this conclusion (185). This 17-trial meta-analysis excluded trials shorter than 1 year because initial, maximal weight loss after approximately 6 months is often followed by weight regain (185). It showed that the weight loss exhibited by the low-fat groups was specific to comparisons with groups consuming ‘usual diet’ and receiving less attention in the form of dietary instruction and/or counselling. Only four of the included trials provided comparable attention to both the low-fat and higher-fat diet groups, and two showed greater weight loss with the low-fat diet while the other two did not. Therefore, the authors of this meta-analysis concluded that evidence from RCTs does not support low-fat diets over other dietary interventions for beneficial effects on energy balance (185).
Thus, the effects of high-CHO/lower-fat diets compared with low-CHO/higher-fat diets on energy balance continues to be a subject of controversy owing to the lack of well-controlled studies of sufficient duration. The challenges involved in filling this gap do not consist of just ensuring that the attention provided to the intervention and control groups are equal. Study participants often fail to meet the goals of their dietary assignments, especially in the later months of the intervention period, even when equal, and even very intensive (191), dietary instructions and counselling are provided (191,192). This suggests that the better way to compare the effects of ad libitum high-CHO/low-fat diets with low-CHO/high-fat diets on energy intake and body weight gain is to provide the participants with ample food and meals formulated to the specification of the assigned diet and prohibit the consumption of any other foods. This is an expensive protocol that will still be limited by potential non-compliance (36) and failure to report the non-compliance (37). Future technological advances may address the compliance and reporting limitations with, for example, wearable food intake monitoring devices (193). However, even if/when perfect compliance and accurate reporting are achieved, there still remains the possibility that provided study diets lead to reductions in ad libitum energy intake because of monotony and curtailment of freedom of dietary choice, rather than to the specific dietary manipulations. Unintended weight loss, which could have been related to the lack of dietary choice rather than dietary composition, confounded the results of a recent crossover study that provided subjects with high-CHO meals for 4 weeks and then very-low-CHO meals for 4 weeks (194). The lack of freedom of choice is also a potential confounder for long-term inpatient protocols, especially ones in which the change of energy intake is the primary outcome. Restrictions related to dietary freedom can reduce energy intake, but inpatient confinement can lead to boredom and/or depression and increase energy intake.
Possibly, the protocol that best minimizes the monotony and the freedom of dietary choice issues for studies investigating effects of ad libitum diets on energy intake and body weight gain is the ‘shop’ model utilized in studies conducted in Europe for 6-month interventions (195). This protocol provides free foods that are appropriate to the formulations of the assigned experimental diets in a grocery shop setting. At the check-out stand, the foods selected by the participants are scanned to ensure that the selections in total meet the specifications for the assigned diet and provide at least 100% of daily Ereq. The uneaten foods are returned and re-scanned for calculation of energy and nutrient intake. The feasibility of utilizing this protocol for 1-year interventions is limited by the expense.
In addition to controlling the potential effects that restricted dietary choice and monotony may have on energy intake, the optimal protocol for comparing the effect of high-CHO versus high-fat diets on weight gain must also control for other dietary components that can affect satiety and energy intake. This includes protein, which has been shown to dose-dependently increase postprandial fullness, decrease postprandial hunger and affect homeostatic hormones involved in the regulation of energy intake (196). There is also evidence to suggest that a high-protein diet reduces reward-driven eating behaviour (197,198). Fibre also affects satiety (199) by mechanisms that may include energy density, decreasing and slowing nutrient absorption from the intestine or triggering signals related to fullness by causing water absorption and distention in the stomach (200). Because whole grains contain more fibre and have a lower energy density than refined grain, it is not surprising that most short-term studies suggest they promote greater satiety (201). It also then would not be surprising if a study comparing a high-fat diet with a high-CHO diet with whole grains yielded different results than a study comparing a high-fat diet with a high-CHO diet with refined grains. The type of fat to be studied may also affect results as there is evidence to suggest that n-3 fatty acids increase satiety (202).
Thus, the challenges involved in resolving the high-CHO versus high-fat diet debate are immense. Research effort and funds may be better directed to determining the optimal ad libitum diet for promoting satiety and reduced energy in-take. This was recently undertaken by Arguin et al. (200) who compared an ad libitum diet consisting of food components (protein > 20% of energy, whole grain, whole fruit and vegetable, n-3 fatty acids, chilli peppers with capsaicinoids (203) and calcium (204)) known to have satiety-enhancing properties (e.g. low-energy density and fibre) with an ad libitum control diet based the Canadian Food Guide for Healthy Eating for 16 weeks. Fat mass was significantly decreased, and the satiety quotient for hunger, fullness and prospective food consumption was significantly increased in male participants who consumed the diet with the satiety-enhancing food components compared with those who consumed the control diet. However, an important limitation of the dietary protocol that may have affected subject retention rates (satiety diet: 91.4%; control diet: 55.9%) and study results was the provision of one pre-prepared meal per day to the high-satiety diet group, but none to the control diet group (200). Nevertheless, the advantages of changing focus from diets designed around macronutrient groups to diets designed around high-nutrient, satiating food components that have the palatability to compete with low-nutrient, energy-dense processed foods warrant exploration.
Preventing weight regain in weight-reduced subjects
Another area that warrants more focus is how to prevent weight regain in subjects who have lost weight on energy-restricted diets. Numerous studies show that the energy homeostatic systems regulating energy intake, energy expenditure, neuroendocrine function and autonomic function in weight-reduced subjects conspire to oppose reduced weight maintenance (205–208), in individuals with or without obesity (207). These adaptations, rather than the comparative efficacy of various weight-loss diets or treatments, are responsible for the depressingly unsuccessful clinical attempts to reverse the obese or overweight states. Investigations focusing on the leptin signalling pathways may have the most potential to yield strategies to prevent weight regain. Leptin administration, which has little effect on subjects at their usual body weight or on subjects consuming energy-restricted diets, has been shown to, at least partially, reverse many of the metabolic, autonomic, neuroendocrine and behavioural adaptations that lead to weight regain in weight-reduced subjects (209).
The weight regain period following hypocaloric diets may represent a specific scenario during which an ad libitum high-CHO diet does promote more weight gain than a low-CHO diet. A recent study showed that weight loss was not different in men consuming low-calorie diets containing either a high glycaemic load (65% of energy as CHO with a high glycaemic index) or lower glycaemic load (50% of energy as CHO with a low glycaemic index) (210), in agreement with the conclusions from the meta-analyses cited above (184,185). However, during the follow-up 3-week overfeeding period, the same men consuming 150% Ereq as the high-CHO/high-glycaemic-load diet gained 1 kg more body weight than the men consuming 150% Ereq as the low-CHO/low-glycaemic-load diet (210). Fat regain among all subjects was inversely associated with fasting fat oxidation (210). Two other investigations in weight-reduced subjects provide evidence that high-CHO/high-glycaemic-load diets promote greater weight regain (211) or lower energy expenditure (212) than lower-CHO/lower-glycaemic-load diets.
Energy restriction and weight reduction are associated with improved insulin sensitivity (213,214). However, with cessation of energy restriction, it may be possible that the augmented insulin secretion caused by high-glycaemic-load diets selectively impairs insulin sensitivity in muscle while maintaining it in white adipose tissue, thus resulting in lowered fat oxidation and increased fat storage. This mechanism, lowered muscle insulin sensitivity and increased adipose sensitivity, has been well documented in rodents exposed to hyperinsulinaemia (215–217), including in a rat model of weight recovery (218). Conversely, it was demonstrated that mice lacking insulin receptors in adipose tissue had normal whole-body glucose metabolism, but the insulin-stimulated glucose uptake in their adipocytes was reduced by 90%. This resulted in mice with reduced adipose tissue mass and increased longevity (219,220).
Other conditions associated with positive energy balance and lowered insulin sensitivity in skeletal muscle relative to adipose tissue could also lead to a disproportional gain in fat mass in response to a high-glycaemic-load diet. One example may be physical inactivity, which specifically lowers muscle insulin sensitivity (221). In support of this, young healthy men displayed lowered whole-body glucose utilization during a hyperinsulinaemic euglycaemic clamp after 10 d of bed rest than before bed rest (222). This suggests decreased insulin sensitivity primarily in their skeletal muscle. In contrast, the glucose uptake in their subcutaneous abdominal adipose tissue was increased after bed rest (222). Lowered insulin sensitivity in skeletal muscle relative to adipose tissue may also occur in metabolically healthy obesity (223), a transient state (224) that may be explained by high adipose insulin sensitivity promoting adipose expansion over ectopic lipid deposition (225).
Conclusions
At comparable levels of energy restriction, high-CHO/low-fat weight loss diets do not impede fat loss compared with low-CHO/high-fat diets. More well-controlled trials lasting at least 1 year are needed to determine the effects of ad libitum high-CHO/low-fat diets compared with ad libitum low-CHO/high-fat diets on energy balance. Emerging evidence suggests that, following weight loss on energy-restricted diets, ad libitum consumption of a high-CHO/high-glycaemic-load diet may, via increased insulin exposure, decrease insulin sensitivity in muscle and increase insulin sensitivity in adipose, thus increasing susceptibility to weight regain. More clinical studies are needed to test this hypothesis, but also to investigate other strategies that may affect weight regain following otherwise successful non-surgical weight loss.
High-carbohydrate/high-glycaemic-load diet and metabolic status: Arne Astrup
A recent publication provides evidence to suggest that susceptibility to weight gain on a high-CHO diet may be influenced by the metabolic status of the individual (226). The investigators reanalysed data from three dietary intervention studies in which diets that differed in CHO content (227,228) or glycaemic load (192) were compared for their effects on weight loss or weight regain. The reported effects of diet group on body weight in the total population of individuals with obesity were quite small (weight-reduced subjects on low-glycaemic-load diet regained 1.9 kg less than those on the high-glycaemic-load diet over 6 months (192)) or undetectable (227,228). However, when the subjects were divided into subgroups based on their baseline fasting glucose and insulin levels, consistent and more pronounced effects of the different diets were detected (226). In all three studies, participants with high baseline fasting glucose and low fasting insulin exhibited a greater loss of body weight on the diets with a low CHO content or glycaemic load than participants with the same glucose and insulin profile on diets with higher CHO content (Fig. 4A) or glycaemic load (Fig. 4B). These analyses suggest that insulin-resistant obese individuals, with a low capacity to increase insulin secretion and overcome the resistance, achieve more satiety and weight loss on lower-CHO/lower-glycaemic-load diets.
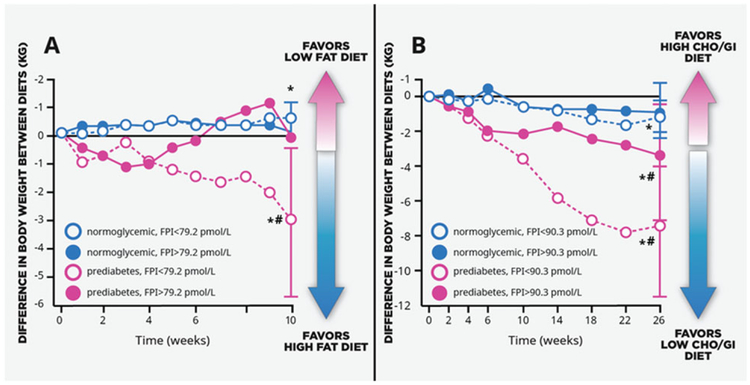
Figure 4.
(A) Reanalysed results from the NUGENOB Study: subjects with obesity, prediabetes and low fasting insulin lost more weight on a high-fat vs. low-fat diet. (B) Reanalysed results from the DioGenes Study: subjects with obesity, prediabetes and low fasting insulin regained three to four times less weight on a low–carbohydrate (CHO)/low-glycaemic-index (GI) diet than subjects with normal glycaemia and obesity. *P < 0.05 from zero; #P < 0.05 between glycaemic/insulinaemic groups. Fasting plasma insulin (FPI). Modified from Hjorth et al. (226).
