Abstract
Purpose of review
Since the discovery of human leukocyte antigen (HLA) in the 1950s, there has been great interest in the role of antibodies in posttransplant rejection. The development of the lymphocyte toxicity test by Terasaki et al. in the 1960s was the first step toward understanding the role of antibodies in posttransplant rejection.
Recent findings
Subsequently, various organs have been transplanted and improving posttransplant outcomes have become a focus of research. In particular, methods to measure antibodies that affect posttransplant outcomes, including anti-HLA antibodies, and methods to desensitize patients from specific antibodies have been explored. One recent method for measuring antibodies is called the solid-phase assay, which uses purified HLA fixed to microbeads. This assay does not use donor lymphocytes and allows clinicians to test the reactivity of patient serum against a panel of antibodies. It has also enabled the identification of specific anti-HLA antibodies using a single HLA.
Summary
In addition to advances in methods to measure and analyze anti-HLA antibodies, the clinical impact of non-HLA antibodies has also received much attention recently.
Keywords: crossmatching, donor-specific antibodies, nonhuman leukocyte antigen antibodies, sensitization, solid-phase assay
INTRODUCTION
To properly discuss antihuman leukocyte antigen (HLA) antibodies in the context of pretransplant antibodies, a brief historical account is necessary. Dausset discovered HLA antigens in the 1950s as a type of protein on leukocytes. HLA antibodies were subsequently found to be a cause of rejection in patients with kidney transplants [1].
In the 1960s, Terasaki and McClelland developed the lymphocyte cytotoxicity test (LCT) to detect anti-HLA antibodies [2]. The LCT is a useful method of detection that is still currently used [2]. Subsequently, various other methods of detection that do not rely on donor lymphocytes have been developed, including flow cytometry crossmatching (FCM) in the 1980s, which uses flow cytometry to crossmatch lymphocytes with high sensitivity; enzyme-linked immunosorbent assay in the 1990s, which uses purified HLA antigens fixed to microplates; and solid-phase assay, which uses purified HLA antigens fixed to microbeads [3].
Box 1.
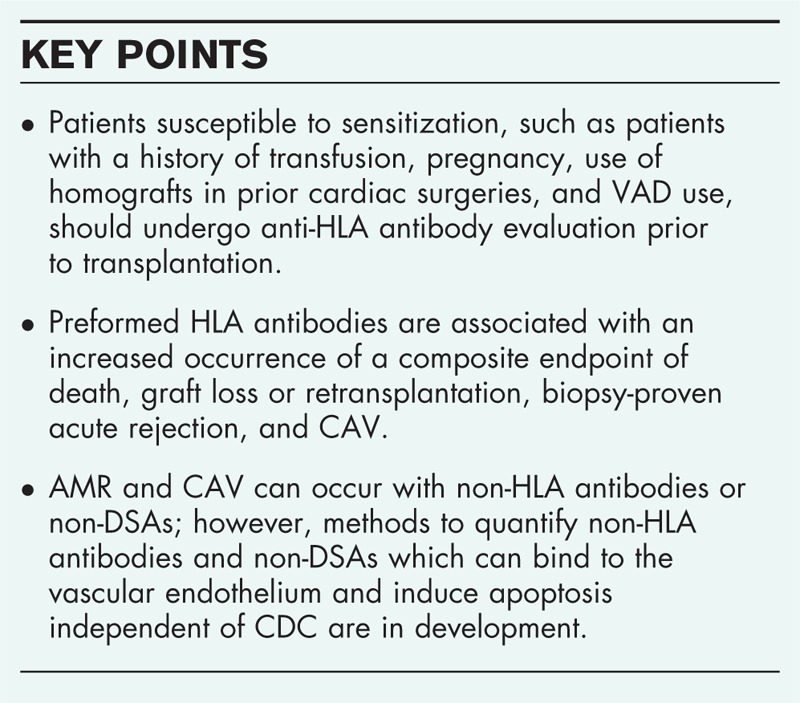
no caption available
MECHANISM OF ANTIBODY PRODUCTION AND ALLOGRAFT INJURY BY ANTIBODIES IN SENSITIZED PATIENTS
HLA molecules are categorized as class I (A, B, C) or class II (DR, DQ, DP). They are located in different regions of chromosome 6. HLA expression on the cell surface enables recognition of self from nonself. Class I HLA molecules are constitutively expressed on all nucleated cells, whereas class II HLA expression is restricted to B cells, activated T cells, and antigen-presenting cells (APCs). Class II expression may be induced on certain cell types, such as endothelial cells, under the influence of cytokine activation, which occurs with ischemia–reperfusion injury. HLA class I or II expression on allograft vascular endothelial cells can account for rejection occurring in the presence of class I or class II donor-specific antibodies (DSAs).
