Abstract
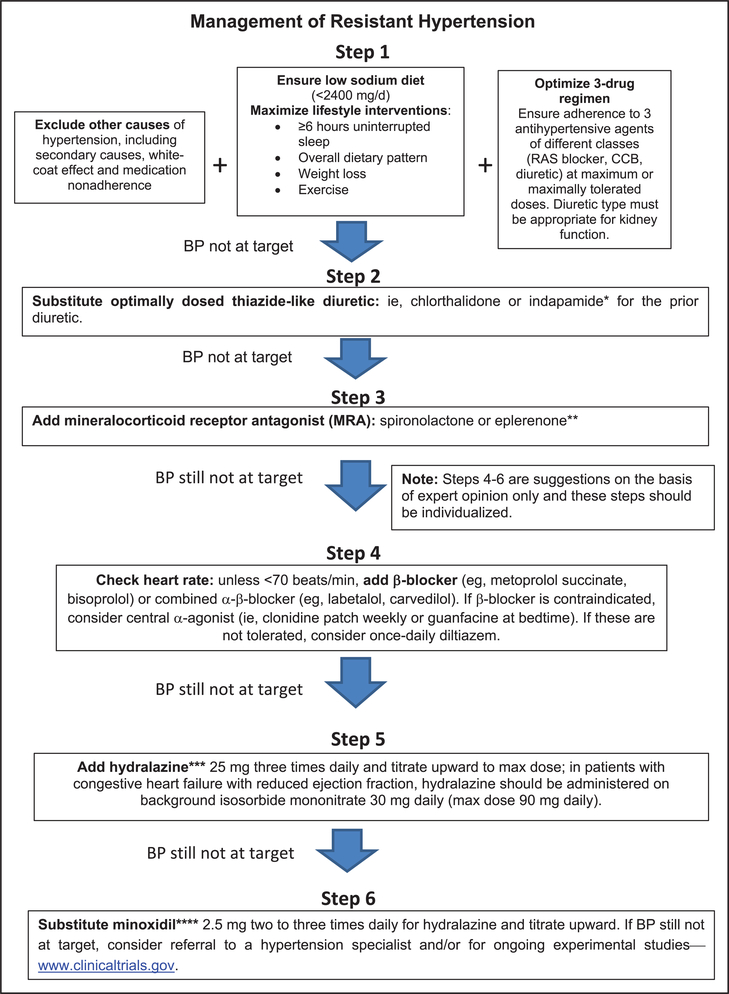
Resistant hypertension (RH) is defined as above-goal elevated blood pressure (BP) in a patient despite the concurrent use of 3 antihypertensive drug classes, commonly including a long-acting calcium channel blocker, a blocker of the renin-angiotensin system (angiotensin-converting enzyme inhibitor or angiotensin receptor blocker), and a diuretic. The antihypertensive drugs should be administered at maximum or maximally tolerated daily doses. RH also includes patients whose BP achieves target values on ≥4 antihypertensive medications. The diagnosis of RH requires assurance of antihypertensive medication adherence and exclusion of the “white-coat effect” (office BP above goal but out-of-office BP at or below target). The importance of RH is underscored by the associated risk of adverse outcomes compared with non-RH. This article is an updated American Heart Association scientific statement on the detection, evaluation, and management of RH. Once antihypertensive medication adherence is confirmed and out-of-office BP recordings exclude a white-coat effect, evaluation includes identification of contributing lifestyle issues, detection of drugs interfering with antihypertensive medication effectiveness, screening for secondary hypertension, and assessment of target organ damage. Management of RH includes maximization of lifestyle interventions, use of long-acting thiazide-like diuretics (chlorthalidone or indapamide), addition of a mineralocorticoid receptor antagonist (spironolactone or eplerenone), and, if BP remains elevated, stepwise addition of antihypertensive drugs with complementary mechanisms of action to lower BP. If BP remains uncontrolled, referral to a hypertension specialist is advised.
Keywords: AHA Scientific Statements, antihypertensive agents, hypertension, hypertension resistant to conventional therapy
Hypertension is the world’s leading risk factor for cardiovascular disease (CVD), stroke, disability, and death. Even with steady improvement during the past 30 years in hypertension awareness, treatment, and control rates, a large proportion of hypertensive adults, despite conscientious clinical management, still fail to achieve their recommended blood pressure (BP) treatment targets on 3 antihypertensive medications or require ≥4 medications to achieve their targets. These individuals, designated as having treatment-resistant hypertension (RH), remain at increased risk for target organ damage, morbidity, and mortality despite ongoing antihypertensive drug therapy.
New recommendations for the detection, evaluation, and management of hypertension have been published in the 2017 American College of Cardiology/American Heart Association (AHA) clinical practice guideline for the prevention, detection, evaluation, and management of high BP in adults.1 Among its recommendations, the 2017 guideline reduces both the BP threshold for initiating antihypertensive therapy to ≥130/80 mm Hg for adults with existing CVD or 10-year atherosclerotic CVD risk ≥10% and the BP goal of treatment to <130/80 mm Hg for most individuals. These recommendations affect the BP threshold for diagnosis of RH and thus will increase its prevalence in the hypertensive population. The current scientific statement is consistent with the 2017 American College of Cardiology/AHA guideline.1
In 2008, the AHA issued its first scientific statement on RH that included recommendations for diagnosis, evaluation, and treatment.2 Since 2008, a large number of studies of RH have improved our understanding of its pathogenesis, evaluation, and treatment. This first revision of the AHA scientific statement2 is intended to place this new evidence into the context of our understanding of RH from prior literature and to identify gaps in knowledge requiring additional research in the future.
Definitions of RH
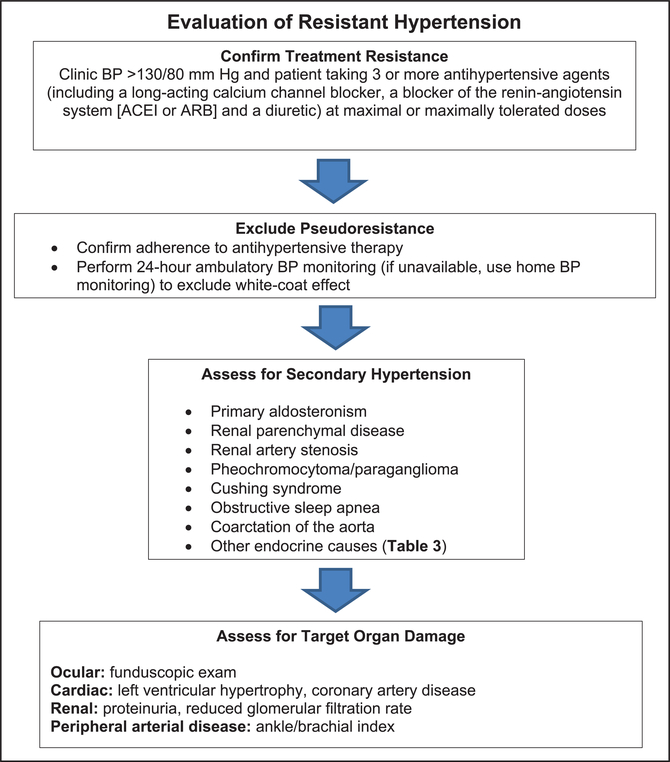
RH is defined as the BP of a hypertensive patient that remains elevated above goal despite the concurrent use of 3 antihypertensive agents of different classes, commonly including a long-acting calcium channel blocker (CCB), a blocker of the renin-angiotensin system (angiotensin-converting enzyme [ACE] inhibitor or angiotensin receptor blocker [ARB]), and a diuretic. All agents should be administered at maximum or maximally tolerated doses and at the appropriate dosing frequency. Albeit arbitrary with respect to the number of medications required, RH is defined in this manner to identify patients who are at higher risk for morbid CVD events and death. Moreover, they are more likely to have medication adverse effects, more likely to have a secondary cause of hypertension compared with hypertensive patients without drug resistance, and may benefit from special diagnostic or therapeutic approaches to control their BP. RH also includes patients whose BP achieves target values on ≥4 antihypertensive medications, a condition that has been referred to in the literature as controlled RH. Thus, the term RH refers to hypertension with both uncontrolled and controlled BP, depending on the number of antihypertensive agents used.
Errors in BP measurement can account for the misdiagnosis of RH. The preparation of the patient, environmental conditions, cuff size, and technique of BP measurement can have a substantial influence on BP results.1,3 In particular, inherent BP variability dictates that diagnostic BP recordings include an average of at least 2 readings obtained on at least 2 separate occasions.1,3 Therefore, before the diagnosis of RH is made, it is critical to ensure accurate BP measurement. Similarly, out-of-office BP and self-monitored BP require proper technique.1,2,4 BP should be measured at any site according to current guidelines.1
The “white-coat effect” is defined as office BP above goal but out-of-office BP levels measured by ambulatory BP monitoring (ABPM) (or, if ABPM is unavailable, by home BP monitoring) below goal in a patient on ≥3 antihypertensive agents. The risk of CVD complications in patients with a white-coat effect is similar to the risk in hypertensive patients with controlled BP.5–7 Out-of-office BP monitoring is generally required to make the diagnosis of true RH.
Nonadherence in taking prescribed antihypertensive medications must also be excluded before RH is diagnosed. Medication nonadherence is highly prevalent in patients with apparent RH.8,9 It has been estimated that as many as 50% to 80% of hypertensive patients prescribed antihypertensive medications demonstrate suboptimal adherence.10 This relatively high proportion with nonadherence that may mimic RH is related, at least in part, to the large pill burden, dosing complexity, expense, high frequency of adverse reactions with multidrug antihypertensive regimens, poor patient-clinician relationship, and clinician inertia with reduced insistence on adherence when patients are consistently nonadherent.11 Exclusion of nonadherence should include frank and nonjudgmental clinician-patient discussion, monitoring of prescription refills and pill counts, and, if available, biochemical assay of drugs or their metabolites in urine or plasma.
In summary, the definition of RH has been modified from that of the 2008 AHA scientific statement in 4 important ways: (1) BP should be measured and the BP threshold for diagnosis and treatment goals should be in accord with current clinical practice guidelines1; (2) patients should be taking ≥3 antihypertensive agents, commonly including a long-acting CCB, a blocker of the renin-angiotensin system (ACE inhibitor or ARB), and a diuretic at maximum or maximally tolerated doses; (3) patients with the white-coat effect should not be included in the definition of RH; and (4) the diagnosis of RH requires the exclusion of antihypertensive medication nonadherence.
Prevalence of RH
As stated, RH requires patient adherence to prescribed medications and that the uncontrolled subset has elevated BP outside the office setting. The term apparent treatment RH (aTRH) is used when ≥1 of the following data elements are missing: medication dose, adherence, or out-of-office BP; thus, pseudoresistance cannot be excluded.12 Among treated adults with hypertension, prevalent aTRH occurs in ≈12% to 15% of population-based13–16 and 15% to 18% of clinic-based reports.17–20 Prevalent aTRH occurs in a higher percentage of the population- and clinic-based samples when an at-risk group is selected, for example, patients with treated hypertension and chronic kidney disease (CKD).16,19 The higher prevalence of aTRH among treated hypertensive adults in clinical trials (34%−39%) is likely explained by the selection of patients with demographic and comorbidity characteristics that place them at high risk for the fatal and nonfatal CVD outcomes of interest.21–26 Moreover, in population- and clinic-based studies, some RH cases may go unrecognized because patients are not prescribed ≥3 drugs at maximal doses despite uncontrolled BP. In contrast, clinical trials usually include forced titration schemes that unmask RH by reducing the prevalence of suboptimal treatment.12
The prevalence of aTRH estimated from selected population-based, clinic-based, and clinical trial-based reports is shown in Table 1 (for details, see the Data Supplement).
Table 1.
Prevalence of aTRH in Adults With Treated Hypertension as Reported From Selected Population-, Clinic-, and Intervention-Based Studies
| Population Based | Time Period | n | Uncontrolled With ≥3 BP Medications, % | Controlled With ≥4 BP Medications, % | aTRH, % |
|---|---|---|---|---|---|
| NHANES13 | 1988–1994 | 2755 | 8.3 | 1.1 | 9.4 |
| NHANES13 | 1999–2004 | 3031 | 8.8 | 2.9 | 11.7 |
| NHANES14 | 2003–2008 | 3710 | 12.8 | ||
| NHANES13 | 2005–2008 | 2586 | 9.7 | 4.8 | 14.5 |
| REGARDS15 | 2003–2007 | 14 731 | 9.1 | 5.0 | 14.1 |
| REGARDS16 (CKD)* | 2003–2007 | 3134 | 28.1 | ||
| Clinic based | |||||
| EURIKA17 (diabetes mellitus) | 2009–2010 | 5220 | 13.0† | 3.1 | 16.1 |
| Spanish ABPM18 | 2004–2009 | 68 045 | 12.2 | 2.6 | 14.8 |
| CRIC (CKD)19‡ | 2003–2008 | 3939 | 21.2 | 19.2 | 40.4 |
| South Carolina20§ | 2007–2010 | 468877 | 9.5 | 8.4 | 17.9 |
| Clinical trials | |||||
| ALLHAT21 | 1994–2002∥ | 14 684 | 11.5 | 1.2 | 12.7 |
| ASCOT22 | 1998–2005 | 19 527 | 48.5 | ||
| ACCOMPLISH25 | 2003–2006¶ | 10 704 | 39 | ||
| INVEST26 | 1997–2003# | 17 190 | 25.1 | 12.6 | 37.8 |
Uncontrolled aTRH is defined as BP ≥140 mm Hg systolic and/or ≥90 mm Hg diastolic on ≥3 BP medications. Controlled aTRH is defined as BP <140 mm Hg systolic and <90 mm Hg diastolic on ≥4 BP medications unless otherwise specified.
ABPM indicates Ambulatory Blood Pressure Monitoring Registry; ACCOMPLISH, Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension; ALLHAT, Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial; ASCOT, Anglo-Scandinavian Cardiac Outcome Trial; aTRH, apparent treatment-resistant hypertension; BP, blood pressure; CKD, chronic kidney disease; CRIC, Chronic Renal Insufficiency Cohort; EURIKA, European Study on Cardiovascular Risk Prevention and Management in Usual Daily Practice; INVEST, International Verapamil-Trandolapril Study; NHANES, National Health and Nutrition Examination Survey; and REGARDS, Reasons for Geographic and Racial Differences in Stroke.
Mean estimated glomerular filtration rate in adults with CKD and aTRH: 60.8 mL⋅min−1⋅1.73 m−2.
aTRH defined as BP >130/>80 mm Hg.
Mean estimated glomerular filtration rate in adults with CKD and aTRH: 38.9 mL⋅min−1⋅1.73 m−2.
Includes untreated hypertensive patients.
Excluded 7628 patients with uncontrolled hypertension on <3 BP medications.
Prevalent uncontrolled aTRH estimated from report on BP control 6 months after randomization.
Excluded 5386 treated participants with BP <130/<80 mm Hg.
Prognosis of RH
Observational studies using the 2008 criteria have shown that patients with RH are at higher risk for poor outcomes compared with patients without RH.23,27–30 In a retrospective study of >200 000 patients with incident hypertension, those with RH were 47% more likely to suffer the combined outcomes of death, myocardial infarction, heart failure, stroke, or CKD over the median 3.8 years of follow-up.23 Differences in CVD events in this study were driven largely by a higher risk for the development of CKD.23 In another study of >400 000 patients, compared with patients without RH, patients with RH had a 32% increased risk of developing end-stage renal disease, a 24% increased risk of an ischemic heart event, a 46% increased risk of heart failure, a 14% increased risk of stroke, and a 6% increased risk of death.29 Prospective studies using ABPM have suggested an almost 2-fold increased risk of CVD events in patients with true RH compared with those with hypertension responsive to treatment.30–33 Together, these studies suggest that RH is associated with an increased risk of adverse outcomes and represents an important public health problem.
RH is associated with worse outcomes among patients with some comorbid conditions. In patients with CKD, RH is associated with higher risk of myocardial infarction, stroke, peripheral arterial disease, heart failure, and all-cause mortality compared with patients without RH.19 Similarly, in patients with ischemic heart disease, RH is associated with higher rates of adverse events, including death, myocardial infarction, and stroke.26,34,35 Conversely, RH is not associated with increased adverse clinical events in patients with heart failure with reduced ejection fraction and may lower the risk for heart failure-related rehospitalization.36
Among patients with RH, lower BP is associated with reduced risk for some cardiovascular events.28,37 In the REGARDS study (Reasons for Geographic and Racial Differences in Stroke), uncontrolled RH was associated with a 2-fold increased risk of coronary heart disease compared with controlled RH. Control status was not associated with differences in stroke or mortality.28 In another study of >118 000 treated hypertensive adults, including >40 000 individuals with RH and 460 000 observation-years, BP control was associated with significantly lower rates of incident stroke and coronary heart disease with no difference in rates of incident heart failure.37 BP control reduced the risk of incident stroke, coronary heart disease, or heart failure by 13% among those with RH compared with a 31% lower risk of these outcomes among patients without RH.37 Although BP control is associated with a lower risk for some CVD outcomes, it is possible that the benefit of BP lowering may be less in patients with RH compared with patients with non-RH.
Patient Characteristics
Demographic correlates of RH include black race, older age, and male sex.38 RH is characterized by the variable clustering of distinct demographics, comorbidities, physiological aberrations, and metabolic abnormalities. However, these factors are not mutually exclusive because, in fact, they can be substantially interdependent (eg, nondipping or reverse dipping BP and sympathetic nervous system overactivity, visceral obesity, and excess aldosterone).
Multiple comorbidities have been associated with RH. Obesity,39–41 left ventricular hypertrophy,42 albuminuria,14,43 diabetes mellitus,14,38,39 CKD,38,39,44 higher Framingham 10-year risk score,39 and obstructive sleep apnea (OSA)45,46 are more common in RH than non-RH. Very high proportions (60%−84%) of individuals with RH have sleep apnea.33,47–49 Other sleep abnormalities are also manifest in RH (relative to those with controlled hypertension or normotensives), including shorter sleep duration, reduced sleep efficiency, and less rapid eye movement sleep.50
Physiological aberrations in RH include vascular disease/dysfunction as evidenced by high rates of peripheral39 and carotid artery atherosclerosis,42 impaired endothelial function,51,52 reduced arterial compliance, and raised systemic vascular resistance,40 all of which may be more pronounced in RH compared with non-RH. The normal nocturnal decline in BP is also attenuated in a high proportion (43%−65%) of individuals with RH.32,53,54 The attenuated nocturnal decline in BP is even more pronounced in uncontrolled compared with controlled RH.52 Nondipping ambulatory BP in individuals with RH has been linked to reduced heart rate variability, a marker of sympathetic nervous system overactivity.53 Reverse dipping, in which nocturnal BP paradoxically rises, may be associated with increased subclinical organ damage and possibly CVD events. Reverse dipping is also associated with increased sympathetic nervous system activity at night.55 In adults with severe uncontrolled RH and self-reported sleep apnea in the SYMPLICITY HTN-3 trial (Renal Denervation in Patients With Uncontrolled Hypertension), renal denervation lowered office systolic BP (SBP) more effectively relative to sham controls at 6 months (−17.0 mm Hg versus −6.3 mm Hg; P<0.01).56 Renal denervation did not lower office SBP in patients without sleep apnea.
RH has also been linked to metabolic derangements, including hyperuricemia,17 aldosterone excess,47 and suppressed circulating renin levels (≈60% of those with RH have suppressed renin levels).57 In general, RH is characterized by exquisite salt sensitivity of BP. Reducing dietary sodium intake to levels significantly below the level of usual intake in Western societies (eg, 50 mmol/d) promptly and impressively lowered BP in many individuals with RH.58 Moreover, the severity of sleep apnea in those with RH is positively related to dietary sodium intake, at least in those with hyperaldosteronism.49 Plasma osmolality-adjusted copeptin concentrations, a surrogate marker for vasopressin release, are almost twice as high in individuals with RH compared with those with nonresistant, controlled BP.59
There are distinct patterns of antihypertensive drug prescribing and administration in individuals with RH. Suboptimal antihypertensive drug regimens substantively contribute to the likelihood of being diagnosed with RH.41,60,61 Accordingly, drug treatment regimens in RH infrequently include either spironolactone or chlorthalidone, 2 highly effective BP-lowering agents, in this high-risk group of hypertensives.60,61 Bedtime dosing of once-daily antihypertensive agents (relative to morning or twice-daily dosing) appears to significantly affect diurnal BP patterns because there are fewer patients with nondipping of BP and >24-hour BP control and higher rates of nocturnal normalization of BP62 with this dosing strategy.
Genetics/Pharmacogenetics
BP has a genetic basis, as evidenced by its heritability in family-based studies,63–65 with estimates for long-term average BP that as much as 50% to 60% of BP variability can be attributed to additive genetic factors.66 However, there has been limited study of the heritability of RH or of the BP-lowering response to specific antihypertensive agents. A disproportionately higher prevalence of RH among blacks67,68 has been suggested to reflect a contribution by genetic factors, but environmental or psychosocial determinants of BP control are also possible.69
Common genetic variants influencing BP have been identified at >300 independent loci but require scores to hundreds of thousands of individuals for their detection.70–91 These genetic variants typically have effects on the order of 1.0-mm Hg SBP and 0.5-mm Hg diastolic BP per BP-raising allele. Individually, these variants explain ≤0.1% of BP variability and, in aggregate, only 3% of total BP variance. This is consistent with the complex nature of BP regulation, involving multiple compensatory systems such as vascular tone, sodium excretion and plasma volume, autonomic nervous system function, and myocardial performance, as well as many as-yet unknown factors.
The majority of genetic studies of RH have been limited to candidate genes and have lacked adequate sample sizes to survive the multiple testing burden across the many such candidate gene studies performed.92–96 Much larger studies of well-characterized individuals with RH and of individuals before and after treatment with specific antihypertensive therapies are needed to better define the role of common and rare genetic variation in causing RH and modulation of drug response. For a more detailed discussion of genetics and pharmacogenetics in RH, please see the Data Supplement.
Diagnosing RH
Identifying and Correcting Medication Nonadherence
Overview
The term adherence is defined as remaining attached (to the medication regimen) and is distinct from compliance, which means submitting to a request, wish, or demand. Medication adherence is an important determinant of hypertension control. However, a quarter of patients who are newly initiated on antihypertensive therapy fail to fill their initial prescription.97,98 During the first year of treatment, the average patient has possession of antihypertensive medications only 50% of the time, and only 1 in 5 patients has sufficiently high adherence to achieve the benefits observed in clinical trials.98,99 Nevertheless, the trend to improved BP control (<140/90 mm Hg) rates among those on antihypertensive treatment to >70% suggests that recent strategies to improve antihypertensive medication adherence and care have been successful.100
Assessing and ensuring optimal medication adherence are essential steps in the evaluation and management of patients with RH. By definition, these patients are taking ≥3 antihypertensive drugs, and information on the level of adherence to the prescribed regimen is crucial to guide clinical reasoning and decision making. A major criterion of success is the ability of the patient to follow the recommendations on a daily basis (adherence) and to stay on therapy (persistence), with the latter aspect most critical in clinical practice.101 In fact, the diagnosis of RH is predicated on the patient’s adherence to the prescribed regimen, and failure to identify inadequate adherence contributes to overestimation of the prevalence of “true” RH. Adequate adherence necessary for patients to experience benefit from full exposure to prescribed pharmacological therapy is generally defined as taking at least 80% of doses, although the scientific basis for this cutoff is unclear.102 Although data are limited, a review of studies of medication adherence in aTRH identified rates of nonadherence in this population ranging from 7% using pharmacy refill records in a managed care population to >60% using serum drug levels in a referral clinic.102
Assessment of Adherence
Identification of patients with inadequate adherence among those with aTRH will avoid unnecessary and potentially harmful treatment intensification and allow implementation of strategies to improve adherence and more cost-effective allocation of health resources. Furthermore, assessment of change in adherence as a major potential confounder is essential in trials assessing new treatment modalities of RH.103 Thus, a systematic approach with reliable, practical methods for measuring medication adherence would facilitate providers’ abilities to optimize their antihypertensive regimens.
Although counterintuitive, 1 analysis found that the SBP response to therapeutic intensification for uncontrolled hypertension was not different across quintiles of adherence.104 Specifically, SBP fell an average of 2.1 mm Hg in the highest adherence quintile (≥98% adherence) and 2.4 mm Hg in the lowest adherence quintile (<80% adherence; mean, 62%) with each therapeutic intensification. Clinicians are less likely to intensify medications when their assessment suggests that the patient is nonadherent. However, clinician assessment of adherence is poor.105 Thus, although work continues to optimize methods for assessing medication adherence, clinicians should be cautious when deciding against therapeutic intensification in a patient with uncontrolled hypertension whom they perceive to be nonadherent. It is important for providers to use effective strategies for improving adherence such as those described subsequently.
Because of the complex and dynamic nature of adherence, it is difficult to measure, particularly by any single assessment. Clinician impression and techniques such as pill count often are inaccurate. However, increasingly reliable and sophisticated indirect and direct methods are available for assessing medication adherence.106 Indirect methods, including patient self-report medication adherence assessment tools such as the Morisky Medication Adherence Scale107 and the Hill-Bone Compliance Scale,108 have demonstrated predictive validity in a variety of hypertensive populations and identify adherence risk factors that can be used to tailor messages targeting improved adherence. Using a nonthreatening, nonaccusatory approach, which requires excellent communication skills, is preferred when interviewing patients about adherence. An example is the following: “When taking multiple medications, it is common to miss doses throughout the week. How many times do you miss taking your BP medication in a week?”
Pharmacy databases for medication possession and refills provide a valid measure of adherence. Measurement of pharmacodynamic parameters (eg, heart rate for β-blockers, lack of rise of plasma renin activity [PRA] for renin-angiotensin inhibitors, N-acetyl-seryl-aspartyl-lysyl-proline measurements for ACE inhibitors) may have limited specificity. Direct methods include witnessed drug intake, the Medication Event Monitoring System, and drug monitoring in body fluids. Witnessed medication taking followed by monitoring of effect on BP has been effective in identifying suspected nonadherence in trials of novel treatment modalities, although this approach has not been widely used in general practice. Medication Event Monitoring System monitoring of the date and time that a medication bottle is opened and closed has superior sensitivity compared with other methods, although ingestion of the medication is not confirmed. Urine or blood measurement of drug or metabolites with mass spectroscopy reliably determines whether a drug is present or absent but does not determine whether it was taken regularly or at a therapeutic level. A novel approach, urine fluorometry, recently was reported as a safe, easy, and reliable method to assess adherence.109 In addition, a new technology moving toward clinical application involves a sensor in the pill that emits a signal on interacting with gastric acid, which is detected by a sensor on the skin.110
There is no gold standard for measuring adherence. Indirect methods such as pill count, self-report, and prescription refill data are simple, inexpensive, and widely used. However, they can easily be manipulated to overestimate adherence. A direct method such as urine or blood measurement of drug or metabolites is considered more robust but is relatively expensive, is of limited availability, and does not perfectly reflect level of adherence. All methods have limitations, and ideally, accurate assessment of adherence should involve a combination of approaches.
Multisystem Intervention to Improve Adherence
Factors contributing to poor adherence are myriad and multilevel. Barriers to adherence exist at the levels of patient (eg, multiple comorbid conditions, resource constraints, suboptimal health literacy, lack of involvement in the treatment decision-making process), clinician (eg, prescription of complex drug regimens, communication barriers, ineffective communication of information about adverse effects, provision of care by multiple providers, clinician inertia), and healthcare system (eg, office visit time limitations, limited access to care, lack of team-based approaches and health information technology).107 Because barriers to medication adherence are complex and varied, solutions to improve adherence at the population level must be multifactorial.108,111,112
Several systematic reviews and meta-analyses have assessed the impact of interventions, including modification of antihypertensive therapy, on adherence to antihypertensive medications.112–120 Evidence specific to improving adherence in the RH population is sparse. However, evidence-based approaches effective in the general hypertension population can be applied. No single intervention is uniquely effective, and a sustained approach using multiple strategies, including health system solutions and those that target the individual patient’s barriers to adherence, is likely to be most effective. Interventions demonstrated to be effective in improving adherence are outlined here and organized by health system, clinician, and patient levels.
Health system-level interventions to improve the quality of hypertension care, medication adherence, and BP control use multidisciplinary team-based care.121 A variety of patient-centered, team-based hypertension care models have been demonstrated to increase the proportion of individuals with controlled BP.121–125 An effective, multifaceted approach often includes systems support for clinical decision making (ie, treatment algorithms), collaboration between clinician and patient, medication adherence, BP monitoring, and patient self-management.126,127 Further systems-level support such as use of electronic health records, registries, clinical decision support (ie, treatment algorithms), technology-based remote monitoring, self-management support tools, and monitoring of performance augments and intensifies team-based care efforts.128–132
Effective strategies for improving adherence to antihypertensive medications include (1) using agents that are dosed once daily over those that require multiple daily doses and using fixed-dose combination agents when available113,116–120,133; (2) using low-cost and generic antihypertensives, particularly when cost of care is a barrier134 (patients with RH often have multiple chronic conditions requiring pharmacotherapy); and (3) consolidating refills, that is, minimizing the number of trips to the pharmacy to obtain all prescribed medications.134
Patient-centered care and patient engagement in deciding which antihypertensive medications are included in their treatment regimen (patient-centered decision making) improve adherence.135,136 In addition, a patient-centered approach to consider overall adverse effect profile and preferential use of agents that are well tolerated may help. Medication adherence scales may be useful.107,108 Many clinicians may benefit from training to enhance communication skills and to increase cultural competence in their interactions with patients.
