Abstract
Rationale:
Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis is the most frequent autoimmune encephalitis in children, and its presentation is various. The disease can be triggered by various infections.
Patient concerns:
Case 1 was a 7-year-old female with the presentation of seizure, repeated fever, language disorder, and decreased muscle strength of the right limbs; Case 2 was a 7-year-old male with the manifestation of repeated emesis, headache, involuntary movement, altered personality, seizures, and cognitive impairment; Case 3 was a 2-year-old female with repeated fever, emesis, seizures, coma, and decreased muscle strength of limbs. Anti-NMDAR antibody was identified in cerebrospinal fluid (CSF) in the 3 cases, confirming the diagnosis of anti-NMDAR encephalitis. Pathogenic examinations revealed positive serum Epstein–Barr virus (EBV)-nuclear antigen and EBV-capsid antigen (CA)-IgG antibodies in the 3 cases, as well as positive EBV-early antigen (EA)-IgG antibody in CSF. Case 1 also had positive EBV-CA-IgA antibody; Case 3 also had positive EBV-CA-IgA and EBV-CA-IgG antibodies.
Diagnoses:
Anti-NMDAR antibody and EBV-EA-IgG antibody in CSF were tested positive in the 3 cases. Thus, they were diagnosed as anti-NMDAR encephalitis associated with reactivated EBV infection.
Interventions:
All of the 3 cases received immunoglobulin, corticosteroid, and ganciclovir treatment. Cases 2 and 3 also received antiepileptic drugs due to repeated seizures. In addition, Case 3 also received assistant respiration, plasma exchange, and rituximab.
Outcomes:
The 3 cases were substantially recovered after treatment. Repeat CSF analysis showed decreased titer of the anti-NMDAR antibody.
Lessons:
Reactivated EBV infection may trigger anti-NMDAR encephalitis in children, which has not been reported previously. Related possible virology tests should be completed while diagnosing the disease.
Keywords: anti-N-methyl-d-aspartate receptor encephalitis, children, Epstein–Barr virus-early antigen-IgG, reactivated Epstein–Barr virus infection
1. Introduction
Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis was 1st described in 2007, which is mediated by antibody recognizing the NR1 subunit of NMDAR. A prospective study showed that anti-NMDAR encephalitis accounted for approximately 4% of all causes of encephalitis.[1] This disease has various clinical symptoms, mainly including movement disorders, seizures, altered consciousness, cognitive impairment, and psychiatric symptoms.[2] Although approximately 75% to 85% of the patients can recover, the others may remain severely disabled or died.[1,2]
The etiology and mechanism of anti-NMDAR encephalitis is not entirely clear. Previous studies reported that the autoimmune process could be triggered by infection (herpes simplex virus [HSV], toxoplasma gondii), vaccination, or occult neoplasm.[3–5] In this context, we report 3 cases of pediatric anti-NMDAR encephalitis. In the 3 cases, the Epstein–Barr virus (EBV)-early antigen (EA)-IgG antibody in cerebrospinal fluid (CSF) was detected positive, which suggested reactivated EBV infection according to the laboratory results,[6,7] and has not been reported previously. Thus, in this report, we presented 3 pediatric cases who were diagnosed as anti-NMDAR encephalitis associated with reactivated EBV infection.