Antibody production begins with the exposure of naïve B cells to an antigen in the presence of APCs or T-helper cells in secondary lymphoid tissues. These stimulated B cells become either plasmablasts secreting low-affinity antibodies or activated B cells that interact with follicular dendritic and T-helper follicular cells to form germinal centers [4]. B cells undergo proliferation, hypermutation, and affinity maturation to become memory B cells or differentiated plasma cells that secrete high-affinity antibodies. Memory B cells circulate through secondary lymphoid organs and in the peripheral circulation. Memory B cells rapidly proliferate upon reexposure to an antigen and differentiate into plasma cells that produce high-affinity antibodies.
Allograft injury by antibodies occurs predominantly through complement activation. Binding of antibodies to HLA antigen results in activation of C1q and the complement cascade. Complement-independent injury by DSA also occurs through Fc receptor recruitment of inflammatory cells and the release of inflammatory mediators. Antimajor histocompatibility complex (MHC) antibodies may either result in direct injury to the capillary endothelium or indirect injury via complement fixation or recruitment of inflammatory cells with Fc receptors [5]. As a result, cellular inflammation, thrombosis, hemorrhage, and lysis cause cardiac allograft dysfunction.
RISK FACTORS FOR SENSITIZATION
Risk factors for sensitization include blood transfusion, prior transplantation, pregnancy, use of homografts in prior cardiac surgeries, and ventricular assist device (VAD) use prior to transplantation [6–10,11▪▪].
Among transfusion recipients, 20% demonstrate sensitization, compared with 3% who do not [12]. Multiparous women are at risk of sensitization to paternal antigens [13]. VADs increase the risk of sensitization because of the higher likelihood of needing blood transfusions, although biomaterials and textured surfaces have also been implicated in increasing immunologic risk through allosensitization. Half of all patients currently undergoing heart transplantation are now bridged with VADs [14]. Sensitization occurs most frequently before transplantation when the underlying condition is myocarditis or repeated infections during VAD use. Sensitization after VAD implantation increases the risk of posttransplant rejection, including hyperacute rejection and primary graft dysfunction [15,16].
CLINICAL IMPLICATIONS OF SENSITIZATION
In heart transplant patients, patients with panel-reactive antibody (PRA) more than 10% have been shown to be at risk for earlier and more severe rejection as well as worse survival [17–21]. In a study involving 8160 heart transplant recipients, Nwakanma et al.[22] demonstrated that sensitization defined as PRA more than 25% is associated with a significant decrease in survival up to 5 years after heart transplantation (P < 0.007) and a higher incidence of rejection (P = 0.017). At 5 years, survival in patients with PRA more than 25% was 65% compared with 74% in patients with PRA less than equal to 25%. More recently, Potena et al.[23] demonstrated that pretransplantation HLA antibodies are associated with worse survival (65 ± 9% versus 82 ± 3%; P = 0.02). Based on these studies, sensitization prior to cardiac transplantation is a well-established risk factor for survival.
The most severe conditions associated with DSAs in heart transplantation are hyperacute rejection and acute posttransplant heart failure, which can occur within minutes to hours after transplantation. Severe posttransplant cardiac dysfunction occurs when a high titer of DSAs reacts with the vascular endothelium of the transplanted heart in the recipient even during transplantation, which initiates a complement-binding reaction in blood vessels leading to cell death, activation of inflammatory cells, and platelet aggregation within minutes to hours after reperfusion. Ultimately, this causes diffuse ischemia and necrosis in the transplanted heart, which is life-threatening [24–27].