At the individual patient level, it is essential to educate patients, their families, and caregivers about hypertension, the consequences of hypertension, and the possible adverse effects of medications. An informed patient is better able to collaborate to establish shared goals of therapy and a plan of care. To maintain contact with patients for ongoing follow-up and monitoring, telehealth or mobile communication approaches may be helpful.132,137 Patients must integrate pill taking into their routine activities of daily living with the use of adherence support tools such as reminders, pillboxes, packaging, or other aids. Individual barriers to adherence such as low health literacy can be difficult to identify in a busy practice setting. Moreover, only 12% of US adults have the health literacy skills needed to manage the demands of our complex health system.138 The Health Literacy Universal Precautions Toolkit recommends assuming that all patients may have difficulty understanding and creating an environment with supportive systems where all patients receive written and oral communication in plain language that promotes empowerment and self-management.138 Key recommendations include using visual, interactive education and providing a medication list or pictorial medication schedule. Another approach, the teach-back method, offers clinicians a nonthreatening way to confirm whether patients understand what has been explained to them. If a patient understands, he/she is able to “teach back” the information accurately. Motivation interventions are also effective in supporting medication adherence and lifestyle modification efforts.139 The creation of an encouraging, blame-free environment in which patients are recognized for progress toward treatment goals, empowered to ask questions, and given “permission” to answer questions related to their treatment honestly is essential to identify and address nonadherence.
Poor BP Measurement Technique
Inaccurate measurement of BP can result in the appearance of treatment resistance. In a study comparing standard triage BP measurements by clinic staff with an automated device obtaining up to 6 BP measurements 1 minute apart while the patient was alone and seated in a quiet room, triage SBPs were a median of 17 mm Hg higher, and the difference was highest in the group of patients with initial SBPs >160 mm Hg.140 In this study of patients referred for RH, inaccurate BP measurement was estimated at 33%.140
Certain aspects of BP measurement technique have been standardized with the widespread acceptance of oscillometric devices to measure BP in the hospital, workplace, and home. For example, potential measurement confounders such as observer bias, end-digit preference, and the presence of an auscultatory gap are not an issue with nonauscultatory methods.3 However, cuff size, body and arm positions, and measurement environment are common features of auscultatory and oscillometric methods that affect the accuracy of BP measurement. Proper BP measurement technique entails (1) preparing the individual by emptying a full urinary bladder and then sitting with legs uncrossed and back, arm, and feet supported in a quiet room, ideally 5 minutes before the first reading is obtained; (2) choosing a BP cuff with a bladder length of at least 80% and width of at least 40% of the arm circumference; (3) placing the cuff directly on the skin of the upper arm at the level of the heart on the supported arm; and (4) obtaining a minimum of 2 readings 1 minute apart.1,3
Cuff-measured BP may differ from intra-arterial pressure. Severe arterial stiffness or medial calcification of the brachial artery may result in an inaccurate detection of Korotkoff sounds when BP is measured with the auscultatory method. The inappropriately elevated cuff pressure in patients with severe arterial disease has been called pseudohypertension.141 The degree to which arterial stiffness or brachial artery calcification affects BP measurement with oscillometric devices has not been established but is suspected to be less pronounced. In addition, the auscultatory method has been shown to overestimate diastolic BP among hypertensive individuals, likely unrelated to calcified arteries.142
White-Coat Effect
The white-coat effect is the observation of repeated BP elevations in the office with controlled or significantly lower BP outside the office in a hypertensive patient on medication. The white-coat effect has been attributed to an alerting reflex triggered by the healthcare provider or the clinic environment that activates the sympathetic nervous system.143 Some degree of BP rise is seen with in-office measurement in the majority of people; however, the white-coat effect can be magnified in individuals diagnosed with hypertension, women, and older individuals.144 A clinically significant white-coat effect may be present in 28% to 39% of individuals with aTRH by office BP measurement.18,32,145 Short duration of hypertension and the absence of diabetes mellitus or CKD are also associated with pseudoresistance from a white-coat effect.
In RH populations, the CVD risk of overdiagnosing RH from the white-coat effect is comparable to that in treated controlled hypertensive patients.31 In a Brazilian cohort of patients with RH, ambulatory BP was an independent predictor of all-cause mortality and a composite outcome of CVD events, whereas office BP showed no prognostic value.32 These findings are consistent with outcome studies in the general population. An analysis of the International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes, which included >12 000 participants from across the world, found that after age was accounted for, the white-coat effect did not contribute to CVD risk.7 Recognizing the risk disparity between white-coat-related BP elevation and RH, randomized clinical trials in RH have excluded patients whose BP is controlled by measurement with either ABPM or home BP monitoring.20,145
The white-coat effect can be easily identified by 24-hour ABPM. However, ABPM is not readily available in all countries and, because of limitations in insurance reimbursement, is not even commonly used in the United States.146 Oscillometric digital devices that can automatically record 3 to 6 BP measurements without a clinician in the examination room are now available for clinical use, a process called automated office BP. BP measurement by automated office BP attenuates the white-coat effect. Self-measured home BPs (with appropriate instruction in the BP measurement technique) correlate with average daytime BPs measured by 24-hour ABPM and can be used to identify the white-coat effect.146 However, it is important to consider that individuals may alter their BP logs or underreport high or low BP values. In general, upper arm cuff-based home BP monitors are preferred over wrist BP monitors. Although wrist monitors may be convenient, particularly for obese patients who require very large cuff sizes, they have the potential for measurement error in individuals with arrhythmias, during arm movement, or when the wrist is not placed at heart level.1,3 Finger BP monitors are not recommended because of error and artifactual readings. Although BP control is <130/80 mm Hg in the office, control by 24-hour ABPM is defined as <125/75 mm Hg.
Treatment Inertia
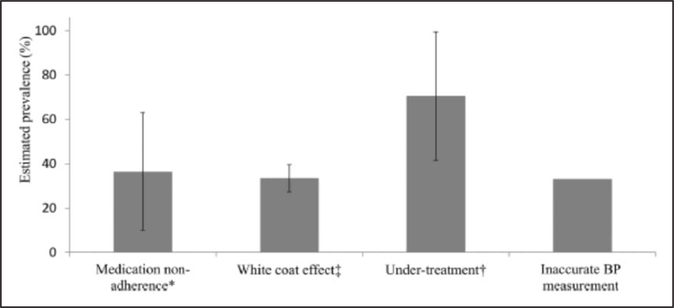
Suboptimal antihypertensive therapy accounts for a large subset of patients not achieving BP targets (Figure 1).140 During 2007 to 2010, only 49.6% of patients with uncontrolled aTRH identified in a community-based practice network in the United States were prescribed an optimal antihypertensive regimen.20 More than 90% of the 84 193 patients with aTRH were appropriately prescribed a diuretic; however, antihypertensive medications were administered at <50% of their maximally recommended dose in 42.1% of patients with uncontrolled aTRH. Patients who were more likely to be prescribed an optimal regimen were black or were diagnosed with CKD, diabetes mellitus, or coronary heart disease.20
Figure 1.
Estimated prevalence of each of the causes of pseudoresistant hypertension. BP indicates blood pressure. Modified from Bhatt et al140 with permission from the American Society of Hypertension. Copyright © 2016, American Society of Hypertension. *Indicates reference 102. †indicates references 20 and 140. ‡indicates references 18, 32, and 145.
Overcoming clinician treatment inertia can be accomplished through an integrated health system model of care. Hypertension control rates exceed the national average in the Kaiser Permanente and Veterans Affairs health systems, where the approach to BP control is systematic and multidisciplinary. Identifying patients with hypertension, standardizing BP measurements, and using a stepwise treatment algorithm have led to an increase in BP control rates from 54% in 2004 to 84% in 2010 in the Kaiser Permanente Southern California health system.147
Lifestyle Factors
The 2017 American College of Cardiology/AHA clinical practice guideline for the prevention, detection, evaluation, and management of high BP in adults provides a detailed discussion of the relationships between several lifestyle factors and high BP.1 The following sections focus specifically on the role of behavioral activities in the pathogenesis of RH.
Obesity
Excess body fat ranks among the most important factors responsible for the increasing prevalence of hypertension.148 Visceral adiposity in particular plays a fundamental role in causing high BP through a variety of mechanisms culminating in enhanced salt sensitivity, vascular dysfunction, and activation of the sympathetic nervous system and renin-angiotensin system.148,149 Mounting evidence further supports the importance of heightened mineralocorticoid activity in RH.149–151
Recent findings from the NHANES (National Health and Nutrition Examination Survey) of 13 375 hypertensive adults demonstrate that body mass index (BMI) ≥30 kg/m2 approximately doubles the risk for aTRH.13 In 14 461 patients with RH in the Spanish Ambulatory Blood Pressure Monitoring Registry, a BMI ≥30 kg/m2 was also an independent risk factor for RH (odds ratio, 1.62; 95% CI, 1.32–1.99).152 Even among 3367 hypertensives with CKD, increasing levels of obesity were independently associated with higher risks of aTRH, ranging from an odds ratio of 1.52 (BMI ≥30 kg/m2) to 2.26 (BMI ≥40 kg/m2).19 Finally, in a large data set (>470 000) from the Kaiser Permanente Southern California health system, obesity (BMI ≥30 kg/m2) was also found to be an independent risk factor for RH (odds ratio, 1.62; 95% CI, 1.42–1.51).38 Although obesity has the potential to promote spurious elevations in BP (eg, use of a smaller-than-optimal BP cuff),148 these findings nonetheless strongly support a direct role for excess adiposity in promoting RH.
Dietary Sodium
Higher dietary sodium intake is incontrovertibly linked to increases in arterial BP.153–155 However, relatively large interindividual variations exist in “salt sensitivity” of BP. The mechanisms are complex and likely involve a host of responses beyond excess volume retention, including vascular dysfunction, arterial stiffness, sympathetic activation, impaired renin-angiotensin axis suppression, mineralocorticoid receptor activation, and immune cell modulation.156,157
It is more difficult to precisely quantify the importance of excess dietary sodium specifically in regard to causing RH for several reasons, including the complexities in accurately characterizing routine intake in the relevant populations.158 However, a number of recently published studies suggest a role. Among 279 patients with RH, brain natriuretic peptide was higher and PRA was lower than in control patients, supporting a component of relative volume excess (although 24-hour urinary sodium levels were not increased).159 In a study of 204 patients with true RH confirmed by ambulatory monitoring, one-third displayed evidence for excess sodium intake (ie, 24-hour urine levels >200 mEq).160 Further indirect evidence can be derived from large population studies in which chronic renal insufficiency, a well-established cause of heightened salt sensitivity of BP, has been repetitively linked to aTRH.13,38,152 Perhaps the most compelling support comes from the few clinical trials demonstrating marked reductions in BP among patients with RH following a reduced sodium diet.58,161
Alcohol
Alcohol intake has been linked to increases in BP and the risk for developing hypertension.162–164 The dose-response association may differ between men (linear) and women (J shaped)164 and can be modified by metabolic genes.163 Nonetheless, heavy alcohol intake (>30–50 g/d) is a well-established risk factor for hypertension.165
Physical Inactivity
Both reduced physical activity and lower physical fitness are independent risk factors for hypertension.166–171 Graded inverse associations between activity and fitness level with the risk for developing high BP have been shown in young adults,168 men,169 and women.170 There is further evidence that a sedentary lifestyle itself (eg, more television watching) independently promotes the onset of hypertension.167,172 Conversely, there is a paucity of studies that have reported associations between physical inactivity and RH. Along with other poor lifestyle factors, self-reported inactivity was not predictive of RH among patients in the REGARDS cohort.173 On the other hand, indirect support for the important role of activity comes from a randomized study demonstrating that a thrice-weekly treadmill walking exercise program for 8 to 12 weeks significantly lowered daytime ambulatory BP (6±12/3±7 mm Hg; P=0.03) among 50 treated patients with RH.173
Dietary Pattern and Other Risk Factors
The Dietary Approaches to Stop Hypertension (DASH) eating pattern is well established to reduce BP, by 6.7/3.5 mm Hg in a recent meta-analysis.174,175 Nonetheless, as with other lifestyle factors in the REGARDS cohort, a low DASH diet questionnaire score was not independently associated with aTRH.166 Further studies are needed to clarify the precise role of poor diet in the pathogenesis of RH. Psychosocial stressors (eg, occupational stress, low social support), negative personality traits (anxiety, anger, depression), and reduced sleep duration/quality have also been associated with high BP176–178; however, data linking them specifically to RH are lacking. Finally, a variety of environmental exposures, including loud noises (eg, traffic), colder temperatures (eg, winter), higher altitudes, and air pollutants, promote high BP.179 Future studies are required to elucidate the potential impact of these commonly encountered environmental factors on RH.
Drug-Related RH
Several classes of pharmacologic agents can increase BP and contribute to drug-induced RH (Table 2). However, the effects of these agents are found to be highly individualized, with the majority of individuals manifesting little or no effect and others demonstrating severe elevations in BP levels.
Table 2.
Drugs and Other Substances With Potential to Induce or Exacerbate Elevated BP and Hypertension
| NSAIDs |
| Oral contraceptives |
| Sympathomimetic |
| Cyclosporine, tacrolimus |
| Erythropoietin |
| VEGF inhibitors |
| Alcohol |
| Cocaine |
| Amphetamines |
| Antidepressants |
| Glucocorticoids, mineralocorticoids |
BP indicates blood pressure; NSAIDs, nonsteroidal anti-inflammatory drugs; and VEGF, vascular endothelial growth factor.
Nonsteroidal Anti-Inflammatory Agents
Nonsteroidal anti-inflammatory agents (NSAIDs) increase BP by reducing the production of prostaglandins E2 and I2, leading to reduced vasodilation and sodium excretion. With their widespread use, NSAIDs are considered one of the most common offending agents affecting BP control.180,181 In general, all nontopical NSAIDs in doses adequate to reduce inflammation and pain can affect BP levels in both normotensive and hypertensive individuals.182 The results of meta-analyses have indicated that the average increase in mean arterial pressure is highly variable, with a range of reports from 2 to 5 mm Hg.183 Furthermore, NSAIDs affect the treatment effect of several antihypertensive medication classes, including diuretics, ACE inhibitors, ARBs, and β-blockers.184,185 It is important to note that antihypertensive medication interactions have typically not been shown with CCBs,186 and the effect of NSAID use on α- and β-blocker efficacy might also be minimal.187
Although the effect of NSAIDs on the incidence of hypertension has been reported as described earlier, there are significant variations in the magnitude of changes in BP in hypertensive patients taking antihypertensive medications.188–192 Several studies found no effect of NSAIDs on BP in patients who were using diuretics.193–195 The SBP increase with NSAIDs in ACE inhibitor users ranged from 5 to 10 mm Hg.196–199 The inhibition of prostaglandins by NSAIDs is proposed as the mechanism that explains the loss of the BP-lowering effect of ACE inhibitors.200,201
BP effects from the use of NSAIDs vary by type, with selective COX-2 (cyclooxygenase-2) inhibitors such as celecoxib having less BP effect than traditional NSAIDs.192,202 The BP effect188–190 appears to be dose-dependent, involving the inhibition of COX-2 in the kidneys, with a reduction in sodium excretion and an increase in intravascular volume.186 In contrast, low-dose aspirin does not have COX-2–inhibiting or BP-increasing effects, as indicated by results from the HOT study (Hypertension Optimal Treatment).191 However, these conclusions cannot be extrapolated to larger doses of aspirin.
Oral Contraceptives and Hormone Replacement
Oral contraceptives raise BP and induce hypertension by increasing angiotensin biosynthesis.203,204 The Nurses’ Health Study of >60 000 normotensive women followed up for 4 years found that women using oral contraceptives had an 80% higher risk of developing hypertension compared with women not using oral contraception agents.205 However, only 41.5 cases of hypertension per 10 000 person-years could be attributed to oral contraceptive use, and this number rapidly declined with cessation of therapy. Similarly, another study in hypertensive women reported that women taking oral contraceptives had more severe hypertension and poorer BP control rates than women using other contraceptive methods.206 The type of oral contraceptives is important, with combined oral contraceptives (progestin and estradiol) associated with BP elevations at greater rates than lower-dose estradiol-only and progestin-only oral contraceptives.207,208 Epidemiological studies of high-dose estrogen use found mean elevations in SBP of 3 to 6 mm Hg, with ≈5% of women developing new hypertension.209 Cessation of estrogen use typically leads to a return to baseline BP within 2 to 12 months, but proteinuria may persist.209,210 The mechanism responsible for the hypertensive effect of oral contraceptives may involve the renin-angiotensin system because estrogen stimulates the production of angiotensinogen.211
Postmenopausal hormone replacement therapy uses much lower estrogen doses than oral contraceptives, combined with progestin for women with an intact uterus. Estrogen replacement therapy and hormone replacement therapy appear to have a neutral effect on BP, as illustrated by the following observations from 2 large randomized trials. The Women’s Health Initiative, a randomized placebo-controlled trial, assessed the effect of estrogen-progestin replacement on outcomes in postmenopausal women211 and found that at 5.2 years hormone replacement therapy produced only a small increase (1.5 mm Hg) in SBP compared with placebo.212 Similar findings were noted in the PEPI trial (Postmenopausal Estrogen/Progestin Interventions) in which estrogen replacement therapy, with or without progestins, did not affect BP after 3 years.213
Sympathomimetic Amines
Sympathetic amines increase BP by activation of the sympathetic nervous system. The association of sympathomimetic amines with dose-related increases in BP has been well established.214,215 Although sympathomimetic-induced hypertension may not be clinically significant in healthy patients, these BP levels can be concerning in individuals with comorbid conditions.214–217 Sympathomimetic amines include amphetamines and similar compounds such as pseudoephedrine and ephedrine. Historically, these compounds were contained in some over-the-counter cough and cold preparations and appetite suppressants, with several, most notably phenylpropanolamine, taken off the current market.
Immunosuppressive Agents
Cyclosporine
Cyclosporine and tacrolimus increase BP by inducing systemic and renal vasoconstriction and sodium retention. Hypertension is reported in the majority of patients undergoing renal, hepatic, or heart transplantation treated with cyclosporine.218 Cyclosporine is associated with enhanced renal vasoconstriction leading to volume-dependent (low renin) hypertension.219,220 Cyclosporine-induced hypertension is usually treated with vasodilatory CCBs. Although diuretics are effective treatment, they may exacerbate prerenal azotemia. Another immunosuppressive agent, tacrolimus, is thought to have some hypertensive effects.
Other Agents
Recombinant Human Erythropoietin
Recombinant human erythropoietin can increase BP by complex mechanisms in a dose-dependent fashion, resulting in hypertension in 20% to 33% of recipients.221 Long-term use of erythropoietin promotes vascular smooth muscle cell growth, vascular remodeling, and medial hypertrophy, with maintained elevated BP.222,223 Erythropoietin-induced hypertension can be controlled with antihypertensive medications, often with a single agent.224
Tyrosine Kinase Inhibitors
In patients being treated for malignancies, antineoplastic drugs that target the VEGF (vascular endothelial growth factor) pathway have emerged as inducers of hypertension.225–227 As an example, hypertension frequently developed in patients receiving treatment with VEGF inhibitors.228 Multiple mechanisms are proposed, including a reduction of nitric oxide and prostacyclin bioavailability, an increase of systemic vascular resistance, and vascular stiffness, which has been observed in animals treated with tyrosine kinase inhibitors of VEGF.229–237
Cocaine
Tachycardia and BP elevation are common clinical manifestations of cocaine use.238 BP elevation is caused by increased central sympathetic outflow and blockade of neuronal norepinephrine reuptake, resulting in neurotransmitter accumulation in the synaptic cleft with resulting intense sympathetic activation.239
Amphetamines
Amphetamines increase norepinephrine production, augmenting sympathetic nervous system activation. The complications of amphetamines are comparable to those of cocaine and include hypertension and tachycardia.240 Methylphenidate has been implicated in the development of hypertension in children treated for attention deficit disorder.241 Mescaline has effects very similar to those of amphetamines and can increase BP.240
Antidepressants
Monoamine oxidase inhibitors may lead to severely elevated BP in individuals who consume foods containing tyramine.242,243 Although the drugs themselves can exacerbate hypertension by increasing the half-life of norepinephrine at sympathetic nerve terminals, the effect is often magnified when amine precursors are also consumed.243 Tranylcypromine is the most likely of the agents to raise BP compared with moclobemide and brofaromine.244,245 Tricyclic antidepressants have been found to increase BP in patients with panic disorders.246 Likewise, dose-dependent increases in BP have been reported in patients receiving therapeutic doses of venlafaxine.247 Severe hypertension has also been identified in patients treated with antidepressants such as fluoxetine.248
Sleep Disorders and Pseudopheochromocytoma
Sleep Deprivation and Pseudopheochromocytoma
Contributing causes of RH are sleep disorders and a related problem called pseudopheochromocytoma. The term, which originated in 1999, describes a pheochromocytoma-like syndrome. Once true pheochromocytoma is excluded, one should consider pseudopheochromocytoma, a syndrome characterized by the presence of paroxysmal hypertension with 3 distinct features: abrupt elevation of BP, equally abrupt onset of distressful physical symptoms, and absence of reported fear or panic at the onset of attacks.249 The original description involved patients with a history of emotional trauma from an event that they had denied; many improved with antidepressant therapy, β-blockers, and counseling. Since that report, there have been numerous case reports of pseudopheochromocytoma with a recent update summarizing the syndrome.250
A related problem in many people with pseudopheochromocytoma is poor sleep quality. Poor sleep quality, if long term, yields the same symptomology of paroxysmal hypertension and elevated BP, especially during the afternoon and evening hours. Poor sleep quality is not the result of just OSA but a host of sleep disorders, including restless leg syndrome and insomnia of various causes.251 Many times, these different sleep disorders coexist and prevent the patient from achieving proper sleep.
The mechanism of poor sleep quality contributing to elevated BP and paroxysmal bouts of very high BP relates to activation of both the sympathetic nervous system and the renin-angiotensin-aldosterone system.252–255 Sympathetic activity is also increased in sleep deprivation, restless leg syndrome, and OSA.252–254 To further support this in RH, data from a post hoc analysis of the SYMPLICITY HTN-3 trial evaluated the effect of renal denervation versus sham control on office and ambulatory (including nocturnal) SBP in patients with OSA. Compared with sham control, renal denervation reduced the 6-month office SBP in subjects with OSA.56
Patients without OSA who suffer from sleep deprivation, defined as less than a minimum of 6 hours of uninterrupted sleep, also have increased sympathetic activity. In this case, it is a consequence of reduced time in non-rapid eye movement or slow-wave sleep that also affects the nocturnal dip in BP.252 This supports the hypothesis that disturbed non-rapid eye movement sleep quantity or quality is a mechanism by which sleep deprivation or restless leg syndrome leads to an increase in sympathetic tone.
SBP, diastolic BP, and heart rate increase the day after a sleep-deprived night compared with the day after a normal sleep; urinary excretion of norepinephrine is also increased.56,253–256 In addition, BP, heart rate, and peripheral vascular resistance progressively decrease in non-rapid eye movement sleep. Thus, any deterioration in sleep quality or quantity may be associated with an increase in nocturnal BP, which could contribute to the development of poor hypertension control.
In contrast to reduced sleep time and quality, the increase in sympathetic activity associated with OSA is a function of intermittent hypoxia; the short-term rise in BP parallels the severity of oxygen desaturation at night.256,257 Indeed, increased sympathetic activity is seen in both animal models subjected exclusively to intermittent hypoxia258 and healthy human volunteers exposed to intermittent hypoxia in a “tent.”259 Respiratory event-related intermittent hypoxia is caused not only by overactivity of the adrenergic system but also by the renin-angiotensin system, as further supported by a meta-analysis.255 It is also important to note that OSA-associated hypertension is only slightly reduced by continuous positive airway pressure (CPAP) treatment.260 Nevertheless, addressing sleep disorders or sleep habits is relevant when considering the risk of either developing or controlling preexistent hypertension.
Poor BP control is strongly associated with OSA, especially in those patients with an apnea/hypopnea index of at least 30 events per hour.261 Correction of sleep apnea with CPAP reduces BP in those who are adherent; however, because the SBP reduction is only from 2 to 5 mm Hg, adjunctive medications are almost always needed.260 Use of ARBs and some β-blockers,262 as well as central α-agonists such as clonidine or guanfacine, a longer-acting agent with comparable activity, at bedtime improves BP control.263 Failure to achieve a sufficient amount of time in the deep stages of sleep increases the risk of developing hypertension.
The relationship between self-reported sleep duration and prevalent hypertension follows a U-shaped association with the nadir of the U being between 7 and 8 hours of uninterrupted sleep per night.256,264,265 As sleep time is either reduced or increased from this range, there is higher prevalence of hypertension.56,266 Moreover, sleep duration of <5 hours is associated with incident hypertension in subjects <60 years of age, whereas sleep duration of >9 hours per night is associated with incident hypertension in subjects >60 years. These early observations have been further confirmed with larger, more recent studies and meta-analyses.256,264,267,268
In summary, sleep quality and duration are critically important in the control of BP in RH. Clinicians should ask frequently about sleep quality and duration because they clearly affect BP control by activating both the sympathetic and renin-angiotensin systems and will further mitigate against controlling BP in RH. Many of these patients will have elevated heart rate disproportionate to their BP, a clinical clue that may be related to sleep quality.269,270 In this setting, reinstating proper sleep patterns is critical, as is using agents that block the systems involved in the problem. Note that if the patient is receiving diuretics and is not adherent to a low-sodium diet, this will produce nocturnal micturition and result in interrupted sleep. Moreover, diuretics increase sympathetic tone; spironolactone blunts this activation, but ARBs do not.271
Obstructive Sleep Apnea
OSA is extremely common in patients with RH, with prevalence rates as high as 70% to 90%, and when present, OSA is often severe.272–277 The high occurrence of OSA in patients with RH has been attributed to increased fluid retention and accompanying upper airway edema, as suggested by studies positively relating the presence and severity of OSA to aldosterone excess and high dietary sodium intake.47,49,277–279 The role of aldosterone in promoting OSA is additionally supported by studies demonstrating that mineralocorticoid receptor antagonists reduce the severity of OSA in patients with RH.280–282 Other studies implicate excessive accumulation of fluid centrally, including in the neck, as an important contributor to OSA severity in patients with RH by demonstrating increased shifting of fluid from the lower extremities into the upper body during supine sleep.283 This central accumulation of fluid has been shown to contribute to upper airway edema with associated increases in upper airway resistance, thereby worsening OSA.284,285 This effect is blunted by intensification of diuretic therapy, including the use of spironolactone.286
Treatment of patients with OSA and RH with CPAP induces significant but generally modest reductions in BP. In a randomized evaluation of treatment of moderate to severe OSA with CPAP versus no CPAP in patients with RH, treatment with CPAP reduced mean 24-hour SBP and diastolic BP by 3.1 and 3.2 mm Hg, respectively.287 The treatment effect, however, was larger with increasing CPAP adherence. When analyzed separately, patients using CPAP at least 4 hours per night had reductions in 24-hour SBP and diastolic BP of 4.4 and 4.1 mm Hg, respectively, and reductions in nocturnal SBP and diastolic BP of 7.1 and 4.1 mm Hg, respectively.287 However, a well-conducted randomized controlled trial recently demonstrated that CPAP plus usual care, compared with usual care alone, did not prevent CVD events in patients with moderate to severe OSA and established CVD.288
Routine evaluation by polysomnography is not indicated for all patients with RH. However, given the high prevalence of often severe OSA in patients with RH and the potential benefit of CPAP to enhance BP control, clinicians should vigorously screen such patients for symptoms of OSA (loud snoring, frequent nocturnal arousals, witnessed apnea, and excessive daytime sleepiness) and have a low threshold for referral for definitive evaluation and treatment.
Secondary Hypertension: Diagnosis and Management
Primary Aldosteronism
Primary aldosteronism is defined as a group of disorders in which aldosterone production is inappropriately high, relatively autonomous, and independent of the renin-angiotensin system and in which aldosterone secretion is not suppressed by sodium loading.289 The disorder includes hypertension caused by volume expansion and sympathetic nervous system activation, hypokalemia, metabolic alkalosis, and advanced cardiovascular and renal disease. Because of the toxic effects of aldosterone on heart and blood vessels, primary aldosteronism is accompanied by a major increase in CVD and events compared with those observed in primary hypertension, including stroke (4.2-fold), myocardial infarction (6.5-fold), and atrial fibrillation (12.1-fold).290 Primary aldosteronism is also associated with left ventricular hypertrophy, diastolic dysfunction and heart failure, large artery stiffness, oxidative stress, widespread tissue inflammation and fibrosis, and increased resistance vessel remodeling compared with primary hypertension.291–293
The majority of studies indicate that primary aldosteronism is a particularly common cause of RH. Observational studies from many different countries have demonstrated a prevalence rate of primary aldosteronism of ≈20% in patients with confirmed RH.57,160,294–296 This relatively high prevalence contrasts with the ≈8% overall prevalence of primary aldosteronism in primary hypertension.289 However, the prevalence of confirmed primary aldosteronism among 1656 hypertensive Chinese patients with RH was only 7.1%, indicating significant variation in prevalence in different populations.297 When considering the frequency of primary aldosteronism, it is important not simply to accept a positive plasma aldosterone:renin ratio as diagnostic of the disease but to confirm the results of this screening test with 1 of 4 recommended confirmatory tests.289 In the Chinese study, saline infusion was performed in all patients to confirm the diagnosis.297
Given the relatively high prevalence of primary aldosteronism in patients with RH, all such patients should be screened.289 Screening for primary aldosteronism should be conducted with the ratio of plasma aldosterone concentration to PRA (aldosterone/renin ratio [ARR]) from a blood sample obtained in the morning with the patient in a seated position for at least 30 minutes before sampling.289,298 A positive screening test requires an ARR of >30 or >20 if plasma aldosterone concentration is ≥16 ng/dL.289 The validity of the screening test depends critically on appropriate preparation of the patient before the test. Hypokalemia, which reduces aldosterone secretion, should be corrected with oral K+ supplements before testing. Pharmacological agents that markedly affect plasma ARR (spironolactone, eplerenone, and high-dose amiloride) should be withdrawn at least 1 month before testing.289 It is also important to recognize that antihypertensive medications other than spironolactone, eplerenone, and amiloride can alter screening test results. For example, β-adrenergic receptor blockers, central α2-receptor agonists, and renin inhibitors suppress PRA, and ACE inhibitors, ARBs, non-potassium-sparing diuretics, and dihydropyridine CCBs increase PRA, thus altering plasma ARR values. If the initial screening test results are not convincing, these medications can be selectively withdrawn for at least 2 weeks while BP is controlled with other agents that do not influence the renin-angiotensin-aldosterone system such as slow-release verapamil, hydralazine, or an α1-adrenergic receptor antagonist (prazosin, doxazosin, or terazosin).289
If the screening test for primary aldosteronism is positive, the patient is usually referred for further evaluation to an endocrinologist or hypertension specialist. Further steps include administration of a confirmatory test (saline suppression test, oral salt-loading test, captopril test, or fludrocortisone suppression test). If primary aldosteronism is confirmed with one of these tests, subtype classification is usually performed with adrenal venous sampling. Patients with unilateral disease (50%; aldosterone-producing adenoma or, much less frequently, unilateral hyperplasia) respond to unilateral laparoscopic adrenalectomy with complete cure (≈50%) or improvement (≈50%) in BP control. Patients with bilateral disease (idiopathic hyperaldosteronism) usually have marked improvement in hypertension control with spironolactone or eplerenone.289
Renal Parenchymal Disease
CKD is both a cause and a complication of poorly controlled hypertension. Reduced kidney function results in impaired salt excretion, overactivation of the renin-angiotensin-aldosterone system, increased sympathetic nervous system activity, and altered medication efficacy. Treatment resistance in patients with CKD is undoubtedly related in large part to increased sodium and fluid retention and consequential intravascular volume expansion. An excess of total body salt and water limits the efficacy of antihypertensive medication classes that lack a natriuretic effect. In addition, salt may have a direct effect on the vasculature, hastening arteriosclerosis and blunting the vascular response to medication.