In another study, 90 participants who consumed the New Nordic Diet (more calories from local and wild countryside plant foods, more seafood and seaweed from local seas and lakes and fewer calories from meat (229)) for 6 months lost 3 kg more body weight than 56 participants who consumed the average Danish diet (230). Subsequent analysis showed that participants with high fasting glucose/low fasting insulin lost 6 kg more body weight consuming the New Nordic Diet than participants with the same glucose and insulin profile consuming the average Danish diet (226). It is important to note that food records suggest that the two groups consumed comparable amounts of available CHO, but the New Nordic diet group consumed 15 g d−1 more fibre (230) and more whole grain. This suggests that the glycaemic index/load (not assessed in the study, but likely lower in the New Nordic diet due to the fibre and whole grain) rather than CHO content is involved in the body weight differences between the participants with high fasting glucose/low fasting insulin on the two diets. However, a recent report from the study investigators also suggests an influence of gut microbiota on diet response (231). Faecal samples were collected from a subgroup of the subjects before starting the New Nordic or average Danish diets, and the ratio of the relative abundance of Prevotella spp. to Bacteroides spp. (P/B) was determined. Previous reports have identified Prevotella and Bacteroides as two typical bacterial clusters present in humans (232), with the relative abundance of Prevotella associated positively with host diet (vegetarian and fibre) (233,234) and negatively with cardiometabolic risk factors (234). Among individuals with a high P/B ratio, the New Nordic Diet resulted in a 3.2 kg larger body fat loss compared with the average Danish diet, while no difference in body fat loss was observed between the Nordic diet and average Danish diet among individuals with low P/B. Among individuals on the New Nordic diet, those with a high P/B ratio lost significantly more body fat than those with a low P/B ratio, while body fat loss was not different between high-P/B and low-P/B groups consuming the average Danish diet (231). Recently, dietary-fibre-induced improvements in postpran-dial blood glucose and insulin were found to be positively associated with the abundance of Prevotella (235). Therefore, the importance of baseline fasting glucose and insulin (i.e. insulin sensitivity) for diet response with regard to weight loss may be linked to gut microbiota and dietary fibre content (231).
Grouping subjects by a combination of fasting glucose and fasting insulin was found to be superior to other various indices of insulin resistance, such as the 30-min response to a glucose dose, for detecting group-by-diet interactions. A good prediction is also achieved when using fasting glucose alone. Patients with T2D or with three or more CVD risk factors were assigned to consume a low-fat Mediterranean diet or a Mediterranean diet with extra-virgin olive oil, and after 5 years, the weight loss difference between the two diet groups was less than 0.5 kg (39). However, when regrouped by fasting glucose, the subjects with higher glucose levels on the Mediterranean diet lost ~2 kg more body weight than the subjects with higher glucose levels on the lower-fat diet.
Conclusions
Emerging evidence suggests that high-CHO diets, or more specifically, high-glycaemic-load/low-fibre diets may promote weight gain or impede weight loss in subjects with impaired glucose metabolism/insulin resistance. Pretreatment fasting glucose and insulin measurements could be useful for identifying individuals who would benefit most from a low–glycaemic-load/high-fibre diet that may improve their weight loss and/or weight maintenance success.
Non-nutritive sweeteners: Allison Sylvetsky
Because NNSs such as aspartame, sucralose, saccharin, acesulfame K and steviol glycosides such as rebaudioside A (extracted from the stevia plant) contain zero or negligible calories, they do not directly contribute to the energy intake side of the energy balance equation. However, it has been suggested that NNS consumption, through several potential mechanisms, may indirectly affect energy balance (236–238). A meta-analysis of nine prospective cohort studies of adults and children demonstrated a positive association between NNS consumption and BMI (0.03 kg m−2, 95% confidence interval: 0.01, 0.06) (239). Epidemiological studies have also shown positive associations between NNS and metabolic syndrome (240–242) and T2D (241,243–248). These studies do not demonstrate causation and are limited by the possibility of residual confounding and reverse causality. Specifically, individuals with higher BMI who are concerned about their weight or patients with T2D may then choose to consume NNS instead of caloric sweeteners. However, studies in which rodents gained more weight (249,250) or exhibited inflammation (251) or glucose intolerance (252) consuming NNS compared with rodents consuming glucose (249) or sucrose (250) provide support for potential cause and effect relationships.
Mechanisms such as sweet taste receptor activation, disturbance of the expected relationship between sweetness and calories, changes in taste preferences and alteration of gut microbiota may explain these associations (236–238). It is also possible that caloric compensation occurs, negating calories ‘saved’ by using NNS. This compensation could be psychological, whereby one’s knowledge of consuming a lower-calorie NNS-containing alternative may lead to giving oneself permission for greater calorie ingestion at subsequent meals. Compensation could be physiological, in which consumption of lower-calorie NNS-containing alternatives promotes heightened hunger and subsequently higher calorie intake.
However, it has been questioned whether any of the above mechanisms occur in humans and whether they occur consistently in rodents. Based on a systematic review, it was reported that in 62 of 90 animal studies, NNS did not increase body weight (253), and a more recent meta-analysis of 12 prospective cohort studies did not support an association between NNS consumption and BMI (0.002 kg m−2; 95% confidence interval: −0.009, 0.005) (253). Further-more, two separate meta-analyses consisting of 10 (239) and eight (253) RCTs both indicated that substituting NNS for sugar resulted in a modest weight loss in adults. Findings also favoured weight loss when NNS was compared with water, but this meta-analysis only included three trials (253). Because the benefits of substituting NNS for sugar is a very different question than the benefits of substituting NNS for water, more studies comparing NNS and water are warranted. This is especially true for the sucralose, saccharin, acesulfame K and steviol glycosides, which have been much less studied than aspartame. Aspartame was utilized in nine of the studies included in the meta-analyses (239,253), and three of these nine studies administered aspartame in commercially available beverages, consistent with the manner in which aspartame is frequently consumed (254).
It does not appear that any of these RCTs revealed adverse effects of NNS consumption on risk factors for cardio-metabolic disease (255). Therefore, NNS may be a useful tool for increasing adherence to behavioural weight loss regimens. The longest intervention study conducted to date included 163 obese women who were randomly assigned to consume or to abstain from aspartame-sweetened foods and beverages during 16 weeks of a 19-week weight reduction programme (active weight loss), a 1-year maintenance programme and a 2-year follow-up period. The aspartame group lost significantly more weight overall and regained significantly less weight during the 1-year maintenance and the 2-year follow-up than the no-aspartame group (256). During a more recent study in which 303 men and women who habitually consumed diet (NNS) beverages participated in a weight loss programme that included a randomized assignment to either stop drinking NNS beverages and consume 24 oz (710 mL) of water or to continue drinking NNS beverages daily for 1 year (257), those in the NNS beverage group lost more weight. These results should be interpreted with caution as those randomized to NNS beverages were essentially continuing their usual beverage intake, while those who were assigned to switch to water were required to implement and sustain a behaviour change. Furthermore, consuming NNS in the context of an intensive weight loss programme focused on calorie reduction may promote more weight loss than NNS use in the general population. Nevertheless, several studies that were not conducted as part of weight loss programmes also showed no effects of NNS to increase body weight (125,128,258,259). This includes the 6-month intervention cited above in which overweight/obese subjects consumed 1 L of sucrose-sweetened cola, isocaloric amounts of low-fat milk or 1 L of aspartame-sweetened cola or water per day. There were no significant differences between the effects of aspartame-sweetened cola and water on body weight, visceral adiposity, liver fat and metabolic risk factors including blood TGs, HDL, total cholesterol, fasting plasma glucose, fasting plasma insulin, HOMA-IR and uric acid (124,128). Indeed, there are no clinical intervention studies involving chronic NNS exposure in which NNS induced a weight increase relative to sugar, water or habitual diet (253).
While studies investigating effects of NNS on weight in children are limited, the largest study to date included 641 normal-weight children, aged 4–11 years, who were randomized to groups consuming 8 oz of sucralose-sweetened beverage or SSB daily for 18 months (260). Compared with SSB consumption, consumption of sucralose-sweetened beverage reduced weight gain and fat accumulation. However, this study did not include an unsweetened control. Because children gain weight due to growth over an 18-month period, without a water or attention control group, it cannot be determined whether the sucralose-sweetened beverage had no effect on body weight or just less effect than the SSB.
Conclusions
Randomized controlled trials consistently demonstrate that consumption of NNS may promote decreased energy in-take, particularly when used as part of comprehensive weight loss programmes. Additional well-controlled RCTs are warranted, most specifically for saccharin, acesulfame K and steviol glycosides, which have been studied less frequently than aspartame and for periods no longer than 16 weeks. More studies in children and more studies assessing metabolic and health outcomes beyond body weight, such as effects on glucose tolerance and inflammation, are needed.
Responses in brain regions associated with reward to the palatable Western diet: Eric Stice
Palatable, high-sugar and/or high-fat foods, major components of the typical Western diet, can indirectly affect the energy-in side of the energy balance equation through activation of brain regions associated with reward that promotes overeating. Importantly, it is not the greater reward region response to the tastes of palatable food that predicts future weight gain, but rather the greater reward region response to images of palatable foods (including advertisements) and visual cues (eg. Golden Arches™) that signal impending tastes of palatable foods. Both animal (261,262) and human (263) experiments indicate that after repeated pairings of cues (cartoon picture of chocolate milkshake (263)) that predict palatable food tastes and actual palatable food tastes, dopamine signalling increases in response to predictive cues but decreases in response to actual food tastes. Therefore, excessive caloric intake that results in weight gain is associated with reduced caudate (one of the brain structures that compose the reward system) response to tastes of high-sugar/high-fat foods (264) and an increase in reward region response to visual cues that suggest impending tastes of these foods (265). An apparent result of this process is that exposure to food cues prompts eating in the absence of hunger, which contributes to excessive weight gain. Indeed, prospective studies show that elevated reward responses to television advertisements depicting palatable foods that are high in dietary fats and sugars (266) or to food cues (267) predicted weight gain in adolescent girls and boys (266–268), young women (269) and adults (270) over periods ranging from 6 months to 3 years. Furthermore, interventions that reduce reward region responses to food cues, either through food response training (271,272) or blockage of sweet taste receptors (272,273), promoted weight loss or reduced intake of high-sugar foods.
Functional magnetic resonance imaging (fMRI) is considered to be one of the best tools currently available for the detection of regional brain activations to specific cues or tasks (274). However, correlations from studies utilizing fMRI have been faulted for being artificially inflated mainly due to small sample sizes (275). More recent studies, though, have confirmed the predictive relationship between increases in reward region response to food cues and energy intake or future weight gain using prospective data that establish temporal precedence (265), split-half replication (265,267) and sample sizes of ~150 (265,267,276). Furthermore, in these larger studies (265,267,276), healthy body weight (BMI > 18 and <25 kg m−2) was an inclusion criteria, thus ensuring that prior overeating or obesity did not contribute to the reward region response measured by fMRI at baseline (267,276). Collectively, these studies indicate the potential value of fMRI as a tool to study vulnerabilities to weight gain and also highlight food cues for their likely importance in the obesity epidemic (277).
Conclusions
Elevated reactivity in brain regions associated with reward in response to food cues predicts future weight gain. Regular in-take of palatable foods that are high in fat and sugar increases responses to food cues in brain regions associated with reward, further increasing overeating. Strategies that reduce responses to food cues in brain regions associated with reward may be effective for promoting weight loss and preventing weight gain. This is an evolving area with high potential to increase knowledge regarding the development of obesity and perhaps help to curb the obesity epidemic.
Emerging evidence about sugar consumption during critical periods and in vulnerable populations: Michael Goran
Elevated reactivity in brain regions associated with reward in response to high-sugar-food cues may explain the positive associations between added sugar and SSB consumption and weight gain in both prospective cohort trials (278–283) and RCTs (281,283). However, it does not explain the emerging evidence that suggests effects of direct and indirect sugar exposure on body composition in very young children.
Vulnerability to second-hand sugar exposure during gestation
Gestation is a critical developmental period during which second-hand sugar exposure may increase susceptibility to obesity in the offspring. Gilman et al. recently reported that in 1,078 mother–child pairs, maternal SSB consumption during the second trimester of pregnancy was positively associated with several indices of obesity in 7-year-old children, including fat mass measured by dual-energy X-ray absorptiometry (284). The associations were not attenuated when adjusted for maternal energy intake or for the number of SSB servings per day consumed by the children. The results also showed no association between maternal intake of NNS beverages and child BMI/adiposity. Both findings are in conflict with the results of a recent study by Zhu et al. who reported a positive association between child BMI z-score at age 7 and maternal intake of NNS beverages during gestation, but no association with maternal intake of SSB (918 mother–child pairs) (285). Study differences that may have contributed to the discordant results include less accurate and objective outcomes (child height and weight reported by the mother (285) versus height, weight and body fat via dual-energy X-ray absorptiometry measured by study staff (284)) and less precise adjustment increments for the child’s intake of SSB and NNS beverages at age 7 (<1 or ≥1 serving per week (285) versus servings per day (284)) in the latter study (285). It is also possible that the association between maternal consumption of NNS beverages and child BMI z-score represented reverse causation. In the Zhu et al. study, pre-pregnancy BMI was higher (27.6 kg m−2) (285) than in the Gilman study (24.6 kg m−2) (284), and it was positively associated with NNS beverage consumption (P < 0.001) (285). However, another recent study also reported positive associations between maternal consumption of NNS during pregnancy and risk of overweight of the off-spring during infancy and no such association with maternal consumption of SSB (3,033 pairs) (286). Further analyses from this study showed the positive associations between maternal consumption of NNS during pregnancy and risk of overweight were specific to male offspring and to infants who were not breastfed for at least 6 months (286). Also, post hoc analyses revealed positive associations between maternal SSB, fruit drinks and fruit juice consumption and risk of over-weight in the offspring (287). As recently reviewed, the evidence concerning the long-term effects of NNS exposure during gestation, infancy and childhood is limited and inconsistent, and more investigation is needed (288).