2. Case presentation
2.1. Case 1
A 7-year-old female was admitted for 2 seizures in 1 month, accompanied by repeated fever and language disorder (only saying simple words). There was no history of other diseases. Neurologic examination revealed decreased muscle strength of right limbs (right upper limb: I–II; right lower limb: IV) and positive ankle clonus. The routine blood test showed the total white blood cell count was 12.38 × 109/L. Laboratory testing revealed normal biochemistry (blood electrolyte, liver function, renal function, blood ammonia, and blood lactic acid). Blood cellular and humoral immunity were normal. Serum viral assay showed positive EBV-nuclear antigen (NA) (>600 IU/mL), EBV-capsid antigen (CA)-IgG, cytomegalovirus (CMV)-IgG, HSV-IgG, and rubella virus (RV)-IgG antibodies (Table 1). CSF analysis revealed elevated levels of white blood cell count (16 × 106/L), IgA (7.67 mg/L), and IgM (3.61 mg/L), as well as normal glucose, protein, and IgG levels. The EBV-EA-IgG, EBV-CA-IgG, and anti-NMDAR antibodies (1:10) were tested positive in CSF, as shown in Table 2. As shown in Figure 1A, brain magnetic resonance imaging (MRI) showed diffuse high T2 signal in the subcortex of bilateral cerebellar hemispheres and local high T2 signal in the left paraventricular. Electroencephalography (EEG) result was normal. Based on the clinical presentation and positive anti-NMDAR antibody in CSF, the patient was diagnosed with anti-NMDAR encephalitis. The patient received immunoglobulin on day 2 after admission (1 g/kg/d, for 2 days), followed by methylprednisolone (20 mg/kg/d for 3 days and then the dose was decreased gradually). The patient also received ganciclovir treatment (from day 6 on) and neurotrophic therapy. However, repeat blood biochemistry result showed impaired liver function (alanine transaminase: 137 IU/L) 1 week later. Thus, the ganciclovir was stopped and liver protection treatment was applied. One week later, repeat alanine transaminase result was normal.
Table 1.
The laboratory results of serum virology from the 3 patients.
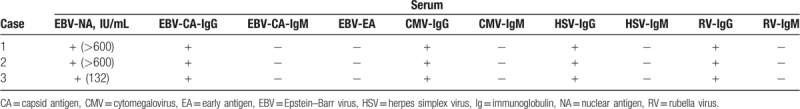
Table 2.
The laboratory results of CSF from the 3 patients.
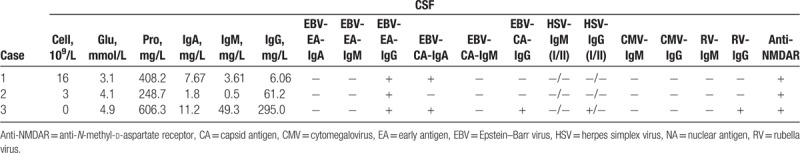
Figure 1.
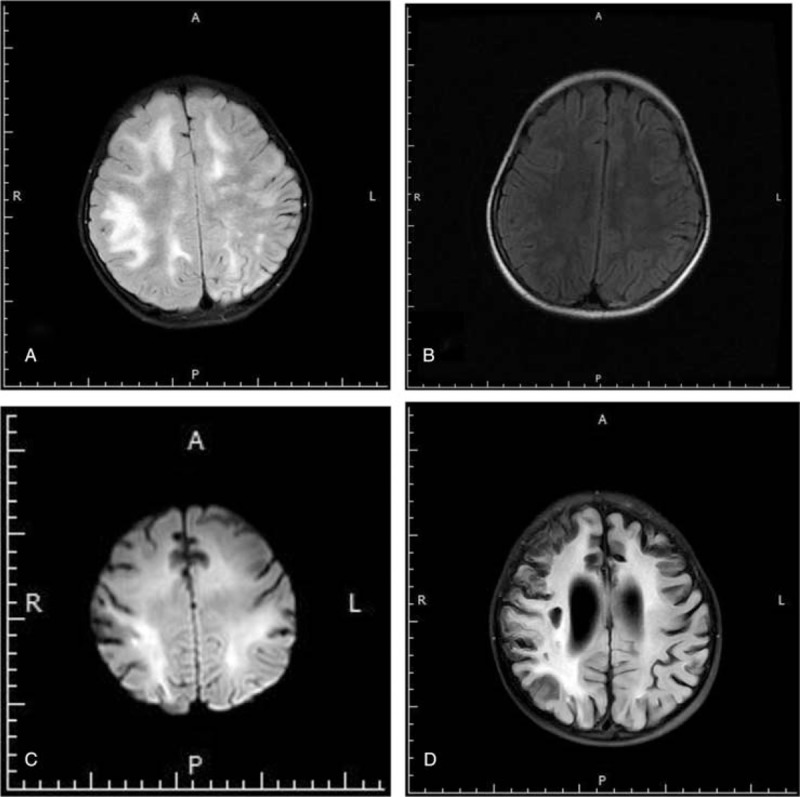
(A) The brain magnetic resonance imaging (MRI) of Case 1 after admission showed diffuse high T2 signal in the subcortex of bilateral cerebellar hemispheres and local high T2 signal in the left paraventricular. (B) MRI (5 months after discharge of Case 1) showed local increased T2 signal in the frontal subcortex and left paraventricular. (C) MRI of Case 3 after admission showed abnormal white matter signal in bilateral cerebral hemisphere, and local cerebral atrophy. (D) MRI (8 months after discharge of Case 3) showed abnormal white matter signal in bilateral cerebral hemisphere and cerebral atrophy.