As the population ages, the prevalence of CKD is estimated to rise, and correspondingly, the number of individuals with RH will increase. From data from NHANES 1999 to 2010, the lifetime risk of developing (or “progressing to”) moderate-stage CKD from normal baseline kidney function is 54% (age, 30–49 years), 52% (age, 50–64 years), and 42% (age ≥65 years).299 An alternative simulation for a 30-year-old individual estimated a residual lifetime risk of 59.1% for estimated glomerular filtration rate (eGFR) <60 mL⋅min−1⋅1.73 m−2.300 Although most of the CKD population will ultimately require antihypertensive drug therapy (30.2% with stage 1 CKD increasing to 78.9% with stage 4 CKD), achievement of current BP goals (≤130/80 mm Hg) declines with higher CKD stage from 49.5% at stage 1 to 30.2% at stage 4 (overall rate, 44.6%) despite greater use of antihypertensive medications. Even when a higher goal of ≤140/90 mm Hg was used, only 66.5% overall reached this target.301
Difficulty controlling BP in late stages of CKD was also seen in CRIC (Chronic Renal Insufficiency Cohort), in which 88% of participants had stage 3 or 4 CKD. Of the 86% with hypertension, 67% were controlled to <140/90 mm Hg and 46% were controlled to <130/80 mm Hg, with a higher prevalence of uncontrolled hypertension in those with lower eGFR.302,303 aTRH was present in 40% and was associated with higher cardiovascular event rates and mortality whether BP was uncontrolled (≥140/90 mm Hg) with 3 antihypertensive agents (52.5%) or controlled with ≥4 agents (47.5%).19 aTRH was more common in patients with lower eGFR, including 22.3% with eGFR >60 mL⋅min−1⋅1.73 m−2, 39.4% with eGFR of 0 to 60 mL⋅min−1⋅1.73 m−2, and 54.2% in those with eGFR <30 mL⋅min−1⋅1.73 m−2. This trend was quantified as 14% higher odds of having aTRH for every 5–mL⋅min−1⋅1.73 m−2 decrease in eGFR (adjusted odds ratio, 1.14; 95% CI, 1.10–1.17). Doubling of proteinuria was associated with a higher likelihood of aTRH with an adjusted odds ratio of 1.28 (95% CI, 1.16–1.42). aTRH was common among CRIC participants, although 72% were receiving nephrology care.
Other data confirm that even when patients with CKD were followed up in nephrology clinics, <15% had their BP controlled to <130/80 mm Hg and <40% achieved a BP of <140/90 mm Hg despite the use of 3 different antihypertensive agents.304 In ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial), CKD as indicated by a serum creatinine of >1.5 mg/dL was a strong predictor of failure to achieve goal BP. Thus, patients with CKD and aTRH are at higher risk for CVD events and renal events (50% decrease in eGFR or incident end-stage renal disease) compared with patients with CKD without aTRH.67
Evaluation of the patient with CKD and RH includes consideration of other secondary causes that may coexist, including renal artery stenosis, primary aldosteronism, or other endocrine causes. Special attention should be focused on appropriate dietary sodium restriction because reduced salt intake may improve the efficacy of antihypertensive medications. Effective control generally requires a diuretic in the regimen with evolution to more potent agents, either thiazidetype agents at higher doses or loop diuretics, as renal function declines.
Renal Artery Stenosis
Hypertension accelerated or worsened by renal artery stenosis remains among the most common causes of RH, particularly in older age groups.305 Among a series of 4494 patients referred to hypertension specialty clinics, 12.7% had secondary causes overall, of which 35% were associated with occlusive renovascular disease.306 More recent series indicate that 24% of older subjects (mean age, 71 years) with RH have significant renal arterial disease.307 Most cases are caused by atherosclerotic disease, but the syndrome of renovascular hypertension can result from other less common obstructive lesions, including a variety of fibromuscular dysplasias, renal artery dissection or infarction, Takayasu arteritis, radiation fibrosis, and renal artery obstruction from aortic endovascular stent grafts. Renal artery stenosis is recognized to produce a wide spectrum of manifestations ranging from an incidental, asymptomatic finding to accelerated hypertension and renal insufficiency. The latter often reflects disease progression.308 Moderate degrees of renovascular hypertension most often can be managed with medical therapy, particularly with the use of agents that block the renin-angiotensin system (ACE inhibitors/ARBs), as several prospective randomized trials have demonstrated.309,310 As a result, current practice has shifted to optimizing antihypertensive drug therapy as the primary treatment for patients with identified renal artery stenosis before embarking on complex imaging or interventional strategies. Most patients with renovascular disease tolerate ACE inhibitor or ARB therapy without adverse renal effects,311 but a modest fraction (10%−20%) will develop an unacceptable rise in serum creatinine, particularly with volume depletion.312 A subset of medically treated patients develop progressive disease syndromes with worsening hypertension, renal insufficiency, or circulatory congestion (“flash pulmonary edema”), which carry high mortality risks. Observational series have repeatedly demonstrated that BP control and mortality can be improved substantially after successful revascularization.313,314 Post hoc analysis of the CORAL trial (Cardiovascular Outcomes With Renal Artery Lesions) data suggests a mortality benefit of revascularization compared with medical therapy for atherosclerotic renal artery stenosis in patients without proteinuria.315
Management of renal artery stenosis can be particularly challenging when bilateral lesions are present because there are considerable risks both with intervention and if intervention is not performed. Diagnosis of this disorder depends on clinical suspicion and consideration for arterial imaging for subjects with unexplained progressive hypertension or renal dysfunction. Duplex imaging to identify increased peak systolic velocity in the renal arteries is most commonly used, often with confirmation by computed tomography angiography or magnetic resonance angiography before invasive studies. Although initial treatment usually includes systematically advancing medical therapy, restoring main renal artery patency with endovascular stenting is also likely to reduce arterial BP, albeit with a residual requirement for antihypertensive drug therapy.316 The most reliable predictor for effective BP reduction after revascularization remains a short duration of pressure elevation.
Medical treatment options for renal artery stenosis have been enhanced with the availability of effective blockade of the renin-angiotensin system and potent CCBs. Large data registries indicate that ACE inhibitor or ARB treatment in patients with identified renal artery stenosis confers a long-term mortality benefit.317 Those individuals who experience a rise in creatinine during ACE inhibitor or ARB treatment usually tolerate restarting the medication after successful revascularization. The rise in serum creatinine during treatment in patients with renal artery stenosis or CKD is often transient and related to sluggish renal autoregulation when BP falls, with the fall being even greater if intravascular volume drops with diuretics or renin-angiotensin system blockers, which dilate the renal efferent more than the afferent arteriole. Patients failing antihypertensive drug therapy or those with bilateral renal artery disease with loss of renal function or episodes of pulmonary edema should be considered for revascularization.318 Most patients with RH and atherosclerotic renal artery stenosis failing medical therapy can be treated with endovascular procedures such as stenting. Restenosis may develop in 15% to 24% of treated patients but may not always be associated with worsening hypertension or kidney function.319 Surgical revascularization is reserved most often for subjects with complex anatomy or associated aortic disease.
Pheochromocytoma/Paraganglioma
The chromaffin cell tumors, pheochromocytoma (adrenal catecholamine producing, 90%) and paraganglioma (extra-adrenal, sympathetic/parasympathetic derived, 10%), are rare even in the hypertensive population, with a prevalence estimated at 0.01% to 0.2%. The prevalence is likely higher in patients referred for RH (eg, up to 4%).320 Symptoms include paroxysmal hypertension (which may be sustained in up to 50% of those with high norepinephrine production or orthostatic in epinephrine-predominant tumors) associated with headache, palpitations, pallor, and piloerection (“cold sweat”). Given the high morbidity and mortality of not treating these tumors, the usual 3-year delay in diagnosis, and the fact that one-third are inherited, it is essential to consider the diagnosis in anyone referred for RH.
The screening test of choice for pheochromocytoma/paraganglioma is measurement of circulating catecholamine metabolites. Catechol 0-methyl transferase releases normetanephrine and metanephrine from the tumors, measured as plasma free (sensitivity, 96%−100%; specificity, 89%−98%) or urinary fractionated (sensitivity, 86%−97%; specificity, 86%−95%) metanephrines.321 Hypertensive patients frequently have elevated levels of catecholamine metabolites, especially in the presence of obesity and OSA and with the use of certain drugs such as tricyclic antidepressants. The levels are usually <4 times the upper limit of normal, but assessment should be repeated. If still elevated, they can be further evaluated as false positives by clonidine-suppression testing, with 100% specificity and 96% sensitivity of failure to reduce plasma metanephrines by 40%.322
Imaging should be pursued only after biochemical evidence for a pheochromocytoma has been obtained. The recent Endocrine Society guideline recommends starting with computed tomography, with magnetic resonance imaging as an alternative and metaiodobenzylguanidine scanning to further evaluate suspected metastatic disease.323 Once the tumor has been confirmed, the patient should be prepared to undergo surgery by an experienced team (endocrinologist or hypertension specialist, surgeon, and anesthesiologist). Treatment with α-adrenergic blockade, a high-sodium diet, and fluid intake are recommended for at least a week before surgery to prevent intraoperative BP instability and to reverse volume contraction from pressure-natriuresis.323 Postoperatively, patients should be followed up for the possibility of recurrence or metastasis, especially with the inherited forms.
Cushing Syndrome
Cushing syndrome is a relatively uncommon endocrine disorder caused by chronic excessive glucocorticoid exposure from endogenous or exogenous sources. This leads to a constellation of classic symptoms (mood disorders, menstrual irregularities, muscle weakness) and signs (weight gain, abdominal striae, hirsutism, dorsal and supraclavicular fat, fragile skin). Glucose abnormalities and hypertension are also common; thus, the disorder mimics a severe form of the metabolic syndrome. Although a variety of relatively small cohort studies suggest that high BP is highly prevalent (often exceeding 80%),324,325 the overall frequency of hypertension, mild or severe, among patients with Cushing syndrome remains to be accurately characterized. Since the earlier AHA statement,2 there has also been little progress in better understanding the degree to which Cushing syndrome plays a role in RH. However, the available evidence does not support it as a common cause. In a recent study of 423 individuals with RH, a systematic evaluation for Cushing syndrome found no overt cases.326 This suggests that although high BP (perhaps even severe forms) may be common among patients with Cushing syndrome, it is unlikely that it is prevalent in the overall population of patients with RH. This is likely principally a result of its rarity; however, the possibility should be recognized that some unknown percentage of patients with RH may be undiagnosed given the high prevalence of the metabolic syndrome, potentially masking occult disease.
Detailed guidelines on whom to screen, the diagnostic algorithm, and the treatment of patients with various forms of Cushing syndrome have been published.327,328 Given the high risk for CVD events, the guidelines explicitly recognize the importance of aggressively treating hypertension.328 Prior reviews outlining the underlying mechanisms of high BP in Cushing syndrome324,325 suggest that blocking the mineralo-corticoid actions of excess cortisol that increase renal sodium absorption with agents such as spironolactone or eplerenone is likely a sensible strategy. However, it is also recognized the hypercortisolism can promote hypertension through a variety of additional pathways (eg, activating the renin-angiotensin system, sensitizing the vasculature to catecholamines, and impairing endogenous nitric oxide bioavailability).324,325 The optimal antihypertensive regimen in patients with Cushing syndrome therefore remains to be adequately described; there have been no randomized controlled trials in this area. Nevertheless, it is probable that excessive activation of mineralocorticoid receptors plays a prominent role and thus adequate diuretic therapy will likely prove to be a cornerstone of successful therapy. Further details on the overall treatment and surgical management of Cushing syndrome are provided in the recent guidelines.328
Coarctation of the Aorta
Patients with operated coarctation of the aorta are likely to have hypertension in adulthood and are at risk for premature CVD, including myocardial infarction, aortic aneurysm (ascending or descending), stroke, and heart failure.329–331 Lifetime BP load is likely higher in patients with repaired coarctation compared with those with normal aortas because they have an exaggerated BP response to exercise. This response may also predict future hypertension.332 All patients with a history of coarctation repair and hypertension should be evaluated for residual aortic arch obstruction with computed tomography angiography, particularly if a gradient between the right arm and leg is present and other sequelae of surgery such as descending aortic aneurysm are present.331 If hypertension is resistant to treatment, surgical or catheter-based intervention should be considered if the risk/benefit ratio for the contemplated procedure is favorable. Because persistent hypertension may be secondary to increased sympathetic tone, β-blockers may be most useful for BP control.333 Antihypertensive therapy typically also includes an ACE inhibitor or an ARB.
Other Causes of Secondary Hypertension
In addition to the above, other unusual/rare endocrine causes of secondary hypertension are included in Table 3.
Table 3.
Other Endocrine Causes of Secondary Hypertension
| Disorder | Major Clinical Findings | Physical Examination | Screening Tests | Additional/Confirmatory Tests |
|---|---|---|---|---|
| Hypothyroidism | Dry skin; cold intolerance; constipation; hoarseness; weight gain | Delayed ankle reflex; periorbital puffiness; coarse skin; cold skin; slow movement; goiter | High TSH; low or normal fT4 | |
| Hyperthyroidism | Warm, moist skin; heat intolerance; nervousness; tremulousness; insomnia; weight loss; diarrhea; proximal muscle weakness | Lid lag; fine tremor of the outstretched hands; warm, moist skin | Low TSH; high or normal fT4 and T3 | Radioactive iodine uptake and scan |
| Hypercalcemia and primary hyperparathyroidism | Hypercalcemia | Usually none | Serum calcium | Serum parathyroid hormone |
| Congenital adrenal hyperplasia (excess DOC) | Hypertension and hypokalemia; virilization (11-β-OH deficiency); incomplete masculinization in males and primary amenorrhea in females (17-α-OH deficiency) | Signs of virilization (11β) or incomplete masculinization (17α) | Hypertension and hypokalemia with low or normal aldosterone and renin | 11-β-OH: elevated DOC, 11-deoxycortisol and androgens; 17-α-OH: decreased androgens and estrogen; elevated DOC and corticosterone |
| Other mineralocorticoid excess syndromes caused by DOC | Early-onset hypertension, hypokalemia | Arrhythmias (with hypokalemia) | Low aldosterone and renin | DOC; urinary cortisol metabolites; genetic testing |
| Acromegaly | Acral features; enlarging shoe, glove, or hat size; headache; visual disturbances; diabetes mellitus | Acral features; large hands and feet; frontal bossing | Serum growth hormone ≥1 ng/mL during oral glucose load | Elevated age- and sex-matched IGF-1 level; MRI scan of the pituitary |
DOC indicates deoxycorticosterone; fT4, free thyroxine; IGF-1, insulin-like growth factor-1; MRI, magnetic resonance imaging; OH, hydroxylase; T3, triiodothyronine; and TSH, thyroid-stimulating hormone.
Evaluation of RH
The evaluation of patients with RH should be directed toward confirmation of true treatment resistance, identification of causes contributing to resistance (including secondary causes of hypertension), and documentation of complications of the hypertensive disease process (see the evaluation algorithm in Figure 2). Assessment for treatment adherence and use of ABPM (or home BP monitoring if ABPM is unavailable) are needed to confirm RH. True RH usually has multiple causes, including excessive dietary salt intake, obesity, CKD, and OSA. Assessment for comorbidities and hypertensive complications is relevant because it will influence antihypertensive therapy in terms of the class of pharmacological agent selected and BP goals.334
Figure 2.
Algorithm depicting the evaluation of resistant hypertension. ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; and BP, blood pressure.
Medical History
Documentation of the duration, severity, and behavior of the hypertension is important, along with adherence to medical appointments and antihypertensive treatment, clinical and adverse event responses to prior antihypertensive medications, and current prescription and over-the-counter medications that may elevate BP or interfere with antihypertensive drug effects. In the clinical setting, accurate medication adherence is not usually self-reported. Hence, clinicians must diplomatically inquire about how often patients might miss their medication doses per week, whether adverse effects might be playing a role in nonadherence, or whether patients have financial challenges affecting their ability to pay out-of-pocket expenses for their antihypertensive drugs.
The medical interview should also be directed toward identifying secondary causes of hypertension (for details, see the Secondary Hypertension: Diagnosis and Management section).
Physical Examination
The examination of patients with RH should be oriented toward ascertainment of target organ damage and possible secondary causes. BP should be measured as described previously. The presence of vascular disease, including in the retina, should be pursued with fundoscopic evaluation and careful examination of the quality of peripheral and carotid pulses and auscultation for bruits. Although not specific, abdominal bruits increase the possibility that obstructive renal artery disease exists. BP should be measured in both arms and in the thigh (in patients <30 years of age) if aortic occlusive disease or aortic coarctation is suspected. If the thigh SBP is >10 mm Hg lower than the arm SBP while the patient is supine, imaging studies for coarctation should be performed.3 Hypercortisolism may be suspected in patients with obesity, diabetes mellitus, hypertension, depression, and bone loss accompanied by abdominal striae, “moon” facies, and intrascapular fat deposition.
Out-of-Office BP Monitoring
Disparities between in-office and out-of-office BP should be adjudicated.334–339 Unattended automated office measurements, self-measurements (home or work), and ABPM have utility in determining whether there might be a white-coat effect that creates the appearance of treatment resistance. Of these methods, ABPM is the most objective and robust means to define the white-coat effect because it has been shown to be a stronger predictor of CVD morbidity and mortality than clinic measurements340 and has received recommendations for use in the diagnosis of hypertension in both the United Kingdom and the United States.1,341,342
A white-coat effect (office BP above goal but ambulatory BP below goal) should be considered in patients with normal or lower self-measured BP, in those lacking target organ involvement (eg, retinopathy, CKD, left ventricular hypertrophy), and when there are symptoms of excessive antihypertensive therapy. If a white-coat effect is confirmed, out-of-office measurements (ambulatory or self-measured values if ABPM is not available) should be used to confirm the achievement of BP goals and to adjust drug therapy.339,343
Biochemical Evaluation
Laboratory examination of the patient with RH should include a basic metabolic profile (serum sodium, potassium, chloride, bicarbonate, glucose, blood urea nitrogen, and creatinine), urinalysis, and a paired morning plasma aldosterone and PRA to screen for primary aldosteronism. Interpretation of the ARR is diminished in the setting of certain antihypertensive agents such as mineralocorticoid receptor antagonists (raise aldosterone levels) or direct renin inhibitors and β-adrenergic- blocking drugs (lower renin levels). The ratio is an effective screening test because it has a high negative predictive value for screening of primary aldosteronism.344,345 The specificity for a high ARR is low as a result of the common occurrence of low-renin states (eg, volume expansion, dietary salt excess or sensitivity). A high ratio (> 20) when the serum aldosterone is >16 ng/dL and PRA is <0.6 ng/mL per hour is suggestive of primary aldosteronism, particularly in a patient taking an ACE inhibitor or ARB (these drugs elevate the PRA; hence, if renin is still suppressed, it increases the sensitivity of the ratio), but further assessments are required to confirm the diagnosis.
Measurement of plasma metanephrines or 24-hour urinary metanephrines is an effective screening test for patients in whom paraganglioma or pheochromocytoma is suspected.346
Noninvasive Imaging
Imaging for the evaluation of renal artery stenosis in patients with RH should be reserved for those with a high likelihood of renovascular disease: young patients in whom fibromuscular dysplasia could be present and older patients with a history of smoking or vascular disease who have increased risk for atherosclerosis. Calculation of aortic and renal artery velocities by duplex ultrasonography of the renal arteries is the usual initial test and is preferred over computerized tomography and magnetic resonance angiography as a screening tool, particularly when patients have CKD (eg, eGFR <60 mL⋅min−1⋅1.73 m−2). Direct renal arteriography in the absence of suspicious noninvasive imaging is not recommended. Abdominal imaging of the adrenal glands by high-resolution computerized tomography or magnetic resonance imaging is indicated only if there is biochemical evidence of hormonally active tumors (eg, elevated aldosterone, catecholamines, or cortisol).
Management of RH
An algorithm for managing RH is depicted in Figure 3.
Figure 3.
Algorithm depicting the management of resistant hypertension. BP indicates blood pressure; CCB, calcium channel blocker; and RAS, renin-angiotensin system. *These diuretics maintain efficacy down to estimated glomerular filtration rates (eGFRs) of 30 mL⋅min−1⋅1.73 m−2. **Use caution if eGFR is <30 mL⋅min−1⋅1.73 m−2. ***Requires concomitant use of a β-blocker and diuretic. ****Requires the concomitant use of a β-blocker and loop diuretic.
Lifestyle Interventions
Weight Loss
Weight-reducing diets are well established to lower BP among patients with hypertension, by 4.5/3.2 mm Hg in the most recent meta-analysis.347 On the other hand, the effect of pharmacologically aided weight loss is mixed, with some medications showing modest benefits (eg, orlistat), whereas others (sibutramine) may even raise BP.348 Although obesity clearly increases the risk for RH, trials demonstrating the efficacy of weight loss among these patients are lacking. Guidelines consistently promulgate a healthy lifestyle (including caloric restriction), aiming for a >5% to −10% body weight loss among overweight and obese adults to help lower BP.1,174 These same recommendations should also apply to patients with RH (despite the lack of published studies among these patients), particularly in light of the fact that weight loss can lead to the successful withdrawal of medications, thereby easing hypertension control.174,348 However, it is important to note that the long-term persistence of weight loss (>1 year) and the associated BP reduction are poorly described and warrant future investigation.
Dietary Salt Restriction
A reduced-sodium diet is well proven to decrease BP.174,349 In a recent meta-analysis, an estimated 1-g (43.5-mmol) reduction in daily sodium intake produces a 2.1− and 1.2–mm Hg decrease in SBP among hypertensive and normotensive patients, respectively.349 In addition to hypertensives, other subgroups of individuals (eg, those with CKD350 and obesity, blacks) are often more sensitive and can derive particularly robust benefits from sodium restriction.153,155 In patients with RH, 2 recent small studies have demonstrated the efficacy of a reduced dietary sodium intake for BP lowering.58,161 In 12 patients with true RH (taking 3.4±0.5 antihypertensive medications), a low-sodium (50 mmol/d) versus a high-sodium (250 mmol/d) diet resulted in a profound reduction in average office BP (−22.7/−9.1 mm Hg) over a 7-day period.58 The low-sodium diet also led to significant reductions in daytime, nocturnal, and 24-hour BP levels (−20.1/−9.8 mm Hg). In a 6-week-long double-blind placebo-controlled crossover trial of 20 patients with stage 3 to 4 CKD, many of whom had RH (average, 3.2 ± 1.1 antihypertensive medications), a low-sodium (75 mmol/24-hour urine) versus a high-sodium (168 mmol/24-hour urine) diet significantly reduced 24-hour ambulatory BP (−9.7/−3.9 mm Hg).161
At present, debate remains about the optimal intake of sodium for the prevention of CVD morbidity and mortality.153–155,349 There is concern that a U-shaped relationship may exist, with the greatest risk reductions being achieved by following only moderate restriction in sodium intake of 173 to 217 mmol/d (4–5 g/d) despite the fact that BP continues to decrease in a linear fashion with even further reductions to <65 mmol/d (1.5 g/d).349 It has been posited that sympathetic nervous system and renin-angiotensin system activation may counter the health benefits derived from the additional BP lowering at the lower range of sodium intake <130 mmol/d (3 g/d). AHA recommendations155 have continued to promulgate that an ideal daily sodium intake is <65 mmol/d (1.5 g/d), particularly among high-risk populations. Moreover, it is possible that the meta-analysis findings do not apply specifically to individuals with RH and that the additional BP lowering achieved through sodium restriction may offset putative risks of achieving low levels of sodium intake. Evidence-based recommendations on the optimal sodium intake among patients with RH must await more definitive clinical trial data. For the time being, the available studies (albeit of short-term duration) support that sodium restriction, even to low levels <65 to 100 mmol/d (1.5–3.0 g/d), yields significant linear reductions in BP among patients with high BP153–155,349 and RH.58,161 Therefore, it seems prudent to continue to support the prior AHA recommendations for patients with RH1 to reduce daily sodium intake to <100 mmol/d (2.3 g/d) and to consider more stringent restrictions to <65 mmol/d (1.5 g/d) on a case-by-case basis. Future trials testing the efficacy of this approach, particularly over longer-term durations (>6–12 months), are vital.
DASH Diet and Other Dietary Factors
A DASH-style eating pattern,174,175 alcohol restriction to <10 g/d (women) and <20 g/d (men),162–165 and a variety of other dietary modifications174 can each yield significant reductions in BP among patients with hypertension. Diet intervention trials specifically among patients with RH are absent. In light of their safety and proven efficacy to lower BP in the general hypertensive population, patients with RH should follow the established dietary recommendations promoted by the AHA1,351 and American Society of Hypertension, as outlined in detail elsewhere.174 Caution should be exercised in adopting a potassium-rich DASH diet in patients with more advanced (stage 4 and 5) CKD because these individuals are at risk for severe hyperkalemia and its consequences.
Exercise
Numerous clinical trials demonstrate that exercise can effectively lower BP, particularly among patients with preexisting hypertension.352,353 In the largest meta-analysis to date, BP was lowered by 8.3/5.2 mm Hg over several weeks by a variety of endurance (aerobic) exercise programs in patients with hypertension.353 Moreover, dynamic resistance exercise was also shown to reduce BP by 1.8/3.2 mm Hg among hypertensive patients. As previously outlined, a treadmill walking exercise program in 1 study was safe and effective for lowering BP in patients with RH.173 A recent small pilot study (n=16) also demonstrated that a water-based aerobic exercise program may be safe and effective for lowering BP in this population.354 Although limited data are available, it seems appropriate that patients with RH should follow current exercise recommendations as promoted by numerous expert guidelines, including the 2017 American College of Cardiology/AHA guideline,1 to reduce BP. In general, this involves ≥150 min/wk (in 3–5 sessions of 30–40 minutes) of moderate to intense aerobic activity, optimally supplemented with 2 to 3 sessions of light resistance training per week. Because RH is more common in obese and older adults, some may be unable to achieve moderate to intense aerobic activity. Low-intensity physical activity, 6 minutes hourly over an 8-hour period, in sedentary individuals lowered BP (14/8 mm Hg) and can improve metabolic syndrome variables.355–359 Moreover, evidence suggests that low-intensity physical activity may be similarly as effective as moderate to intense physical activity in preventing type 2 diabetes mellitus,360 which is a concern in nondiabetic adults with RH.
Alternative Approaches
In a recent scientific statement from the AHA, the evidence supporting the BP-lowering efficacy of alternative approaches was reviewed.361 Although many treatments had inadequate scientific support (eg, acupuncture, yoga), several modalities, including transcendental meditation, device-guided slow breathing, and isometric handgrip exercise, were felt to hold promise for clinical practice. More recent evidence continues to support the efficacy of isometric handgrip in particular. The latest meta-analysis demonstrates that isometric handgrip, typically performed for 12 minutes 3 to 5 times per week, lowers BP by 5.2/3.9 mm Hg.362 However, no trials among patients with RH have been performed. The usefulness of any alternative approach as an adjuvant to control BP in this population requires further investigation. Furthermore, the efficacy of intervening on other lifestyle factors that have been linked to high BP or RH, such as improving sleep quality and mitigating environmental triggers (eg, cold, noise, pollution), remains unknown.