Vulnerability to second-hand sugar exposure during lactation
Lactation is also a critical developmental period during which second-hand sugar exposure may increase susceptibility to obesity in the offspring. A recent study of 25 mother–child pairs provided evidence that the fructose concentration in breast milk is associated with infant body composition, including fat mass (289). Breast milk samples collected at 1 and 6 months post-partum contained fructose, but at much lower concentrations than glucose and lactose (means = 0.0037, 0.14, and 421 mmol L−1, respectively). However, the concentrations of fructose were quite variable, and it was the only component of breast milk that was positively associated with infant body weight, fat mass, lean mass and bone mineral content at 6 months of age (289). While this level of fructose has been shown to enhance transcription factors involved in adipocyte differentiation (peroxisome proliferator-activated receptor γ and CCAAT/enhancer-binding protein α) and increase glucose transporter 4 expression in preadipocyte culture (290), more research will be needed to determine whether it has in vivo effects in breastfed infants.
Vulnerability to first-hand sugar exposure during infancy and early childhood
If infants are susceptible to the effects of second-hand fructose exposure during gestation and lactation, then it follows that they are also susceptible to direct effects of fructose consumption. In support of this, the prevalence of obesity in a study of 1,189 6-year-old children was twice as high in those who consumed SSB during infancy (0–12 months of age) compared with those who consumed none (17.0% vs. 8.6%) (291). Adjustment for SSB intake at age 6 did not eliminate this association (291). Importantly, consumption of any SSB during infancy also doubled the risk that these children would be consuming at least one SSB per day at age 6 (292).
Added sugar consumption may contribute to the susceptibility of Hispanic children to early-onset obesity within the first few years of life. The prevalence of obesity in 1,483 Hispanic children, 2–4 years of age, from the Los Angeles County Women, Infants and Child programme, was 15% (293) compared with the average of 10% in 2–5-year-old NHANES children born in the same years (294). Based on caregiver reports, 18% of the Hispanic children consumed two or more servings of SSB per day (serving = 12 oz), 25% consumed one serving and 57% consumed zero SSB serving per day. Overall, this a 50% higher level of SSB consumption than that reported for 2- to 3-year-old children in NHANES 2003–2004 (295). SSB consumption by the young Hispanic children was associated with BMI, with the prevalence of obesity exceeding 20% among children who consumed two or more SSB servings per day (293). On the positive side, it was also observed that the prevalence of obesity was reduced to 10% among the children consuming two or more SSB servings per day who had been breastfed for ≥1 year, resulting in a significant interaction between SSB intake and breastfeeding (P = 0.005) (293). More study of young Hispanic children will be needed to determine the contributions of added sugar consumption, socio-economic status, cultural factors and genetics to their high prevalence of obesity and to examine the mechanisms underlying the protective effects of longer-term breastfeeding.
Sugar consumption in vulnerable populations
Sugar exposure may have unique effects in certain populations. Hispanics for example may be particularly vulnerable to the adverse effects of sugar exposure because they are disproportionate carriers of the variant allele of the patatin-like phospholipase domain-containing protein 3 (PNPLA3) gene. The PNPLA3 variant allele has a frequency rate of about 50% in Hispanics compared with <10% in other populations (296). The PNPLA3 variant predisposes to NAFLD (297) and is associated with increased liver fat (296,298), elevated plasma alanine transaminase levels (299,300) and cirrhosis/advanced fibrosis (301) in the Hispanic population. Hispanic children with obesity, ranging in age from 8 to 18 years, also exhibited significantly increased liver fat as a function of PNPLA3 risk allele number, with 64% of the homozygous carriers having a liver fat content greater than 5.5% (302) (>5% liver fat = NAFLD).
The effects of the PNPLA3 variant allele may be exacerbated in the context of a high-sugar diet (303). PNPLA3 variant allele knock-in mice had normal levels of hepatic fat on a chow diet, but when challenged with a high-sucrose diet, their liver fat levels increased twofold to threefold compared with wild-type littermates consuming a high-sucrose diet (297). Obese Hispanic children with two copies of the PNPLA3 variant were particularly susceptible to increased liver fat when consuming a diet high in added sugar (302). Conversely, when adults homozygous for the variant allele were placed on a low-calorie, low-CHO diet, they lost 3 kg of body weight and reduced liver fat by 45% in only 6 d. Control subjects, who were homozygous for the wild-type gene and matched for BMI and liver fat, lost the same amount of weight, but their reduction in liver fat (18%) was significantly lower (304). More studies are needed to confirm a gene * diet interaction between the PNPLA3 gene variant and dietary sugar intake.
Conclusions
Added sugar consumption in early life is associated with higher obesity in childhood. More research is needed to determine if first-hand and second-hand sugar exposure during critical developmental periods, specifically gestation, lactation and infancy, is associated with higher risk of obesity. The greater frequency of the PNPLA3 gene variant in Hispanics and its potential interaction with dietary sugar may make Hispanic children and adults particularly susceptible to the negative health effects of high-sugar diets.
Contributions of the gut microbiome to diet-induced obesity: Peter Turnbaugh
Consumption of a high-fat, high-sugar Western diet may affect one or both sides of the energy balance equation by shifting the types of microbes found within the gastrointestinal tract (the gut microbiome) and their metabolic activity. Consumption of the Western diet results in a significant change to the gut microbiome (often increased Firmicutes and decreased Bacteroidetes) (305), a microbial signature that is remarkably reproducible across inbred and outbred mice of diverse genotypes and mice with defects in immunity and metabolism (306). Gut microbes from mice with Western-diet-induced obesity or from lean mice consuming a low-fat, plant-polysaccharide-rich diet were transplanted into germ-free (GF) recipients. The GF mice receiving the microbiome from mice with Western-diet-induced obesity gained more body fat than the mice receiving the microbiome from the lean donors (305), demonstrating that differences in the mouse gut microbiome can affect host energy balance.
Human studies also support causal links between diet, the gut microbiome and host energy balance (307–309). Short-term dietary interventions revealed that consumption of a high-fat diet rich in animal products reshapes the human gut microbiome within a single day (310). Transplantation of the stool microbiome from human twins discordant for obesity demonstrated that the mice receiving the microbiomes of the twins with obesity gained more body fat than mice colonized with the microbiomes of lean twins (308).
Multiple mechanisms have been proposed through which changes in the structure and function of the gut microbiome influence host adiposity, encompassing both sides of the energy balance equation (311). One possible mechanism by which gut bacteria may increase ‘energy in’ is by liberating additional calories from the diet and/or altering the intestinal absorption of nutrients. Compared with lean controls, the distal gut microbiomes from obese mice were enriched for genes that encode enzymes that break down substrates such as plant polysaccharides that cannot be metabolized by human enzymes (312). Consistent with studies of the Western-diet-associated microbiome (305), transplantation of the distal gut microbiome from obese mice resulted in a significant increase in host adiposity (312). Other groups have identified single bacterial strains and even specific proteins that are sufficient to ameliorate diet-induced obesity. Colonization with the gut Verrucomicrobium Akkermansia muciniphila led to increased stool calories (consistent with altered intestinal metabolism and/or absorption), normalization of plasma lipopolysaccharide concentration and decreased insulin resistance and triglyceridaemia in high-fat-fed mice (313). These effects appeared to be partly mediated by activation of toll-like receptor 2 by a specific protein present on the outer membrane of A. muciniphila (313,314). Recent studies have also suggested that gut bacterial metabolites may increase appetite through the activation of the parasympathetic nervous system (315).
On the other hand, there is emerging evidence to suggest that gut bacteria can also affect energy output (316). An example of one potential mechanism involves microbiota-induced changes in bile acid composition, which can alter signalling to the bile acid nuclear receptor, farnesoid X receptor (FXR) (317–319). A recent study in conventionally raised (CONVR) or GF mice that were wild type (FXR+/+) or FXR knockout (FXR−/−) suggested that the gut microbiota impacts diet-induced obesity via FXR signalling. While consuming a high-fat diet for 10 weeks, CONVR FXR+/+ mice gained more body weight and had higher levels of liver fat than CONVR FXR−/− mice, GF FXR+/+ mice or GF FXR−/− mice (320)). Previous studies in mice treated with an FXR inhibitor have shown that the decreases in liver fat were accompanied by unaffected energy intake and increased energy expenditure (321).
Conclusions
Consumption of a processed high-fat, high-sugar diet alters the structure and function of the mouse gut microbiome (322). Data from animal models suggest that the gut microbiome can shape both sides of the energy balance equation, altering energy intake and expenditure. More research is needed to better understand the mechanisms responsible, the role of diet-induced changes to the gut microbiome in the pathophysiology of cardiometabolic disease and the translational relevance of these findings for the treatment of human obesity.
Overall conclusions
The overall purpose of the 2017 CrossFit Foundation Academic Conference was to address the following question: are all calories equal with regard to effects on cardio-metabolic disease and obesity? It was the first objective of the conference to consider whether certain dietary components, specifically fat and sugar, increase risk for cardiometabolic disease by metabolic mechanisms that are not mediated solely by positive energy balance and fat gain. Although this is a complex area involving the need to account for many inter-related factors, including the challenges related to manipulating and/or documenting dietary intake in human research subjects, the authors agree on the following points:
Evidence suggests that consumption of n-6 fatty acids results in lower cardiometabolic risk factors/risk compared with isocaloric amounts of SFA. However, differences exist between individual SFA, and the food matrix needs to be considered; e.g. dairy foods such as cheese and yogurts are associated with reduced cardiometabolic risk. More research is needed to clarify the differences among the individual SFA and SFA-containing foods.
Evidence strongly suggests that consumption of fructose-sweetened, HFCS-sweetened or sucrose-sweetened beverages increases cardiometabolic risk factors/risk compared with isocaloric amounts of starch. More research is needed comparing the metabolic effects of SSB versus sugar in solid food and sugar in solid food versus refined or whole grain starch.
Under the second objective of the conference, it was considered whether all calories were equal with regard to their potential to promote fat gain and obesity. More specifically, evidence was presented suggesting that certain dietary patterns or components have the potential to promote fat gain via mechanisms that are in addition to their specific contribution of calories to the ‘energy in’ side of the energy balance equation. The authors agree on the following points:
There is currently insufficient evidence that a high-CHO diet affects weight gain or weight loss to a different extent than a high-fat diet. Susceptibility to weight gain when consuming diets high in refined CHO/glycaemic load may be affected by the metabolic status of the individual (i.e. glucose tolerance/insulin sensitivity). More studies focused on strategies to prevent weight regain in weight-reduced subjects are needed.
RCTs ranging from 4 weeks to 3 years in duration demonstrate that consumption of aspartame does not promote body weight gain in adults. Well-controlled and long-term RCTs in adults are warranted to assess the effects of saccharin, acesulfame K and steviol glyco-sides on body weight and other health outcomes. More studies to assess the effects of all types of NNSs in children are needed.
- Continued research on the following topics could provide important insights and strategies for slowing the obesity epidemic.
- The high-sugar, high-fat palatable Western diet could be perturbing both sides of the energy balance equation through effects on brain regions associated with reward and/or on the gut microbiome.
- Susceptibility to weight gain may be affected by exposure to sugar and/or NSS during critical periods of development from pre-conception to adult life.
Nutrition and public health
Rates of obesity and cardiometabolic disease continue to climb globally (7,323–326). This is clearly a multifactorial problem that involves genetic, metabolic, behavioural and environmental factors, and progress towards a resolution will require consideration of all these factors (327). Regarding nutrition, the speakers and delegates at this conference agree that strategies to solve the complex problems associated with obesity should include consideration of the above conclusions regarding added sugar and specific high-SFA foods. Also, we need more research on the effects of diet in vulnerable populations and during critical periods of development, and the ways that responses to diet may be mediated by the genotype or metabolic status of the individual, by the responsiveness of brain regions associated with reward to food cues or by the microbiome. Such research may provide new strategies for attenuating the obesity crisis by developing more individual-based/precision-based nutrition approaches. Therefore, the search for solutions must include support for research to promote the potential of these new strategies and to fill knowledge gaps. And, finally, they also agree that the scientific evidence related to any single food or macronutrient or mechanism is less likely to impact the global epidemics of obesity and cardiometabolic disease as significantly as solutions related to the following:
Prevention: The food environment needs to be improved. Food policies that will improve the availability and reduce the costs of healthy foods compared with high-calorie, palatable foods are needed. Lowering the plethora of food cues that may promote overeating of high-sugar, high-fat palatable foods could also be an effective strategy.
Healthy dietary patterns: Strategies that emphasize the health benefits and palatability of dietary patterns consisting of whole grains, fruits, vegetables and healthy fats, rather than the negative consequences of a single food or food group, are likely a better approach to prevention.