Although HLA class II antigens are not normally expressed in the transplanted heart, expression can be induced through inflammation and dysfunction introduced during organ extraction and storage [28▪]. Furthermore, non-HLA antibodies can also cause acute antibody-mediated rejection (AMR) [29].
Given that positive DSA status results in poor postheart transplant prognosis, many countries have instituted screening tests for HLA antibodies in patients waiting for transplantation. As a result, the incidence of hyperacute rejection has decreased dramatically [21,30]. However, AMR is now known to contribute to the progression of cardiac allograft vasculopathy (CAV) [31]. It has recently become a focus of attention more than 10 years after it was first described [32].
Regarding the timing of sensitization, Ho et al.[33] reported that the 15-year survival rate was 70% in patients negative for anti-HLA antibodies, 71% in patients positive for anti-HLA antibodies only before transplantation, and 56% in patients positive for anti-HLA antibodies before and after transplantation. Patients with de novo antibodies that appear more than one year after transplantation had the worst prognosis, with a 15-year survival rate of 47%. Recent data demonstrated that preformed HLA antibodies are associated with an increased occurrence of a composite endpoint of death, graft loss or retransplantation, biopsy-proven acute rejection, and CAV (P < 0.04). HLA class I antibodies were more prevalent among those who reached this endpoint than those who did not (P < 0.01), but there were no differences in anticlass II antibodies between patients who met the endpoint and those who did not [34].
CAV has been reported to progress rapidly in patients with HLA class II DSA [35]. Tran et al.[36] found that 88% of prolonged DSA expression was HLA class II antibodies in pediatric transplant patients. The presence of DQ antibodies resulted in the worst prognosis [37▪].
Recent strategies to combat DSAs include desensitization therapy followed by a short waiting time to transplantation [38,39]. Other measures such as minimizing transfusions or reducing exposure to HLA antigens through radiation therapy or leukocyte removal are also important [26,40].
METHODS TO ASSESS HUMAN LEUKOCYTE ANTIGEN ANTIBODIES AND CROSSMATCHING
In 1969, crossmatching based on complement-dependent cytotoxicity (CDC) was used for detecting DSAs associated with acute rejections. CDC detects complement-dependent antibodies in patient serum using donor lymphocytes as the standard. Thus, it is sometimes called LCT. Since it was first described, LCT has become widely used. Its sensitivity has improved through the addition of antihuman globulins (AHGs) [41]. However, an adequate correlation between LCT results and clinical symptoms has not been well described, and other highly sensitive methods of detecting antibodies such as FCM and solid-phase assays using purified HLA antigens have been developed Table 1.
Table 1.
Anti-HLA antibody detection methods
 |
|||
| Method | CDC (LCT) | FCM | PRA (solid-phase assay) |
| Target | Donor lymphocytes | Donor lymphocytes | Purified HLA antigens |
| Antibody specificity | Donor-specific complement-binding antibodies | Donor-specific complement-binding/nonbinding antibodies | Known HLA antibodies Non-HLA antibodies |
| Sensitivity | Low Detects only complement-binding antibodies | High Detects both complement-binding and nonbinding antibodies | High Detects both complement-binding and nonbinding antibodies |
| Effects of medications | Influenced by cytotoxic antibodies (ATG, rituximab, and so on) | Influenced by antibody therapies (high-dose IVIG, ATG, rituximab) | Influenced by antibody therapies (high-dose IVIG, ATG, rituximab) |
ATG, Antithymocyte globulin; CDC, complement-dependent cytotoxicity; FCM, flow cytometry crossmatching; IVIG, intravenous immunoglobulin; LCT, lymphocyte cytotoxicity test; PRA, panel-reactive antibody.
Crossmatching can be subdivided into two major types: direct crossmatching, which involves direct reactions between patient serum and donor lymphocytes, and virtual crossmatching, which uses immobilized HLA antigens instead of donor lymphocytes. The most effective crossmatching method is to first use highly sensitive virtual crossmatching followed by direct crossmatching as a confirmatory test.
The possibility of false-positive reactions exists in patients waiting for organ transplants, as they are sometimes treated with antibody therapies such as rituximab. Therefore, antibody detection tests should be performed before antibody-based therapy.
