Abstract
Circulating tumor cells (CTCs) have received enormous attention as a novel biomarker in various malignant diseases. We investigated the clinical association between the presence of perioperative CTCs and survival outcomes in women with ovarian cancer. In a total of 30 women who were scheduled to undergo a surgical treatment for ovarian cancer, peripheral blood samples were obtained before and after surgery. CTCs were isolated and counted using the optimized tapered-slit filter (TSF) platform. The association between the presence of perioperative CTCs and tumor features was evaluated. The impact of the presence of perioperative CTCs on progression-free survival (PFS) and overall survival (OS) rates were analyzed using a Kaplan–Meier method. The median age was 58 (range, 24–77) years, and the median follow-up period was 31.5 (range, 1–41) months. Overall, the CTC detection rate was not significantly different before and after surgery (76.7% vs 57.1%, P = .673). The presence of postoperative CTCs was not significantly associated with 3-year PFS (29.1% vs 58.3%, P = .130) and OS (84.4% vs 80.0%, P = .559) rates in the whole study population. In advanced stage, PFS rate in patients with postoperative CTCs had lower PFS rates than those without postoperative CTCs, although there was no statistical significance (18.8% vs 57.1%, P = .077). Postoperative CTC was more frequently detected in women who had lymph node involvement than those who did not (7/7 [100%] vs 3/10 [30.0%], P = .010). The presence of postoperative CTCs as detected using the TSF platform seems to be associated with poorer PFS rates in women with ovarian cancer of advanced stage. Further study with a larger population is warranted to validate our study findings.
Keywords: circulating tumor cell, ovarian cancer, prognosis, tapered-slit filter platform
1. Introduction
The prognosis of patients with ovarian cancer is relatively poor compared with that of patients with other gynecologic cancers.[1] The disease is often diagnosed in advanced stages, owing to the lack of perceptible signs and symptoms and an effective screening program. Despite the development of surgical approaches and chemotherapy regimens, nearly 80% patients relapse within 5 years.[2] Cancer antigen-125 (CA-125) tests with imaging, such as computed tomography (CT), are routinely used as follow-up tests for diagnosing recurrences after surgery or chemotherapy. However, the sensitivity of CA-125 tests for levels >35 U/ml, which indicates a diagnosis of recurrence in ovarian cancer, is below 70%.[3,4] The low sensitivity of CA-125 tests may be because the levels of antigen from small recurrent tumors may be too low to activate an antibody response. Therefore, CA-125 tests and CT are not useful for predicting prognosis immediately after debulking surgery, and the identification of new biomarkers reflecting current disease activity is urgently needed for ovarian cancer.
Circulating tumor cells (CTCs) have received enormous attention as a novel biomarker in various malignant diseases, including ovarian cancer, over the past decade. CTCs are individual cells or clusters of cancer cells that enter into the bloodstream through intravasation from primary tumors and reach a distant organ, where they can eventually grow into an overt metastasis.[5–7] CTCs have been demonstrated as a main source of metastatic spread. Metastatic spread is considered the most critical process for cancer-associated outcomes of survivors, therefore, enrichment and isolation of CTCs is a subject of active investigation in the cancer research field. The phenomenon of patients with positive CTCs indicating worse prognoses than those with negative CTCs has already been confirmed in studies of metastatic breast, colorectal, and prostate cancer. Several reports have suggested that CTCs are also predictive of a shorter progression-free survival (PFS) and overall survival (OS) in ovarian cancer.[8–12] However, the results were negative for PFS and OS in some studies.[13–15]
Regarding prognosis, if CTCs could optimize predictions of disease activity or treatment response, more optimal individual treatment strategies could be established. Therefore, we aimed to evaluate the potential value of CTCs for predicting the prognosis of ovarian cancer. In this prospective observational study of 30 patients with ovarian cancer, we used a novel strategy for isolating CTCs in peripheral blood.
2. Methods
2.1. Study population
A total of 30 women who were scheduled to undergo a staging operation for ovarian cancer in single institution were prospectively recruited between May 2015 and April 2016. Patients with a prior malignancy less than 5 years from enrollment were excluded. All patients gave written informed consent, and this study was approved by the institutional review board (B-1408/263-003).