From day 5 on after admission, the patient's muscle strength of the right limbs started to recover. She began to have more fluent speech and communication with her parents on day 7. There was no seizure since her admission. On day 20, repeat MRI revealed the range of lesion was obviously reduced, and CSF test showed normal. She was discharged on day 22, with enhanced muscle strength of right limbs (IV) and more fluent speech. The patient was followed up for 16 months. Fifteen days after discharge, the patient had normal speech, and her muscle strength of the right limbs recovered fully 15 days later. Head MRI for 5 times indicated the lesion was improved gradually. The recent MRI (5 months after discharge) showed local increased T2 signal in the frontal subcortex and left paraventricular, which was improved markedly, as shown in Figure 1B.
2.2. Case 2
A 7-year-old male was admitted with the presentation of 1-month history of repeated emesis, headache, involuntary movement (involuntary tongue movement, hand rubbing), and cognitive impairment. He also had altered personality (irritability, delirium) and repeated seizures for 9 days. There was no history of other diseases before. The neurologic examination was unremarkable. On day 1 after admission, the patient appeared altered consciousness and language dysfunction. On day 6, he developed positive bilateral Babinski signs and ankle clonus. The routine blood test was normal. Laboratory testing revealed normal biochemistry (blood electrolyte, liver function, renal function, blood ammonia, and blood lactic acid). Blood cellular and humoral immunity were also normal. Serum viral assay showed positive EBV-NA (>600 IU/mL), EBV-CA-IgG, CMV-IgG, HSV-IgG, and RV-IgG antibodies (Table 1). CSF analysis revealed normal white cell count, glucose, protein, IgA, IgM, and IgG. The EBV-EA-IgG and anti-NMDAR antibodies (1:10) were tested positive in CSF (Table 2). Brain MRI showed normal and EEG result indicated δ brush. Based on the clinical presentation and positive anti-NMDAR antibody in CSF, the patient was diagnosed with anti-NMDAR encephalitis. The patient received treatment of immunoglobulin (2 g/kg, divided into 2 days; on days 3–4, 15–16, 38–39), methylprednisolone (from day 4 onwards, the initial does was 20 mg/kg/d and then the dose was decreased gradually) and ganciclovir (from day 5 onwards, for 4 weeks). However, he also received assistant respiration (from day 13 to 16) because of hypoventilation, and then received plasma exchange for 5 times (on days 17, 20, 27, 32, 35) as well as rituximab for 4 times (300 mg/d; on days 40, 47, 54, 61) due to lack of obvious improvement. The patient still had repeated seizures after admission; hence, he was treated with clonazepam and sodium valproate. On day 44, his altered personality started to recover gradually. From day 47 onwards, he began to have decreased involuntary movement and improved cognitive function. On day 49, his Babinski sign and ankle clonus turned negative. On day 67, the NMDAR antibody titer in CSF had decreased to 1:3.2. Seventy-four days following admission, the patient was discharged, with no seizure and no involuntary movement. However, he still could not speak. The patient was followed up for 10 months. During the period, he received oral corticosteroid, sodium valproate, and neurotrophic therapy. His language started to recover 5 months after discharge, which was almost fully recovered in the next 3 months. Until now, his cognitive function has been significantly improved, and he could attend school normally. Repeat MRI and EEG results were normal.