Direct crossmatching
Complement-dependent cytotoxicity or lymphocyte cytotoxicity test method
The method involves reacting patient serum with donor lymphocytes, adding rabbit complement, and visualizing cell damage because of the antigen–antibody reactions using a dye such as eosin. The advantages of this method include the ability to detect cell damage caused by non-HLA antibodies because the reaction conditions closely mimic in-vivo conditions. In contrast, disadvantages include difficulties in preparing donor lymphocytes and variations in results on the basis of technical expertise and experience, as part of the method involves visualization. Nonspecific reactions due to IgM antibodies may also occur, hampering detection and necessitating care when using this method. Although attempts at increasing the sensitivity of antibody detection have been made, for example, by introducing AHGs before the addition of the rabbit complement (AHG–LCT method), its sensitivity is lower compared with FCM method and solid-phase assay.
Flow cytometry crossmatching method
The method involves reacting patient serum with donor lymphocytes and adding a fluorescent-labeled antihuman immunoglobulin secondary antibody, which labels the antibodies bound to lymphocytes. FCM can then be used to detect shifts in the distribution of fluorescence signals. As FCM involves counting bound antibodies and lymphocytes, this method can be used to detect anti–non-HLA antibodies; however, it cannot be used to assess complement-binding antibodies. FCM is more sensitive at detecting physiological reactions than LCT. Consequently, this method is widely used for crossmatching in countries with more patients requiring organ transplant.
Virtual crossmatching
The method involves reacting patient serum with HLA antigens fixed onto microbeads and detecting signals from bound anti-HLA antibodies using a specialized device (solid-phase assay). The platform for detection includes a flow cytometer [42] or Luminex [43]. Depending on the type of the purified HLA antigen on the beads, panel reactive antibodies (PRAs) or the single-antigen bead (SAB) method can be used.
Panel-reactive antibodies
Traditionally, PRA was conducted used human lymphocytes; however, currently, the microbeads on the panel are coated with purified HLA antigens from human lymphocytes.
Single-antigen bead assay
The microbeads are coated with purified HLA antigens synthesized through genetic engineering. A specific anti-HLA antibody can be detected using a single HLA antigen of interest. SAB assays are now commonly deployed for determination of multiple antibody specificities and antibody quantification.
MEAN FLUORESCENCE INTENSITY
In the Luminex method, purified HLA antigens are fixed onto fluorescent polystyrene Luminex beads and reacted with anti-HLA antibodies in patient serum. The antibody–bead complex is detected with a fluorescent phycoerythrin-labeled antihuman immunoglobulin as a secondary antibody via the Luminex platform. Fluorescent-labeled detection occurs via a red laser that detects the type of Luminex bead, and therefore the HLA antigen on the bead, and a green laser that detects phycoerythrin on the secondary antibody.
Mean fluorescence intensity (MFI) is used as a readout of Luminex fluorescence values and represents the fluorescence intensity of the signal emitted by the phycoerythrin of each Luminex bead. Laboratories will typically perform validation studies to determine the relationship between antibody levels determined using SABs and flow crossmatching. For this purpose, the strength of antibody binding on SABs as represented by MFI is compared with the degree of flow crossmatching.
In general, MFI levels more than 1000 or more than 5000 are used for reporting positive results; levels more than 8000 or more than 10,000 might correspond to cytotoxic antibodies and are clinically significant. MFI is affected by several technical and biologic factors. Antigen density on beads may vary depending on HLA molecule type and assay manufacturer or batch. Antigen density on beads may not reflect the natural expression of HLA molecules on cells in vivo. Therefore, HLA antibodies may remain undetected if they are unable to bind to the distorted HLA molecule. Conversely, clinically irrelevant antibodies that bind to denatured but not intact antigen may be detected.
C1q ASSAY
Conventional solid-phase assays do not discriminate between complement-fixing and non-complement-fixing antibodies and the intensity of antibodies by MFI may not be the best test of potential cytotoxicity because not all antibodies at high intensity may be detrimental to graft function. A novel C1q assay, based on the binding of HLA antibodies to C1q, which is required for the activation of the complement cascade through the classical pathway that leads to cell injury and death, has been developed [44]. The C1q assay appears to be much more sensitive than the standard CDC assay at detecting complement-fixing antibodies; however, they reported that C1q fixation is independent of MFI values [45]. In addition, Schaub et al.[46] have demonstrated that a negative C1q assay result does not always indicate the absence of strong complement-binding IgG subclasses.