A least 1 ongoing randomized clinical trial (TRIUMPH [Triple Pill vs Usual Care Management for Patients With Mild-to-Moderate Hypertension]) is investigating the effectiveness of an intense multifaceted lifestyle intervention program versus routine care in patients with RH.363
Pharmacological Treatment of RH
General Principles
Once all identifiable forms of hypertension, particularly endocrine causes, have been excluded and contributions from the white-coat effect (office BP at least 20/10 mm Hg higher than home or ABPM measurements) and masked uncontrolled hypertension (office BP measurements suggesting adequate BP control but out-of-office readings elevated above goal) are considered, therapeutic approaches for improved BP control in RH can begin. Three mechanistically complementary antihypertensive agents, commonly including a long-acting CCB, a blocker of the renin-angiotensin system (ACE inhibitor or ARB), and a diuretic, should already be prescribed and taken by the patient to ensure a proper diagnosis of RH (Figure 3, step 1). Hydrochlorothiazide does not induce a predictable natriuresis below an GFR of 45 mL⋅min−1⋅1.73 m−2, but chlorthalidone induces natriuresis down to an eGFR of 30 mL⋅min−1⋅1.73 m−2. Thiazide or thiazide-like diuretics are appropriate down to an eGFR of 25 to 30 mL⋅min−1⋅1.73 m−2.364 Below this eGFR level or in hypoalbuminemic states (ie, serum albumin < 3.0 g/L), a long-acting loop diuretic such as torsemide should be used over shorter-acting agents such as bumetanide or furosemide.365 These 3 separate pharmacological classes of antihypertensive agents must be given at maximally tolerated doses such as amlodipine 10 mg, chlorthalidone 25 mg, and one of the ACE inhibitors or ARBs at maximal dose.366 Suboptimal medication regimens are common in patients with hypertension resistant to antihypertensive therapy.21,60,367
Specific Therapeutic Regimens
Because most cases of RH are linked to either volume excess, especially in CKD, or high sympathetic tone, establishment of the pathogenesis will optimally facilitate the choice of the fourth drug. In addition, ensuring that the most effective antihypertensive agent from certain pharmacological classes is selected is important when a fourth agent is added (Table 4).
Table 4.
Specific Clinical Issues Associated With Treatment Resistance*
| Issue Associated With Treatment Resistance | Management Consideration(s) |
|---|---|
| Volume control, edema resolution | Thiazide→chlorthalidone→loop diuretic |
| Heart rate control inadequate | β-Blocker, α,β-blocker, verapamil, diltiazem |
| Renin and aldosterone levels low | Low-salt diet, avoid nighttime shift work, amiloride |
| Renin low, aldosterone normal to high normal | Mineralocorticoid receptor antagonist |
| Would split dosing of medications improve control? | Evaluate BP pattern according to home and ambulatory BP monitoring |
| Medication adherence questionable | Initiate indirect or direct methods to detect nonadherence; if nonadherence is documented (partial or complete), discuss frankly, nonjudgmentally with patient and family |
| Pattern of BP response to medications outside clinician visit times unknown | Identify meal effects on BP, duration of medication effect, relationship of BP to side effects using out-of-office BP monitoring |
| Sleep disordered breathing; significant anxiety associated with highly variable hypertension | Initiate nondrug strategies concurrently with or separately from antihypertensive drug therapy |
BP indicates blood pressure.
Modified from White et al334 with permission from the American Society of Hypertension.
Diuretic choices (Figure 3, step 2, and Table 4) are important because volume excess and failure to adhere to low-sodium diets are very common causes of RH. The diuretics with the greatest evidence base for reducing cardiovascular outcomes are the thiazide-like diuretics chlorthalidone and indapamide.368,369 Comparative studies show an additional SBP reduction of 7 to 8 mm Hg simply by switching from hydrochlorothiazide to the same daily dose of chlorthalidone.370–372
Recent reports document the efficacy of mineralocorticoid receptor blockade (Figure 3, step 3, and Table 4) to improve BP in patients with RH.373–377 In patients who are not overtly volume overloaded but who have evidence of low renin status or salt sensitivity of BP, mineralocorticoid receptor antagonists (spironolactone or eplerenone) are more successful than α- or β-blockers.373,377 Spironolactone has the advantage of once-daily administration and can be initiated at doses of as little as 12.5 to 25 mg daily. However, the use of spironolactone as a fourth drug is limited by tolerability issues in some patients, including the development of hyperkalemia in those with CKD with an eGFR <45 mL⋅min−1⋅1.73 m−2 or baseline serum potassium >4.5 mEq/L.378 Approximately 70% of adults with RH are candidates for mineralocorticoid receptor antagonists based on eGFR and serum K+, yet only a small fraction receive these effective agents.379 With prolonged use at higher doses, gynecomastia and erectile dysfunction in men and menstrual irregularities in women may limit the use of spironolactone. In such cases, eplerenone may be used successfully.380 Because of its shorter half-life compared with spironolactone, eplerenone should be administered twice daily for optimal effect.
The choice of ARB may also be important. Studies comparing various ARBs demonstrate clear advantages of certain agents in BP reduction over others. Specifically, 24-hour ABPM studies demonstrate that azilsartan medoximil provides on average an additional 4 to 8 mm Hg further SBP reduction over other ARBs (eg, valsartan and olmesartan) or the ACE inhibitor ramipril.381–383
Dihydropyridine CCBs such as amlodipine and nifedipine extended release are the most studied in the setting of hypertension. Other drugs in this class such as nicardipine and isradipine are administered 2 or 3 times daily and do not lend themselves to optimal adherence in RH. Some data suggest that long-acting formulations of nifedipine may have slightly greater antihypertensive actions than amlodipine but are associated with more edema.384–386 Nondihydropyridine CCBs such as verapamil also may be useful, but the evidence supporting their use in RH is limited.
Alteration of the dosing times (eg, to include a nocturnal dose) or using divided doses of drugs with half-lives of <12 to 15 hours may also improve BP control even when the drug theoretically has a pharmacodynamic effect of up to 24 hours in duration.387,388 Dosing at night of certain agents, for example, guanfacine, also helps reduce adverse effects such as drowsiness and may aid in sleeping.
The choice of a fifth drug (to add) depends on sympathetic drive as assessed in part by heart rate (Figure 3, step 4). In 2 post hoc analyses from large outcome trials, patients with heart rates >80 bpm had higher mortality.389,390 Thus, agents such as β-blockers or, if medically contraindicated, central α−2 agonists such as transdermal clonidine or guanfacine should be considered (Figure 3, step 4). Clonidine tablets should be avoided because of the need for frequent administration and the risk of rebound hypertension during periods of nonadherence and after discontinuation.
If BP is still not controlled with the above-described measures, the addition of hydralazine should be considered and combined with nitrates in cases of heart failure391,392 (Figure 3, step 5). Nitrates are preferred in this setting because they help restore calcium (Ca2+) cycling and cardiac contractile performance and control superoxide production in isolated cardiomyocytes.393 Moreover, hydralazine reduces nitrate tolerance in this setting.394 Note that hydralazine causes increased sympathetic tone and sodium avidity and therefore should be used in the presence of background appropriate diuretic and β-blocker therapy (Figure 3, step 5). Total daily doses of hydralazine should be <150 mg to avoid drug-induced systemic lupus erythematosus.
Lastly, minoxidil may be tried if hydralazine fails (Figure 3, step 6). Minoxidil is not well tolerated. It induces hirsutism, which in women can lead to discontinuation of the agent. Minoxidil must be given a minimum of twice daily and causes profound sodium avidity with fluid retention and increased sympathetic tone. Thus, a loop diuretic and β-blocker are required in virtually all cases. In this setting, however, minoxidil lowers BP effectively in most cases.395
Hypertension Specialist
The American Hypertension Specialists Certification Program has developed criteria for physicians to become Certified Hypertension Specialists (physicians only) and Certified Hypertension Clinicians (physicians and allied health providers). The requirements for both certification programs may be found at online.396
Hypertension specialists and clinicians have greater experience and knowledge managing the RH patient compared with most healthcare professionals. Retrospective studies have demonstrated improved BP control by hypertension specialists for patients referred for uncontrolled hypertension.366,397 When BP control is not progressing the way the primary care provider thinks it should, the patient should be referred.
Device-Based Treatment of RH
Human sympathetic nervous system dysfunction plays an important role in the development and progression of hypertension, heart failure, and CKD.398,399 In the 1940s, surgical sympathectomy demonstrated dramatic improvement in BP control and accompanying reduction in cardiac size, improved renal function, and a decrease in the rate of cerebrovascular events before the availability of antihypertensive drugs.400 These successes were soon diminished by the occurrence of severe orthostatic hypotension, as well as erectile dysfunction and bowel and bladder incontinence, and the increasing availability of antihypertensive agents that eventually led to the general discontinuation of lumbar sympathectomy in clinical practice by the mid-1970s. Nevertheless, these early studies formed the basis for research on modern devices for the treatment of RH, the majority of which reduce sympathetic nervous system outflow.
Renal Nerve Ablation
Renal nerve ablation has been studied and used in clinical practice outside the United States for several years; hence, until recently, most data reported on its effects have come from centers in Australia and Europe. Early studies in this field were quite promising and showed large reductions of clinic BP in patients failing to be controlled with 4 or 5 antihypertensive drugs.401,402 However, these studies were uncontrolled, and most did not study ambulatory BP as the primary end point. Later research showed that in uncontrolled studies, effect sizes using ABPM were much less (one-third) than the changes measured in the clinic setting.403
The first sham-controlled prospective randomized study in the field of renal ablation therapy, SYMPLICITY HTN-3, showed little to no effect of renal denervation therapy in a severely drug treatment-resistant population.404 The failure of this most rigorously designed randomized sham-control study to meet the efficacy end points raised several methodological concerns, including alterations in patient behavior in adherence to their multidrug pharmacological regimens and inadequate denervation resulting from technical failure of the catheter, the interventional proceduralist, or both.405
The methodological concerns arising from SYMPLICITY HTN-3 have led to several new catheters and new trial designs to evaluate the efficacy of more extensive renal denervation. Several studies are ongoing, but there are no conclusions at present. Data from animal studies clearly document a reduction in norepinephrine spillover rate and lowering of BP with both radiofrequency and ultrasonic catheters to induce renal artery denervation. Post hoc analyses from SYMPLICITY HTN-3 suggest that BP fell in patients with more complete ablation compared with those with lesser degrees of renal nerve ablation. In addition, the DENERHTN trial (Renal Denervation for Hypertension) recently demonstrated a statistically significant reduction in daytime ambulatory SBP (−5.9 mm Hg; P=0.03) in patients on 3 antihypertensive drugs randomized to renal denervation versus those on 4 antihypertensive drugs.406 At present, renal denervation procedures to treat RH have been discontinued in most countries unless patients are in research programs.
More recent data from the SPYRAL HTN-OFF MED study using a different catheter providing more extensive renal denervation showed a significant reduction in BP.407 However, the patients enrolled in this study did not have RH, and at best, this represents a proof-of-principle study demonstrating the role of the renal sympathetic nervous system in hypertension.
Carotid Baroreceptor Activation Therapy
Carotid baroreceptor activation therapy is a system that consists of baroreflex activation leads placed adjacent to the carotid sinus, an implantable pulse generator, and an external programming system. This modality electronically activates baroreceptors that signal the brain to orchestrate a multisystemic response for disorders associated with sympathetic overactivity such as hypertension, heart failure, and arrhythmias.408,409 Consequences of carotid sinus stimulation include reduced sympathetic nervous system activity and enhanced vagal activity. Hence, the heart rate slows, allowing greater left ventricular filling time and reducing cardiac workload and energy demands. In addition, arterial dilation occurs, reducing cardiac afterload and improving renal blood flow, which augments natriuresis. The degree of stimulation can be titrated in ≈4-minute periods to meet individual patient hemodynamic requirements.
The first results from a large randomized trial in 322 participants with RH410 failed to meet the primary end point of the trial, a composite of 5 individual efficacy and safety end points. However, the device was considered safe and efficacious long term in RH. This has led to refinements of the device and further research with single-sided catheters and a new Rheos system.408
The MobiusHD carotid bulb expansion device is a small endovascular implantable device that works by stretching the carotid artery at the bulb and activates baroreceptors to lower BP. Studies in Europe, CALM-FIM_EUR411 (Controlling and Lowering Blood Pressure With the MOBIUSHD), which was completed, and in the United States, CALM-FIM_US,412 which is ongoing, should provide results in 1 to 2 years. CALM-FIM_EUR has recently demonstrated in patients with RH that endovascular baroreflex amplification with the MobiusHD device substantially lowered BP with an acceptable safety profile.413
Devices on the Horizon for RH
In addition to renal nerve ablation therapy and baroreceptor activation therapy, other novel experimental devices are being explored for the treatment of RH with varying degrees of success and experience in patients. To date, most of the studies with these devices are uncontrolled and have small sample sizes. These include the central arteriovenous anastomosis ROX coupler device,414 the ReCor endovascular ultrasonic renal denervation device,415 and median nerve stimulation that uses an electro-acupuncture technique to reduce sympathetic outflow.416
In conclusion, device therapy for RH is investigational at present. Future research and development is encouraged because novel devices that are effective may be applicable to patients who are refractory to drug therapy or who have difficulty tolerating efficacious drugs.405
Research Challenges and Needs
Knowledge of the prevalence of true RH that accounts for inaccurate BP measurement, white-coat effect, and medication nonadherence is urgently needed. Reducing the BP target in the general hypertensive population will no doubt increase the prevalence of RH. Coupled with the documentation of prevalence, the prognosis of RH needs to be reconfirmed. It is probable that the characteristics of patients with RH may change with refinement of the definition using lower BP thresholds. Better understanding of the optimal BP target for RH is needed.
The pathophysiology of RH is not always clear in individual patients and urgently needs elucidation. This includes environmental influences, especially the role of dietary salt intake and the salt sensitivity of BP.417 Despite the sophisticated genetics technologies available today, the genetic basis for hypertension and RH continues to defy clear understanding. Except for the rare monogenetic causes, the pathophysiology of primary hypertension appears to be governed by a multiplicity of genes, each contributing only weak effects. Potential phenotypes to be explored by genetic analysis include RH at a young age, obesity-induced hypertension, salt-sensitive hypertension, and hypertension related to dysregulation of selected hormonal systems such as the renin-angiotensin-aldosterone system and the sympathetic nervous system. Identifying specific phenotypes of RH, which could be explored in greater depth for genetic contributions, would likely enhance our understanding of the origins of this complex disease process.
Once the prevalence of true RH is known, strategies for prevention should be considered. These might include earlier pharmacological treatment in the life course of those at high risk for RH and to prevent comorbidities that might make hypertension more difficult to treat such as vascular stiffening, arteriolar hypertrophy, and loss of renal function. Nonpharmacological strategies might include behavioral and sleep interventions.
Currently, the approach to BP control in RH has focused on the addition or substitution of drugs or drug classes based on meaningful pharmacological principles that are only partially successful in true RH. Future research should seek to improve personalized antihypertensive drug selection in patients with RH. For example, pharmacogenetics and pharmacogenomics are expected to provide a more rational and targeted approach to treatment. However, a prerequisite is to define in selected populations the role of genetic/genomic variation in BP responses to different pharmacological classes. This will take large well-controlled studies in which the participants are as well characterized phenotypically as possible. In the meantime, the development of new classes of pharmacological agents will be important to improve the currently limited selection of drug alternatives in RH. It will also be important to broaden the approach to treatment to better ways of effecting long-term beneficial lifestyle change, detecting and reversing nonadherence, and using team-based care and telehealth to reduce BP. Alternative approaches such as improving sleep quality may also help reduce the prevalence of RH.
Several studies suggest that the benefits of BP control are less in patients with RH than those without RH. The reason for the lesser efficacy of hypertension control in RH is unclear but could be related to differences in the 24-hour BP profile (eg, less nocturnal dipping or greater BP variability), differences in pathophysiological mechanisms, or greater degrees of subclinical target organ injury.
The role of device-based sympatholytic treatments, as with renal denervation and baroreceptor stimulation, awaits clarification. Although previously approved and used in different countries worldwide, validation of true benefit has not been confirmed in rigorous, double-blind comparisons with sham intervention. Such studies are ongoing with preliminary results expected in 2018. If independent benefit is confirmed, incorporation of device-based therapies into current treatment algorithms based on lifestyle and pharmacological therapies would be appropriate.
Disclosures
Writing Group Disclosures
| Writing Group Member | Employment | Research Grant | Other Research Support | Speakers’ Bureau/ Honoraria | Expert Witness | Ownership Interest | Consultant/ Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Robert M. Carey | University of Virginia Health System | NIH (2 research grants on signaling mechanisms of salt sensitivity of blood pressure and hypertension)† | None | None | None | None | None | University of Virginia (professor of medicine)† |
| David A. Calhoun | University of Alabama at Birmingham | ReCor Medical† | None | None | None | None | Novartis*; Mitsubishi Tanabe* | None |
| George L. Bakris | University of Chicago, ASH Comprehensive Hypertension Center | Bayer (International PI on renal outcome trial FIDELIO; all monies go to University of Chicago)*; Janssen (on steering committee of renal outcome trial CREDENCE; all monies go to University of Chicago)*; Vascular Dynamics (National Clinical Trial Steering Committee Member; all monies go to University of Chicago Medicine)† | None | None | None | None | Merck*; NovoNordisk* | None |
| Robert D. Brook | University of Michigan Medical School | None | None | None | None | None | None | None |
| Stacie L. Daugherty | University of Colorado School of Medicine | AHA†; NHLBI (R01 grant)† | None | None | None | None | None | None |
| Cheryl R. Dennison-Himmelfarb | Johns Hopkins University School of Nursing | Helene Fuld Health Trust (payment to her institution [JHU])†; NIH (payment to her institution [JHU])† | Preventive Cardiovascular Nurses Association (member Board of Director [unpaid])* | None | None | None | None | Johns Hopkins University (professor; associate dean for research)† |
| Brent M. Egan | University of South Carolina School of Medicine-Greenville Care Coordination Institute | None | None | Merck Sorono* | None | Pfizer (immediate family members)† | Medtronic* | None |
| John M. Flack | Southern Illinois University School of Medicine | Bayer (CKD/CVD prevention)†; Glaxo Smith-Kline (CKD)†; Indorsia, Quantam Genomics, ReCor Medical (hypertension)†; Vascular Dynamics (resistant hypertension)† | American College of Physicians (Board of Regents)†; American Hypertension Specialist Certification Program (president)† | None | None | None | Back Beat Hypertension*; Forest (unpaid)*; NuSirt Bipharma (unpaid)* | None |
| Samuel S. Gidding | Dr Samuel Gidding Familial Hypercholesterolemia Foundation Advocacy | NIH (research grant on which hypertension is studied)† | None | None | None | None | None | None |
| Eric Judd | University of Alabama at Birmingham School of Medicine | Baxter (PI for UAB site in prismacitrate 18 clinical trial)*; Bayer (PI for UAB site in FIDELIO clinical trial)*; NIH (recipient of a K23 grant from NIDDK)† | None | None | None | None | None | None |
| Daniel T. Lackland | Medical University of South Carolina | None | None | None | None | None | None | None |
| Cheryl L. Laffer | Vanderbilt University School of Medicine | NHLBI (coinvestigator on HL129941–02)* | None | None | None | None | None | NHLBI (coinvestigator on HL129941–02 grant)* |
| Christopher Newton-Cheh | Massachusetts General Hospital/ Harvard Medical School, Cardiovascular Research Center and Center for Human Genetic Research | None | None | None | None | None | None | None |
| Steven M. Smith | University of Florida College of Pharmacy | NHLBI (K01 Career Development Award [2018–2023])† | None | None | None | None | None | None |
| Sandra J. Taler | Mayo Clinic | None | None | None | None | None | None | None |
| Stephen C. Textor | Mayo Clinic | None | None | None | None | None | None | None |
| Tanya N. Turan | Medical University of South Carolina, MUSC Stroke Center | None | None | None | None | None | Boehringer ingelheim†;Pfizer† | None |
| William B. White | University of Connecticut, School of Medicine | None | None | None | Abbvie, inc† | None | Novartis Pharmaceuticals, inc* | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/ Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Frank C. Brosius | University of Arizona | None | None | None | None | None | None | None |
| Philip B. Gorelick | Michigan State University | None | None | DSMB for LCZ 696 in heart failure patients for preservation of cognition*; AbbVie (member, cardiovascular adjudication committee for SONAR study of blood pressure control in diabetic renal disease)* | None | None | None | None |
| Donald D. Heistad | University of Iowa | None | None | None | None | None | None | None |
| Rhian M. Touyz | University of Glasgow (United Kingdom) | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Supplementary Material
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
Publisher's Disclaimer: The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit http://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published correction appears in Hypertension. 2018;71:e140-e144]. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 2.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141 [DOI] [PubMed] [Google Scholar]
- 3.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals, part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6 [DOI] [PubMed] [Google Scholar]
- 4.Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, Kario K, Lurbe E, Manolis A, Mengden T, O’Brien E, Ohkubo T, Padfield P, Palatini P, Pickering TG, Redon J, Revera M, Ruilope LM, Shennan A, Staessen JA, Tisler A, Waeber B, Zanchetti A, Mancia G; ESH Working Group on Blood Pressure Monitoring. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779–785. doi: 10.1038/jhh.2010.54 [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, Bombelli M, Brambilla G, Facchetti R, Sega R, Toso E, Grassi G. Long-term prognostic value of white coat hypertension: an insight from diagnostic use of both ambulatory and home blood pressure measurements. Hypertension. 2013;62:168–174. doi: 10.1161/HYPERTENSIONAHA.111.00690 [DOI] [PubMed] [Google Scholar]
- 6.Stergiou GS, Asayama K, Thijs L, Kollias A, Niiranen TJ, Hozawa A, Boggia J, Johansson JK, Ohkubo T, Tsuji I, Jula AM, Imai Y, Staessen JA; on behalf of the International Database on HOme blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63:675–682. doi: 10.1161/HYPERTENSIONAHA.113.02741 [DOI] [PubMed] [Google Scholar]
- 7.Franklin SS, Thijs L, Asayama K, Li Y, Hansen TW, Boggia J, Jacobs L, Zhang Z, Kikuya M, Björklund-Bodegård K, Ohkubo T, Yang WY, Jeppesen J, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Filipovský J, Imai Y, Wang JG, O’Brien E, Staessen JA; IDACO Investigators. The cardiovascular risk of white-coat hypertension. J Am Coll Cardiol. 2016;68:2033–2043. doi: 10.1016/j.jacc.2016.08.035 [DOI] [PubMed] [Google Scholar]