2.3. Case 3
A 2-year-old female was admitted for repeated fever, emesis, seizures for 1 month, and coma for 1 day. There was no history of other diseases before. Physical examination showed Glasgow score was 7, involuntary movement (limbs, eyes, and orofacial abnormal movement), decreased muscle strength of limbs (I–II), positive bilateral Babinski sign, and ankle clonus. The routine blood result showed normal. Laboratory testing revealed normal biochemistry (blood electrolyte, liver function, renal function, blood ammonia, and blood lactic acid). Blood cellular and humoral immunity were also normal. Serum viral test showed positive EBV-NA, EBV-CA-IgG, CMV-IgG, HSV-IgG, and RV-IgG antibodies (Table 1). CSF analysis revealed high levels of protein (606.3 mg/L), IgA (11.2 mg/L), IgM (49.3 mg/L), and IgG (295 mg/L). The white blood cell count and glucose in CSF were normal. CSF test showed positive EBV-EA-IgG, EBV-CA-IgA, EBV-CA-IgG, HSV-I-IgG, RV-IgG, and anti-NMDAR (1:3.2) antibodies (Table 2). Brain MRI (Fig. 1C) showed abnormal white matter signal in bilateral cerebral hemisphere (decreased T1 and increased T2/FLAIR signal), reinforced local region in enhanced brain surface and parenchyma, and local cerebral atrophy. EEG result showed background slow waves. The patient was diagnosed with anti-NMDAR encephalitis according to the clinical presentation and positive anti-NMDAR antibody in CSF. The patient received immunoglobulin (2 g/kg, divided into 2 days; on days 2–3, 28–29, 44–45), methylprednisolone (from day 2 onwards, the initial dose was 20 mg/kg/d and then the dose was decreased gradually) and ganciclovir (from day 6 onwards, for 4 weeks). She was diagnosed with secondary epilepsy, and was also treated with levetiracetam (from day 2 onwards) and oxcarbazepine (from day 30 onwards). On day 18 after admission, her muscle strength of limbs started to recover. On day 23, she had decreased involuntary movement, and her consciousness began to recover gradually 4 days later. On day 21, repeat CSF test showed the protein and IgA levels were normal, as well as decreased IgG (60.1 mg/L) and IgM (2.9 mg/L) levels. On day 46, the anti-NMDAR antibody titer in CSF had decreased to 1:1. She was discharged 57 days after admission. At the same time, she had improved consciousness level (Glasgow score: 13), enhanced muscle strength (upper limbs: III, lower limbs: IV) and decreased involuntary movement. However, she still could not speak. The patient was followed up for 9 months. The patient received oral corticosteroid, anti-epileptic drugs (levetiracetam and oxcarbazepine), and neurotrophic therapy. Repeat head MRI suggested abnormal white matter signals in bilateral cerebral hemisphere (decreased T1 and increased T2/FLAIR signal) and cerebral atrophy, as shown in Figure 1D. Repeat EEG showed epileptic discharge. Until now, her muscle strength of the left upper limb was III, and the other limbs were normal (V). However, she still had language dysfunction and cognitive impairment.
This study was approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine (approval number: XHEC-D-2019-021). Informed written consents were obtained from the patients for publication of the case reports and accompanying images.