All of the enrolled patients had results of CA-125, risk of ovarian malignancy algorithm (ROMA), and risk of malignancy index (RMI) within 1 month before surgery.[16] Ascites was evaluated by CT or magnetic resonance imaging (MRI), and grade 2 to 3 of ascites was counted as positive; grade 2 is moderate ascites causing moderate symmetrical distension of the abdomen, and grade 3 is large ascites causing marked abdominal distension.[17]
Among 30 patients, based on preoperative evaluation, 10 patients received platinum-based chemotherapy before surgery at their physician's discretion. All of the 10 patients who received neoadjuvant chemotherapy were diagnosed with disseminated ovarian cancer based on ascites cytology or an ovary/omentum biopsy before surgery and were not suitable for primary debulking surgery because of unresectable tumor or poor performance status. After surgery, all of the enrolled patients were finally diagnosed with ovarian cancer by gynecologic pathologists. They underwent debulking surgery, categorized as either optimal (residual tumor ≤1.0 cm) or suboptimal (residual tumor >1.0 cm). About 1 week after surgery, the International Federation of Gynecology and Obstetrics (FIGO) stage, histologic type, tumor grade, and tumor size were confirmed by the final pathologic report.
2.2. Blood collection and preparation
Before surgery, 5.0 mL peripheral blood was obtained from the patients’ antecubital vein to isolate CTCs. All blood samples collected in Vacutainer tubes (Becton Dickinson; lithium heparin as anticoagulant) were transferred to Korea Advanced Institute of Science and Technology (KAIST) to identify and count CTCs. To avoid cell destruction during delivery, the collection tube was packed in a foam plastic box with ice pack, and delivery was completed within 6 hours from sampling. In all 30 enrolled patients, 5.0 mL peripheral blood was obtained again within 1 month after surgery and before the start of first adjuvant chemotherapy to isolate CTCs.
2.3. Identification and counting of CTC
CTC isolation and enumeration was performed by two researchers (J Bu, YT Kang) using the previously reported tapered-slit filter (TSF) platform.[18] The TSF consists of three layers: two chambers, top and bottom, for flow guidance and a filter for filtration of CTCs. The size of each layer was 16 × 12 mm and the effective filter region was 10 × 10 mm. Those three layers were assembled by a zig. The supporting force compensates for the hydraulic force acting on a cell passing through the tapered-slit structure. The TSF isolates CTCs based on their physical features including size and deformability, unrelated to their surface protein expression. Because the TSF platform was designed with a wide entrance and narrower slit exits, it is capable to increase sample flow rate and to minimize cell stress. Five milliliters blood from patients were diluted in 10.0 mL phosphate-buffered saline (PBS) solution without any pretreatment, such as cell fixation, erythrocyte lysis, or Ficoll separations, and directly processed into the TSF platform using the withdraw mode of a syringe pump. The cells captured were gently released by a reverse flow of PBS solution, and the released cells were mounted on a slide glass using a cytocentrifuge, Shandon Cytospin III (Thermo Scientific, Wilmington, DE). The immunostaining protocol described in our previous research was optimized for TSF.[19] The cell-mounted glass slides were first fixed, permeabilized, and blocked, then stained for immunofluorescence. Afterwards, fluorescent images were acquired and quantified using MetaMorph software (Molecular Devices, Sunnyvale, CA). All immunostained cells were carefully examined and enumerated as CTCs considering both (a) staining criteria: 4′,6-diamidino-2-phenylindole (DAPI)-positive, cluster of differentiation 45 (CD45)-negative, cytokeratin (CK) 9-positive, and epithelial cell adhesion molecule (EpCAM)-positive, and (b) morphological criteria, such as higher nucleus-to-cytoplasm ratio, larger size, and higher degree of irregularity than was observed in background blood cells (Fig. 1).
Figure 1.