- 8.Hameed MA, Tebbit L, Jacques N, Thomas M, Dasgupta I. Non-adherence to antihypertensive medication is very common among resistant hypertensives: results of a directly observed therapy clinic. J Hum Hypertens. 2016;30:83–89. doi: 10.1038/jhh.2015.38 [DOI] [PubMed] [Google Scholar]
- 9.Schulz M, Krueger K, Schuessel K, Friedland K, Laufs U, Mueller WE, Ude M. Medication adherence and persistence according to different antihypertensive drug classes: a retrospective cohort study of 255,500 patients. Int J Cardiol. 2016;220:668–676. doi: 10.1016/j.ijcard.2016.06.263 [DOI] [PubMed] [Google Scholar]
- 10.Elliott WJ. What factors contribute to the inadequate control of elevated blood pressure? J Clin Hypertens (Greenwich). 2008;10(suppl 1):20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Laan DM, Elders PJM, Boons CCLM, Beckeringh JJ, Nijpels G, Hugtenburg JG Factors associated with antihypertensive medication nonadherence: a systematic review. J Hum Hypertens. 2017;31:687–694. doi: 10.1038/jhh.2017.48 [DOI] [PubMed] [Google Scholar]
- 12.Pimenta E, Calhoun DA. Resistant hypertension: incidence, prevalence, and prognosis. Circulation. 2012;125:1594–1596. doi: 10.1161/CIRCULATIONAHA.112.097345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988–2008. Circulation. 2011:124;1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011:57;1076–1080. [DOI] [PubMed] [Google Scholar]
- 15.Diaz KM, Booth JN 3rd, Calhoun DA, Irvin MR, Howard G, Safford MM, Muntner P, Shimbo D. Healthy lifestyle factors and risk of cardiovascular events and mortality in treatment-resistant hypertension: the Reasons for Geographic and Racial Differences in Stroke study. Hypertension. 2014;64:465–471. doi: 10.1161/HYPERTENSIONAHA.114.03565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanner RM, Calhoun DA, Bell EK, Bowling CB, Gutiérrez OM, Irvin MR, Lackland DT, Oparil S, Warnock D, Muntner P. Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol. 2013;8:1583–1590. doi: 10.2215/CJN.00550113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borghi C, Tubach F, De Backer G, Dallongeville J, Guallar E, Medina J, Perk J, Roy C, Banegas JR, Rodriguez-Artalejo F, Halcox JP. Lack of control of hypertension in primary cardiovascular disease prevention in Europe: results from the EURIKA study. Int J Cardiol. 2016;218:83–88. doi: 10.1016/j.ijcard.2016.05.044 [DOI] [PubMed] [Google Scholar]
- 18.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A, Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011:57;898–902. [DOI] [PubMed] [Google Scholar]
- 19.Thomas G, Xie D, Chen HY, Anderson AH, Appel LJ, Bodana S, Brecklin CS, Drawz P, Flack JM, Miller ER 3rd, Steigerwalt SP, Townsend RR, Weir MR, Wright JT Jr, Rahman M; CRIC Study Investigators. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the Chronic Renal Insufficiency Cohort Study. Hypertension. 2016;67:387–396. doi: 10.1161/HYPERTENSIONAHA.115.06487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan BM, Zhao Y, Li J, Brzezinski WA, Todoran TM, Brook RD, Calhoun DA. Prevalence of optimal treatment regimens in patients with apparent treatment-resistant hypertension based on office blood pressure in a community-based practice network. Hypertension. 2013;62:691–697. doi: 10.1161/HYPERTENSIONAHA.113.01448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, Black HR, Kostis JB, Probstfield JL, Whelton PK, Rahman M; for the ALLHAT Collaborative Research Group. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014;64:1012–1021. doi: 10.1161/HYPERTENSIONAHA.114.03850 [DOI] [PubMed] [Google Scholar]
- 22.Gupta AK, Nasothimiou EG, Chang CL, Sever PS, Dahlof B, Poulter NR; ASCOT Investigators. Baseline predictors of resistant hypertension in the Anglo-Scandinavian Cardiac Outcome Trial (ASCOT): a risk score to identify those at high-risk. J Hypertens. 2011;29:2004–2013. doi: 10.1097/HJH.0b013e32834a8a42 [DOI] [PubMed] [Google Scholar]
- 23.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, O’Connor PJ, Selby JV, Ho PM. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182 [DOI] [PubMed] [Google Scholar]
- 25.Jamerson K, Bakris GL, Dahlof B, Pitt B, Velazquez E, Gupte J, Lefkowitz M, Hester A, Shi V, Kjeldsen SE, Cushman W, Papademetriou V, Weber M; ACCOMPLISH Investigators. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Press. 2007;16:80–86. doi: 10.1080/08037050701395571 [DOI] [PubMed] [Google Scholar]
- 26.Smith SM, Gong Y, Handberg E, Messerli FH, Bakris GL, Ahmed A, Bavry AA, Pepine CJ, Cooper-Dehoff RM. Predictors and outcomes of resistant hypertension among patients with coronary artery disease and hypertension. J Hypertens. 2014;32:635–643. doi: 10.1097/HJH.0000000000000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung CY, Wang KY, Wu TJ, Hsieh YC, Huang JL, Loh el-W, Lin CH. Resistant hypertension, patient characteristics, and risk of stroke. PLoS One. 2014;9:e104362. doi: 10.1371/journal.pone.0104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irvin MR, Booth JN 3rd, Shimbo D, Lackland DT, Oparil S, Howard G, Safford MM, Muntner P, Calhoun DA. Apparent treatment-resistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. J Am Soc Hypertens. 2014;8:405–413. doi: 10.1016/j.jash.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim JJ, Bhandari SK, Shi J, Reynolds K, Calhoun DA, Kalantar-Zadeh K, Jacobsen SJ. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015;88:622–632. doi: 10.1038/ki.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsioufis C, Kasiakogias A, Kordalis A, Dimitriadis K, Thomopoulos C, Tsiachris D, Vasileiou P, Doumas M, Makris T, Papademetriou V, Kallikazaros I, Bakris G, Stefanadis C. Dynamic resistant hypertension patterns as predictors of cardiovascular morbidity: a 4-year prospective study. J Hypertens. 2014;32:415–422. doi: 10.1097/HJH.0000000000000023 [DOI] [PubMed] [Google Scholar]
- 31.Pierdomenico SD, Lapenna D, Bucci A, Di Tommaso R, Di Mascio R, Manente BM, Caldarella MP, Neri M, Cuccurullo F, Mezzetti A. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18:1422–1428. doi: 10.1016/j.amjhyper.2005.05.014 [DOI] [PubMed] [Google Scholar]
- 32.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168:2340–2346. doi: 10.1001/archinte.168.21.2340 [DOI] [PubMed] [Google Scholar]
- 33.Muxfeldt ES, Cardoso CR, Salles GF. Prognostic value of nocturnal blood pressure reduction in resistant hypertension. Arch Intern Med. 2009;169:874–880. doi: 10.1001/archinternmed.2009.68 [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Huo T, Delia Johnson B, Bittner V, Kelsey SF, Vido Thompson D, Noel Bairey Merz C, Pepine CJ, Cooper-Dehoff RM. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: a report from the NHLBI-sponsored WISE Study. J Am Heart Assoc. 2014;3:e000660. doi: 10.1161/JAHA.113.000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bangalore S, Fayyad R, Laskey R, Demicco DA, Deedwania P, Kostis JB, Messerli FH; Treating to New Targets Steering Committee and Investigators. Prevalence, predictors, and outcomes in treatment-resistant hypertension in patients with coronary disease. Am J Med. 2014;127:71–81.e1. doi: 10.1016/j.amjmed.2013.07.038 [DOI] [PubMed] [Google Scholar]
- 36.Jin CN, Liu M, Sun JP, Fang F, Wen YN, Yu CM, Lee AP. The prevalence and prognosis of resistant hypertension in patients with heart failure. PLoS One. 2014;9:e114958. doi: 10.1371/journal.pone.0114958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egan BM, Kai B, Wagner CS, Henderson JH, Chandler AH, Sinopoli A. Blood pressure control provides less cardiovascular protection in adults with than without apparent treatment-resistant hypertension. J Clin Hypertens (Greenwich). 2016;18:817–824. doi: 10.1111/jch.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sim JJ, Bhandari SK, Shi J, Liu IL, Calhoun DA, McGlynn EA, Kalantar-Zadeh K, Jacobsen SJ. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc. 2013;88:1099–1107. doi: 10.1016/j.mayocp.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a Veterans Affairs population. J Clin Hypertens (Greenwich). 2014;16:741–745. doi: 10.1111/jch.12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakhtar O, Ference BA, Hedquist LA, Levy PD, Flack JM. Relationship of resistant hypertension and treatment outcomes with total arterial compliance in a predominantly African American hypertensive cohort. J Clin Hypertens (Greenwich). 2012;14:618–622. doi: 10.1111/j.1751-7176.2012.00653.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holecki M, Duława J, Chudek J. Resistant hypertension in visceral obesity. Eur J Intern Med. 2012;23:643–648. doi: 10.1016/j.ejim.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 42.Cuspidi C, Macca G, Sampieri L, Michev I, Salerno M, Fusi V, Severgnini B, Meani S, Magrini F, Zanchetti A. High prevalence of cardiac and extracardiac target organ damage in refractory hypertension. J Hypertens. 2001;19:2063–2070. [DOI] [PubMed] [Google Scholar]
- 43.Oliveras A, Armario P, Hernández-Del Rey R, Arroyo JA, Poch E, Larrousse M, Roca-Cusachs A, de la Sierra A. Urinary albumin excretion is associated with true resistant hypertension. J Hum Hypertens. 2010;24:27–33. doi: 10.1038/jhh.2009.35 [DOI] [PubMed] [Google Scholar]
- 44.Oliveras A, de la Sierra A. Resistant hypertension: patient characteristics, risk factors, co-morbidities and outcomes. J Hum Hypertens. 2014;28:213–217. doi: 10.1038/jhh.2013.77 [DOI] [PubMed] [Google Scholar]
- 45.Demede M, Pandey A, Zizi F, Bachmann R, Donat M, McFarlane SI, Jean-Louis G, Ogedegbe G. Resistant hypertension and obstructive sleep apnea in the primary-care setting. Int J Hypertens. 2011;2011:340929. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhandari SK, Shi J, Molnar MZ, Rasgon SA, Derose SF, Kovesdy CP, Calhoun DA, Kalantar-Zadeh K, Jacobsen SJ, Sim JJ. Comparisons of sleep apnoea rate and outcomes among patients with resistant and nonresistant hypertension. Respirology. 2016;21:1486–1492. doi: 10.1111/resp.12840 [DOI] [PubMed] [Google Scholar]
- 47.Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, Oparil S, Cofield SS, Calhoun DA. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–368. [PMC free article] [PubMed] [Google Scholar]
- 48.Pedrosa RP, Drager LF, Gonzaga CC, Sousa MG, de Paula LK, Amaro AC, Amodeo C, Bortolotto LA, Krieger EM, Bradley TD, Lorenzi-Filho G. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788 [DOI] [PubMed] [Google Scholar]
- 49.Pimenta E, Stowasser M, Gordon RD, Harding SM, Batlouni M, Zhang B, Oparil S, Calhoun DA. Increased dietary sodium is related to severity of obstructive sleep apnea in patients with resistant hypertension and hyperaldosteronism. Chest. 2013;143:978–983. doi: 10.1378/chest.12-0802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman O, Bradley TD, Ruttanaumpawan P, Logan AG. Independent association of drug-resistant hypertension to reduced sleep duration and efficiency. Am J Hypertens. 2010;23:174–179. doi: 10.1038/ajh.2009.220 [DOI] [PubMed] [Google Scholar]
- 51.De la Sierra A, Larrousse M, Oliveras A, Armario P, Hernàndez-del Rey R, Poch E,Roca-Cusachs A. Abnormalities of vascular function in resistant hypertension. Blood Press. 2012;21:104–109. [DOI] [PubMed] [Google Scholar]
- 52.Quinaglia T, Martins LC, Figueiredo VN, Santos RC, Yugar-Toledo JC, Martin JF, Demacq C, Pimenta E, Calhoun DA, Moreno H Jr. Nondipping pattern relates to endothelial dysfunction in patients with uncontrolled resistant hypertension. J Hum Hypertens. 2011;25:656–664. doi: 10.1038/jhh.2011.43 [DOI] [PubMed] [Google Scholar]
- 53.Salles GF, Ribeiro FM, Guimarâes GM, Muxfeldt ES, Cardoso CR. A reduced heart rate variability is independently associated with a blunted nocturnal blood pressure fall in patients with resistant hypertension. J Hypertens. 2014;32:644–651. doi: 10.1097/HJH.0000000000000068 [DOI] [PubMed] [Google Scholar]
- 54.Kansui Y, Matsumura K, Kida H, Sakata S, Ohtsubo T, Ibaraki A, Kitazono T Clinical characteristics of resistant hypertension evaluated by ambulatory blood pressure monitoring. Clin Exp Hypertens. 2014;36:454–458. doi: 10.3109/10641963.2013.846360 [DOI] [PubMed] [Google Scholar]
- 55.Cuspidi C, Sala C, Tadic M, Gherbesi E, De Giorgi A, Grassi G, Mancia G. Clinical and prognostic significance of a reverse dipping pattern on ambulatory monitoring: an updated review. J Clin Hypertens (Greenwich). 2017;19:713–721. doi: 10.1111/jch.13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kario K, Bhatt DL, Kandzari DE, Brar S, Flack JM, Gilbert C, Oparil S, Robbins M, Townsend RR, Bakris G. Impact of renal denervation on patients with obstructive sleep apnea and resistant hypertension: insights from the SYMPLICITY HTN-3 trial. Circ J. 2016;80:1404–1412. doi: 10.1253/circj.CJ-16-0035 [DOI] [PubMed] [Google Scholar]
- 57.Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–2226. [DOI] [PubMed] [Google Scholar]
- 58.Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ, Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension. Hypertension. 2009;54:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mendes M, Dubourg J, Blanchard A, Bergerot D, Courand PY, Forni V, Frank M, Bobrie G, Menard J, Azizi M. Copeptin is increased in resistant hypertension. J Hypertens. 2016;34:2458–2464. doi: 10.1097/HJH.0000000000001106 [DOI] [PubMed] [Google Scholar]
- 60.Hwang AY, Dave C, Smith SM. Trends in antihypertensive medication use among US patients with resistant hypertension, 2008 to 2014. Hypertension. 2016;68:1349–1354. doi: 10.1161/HYPERTENSIONAHA.116.08128 [DOI] [PubMed] [Google Scholar]
- 61.Grigoryan L, Pavlik VN, Hyman DJ. Characteristics, drug combinations and dosages of primary care patients with uncontrolled ambulatory blood pressure and high medication adherence. J Am Soc Hypertens. 2013;7:471–476. doi: 10.1016/j.jash.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hermida RC, Ríos MT, Crespo JJ, Moyá A, Domínguez-Sardiña M, Otero A, Sánchez JJ, Mojón A, Fernández JR, Ayala DE; Hygia Project Investigators. Treatment-time regimen of hypertension medications significantly affects ambulatory blood pressure and clinical characteristics of patients with resistant hypertension. Chronobiol Int. 2013;30:192–206. doi: 10.3109/07420528.2012.701460 [DOI] [PubMed] [Google Scholar]
- 63.Hottenga JJ, Boomsma DI, Kupper N, Posthuma D, Snieder H, Willemsen G, de Geus EJ. Heritability and stability of resting blood pressure. Twin Res Hum Genet. 2005;8:499–508. doi: 10.1375/183242705774310123 [DOI] [PubMed] [Google Scholar]
- 64.Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41:1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D [DOI] [PubMed] [Google Scholar]
- 65.Kupper N, Willemsen G, Riese H, Posthuma D, Boomsma DI, de Geus EJ. Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension. 2005;45:80–85. doi: 10.1161/01.HYP.0000149952.84391.54 [DOI] [PubMed] [Google Scholar]
- 66.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, Cupples LA, Myers RH. Evidence for a gene influencing blood pressure on chromosome 17: genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham Heart Study. Hypertension. 2000;36:477–483. [DOI] [PubMed] [Google Scholar]
- 67.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM; ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404. [DOI] [PubMed] [Google Scholar]
- 68.Howard VJ, Tanner RM, Anderson A, Irvin MR, Calhoun DA, Lackland DT, Oparil S, Muntner P. Apparent treatment-resistant hypertension among individuals with history of stroke or transient ischemic attack. Am J Med. 2015;128:707–714.e2. doi: 10.1016/j.amjmed.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shallcross AJ, Butler M, Tanner RM, Bress AP, Muntner P, Shimbo D, Ogedegbe G, Sims M, Spruill TM. Psychosocial correlates of apparent treatment-resistant hypertension in the Jackson Heart Study. J Hum Hypertens. 2017;31:474–478. doi: 10.1038/jhh.2016.100 [DOI] [PubMed] [Google Scholar]
- 70.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Döring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O’Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O’Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvänen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dörr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Völker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Völzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB; Wellcome Trust Case Control Consortium. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Köttgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O’Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.International Consortium for Blood Pressure Genome-Wide Association, Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sober S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjögren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimäki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND; CARDIoGRAM Consortium; CKDGen Consortium; KidneyGen Consortium; EchoGen Consortium; CHARGE-HF Consortium, Aspelund T, Garcia M, Chang YP, O’Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kähönen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Köttgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grässler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stančáková A, Raffel LJ, Yao J, Kathiresan S, O’Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG,Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikäinen LP, Soininen P, Tukiainen T, Würtz P, Ong RT, Dörr M, Kroemer HK, Völker U, Völzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Järvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franceschini N, Fox E, Zhang Z, Edwards TL, Nalls MA, Sung YJ, Tayo BO, Sun YV, Gottesman O, Adeyemo A, Johnson AD, Young JH, Rice K, Duan Q, Chen F, Li Y, Tang H, Fornage M, Keene KL, Andrews JS, Smith JA, Faul JD, Guangfa Z, Guo W, Liu Y, Murray SS, Musani SK, Srinivasan S, Velez Edwards DR, Wang H, Becker LC, Bovet P, Bochud M, Broeckel U, Burnier M, Carty C, Chasman DI, Ehret G, Chen WM, Chen G, Chen W, Ding J, Dreisbach AW, Evans MK, Guo X, Garcia ME, Jensen R, Keller MF, Lettre G, Lotay V, Martin LW, Moore JH, Morrison AC, Mosley TH, Ogunniyi A, Palmas W, Papanicolaou G, Penman A, Polak JF, Ridker PM, Salako B, Singleton AB, Shriner D, Taylor KD, Vasan R, Wiggins K, Williams SM, Yanek LR, Zhao W, Zonderman AB, Becker DM, Berenson G, Boerwinkle E, Bottinger E, Cushman M, Eaton C, Nyberg F, Heiss G, Hirschhron JN, Howard VJ, Karczewsk KJ, Lanktree MB, Liu K, Liu Y, Loos R, Margolis K, Snyder M, Psaty BM, Schork NJ, Weir DR, Rotimi CN, Sale MM, Harris T, Kardia SL, Hunt SC, Arnett D, Redline S, Cooper RS, Risch NJ, Rao DC, Rotter JI, Chakravarti A, Reiner AP, Levy D, Keating BJ, Zhu X; Asian Genetic Epidemiology Network Consortium. Genome-wide association analysis of blood-pressure traits in African-ancestry individuals reveals common associated genes in African and non-African populations. Am J Hum Genet. 2013;93:545–554. doi: 10.1016/j.ajhg.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, Tay WT, Chen CH, Zhang Y, Yamamoto K, Katsuya T, Yokota M, Kim YJ, Ong RT, Nabika T, Gu D, Chang LC, Kokubo Y, Huang W, Ohnaka K, Yamori Y, Nakashima E, Jaquish CE, Lee JY, Seielstad M, Isono M, Hixson JE, Chen YT, Miki T, Zhou X, Sugiyama T, Jeon JP, Liu JJ, Takayanagi R, Kim SS, Aung T, Sung YJ, Zhang X, Wong TY, Han BG, Kobayashi S, Ogihara T, Zhu D, Iwai N, Wu JY, Teo YY, Tai ES, Cho YS, He J. Metaanalysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43:531–538. doi: 10.1038/ng.834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fox ER, Young JH, Li Y, Dreisbach AW, Keating BJ, Musani SK, Liu K, Morrison AC, Ganesh S, Kutlar A, Ramachandran VS, Polak JF, Fabsitz RR, Dries DL, Farlow DN, Redline S, Adeyemo A, Hirschorn JN, Sun YV, Wyatt SB, Penman AD, Palmas W, Rotter JI, Townsend RR, Doumatey AP, Tayo BO, Mosley TH Jr, Lyon HN, Kang SJ, Rotimi CN, Cooper RS, Franceschini N, Curb JD, Martin LW, Eaton CB, Kardia SL, Taylor HA, Caulfield MJ, Ehret GB, Johnson T, Chakravarti A, Zhu X, Levy D; International Consortium for Blood Pressure Genome-Wide Association Studies (ICBP-GWAS). Association of genetic variation with systolic and diastolic blood pressure among African Americans: the Candidate Gene Association Resource study. Hum Mol Genet. 2011;20:2273–2284. doi: 10.1093/hmg/ddr092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganesh SK, Chasman DI, Larson MG, Guo X, Verwoert G, Bis JC, Gu X, Smith AV, Yang ML, Zhang Y, Ehret G, Rose LM, Hwang SJ, Papanicolau GJ, Sijbrands EJ, Rice K, Eiriksdottir G, Pihur V, Ridker PM, Vasan RS, Newton-Cheh C, Raffel LJ, Amin N, Rotter JI, Liu K, Launer LJ, Xu M, Caulfield M, Morrison AC, Johnson AD, Vaidya D, Dehghan A, Li G, Bouchard C, Harris TB, Zhang H, Boerwinkle E, Siscovick DS, Gao W, Uitterlinden AG, Rivadeneira F, Hofman A, Willer CJ, Franco OH, Huo Y, Witteman JC, Munroe PB, Gudnason V, Palmas W, van Duijn C, Fornage M, Levy D, Psaty BM, Chakravarti A; Global Blood Pressure Genetics Consortium. Effects of long-term averaging of quantitative blood pressure traits on the detection of genetic associations. Am J Hum Genet. 2014;95:49–65. doi: 10.1016/j.ajhg.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Padmanabhan S, Melander O, Johnson T, Di Blasio AM, Lee WK, Gentilini D, Hastie CE, Menni C, Monti MC, Delles C, Laing S, Corso B, Navis G, Kwakernaak AJ, van der Harst P, Bochud M, Maillard M, Burnier M, Hedner T, Kjeldsen S, Wahlstrand B, Sjögren M, Fava C, Montagnana M, Danese E, Torffvit O, Hedblad B, Snieder H, Connell JM, Brown M, Samani NJ, Farrall M, Cesana G, Mancia G, Signorini S, Grassi G, Eyheramendy S, Wichmann HE, Laan M, Strachan DP, Sever P, Shields DC, Stanton A, Vollenweider P, Teumer A, Völzke H, Rettig R, Newton-Cheh C, Arora P, Zhang F, Soranzo N, Spector TD, Lucas G, Kathiresan S, Siscovick DS, Luan J, Loos RJ, Wareham NJ, Penninx BW, Nolte IM, McBride M, Miller WH, Nicklin SA, Baker AH, Graham D, McDonald RA, Pell JP, Sattar N, Welsh P, Munroe P, Caulfield MJ, Zanchetti A, Dominiczak AF; Global BPgen Consortium. Genomewide association study of blood pressure extremes identifies variant near UMOD associated with hypertension. PLoS Genet. 2010;6:e1001177. doi: 10.1371/journal.pgen.1001177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salvi E, Kutalik Z, Glorioso N, Benaglio P, Frau F, Kuznetsova T, Arima H, Hoggart C, Tichet J, Nikitin YP, Conti C, Seidlerova J, Tikhonoff V, Stolarz-Skrzypek K, Johnson T, Devos N, Zagato L, Guarrera S, Zaninello R, Calabria A, Stancanelli B, Troffa C, Thijs L, Rizzi F, Simonova G, Lupoli S, Argiolas G, Braga D, D’Alessio MC, Ortu MF, Ricceri F, Mercurio M, Descombes P, Marconi M, Chalmers J, Harrap S, Filipovsky J, Bochud M, Iacoviello L, Ellis J, Stanton AV, Laan M, Padmanabhan S, Dominiczak AF, Samani NJ, Melander O, Jeunemaitre X, Manunta P, Shabo A, Vineis P, Cappuccio FP, Caulfield MJ, Matullo G, Rivolta C, Munroe PB, Barlassina C, Staessen JA, Beckmann JS, Cusi D. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension. 2012;59:248–255. doi: 10.1161/HYPERTENSIONAHA.111.181990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, Bochud M, Rice KM, Henneman P, Smith AV, Ehret GB, Amin N, Larson MG, Mooser V, Hadley D, Dörr M, Bis JC, Aspelund T, Esko T, Janssens AC, Zhao JH, Heath S, Laan M, Fu J, Pistis G, Luan J, Arora P, Lucas G, Pirastu N, Pichler I, Jackson AU, Webster RJ, Zhang F, Peden JF, Schmidt H, Tanaka T, Campbell H, Igl W, Milaneschi Y, Hottenga JJ, Vitart V, Chasman DI, Trompet S, Bragg-Gresham JL, Alizadeh BZ, Chambers JC, Guo X, Lehtimäki T, Kühnel B, Lopez LM, Polasek O, Boban M, Nelson CP, Morrison AC, Pihur V, Ganesh SK, Hofman A, Kundu S, Mattace- Raso FU, Rivadeneira F, Sijbrands EJ, Uitterlinden AG, Hwang SJ, Vasan RS, Wang TJ, Bergmann S, Vollenweider P, Waeber G, Laitinen J, Pouta A, Zitting P, McArdle WL, Kroemer HK, Völker U, Völzke H, Glazer NL, Taylor KD, Harris TB, Alavere H, Haller T, Keis A, Tammesoo ML, Aulchenko Y, Barroso I, Khaw KT, Galan P, Hercberg S, Lathrop M, Eyheramendy S, Org E, Sober S, Lu X, Nolte IM, Penninx BW, Corre T, Masciullo C, Sala C, Groop L, Voight BF, Melander O, O’Donnell CJ, Salomaa V, d’Adamo AP, Fabretto A, Faletra F, Ulivi S, Del Greco F, Facheris M, Collins FS, Bergman RN, Beilby JP, Hung J, Musk AW, Mangino M, Shin SY, Soranzo N, Watkins H, Goel A, Hamsten A, Gider P, Loitfelder M, Zeginigg M, Hernandez D, Najjar SS, Navarro P, Wild SH, Corsi AM, Singleton A, de Geus EJ, Willemsen G, Parker AN, Rose LM, Buckley B, Stott D, Orru M, Uda M, van der Klauw MM, Zhang W, Li X, Scott J, Chen YD, Burke GL, Kähönen M, Viikari J, Döring A, Meitinger T, Davies G, Starr JM, Emilsson V, Plump A, Lindeman JH, Hoen PA, König IR, Felix JF, Clarke R, Hopewell JC, Ongen H, Breteler M, Debette S, Destefano AL, Fornage M, Mitchell GF, Smith NL, Holm H, Stefansson K, Thorleifsson G, Thorsteinsdottir U, Samani NJ, Preuss M, Rudan I, Hayward C, Deary IJ, Wichmann HE, Raitakari OT, Palmas W, Kooner JS, Stolk RP, Jukema JW, Wright AF, Boomsma DI, Bandinelli S, Gyllensten UB, Wilson JF, Ferrucci L, Schmidt R, Farrall M, Spector TD, Palmer LJ, Tuomilehto J, Pfeufer A, Gasparini P, Siscovick D, Altshuler D, Loos RJ, Toniolo D, Snieder H, Gieger C, Meneton P, Wareham NJ, Oostra BA, Metspalu A, Launer L, Rettig R, Strachan DP, Beckmann JS, Witteman JC, Erdmann J, van Dijk KW, Boerwinkle E, Boehnke M, Ridker PM, Jarvelin MR, Chakravarti A, Abecasis GR, Gudnason V, Newton-Cheh C, Levy D, Munroe PB, Psaty BM, Caulfield MJ, Rao DC, Tobin MD, Elliott P, van Duijn CM; LifeLines Cohort Study; EchoGen consortium; AortaGen Consortium; CHARGE Consortium Heart Failure Working Group; KidneyGen Consortium; CKDGen Consortium; Cardiogenics Consortium; CardioGram. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–1011. doi: 10.1038/ng.922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ganesh SK, Tragante V, Guo W, Guo Y, Lanktree MB, Smith EN, Johnson T, Castillo BA, Barnard J, Baumert J, Chang YP, Elbers CC, Farrall M, Fischer ME, Franceschini N, Gaunt TR, Gho JM, Gieger C, Gong Y, Isaacs A, Kleber ME, Mateo Leach I, McDonough CW, Meijs MF, Mellander O, Molony CM, Nolte IM, Padmanabhan S, Price TS, Rajagopalan R, Shaffer J, Shah S, Shen H, Soranzo N, van der Most PJ, Van Iperen EP, Van Setten J, Van Setten JA, Vonk JM, Zhang L, Beitelshees AL, Berenson GS, Bhatt DL, Boer JM, Boerwinkle E, Burkley B, Burt A, Chakravarti A, Chen W, Cooper-Dehoff RM, Curtis SP, Dreisbach A, Duggan D, Ehret GB, Fabsitz RR, Fornage M, Fox E, Furlong CE, Gansevoort RT, Hofker MH, Hovingh GK, Kirkland SA, Kottke-Marchant K, Kutlar A, Lacroix AZ, Langaee TY, Li YR, Lin H, Liu K, Maiwald S, Malik R, Murugesan G, Newton-Cheh C, O’Connell JR, Onland-Moret NC, Ouwehand WH, Palmas W, Penninx BW, Pepine CJ, Pettinger M, Polak JF, Ramachandran VS, Ranchalis J, Redline S, Ridker PM, Rose LM, Scharnag H, Schork NJ, Shimbo D, Shuldiner AR, Srinivasan SR, Stolk RP, Taylor HA, Thorand B, Trip MD, van Duijn CM, Verschuren WM, Wijmenga C, Winkelmann BR, Wyatt S, Young JH, Boehm BO, Caulfield MJ, Chasman DI, Davidson KW, Doevendans PA, Fitzgerald GA, Gums JG, Hakonarson H, Hillege HL, Illig T, Jarvik GP, Johnson JA, Kastelein JJ, Koenig W, März W, Mitchell BD, Murray SS, Oldehinkel AJ, Rader DJ, Reilly MP, Reiner AP, Schadt EE, Silverstein RL, Snieder H, Stanton AV, Uitterlinden AG, van der Harst P, van der Schouw YT, Samani NJ, Johnson AD, Munroe PB, de Bakker PI, Zhu X, Levy D, Keating BJ, Asselbergs FW; CARDIOGRAM, METASTROKE; LifeLines Cohort Study. Loci influencing blood pressure identified using a cardiovascular gene-centric array. Hum Mol Genet. 2013;22:1663–1678. doi: 10.1093/hmg/dds555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho JE, Levy D, Rose L, Johnson AD, Ridker PM, Chasman DI. Discovery and replication of novel blood pressure genetic loci in the Women’s Genome Health Study. J Hypertens. 2011;29:62–69. doi: 10.1097/HJH.0b013e3283406927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson AD, Newton-Cheh C, Chasman DI, Ehret GB, Johnson T, Rose L, Rice K, Verwoert GC, Launer LJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, Caulfield M, van Duijn CM, Ridker PM, Munroe PB, Levy D; on behalf of the Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium; Global BPgen Consortium; Women’s Genome Health Study. Association of hypertension drug target genes with blood pressure and hypertension in 86,588 individuals. Hypertension. 2011;57:903–910. doi: 10.1161/HYPERTENSIONAHA.110.158667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson T, Gaunt TR, Newhouse SJ, Padmanabhan S, Tomaszewski M, Kumari M, Morris RW, Tzoulaki I, O’Brien ET, Poulter NR, Sever P, Shields DC, Thom S, Wannamethee SG, Whincup PH, Brown MJ, Connell JM, Dobson RJ, Howard PJ, Mein CA, Onipinla A, Shaw-Hawkins S, Zhang Y, Davey Smith G, Day IN, Lawlor DA, Goodall AH; Cardiogenics Consortium, Fowkes FG, Abecasis GR, Elliott P, Gateva V; Global BPgen Consortium, Braund PS, Burton PR, Nelson CP, Tobin MD, van der Harst P, Glorioso N, Neuvrith H, Salvi E, Staessen JA, Stucchi A, Devos N, Jeunemaitre X, Plouin PF, Tichet J, Juhanson P, Org E, Putku M, Sober S, Veldre G, Viigimaa M, Levinsson A, Rosengren A, Thelle DS, Hastie CE, Hedner T, Lee WK, Melander O, Wahlstrand B, Hardy R, Wong A, Cooper JA, Palmen J, Chen L, Stewart AF, Wells GA, Westra HJ, Wolfs MG, Clarke R, Franzosi MG, Goel A, Hamsten A, Lathrop M, Peden JF, Seedorf U, Watkins H, Ouwehand WH, Sambrook J, Stephens J, Casas JP, Drenos F, Holmes MV, Kivimaki M, Shah S, Shah T, Talmud PJ, Whittaker J, Wallace C, Delles C, Laan M, Kuh D, Humphries SE, Nyberg F, Cusi D, Roberts R, Newton-Cheh C, Franke L, Stanton AV, Dominiczak AF, Farrall M, Hingorani AD, Samani NJ, Caulfield MJ, Munroe PB. Blood pressure loci identified with a gene-centric array. Am J Hum Genet. 2011;89:688–700. doi: 10.1016/j.ajhg.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takeuchi F, Isono M, Katsuya T, Yamamoto K, Yokota M, Sugiyama T, Nabika T, Fujioka A, Ohnaka K, Asano H, Yamori Y, Yamaguchi S, Kobayashi S, Takayanagi R, Ogihara T, Kato N. Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation. 2010;121:2302–2309. doi: 10.1161/CIRCULATIONAHA.109.904664 [DOI] [PubMed] [Google Scholar]