Personalized nutrition: Individual variability needs to be considered when defining the healthy diet. For example, the optimal diet composition for weight control may depend on the individual’s glucose metabolism. In addition, genetics, the microbiome, life stages (pregnancy, infancy and early childhood), culture, dietary preferences and weaknesses (i.e. ‘Is the brain reward region more activated by sweet candy bars or salty potato chips?’) should all be considered when determining the optimal diet pattern for an individual or susceptible segment of the population.
Acknowledgements
The CrossFit Foundation Sports and Health Sciences Institute’s 2017 Academic Conference ‘Diet and Cardiometabolic Health – Beyond Calories’ was funded by the CrossFit Foundation primarily through a grant provided by the Laura and John Arnold Foundation. Any views expressed in this publication do not necessarily reflect the views of the funder(s).
The only role of the funders in the development of the scientific content of the conference was providing the overall topic and selection of the academic organizer, Dr Kimber Stanhope. The funders had no role in the selection of speakers and the development of the agenda. There was no input by the funders into the content of the manuscript, which was not reviewed at any stage by the funders until after it was submitted for publication.
Graphics credit: Erick Diaz-Soto, Art and Motion Graphics Director, CrossFit, Inc.; Alison Wice, Jr, Motion Graphics Designer, CrossFit, Inc.
Abbreviations:
- apo
apolipoprotein
- BMI
body mass index
- BMIZ
BMI z-score
- CHD
coronary heart disease
- CHO
carbohydrate
- CVD
cardiovascular disease
- DNL
de novo lipogenesis
- Ereq
energy requirement
- FFA
free fatty acids
- fMRI
functional magnetic resonance imaging
- FPI
fasting plasma insulin
- FXR
farnesoid X receptor
- HDL
high-density lipoprotein
- HFCS
high-fructose corn syrup
- HOMA-IR
homeostatic model assessment insulin resistance
- LDL-C
low-density lipoprotein cholesterol
- NAFLD
non-alcoholic fatty liver disease
- NHANES
National Health and Nutrition Examination Survey
- NIH-AARP
National Institutes of Health–American Association of Retired Persons
- NNS
non-nutritive sweetener
- PNPLA3
patatin-like phospholipase domain-containing protein 3
- P/B
Prevotella spp. to Bacteroides spp.
- PUFA
polyunsaturated fatty acid
- RCT
randomized controlled trial
- sdLDL
small dense low-density lipoprotein
- SFA
saturated fatty acid
- SSB
sugar-sweetened beverages
- TG
triglyceride
- T2D
type 2 diabetes
- USDA
United States Department of Agriculture
- VLDL
very-low-density lipoprotein
Footnotes
Conflict of interest statement
The following authors have no conflicts of interest to report: Drs Schmidt, Turnbaugh and Bray.
The following authors acknowledge these conflicts of interest.
Dr Stanhope received honoraria from the CrossFit Foundation for serving as a Conference Academic Organizer and for writing the manuscript.
Dr Goran received honorarium from the CrossFit Foundation for serving on the Advisory Committee and travel reimbursement from the CrossFit Foundation for attending the conference in July 2017.
Drs Bosy-Westphal, King, Schwarz, Sylvetsky, Gardner, Mason, Rosenbaum and Allister-Price and Ms Sigala received travel reimbursement from the CrossFit Foundation for attending the conference in July 2017.
Dr Malik received travel reimbursement from the CrossFit Foundation for attending the conference in July 2017. She was paid for consulting services by the City of San Francisco for litigation related to health warning labels of soda and is on a pro bono retainer to The Center for Science in the Public Interest for expert support in litigation related to sugar-sweetened beverages.
Dr Havel received travel reimbursement from the CrossFit Foundation for attending the conference in July 2017. He has received research grants from Bristol Myers Squibb and Arrowhead Pharmaceuticals.
Dr Stice received travel reimbursement from the CrossFit Foundation for attending the conference in July 2017. His institution has received research funding from Crave Crush, and he has received reimbursement for travel from Crave Crush.
Dr Ravussin received travel reimbursement from the CrossFit Foundation for attending the conference in July 2017. He serves on the Scientific Advisory Board to the Nutrilite Health Institute with Amway and for the Institute of Cardiometabolism and Nutrition in Paris, France; has a consultant contract with Janssen and with Nutrilite Health Institute with Amway; gives lectures at the Open Academy in Venice; and is a lecturer/advisor for the Center for Medical Weight Loss. He has received research grants or unrestricted gifts from Amway, Nestle, the Nutrition Science Initiative, Novartis, Sanofi-Aventis, Weight Watchers and Ethicon Surgery. He has a patent for ‘Night Moderate Hypoxia to Treat Insulin Resistance and Cardiometabolic Syndrome’.
Dr Welsh received travel reimbursement from the CrossFit Foundation for attending the conference in July 2017. She received payment from the Sugar Foundation for an analysis and presentation on sugar consumption in toddlers.
Dr Greenwood received honorarium for serving as Chairperson of the manuscript planning meeting and travel reimbursement from the CrossFit Foundation for attending the conference in July 2017.
Dr Astrup received honorarium from CrossFit Foundation for serving on the Advisory Committee and travel reimbursement from CrossFit Foundation for attending the conference in July 2017. He reports personal fees from Dutch Beer Institute, NL; Feast Kitchen A/S, Denmark; Groupe Éthique et Santé, France; McCain Foods Limited, USA; Nestlé Research Center, Switzerland; Weight Watchers, USA; BioCare Copenhagen, Zaluvida, Switzerland; Basic Research, USA; Beachbody, USA; Danish Agriculture & Food Council, Novo Nordisk, Denmark; Pfizer, Germany; Saniona, Denmark; Sanofi-Aventis, Germany; S-Biotek, Denmark; Scandinavian Airlines System, Denmark; and Tetra Pak, Sweden; personal fees and other from Gelesis, USA; grants from Arla Foods, DK; Danish Dairy Research Council; and Gelesis, USA outside the submitted work. In addition, Dr Astrup has a patents pending to the University of Copenhagen ‘Methods of inducing weight loss, treating obesity and preventing weight gain’ (licensee Gelesis, USA) and ‘Biomarkers for predicting degree of weight loss’ (licensee Nestec SA, CH), and he is a co-inventor of a number of other patents owned by the University in accordance with Danish law. Astrup receives royalties for the books Verdens Bedste Kur, Politikens Forlag, Denmark, 2012 (subsequently published in English as World’s Best Diet, Penguin, Australia, and The Nordic Way, Random House, USA), and Spis dig slank efter dit blodsukker (Eat according to your blood sugar and be slim), Politikens Forlag, Denmark, 2017. He is a co-author of several books in the pipeline about personalized nutrition for weight loss; a co-owner and member of the board of the consultancy company Dentacom Aps, Denmark, co-founder; and a co-owner of UCPH spin-outs Mobile Fitness A/S & Flaxslim ApS (where he is also a member of the board, 2015–present) and Personalized Weight Management Research Consortium ApS (Gluco-diet.dk/2017–present).
Dr Krauss received honorarium from CrossFit Foundation for serving on the Advisory Committee and travel reimbursement from CrossFit Foundation for attending the conference in July 2017. He has received payment for services on the Scientific Advisory Board of Virta Health and from Quest Diagnostics for services on speakers bureau. He has received research grants from the Almond Board of California and The Dairy Research Institute. He has a patent on lipoprotein particle analysis.
References
- 1.Sun J, Buys NJ, Hills AP. Dietary pattern and its association with the prevalence of obesity, hypertension and other cardiovascular risk factors among Chinese older adults. Int J Environ Res Public Health 2014; 11: 3956–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Ramirez S, Mundo-Rosas V, Garcia-Guerra A, Shamah-Levy T. Dietary patterns are associated with overweight and obesity in Mexican school-age children. Arch Latinoam Nutr 2011; 61: 270–278. [PubMed] [Google Scholar]
- 3.Naja F, Nasreddine L, Itani L et al. Dietary patterns and their association with obesity and sociodemographic factors in a national sample of Lebanese adults. Public Health Nutr 2011; 14: 1570–1578. [DOI] [PubMed] [Google Scholar]
- 4.Naja F, Hwalla N, Itani L, Karam S, Sibai AM, Nasreddine L. A Western dietary pattern is associated with overweight and obesity in a national sample of Lebanese adolescents (13–19 years): a cross-sectional study. Br J Nutr 2015; 114: 1909–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen MD, Ryan DH, Apovian CM et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129: S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012; 307: 491–497. [DOI] [PubMed] [Google Scholar]
- 7.Hale CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016 In: NCHS Data Brief. National Center for Health Statistics: Hyattsville, MD, 2017. [PubMed] [Google Scholar]
- 8.Balch H, Splitter S, Flynn P, Kinsell LW. The relationship of dietary fat to atherosclerotic disease. Calif Med 1958; 89: 165–168. [PMC free article] [PubMed] [Google Scholar]
- 9.Farvid MS, Ding M, Pan A et al. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation 2014; 130: 1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury R, Warnakula S, Kunutsor S et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 2014; 160: 398–406. [DOI] [PubMed] [Google Scholar]
- 11.Knopp RH, Walden CE, Retzlaff BM et al. Long-term cholesterol-lowering effects of 4 fat-restricted diets in hypercholesterolemic and combined hyperlipidemic men. The Dietary Alternatives Study. JAMA 1997; 278: 1509–1515. [PubMed] [Google Scholar]
- 12.Lee HA, Hwang HJ, Oh SY et al. The differential effects of changes in individual macronutrient intake on changes in lipid concentrations during childhood: from the Ewha Birth & Growth Cohort. Clin Nutr 2017: 30156. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveira Otto MC, Mozaffarian D, Kromhout D et al. Dietary intake of saturated fat by food source and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr 2012; 96: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorning TK, Bertram HC, Bonjour JP et al. Whole dairy matrix or single nutrients in assessment of health effects: current evidence and knowledge gaps. Am J Clin Nutr 2017; 105: 1033–1045. [DOI] [PubMed] [Google Scholar]
- 15.Biong AS, Muller H, Seljeflot I, Veierod MB, Pedersen JI. A comparison of the effects of cheese and butter on serum lipids, haemostatic variables and homocysteine. Br J Nutr 2004; 92: 791–797. [DOI] [PubMed] [Google Scholar]
- 16.Hjerpsted J, Leedo E, Tholstrup T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am J Clin Nutr 2011; 94: 1479–1484. [DOI] [PubMed] [Google Scholar]
- 17.Nestel PJ, Chronopulos A, Cehun M. Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects. Eur J Clin Nutr 2005; 59: 1059–1063. [DOI] [PubMed] [Google Scholar]
- 18.Tholstrup T, Hoy CE, Andersen LN, Christensen RD, Sandstrom B. Does fat in milk, butter and cheese affect blood lipids and cholesterol differently? J Am Coll Nutr 2004; 23: 169–176. [DOI] [PubMed] [Google Scholar]
- 19.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010; 121: 2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnard ND, Willett WC, Ding EL. The misuse of meta-analysis in nutrition research. JAMA 2017; 318: 1435–1436. [DOI] [PubMed] [Google Scholar]
- 21.Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev 2015: CD011737. [DOI] [PubMed] [Google Scholar]
- 22.Dehghan M, Mente A, Zhang X et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017; 390: 2050–2062. [DOI] [PubMed] [Google Scholar]
- 23.Jakobsen MU, Dethlefsen C, Joensen AM et al. Intake of carbohydrates compared with intake of saturated fatty acids and risk of myocardial infarction: importance of the glycemic index. Am J Clin Nutr 2010; 91: 1764–1768. [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Lichtenstein AH, Wu JHY et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation 2017; 136: e1–e23. [DOI] [PubMed] [Google Scholar]
- 25.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr 2010; 91: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebman BF, Katan MB, Jacobson MF. Association of dietary, circulating, and supplement fatty acids with coronary risk. Ann Intern Med 2014; 161: 454–455. [DOI] [PubMed] [Google Scholar]
- 27.Woodhill JM, Palmer AJ, Leelarthaepin B, McGilchrist C, Blacket RB. Low fat, low cholesterol diet in secondary prevention of coronary heart disease. Adv Exp Med Biol 1978; 109: 317–330. [DOI] [PubMed] [Google Scholar]
- 28.Siri-Tarino PW, Chiu S, Bergeron N, Krauss RM. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu Rev Nutr 2015; 35: 517–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsden CE, Zamora D, Leelarthaepin B et al. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013; 346: e8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turpeinen O, Karvonen MJ, Pekkarinen M, Miettinen M, Elosuo R, Paavilainen E. Dietary prevention of coronary heart disease: the Finnish Mental Hospital Study. Int J Epidemiol 1979; 8: 99–118. [DOI] [PubMed] [Google Scholar]
- 31.Leren P The Oslo Diet-Heart study. Eleven-year report. Circulation 1970; 42: 935–942. [DOI] [PubMed] [Google Scholar]
- 32.Dayton S, Pearce ML, Hashimot S, Dixon WJ, Tomiyasu U. A controlled clinical trial of a diet high in unsaturated fat in preventing complications of atherosclerosis. Circulation 1969; 40: II-1–II-63. [Google Scholar]
- 33.Controlled trial of soya-bean oil in myocardial infarction. Lancet 1968; 2: 693–699. [PubMed] [Google Scholar]