3. Discussion
In the 3 cases, the diagnosis of anti-NMDAR encephalitis was confirmed based on their symptoms and positive anti-NMDAR antibody in CSF. Interestingly, we found positive EBV-EA-IgG antibody in CSF. Several studies suggested that EBV-EA-IgG could present in both acute and reactivated EBV infection.[6,7] Thus, the finding in this context indicated reactivated EBV infection according to the laboratory results, which was significative and has not been reported before.
Since anti-NMDAR encephalitis was 1st described in 2007, there have been increasing numbers of reports regarding it worldwide. It was reported that behavior problems, seizures, and movement disorders were the most common symptoms in children.[1] In this context, we found that the 3 patients developed the symptoms of language dysfunction, seizures, poor comprehension, altered personality, cognitive impairment, and involuntary movement. Thus, more data are required to assess whether the patients with reactivated EBV infection may exhibit specific symptoms that differ from those without EBV infection.
Previous studies suggested that anti-NMDAR encephalitis might be associated with postinfectious immune response. It has been reported that anti-NMDAR encephalitis was complicated with acute influenza, vaccines, and mycoplasma infections.[5,8,9] Toxoplasma gondii and HSV infection could trigger anti-NMDAR encephalitis, especially HSV, which has been widely studied in recent years. It was reported that 30% of the patients with HSV encephalitis had positive anti-NMDAR antibodies in serum or CSF.[10] Xu et al reported a case of one 67-year-old female diagnosed with anti-NMDAR encephalitis in 2011, and serum EBV-IgM was detected positive.[11] These studies suggested that infectious agents might damage blood–brain barrier and trigger a synaptic inflammatory response. However, the mechanism that explains the autoimmune process is currently unclear. In our context, we found positive EBV-EA-IgG antibody in CSF, which suggested reactivated EBV infection, and the possibility that reactivated EBV infection may trigger pediatric anti-NMDAR encephalitis has not been reported previously.
The majority of patients with primary EBV infection are usually asymptomatic, and only 0.37% to 7.3% of them exhibit neurologic complications, such as meningoencephalitis or meningitis.[12] EBV infection is associated with some autoimmune diseases, such as multiple sclerosis and rheumatoid arthritis, in which the infection can induce B-cell activation, damage blood–brain barrier, and lead to a strong serum antibody response to EBV-NA.[13–15] Elevated levels of serum antibodies against EBV-NA, CA, and EA were detected in patients with rheumatoid arthritis.[15] In our 3 cases, based on the laboratory testing, the diagnosis of EBV encephalitis was unconfirmed due to the lack of polymerase chain reaction for EBV in CSF. We speculate that the infection may damage blood–brain barrier, invade central nervous system, and participate in the secondary immune process. The possible mechanism may be that virus infection can destroy neurons, and then initiate a primary autoimmune response against NMDAR by presenting tissue which is normally shielded from systemic immunity.[10] The cases exhibited neurologic and psychiatric symptoms, and the latter could appear in immunocompromised patients with EBV-induced encephalitis, which also suggested that immune system might participate in psychiatric disorders induced by EBV infection.[16]
A study reported 577 cases of anti-NMDAR encephalitis, and about 70% had a good outcome.[17] In our cases, the clinical symptoms were obviously improved after treatment, and the antibody titer of anti-NMDAR was decreased obviously.
Taken together, anti-NMDAR encephalitis can be triggered by various infections. We found positive EBV-EA-IgG antibody in the 3 cases, and there may be a link between reactivated EBV infection and pediatric anti-NMDAR encephalitis.
4. Conclusion
In conclusion, reactivated EBV infection is very likely to be associated with pediatric anti-NMDAR encephalitis. Related possible serum and CSF virology tests should be completed while diagnosing the disease, which can help ascertain patients’ partial etiology and facilitate prompt etiologic treatment. Further studies should be conducted regarding the mechanism underlying the association between the reactivated EBV infection and anti-NMDAR encephalitis.