- 85.Tomaszewski M, Debiec R, Braund PS, Nelson CP, Hardwick R, Christofidou P, Denniff M, Codd V, Rafelt S, van der Harst P, Waterworth D, Song K, Vollenweider P, Waeber G, Zukowska-Szczechowska E, Burton PR, Mooser V, Charchar FJ, Thompson JR, Tobin MD, Samani NJ. Genetic architecture of ambulatory blood pressure in the general population: insights from cardiovascular gene-centric array. Hypertension. 2010;56:1069–1076. doi: 10.1161/HYPERTENSIONAHA.110.155721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tragante V, Barnes MR, Ganesh SK, Lanktree MB, Guo W, Franceschini N, Smith EN, Johnson T, Holmes MV, Padmanabhan S, Karczewski KJ, Almoguera B, Barnard J, Baumert J, Chang YP, Elbers CC, Farrall M, Fischer ME, Gaunt TR, Gho JM, Gieger C, Goel A, Gong Y, Isaacs A, Kleber ME, Mateo Leach I, McDonough CW, Meijs MF, Melander O, Nelson CP, Nolte IM, Pankratz N, Price TS, Shaffer J, Shah S, Tomaszewski M, van der Most PJ, Van Iperen EP, Vonk JM, Witkowska K, Wong CO, Zhang L, Beitelshees AL, Berenson GS, Bhatt DL, Brown M, Burt A, Cooper-DeHoff RM, Connell JM, Cruickshanks KJ, Curtis SP, Davey-Smith G, Delles C, Gansevoort RT, Guo X, Haiqing S, Hastie CE, Hofker MH, Hovingh GK, Kim DS, Kirkland SA, Klein BE, Klein R, Li YR, Maiwald S, Newton-Cheh C, O’Brien ET, Onland-Moret NC, Palmas W, Parsa A, Penninx BW, Pettinger M, Vasan RS, Ranchalis JE, Ridker PM, Rose LM, Sever P, Shimbo D, Steele L, Stolk RP, Thorand B, Trip MD, van Duijn CM, Verschuren WM, Wijmenga C, Wyatt S, Young JH, Zwinderman AH, Bezzina CR, Boerwinkle E, Casas JP, Caulfield MJ, Chakravarti A, Chasman DI, Davidson KW, Doevendans PA, Dominiczak AF, FitzGerald GA, Gums JG, Fornage M, Hakonarson H, Halder I, Hillege HL, Illig T, Jarvik GP, Johnson JA, Kastelein JJ, Koenig W, Kumari M, März W, Murray SS, O’Connell JR, Oldehinkel AJ, Pankow JS, Rader DJ, Redline S, Reilly MP, Schadt EE, Kottke-Marchant K, Snieder H, Snyder M, Stanton AV, Tobin MD, Uitterlinden AG, van der Harst P, van der Schouw YT, Samani NJ, Watkins H, Johnson AD, Reiner AP, Zhu X, de Bakker PI, Levy D, Asselbergs FW, Munroe PB, Keating BJ. Gene-centric meta-analysis in 87,736 individuals of European ancestry identifies multiple blood-pressure-related loci. Am J Hum Genet. 2014;94:349–360. doi: 10.1016/j.ajhg.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu X, Wang L, Lin X, Huang J, Charles Gu C, He M, Shen H, He J, Zhu J, Li H, Hixson JE, Wu T, Dai J, Lu L, Shen C, Chen S, He L, Mo Z, Hao Y, Mo X, Yang X, Li J, Cao J, Chen J, Fan Z, Li Y, Zhao L, Li H, Lu F, Yao C, Yu L, Xu L, Mu J, Wu X, Deng Y, Hu D, Zhang W, Ji X, Guo D, Guo Z, Zhou Z, Yang Z, Wang R, Yang J, Zhou X, Yan W, Sun N, Gao P, Gu D. Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet. 2015;24:865–874. doi: 10.1093/hmg/ddu478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu C, Kraja AT, Smith JA, Brody JA, Franceschini N, Bis JC, Rice K, Morrison AC, Lu Y, Weiss S, Guo X, Palmas W, Martin LW, Chen YD, Surendran P, Drenos F, Cook JP, Auer PL, Chu AY, Giri A, Zhao W, Jakobsdottir J, Lin LA, Stafford JM, Amin N, Mei H, Yao J, Voorman A, Larson MG, Grove ML, Smith AV, Hwang SJ, Chen H, Huan T, Kosova G, Stitziel NO, Kathiresan S, Samani N, Schunkert H, Deloukas P, Li M, Fuchsberger C, Pattaro C, Gorski M, Kooperberg C, Papanicolaou GJ, Rossouw JE, Faul JD, Kardia SL, Bouchard C, Raffel LJ, Uitterlinden AG, Franco OH, Vasan RS, O’Donnell CJ, Taylor KD, Liu K, Bottinger EP, Gottesman O, Daw EW, Giulianini F, Ganesh S, Salfati E, Harris TB, Launer LJ, Dörr M, Felix SB, Rettig R, Völzke H, Kim E, Lee WJ, Lee IT, Sheu WH, Tsosie KS, Edwards DR, Liu Y, Correa A, Weir DR, Völker U, Ridker PM, Boerwinkle E, Gudnason V, Reiner AP, van Duijn CM, Borecki IB, Edwards TL, Chakravarti A, Rotter JI, Psaty BM, Loos RJ, Fornage M, Ehret GB, Newton-Cheh C, Levy D, Chasman DI; CHD Exome+ Consortium; ExomeBP Consortium; GoT2DGenes Consortium; T2D-GENES Consortium; Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia; CKDGen Consortium. Metaanalysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat Genet. 2016;48:1162–1170. doi: 10.1038/ng.3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Surendran P, Drenos F, Young R, Warren H, Cook JP, Manning AK, Grarup N, Sim X, Barnes DR, Witkowska K, Staley JR, Tragante V, Tukiainen T, Yaghootkar H, Masca N, Freitag DF, Ferreira T, Giannakopoulou O, Tinker A, Harakalova M, Mihailov E, Liu C, Kraja AT, Fallgaard Nielsen S, Rasheed A, Samuel M, Zhao W, Bonnycastle LL, Jackson AU, Narisu N, Swift AJ, Southam L, Marten J, Huyghe JR, Stancakova A, Fava C, Ohlsson T, Matchan A, Stirrups KE, Bork-Jensen J, Gjesing AP, Kontto J, Perola M, Shaw-Hawkins S, Havulinna AS, Zhang H, Donnelly LA, Groves CJ, Rayner NW, Neville MJ, Robertson NR, Yiorkas AM, Herzig KH, Kajantie E, Zhang W, Willems SM, Lannfelt L, Malerba G, Soranzo N, Trabetti E, Verweij N, Evangelou E, Moayyeri A, Vergnaud AC, Nelson CP, Poveda A, Varga TV, Caslake M, de Craen AJ, Trompet S, Luan J, Scott RA, Harris SE, Liewald DC, Marioni R, Menni C, Farmaki AE, Hallmans G, Renström F, Huffman JE, Hassinen M, Burgess S, Vasan RS, Felix JF, Uria-Nickelsen M, Malarstig A, Reily DF, Hoek M, Vogt T, Lin H, Lieb W, Traylor M, Markus HF, Highland HM, Justice AE, Marouli E, Lindström J, Uusitupa M, Komulainen P, Lakka TA, Rauramaa R, Polasek O, Rudan I, Rolandsson O, Franks PW, Dedoussis G, Spector TD, Jousilahti P, Männistö S, Deary IJ, Starr JM, Langenberg C, Wareham NJ, Brown MJ, Dominiczak AF, Connell JM, Jukema JW, Sattar N, Ford I, Packard CJ, Esko T, Mägi R, Metspalu A, de Boer RA, van der Meer P, van der Harst P, Gambaro G, Ingelsson E, Lind L, de Bakker PI, Numans ME, Brandslund I, Christensen C, Petersen ER, Korpi-Hyövälti E, Oksa H, Chambers JC, Kooner JS, Blakemore AI, Franks S, Jarvelin MR, Husemoen LL, Linneberg A, Skaaby T, Thuesen B, Karpe F, Tuomilehto J, Doney AS, Morris AD, Palmer CN, Holmen OL, Hveem K, Willer CJ, Tuomi T, Groop L, Käräjämäki A, Palotie A, Ripatti S, Salomaa V, Alam DS, Shafi Majumder AA, Di Angelantonio E, Chowdhury R, McCarthy MI, Poulter N, Stanton AV, Sever P, Amouyel P, Arveiler D, Blankenberg S, Ferrières J, Kee F, Kuulasmaa K, Müller-Nurasyid M, Veronesi G, Virtamo J, Deloukas P, Elliott P, Zeggini E, Kathiresan S, Melander O, Kuusisto J, Laakso M, Padmanabhan S, Porteous D, Hayward C, Scotland G, Collins FS, Mohlke KL, Hansen T, Pedersen O, Boehnke M, Stringham HM, Frossard P, Newton-Cheh C, Tobin MD, Nordestgaard BG, Caulfield MJ, Mahajan A, Morris AP, Tomaszewski M, Samani NJ, Saleheen D, Asselbergs FW, Lindgren CM, Danesh J, Wain LV, Butterworth AS, Howson JM, Munroe PB; CHARGE-Heart Failure Consortium; EchoGen Consortium; METASTROKE Consortium; GIANT Consortium; EPIC-InterAct Consortium; Lifelines Cohort Study; Wellcome Trust Case Control Consortium; Understanding Society Scientific Group; EPIC-CVD Consortium; CHARGE+ Exome Chip Blood Pressure Consortium; T2D-GENES Consortium; GoT2DGenes Consortium; ExomeBP Consortium; CHD Exome+ Consortium. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet. 2016;48:1151–1161. doi: 10.1038/ng.3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ehret GB, Ferreira T, Chasman DI, Jackson AU, Schmidt EM, Johnson T, Thorleifsson G, Luan J, Donnelly LA, Kanoni S, Petersen AK, Pihur V, Strawbridge RJ, Shungin D, Hughes MF, Meirelles O, Kaakinen M, Bouatia-Naji N, Kristiansson K, Shah S, Kleber ME, Guo X, Lyytikäinen LP, Fava C, Eriksson N, Nolte IM, Magnusson PK, Salfati EL, Rallidis LS, Theusch E, Smith AJP, Folkersen L, Witkowska K, Pers TH, Joehanes R, Kim SK, Lataniotis L, Jansen R, Johnson AD, Warren H, Kim YJ, Zhao W, Wu Y, Tayo BO, Bochud M, Absher D, Adair LS, Amin N, Arking DE, Axelsson T, Baldassarre D, Balkau B, Bandinelli S, Barnes MR, Barroso I, Bevan S, Bis JC, Bjornsdottir G, Boehnke M, Boerwinkle E, Bonnycastle LL, Boomsma DI, Bornstein SR, Brown MJ, Burnier M, Cabrera CP, Chambers JC, Chang IS, Cheng CY, Chines PS, Chung RH, Collins FS, Connell JM, Döring A, Dallongeville J, Danesh J, de Faire U, Delgado G, Dominiczak AF, Doney ASF, Drenos F, Edkins S, Eicher JD, Elosua R, Enroth S, Erdmann J, Eriksson P, Esko T, Evangelou E, Evans A, Fall T, Farrall M, Felix JF, Ferrières J, Ferrucci L, Fornage M, Forrester T, Franceschini N, Duran OHF, Franco-Cereceda A, Fraser RM, Ganesh SK, Gao H, Gertow K, Gianfagna F, Gigante B, Giulianini F, Goel A, Goodall AH, Goodarzi MO, Gorski M, Gräßler J, Groves C, Gudnason V, Gyllensten U, Hallmans G, Hartikainen AL, Hassinen M, Havulinna AS, Hayward C, Hercberg S, Herzig KH, Hicks AA, Hingorani AD, Hirschhorn JN, Hofman A, Holmen J, Holmen OL, Hottenga JJ, Howard P, Hsiung CA, Hunt SC, Ikram MA, Illig T, Iribarren C, Jensen RA, Kähönen M, Kang H, Kathiresan S, Keating BJ, Khaw KT, Kim YK, Kim E, Kivimaki M, Klopp N, Kolovou G, Komulainen P, Kooner JS, Kosova G, Krauss RM, Kuh D, Kutalik Z, Kuusisto J, Kvaløy K, Lakka TA, Lee NR, Lee IT, Lee WJ, Levy D, Li X, Liang KW, Lin H, Lin L, Lindström J, Lobbens S, Männistö S, Müller G, Müller-Nurasyid M, Mach F, Markus HS, Marouli E, McCarthy MI, McKenzie CA, Meneton P, Menni C, Metspalu A, Mijatovic V, Moilanen L, Montasser ME, Morris AD, Morrison AC, Mulas A, Nagaraja R, Narisu N, Nikus K, O’Donnell CJ, O’Reilly PF, Ong KK, Paccaud F, Palmer CD, Parsa A, Pedersen NL, Penninx BW, Perola M, Peters A, Poulter N, Pramstaller PP, Psaty BM, Quertermous T, Rao DC, Rasheed A, Rayner NWNWR, Renström F, Rettig R, Rice KM, Roberts R, Rose LM, Rossouw J, Samani NJ, Sanna S, Saramies J, Schunkert H, Sebert S, Sheu WH, Shin YA, Sim X, Smit JH, Smith AV, Sosa MX, Spector TD, Stančáková A, Stanton A, Stirrups KE, Stringham HM, Sundstrom J, Swift AJ, Syvänen AC, Tai ES, Tanaka T, Tarasov KV, Teumer A, Thorsteinsdottir U, Tobin MD, Tremoli E, Uitterlinden AG, Uusitupa M, Vaez A, Vaidya D, van Duijn CM, van Iperen EPA, Vasan RS, Verwoert GC, Virtamo J, Vitart V, Voight BF, Vollenweider P, Wagner A, Wain LV, Wareham NJ, Watkins H, Weder AB, Westra HJ, Wilks R, Wilsgaard T, Wilson JF, Wong TY, Yang TP, Yao J, Yengo L, Zhang W, Zhao JH, Zhu X, Bovet P, Cooper RS, Mohlke KL, Saleheen D, Lee JY, Elliott P, Gierman HJ, Willer CJ, Franke L, Hovingh GK, Taylor KD, Dedoussis G, Sever P, Wong A, Lind L, Assimes TL, Njøstad I, Schwarz PE, Langenberg C, Snieder H, Caulfield MJ, Melander O, Laakso M, Saltevo J, Rauramaa R, Tuomilehto J, Ingelsson E, Lehtimäki T, Hveem K, Palmas W, März W, Kumari M, Salomaa V, Chen YI, Rotter JI, Froguel P, Jarvelin MR, Lakatta EG, Kuulasmaa K, Franks PW, Hamsten A, Wichmann HE, Palmer CNA, Stefansson K, Ridker PM, Loos RJF, Chakravarti A, Deloukas P, Morris AP, Newton-Cheh C, Munroe PB; CHARGE-EchoGen Consortium; CHARGE-HF Consortium; Wellcome Trust Case Control Consortium. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet. 2016;48:1171–1184. doi: 10.1038/ng.3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffmann TJ, Ehret GB, Nandakumar P, Ranatunga D, Schaefer C, Kwok PY, Iribarren C, Chakravarti A, Risch N. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat Genet. 2017;49:54–64. doi: 10.1038/ng.3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yugar-Toledo JC, Martin JF, Krieger JE, Pereira AC, Demacq C, Coelho OR, Pimenta E, Calhoun DA, Júnior HM. Gene variation in resistant hypertension: multilocus analysis of the angiotensin 1-converting enzyme, angiotensinogen, and endothelial nitric oxide synthase genes. DNA Cell Biol. 2011;30:555–564. doi: 10.1089/dna.2010.1156 [DOI] [PubMed] [Google Scholar]
- 93.Oliveira-Paula GH, Lacchini R, Coeli-Lacchini FB, Junior HM, Tanus-Santos JE. Inducible nitric oxide synthase haplotype associated with hypertension and responsiveness to antihypertensive drug therapy. Gene. 2013;515:391–395. doi: 10.1016/j.gene.2012.12.059 [DOI] [PubMed] [Google Scholar]
- 94.Lynch AI, Irvin MR, Davis BR, Ford CE, Eckfeldt JH, Arnett DK. Genetic and adverse health outcome associations with treatment resistant hypertension in GenHAT. Int J Hypertens. 2013;2013:578578. doi: 10.1155/2013/578578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fontana V, McDonough CW, Gong Y, El Rouby NM, Sá AC, Taylor KD, Chen YD, Gums JG, Chapman AB, Turner ST, Pepine CJ, Johnson JA, Cooper-DeHoff RM. Large-scale gene-centric analysis identifies polymorphisms for resistant hypertension. J Am Heart Assoc. 2014;3:e001398. doi: 10.1161/JAHA.114.001398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, Guiducci C, Kathiresan S, Benjamin EJ, Struck J, Morgenthaler NG, Bergmann A, Blankenberg S, Kee F, Nilsson P, Yin X, Peltonen L, Vartiainen E, Salomaa V, Hirschhorn JN, Melander O, Wang TJ. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41:348–353. doi: 10.1038/ng.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holland N, Segraves D, Nnadi VO, Belletti DA, Wogen J, Arcona S. Identifying barriers to hypertension care: implications for quality improvement initiatives. Dis Manag. 2008;11:71–77. doi: 10.1089/dis.2008.1120007 [DOI] [PubMed] [Google Scholar]
- 98.Gwadry-Sridhar FH, Manias E, Lal L, Salas M, Hughes DA, Ratzki-Leewing A, Grubisic M. Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: a systematic review by the ISPOR medication adherence and persistence special interest group. Value Health. 2013;16:863–871. doi: 10.1016/j.jval.2013.03.1631 [DOI] [PubMed] [Google Scholar]
- 99.Petrilla AA, Benner JS, Battleman DS, Tierce JC, Hazard EH. Evidence-based interventions to improve patient compliance with antihypertensive and lipid-lowering medications. Int J Clin Pract. 2005;59:1441–1451. doi: 10.1111/j.1368-5031.2005.00704.x [DOI] [PubMed] [Google Scholar]
- 100.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association [published correction appears in Circulation. 2016;133:e599]. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 101.Burnier M, Wuerzner G. Ambulatory blood pressure and adherence monitoring: diagnosing pseudoresistant hypertension. Semin Nephrol. 2014;34:498–505. doi: 10.1016/j.semnephrol.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 102.Hyman DJ, Pavlik V. Medication adherence and resistant hypertension. J Hum Hypertens. 2015;29:213–218. doi: 10.1038/jhh.2014.73 [DOI] [PubMed] [Google Scholar]
- 103.Berra E, Azizi M, Capron A, Høieggen A, Rabbia F, Kjeldsen SE, Staessen JA, Wallemacq P, Persu A. Evaluation of adherence should become an integral part of assessment of patients with apparently treatment-resistant hypertension. Hypertension. 2016;68:297–306. doi: 10.1161/HYPERTENSIONAHA.116.07464 [DOI] [PubMed] [Google Scholar]
- 104.Rose AJ, Berlowitz DR, Manze M, Orner MB, Kressin NR. Intensifying therapy for hypertension despite suboptimal adherence. Hypertension. 2009;54:524–529. doi: 10.1161/HYPERTENSIONAHA.109.133389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meddings J, Kerr EA, Heisler M, Hofer TP. Physician assessments of medication adherence and decisions to intensify medications for patients with uncontrolled blood pressure: still no better than a coin toss. BMC Health Serv Res. 2012;12:270. doi: 10.1186/1472-6963-12-270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eskås PA, Heimark S, Eek Mariampillai J, Larstorp AC, Fadl Elmula FE, Høieggen A. Adherence to medication and drug monitoring in apparent treatment-resistant hypertension. Blood Press. 2016;25:199–205. doi: 10.3109/08037051.2015.1121706 [DOI] [PubMed] [Google Scholar]
- 107.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Prog Cardiovasc Nurs. 2000;15:90–96. [DOI] [PubMed] [Google Scholar]
- 109.Correa NB, de Faria AP, Ritter AM, Sabbatini AR, Almeida A, Brunelli V, Calhoun DA, Moreno H, Modolo R. A practical approach for measurement of antihypertensive medication adherence in patients with resistant hypertension. J Am Soc Hypertens. 2016;10:510–516.e1. doi: 10.1016/j.jash.2016.03.194 [DOI] [PubMed] [Google Scholar]
- 110.Proteus Digital Health. U.S. FDA accepts first digital medicine new drug application for Otsuka and Proteus Digital Health. http://www.proteus.com/press-releases/u-s-fda-accepts-first-digital-medicine-new-drug-application-for-otsuka-and-proteus-digital-health/. September 10, 2015. Accessed January 5, 2016.
- 111.Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86:304–314. doi: 10.4065/mcp.2010.0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, Agoritsas T, Mistry N, Iorio A, Jack S, Sivaramalingam B, Iserman E, Mustafa RA, Jedraszewski D, Cotoi C, Haynes RB. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014:CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–719. doi: 10.1016/j.amjmed.2006.08.033 [DOI] [PubMed] [Google Scholar]
- 114.Gwadry-Sridhar FH, Manias E, Lal L, Salas M, Hughes DA, Ratzki-Leewing A, Grubisic M. Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: a systematic review by the ISPOR Medication Adherence and Persistence Special Interest Group. Value Health. 2013;16:863–871. doi: 10.1016/j.jval.2013.03.1631 [DOI] [PubMed] [Google Scholar]
- 115.Peacock E, Krousel-Wood M. Adherence to antihypertensive therapy. Med Clin North Am. 2017;101:229–245. doi: 10.1016/j.mcna.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. [DOI] [PubMed] [Google Scholar]
- 117.Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55:399–407. doi: 10.1161/HYPERTENSIONAHA.109.139816 [DOI] [PubMed] [Google Scholar]
- 118.Iskedjian M, Einarson TR, MacKeigan LD, Shear N, Addis A, Mittmann N, Ilersich AL. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysis. Clin Ther. 2002;24:302–316. [DOI] [PubMed] [Google Scholar]
- 119.Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004;164:722–732. doi: 10.1001/archinte.164.7.722 [DOI] [PubMed] [Google Scholar]
- 120.Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13:898–909. doi: 10.1111/j.1751-7176.2011.00550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Proia KK, Thota AB, Njie GJ, Finnie RK, Hopkins DP, Mukhtar Q, Pronk NP, Zeigler D, Kottke TE, Rask KJ, Lackland DT, Brooks JF, Braun LT, Cooksey T; Community Preventive Services Task Force. Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;47:86–99. doi: 10.1016/j.amepre.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brownstein JN, Chowdhury FM, Norris SL, Horsley T, Jack L Jr, Zhang X, Satterfield D. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32:435–447. doi: 10.1016/j.amepre.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 123.Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169:1748–1755. doi: 10.1001/archinternmed.2009.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Clark CE, Smith LF, Taylor RS, Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Santschi V, Chiolero A, Colosimo AL, Platt RW, Taffé P, Burnier M, Burnand B, Paradis G. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718. doi: 10.1161/JAHA.113.000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brush JE Jr, Handberg EM, Biga C, Birtcher KK, Bove AA, Casale PN, Clark MG, Garson A Jr, Hines JL, Linderbaum JA, Rodgers GP, Shor RA, Thourani VH, Wyman JF. 2015 ACC health policy statement on cardiovascular team-based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118–2136. doi: 10.1016/j.jacc.2015.03.550 [DOI] [PubMed] [Google Scholar]
- 127.Thomas KL, Shah BR, Elliot-Bynum S, Thomas KD, Damon K, Allen LaPointe NM, Calhoun S, Thomas L, Breathett K, Mathews R, Anderson M, Califf RM, Peterson ED. Check it, change it: a community-based, multifaceted intervention to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2014;7:828–834. doi: 10.1161/CIRCOUTCOMES.114.001039 [DOI] [PubMed] [Google Scholar]
- 128.Samal L, Linder JA, Lipsitz SR, Hicks LS. Electronic health records, clinical decision support, and blood pressure control. Am J Manag Care. 2011;17:626–632. [PubMed] [Google Scholar]
- 129.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. doi: 10.1001/jama.2013.108769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jaffe MG, Young JD. The Kaiser Permanente Northern California Story: improving hypertension control from 44% to 90% in 13 years (2000 to 2013). J Clin Hypertens (Greenwich). 2016;18:260–261. doi: 10.1111/jch.12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Go AS, Bauman MA, Coleman King SM, Fonarow GC, Lawrence W, Williams KA, Sanchez E. An effective approach to high blood pressure control: a science advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention [published correction appears in Hypertension. 2014;63:e175]. Hypertension. 2014;63:878–885. doi: 10.1161/HYP.0000000000000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Omboni S, Ferrari R. The role of telemedicine in hypertension management: focus on blood pressure telemonitoring. Curr Hypertens Rep. 2015;17:535. doi: 10.1007/s11906-015-0535-3 [DOI] [PubMed] [Google Scholar]
- 133.Yang W, Chang J, Kahler KH, Fellers T, Orloff J, Wu EQ, Bensimon AG. Evaluation of compliance and health care utilization in patients treated with single pill vs. free combination antihypertensives. Curr Med Res Opin. 2010;26:2065–2076. doi: 10.1185/03007995.2010.494462 [DOI] [PubMed] [Google Scholar]
- 134.Choudhry NK, Denberg TD, Qaseem A; Clinical Guidelines Committee of American College of Physicians. Improving adherence to therapy and clinical outcomes while containing costs: opportunities from the greater use of generic medications: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2016;164:41–49. doi: 10.7326/M14-2427 [DOI] [PubMed] [Google Scholar]
- 135.Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215–1221. doi: 10.1001/jamainternmed.2013.6172 [DOI] [PubMed] [Google Scholar]
- 136.Roumie CL, Greevy R, Wallston KA, Elasy TA, Kaltenbach L, Kotter K, Dittus RS, Speroff T. Patient centered primary care is associated with patient hypertension medication adherence. J Behav Med. 2011;34:244–253. doi: 10.1007/s10865-010-9304-6 [DOI] [PubMed] [Google Scholar]
- 137.Burke LE, Ma J, Azar KMJ, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B, Turan TN, Spring B, Steinberger J, Quinn CC; on behalf of the American Heart Association Publications Committee of the Council on Epidemiology and Prevention, Behavior Change Committee of the Council on Cardiometabolic Health, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, Council on Quality of Care and Outcomes Research, and Stroke Council. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association [published correction appears in Circulation. 2015;132:e233]. Circulation. 2015;132:1157–1213. doi: 10.1161/CIR.0000000000000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.AHRQ. AHRQ Health Literacy Universal Precautions Toolkit. https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/index.html. Updated November 2016 Accessed January 5, 2017.