- 34.Institute of Medicine (US) Committee to Review the NIH Women’s Health Initiative. An assessment of the NIH Women’s Health Initiative In: Thaul S, Hotra D (eds). The National Academies Collection: Reports Funded by National Institutes of Health. National Academies Press: Washington (DC), 1993, pp. 25–76. [PubMed] [Google Scholar]
- 35.Howard BV, Van Horn L, Hsia J et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006; 295: 655–666. [DOI] [PubMed] [Google Scholar]
- 36.Robiner WN. Enhancing adherence in clinical research. Contemp Clin Trials 2005; 26: 59–77. [DOI] [PubMed] [Google Scholar]
- 37.Dhurandhar NV, Schoeller D, Brown AW et al. Energy balance measurement: when something is not better than nothing. Int J Obes (Lond) 2015; 39: 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estruch R, Ros E, Salas-Salvado J et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013; 368: 1279–1290. [DOI] [PubMed] [Google Scholar]
- 39.Estruch R, Martinez-Gonzalez MA, Corella D et al. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol 2016; 4: 666–676. [DOI] [PubMed] [Google Scholar]
- 40.Institute of Medicine. Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease. Natl. Acad. Press: Washington, DC, 2010. [PubMed] [Google Scholar]
- 41.Krauss RM. All low-density lipoprotein particles are not created equal. Arterioscler Thromb Vasc Biol 2014; 34: 959–961. [DOI] [PubMed] [Google Scholar]
- 42.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 2002; 43: 1363–1379. [DOI] [PubMed] [Google Scholar]
- 43.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr 2006; 83: 1025–1031 quiz 205. [DOI] [PubMed] [Google Scholar]
- 44.Mente A, Dehghan M, Rangarajan S et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: a cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol 2017; 5(10): 774–787. [DOI] [PubMed] [Google Scholar]
- 45.Fredenrich A Role of apolipoprotein CIII in triglyceride-rich lipoprotein metabolism. Diabetes Metab 1998; 24: 490–495. [PubMed] [Google Scholar]
- 46.Kawakami A, Aikawa M, Alcaide P, Luscinskas FW, Libby P, Sacks FM. Apolipoprotein CIII induces expression of vascular cell adhesion molecule-1 in vascular endothelial cells and increases adhesion of monocytic cells. Circulation 2006; 114(7): 681. [DOI] [PubMed] [Google Scholar]
- 47.Shin MJ, Krauss RM. Apolipoprotein CIII bound to apoB-containing lipoproteins is associated with small, dense LDL independent of plasma triglyceride levels in healthy men. Atherosclerosis 2010; 211: 337–341. [DOI] [PubMed] [Google Scholar]
- 48.Furtado JD, Campos H, Appel LJ et al. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. Am J Clin Nutr 2008; 87: 1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Hruby A, Bernstein AM et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease. A Prospective Cohort Study J Am Coll Cardiol 2015; 66: 1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng J, Huang T, Yu Y, Hu X, Yang B, Li D. Fish consumption and CHD mortality: an updated meta-analysis of seventeen cohort studies. Public Health Nutr 2012; 15: 725–737. [DOI] [PubMed] [Google Scholar]
- 51.Siscovick DS, Barringer TA, Fretts AM et al. Omega-3 polyun-saturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation 2017; 135: e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basaranoglu M, Basaranoglu G, Bugianesi E. Carbohydrate in-take and nonalcoholic fatty liver disease: fructose as a weapon of mass destruction. Hepatobiliary Surg Nutr 2015; 4: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson RJ, Sanchez-Lozada LG, Andrews P, Lanaspa MA. Perspective: a historical and scientific perspective of sugar and its relation with obesity and diabetes. Adv Nutr 2017; 8: 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malik VS, Hu FB. Fructose and cardiometabolic health: what the evidence from sugar-sweetened beverages tells us. J Am Coll Cardiol 2015; 66: 1615–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rippe JM, Marcos A. Controversies about sugars consumption: state of the science. Eur J Nutr 2016; 55: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sievenpiper JL. Sickeningly sweet: does sugar cause chronic disease? No. Can J Diabetes 2016; 40: 287–295. [DOI] [PubMed] [Google Scholar]
- 57.Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci 2016; 61: 1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanhope KL. Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci 2016; 53: 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xi B, Huang Y, Reilly KH et al. Sugar-sweetened beverages and risk of hypertension and CVD: a dose-response meta-analysis. Br J Nutr 2015; 113: 709–717. [DOI] [PubMed] [Google Scholar]
- 60.Wang DD, Sievenpiper JL, de Souza RJ et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014; 232: 125–133. [DOI] [PubMed] [Google Scholar]
- 61.Wang DD, Sievenpiper JL, de Souza RJ et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr 2012; 142: 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr 2014; 100: 65–79. [DOI] [PubMed] [Google Scholar]
- 63.Kelishadi R, Mansourian M, Heidari-Beni M. Association of fructose consumption and components of metabolic syndrome in human studies: a systematic review and meta-analysis. Nutrition 2014; 30: 503–510. [DOI] [PubMed] [Google Scholar]
- 64.Jayalath VH, Sievenpiper JL, de Souza RJ et al. Total fructose intake and risk of hypertension: a systematic review and meta-analysis of prospective cohorts. J Am Coll Nutr 2014; 33: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imamura F, O’Connor L, Ye Z et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. Br J Sports Med 2016; 50: 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.U.S. Food and Drug Administration. Changes to the Nutrition Facts Label. 2017.
- 67.World Health Organization. Guideline: Sugars Intake for Adults and Children. WHO: Geneva, 2015. [PubMed] [Google Scholar]
- 68.Joshipura KJ, Hu FB, Manson JE et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med 2001; 134: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 69.Dauchet L, Ferrieres J, Arveiler D et al. Frequency of fruit and vegetable consumption and coronary heart disease in France and Northern Ireland: the PRIME study. Br J Nutr 2004; 92: 963–972. [DOI] [PubMed] [Google Scholar]
- 70.Dauchet L, Amouyel P, Dallongeville J. Fruit and vegetable consumption and risk of stroke: a meta-analysis of cohort studies. Neurology 2005; 65: 1193–1197. [DOI] [PubMed] [Google Scholar]
- 71.Yamada T, Hayasaka S, Shibata Y et al. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J Epidemiol 2011; 21: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagura J, Iso H, Watanabe Y et al. Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: the JACC Study. Br J Nutr 2009; 102: 285–292. [DOI] [PubMed] [Google Scholar]
- 73.Iso H, Kubota Y. Japan Collaborative Cohort Study for Evaluation of C. Nutrition and disease in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev 2007; 8: 35–80. [PubMed] [Google Scholar]
- 74.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet 1993; 342: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 75.Bazzano LA, He J, Ogden LG et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr 2002; 76: 93–99. [DOI] [PubMed] [Google Scholar]
- 76.Steffen LM, Jacobs DR Jr, Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003; 78: 383–390. [DOI] [PubMed] [Google Scholar]
- 77.Liu S, Manson JE, Lee IM et al. Fruit and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. Am J Clin Nutr 2000; 72: 922–928. [DOI] [PubMed] [Google Scholar]
- 78.Hung HC, Joshipura KJ, Jiang R et al. Fruit and vegetable in-take and risk of major chronic disease. J Natl Cancer Inst 2004; 96: 1577–1584. [DOI] [PubMed] [Google Scholar]
- 79.Gillman MW, Cupples LA, Gagnon D et al. Protective effect of fruits and vegetables on development of stroke in men. JAMA 1995; 273: 1113–1117. [DOI] [PubMed] [Google Scholar]
- 80.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol 2004; 160: 1223–1233. [DOI] [PubMed] [Google Scholar]
- 81.Wang PY, Fang JC, Gao ZH, Zhang C, Xie SY. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: a meta-analysis. J Diabetes Investig 2016; 7: 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuzma JN, Schmidt KA, Kratz M. Prevention of metabolic diseases: fruits (including fruit sugars) vs. vegetables. Curr Opin Clin Nutr Metab Care 2017; 20: 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hartley L, Igbinedion E, Holmes J et al. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev 2013: CD009874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alissa EM, Ferns GA. Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr 2017; 57: 1950–1962. [DOI] [PubMed] [Google Scholar]
- 85.Morand C, Dubray C, Milenkovic D et al. Hesperidin contributes to the vascular protective effects of orange juice: a randomized crossover study in healthy volunteers. Am J Clin Nutr 2011; 93: 73–80. [DOI] [PubMed] [Google Scholar]
- 86.Simpson EJ, Mendis B, Macdonald IA. Orange juice consumption and its effect on blood lipid profile and indices of the metabolic syndrome; a randomised, controlled trial in an at-risk population. Food Funct 2016; 7: 1884–1891. [DOI] [PubMed] [Google Scholar]
- 87.Dohadwala MM, Hamburg NM, Holbrook M et al. Effects of Concord grape juice on ambulatory blood pressure in prehypertension and stage 1 hypertension. Am J Clin Nutr 2010; 92: 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR Jr, Popkin BM. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr 2010; 92: 954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mattei J, Malik V, Hu FB, Campos H. Substituting homemade fruit juice for sugar-sweetened beverages is associated with lower odds of metabolic syndrome among Hispanic adults. J Nutr 2012; 142: 1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng Y, Li Y, Huang T, Cheng HL, Campos H, Qi L. Sugar-sweetened beverage intake, chromosome 9p21 variants, and risk of myocardial infarction in Hispanics. Am J Clin Nutr 2016; 103: 1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eshak ES, Iso H, Mizoue T, Inoue M, Noda M, Tsugane S. Soft drink, 100% fruit juice, and vegetable juice intakes and risk of diabetes mellitus. Clin Nutr 2013; 32: 300–308. [DOI] [PubMed] [Google Scholar]
- 92.Schulze MB, Manson JE, Ludwig DS et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA 2004; 292: 927–934. [DOI] [PubMed] [Google Scholar]
- 93.Xi B, Li S, Liu Z et al. Intake of fruit juice and incidence of type 2 diabetes: a systematic review and meta-analysis. PLoS One 2014; 9: e93471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao X, Qi L, Qiao N et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension 2007; 50: 306–312. [DOI] [PubMed] [Google Scholar]
- 95.Leung CW, Laraia BA, Needham BL et al. Soda and cell aging: associations between sugar-sweetened beverage consumption and leukocyte telomere length in healthy adults from the National Health and Nutrition Examination Surveys. Am J Public Health 2014; 104: 2425–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshida M, McKeown NM, Rogers G et al. Surrogate markers of insulin resistance are associated with consumption of sugar-sweetened drinks and fruit juice in middle and older-aged adults. J Nutr 2007; 137: 2121–2127. [DOI] [PubMed] [Google Scholar]
- 97.Ferreira-Pego C, Babio N, Bes-Rastrollo M et al. Frequent consumption of sugar- and artificially sweetened beverages and natural and bottled fruit juices is associated with an increased risk of metabolic syndrome in a Mediterranean population at high cardiovascular disease risk. J Nutr 2016; 146: 1528–1536. [DOI] [PubMed] [Google Scholar]
- 98.Odegaard AO, Koh WP, Arakawa K, Yu MC, Pereira MA. Soft drink and juice consumption and risk of physician-diagnosed incident type 2 diabetes: the Singapore Chinese Health Study. Am J Epidemiol 2010; 171: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pan A, Malik VS, Schulze MB, Manson JE, Willett WC, Hu FB. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am J Clin Nutr 2012; 95: 1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiavaroli L, de Souza RJ, Ha V et al. Effect of fructose on established lipid targets: a systematic review and meta-analysis of controlled feeding trials. J Am Heart Assoc 2015; 4: e001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ha V, Sievenpiper JL, de Souza RJ et al. Effect of fructose on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Hypertension 2012; 59: 787–795. [DOI] [PubMed] [Google Scholar]
- 102.Sievenpiper JL, de Souza RJ, Mirrahimi A et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med 2012; 156: 291–304. [DOI] [PubMed] [Google Scholar]
- 103.Madero M, Arriaga JC, Jalal D et al. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metabolism 2011; 60: 1551–1559. [DOI] [PubMed] [Google Scholar]
- 104.Bechthold A, Boeing H, Schwedhelm C et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr 2017: 1–20. [DOI] [PubMed] [Google Scholar]
- 105.Huang C, Huang J, Tian Y, Yang X, Gu D. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta-analysis of prospective studies. Atherosclerosis 2014; 234: 11–16. [DOI] [PubMed] [Google Scholar]
- 106.Imamura F, O’Connor L, Ye Z et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015; 351: h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Greenwood DC, Threapleton DE, Evans CE et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr 2014; 112: 725–734. [DOI] [PubMed] [Google Scholar]
- 108.Wang M, Yu M, Fang L, Hu RY. Association between sugar-sweetened beverages and type 2 diabetes: a meta-analysis. J Diabetes Investig 2015; 6: 360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schwingshackl L, Hoffmann G, Lampousi AM et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2017; 32: 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010; 33: 2477–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jayalath VH, , Ha V et al. Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr 2015; 102: 914–921. [DOI] [PubMed] [Google Scholar]