NONHUMAN LEUKOCYTE ANTIGEN ANTIBODIES
AMR can occur with non-DSA or non-HLA antibodies. These antibodies can bind to the vascular endothelium and induce apoptosis independent of CDC. Representative examples include MHC I chain-related gene A (MICA) [47,48], angiotensin II type I receptor (AT1R) [49,50▪▪], endothelin-1 type A receptor (ETAR) [51], endothelial cell antigens [52], vimentin [53], K-α-1-tubulin [54], collagen-V [55], anticardiac myosin antibody [56], and other non-HLA IgM antibodies. In cardiac transplantation, anti-MICA and antiendothelial antibodies have been associated with increased AMR [57] and the development of CAV [47,56]. AT1R and ETAR have been implicated in the development of acute cellular rejection, AMR, and early-onset microvasculopathy [51].
These non-HLA antibodies have been reported to cause posttransplant AMR and CAV and worsen survival [54,58–60]. However, methods to quantify non-DSA and non-HLA antibodies are in development. Currently, there are no established desensitization therapies for non-DSA and non-HLA antibodies.
MONITORING OF SENSITIZED PATIENTS AWAITING TRANSPLANTATION
Circulating antibodies need to be periodically monitored while awaiting heart transplantation. If PRAs increase to above 10%, further evaluations are required. For example, details such as the complement-binding ability of the detected antibodies and specificity toward anti-HLA class I and II (such as HLA A, B, Cw, DR, and DQ antigens) are required, in addition to determining DSA levels, which might increase the risk of rejection [38,61]. Patients susceptible to sensitization, such as patients with a history of PRA-positive assay results, transfusion, pregnancy, homograft transplantation, transplantation, and VAD use, should undergo anti-HLA antibody evaluation prior to transplantation [61].
Anti-HLA antibodies should be periodically monitored in patients undergoing desensitization therapy until a compatible donor can be found. Anti-HLA antibody testing should be performed every 6 months for stable nonsensitized patients. Testing should occur 2–4 weeks after transfusion in heart transplant candidates. Currently, there are no recommendations for the timing of anti-HLA antibody testing in patients with infections or using VADs. Each medical institution is responsible for determining its own testing schedules.
CONCLUSION
Since the development of the LCT in the 1960s by Terasaki et al., antibodies have been known to affect posttransplant outcomes. In particular, the solid-phase assay, which involves fixing purified HLA onto microbeads, enabled pretransplant reactivity of patient serum to be determined without using donor lymphocytes. The solid-phase assay has allowed specific anti-HLA antibodies to be identified. Advances in methods to measure and analyze anti-HLA antibodies, as well as the clinical impact of non-HLA antibodies, is becoming a focus of research. Future studies should focus on the impact of non-HLA antibodies on posttransplant prognosis.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Terasaki PI, Kreisler M, Mickey RM. Presensitization and kidney transplant failures. Postgrad Med J 1971; 47:89–100. [PMC free article] [PubMed] [Google Scholar]
- 2.Terasaki PI, McClelland JD. Microdroplet assay of human serum cytotoxins. Nature 1964; 204:998–1000. [DOI] [PubMed] [Google Scholar]
- 3.Pei R, Lee JH, Shih NJ, et al. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation 2003; 75:43–49. [DOI] [PubMed] [Google Scholar]
- 4.Stegall MD, Dean PG, Gloor J. Mechanisms of alloantibody production in sensitized renal allograft recipients. Am J Transplant 2009; 9:998–1005. [DOI] [PubMed] [Google Scholar]
- 5.Djamali AD, Kaufman TM, Ellis W, et al. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant 2014; 14:255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray RA, Harris SB, Josephson CD, et al. Unappreciated risk factors for transplant patients: HLA antibodies in blood components. Hum Immunol 2004; 65:240–244. [DOI] [PubMed] [Google Scholar]
- 7.Lietz K, John R, Kocher A, et al. Increased prevalence of autoimmune phenomena and greater risk for alloreactivity in female heart transplant recipients. Circulation 2001; 104 Suppl 1:I177–I183. [DOI] [PubMed] [Google Scholar]
- 8.Mehra MR, Uber PA, Uber WE, et al. Allosensitization in heart transplantation: implications and management strategies. Curr Opin Cardiol 2003; 18:153–158. [DOI] [PubMed] [Google Scholar]