- 139.Artinian NT, Fletcher GF, Mozaffarian D, Kris-Etherton P, Van Horn L, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS, Meininger JC, Banks J, Stuart-Shor EM, Fletcher BJ, Miller TD, Hughes S, Braun LT, Kopin LA, Berra K, Hayman LL, Ewing LJ, Ades PA, Durstine JL, Houston-Miller N, Burke LE; on behalf of the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:406–441. doi: 10.1161/CIR.0b013e3181e8edf1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bhatt H, Siddiqui M, Judd E, Oparil S, Calhoun D. Prevalence of pseudoresistant hypertension due to inaccurate blood pressure measurement. J Am Soc Hypertens. 2016;10:493–499. doi: 10.1016/j.jash.2016.03.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Messerli FH, Ventura HO, Amodeo C. Osler’s maneuver and pseudohypertension. N Engl J Med. 1985;312:1548–1551. doi: 10.1056/NEJM198506133122405 [DOI] [PubMed] [Google Scholar]
- 142.Nielsen PE, Larsen B, Holstein P, Poulsen HL. Accuracy of auscultatory blood pressure measurements in hypertensive and obese subjects. Hypertension. 1983;5:122–127. [DOI] [PubMed] [Google Scholar]
- 143.Grassi G, Turri C, Vailati S, Dell’Oro R, Mancia G. Muscle and skin sympathetic nerve traffic during the “white-coat” effect. Circulation. 1999;100:222–225. [DOI] [PubMed] [Google Scholar]
- 144.Manios ED, Koroboki EA, Tsivgoulis GK, Spengos KM, Spiliopoulou IK, Brodie FG, Vemmos KN, Zakopoulos NA. Factors influencing whitecoat effect. Am J Hypertens. 2008;21:153–158. [DOI] [PubMed] [Google Scholar]
- 145.Brown MA, Buddle ML, Martin A. Is resistant hypertension really resistant? Am J Hypertens. 2001;14:1263–1269. [DOI] [PubMed] [Google Scholar]
- 146.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sim JJ, Handler J, Jacobsen SJ, Kanter MH. Systemic implementation strategies to improve hypertension: the Kaiser Permanente Southern California experience. Can J Cardiol. 2014;30:544–552. doi: 10.1016/j.cjca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 148.Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, Sowers J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich). 2013;15:14–33. doi: 10.1111/jch.12049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. doi: 10.1161/CIRCRESAHA.116.305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Byrd JB, Brook RD. A critical review of the evidence supporting aldosterone in the etiology and its blockade in the treatment of obesity-associated hypertension. J Hum Hypertens. 2014;28:3–9. doi: 10.1038/jhh.2013.42 [DOI] [PubMed] [Google Scholar]
- 151.Calhoun DA, Sharma K. The role of aldosteronism in causing obesity-related cardiovascular risk. Cardiol Clin. 2010;28:517–527. doi: 10.1016/j.ccl.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.de la Sierra A, Banegas JR, Oliveras A, Gorostidi M, Segura J, de la Cruz JJ, Armario P, Ruilope LM. Clinical differences between resistant hypertensives and patients treated and controlled with three or less drugs. J Hypertens. 2012;30:1211–1216. [DOI] [PubMed] [Google Scholar]
- 153.O’Donnell M, Mente A, Yusuf S. Sodium intake and cardiovascular health. Circ Res. 2015;116:1046–1057. doi: 10.1161/CIRCRESAHA.116.303771 [DOI] [PubMed] [Google Scholar]
- 154.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 155.Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M, Labarthe DR, MacGregor GA, Sacks FM, Stamler J, Vafiadis DK, Van Horn LV. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880–2889. doi: 10.1161/CIR.0b013e318279acbf [DOI] [PubMed] [Google Scholar]
- 156.Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol. 2012;302:H1031–H1049. doi: 10.1152/ajpheart.00899.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oh YS, Appel LJ, Galis ZS, Hafler DA, He J, Hernandez AL, Joe B, Karumanchi SA, Maric-Bilkan C, Mattson D, Mehta NN, Randolph G, Ryan M, Sandberg K, Titze J, Tolunay E, Toney GM, Harrison DG. National Heart, Lung, and Blood Institute Working Group Report on Salt in Human Health and Sickness: building on the current scientific evidence. Hypertension. 2016;68:281–288. doi: 10.1161/HYPERTENSIONAHA.116.07415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M, Appel LJ; on behalf of the American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173–1186. doi: 10.1161/CIR.0000000000000015 [DOI] [PubMed] [Google Scholar]
- 159.Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, Pimenta E, Aban I, Oparil S, Calhoun DA. Characterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med. 2008;168:1159–1164. doi: 10.1001/archinte.168.11.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Florczak E, Prejbisz A, Szwench-Pietrasz E, Sliwiński P, Bieleń P, Klisiewicz A, Michałowska I, Warchoł E, Januszewicz M, Kała M, Witkowski A, Więcek A, Narkiewicz K, Somers VK, Januszewicz A. Clinical characteristics of patients with resistant hypertension: the RESIST-POL study. J Hum Hypertens. 2013;27:678–685. doi: 10.1038/jhh.2013.32 [DOI] [PubMed] [Google Scholar]
- 161.McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, Campbell KL. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24:2096–2103. doi: 10.1681/ASN.2013030285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McFadden CB, Brensinger CM, Berlin JA, Townsend RR. Systematic review of the effect of daily alcohol intake on blood pressure. Am J Hypertens. 2005;18(pt 1):276–286. doi: 10.1016/j.amjhyper.2004.07.020 [DOI] [PubMed] [Google Scholar]
- 163.Chen L, Smith GD, Harbord RM, Lewis SJ. Alcohol intake and blood pressure: a systematic review implementing a mendelian randomization approach. PLoS Med. 2008;5:e52. doi: 10.1371/journal.pmed.0050052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Briasoulis A, Agarwal V, Messerli FH. Alcohol consumption and the risk of hypertension in men and women: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2012;14:792–798. doi: 10.1111/jch.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hussain K, Arisari RA, Ferder L. Alcohol-induced hypertension: mechanism and prevention. World J Cardiol. 2014;26:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shimbo D, Levitan EB, Booth JN 3rd, Calhoun DA, Judd SE, Lackland DT, Safford MM, Oparil S, Muntner P. The contributions of unhealthy lifestyle factors to apparent resistant hypertension: findings from the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Hypertens. 2013;31:370–376. doi: 10.1097/HJH.0b013e32835b6be7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Diaz KM, Shimbo D. Physical activity and the prevention of hypertension. Curr Hypertens Rep. 2013;15:659–668. doi: 10.1007/s11906-013-0386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Carnethon MR, Evans NS, Church TS, Lewis CE, Schreiner PJ, Jacobs DR Jr, Sternfeld B, Sidney S. Joint associations of physical activity and aerobic fitness on the development of incident hypertension: coronary artery risk development in young adults. Hypertension. 2010;56:49–55. doi: 10.1161/HYPERTENSIONAHA.109.147603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chase NL, Sui X, Lee DC, Blair SN. The association of cardiorespiratory fitness and physical activity with incidence of hypertension in men. Am J Hypertens. 2009;22:417–424. doi: 10.1038/ajh.2009.6 [DOI] [PubMed] [Google Scholar]
- 170.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401–411. doi: 10.1001/jama.2009.1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Crump C, Sundquist J, Winkleby MA, Sundquist K. Interactive effects of physical fitness and body mass index on the risk of hypertension. JAMA Intern Med. 2016;176:210–216. doi: 10.1001/jamainternmed.2015.7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pouliou T, Ki M, Law C, Li L, Power C. Physical activity and sedentary behaviour at different life stages and adult blood pressure in the 1958 British cohort. J Hypertens. 2012;30:275–283. doi: 10.1097/HJH.0b013e32834f1915 [DOI] [PubMed] [Google Scholar]
- 173.Dimeo F, Pagonas N, Seibert F, Arndt R, Zidek W, Westhoff TH. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60:653–658. doi: 10.1161/HYPERTENSIONAHA.112.197780 [DOI] [PubMed] [Google Scholar]
- 174.Appel LJ, Giles TD, Black HR, Izzo JL Jr, Materson BJ, Oparil S, Weber MA; American Society of Hypertension Writing Group. ASH position paper: dietary approaches to lower blood pressure. J Clin Hypertens (Greenwich). 2009;11:358–368. doi: 10.1111/j.1751-7176.2009.00136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253–1261. doi: 10.1016/j.numecd.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 176.Trudel-Fitzgerald C, Gilsanz P, Mittleman MA, Kubzansky LD. Dysregulated blood pressure: can regulating emotions help? Curr Hypertens Rep. 2015;17:92. doi: 10.1007/s11906-015-0605-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cuffee Y, Ogedegbe C, Williams NJ, Ogedegbe G, Schoenthaler A. Psychosocial risk factors for hypertension: an update of the literature. Curr Hypertens Rep. 2014;16:483. doi: 10.1007/s11906-014-0483-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gangwisch JE. A review of evidence for the link between sleep duration and hypertension. Am J Hypertens. 2014;27:1235–1242. doi: 10.1093/ajh/hpu071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brook RD, Weder AB, Rajagopalan S. “Environmental hypertensionology” the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens (Greenwich). 2011;13:836–842. doi: 10.1111/j.1751-7176.2011.00543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dedier J, Stampfer MJ, Hankinson SE, Willett WC, Speizer FE, Curhan GC. Nonnarcotic analgesic use and the risk of hypertension in US women. Hypertension. 2002;40:604–608. [DOI] [PubMed] [Google Scholar]
- 181.Forman JP, Stampfer MJ, Curhan GC. Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension. 2005;46:500–507. doi: 10.1161/01.HYP.0000177437.07240.70 [DOI] [PubMed] [Google Scholar]
- 182.Warner TD, Mitchell JA. COX-2 selectivity alone does not define the cardiovascular risks associated with non-steroidal anti-inflammatory drugs. Lancet. 2008;371:270–273. doi: 10.1016/S0140-6736(08)60137-3 [DOI] [PubMed] [Google Scholar]
- 183.Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121:289–300. [DOI] [PubMed] [Google Scholar]
- 184.Radack KL, Deck CC, Bloomfield SS. Ibuprofen interferes with the efficacy of antihypertensive drugs: a randomized, double-blind, placebocontrolled trial of ibuprofen compared with acetaminophen. Ann Intern Med. 1987;107:628–635. [DOI] [PubMed] [Google Scholar]
- 185.Conlin PR, Moore TJ, Swartz SL, Barr E, Gazdick L, Fletcher C, DeLucca P, Demopoulos L. Effect of indomethacin on blood pressure lowering by captopril and losartan in hypertensive patients. Hypertension. 2000;36:461–465. [DOI] [PubMed] [Google Scholar]
- 186.White WB. Cardiovascular effects of the cyclooxygenase inhibitors. Hypertension. 2007;49:408–418. doi: 10.1161/01.HYP.0000258106.74139.25 [DOI] [PubMed] [Google Scholar]
- 187.Ishiguro C, Fujita T, Omori T, Fujii Y, Mayama T, Sato T. Assessing the effects of non-steroidal anti-inflammatory drugs on antihypertensive drug therapy using post-marketing surveillance database. J Epidemiol. 2008;18:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Whelton A, White WB, Bello AE, Puma JA, Fort JG; SUCCESS-VII Investigators. Effects of celecoxib and rofecoxib on blood pressure and edema in patients > or =65 years of age with systemic hypertension and osteoarthritis. Am J Cardiol. 2002;90:959–963. [DOI] [PubMed] [Google Scholar]
- 189.White WB, Kent J, Taylor A, Verburg KM, Lefkowith JB, Whelton A. Effects of celecoxib on ambulatory blood pressure in hypertensive patients on ACE inhibitors. Hypertension. 2002;39:929–934. [DOI] [PubMed] [Google Scholar]
- 190.Messerli FH, Sichrovsky T. Does the pro-hypertensive effect of cyclooxygenase-2 inhibitors account for the increased risk in cardiovascular disease? Am J Cardiol. 2005;96:872–873. doi: 10.1016/j.amjcard.2005.05.038 [DOI] [PubMed] [Google Scholar]
- 191.Zanchetti A, Hansson L, Leonetti G, Rahn KH, Ruilope L, Warnold I, Wedel H. Low-dose aspirin does not interfere with the blood pressure-lowering effects of antihypertensive therapy. J Hypertens. 2002;20:1015–1022. [DOI] [PubMed] [Google Scholar]
- 192.Hörl WH. Nonsteroidal Anti-inflammatory drugs and the kidney. Pharmaceuticals (Basel). 2010;3:2291–2321. doi: 10.3390/ph3072291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Johnson AG, Simons LA, Simons J, Friedlander Y, McCallum J. Nonsteroidal anti-inflammatory drugs and hypertension in the elderly: a community-based cross-sectional study. Br J Clin Pharmacol. 1993;35:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Davies JG, Rawlins DC, Busson M. Effect of ibuprofen on blood pressure control by propranolol and bendrofluazide. J Int Med Res. 1988;16:173–181. doi: 10.1177/030006058801600302 [DOI] [PubMed] [Google Scholar]
- 195.Wright JT, McKenney JM, Lehany AM, Bryan DL, Cooper LW, Lambert CM. The effect of high-dose short-term ibuprofen on antihypertensive control with hydrochlorothiazide. Clin Pharmacol Ther. 1989;46:440–444. [DOI] [PubMed] [Google Scholar]
- 196.Polonia J, Boaventura I, Gama G, Camôes I, Bernardo F, Andrade P, Nunes JP, Brandâo F, Cerqueira-Gomes M. Influence of non-steroidal anti-inflammatory drugs on renal function and 24h ambulatory blood pressure-reducing effects of enalapril and nifedipine gastrointestinal therapeutic system in hypertensive patients. J Hypertens. 1995;13:925–931. [DOI] [PubMed] [Google Scholar]
- 197.Morgan TO, Anderson A, Bertram D. Effect of indomethacin on blood pressure in elderly people with essential hypertension well controlled on amlodipine or enalapril. Am J Hypertens. 2000;13:1161–1167. [DOI] [PubMed] [Google Scholar]
- 198.Fogari R, Zoppi A, Carretta R, Veglio F, Salvetti A; Italian Collaborative Study Group. Effect of indomethacin on the antihypertensive efficacy of valsartan [DOI] [PubMed] [Google Scholar]
- 199.Izhar M, Alausa T, Folker A, Hung E, Bakris GL. Effects of COX inhibition on blood pressure and kidney function in ACE inhibitor-treated blacks and Hispanics. Hypertension. 2004;43:573–577. doi: 10.1161/01.HYP.0000115921.55353.e0 [DOI] [PubMed] [Google Scholar]
- 200.Palmer R, Weiss R, Zusman RM, Haig A, Flavin S, MacDonald B. Effects of nabumetone, celecoxib, and ibuprofen on blood pressure control in hypertensive patients on angiotensin converting enzyme inhibitors. Am J Hypertens. 2003;16:135–139. [DOI] [PubMed] [Google Scholar]
- 201.MacFarlane LL, Orak DJ, Simpson WM. NSAIDs, antihypertensive agents and loss of blood pressure control. Am Fam Physician. 1995;51:849–856. [PubMed] [Google Scholar]
- 202.Ruschitzka F, Borer JS, Krum H, Flammer AJ, Yeomans ND, Libby P, Lüscher TF, Solomon DH, Husni ME, Graham DY, Davey DA, Wisniewski LM, Menon V, Fayyad R, Beckerman B, Iorga D, Lincoff AM, Nissen SE. Differential blood pressure effects of ibuprofen, naproxen, and celecoxib in patients with arthritis: the PRECISION-ABPM (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen or Naproxen Ambulatory Blood Pressure Measurement) Trial. Eur Heart J. 2017;38:3282–3292. doi: 10.1093/eurheartj/ehx508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Prentice RL. On the ability of blood pressure effects to explain the relation between oral contraceptives and cardiovascular disease. Am J Epidemiol 1988;127:213–219. [DOI] [PubMed] [Google Scholar]
- 204.Rosenthal T, Oparil S. Oral contraceptives, hormones replacement therapy, and hypertension In: Lip G, Hall J, eds. Comprehensive Hypertension. New York, NY: Elsevier/Mosby; 2007:865–882. [Google Scholar]
- 205.Chasan-Taber L, Willett WC, Manson JE, Spiegelman D, Hunter DJ, Curhan G, Colditz GA, Stampfer MJ. Prospective study of oral contraceptives and hypertension among women in the United States. Circulation. 1996;94:483–489. [DOI] [PubMed] [Google Scholar]
- 206.Lubianca JN, Faccin CS, Fuchs FD. Oral contraceptives: a risk factor for uncontrolled blood pressure among hypertensive women. Contraception. 2003;67:19–24. [DOI] [PubMed] [Google Scholar]
- 207.White WB, Hanes V, Chauhan V, Pitt B. Effects of a new hormone therapy, drospirenone and 17-beta-estradiol, in postmenopausal women with hypertension. Hypertension. 2006;48:246–253. doi: 10.1161/01.HYP.0000232179.60442.84 [DOI] [PubMed] [Google Scholar]
- 208.ACOG Committee on Practice Bulletins-Gynecology. ACOG practice bulletin: No. 73: use of hormonal contraception in women with coexisting medical conditions: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2006;107:1453–1472. [DOI] [PubMed] [Google Scholar]
- 209.Woods JW. Oral contraceptives and hypertension. Hypertension. 1988;11(pt 2):II11–II15. [DOI] [PubMed] [Google Scholar]
- 210.Lim KG, Isles CG, Hodsman GP. Malignant hypertension in women of childbearing age and its relation to the contraceptive pill. Br Med J. 1987;294:1057–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Goldhaber SZ, Hennekens CH, Spark RF, Evans DA, Rosner B, Taylor JO, Kass EH. Plasma renin substrate, renin activity, and aldosterone levels in a sample of oral contraceptive users from a community survey. Am Heart J. 1984;107:119–122. [DOI] [PubMed] [Google Scholar]
- 212.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 213.Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women: the Postmenopausal Estrogen/ Progestin Interventions (PEPI) Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 214.Fleming GA. The FDA, regulation, and the risk of stroke. N Engl J Med. 2000;343:1886–1887. doi: 10.1056/NEJM200012213432510 [DOI] [PubMed] [Google Scholar]
- 215.Mersfelder TL. Phenylpropanolamine and stroke: the study, the FDA ruling, the implications. Cleve Clin J Med. 2001;68:208–209, 213. [DOI] [PubMed] [Google Scholar]
- 216.Cantu C, Arauz A, Murillo-Bonilla LM, Lopez M, Barinagarrementeria F. Stroke associated with sympathomimetics contained in over-the-counter cough and cold drugs. Stroke. 2003;34:1667–1672. doi: 10.1161/01.STR.0000075293.45936.FA [DOI] [PubMed] [Google Scholar]
- 217.Kernan WN, Viscoli CM, Brass LM, Broderick JP, Brott T, Feldmann E, Morgenstern LB, Wilterdink JL, Horwitz RI. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343:1826–1832. doi: 10.1056/NEJM200012213432501 [DOI] [PubMed] [Google Scholar]
- 218.Porter GA, Bennett WM, Sheps SG. Cyclosporine-associated hypertension: National High Blood Pressure Education Program. Arch Intern Med. 1990;150:280–283. [DOI] [PubMed] [Google Scholar]
- 219.Textor SC, Taler SJ, Canzanello VJ. Cyclosporine, blood pressure and atherosclerosis. Cardiol Rev. 1997;5:141–151. [Google Scholar]
- 220.Chapman JR, Marcen R, Arias M, Raine AE, Dunnill MS, Morris PJ. Hypertension after renal transplantation: a comparison of cyclosporine and conventional immunosuppression. Transplantation. 1987;43:860–864. [PubMed] [Google Scholar]
- 221.Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR. Recombinant human erythropoietin in anemic patients with end-stage renal disease: results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. [DOI] [PubMed] [Google Scholar]
- 222.Vaziri ND. Mechanism of erythropoietin-induced hypertension. Am J Kidney Dis. 1999;33:821–828. [DOI] [PubMed] [Google Scholar]
- 223.Raine AE, Roger SD. Effects of erythropoietin on blood pressure. Am J Kidney Dis. 1991;18(suppl 2):76–83. [PubMed] [Google Scholar]
- 224.Eschbach JW, Aquiling T, Haley NR, Fan MH, Blagg CR. The long-term effects of recombinant human erythropoietin on the cardiovascular system. Clin Nephrol. 1992;38(suppl 1):S98–S103. [PubMed] [Google Scholar]
- 225.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381 [DOI] [PubMed] [Google Scholar]
- 226.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 227.Shinkaruk S, Bayle M, Laïn G, Déléris G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2003;3:95–117. [DOI] [PubMed] [Google Scholar]
- 228.Sica DA. Angiogenesis inhibitors and hypertension: an emerging issue. J Clin Oncol. 2006;24:1329–1331. doi: 10.1200/JCO.2005.04.5740 [DOI] [PubMed] [Google Scholar]
- 229.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–193. doi: 10.1053/j.ajkd.2006.11.039 [DOI] [PubMed] [Google Scholar]
- 230.Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2 [DOI] [PubMed] [Google Scholar]
- 231.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and metaanalysis. Acta Oncol. 2009;48:9–17. doi: 10.1080/02841860802314720 [DOI] [PubMed] [Google Scholar]
- 232.Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM, Vokes EE. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661 [DOI] [PubMed] [Google Scholar]
- 233.Hansen-Smith FM, Morris LW, Greene AS, Lombard JH. Rapid microvessel rarefaction with elevated salt intake and reduced renal mass hypertension in rats. Circ Res. 1996;79:324–330. [DOI] [PubMed] [Google Scholar]
- 234.Hutchins PM, Lynch CD, Cooney PT, Curseen KA. The microcirculation in experimental hypertension and aging. Cardiovasc Res. 1996;32:772–780. [PubMed] [Google Scholar]
- 235.Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab: a crucial role for microcirculation. Ann Oncol. 2008;19:927–934. doi: 10.1093/annonc/mdm550 [DOI] [PubMed] [Google Scholar]
- 236.Steeghs N, Gelderblom H, Roodt JO, Christensen O, Rajagopalan P, Hovens M, Putter H, Rabelink TJ, de Koning E. Hypertension and rarefaction during treatment with telatinib, a small molecule angiogenesis inhibitor. Clin Cancer Res. 2008;14:3470–3476. doi: 10.1158/1078-0432.CCR-07-5050 [DOI] [PubMed] [Google Scholar]
- 237.Veronese ML, Mosenkis A, Flaherty KT, Gallagher M, Stevenson JP, Townsend RR, O’Dwyer PJ. Mechanisms of hypertension associated with BAY 43–9006. J Clin Oncol. 2006;24:1363–1369. doi: 10.1200/JCO.2005.02.0503 [DOI] [PubMed] [Google Scholar]
- 238.Das G Cardiovascular effects of cocaine abuse. Int J Clin Pharmacol Ther Toxicol. 1993;31:521–528. [PubMed] [Google Scholar]
- 239.Gawin FH, Ellinwood EH Jr. Cocaine and other stimulants: actions, abuse, and treatment. N Engl J Med. 1988;318:1173–1182. doi: 10.1056/NEJM198805053181806 [DOI] [PubMed] [Google Scholar]
- 240.Chiang W, Goldfrank L. The medical complications of drug abuse. Med J Aust. 1990;152:83–88. [DOI] [PubMed] [Google Scholar]
- 241.Ballard JE, Boileau RA, Sleator EK, Massey BH, Sprague RL. Cardiovascular responses of hyperactive children to methylphenidate. JAMA. 1976;236:2870–2874. [PubMed] [Google Scholar]
- 242.Liu LX, Rustgi AK. Cardiac myonecrosis in hypertensive crisis associated with monoamine oxidase inhibitor therapy. Am J Med. 1987;82:1060–1064. [DOI] [PubMed] [Google Scholar]
- 243.Fallon B, Foote B, Walsh BT, Roose SP “Spontaneous” hypertensive episodes with monoamine oxidase inhibitors. J Clin Psychiatry. 1988;49:163–165. [PubMed] [Google Scholar]
- 244.Abrams JH, Schulman P, White WB. Successful treatment of a monoamine oxidase inhibitor-tyramine hypertensive emergency with intravenous labetalol. N Engl J Med. 1985;313:52. [PubMed] [Google Scholar]
- 245.Guzzardi L Monoamine oxidase inhibitors In: Haddad LM, Winchester JF, eds. The Clinical Management of Poisoning and Drug Overdose. Philadelphia, PA: WB Saunders; 1983:496–502. [Google Scholar]
- 246.Louie AK, Louie EK, Lannon RA. Systemic hypertension associated with tricyclic antidepressant treatment in patients with panic disorder. Am J Cardiol. 1992;70:1306–1309. [DOI] [PubMed] [Google Scholar]
- 247.Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry. 1998;59:502–508. [DOI] [PubMed] [Google Scholar]
- 248.Amsterdam JD, Garcia-Espana F, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, Beasley C. Blood pressure changes during short-term fluoxetine treatment. J Clin Psychopharmacol. 1999;19:9–14. [DOI] [PubMed] [Google Scholar]
- 249.Mann SJ. Severe paroxysmal hypertension (pseudopheochromocytoma): understanding the cause and treatment. Arch Intern Med. 1999;159:670–674. [DOI] [PubMed] [Google Scholar]
- 250.Mann SJ. Labile and paroxysmal hypertension: common clinical dilemmas in need of treatment studies. Curr Cardiol Rep. 2015;17:99. doi: 10.1007/s11886-015-0646-0 [DOI] [PubMed] [Google Scholar]
- 251.Pepin JL, Borel AL, Tamisier R, Baguet JP, Levy P, Dauvilliers Y Hypertension and sleep: overview of a tight relationship. Sleep Med Rev. 2014;18:509–519. doi: 10.1016/j.smrv.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 252.Sayk F, Teckentrup C, Becker C, Heutling D, Wellhoner P, Lehnert H, Dodt C. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R191–R197. doi: 10.1152/ajpregu.00368.2009 [DOI] [PubMed] [Google Scholar]
- 253.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12(pt 1):63–68. [DOI] [PubMed] [Google Scholar]
- 254.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0 [DOI] [PubMed] [Google Scholar]
- 255.Jin ZN, Wei YX. Meta-analysis of effects of obstructive sleep apnea on the renin-angiotensin-aldosterone system. J Geriatr Cardiol. 2016;13:333–343. doi: 10.11909/j.issn.1671-5411.2016.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Itani O, Jike M, Watanabe N, Kaneita Y Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 257.Guralnick A, Bakris GL. Approaches for targeting blood pressure control in sleep disorders. Curr Opin Nephrol Hypertens. 2012;21:469–474. doi: 10.1097/MNH.0b013e32835623f5 [DOI] [PubMed] [Google Scholar]
- 258.Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Lévy P, Gozal E. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med. 2008;177:227–235. doi: 10.1164/rccm.200702-238OC [DOI] [PubMed] [Google Scholar]
- 259.Tamisier R, Pépin JL, Rémy J, Baguet JP, Taylor JA, Weiss JW, Lévy P. 14 Nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37:119–128. doi: 10.1183/09031936.00204209 [DOI] [PubMed] [Google Scholar]
- 260.Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, Tracy RP, Rueschman M, Blumenthal RS, Lewis EF, Bhatt DL, Redline S. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dernaika TA, Kinasewitz GT, Tawk MM. Effects of nocturnal continuous positive airway pressure therapy in patients with resistant hypertension and obstructive sleep apnea. J Clin Sleep Med. 2009;5:103–107. [PMC free article] [PubMed] [Google Scholar]
- 262.Weichler U, Herres-Mayer B, Mayer J, Weber K, Hoffmann R, Peter JH. Influence of antihypertensive drug therapy on sleep pattern and sleep apnea activity. Cardiology. 1991;78:124–130. doi: 10.1159/000174776 [DOI] [PubMed] [Google Scholar]
- 263.Pawlik MT, Hansen E, Waldhauser D, Selig C, Kuehnel TS. Clonidine premedication in patients with sleep apnea syndrome: a randomized, double-blind, placebo-controlled study. Anesth Analg. 2005;101:1374–1380. doi: 10.1213/01.ANE.0000180194.30741.40 [DOI] [PubMed] [Google Scholar]
- 264.Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin. 2016;11:81–89. doi: 10.1016/j.jsmc.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Guo X, Zheng L, Wang J, Zhang X, Zhang X, Li J, Sun Y. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta-analysis. Sleep Med. 2013;14:324–332. doi: 10.1016/j.sleep.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 266.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. [DOI] [PubMed] [Google Scholar]
- 267.Wang Y, Mei H, Jiang YR, Sun WQ, Song YJ, Liu SJ, Jiang F. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med. 2015;11:1047–1056. doi: 10.5664/jcsm.5024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, Bhatt DL; on behalf of the American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nilsson PM, Nilsson JA, Hedblad B, Berglund G. Sleep disturbance in association with elevated pulse rate for prediction of mortality: consequences of mental strain? J Intern Med. 2001;250:521–529. [DOI] [PubMed] [Google Scholar]
- 270.Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, Demeersman RE, Basner RC. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol (1985). 2005;98:2024–2032. doi: 10.1152/japplphysiol.00620.2004 [DOI] [PubMed] [Google Scholar]
- 271.Raheja P, Price A, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, Vongpatanasin W. Spironolactone prevents chlorthalidone-induced sympathetic activation and insulin resistance in hypertensive patients. Hypertension. 2012;60:319–325. doi: 10.1161/HYPERTENSIONAHA.112.194787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lloberes P, Lozano L, Sampol G, Romero O, Jurado MJ, Rios J, Untoria MD, Tovar JL. Obstructive sleep apnoea and 24-h blood pressure in patients with resistant hypertension. J Sleep Res. 2010;19:597–602. doi: 10.1111/j.1365-2869.2010.00839.x [DOI] [PubMed] [Google Scholar]
- 273.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–2277. [DOI] [PubMed] [Google Scholar]
- 274.Min HJ, Cho YJ, Kim CH, Kim DH, Kim HY, Choi JI, Lee JG, Park S, Cho HJ. Clinical features of obstructive sleep apnea that determine its high prevalence in resistant hypertension. Yonsei Med J. 2015;56:1258–1265. doi: 10.3349/ymj.2015.56.5.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Muxfeldt ES, Margallo VS, Guimarâes GM, Salles GF. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens. 2014;27:1069–1078. doi: 10.1093/ajh/hpu023 [DOI] [PubMed] [Google Scholar]
- 276.Walia HK, Li H, Rueschman M, Bhatt DL, Patel SR, Quan SF, Gottlieb DJ, Punjabi NM, Redline S, Mehra R. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med. 2014;10:835–843. doi: 10.5664/jcsm.3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–459. doi: 10.1378/chest.06-1442 [DOI] [PubMed] [Google Scholar]
- 278.Di Murro A, Petramala L, Cotesta D, Zinnamosca L, Crescenzi E, Marinelli C, Saponara M, Letizia C. Renin-angiotensin-aldosterone system in patients with sleep apnoea: prevalence of primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2010;11:165–172. doi: 10.1177/1470320310366581 [DOI] [PubMed] [Google Scholar]
- 279.Prejbisz A, Florczak E, Klisiewicz A, Dobrowolski P, Janaszek-Sitkowska H, Bieleń P, Szwench-Pietrasz E, Warchoł-Celińska E, Kołodziejczyk-Kruk S, Janas J, Kabat M, Imiela J, Sliwiński P, Januszewicz A. Relationship between primary aldosteronism and obstructive sleep apnoea, metabolic abnormalities and cardiac structure in patients with resistant hypertension. Endokrynol Pol. 2013;64:363–367. doi: 10.5603/EP.2013.0019 [DOI] [PubMed] [Google Scholar]
- 280.Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM, Calhoun DA. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24:532–537. doi: 10.1038/jhh.2009.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Krasińska B, Miazga A, Cofta S, Szczepaniak-Chichel L, Trafas T, Krasiński Z, Pawlaczyk-Gabriel K, Tykarski A. Effect of eplerenone on the severity of obstructive sleep apnea and arterial stiffness in patients with resistant arterial hypertension. Pol Arch Med Wewn. 2016;126:330–339. doi: 10.20452/pamw.3410 [DOI] [PubMed] [Google Scholar]
- 282.Yang L, Zhang H, Cai M, Zou Y, Jiang X, Song L, Liang E, Bian J, Wu H, Hui R. Effect of spironolactone on patients with resistant hypertension and obstructive sleep apnea. Clin Exp Hypertens. 2016;38:464–468. doi: 10.3109/10641963.2015.1131290 [DOI] [PubMed] [Google Scholar]
- 283.Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56:1077–1082. doi: 10.1161/HYPERTENSIONAHA.110.154427 [DOI] [PubMed] [Google Scholar]
- 284.Chiu KL, Ryan CM, Shiota S, Ruttanaumpawan P, Arzt M, Haight JS, Chan CT, Floras JS, Bradley TD. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174:1378–1383. doi: 10.1164/rccm.200607-927OC [DOI] [PubMed] [Google Scholar]