- 112.Micha R, Shulkin ML, Penalvo JL et al. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS One 2017; 12: e0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang Q, Zhang Z, Gregg EW, Flanders WD, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med 2014; 174: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tasevska N, Park Y, Jiao L, Hollenbeck A, Subar AF, Potischman N. Sugars and risk of mortality in the NIH-AARP Diet and Health Study. Am J Clin Nutr 2014; 99: 1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee AK, Binongo JN, Chowdhury R et al. Consumption of less than 10% of total energy from added sugars is associated with increasing HDL in females during adolescence: a longitudinal analysis. J Am Heart Assoc 2014; 3: e000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J, Light K, Henderson M et al. Consumption of added sugars from liquid but not solid sources predicts impaired glucose homeostasis and insulin resistance among youth at risk of obesity. J Nutr 2014; 144: 81–86. [DOI] [PubMed] [Google Scholar]
- 117.Stanhope KL, Schwarz JM, Keim NL et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009; 119: 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stanhope KL, Griffen SC, Bremer AA et al. Metabolic responses to prolonged consumption of glucose- and fructose-sweetened beverages are not associated with postprandial or 24-h glucose and insulin excursions. Am J Clin Nutr 2011; 94: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Asrih M, Jornayvaz FR. Metabolic syndrome and nonalcoholic fatty liver disease: is insulin resistance the link? Mol Cell Endocrinol 2015; 418(Pt 1): 55–65. [DOI] [PubMed] [Google Scholar]
- 120.Cox CL, Stanhope KL, Schwarz JM et al. Consumption of fructose-sweetened beverages for 10 weeks reduces net fat oxidation and energy expenditure in overweight/obese men and women. Eur J Clin Nutr 2012; 66: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cox CL, Stanhope KL, Schwarz JM et al. Consumption of fructose- but not glucose-sweetened beverages for 10 weeks increases circulating concentrations of uric acid, retinol binding protein-4, and gamma-glutamyl transferase activity in overweight/obese humans. Nutr Metab 2012; 9: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ishimoto T, Lanaspa MA, Le MT et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci U S A 2012; 109: 4320–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr 1993; 58: 754S–765S. [DOI] [PubMed] [Google Scholar]
- 124.Bruun JM, Maersk M, Belza A, Astrup A, Richelsen B. Consumption of sucrose-sweetened soft drinks increases plasma levels of uric acid in overweight and obese subjects: a 6-month randomised controlled trial. Eur J Clin Nutr 2015; 69: 949–953. [DOI] [PubMed] [Google Scholar]
- 125.Stanhope KL, Medici V, Bremer AA et al. A dose–response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr 2015; 101: 1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Taskinen MR, Soderlund S, Bogl LH et al. Adverse effects of fructose on cardiometabolic risk factors and hepatic lipid metabolism in subjects with abdominal obesity. J Intern Med 2017; 282: 187–201. [DOI] [PubMed] [Google Scholar]
- 127.Schwarz JM, Noworolski SM, Wen MJ et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J Clin Endocrinol Metab 2015; 100: 2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maersk M, Belza A, Stodkilde-Jorgensen H et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012; 95: 283–289. [DOI] [PubMed] [Google Scholar]
- 129.Sevastianova K, Santos A, Kotronen A et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr 2012; 96: 727–734. [DOI] [PubMed] [Google Scholar]
- 130.Adiels M, Taskinen MR, Packard C et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 2006; 49: 755–765. [DOI] [PubMed] [Google Scholar]
- 131.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008; 28: 1225–1236. [DOI] [PubMed] [Google Scholar]
- 132.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 2006; 55(Suppl 2): S9–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 2002; 87: 3023–3028. [DOI] [PubMed] [Google Scholar]
- 134.Bergheim I, Weber S, Vos M et al. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 2008; 48: 983–992. [DOI] [PubMed] [Google Scholar]
- 135.Kavanagh K, Wylie AT, Tucker KL et al. Dietary fructose induces endotoxemia and hepatic injury in calorically controlled primates. Am J Clin Nutr 2013; 98: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J Nutr Biochem 2007; 18: 1–9. [DOI] [PubMed] [Google Scholar]
- 137.Stanhope KL, Bremer AA, Medici V et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab 2011; 96: E1596–E1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ec-topic fat deposition in muscles. Appl Physiol Nutr Metab 2013; 38: 681–688. [DOI] [PubMed] [Google Scholar]
- 139.Yu Z, Lowndes J, Rippe J. High-fructose corn syrup and sucrose have equivalent effects on energy-regulating hormones at normal human consumption levels. Nutr Res 2013; 33: 1043–1052. [DOI] [PubMed] [Google Scholar]
- 140.Black RN, Spence M, McMahon RO et al. Effect of eucaloric high- and low-sucrose diets with identical macronutrient profile on insulin resistance and vascular risk: a randomized controlled trial. Diabetes 2006; 55: 3566–3572. [DOI] [PubMed] [Google Scholar]
- 141.Groen JJ, Balogh M, Yaron E, Cohen AM. Effect of interchanging bread and sucrose as main source of carbohydrate in a low fat diet on the serum cholesterol levels of healthy volunteer subjects. Am J Clin Nutr 1966; 19: 46–58. [DOI] [PubMed] [Google Scholar]
- 142.Marckmann P, Raben A, Astrup A. Ad libitum intake of low-fat diets rich in either starchy foods or sucrose: effects on blood lipids, factor VII coagulant activity, and fibrinogen. Metabolism 2000; 49: 731–735. [DOI] [PubMed] [Google Scholar]
- 143.Reiser S, Bickard MC, Hallfrisch J, Michaelis OET, Prather ES. Blood lipids and their distribution in lipoproteins in hyperinsulinemic subjects fed three different levels of sucrose. J Nutr 1981; 111: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 144.Reiser S, Hallfrisch J, Michaelis OET, Lazar FL, Martin RE, Prather ES. Isocaloric exchange of dietary starch and sucrose in humans. I. Effects on levels of fasting blood lipids. Am J Clin Nutr 1979; 32: 1659–1669. [DOI] [PubMed] [Google Scholar]
- 145.Mann JI, Truswell AS. Effects of isocaloric exchange of dietary sucrose and starch on fasting serum lipids, postprandial insulin secretion and alimentary lipaemia in human subjects. Br J Nutr 1972; 27: 395–405. [DOI] [PubMed] [Google Scholar]
- 146.Aeberli I, Hochuli M, Gerber PA et al. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 2013; 36: 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aeberli I, Gerber PA, Hochuli M et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011; 94: 479–485. [DOI] [PubMed] [Google Scholar]
- 148.Stanhope KL, Bremer AA, Medici V, Keim NL, Havel PJ. Compared with aspartame, consumption of high fructose corn syrup- and sucrose-sweetened beverages increases triglycerides, cholesterol, non-HDL cholesterol, apolipoprotein-B, and uric acid in young men and women. J Womens Health 2013; 22: 898–899. [Google Scholar]
- 149.Reiser S, Bohn E, Hallfrisch J, Michaelis OET, Keeney M, Prather ES. Serum insulin and glucose in hyperinsulinemic subjects fed three different levels of sucrose. Am J Clin Nutr 1981; 34: 2348–2358. [DOI] [PubMed] [Google Scholar]
- 150.Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr 2000; 72: 1128–1134. [DOI] [PubMed] [Google Scholar]
- 151.Bantle JP, Swanson JE, Thomas W, Laine DC. Metabolic effects of dietary fructose in diabetic subjects. Diabetes Care 1992; 15: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 152.Hallfrisch J, Ellwood K, Michaelis OET, Reiser S, Prather ES. Plasma fructose, uric acid, and inorganic phosphorus responses of hyperinsulinemic men fed fructose. J Am Coll Nutr 1986; 5: 61–68. [DOI] [PubMed] [Google Scholar]
- 153.Hallfrisch J, Ellwood KC, Michaelis OET, Reiser S, O’Dorisio TM, Prather ES. Effects of dietary fructose on plasma glucose and hormone responses in normal and hyperinsulinemic men. J Nutr 1983; 113: 1819–1826. [DOI] [PubMed] [Google Scholar]
- 154.Hallfrisch J, Reiser S, Prather ES. Blood lipid distribution of hyperinsulinemic men consuming three levels of fructose. Am J Clin Nutr 1983; 37: 740–748. [DOI] [PubMed] [Google Scholar]
- 155.Johnston RD, Stephenson MC, Crossland H et al. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 2013; 145: 1016–25 e2. [DOI] [PubMed] [Google Scholar]
- 156.Lewis AS, McCourt HJ, Ennis CN et al. Comparison of 5% versus 15% sucrose intakes as part of a eucaloric diet in overweight and obese subjects: effects on insulin sensitivity, glucose metabolism, vascular compliance, body composition and lipid profile. A randomised controlled trial. Metabolism 2013; 62: 694–702. [DOI] [PubMed] [Google Scholar]
- 157.Swanson JE, Laine DC, Thomas W, Bantle JP. Metabolic effects of dietary fructose in healthy subjects. Am J Clin Nutr 1992; 55: 851–856. [DOI] [PubMed] [Google Scholar]
- 158.Swarbrick MM, Stanhope KL, Elliott SS et al. Consumption of fructose-sweetened beverages for 10 weeks increases postpran-dial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr 2008; 100: 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lustig RH, Mulligan K, Noworolski SM et al. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity (Silver Spring) 2016; 24: 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schwarz JM, Noworolski SM, Erkin-Cakmak A et al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology 2017; 153(3): 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gugliucci A, Lustig RH, Caccavello R et al. Short-term isocaloric fructose restriction lowers apoC-III levels and yields less atherogenic lipoprotein profiles in children with obesity and metabolic syndrome. Atherosclerosis 2016; 253: 171–177. [DOI] [PubMed] [Google Scholar]
- 162.Ibarra-Reynoso LDR, Lopez-Lemus HL, Garay-Sevilla ME, Malacara JM. Effect of restriction of foods with high fructose corn syrup content on metabolic indices and fatty liver in obese children. Obes Facts 2017; 10: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mager DR, Iniguez IR, Gilmour S, Yap J. The effect of a low fructose and low glycemic index/load (FRAGILE) dietary intervention on indices of liver function, cardiometabolic risk factors, and body composition in children and adolescents with nonalcoholic fatty liver disease (NAFLD). JPEN J Parenter Enteral Nutr 2015; 39: 73–84. [DOI] [PubMed] [Google Scholar]
- 164.Campos V, Despland C, Brandejsky V et al. Sugar- and artificially sweetened beverages and intrahepatic fat: a randomized controlled trial. Obesity (Silver Spring) 2015; 23: 2335–2339. [DOI] [PubMed] [Google Scholar]
- 165.Danforth E Jr. Diet and obesity. Am J Clin Nutr 1985; 41: 1132–1145. [DOI] [PubMed] [Google Scholar]
- 166.Acheson KJ, Schutz Y, Bessard T, Ravussin E, Jequier E, Flatt JP. Nutritional influences on lipogenesis and thermogenesis after a carbohydrate meal. Am J Physiol 1984; 246: E62–E70. [DOI] [PubMed] [Google Scholar]
- 167.Schutz Y, Flatt JP, Jequier E. Failure of dietary fat intake to promote fat oxidation: a factor favoring the development of obesity. Am J Clin Nutr 1989; 50: 307–314. [DOI] [PubMed] [Google Scholar]
- 168.Flatt JP, Ravussin E, Acheson KJ, Jequier E. Effects of dietary fat on postprandial substrate oxidation and on carbohydrate and fat balances. J Clin Invest 1985; 76: 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Astrup A, Buemann B, Christensen NJ, Toubro S. Failure to increase lipid oxidation in response to increasing dietary fat content in formerly obese women. Am J Physiol 1994; 266: E592–E599. [DOI] [PubMed] [Google Scholar]
- 170.Schrauwen P, van Marken Lichtenbelt WD, Saris WH, Westerterp KR. Changes in fat oxidation in response to a high-fat diet. Am J Clin Nutr 1997; 66: 276–282. [DOI] [PubMed] [Google Scholar]
- 171.Smith SR, de Jonge L, Zachwieja JJ et al. Fat and carbohydrate balances during adaptation to a high-fat. Am J Clin Nutr 2000; 71: 450–457. [DOI] [PubMed] [Google Scholar]
- 172.Smith SR, de Jonge L, Zachwieja JJ et al. Concurrent physical activity increases fat oxidation during the shift to a high-fat diet. Am J Clin Nutr 2000; 72: 131–138. [DOI] [PubMed] [Google Scholar]
- 173.Glickman N, Mitchell HH et al. The total specific dynamic action of high-protein and high-carbohydrate diets on human subjects. J Nutr 1948; 36: 41–57. [DOI] [PubMed] [Google Scholar]
- 174.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr 2004; 23: 373–385. [DOI] [PubMed] [Google Scholar]
- 175.Segal KR, Albu J, Chun A, Edano A, Legaspi B, Pi-Sunyer FX. Independent effects of obesity and insulin resistance on postpran-dial thermogenesis in men. J Clin Invest 1992; 89: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cotton JR, Burley VJ, Weststrate JA, Blundell JE. Dietary fat and appetite: similarities and differences in the satiating effect of meals supplemented with either fat or carbohydrate. Journal of human nutrition and dietetics: the official journal of the British Dietetic Association 2007; 20: 186–199. [DOI] [PubMed] [Google Scholar]
- 177.Williams RA, Roe LS, Rolls BJ. Assessment of satiety depends on the energy density and portion size of the test meal. Obesity (Silver Spring) 2014; 22: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rouhani MH, Haghighatdoost F, Surkan PJ, Azadbakht L. Associations between dietary energy density and obesity: a systematic review and meta-analysis of observational studies. Nutrition 2016; 32(10): 1037–47. [DOI] [PubMed] [Google Scholar]