- 9.Rebibou JM, Chabod J, Alcalay D, et al. Flow cytometric evaluation of pregnancy-induced anti-HLA immunization and blood transfusion-induced reactivation. Transplantation 2002; 74:537–540. [DOI] [PubMed] [Google Scholar]
- 10.Welters MJ, Oei FB, Witvliet MD, et al. A broad and strong humoral immune response to donor HLA after implantation of cryopreserved human heart valve allografts. Hum Immunol 2002; 63:1019–1025. [DOI] [PubMed] [Google Scholar]
- 11▪▪.See SB, Clerkin KJ, Kennel PJ, et al. Ventricular assist device elicits serum natural IgG that correlates with the development of primary graft dysfunction following heart transplantation. J Heart Lung Transplant 2017; 36:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study shows that VAD support elicits IgG polyreactive natural antibodies reactive to apoptotic cells and oxidized epitopes, and these findings further support the idea of a broad and nonspecific B-cell activation by VAD, resulting in IgG sensitization.
- 12.Leffell MS, Kim D, Vega RM, et al. Red blood cell transfusions and the risk of allosensitization in patients awaiting primary kidney transplantation. Transplantation 2014; 97:525–533. [DOI] [PubMed] [Google Scholar]
- 13.Reed E, Beer AE, Hutcherson H, et al. The alloantibody response of pregnant women and its suppression by soluble HLA antigens and antiidiotypic antibodies. J Reprod Immunol 1991; 20:115–128. [DOI] [PubMed] [Google Scholar]
- 14.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth Adult Heart Transplantation Report—2018; focus theme: multiorgan transplantation. J Heart Lung Transplant 2018; 37:1155–1168. [DOI] [PubMed] [Google Scholar]
- 15.John R, Lietz K, Schuster M, et al. Immunologic sensitization in recipients of left ventricular assist devices. J Thorac Cardiovasc Surg 2003; 125:578–591. [DOI] [PubMed] [Google Scholar]
- 16.Arnaoutakis GJ, George TJ, Kilic A, et al. Effect of sensitization in US heart transplant recipients bridged with a ventricular assist device: update in a modern cohort. J Thorac Cardiovasc Surg 2011; 142:1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itescu S, Tung TC, Burke EM, et al. Preformed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of heart transplantation. Circulation 1998; 98:786–793. [DOI] [PubMed] [Google Scholar]
- 18.Kobashigawa JA, Sabad A, Drinkwater D, et al. Pretransplant panel reactive-antibody screens: are they truly a marker for poor outcome after cardiac transplantation? Circulation 1996; 94 suppl:II294–II297. [PubMed] [Google Scholar]
- 19.Suciu-Foca N, Reed E, Marboe C, et al. The role of anti-HLA antibodies in heart transplantation. Transplantation 1991; 51:716–724. [DOI] [PubMed] [Google Scholar]
- 20.Michaels PJ, Espejo ML, Kobashigawa J, et al. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant 2003; 22:58–69. [DOI] [PubMed] [Google Scholar]
- 21.Svobodova E, Gazdic T, Kubanek M, et al. Novel insights into pretransplant allosensitization in heart transplant recipients in the contemporary era of immunosuppression and rejection surveillance. Transplant Int 2016; 29:63–72. [DOI] [PubMed] [Google Scholar]
- 22.Nwakanma LU, Williams JA, Weiss ES, et al. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg 2007; 84:1556–1562. [DOI] [PubMed] [Google Scholar]
- 23.Potena L, Bontadini A, Iannelli S, et al. Occurrence of fatal and nonfatal adverse outcomes after heart transplantation in patients with pretransplant noncytotoxic HLA antibodies. J Transplant 2013; 2013:519680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindenfeld J, Miller GG, Shakar SF, et al. Drug therapy in the heart transplant recipient: part I: cardiac rejection and immunosuppressive drugs. Circulation 2004; 110:3734–3740. [DOI] [PubMed] [Google Scholar]
- 25.Parham P. The immune system. fourth ed.New York: W. W. Norton & Company; 2014. [Google Scholar]
- 26.Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010; 29:914–956. [DOI] [PubMed] [Google Scholar]
- 27.Cai J, Terasaki Pl. Humoral theory of transplantation: mechanism, prevention, and treatment. Hum Immunol 2005; 66:334–342. [DOI] [PubMed] [Google Scholar]
- 28▪.Clerkin KJ, Farr MA, Restaino SW, et al. Donor-specific anti-HLA antibodies with antibody-mediated rejection and long-term outcomes following heart transplantation. J Heart Lung Transplant 2017; 36:540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study shows that class II donor-specific antibodies provided prognostic information regarding future pathologic antibody-mediated rejection (pAMR), graft dysfunction with pAMR, and graft loss.