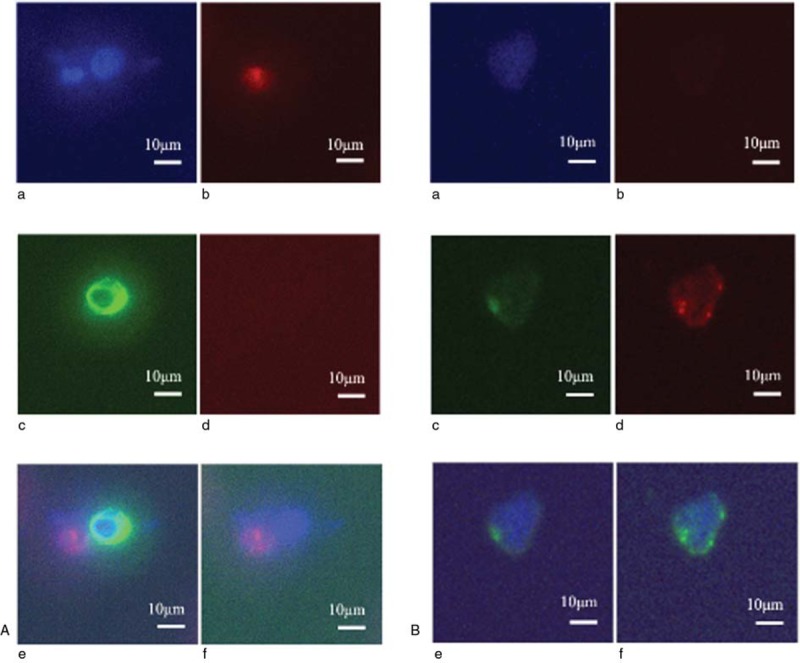
Immunostaining of a white blood cell (A) and a circulating tumor cell (B) in peripheral blood for the following markers: (a) DAPI, (b) CD45, (c) CK9, (d) EpCAM, (e) DAPI+CD45+CK9, and (f) DAPI+CD45+EpCAM. The bar represents 10 μm. DAPI = 4′, 6-diamidino-2-phenylindole, CD45 = cluster of differentiation 45, CK9 = cytokeratin 9, EpCAM = epithelial cell adhesion molecule.
2.4. Statistical analysis
Kaplan–Meier curves were used to evaluate 3-year PFS and OS rates. In addition, associations between detection of perioperative CTCs and clinicopathologic factors were evaluated for statistical significance. Fisher's exact test and the chi-square test were used for comparing categorical variables. A two-sided P-value of <.05 was considered statistically significant. SPSS version 22.0 (IBM Inc., Armonk, NY) was used for statistical analyses.
3. Results
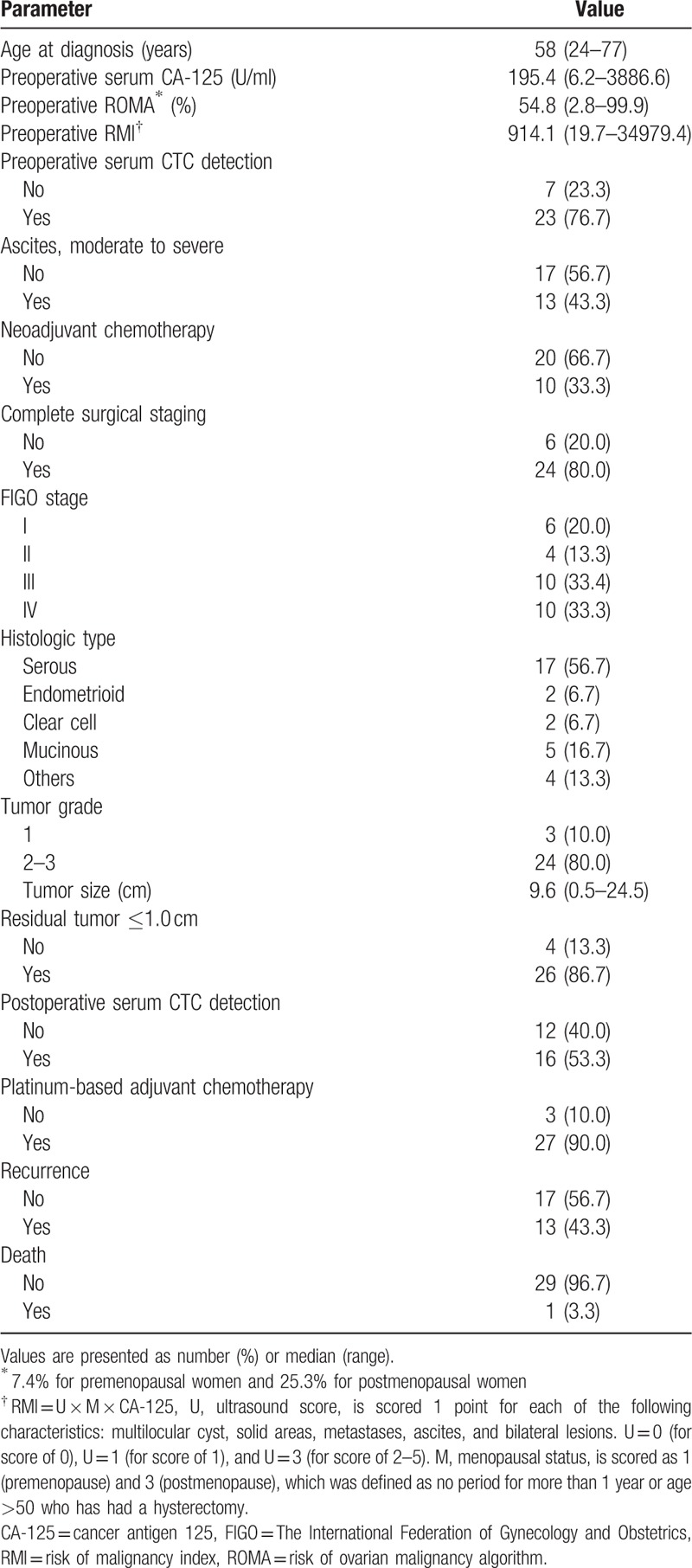
Baseline characteristics of a total 30 patients with ovarian cancer are presented in Table 1. The median age at diagnosis was 58 years (24–77 years), and the median follow-up period was 31.5 months (1–41 months). Overall, the CTC positive rate was not different between preoperative and postoperative peripheral blood samples (23/30 [76.7%] vs 16/28 [57.1%], P = .673). Complete surgical staging, including lymphadenectomy, was performed in 24 (80.0%) patients. Four