- 179.Flatt JP. Issues and misconceptions about obesity. Obesity (Silver Spring) 2011; 19: 676–686. [DOI] [PubMed] [Google Scholar]
- 180.Walker TB, Parker MJ. Lessons from the war on dietary fat. J Am Coll Nutr 2014; 33: 347–351. [DOI] [PubMed] [Google Scholar]
- 181.Havel PJ, Townsend R, Chaump L, Teff K. High-fat meals reduce 24-h circulating leptin concentrations in women. Diabetes 1999; 48: 334–341. [DOI] [PubMed] [Google Scholar]
- 182.Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA 2014; 311: 2167–2168. [DOI] [PubMed] [Google Scholar]
- 183.Hall KD, Bemis T, Brychta R et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab 2015; 22: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: a systematic review and meta-analysis. PLoS One 2014; 9: e100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tobias DK, Chen M, Manson JE, Ludwig DS, Willett W, Hu FB. Effect of low-fat diet interventions versus other diet interventions on long-term weight change in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2015; 3: 968–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Soenen S, Bonomi AG, Lemmens SG et al. Relatively high-protein or ‘low-carb’ energy-restricted diets for body weight loss and body weight maintenance? Physiol Behav 2012; 107: 374–380. [DOI] [PubMed] [Google Scholar]
- 187.Gardner CD, Trepanowski JF, Del Gobbo LC et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in over-weight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA 2018; 319: 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Saris WHM. The search for optimal macronutrient recommendations In: Biesalski H, Drewnowski A, Dwyer J, Strain J, Weber P, Eggersdorfer M (eds). Sustainable nutrition in a changing world. Springer: Cham, 2017. [Google Scholar]
- 189.Astrup A, Grunwald GK, Melanson EL, Saris WH, Hill JO. The role of low-fat diets in body weight control: a meta-analysis of ad libitum dietary intervention studies. Int J Obes Relat Metab Disord 2000; 24: 1545–1552. [DOI] [PubMed] [Google Scholar]
- 190.Hooper L, Abdelhamid A, Bunn D, Brown T, Summerbell CD, Skeaff CM. Effects of Total Fat Intake on Body Weight (Review). John Wiley & Sons, Ltd., 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mirza NM, Palmer MG, Sinclair KB et al. Effects of a low glycemic load or a low-fat dietary intervention on body weight in obese Hispanic American children and adolescents: a randomized controlled trial. Am J Clin Nutr 2013; 97: 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Larsen TM, Dalskov SM, van Baak M et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 2010; 363: 2102–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Vu T, Lin F, Alshurafa N, Xu WY. Wearable food intake monitoring technologies: A comprehensive review. Comput Secur 2017; 6. [Google Scholar]
- 194.Hall KD, Chen KY, Guo J et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in over-weight and obese men. Am J Clin Nutr 2016; 104: 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Skov AR, Toubro S, Raben A, Astrup A. A method to achieve control of dietary macronutrient composition in ad libitum diets consumed by free-living subjects. Eur J Clin Nutr 1997; 51: 667–672. [DOI] [PubMed] [Google Scholar]
- 196.Belza A, Ritz C, Sorensen MQ, Holst JJ, Rehfeld JF, Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr 2013; 97: 980–989. [DOI] [PubMed] [Google Scholar]
- 197.Martens EA, Westerterp-Plantenga MS. Protein diets, body weight loss and weight maintenance. Curr Opin Clin Nutr Metab Care 2014; 17: 75–79. [DOI] [PubMed] [Google Scholar]
- 198.Journel M, Chaumontet C, Darcel N, Fromentin G, Tome D. Brain responses to high-protein diets. Adv Nutr 2012; 3: 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wanders AJ, van den Borne JJ, de Graaf C et al. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes Rev 2011; 12: 724–739. [DOI] [PubMed] [Google Scholar]
- 200.Arguin H, Tremblay A, Blundell JE et al. Impact of a non-restrictive satiating diet on anthropometrics, satiety responsiveness and eating behaviour traits in obese men displaying a high or a low satiety phenotype. Br J Nutr 2017; 118: 750–760. [DOI] [PubMed] [Google Scholar]
- 201.Cooper DN, Martin RJ, Keim NL. Does whole grain consumption alter gut microbiota and satiety? Healthcare (Basel) 2015; 3: 364–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Parra D, Ramel A, Bandarra N, Kiely M, Martinez JA, Thorsdottir I. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite 2008; 51: 676–680. [DOI] [PubMed] [Google Scholar]
- 203.Tremblay A, Arguin H, Panahi S. Capsaicinoids: a spicy solution to the management of obesity? Int J Obes (Lond) 2016; 40: 1198–1204. [DOI] [PubMed] [Google Scholar]
- 204.Gonzalez JT, Green BP, Brown MA, Rumbold PL, Turner LA, Stevenson EJ. Calcium ingestion suppresses appetite and produces acute overcompensation of energy intake independent of protein in healthy adults. J Nutr 2015; 145: 476–482. [DOI] [PubMed] [Google Scholar]
- 205.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 2008; 118: 2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr 2000; 71: 1421–1432. [DOI] [PubMed] [Google Scholar]
- 207.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995; 332: 621–628. [DOI] [PubMed] [Google Scholar]
- 208.Kissileff HR, Thornton JC, Torres MI et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr 2012; 95: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol 2014; 223: T83–T96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kahlhofer J, Lagerpusch M, Enderle J et al. Carbohydrate in-take and glycemic index affect substrate oxidation during a controlled weight cycle in healthy men. Eur J Clin Nutr 2014; 68: 1060–1066. [DOI] [PubMed] [Google Scholar]
- 211.Claessens M, van Baak MA, Monsheimer S, Saris WH. The effect of a low-fat, high-protein or high-carbohydrate ad libitum diet on weight loss maintenance and metabolic risk factors. Int J Obes (Lond) 2009; 33: 296–304. [DOI] [PubMed] [Google Scholar]
- 212.Ebbeling CB, Swain JF, Feldman HA et al. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA 2012; 307: 2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ross R, Dagnone D, Jones PJ et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 2000; 133: 92–103. [DOI] [PubMed] [Google Scholar]
- 214.Assali AR, Ganor A, Beigel Y, Shafer Z, Hershcovici T, Fainaru M. Insulin resistance in obesity: body-weight or energy balance? J Endocrinol 2001; 171: 293–298. [DOI] [PubMed] [Google Scholar]
- 215.Ueno M, Carvalheira JB, Tambascia RC et al. Regulation of insulin signalling by hyperinsulinaemia: role of IRS-1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia 2005; 48: 506–518. [DOI] [PubMed] [Google Scholar]
- 216.Kim JK, Michael MD, Previs SF et al. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J Clin Invest 2000; 105: 1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cusin I, Terrettaz J, Rohner-Jeanrenaud F, Zarjevski N, Assimacopoulos-Jeannet F, Jeanrenaud B. Hyperinsulinemia increases the amount of GLUT4 mRNA in white adipose tissue and decreases that of muscles: a clue for increased fat depot and insulin resistance. Endocrinology 1990; 127: 3246–3248. [DOI] [PubMed] [Google Scholar]
- 218.Cettour-Rose P, Samec S, Russell AP et al. Redistribution of glucose from skeletal muscle to adipose tissue during catch-up fat: a link between catch-up growth and later metabolic syndrome. Diabetes 2005; 54: 751–756. [DOI] [PubMed] [Google Scholar]
- 219.Bluher M, Michael MD, Peroni OD et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell 2002; 3: 25–38. [DOI] [PubMed] [Google Scholar]
- 220.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 2003; 299: 572–574. [DOI] [PubMed] [Google Scholar]
- 221.Gratas-Delamarche A, Derbre F, Vincent S, Cillard J. Physical inactivity, insulin resistance, and the oxidative-inflammatory loop. Free Radic Res 2014; 48: 93–108. [DOI] [PubMed] [Google Scholar]
- 222.Hojbjerre L, Alibegovic AC, Sonne MP et al. Increased lipolysis but diminished gene expression of lipases in subcutaneous adipose tissue of healthy young males with intrauterine growth retardation. J Appl Physiol (1985) 2011; 111: 1863–1870. [DOI] [PubMed] [Google Scholar]
- 223.Bosy-Westphal A, Hagele F, Nas A. Impact of dietary glycemic challenge on fuel partitioning. Eur J Clin Nutr 2017; 71: 327–330. [DOI] [PubMed] [Google Scholar]
- 224.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev 2014; 15: 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Stefan N, Kantartzis K, Machann J et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008; 168: 1609–1616. [DOI] [PubMed] [Google Scholar]
- 226.Hjorth MF, Ritz C, Blaak EE et al. Pretreatment fasting plasma glucose and insulin modify dietary weight loss success: results from 3 randomized clinical trials. Am J Clin Nutr 2017; 106: 499–505. [DOI] [PubMed] [Google Scholar]
- 227.Foster GD, Wyatt HR, Hill JO et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med 2010; 153: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Petersen M, Taylor MA, Saris WH et al. Randomized, multi-center trial of two hypo-energetic diets in obese subjects: high-versus low-fat content. Int J Obes (Lond) 2006; 30: 552–560. [DOI] [PubMed] [Google Scholar]
- 229.Mithril C, Dragsted LO, Meyer C, Blauert E, Holt MK, Astrup A. Guidelines for the new Nordic diet. Public Health Nutr 2012; 15: 1941–1947. [DOI] [PubMed] [Google Scholar]
- 230.Poulsen SK, Due A, Jordy AB et al. Health effect of the New Nordic diet in adults with increased waist circumference: a 6-mo randomized controlled trial. Am J Clin Nutr 2014; 99: 35–45. [DOI] [PubMed] [Google Scholar]
- 231.Hjorth MF, Roager HM, Larsen TM et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int J Obes (Lond) 2018. February; 42(2): 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Arumugam M, Raes J, Pelletier E et al. Enterotypes of the human gut microbiome. Nature 2011; 473: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lim MY, Rho M, Song YM, Lee K, Sung J, Ko G. Stability of gut enterotypes in Korean monozygotic twins and their association with biomarkers and diet. Sci Rep 2014; 4: 7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.de Moraes AC, Fernandes GR, da Silva IT et al. Enterotype may drive the dietary-associated cardiometabolic risk factors. Front Cell Infect Microbiol 2017; 7: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kovatcheva-Datchary P, Nilsson A, Akrami R et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 2015; 22: 971–982. [DOI] [PubMed] [Google Scholar]
- 236.Pepino MY. Metabolic effects of non-nutritive sweeteners. Physiol Behav 2015; 152: 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Shearer J, Swithers SE. Artificial sweeteners and metabolic dysregulation: lessons learned from agriculture and the laboratory. Rev Endocr Metab Disord 2016; 17: 179–186. [DOI] [PubMed] [Google Scholar]
- 238.Fowler SP. Low-calorie sweetener use and energy balance: results from experimental studies in animals, and large-scale prospective studies in humans. Physiol Behav 2016; 164: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014; 100: 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 2008; 117: 754–761. [DOI] [PubMed] [Google Scholar]
- 241.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009; 32: 688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dhingra R, Sullivan L, Jacques PF et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007; 116: 480–488. [DOI] [PubMed] [Google Scholar]
- 243.Sakurai M, Nakamura K, Miura K et al. Sugar-sweetened beverage and diet soda consumption and the 7-year risk for type 2 diabetes mellitus in middle-aged Japanese men. Eur J Nutr 2014; 53: 251–258. [DOI] [PubMed] [Google Scholar]
- 244.O’Connor L, Imamura F, Lentjes MA, Khaw KT, Wareham NJ, Forouhi NG. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia 2015; 58: 1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.InterAct Consortium, Romaguera D, Norat T et al. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia 2013; 56: 1520–1530. [DOI] [PubMed] [Google Scholar]
- 246.Fagherazzi G, Vilier A, Saes Sartorelli D, Lajous M, Balkau B, Clavel-Chapelon F. Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the Etude Epidemiologique aupres des femmes de la Mutuelle Generale de l’Education Nationale-European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 2013; 97: 517–523. [DOI] [PubMed] [Google Scholar]
- 247.de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr 2011; 93: 1321–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bhupathiraju SN, Pan A, Malik VS et al. Caffeinated and caffeine-free beverages and risk of type 2 diabetes. Am J Clin Nutr 2013; 97: 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Swithers SE, Sample CH, Davidson TL. Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. Behav Neurosci 2013; 127: 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Feijo FD, Ballard CR, Foletto KC et al. Saccharin and aspar-tame, compared with sucrose, induce greater weight gain in adult Wistar rats, at similar total caloric intake levels. Appetite 2013; 60: 203–207. [DOI] [PubMed] [Google Scholar]
- 251.Choudhary AK, Pretorius E. Revisiting the safety of aspar-tame. Nutr Rev 2017; 75: 718–730. [DOI] [PubMed] [Google Scholar]
- 252.Suez J, Korem T, Zeevi D et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014; 514: 181–186. [DOI] [PubMed] [Google Scholar]
- 253.Rogers PJ, Hogenkamp PS, de Graaf C et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes (Lond) 2016; 40: 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Franz M Amounts of Sweeteners in Popular Diet Sodas. R.A. Rapaport Publishing, Inc., 2010. [Google Scholar]