- 29.Delgado JF, Sanchez V, de la Calzada CS. Acute rejection after heart transplantation. Expert Opin Pharmacother 2006; 7:1139–1149. [DOI] [PubMed] [Google Scholar]
- 30.Mangiola M, Marrari M, Feingold B, Zeevi A. Significance of anti-HLA antibodies on adult and pediatric heart allograft outcomes. Front Immunol 2017; 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunoda S. Cardiac allograft vasculopathy? Heart transplantation provides insights into pathogenesis and treatment of arteriosclerosis. Circ J 2018; 82:2943–2945. [DOI] [PubMed] [Google Scholar]
- 32.Hammond EH, Yowell RL, Nunoda S, et al. Vascular (humoral) rejection in heart transplantation: pathologic observations and clinical implications. J Heart Transplant 1989; 8:430–443. [PubMed] [Google Scholar]
- 33.Ho EK, Vlad G, Vasilescu ER, et al. Pre and posttransplantation allo-sensitization in heart allograft recipients: major impact of de novo alloantibody production on allograft survival. Hum Immunol 2011; 7:5–10. [DOI] [PubMed] [Google Scholar]
- 34.Starling RC, Stehlik J, Baran DA, et al. Multicenter analysis of immune biomarkers and heart transplant outcomes: results of the clinical trials in organ transplantation-05 study. Am J Transplant 2016; 16:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irving CA, Carter V, Gennery AR, et al. Effect of persistent versus transient donor-specific HLA antibodies on graft outcomes in pediatric cardiac transplantation. J Heart Lung Transplant 2015; 34:1310–1317. [DOI] [PubMed] [Google Scholar]
- 36.Tran A, Fixler D, Huang R, et al. Donor-specific HLA alloantibodies: impact on cardiac allograft vasculopathy, rejection, and survival after pediatric heart transplantation. J Heart Lung Transplant 2016; 35:87–91. [DOI] [PubMed] [Google Scholar]
- 37▪.Cole RT, Gandhi J, Bray RA, et al. De novo DQ donor-specific antibodies are associated with worse outcomes compared to non-DQ de novo donor-specific antibodies following heart transplantation. Clin Transplant 2017; 31:1–7. [DOI] [PubMed] [Google Scholar]; The study compares outcomes in patients developing de novo donor-specific antibodies (dnDSAs) to DQ antigens with those developing non-DQ dnDSAs and those free from dnDSAs, and the patients with dnDSA to DQ antigens following HTx are associated with increased risk of death and graft dysfunction.