- 285.Redolfi S, Arnulf I, Pottier M, Bradley TD, Similowski T. Effects of venous compression of the legs on overnight rostral fluid shift and obstructive sleep apnea. Respir Physiol Neurobiol. 2011;175:390–393. doi: 10.1016/j.resp.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 286.Kasai T, Bradley TD, Friedman O, Logan AG. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J Hypertens. 2014;32:673–680. doi: 10.1097/HJH.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 287.Martínez-García MA, Capote F, Campos-Rodríguez F, Lloberes P, Díaz de Atauri MJ, Somoza M, Masa JF, González M, Sacristán L, Barbé F, Durán-Cantolla J, Aizpuru F, Mañas E, Barreiro B, Mosteiro M, Cebrián JJ, de la Peña M, García-Río F, Maimó A, Zapater J, Hernández C, Grau SanMarti N, Montserrat JM; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–2415. doi: 10.1001/jama.2013.281250 [DOI] [PubMed] [Google Scholar]
- 288.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, Chen G, Du B, McArdle N, Mukherjee S, Tripathi M, Billot L, Li Q, Lorenzi-Filho G, Barbe F, Redline S, Wang J, Arima H, Neal B, White DP, Grunstein RR, Zhong N, Anderson CS; SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 289.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061 [DOI] [PubMed] [Google Scholar]
- 290.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015 [DOI] [PubMed] [Google Scholar]
- 291.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–336. doi: 10.1161/HYPERTENSIONAHA.113.01060 [DOI] [PubMed] [Google Scholar]
- 292.Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, Ghiadoni L, Bernini M, Santoro G, Salvetti A. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008;26:2399–2405. doi: 10.1097/HJH.0b013e32831286fd [DOI] [PubMed] [Google Scholar]
- 293.Stehr CB, Mellado R, Ocaranza MP, Carvajal CA, Mosso L, Becerra E, Solis M, García L, Lavandero S, Jalil J, Fardella CE. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J Hypertens. 2010;28:2120–2126. doi: 10.1097/HJH.0b013e32833d0177 [DOI] [PubMed] [Google Scholar]
- 294.Gallay BJ, Ahmad S, Xu L, Toivola B, Davidson RC. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosterone-renin ratio. Am J Kidney Dis. 2001;37:699–705. [DOI] [PubMed] [Google Scholar]
- 295.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. [DOI] [PubMed] [Google Scholar]
- 296.Strauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens. 2003;17:349–352. doi: 10.1038/sj.jhh.1001554 [DOI] [PubMed] [Google Scholar]
- 297.Sang X, Jiang Y, Wang W, Yan L, Zhao J, Peng Y, Gu W, Chen G, Liu W, Ning G. Prevalence of and risk factors for primary aldosteronism among patients with resistant hypertension in China. J Hypertens. 2013;31:1465–1471. doi: 10.1097/HJH.0b013e328360ddf6 [DOI] [PubMed] [Google Scholar]
- 298.Carey RM. Diagnosing and managing primary aldosteronism in hypertensive patients: a case-based approach. Curr Cardiol Rep. 2016;18:97. doi: 10.1007/s11886-016-0774-1 [DOI] [PubMed] [Google Scholar]
- 299.Hoerger TJ, Simpson SA, Yarnoff BO, Pavkov ME, Ríos Burrows N, Saydah SH, Williams DE, Zhuo X. The future burden of CKD in the United States: a simulation model for the CDC CKD Initiative. Am J Kidney Dis. 2015;65:403–411. doi: 10.1053/j.ajkd.2014.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR, Chow EK, Kasiske BL, Kovesdy CP, Nadkarni GN, Shalev V, Segev DL, Coresh J, Lentine KL, Garg AX; Chronic Kidney Disease Prognosis Consortium. Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med. 2016;374:411–421. doi: 10.1056/NEJMoa1510491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kuznik A, Mardekian J, Tarasenko L. Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults with chronic kidney disease: an analysis of National Health and Nutritional Examination Survey data, 2001–2010. BMC Nephrol 2013;14:132. doi: 10.1186/1471-2369-14-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group. Chronic Renal Insufficiency Cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT Jr; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Saelen MG, Prøsch LK, Gudmundsdottir H, Dyrbekk D, Helge Hunderi O, Arnesen E, Paulsen D, Skjønsberg H, Os I. Controlling systolic blood pressure is difficult in patients with diabetic kidney disease exhibiting moderate-to-severe reductions in renal function. Blood Press. 2005;14:170–176. doi: 10.1080/08037050510008959 [DOI] [PubMed] [Google Scholar]
- 305.Hermann SM, Textor SM. Current concepts in the treatment of renovascular hypertension. Am J Hypertens. 2018;31:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609–615. [DOI] [PubMed] [Google Scholar]
- 307.Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol. 2014;113:687–690. doi: 10.1016/j.amjcard.2013.10.046 [DOI] [PubMed] [Google Scholar]
- 308.Textor SC. Progressive hypertension in a patient with “incidental” renal artery stenosis. Hypertension. 2002;40:595–600. [DOI] [PubMed] [Google Scholar]
- 309.Patel SM, Li J, Parikh SA. Renal artery stenosis: optimal therapy and indications for revascularization. Curr Cardiol Rep. 2015;17:623. doi: 10.1007/s11886-015-0623-7 [DOI] [PubMed] [Google Scholar]
- 310.Raman G, Adam GP, Halladay CW, Langberg VN, Azodo IA, Balk EM. Comparative effectiveness of management strategies for renal artery stenosis: an updated systematic review. Ann Intern Med. 2016;165:635–649. doi: 10.7326/M16-1053 [DOI] [PubMed] [Google Scholar]
- 311.Evans KL, Tuttle KR, Folt DA, Dawson T, Haller ST, Brewster PS, He W, Jamerson K, Dworkin LD, Cutlip DE, Murphy TP, D’Agostino RB Sr, Henrich W, Cooper CJ. Use of renin-angiotensin inhibitors in people with renal artery stenosis. Clin J Am Soc Nephrol. 2014;9:1199–1206. doi: 10.2215/CJN.11611113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chrysochou C, Foley RN, Young JF, Khavandi K, Cheung CM, Kalra PA. Dispelling the myth: the use of renin-angiotensin blockade in atheromatous renovascular disease. Nephrol Dial Transplant. 2012;27:1403–1409. doi: 10.1093/ndt/gfr496 [DOI] [PubMed] [Google Scholar]
- 313.Messerli FH, Bangalore S, Makani H, Rimoldi SF, Allemann Y, White CJ, Textor S, Sleight P Flash pulmonary oedema and bilateral renal artery stenosis: the Pickering syndrome. Eur Heart J. 2011;32:2231–2235. doi: 10.1093/eurheartj/ehr056 [DOI] [PubMed] [Google Scholar]
- 314.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis. 2014;63:186–197. doi: 10.1053/j.ajkd.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 315.Murphy TP, Cooper CJ, Pencina KM, D’Agostino R, Massaro J, Cutlip DE, Jamerson K, Matsumoto AH, Henrich W, Shapiro JI, Tuttle KR, Cohen DJ, Steffes M, Gao Q, Metzger DC, Abernethy WB, Textor SC, Briguglio J, Hirsch AT, Tobe S, Dworkin LD. Relationship of albuminuria and renal artery stent outcomes: results from the CORAL randomized clinical trial (Cardiovascular Outcomes With Renal Artery Lesions). Hypertension. 2016;68:1145–1152. doi: 10.1161/HYPERTENSIONAHA.116.07744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Weinberg I, Keyes MJ, Giri J, Rogers KR, Olin JW, White CJ, Jaff MR. Blood pressure response to renal artery stenting in 901 patients from five prospective multicenter FDA-approved trials. Catheter Cardiovasc Interv. 2014;83:603–609. doi: 10.1002/ccd.25263 [DOI] [PubMed] [Google Scholar]
- 317.Hackam DG, Duong-Hua ML, Mamdani M, Li P, Tobe SW, Spence JD, Garg AX. Angiotensin inhibition in renovascular disease: a population-based cohort study. Am Heart J. 2008;156:549–555. doi: 10.1016/j.ahj.2008.05.013 [DOI] [PubMed] [Google Scholar]
- 318.Parikh SA, Shishehbor MH, Gray BH, White CJ, Jaff MR. SCAI expert consensus statement for renal artery stenting appropriate use. Catheter Cardiovasc Interv. 2014;84:1163–1171. doi: 10.1002/ccd.25559 [DOI] [PubMed] [Google Scholar]
- 319.Boateng FK, Greco BA. Renal artery stenosis: prevalence of, risk factors for, and management of in-stent stenosis. Am J Kidney Dis. 2013;61:147–160. doi: 10.1053/j.ajkd.2012.07.025 [DOI] [PubMed] [Google Scholar]
- 320.Martell N, Rodriguez-Cerrillo M, Grobbee DE, López-Eady MD, Fernández-Pinilla C, Avila M, Fernández-Cruz A, Luque M. High prevalence of secondary hypertension and insulin resistance in patients with refractory hypertension. Blood Press. 2003;12:149–154. [DOI] [PubMed] [Google Scholar]
- 321.Schwartz GL. Screening for adrenal-endocrine hypertension: overview of accuracy and cost-effectiveness. Endocrinol Metab Clin North Am. 2011;40:279–294, vii. doi: 10.1016/j.ecl.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003;88:2656–2666. doi: 10.1210/jc.2002-030005 [DOI] [PubMed] [Google Scholar]
- 323.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr; Endocrine Society. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–1942. doi: 10.1210/jc.2014-1498 [DOI] [PubMed] [Google Scholar]
- 324.Cicala MV, Mantero F. Hypertension in Cushing’s syndrome: from pathogenesis to treatment. Neuroendocrinology. 2010;92(suppl 1):44–49. doi: 10.1159/000314315 [DOI] [PubMed] [Google Scholar]
- 325.Singh Y, Kotwal N, Menon AS. Endocrine hypertension: Cushing’s syndrome. Indian J Endocrinol Metab. 2011;15(suppl 4):S313–S316. doi: 10.4103/2230-8210.86973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Martins LC, Conceição FL, Muxfeldt ES, Salles GF. Prevalence and associated factors of subclinical hypercortisolism in patients with resistant hypertension. J Hypertens. 2012;30:967–973. doi: 10.1097/HJH.0b013e3283521484 [DOI] [PubMed] [Google Scholar]
- 327.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A; Endocrine Society. Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:2807–2831. doi: 10.1210/jc.2015-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Choudhary P, Canniffe C, Jackson DJ, Tanous D, Walsh K, Celermajer DS. Late outcomes in adults with coarctation of the aorta. Heart. 2015;101:1190–1195. doi: 10.1136/heartjnl-2014-307035 [DOI] [PubMed] [Google Scholar]
- 330.Canniffe C, Ou P, Walsh K, Bonnet D, Celermajer D. Hypertension after repair of aortic coarctation: a systematic review. Int J Cardiol. 2013;167:2456–2461. doi: 10.1016/j.ijcard.2012.09.084 [DOI] [PubMed] [Google Scholar]
- 331.Vriend JW, Mulder BJ. Late complications in patients after repair of aortic coarctation: implications for management. Int J Cardiol. 2005;101:399–406. doi: 10.1016/j.ijcard.2004.03.056 [DOI] [PubMed] [Google Scholar]
- 332.Luijendijk P, Bouma BJ, Vriend JW, Vliegen HW, Groenink M, Mulder BJ. Usefulness of exercise-induced hypertension as predictor of chronic hypertension in adults after operative therapy for aortic isthmic coarctation in childhood. Am J Cardiol. 2011;108:435–439. doi: 10.1016/j.amjcard.2011.03.063 [DOI] [PubMed] [Google Scholar]
- 333.Moltzer E, Mattace Raso FU, Karamermer Y, Boersma E, Webb GD, Simoons ML, Danser AH, van den Meiracker AH, Roos-Hesselink JW. Comparison of candesartan versus metoprolol for treatment of systemic hypertension after repaired aortic coarctation. Am J Cardiol. 2010;105:217–222. doi: 10.1016/j.amjcard.2009.08.674 [DOI] [PubMed] [Google Scholar]
- 334.White WB, Turner JR, Sica DA, Bisognano JD, Calhoun DA, Townsend RR, Aronow HD, Bhatt DL, Bakris GL. Detection, evaluation and treatment of severe and resistant hypertension: proceedings from an American Society of Hypertension Interactive Forum held in Bethesda, MA, U.S.S., October 10th 2013. J Am Soc Hypertens. 2014;8:743–757. [DOI] [PubMed] [Google Scholar]
- 335.Myers MG, Kaczorowski J, Paterson JM, Dolovich L, Tu K. Thresholds for diagnosing hypertension based on automated office blood pressure measurements and cardiovascular risk. Hypertension. 2015;66:489–495. doi: 10.1161/HYPERTENSIONAHA.115.05782 [DOI] [PubMed] [Google Scholar]
- 336.Armstrong D, Matangi M, Brouillard D, Myers MG. Automated office blood pressure: being alone and not location is what matters most. Blood Press Monit. 2015;20:204–208. doi: 10.1097/MBP.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 337.SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Myers MG, Campbell NR. Unfounded concerns about the use of automated office blood pressure measurement in SPRINT. J Am Soc Hypertens. 2016;10:903–905. doi: 10.1016/j.jash.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 339.Pickering TG, White WB; American Society of Hypertension Writing Group. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2008;2:119–124. doi: 10.1016/j.jash.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 340.Verdecchia P, Angeli F, Cavallini C. Ambulatory blood pressure for cardiovascular risk stratification. Circulation. 2007;115:2091–2093. doi: 10.1161/CIRCULATIONAHA.107.697086 [DOI] [PubMed] [Google Scholar]
- 341.National Institute for Health and Care Excellence. Clinical guideline 127: hypertension: clinical management of primary hypertension in adults. August 2011. http://guidance.nice.org.uk/CG127/Guidance. Accessed December 11, 2016.
- 342.Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192–204. doi: 10.7326/M14-1539 [DOI] [PubMed] [Google Scholar]
- 343.White WB. Ambulatory blood-pressure monitoring in clinical practice. N Engl J Med. 2003;348:2377–2378. doi: 10.1056/NEJMp030057 [DOI] [PubMed] [Google Scholar]
- 344.Nishizaka MK, Pratt-Ubunama M, Zaman MA, Cofield S, Calhoun DA. Validity of plasma aldosterone-to-renin activity ratio in African American and white subjects with resistant hypertension. Am J Hypertens. 2005;18:805–812. doi: 10.1016/j.amjhyper.2005.01.002 [DOI] [PubMed] [Google Scholar]
- 345.Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005;51:386–394. doi: 10.1373/clinchem.2004.041780 [DOI] [PubMed] [Google Scholar]
- 346.Sawka AM, Jaeschke R, Singh RJ, Young WF Jr. A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab. 2003;88:553–558. doi: 10.1210/jc.2002-021251 [DOI] [PubMed] [Google Scholar]
- 347.Semlitsch T, Jeitler K, Berghold A, Horvath K, Posch N, Poggenburg S, Siebenhofer A. Long-term effects of weight-reducing diets in people with hypertension. Cochrane Database Syst Rev. 2016;3:CD008274. doi: 10.1002/14651858.CD008274.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Siebenhofer A, Jeitler K, Horvath K, Berghold A, Posch N, Meschik J, Semlitsch T. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst Rev. 2016:3:CD007654. [DOI] [PubMed] [Google Scholar]
- 349.Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Li W, Lu Y, Yi S, Rensheng L, Iqbal R, Mony P, Yusuf R, Yusoff K, Szuba A, Oguz A, Rosengren A, Bahonar A, Yusufali A, Schutte AE, Chifamba J, Mann JF, Anand SS, Teo K, Yusuf S; PURE, EPIDREAM and ONTARGET/ TRANSCEND Investigators. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388:465–475. doi: 10.1016/S0140-6736(16)30467-6 [DOI] [PubMed] [Google Scholar]
- 350.McMahon EJ, Campbell KL, Bauer JD, Mudge DW. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev. 2015;18:CD010070. [DOI] [PubMed] [Google Scholar]
- 351.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129(suppl 2):S100-S101 and Circulation. 2015;131:e326]. Circulation. 2014;129(suppl 2):S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1 [DOI] [PubMed] [Google Scholar]
- 352.Pescatello LS, MacDonald HV, Lamberti L, Johnson BT. Exercise for hypertension: a prescription update integrating existing recommendations with emerging research. Curr Hypertens Rep. 2015;17:87. doi: 10.1007/s11900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004473. doi: 10.1161/JAHA.112.004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Guimaräes GV, Cruz LG, Tavares AC, Dorea EL, Fernandes-Silva MM, Bocchi EA. Effects of short-term heated water-based exercise training on systemic blood pressure in patients with resistant hypertension: a pilot study. Blood Press Monit. 2013;18:342–345. doi: 10.1097/MBP.0000000000000000 [DOI] [PubMed] [Google Scholar]
- 355.Dempsey PC, Sacre JW, Larsen RN, Straznicky NE, Sethi P, Cohen ND, Cerin E, Lambert GW, Owen N, Kingwell BA, Dunstan DW. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376–2382. doi: 10.1097/HJH.0000000000001101 [DOI] [PubMed] [Google Scholar]
- 356.Dempsey PC, Larsen RN, Sethi P, Sacre JW, Straznicky NE, Cohen ND, Cerin E, Lambert GW, Owen N, Kingwell BA, Dunstan DW. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care. 2016;39:964–972. doi: 10.2337/dc15-2336 [DOI] [PubMed] [Google Scholar]
- 357.Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, Shaw JE, Bertovic DA, Zimmet PZ, Salmon J, Owen N. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. doi: 10.2337/dc11-1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Larsen RN, Kingwell BA, Sethi P, Cerin E, Owen N, Dunstan DW. Breaking up prolonged sitting reduces resting blood pressure in over-weight/obese adults. Nutr Metab Cardiovasc Dis. 2014;24:976–982. doi: 10.1016/j.numecd.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 359.Pescatello LS, Murphy D, Costanzo D. Low-intensity physical activity benefits blood lipids and lipoproteins in older adults living at home. Age Ageing. 2000;29:433–439. [DOI] [PubMed] [Google Scholar]
- 360.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30:529–542. doi: 10.1007/s10654-015-0056-z [DOI] [PubMed] [Google Scholar]
- 361.Brook RD, Appel LJ, Rubenfire M, Ogedegbe G, Bisognano JD, Elliott WJ, Fuchs FD, Hughes JW, Lackland DT, Staffileno BA, Townsend RR, Rajagopalan S; on behalf of the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Nutrition, Physical Activity. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension. 2013;61:1360–1383. doi: 10.1161/HYP.0b013e318293645f [DOI] [PubMed] [Google Scholar]
- 362.Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta-analysis to optimize benefit. Hypertens Res. 2016;39:88–94. doi: 10.1038/hr.2015.111 [DOI] [PubMed] [Google Scholar]
- 363.Blumenthal JA, Sherwood A, Smith PJ, Mabe S, Watkins L, Lin PH, Craighead LW, Babyak M, Tyson C, Young K, Ashworth M, Kraus W, Liao L, Hinderliter A. Lifestyle modification for resistant hypertension: the TRIUMPH randomized clinical trial. Am Heart J. 2015;170:986–994.e5. doi: 10.1016/j.ahj.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Agarwal R, Sinha AD, Pappas MK, Ammous F. Chlorthalidone for poorly controlled hypertension in chronic kidney disease: an interventional pilot study. Am J Nephrol. 2014;39:171–182. doi: 10.1159/000358603 [DOI] [PubMed] [Google Scholar]
- 365.Goodfriend TL, Ball DL, Oelkers W, Bahr V. Torsemide inhibits aldosterone secretion in vitro. Life Sci. 1998;63:PL45–PL50. [DOI] [PubMed] [Google Scholar]
- 366.Garg JP, Elliott WJ, Folker A, Izhar M, Black HR; RUSH University Hypertension Service. Resistant hypertension revisited: a comparison of two university-based cohorts. Am J Hypertens. 2005;18(pt 1):619–626. doi: 10.1016/j.amjhyper.2004.11.021 [DOI] [PubMed] [Google Scholar]
- 367.Kjeldsen SE, Julius S, Dahlöf B, Weber MA. Physician (investigator) inertia in apparent treatment-resistant hypertension: insights from large randomized clinical trials: Lennart Hansson Memorial Lecture. Blood Press. 2015;24:1–6. doi: 10.3109/08037051.2014.946787 [DOI] [PubMed] [Google Scholar]
- 368.Roush GC, Sica DA. Diuretics for hypertension: a review and update. Am J Hypertens. 2016;29:1130–1137. doi: 10.1093/ajh/hpw030 [DOI] [PubMed] [Google Scholar]
- 369.DiNicolantonio JJ, Bhutani J, Lavie CJ, O’Keefe JH. Evidence-based diuretics: focus on chlorthalidone and indapamide. Future Cardiol. 2015;11:203–217. doi: 10.2217/fca.14.83 [DOI] [PubMed] [Google Scholar]
- 370.Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB, Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. doi: 10.1161/01.HYP.0000203309.07140.d3 [DOI] [PubMed] [Google Scholar]
- 371.Khosla N, Chua DY, Elliott WJ, Bakris GL. Are chlorthalidone and hydrochlorothiazide equivalent blood-pressure-lowering medications? J Clin Hypertens (Greenwich). 2005;7:354–356. [DOI] [PubMed] [Google Scholar]
- 372.Bakris GL, Sica D, White WB, Cushman WC, Weber MA, Handley A, Song E, Kupfer S. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med. 2012;125:1229.e1–1229.e10. doi: 10.1016/j.amjmed.2012.05.023 [DOI] [PubMed] [Google Scholar]
- 373.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society’s PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068. doi: 10.1016/S0140-6736(15)00257-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Zhao D, Liu H, Dong P, Zhao J. A meta-analysis of add-on use of spironolactone in patients with resistant hypertension. Int J Cardiol. 2017;233:113–117. doi: 10.1016/j.ijcard.2016.12.158 [DOI] [PubMed] [Google Scholar]
- 375.Wang C, Xiong B, Huang J. Efficacy and safety of spironolactone in patients with resistant hypertension: a meta-analysis of randomised controlled trials. Heart Lung Circ. 2016;25:1021–1030. doi: 10.1016/j.hlc.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 376.Rosa J, Widimský P, Waldauf P, Lambert L, Zelinka T, Táborský M, Branny M, Toušek P, Petrak O, Čurila K, Bednář F, Holaj R, Štrauch B, Václavík J, Nykl I, Krátká Z, Kocianova E, Jiravský O, Rappová G, Indra T, Widimský J Jr. Role of adding spironolactone and renal denervation in true resistant hypertension: one-year outcomes of randomized PRAGUE-15 study. Hypertension. 2016;67:397–403. doi: 10.1161/HYPERTENSIONAHA.115.06526 [DOI] [PubMed] [Google Scholar]
- 377.de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55:147–152. doi: 10.1161/HYPERTENSIONAHA.109.140988 [DOI] [PubMed] [Google Scholar]
- 378.Lazich I, Bakris GL . Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol. 2014;34:333–339. doi: 10.1016/j.semnephrol.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 379.Egan BM, Li J. Role of aldosterone blockade in resistant hypertension. Semin Nephrol. 2014;34:273–284. doi: 10.1016/j.semnephrol.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 380.Parthasarathy HK, Ménard J, White WB, Young WF Jr, Williams GH, Williams B, Ruilope LM, McInnes GT, Connell JM, MacDonald TM. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J Hypertens. 2011;29:980–990. doi: 10.1097/HJH.0b013e3283455ca5 [DOI] [PubMed] [Google Scholar]
- 381.White WB, Weber MA, Sica D, Bakris GL, Perez A, Cao C, Kupfer S. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57:413–420. doi: 10.1161/HYPERTENSIONAHA.110.163402 [DOI] [PubMed] [Google Scholar]
- 382.Bakris GL, Sica D, Weber M, White WB, Roberts A, Perez A, Cao C, Kupfer S. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. J Clin Hypertens (Greenwich). 2011;13:81–88. doi: 10.1111/j.1751-7176.2010.00425.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Bönner G, Bakris GL, Sica D, Weber MA, White WB, Perez A, Cao C, Handley A, Kupfer S. Antihypertensive efficacy of the angiotensin receptor blocker azilsartan medoxomil compared with the angiotensin-converting enzyme inhibitor ramipril. J Hum Hypertens. 2013;27:479–486. doi: 10.1038/jhh.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Byyny RL, Shannon T, Schwartz LA, Rotolo C, Jungerwirth S. Efficacy and safety of nifedipine coat-core versus amlodipine in patients with mild to moderate essential hypertension: comparison of 24-hour mean ambulatory diastolic blood pressure. J Cardiovasc Pharmacol Ther. 1997;2:77–84. doi: 10.1177/107424849700200201 [DOI] [PubMed] [Google Scholar]
- 385.Kuga K, Xu DZ, Ohtsuka M, Aonuma K, Lau AH, Watanabe Y, Ohtsuka K. Comparison of daily anti-hypertensive effects of amlodipine and nifedipine coat-core using ambulatory blood pressure monitoring: utility of “hypobaric curve” and “hypobaric area.” Clin Exp Hypertens. 2011;33:231–239. doi: 10.3109/10641963.2011.583968 [DOI] [PubMed] [Google Scholar]
- 386.Tanaka T, Miura S, Tanaka M, Uehara Y, Hirano T, Saku K. Efficacies of controlling morning blood pressure and protecting the kidneys by treatment with valsartan and nifedipine CR or valsartan and amlodipine (MONICA study). J Clin Med Res. 2013;5:432–440. doi: 10.4021/jocmr1563w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Sun Y, Yu X, Liu J, Zhou N, Chen L, Zhao Y, Li X, Wang J, Cui L. Effect of bedtime administration of blood-pressure lowering agents on ambulatory blood pressure monitoring results: a meta-analysis. Cardiol J. 2016;23:473–481. doi: 10.5603/CJ.a2016.0027 [DOI] [PubMed] [Google Scholar]
- 388.Szauder I, Csajági E, Major Z, Pavlik G, Ujhelyi G. Treatment of hypertension: favourable effect of the twice-daily compared to the once-daily (evening) administration of perindopril and losartan. Kidney Blood Press Res. 2015;40:374–385. doi: 10.1159/000368513 [DOI] [PubMed] [Google Scholar]
- 389.Julius S, Palatini P, Kjeldsen SE, Zanchetti A, Weber MA, McInnes GT, Brunner HR, Mancia G, Schork MA, Hua TA, Holzhauer B, Zappe D, Majahalme S, Jamerson K, Koylan N. Usefulness of heart rate to predict cardiac events in treated patients with high-risk systemic hypertension. Am J Cardiol. 2012;109:685–692. doi: 10.1016/j.amjcard.2011.10.025 [DOI] [PubMed] [Google Scholar]
- 390.Okin PM, Kjeldsen SE, Julius S, Hille DA, Dahlof B, Devereux RB. Effect of changing heart rate during treatment of hypertension on incidence of heart failure. Am J Cardiol. 2012;109:699–704. doi: 10.1016/j.amjcard.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 391.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN; African- American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934 [DOI] [PubMed] [Google Scholar]
- 392.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, Smith R, Dunkman WB, Loeb H, Wong M. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–310. doi: 10.1056/NEJM199108013250502 [DOI] [PubMed] [Google Scholar]
- 393.Dulce RA, Yiginer O, Gonzalez DR, Goss G, Feng N, Zheng M, Hare JM. Hydralazine and organic nitrates restore impaired excitation-contraction coupling by reducing calcium leak associated with nitroso-redox imbalance. J Biol Chem. 2013;288:6522–6533. doi: 10.1074/jbc.M112.412130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Gogia H, Mehra A, Parikh S, Raman M,Ajit-Uppal J, Johnson JV, Elkayam U. Prevention of tolerance to hemodynamic effects of nitrates with concomitant use of hydralazine in patients with chronic heart failure. J Am Coll Cardiol. 1995;26:1575–1580. doi: 10.1016/0735-1097(95)00368-1 [DOI] [PubMed] [Google Scholar]
- 395.Sica DA. Minoxidil:an underused vasodilator for resistant or severe hypertension. J Clin Hypertens. 2004;6:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Professional Testing Corporation. American Hypertension Specialist Certification Program (AHSCP). http://www.ptcny.com/clients/ahscp. Accessed April 5, 2018.
- 397.Elliott WJ, Egen B, Giles TD, Bakris GL, White WB, Sansone TM. Rationale for establishing a mechanism to increase reimbursement to hypertension specialists. J Clin Hypertens. 2013;16:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041 [DOI] [PubMed] [Google Scholar]
- 399.Grassi G, Bertoli S, Seravalle G. Sympathetic nervous system: role in hypertension and in chronic kidney disease. Curr Opin Nephrol Hypertens. 2012;21:46–51. doi: 10.1097/MNH.0b013e32834db45d [DOI] [PubMed] [Google Scholar]
- 400.Evelyn KA, Alexander F, Cooper SR. Effect of sympathectomy on blood pressure in hypertension; a review of 13 years’ experience of the Massachusetts General Hospital. J Am Med Assoc. 1949;140:592–602. [DOI] [PubMed] [Google Scholar]
- 401.Worthley SG, Tsioufis CP, Worthley MI, Sinhal A, Chew DP, Meredith IT, Malaiapan Y, Papademetriou V. Safety and efficacy of a multielectrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–2140. doi: 10.1093/eurheartj/eht197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmeider RE, Bohm M. Renal sympathetic denervation in patients with treatment resistant hypertension (the Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 403.Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, Bauer A, Ott C, Blessing E, Sobotka PA, Krum H, Schlaich M, Esler M, Bohm M. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132–140. doi: 10.1161/CIRCULATIONAHA.112.000949 [DOI] [PubMed] [Google Scholar]
- 404.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670 [DOI] [PubMed] [Google Scholar]
- 405.White WB, Galis ZS, Henegar J, Kandzari DE, Victor R, Sica D, Townsend RR, Turner JR, Virmani R, Mauri L. Renal denervation therapy for hypertension: pathways for moving development forward. J Am Soc Hypertens. 2015;9:341–350. doi: 10.1016/j.jash.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 406.Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier-Véhier C, Courand PY, Lantelme P, Denolle T, Dourmap-Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–1965. doi: 10.1016/S0140-6736(14)61942-5 [DOI] [PubMed] [Google Scholar]
- 407.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Bohm M; SPYRAL HTN-OFF MED Trial Investigators. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390:2160–2170. doi: 10.1016/S0140-6736(17)32281-X [DOI] [PubMed] [Google Scholar]
- 408.Bisognano JD, Kaufman CL, Bach DS, Lovett EG, de Leeuw P; DEBuT-HT and Rheos Feasibility Trial Investigators. Improved cardiac structure and function with chronic treatment using an implantable device in resistant hypertension: results from European and United States trials of the Rheos system. J Am Coll Cardiol. 2011;57:1787–1788. doi: 10.1016/j.jacc.2010.11.048 [DOI] [PubMed] [Google Scholar]
- 409.Bakris GL, Nadim MK, Haller H, Lovett EG, Schafer JE, Bisognano JD. Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long-term follow-up in the Rheos Pivotal Trial. J Am Soc Hypertens. 2012;6:152–158. doi: 10.1016/j.jash.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 410.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the doubleblind, randomized, placebo-controlled Rheos pivotal trial. J Am Coll Cardiol. 2011;58:765–773. doi: 10.1016/j.jacc.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 411.ClinicalTrials.gov. Controlling and Lowering Blood Pressure With The MOBIUSHD™ (CALM-FIM_EUR). https://clinicaltrials.gov/ct2/show/NCT01911897?term=CALM+FIM+US&rank=1. Accessed April 5, 2018.
- 412.ClinicalTrials.gov. Controlling and Lowering Blood Pressure With The MOBIUSHD™ (CALM-FIM_US). https://clinicaltrials.gov/ct2/show/NCT01831895?term=CALM+FIM+US&rank=2. Accessed April 5, 2018.
- 413.Spiering W, Williams B, Van der Heyden J, van Kleef M, Lo R, Versmissen J, Moelker A, Kroon A, Reuter H, Ansel G, Stone GW, Bates M; CALM-FIM_EUR investigators. Endovascular baroreflex amplification for resistant hypertension: a safety and proof-of-principle clinical study. Lancet. 2017;390:2655–2661. doi: 10.1016/S0140-6736(17)32337-1 [DOI] [PubMed] [Google Scholar]
- 414.Lobo MD, Sobotka PA, Stanton A, Cockcroft JR, Sulke N, Dolan E, van der Giet M, Hoyer J, Furniss SS, Foran JP, Witkowski A, Januszewicz A, Schoors D, Tsioufis K, Rensing BJ, Scott B, Ng GA, Ott C, Schmieder RE; ROX CONTROL HTN Investigators. Central arteriovenous anastomosis for the treatment of patients with uncontrolled hypertension (the ROX CONTROL HTN study): a randomised controlled trial. Lancet. 2015;385:1634–1641. doi: 10.1016/S0140-6736(14)62053-5 [DOI] [PubMed] [Google Scholar]
- 415.Mabin T, Sapoval M, Cabane V, Stemmett J, Iyer M. First experience with endovascular ultrasound renal denervation for the treatment of resistant hypertension. EuroIntervention. 2012;8:57–61. doi: 10.4244/EIJV8I1A10 [DOI] [PubMed] [Google Scholar]
- 416.Li P, Tjen-A-Looi SC, Guo ZL, Longhurst JC. An arcuate-ventrolateral periaqueductal gray reciprocal circuit participates in electroacupuncture cardiovascular inhibition. Auton Neurosci. 2010;158:13–23. doi: 10.1016/j.autneu.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Elijovich F, Weinberger MH, Anderson CA, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL; on behalf of the American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; and Stroke Council. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 2016;68:e7–e46. doi: 10.1161/HYP.0000000000000047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.