premenopausal young women with tumors confined to the unilateral ovary underwent fertility-preserving surgery. Two patients with distant metastasis underwent palliative debulking surgery without lymphadenectomy. Twenty (66.7%) patients were diagnosed with advanced stage (FIGO stage III–IV) after surgery. Optimal debulking was achieved in 26 (86.7%) patients. Twenty-seven patients received platinum-based adjuvant chemotherapy within 1 month after surgery. A total of 15 (50.0%) patients experienced recurrence, and two who had platinum-resistance recurred within 6 months after the last chemotherapy. Three (10.0%) patient died in the study period.
Table 1.
Baseline characteristics (n = 30).
3.1. Association between baseline characteristics and the presence of CTC
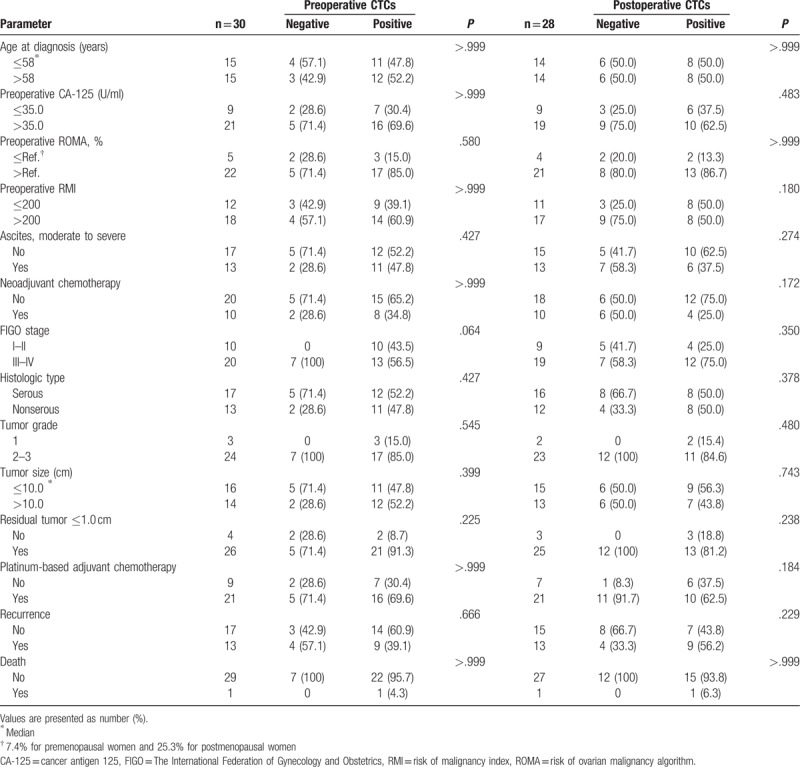
In all study subjects, neither preoperative (serum CA-125, ROMA, RMI, ascites, and neoadjuvant chemotherapy) nor postoperative (FIGO stage, histologic type, tumor grade, and tumor size) clinicopathologic characteristics were associated with presence of any of either preoperative or postoperative peripheral CTCs. (Table 2.) In addition, there was no significant association between optimal debulking with residual tumor ≤1.0 cm and pre- and postoperative presence of peripheral CTCs. Recurrence and death were also similar between patients with and without perioperative CTCs.