- 38.Kobashigawa J, Mehra M, West L, et al. Report from a consensus conference on the sensitized patient awaiting heart transplantation. J Heart Lung Transplant 2009; 28:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobashigawa JA, Patel JK, Kittleson MM, et al. The long-term outcome of treated sensitized patients who undergo heart transplantation. Clin Transplant 2011; 25:E61–E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colvin MM, Cook JL, Chang P, et al. Antibody-mediated rejection in cardiac transplantation: emerging knowledge in diagnosis and management: a scientific statement from the American Heart Association. Circulation 2015; 131:1608–1639. [DOI] [PubMed] [Google Scholar]
- 41.Schlaf G, Pollok-Kopp B, Manzke T, et al. Novel solid phase-based ELISA assays contribute to an improved detection of anti-HLA antibodies and to an increased reliability of pre and posttransplant crossmatching. NDT Plus 2010; 3:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Won DI, Jung HD, Jung OJ, et al. Flow cytometry PRA using lymphocyte pools from random donors. Clin Cytom 2007; 72:256–264. [DOI] [PubMed] [Google Scholar]
- 43.Smith JD, Hamour IM, Banner NR, Rose ML. C4d fixing, luminex binding antibodies: a new tool for prediction of graft failure after heart transplantation. Am Soc Transplant Surg 2007; 7:2809–2815. [DOI] [PubMed] [Google Scholar]
- 44.Chin C, Chen G, Sueria F, Berry G, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant 2011; 30:158–163. [DOI] [PubMed] [Google Scholar]
- 45.Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum Immunol 2011; 72:849–858. [DOI] [PubMed] [Google Scholar]
- 46.Schaub SH, Hönger G, Koller MT, et al. Determinants of C1q binding in the single antigen bead assay. Transplantation 2014; 98:387–393. [DOI] [PubMed] [Google Scholar]
- 47.Kauke T, Kaczmarek I, Dick A, et al. Anti-MICA antibodies are related to adverse outcome in heart transplant recipients. J Heart Lung Transplant 2009; 28:305–311. [DOI] [PubMed] [Google Scholar]
- 48.Angaswamy N, Saini D, Ramachandran S, et al. Development of antibodies to human leukocyte antigen precedes development of antibodies to major histocompatibility class I-related chain A and are significantly associated with development of chronic rejection after human lung transplantation. Hum Immunol 2010; 71:560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao K, Lai C-H, Flores SV, et al. AT1R antibodies together with anti-HLA donor specific antibodies (HLA-DSA) identify patients at risk for immune complication in heart transplant. J Heart Lung Transplant 2012; 31:S163. [Google Scholar]
- 50▪▪.Randhawa PK, Philogene MC, Jeresano M, et al. Management of heart transplant recipients with hemodynamically significant clinical rejection in the presence of antibodies against angiotensin II type 1 receptor: a retrospective study. Trends Transplant 2018; 11:1–6. [Google Scholar]; The study shows that AT1R-Ab in heart transplant recipients appears to be associated with hemodynamic significant rejection and the use of angiotensin receptor blockade can be considered in the therapy of these patients.
- 51.Hiemann NE, Meyer R, Wellnhofer E, et al. Non-HLA antibodies targeting vascular receptors enhance alloimmune response and microvasculopathy after heart transplantation. Transplantation 2012; 94:919–924. [DOI] [PubMed] [Google Scholar]
- 52.Faulk WP, Rose M, Meroni PL, et al. Antibodies to endothelial cells identify myocardial damage and predict development of coronary artery disease in patients with transplanted hearts. Hum Immunol 1999; 60:826–832. [DOI] [PubMed] [Google Scholar]
- 53.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation 2001; 71:886–892. [DOI] [PubMed] [Google Scholar]
- 54.Goers TA, Ramachandran S, Aloush A, et al. De novo production of K-alpha 1 tubulin-specific antibodies: role in chronic lung allograft rejection. J Immunol 2008; 180:4487–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwata T, Philipovskiy A, Fisher AJ, et al. Antitype V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol 2008; 181:5738–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalache S, Dinavahi R, Pinney S, et al. Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients. J Immunol 2011; 187:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Q, Cecka JM, Gjertson DW, et al. HLA and MICA: targets of antibody-mediated rejection in heart transplantation. Transplantation 2011; 91:1153–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobashigawa J, Crespo-Leiro MG, Ensminger SM, et al. Report from a consensus conference on antibody-mediated rejection in heart transplantation. J Heart Lung Transplant 2010; 30:252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nath DS, Bias Basha H, Tiriveedhi V, et al. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant 2010; 29:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fredrich R, Toyoda M, Czer LS, et al. The clinical significance of antibodies to human vascular endothelial cells after cardiac transplantation. Transplantation 1999; 67:385–391. [DOI] [PubMed] [Google Scholar]
- 61.Takemoto SK, Zeevi A, Feng S, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant 2004; 4:1033–1041. [DOI] [PubMed] [Google Scholar]