Author contributions
Conceptualization: Ling Li.
Data curation: Ruolin Hou.
Investigation: Ruolin Hou, Jing Wu, Dake He, Yumei Yan.
Methodology: Ruolin Hou, Jing Wu, Dake He, Yumei Yan, Ling Li.
Supervision: Dake He, Ling Li.
Writing – original draft: Ruolin Hou.
Writing – review & editing: Ling Li.
Footnotes
Abbreviations: Anti-NMDAR = anti-N-methyl-d-aspartate receptor, CA = capsid antigen, CMV = cytomegalovirus, CSF = cerebrospinal fluid, EA = early antigen, EBV = Epstein–Barr virus, EEG = electroencephalography, HSV = herpes simplex virus, MRI = magnetic resonance imaging, NA = nuclear antigen, RV = rubella virus.
Funding: Shanghai Hospital Development Center (SHDC12015113).
The authors have no conflicts of interest to disclose.
References
- [1].Barbagallo M, Vitaliti G, Pavone P, et al. Pediatric autoimmune encephalitis. J Pediatr Neurosci 2017;12:130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Geoghegan S, Walsh A, King MD, et al. Anti-N-methyl-d-aspartate receptor antibody mediated neurologic relapse post herpes simplex encephalitis: a case series. Pediatr Infect Dis J 2016;35:e258–61. [DOI] [PubMed] [Google Scholar]
- [4].Cai XT, Zhou H, Xie Y, et al. Anti-N-methyl-d-aspartate receptor encephalitis associated with acute toxoplasma gondii infection: a case report. Medicine 2018;97:e9924–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang H. Anti-NMDA receptor encephalitis and vaccination. Int J Mol Sci 2017;18:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Buisson M, Fleurent B, Mak M, et al. Novel immunoblot assay using four recombinant antigens for diagnosis of Epstein-Barr virus primary infection and reactivation. J Clin Microbiol 1999;37:2709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Crowley A, Connell J, Schaffer K, et al. Is there diagnostic value in detection of immunoglobuling antibodies to the Epstein-Barr virus early antigen? Biores Open Access 2012;1:291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baltagi S, Shoykhet M, Felmet K, et al. Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Ped Crit Car Med 2010;11:179–84. [DOI] [PubMed] [Google Scholar]
- [9].Gable SM, Gavali S, Radner A, et al. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis 2009;28:1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pruss H, Finke C, Holtje M, et al. N-Methyl-d-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol 2012;16:902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu CL, Liu L, Zhao WQ, et al. Anti-N-methyl-d-aspartate receptor encephalitis with serum anti-thyroid antibodies and IgM antibodies against Epstein-Barr virus viral capsid antigen: a case report and one year follow-up. BMC Neurol 2011;29:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tselis AC. Epstein-Barr virus infections of the nervous system. Handb Clin Neurol 2014;123:285–305. [DOI] [PubMed] [Google Scholar]
- [13].Laurence M, Benito-León J. Epstein-Barr virus and multiple sclerosis: updating Pender's hypothesis. Mult Scler Relat Disord 2017;16:8–14. [DOI] [PubMed] [Google Scholar]
- [14].Draborg AH, Duus K, Houen G. Epstein-Barr virus in systemic autoimmune diseases. Clin Dev Immunol 2013;2013:535738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Blaschke S, Schwarz G, Moneke D, et al. Epstein-Barr virus infection in peripheral blood mononuclear cells, synovial fluid cells, andsynovial membranes of patients with rheumatoid arthritis. J Rheumatol 2000;27:866–73. [PubMed] [Google Scholar]
- [16].Behr J, Schaefer M, Littmann E, et al. Psychiatric symptoms and cognitive dysfunction caused by Epstein-Barr virus-induced encephalitis. Eur Psychiatry 2006;21:521–2. [DOI] [PubMed] [Google Scholar]
- [17].Titular MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]