Table 2.
Association of circulating tumor cells (CTCs) with baseline characteristics in all study population (n = 30).
3.2. Postoperative CTCs in patients with advanced stage ovarian cancer
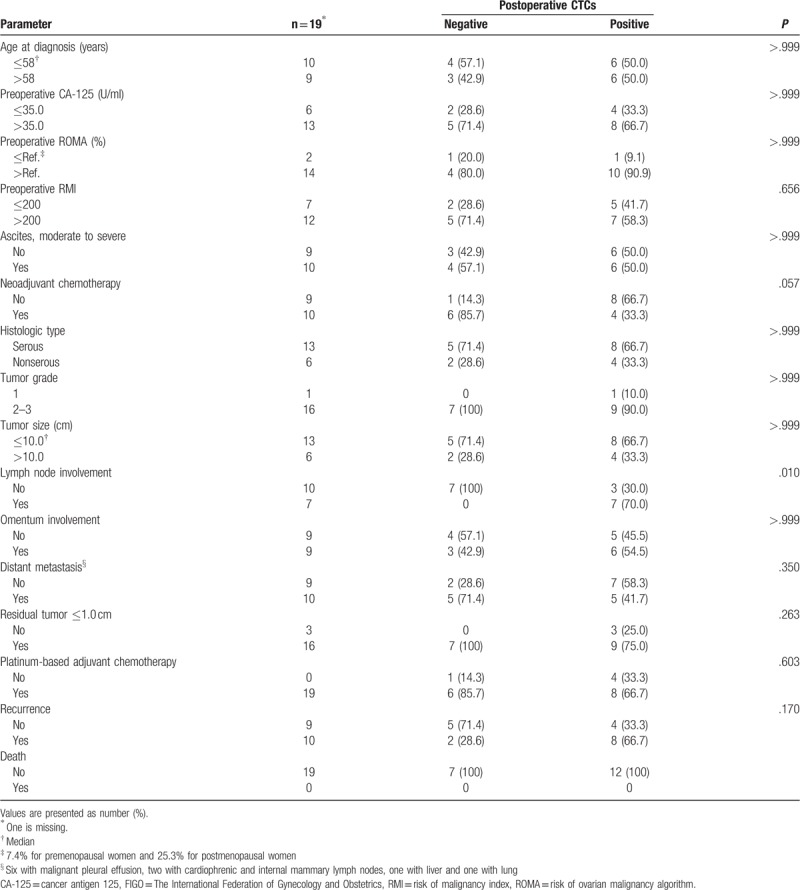
Table 3 shows the association of postoperative CTCs with clinicopathologic characteristics in the patients with advanced stage. As in the whole study population, most of the evaluated characteristics were not associated with the presence of postoperative CTCs. However, postoperative CTCs were detected more frequently in patients who had lymph node involvement than in those who did not (7/7 [100%] vs 3/10 [30.0%], P = .010). Otherwise, the presence of postoperative CTCs was associated neither with omental nor with distant organ metastasis. Regarding the presence of preoperative CTCs, none of the three sites were significantly associated with a positive CTC rate (data not shown).
Table 3.
Association between clinicopathologic characteristics and postoperative circulating tumor cells (CTCs) in patients with advanced stage (n = 20).
3.3. Survival outcomes in patients with vs without perioperative CTCs
There was no difference in 3-year PFS (44.7% vs 33.3%, P = .342) and OS (73.4% vs 100%, P = .320) rates between patients with and without preoperative CTCs in the whole study population (data not shown). The presence of postoperative CTCs was also not significantly associated with PFS (29.1% vs 58.3%, P = .130) and OS (84.4% vs 80.0%, P = .559) rates in the whole study population (Fig. 2). Upon stratified analysis by stage, PFS rate in patients with postoperative CTCs had lower PFS rates than those without postoperative CTCs in advanced stage disease, although there was no statistical significance (18.8% vs 57.1%, P = .077). There was no difference in OS rates between advanced stage patients with and without preoperative CTCs (87.5% vs 66.7%, P = .869).
Figure 2.