- 255.Azad MB, Abou-Setta AM, Chauhan BF et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ 2017; 189: E929–E939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr 1997; 65: 409–418. [DOI] [PubMed] [Google Scholar]
- 257.Peters JC, Beck J, Cardel M et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: a randomized clinical trial. Obesity (Silver Spring) 2016; 24: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002; 76: 721–729. [DOI] [PubMed] [Google Scholar]
- 259.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr 1990; 51: 963–969. [DOI] [PubMed] [Google Scholar]
- 260.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 2012; 367: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 261.Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of Pavlovian cues and reward: population and rate codes. J Neurosci 2004; 24: 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science 2005; 307: 1642–1645. [DOI] [PubMed] [Google Scholar]
- 263.Burger KS, Stice E. Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. NeuroImage 2014; 99: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci 2010; 30: 13105–13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Stice E, Yokum S. Gain in body fat is associated with increased striatal response to palatable food cues, whereas body fat stability is associated with decreased striatal response. J Neurosci 2016; 36: 6949–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring) 2014; 22: 2544–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Stice E, Burger KS, Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. J Neurosci 2015; 35: 10316–10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 2011; 19: 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci 2012; 32: 5549–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Geha PY, Aschenbrenner K, Felsted J, O’Malley SS, Small DM. Altered hypothalamic response to food in smokers. Am J Clin Nutr 2013; 97: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Stice E, Yokum S, Burger K, Rohde P, Shaw H, Gau JM. A pilot randomized trial of a cognitive reappraisal obesity prevention program. Physiol Behav 2015; 138: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Stice E, Yokum S, Veling H, Kemps E, Lawrence NS. Pilot test of a novel food response and attention training treatment for obesity: brain imaging data suggest actions shape valuation. Behav Res Ther 2017; 94: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Stice E, Yokum S, Gau JM. Gymnemic acids lozenge reduces short term consumption of high-sugar food: a placebo controlled experiment. J Psychopharmacol 2017; 31(11): 1496–1502. [DOI] [PubMed] [Google Scholar]
- 274.Farr OM, Li CS, Mantzoros CS. Central nervous system regulation of eating: insights from human brain imaging. Metabolism 2016; 65: 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yarkoni T Big correlations in little studies: inflated fMRI correlations reflect low statistical power – commentary on Vul et al. (2009). Perspect Psychol Sci 2009; 4: 294–298. [DOI] [PubMed] [Google Scholar]
- 276.Burger KS, Stice E. Elevated energy intake is correlated with hyperresponsivity in attentional, gustatory, and reward brain regions while anticipating palatable food receipt. Am J Clin Nutr 2013; 97: 1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Ziauddeen H, Alonso-Alonso M, Hill JO, Kelley M, Khan NA. Obesity and the neurocognitive basis of food reward and the control of intake. Adv Nutr 2015; 6: 474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Berkey CS, Rockett HR, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res 2004; 12: 778–788. [DOI] [PubMed] [Google Scholar]
- 279.Lee AK, Chowdhury R, Welsh JA. Sugars and adiposity: the long-term effects of consuming added and naturally occurring sugars in foods and in beverages. Obes Sci Pract 2015; 1: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet 2001; 357: 505–508. [DOI] [PubMed] [Google Scholar]
- 281.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013; 98: 1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond) 2013; 37(10): 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2013; 346: e7492. [DOI] [PubMed] [Google Scholar]
- 284.Gillman MW, Rifas-Shiman SL, Fernandez-Barres S, Kleinman K, Taveras EM, Oken E. Beverage intake during pregnancy and childhood adiposity. Pediatrics 2017; 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zhu Y, Olsen SF, Mendola P et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: a prospective cohort study. Int J Epidemiol 2017; 46(5): 1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Azad MB, Sharma AK, de Souza RJ et al. Association Between Artificially Sweetened Beverage Consumption During Pregnancy and Infant Body Mass Index. JAMA Pediatr 2016; 170: 662–670. [DOI] [PubMed] [Google Scholar]
- 287.Azad MB, de Souza RJ, Sharma AK. Artificially sweetened beverage consumption during pregnancy and infant body mass index – reply. JAMA Pediatr 2016; 170: 1117–1119. [DOI] [PubMed] [Google Scholar]
- 288.Reid AE, Chauhan BF, Rabbani R et al. Early exposure to nonnutritive sweeteners and long-term metabolic health: a systematic review. Pediatrics 2016; 137: e20153603. [DOI] [PubMed] [Google Scholar]
- 289.Goran MI, Martin AA, Alderete TL, Fujiwara H, Fields DA. Fructose in breast milk is positively associated with infant body composition at 6 months of age. Nutrients 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Du L, Heaney AP. Regulation of adipose differentiation by fructose and GluT5. Mol Endocrinol 2012; 26: 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pan L, Li R, Park S, Galuska DA, Sherry B, Freedman DSA. Longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics 2014; 134(Suppl 1): S29–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Park S, Pan L, Sherry B, Li R. The association of sugar-sweetened beverage intake during infancy with sugar-sweetened beverage intake at 6 years of age. Pediatrics 2014; 134(Suppl 1): S56–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Davis JN, Whaley SE, Goran MI. Effects of breastfeeding and low sugar-sweetened beverage intake on obesity prevalence in Hispanic toddlers. Am J Clin Nutr 2012; 95: 3–8. [DOI] [PubMed] [Google Scholar]
- 294.Ogden CL, Carroll MD, Lawman HG et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 2016; 315: 2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc 2010; 110: 1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Romeo S, Kozlitina J, Xing C et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008; 40: 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Smagris E, BasuRay S, Li J et al. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology 2015; 61: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Goran MI, Walker R, Le KA et al. Effects of PNPLA3 on liver fat and metabolic profile in Hispanic children and adolescents. Diabetes 2010; 59: 3127–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Li Q, Qu HQ, Rentfro AR et al. PNPLA3 polymorphisms and liver aminotransferase levels in a Mexican American population. Clin Invest Med 2012; 35: E237–E245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Larrieta-Carrasco E, Acuna-Alonzo V, Velazquez-Cruz R et al. PNPLA3 I148M polymorphism is associated with elevated alanine transaminase levels in Mexican indigenous and mestizo populations. Mol Biol Rep 2014; 41: 4705–4711. [DOI] [PubMed] [Google Scholar]
- 301.Jiao J, Watt GP, Lee M et al. Cirrhosis and advanced fibrosis in Hispanics in Texas: the dominant contribution of central obesity. PLoS One 2016; 11: e0150978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Davis JN, Le KA, Walker RW et al. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am J Clin Nutr 2010; 92: 1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Goran MI, Walker R, Allayee H. Genetic-related and carbohydrate-related factors affecting liver fat accumulation. Curr Opin Clin Nutr Metab Care 2012; 15: 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sevastianova K, Kotronen A, Gastaldelli A et al. Genetic variation in PNPLA3 (adiponutrin) confers sensitivity to weight loss-induced decrease in liver fat in humans. Am J Clin Nutr 2011; 94: 104–111. [DOI] [PubMed] [Google Scholar]
- 305.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008; 3: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Carmody RN, Gerber GK, Luevano JM Jr et al. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015; 17: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis 2015; 2: ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ridaura VK, Faith JJ, Rey FE et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013; 341: 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Vrieze A, Van Nood E, Holleman F et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012; 143: 913–6 e7. [DOI] [PubMed] [Google Scholar]
- 310.David LA, Maurice CF, Carmody RN et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Boulange CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 2016; 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- 313.Plovier H, Everard A, Druart C et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 2017; 23: 107–113. [DOI] [PubMed] [Google Scholar]
- 314.Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol 2017; 8: 1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Perry RJ, Peng L, Barry NA et al. Acetate mediates a microbiome–brain–beta-cell axis to promote metabolic syndrome. Nature 2016; 534: 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Backhed F, Ding H, Wang T et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004; 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Li F, Jiang C, Krausz KW et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 2013; 4: 2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Jiang C, Xie C, Li F et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest 2015; 125: 386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Sayin SI, Wahlstrom A, Felin J et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013; 17: 225–235. [DOI] [PubMed] [Google Scholar]
- 320.Parseus A, Sommer N, Sommer F et al. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017; 66: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Jiang C, Xie C, Lv Y et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun 2015; 6: 10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Turnbaugh PJ. Microbes and diet-induced obesity: fast, cheap, and out of control. Cell Host Microbe 2017; 21: 278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017; 390: 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ogurtsova K, da Rocha Fernandes JD, Huang Y et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017; 128: 40–50. [DOI] [PubMed] [Google Scholar]
- 325.George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol 2017; 74: 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Stokes A, Preston SH. The contribution of rising adiposity to the increasing prevalence of diabetes in the United States. Prev Med 2017; 101: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Yanovski SZ, Yanovski JA. Toward precision approaches for the prevention and treatment of obesity. JAMA 2018; 319: 223–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ebbert JO, Jensen MD. Fat depots, free fatty acids, and dyslipidemia. Nutrients 2013; 5: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ryden M, Arner P. Cardiovascular risk score is linked to sub-cutaneous adipocyte size and lipid metabolism. J Intern Med 2017; 282: 220–228. [DOI] [PubMed] [Google Scholar]
- 330.Roden M, Price TB, Perseghin G et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 1996; 97: 2859–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes 1995; 44: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 332.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med 2014; 371: 1131–1141. [DOI] [PubMed] [Google Scholar]
- 333.Ryden M, Arner P. Subcutaneous adipocyte lipolysis contributes to circulating lipid levels. Arterioscler Thromb Vasc Biol 2017; 37: 1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Andersen IR, Sondergaard E, Sorensen LP et al. Increased VLDL-TG fatty acid storage in skeletal muscle in men with type 2 diabetes. J Clin Endocrinol Metab 2017; 102: 831–839. [DOI] [PubMed] [Google Scholar]
- 335.Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metab 2012; 15: 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev 2002; 23: 201–229. [DOI] [PubMed] [Google Scholar]
- 337.Christian P, Sacco J, Adeli K. Autophagy: emerging roles in lipid homeostasis and metabolic control. Biochim Biophys Acta 2013; 1831: 819–824. [DOI] [PubMed] [Google Scholar]
- 338.Fisher EA. The degradation of apolipoprotein B100: multiple opportunities to regulate VLDL triglyceride production by different proteolytic pathways. Biochim Biophys Acta 2012; 1821: 778–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol 2012; 23: 206–212. [DOI] [PubMed] [Google Scholar]
- 340.Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyper-uricemia as new marker for metabolic syndrome. ISRN Rheumatol 2014; 2014: 852954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Cai W, Wu X, Zhang B et al. Serum uric acid levels and non-alcoholic fatty liver disease in Uyghur and Han ethnic groups in northwestern China. Arq Bras Endocrinol Metabol 2013; 57: 617–622. [DOI] [PubMed] [Google Scholar]
- 342.Viazzi F, Garneri D, Leoncini G et al. Serum uric acid and its relationship with metabolic syndrome and cardiovascular risk profile in patients with hypertension: insights from the I-DEMAND study. Nutr Metab Cardiovasc Dis 2014; 24: 921–927. [DOI] [PubMed] [Google Scholar]
- 343.Cox CL, Stanhope KL, Schwarz JM et al. Circulating concentrations of monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J Clin Endocrinol Metab 2011; 96: E2034–E2038. [DOI] [PMC free article] [PubMed] [Google Scholar]