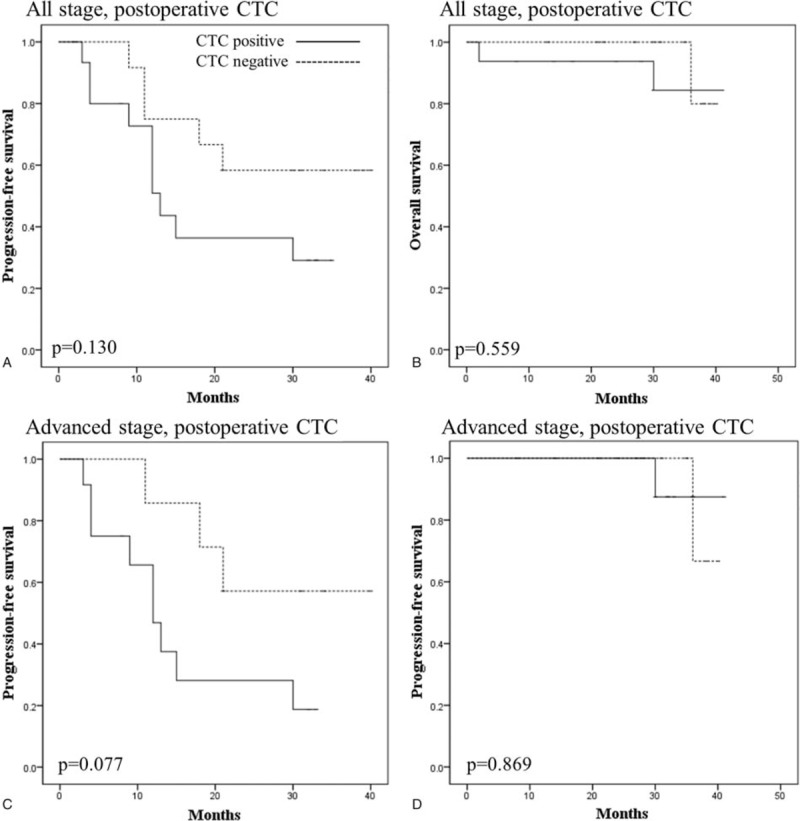
Kaplan–Meier curves comparing (A) PFS and (B) OS between the patients with a negative (dotted line) and positive (solid line) presence of postoperative CTCs in all study populations. (C) PFS and (D) OS according to the presence of postoperative CTCs in patients with advanced stage. CTCs = circulating tumor cells, OS = overall survival, PFS = progression-free survival.
4. Discussion
Our findings were based on the TSF platform for isolating CTCs in peripheral blood in patients with ovarian cancer. We successfully isolated CTCs, and demonstrated that the presence of perioperative CTCs was not associated with tumor histology and grade in this study. FIGO stage and optimal debulking also did not affect the CTC-positive rate. However, there were several notable findings in the stratified analysis by FIGO stage. Firstly, the presence of postoperative CTCs seems to be associated with 3-year PFS rates in advanced stage as shown in Figure 2D (3-year PFS rate; 57.1% vs 18.8%, P = .077). Secondly, postoperative CTCs were more frequently detected in patients with lymph node involvement than in patients without (100% vs 30.0%, P = .010).
In the early 2000s, ovarian cancer was thought to spread primarily by direct cell seeding throughout the abdominal cavity; therefore, the clinical utility of identifying CTCs was not defined.[13,14] However, several researchers recently reported that CTCs are significantly associated with PFS and OS rates in patients with ovarian cancer.[8–10,20] Among them, Obermayr et al showed that the presence of CTCs was more related to PFS and OS rates after primary treatment rather than at the first diagnosis in 93 follow-up patients.[10] It was consistent with our study findings that postoperative CTCs could have a potential prognostic value in advanced stage ovarian cancer. This approach might help to identify current disease activity, especially after completion of surgery or adjuvant chemotherapy, for additional alternative therapeutic plans.
In our study, however, some results were inconsistent with those of previous reports. Fan et al found that higher CTC counts was associated with advanced stage and higher CA-125 levels.[9] Obermayr et al suggested that the presence of CTCs may reflect suboptimal debulking and poor response to therapy.[10] However, the results of our study did not show any significant findings of CTCs in patients with advanced stage, higher CA-125 levels, suboptimal debulking, or platinum resistance. We inferred that the probable reason was the restricted number of our study population and possibly the use of a different CTC-detecting platform. Of note, in the patients with advanced stage disease, postoperative CTCs were more frequently detected in patients who had lymph node metastasis than those who did not (7/7 [100%] vs 3/10 [30.0%], P = .010), in contrast to cases with omental (77.8%–66.7%) and distant metastasis (90.0%–50.0%). One of the plausible explanations for this finding is the possibility of dissemination of cancer cells into the circulation during surgical manipulation of metastatic lymph nodes. Cancer cells are already disseminated before surgery in advanced stage disease; therefore, the impact of further dissemination by surgical manipulation may be insignificant. However, a transient increase in cancer cell dissemination hypothetically can occur after surgery, and our findings in patients with lymph node metastasis might also be a result of nodal disruption and lymphatic chain break.[21] Some authors previously reported that CTCs might not be significantly associated with lymph node metastasis in ovarian cancer.[10,22] However, in many studies of other diseases, including metastatic breast and colorectal cancer, it is suggested that the presence of CTCs is associated with increased lymph node involvement.[23–26]
The present study has some limitations. First, this study represents a single center's work, and its small sample size might lower the statistical power of the analysis. Especially, number of the patients with early stage, low-grade and nonserous histology is too small to analyze by stratified subgroup. These clinical factors can affect the survival outcomes, therefore, further study with larger population is needed. Together with the small sample size, the short follow-up period did not allow us to reach mature survival outcomes. Second, blood sampling in patients after postoperative adjuvant chemotherapy was too sparse to collect a sufficient number of samples to make firm results about the value of CTCs as a marker for response of chemotherapy and long-term prognosis. Third, we used our own CTC-detecting method using the TSF platform, of which the reliability has not yet been established, despite its many advantages over existing methods. CTC detection method using a combination of TSF platform and surface marker expression with confirmatory morphologic criteria should be validated in further studies with a larger sample size. The difficulty in capturing heterogeneous CTCs in ovarian cancers by EpCAM-based methods may be due to its down-regulation to negligible levels after epithelial-mesenchymal transition (EMT). To overcome this limitation in the future, label-free CTC isolation methodologies which have shown comparable or even higher detection sensitivity in detecting CTCs can be applied. Development of a universally accepted method of isolating CTCs is important to work towards a consensus in this field.
5. Conclusion
Most of the results from relevant studies agree that CTCs might have a promising value in various experimental and clinical fields. Although further study with large population is necessary to confirm the results of this study, postoperative CTCs detected using the TSF platform seem to be a potential predictor of the prognosis in patients with advanced stage ovarian cancer. Isolation of postoperative CTCs may provide physicians with useful information for identifying poor treatment responses earlier, and a guide for additional treatment options in the future.
Author contributions
Conceptualization: Miseon Kim, Dong Hoon Suh, Yong Beom Kim, Young-Ho Cho.
Data curation: Miseon Kim, Jin Young Choi, Jiyoon Bu, Yoon-Tae Kang.
Formal analysis: Miseon Kim, Dong Hoon Suh, Jin Young Choi, Jiyoon Bu, Yoon-Tae Kang.
Funding acquisition: Yong Beom Kim, Young-Ho Cho.
Methodology: Jiyoon Bu, Yoon-Tae Kang.
Resources: Dong Hoon Suh, Kidong Kim, Jae Hong No, Yong Beom Kim, Young-Ho Cho.
Software: Jiyoon Bu, Yoon-Tae Kang.
Supervision: Kidong Kim, Jae Hong No, Yong Beom Kim, Young-Ho Cho.
Writing – original draft: Miseon Kim, Jin Young Choi.
Writing – review & editing: Miseon Kim, Dong Hoon Suh, Kidong Kim, Jae Hong No, Yong Beom Kim, Young-Ho Cho.
Footnotes
Abbreviations: CA-125 = cancer antigen-125, CD45 = cluster of differentiation 45, CK = cytokeratin, CT = computed tomography, CTC = circulating tumor cell, DAPI = 4′,6-diamidino-2-phenylindole, EpCAM = epithelial cell adhesion molecule, FIGO = International Federation of Gynecology and Obstetrics, MRI = magnetic resonance imaging, OS = overall survival, PBS = phosphate-buffered saline, PFS = progression-free survival, RMI = risk of malignancy index, ROMA = risk of ovarian malignancy algorithm, TSF = tapered-slit filter.
This research was supported by the Converging Research Center Program, funded by the Ministry of Science, ICT, and Future Planning of Korea (No. NRF-2014M3C1A8048780).
The authors have no conflicts of interest to disclose.
The authors of this work have nothing to disclose.
References
- [1].Baldwin LA, Huang B, Miller RW, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol 2012;120:612–8. [DOI] [PubMed] [Google Scholar]
- [2].Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer 2009;9:167–81. [DOI] [PubMed] [Google Scholar]
- [3].Yang Z, Zhao B, Li L. The significance of the change pattern of serum CA125 level for judging prognosis and diagnosing recurrences of epithelial ovarian cancer. J Ovarian Res 2016;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [4].Guo N, Peng Z. Does serum CA125 have clinical value for follow-up monitoring of postoperative patients with epithelial ovarian cancer? Results of a 12-year study. J Ovarian Res 2017;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426–37. [DOI] [PubMed] [Google Scholar]
- [6].Masuda T, Hayashi N, Iguchi T, et al. Clinical and biological significance of circulating tumor cells in cancer. Mol Oncol 2016;10:408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Poveda A, Kaye SB, McCormack R, et al. Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol 2011;122:567–72. [DOI] [PubMed] [Google Scholar]
- [9].Fan T, Zhao Q, Chen JJ, et al. Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol Oncol 2009;112:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Obermayr E, Castillo-Tong DC, Pils D, et al. Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance - A study of the OVCAD consortium. Gynecol Oncol 2013;128:15–21. [DOI] [PubMed] [Google Scholar]
- [11].Zeng L, Liang X, Liu Q, et al. The predictive value of circulating tumor cells in ovarian cancer a meta analysis. Int J Gynecol Cancer 2017;27:1109–17. [DOI] [PubMed] [Google Scholar]
- [12].Cui L, Kwong J, Wang CC. Prognostic value of circulating tumor cells and disseminated tumor cells in patients with ovarian cancer: a systematic review and meta-analysis. J Ovarian Res 2015;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Judson P, Geller M, Bliss R, et al. Preoperative detection of peripherally circulating cancer cells and its prognostic significance in ovarian cancer. Gynecol Oncol 2003;91:389–94. [DOI] [PubMed] [Google Scholar]
- [14].Marth C, Kisic J, Kaern J, et al. Circulating tumor cells in the peripheral blood and bone marrow of patients with ovarian carcinoma do not predict prognosis cancer. Cancer 2002;94:707–12. [DOI] [PubMed] [Google Scholar]
- [15].Behbakht K, Sill M, Darcy K, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol 2011;123:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Karlsen M, Sandhu N, Høgdall C, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2012;127:379–83. [DOI] [PubMed] [Google Scholar]
- [17].Moore K, Aithal G. Guidelines on the management of ascites in cirrhosis. Gut 2006;55Suppl 6:vi1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kang YT, Doh I, Cho YH. Tapered-slit membrane filters for high-throughput viable circulating tumor cell isolation. Biomed Microdevices 2015;17:45. [DOI] [PubMed] [Google Scholar]
- [19].Seo H, Hwang Y, Choe K, et al. In vivo quantitation of injected circulating tumor cells from great saphenous vein based on video-rate confocal microscopy. Biomed Opt Express 2015;6:2158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aktas B, Kasimir-Bauer S, Heubner M, et al. Molecular profiling and prognostic relevance of circulating tumor cells in the blood of ovarian cancer patients at primary diagnosis and after platinum-based chemotherapy. Int J Gynecol Cancer 2011;21:822–30. [DOI] [PubMed] [Google Scholar]
- [21].Camara O, Kavallaris A, Nöschel H, et al. Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells. World J Surg Oncol 2006;4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Banys M, Solomayer EF, Becker S, et al. Disseminated tumor cells in bone marrow may affect prognosis of patients with gynecologic malignancies. Int J Gynecol Cancer 2009;19:948–52. [DOI] [PubMed] [Google Scholar]
- [23].Maltoni R, Fici P, Amadori D, et al. Circulating tumor cells in early breast cancer: a connection with vascular invasion. Cancer Lett 2015;367:43–8. [DOI] [PubMed] [Google Scholar]
- [24].van Dalum G, Stam G, Scholten L, et al. Importance of circulating tumor cells in newly diagnosed colorectal cancer. Int J Oncol 2015;46:1361–8. [DOI] [PubMed] [Google Scholar]
- [25].Janni W, Rack B, Terstappen L, et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res 2016;22:2583–3593. [DOI] [PubMed] [Google Scholar]
- [26].Hristozova T, Konschak R, Stromberger C, et al. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann Oncol 2011;22:1878–85. [DOI] [PubMed] [Google Scholar]


