Abstract
This study aimed to investigate the possible changes in anterior chest tightness after breast cancer surgery. We also try to investigate whether anterior chest tightness is associated with upper-limb dysfunction after breast cancer surgery. Eighty-three women who underwent breast cancer surgery were evaluated before and 2 weeks, 3 months, and 9 months after surgery. Anterior chest tightness was measured using the length of the pectoralis minor muscle through 2 methods (length from the coracoid process to the fourth rib and linear distance from the table to the posterior acromion with supine position). Shoulder range of motion and the K-DASH (Korean version of Disability Arm and Shoulder Questionnaire) score were measured to quantify functional performance of upper limb. Anterior chest tightness of patients with breast cancer significantly increased after surgery. Upper limb dysfunction was observed such as reduced shoulder range-of-motion and increased K-DASH score over time. Increase in chest tightness was correlated with shoulder range-of-motion reduction. Chest tightness was not correlated with K-DASH score directly. However, shoulder range-of-motion reduction was significantly correlated with K-DASH score. Chest tightness and upper limb dysfunction increased in breast cancer survivor. Increase in chest tightness after surgery is associated with upper limb dysfunction and careful attention is required.
Keywords: articular, breast neoplasms, pectoralis muscles, range of motion, upper extremity
1. Introduction
Many breast cancer survivors experience shoulder and arm dysfunction.[1] Shoulder and arm pain, restricted motion, upper-limb weakness and sensory change, and edema are common complaints.[2–6] Operations and radiation that involve the breast and axilla are routinely performed for the treatment of breast cancer, and these procedures directly involve the neuromusculoskeletal tissues around the shoulder girdle regions.[7] Those injuries as well as postsurgical pain, scar tissue formation, and protective posturing may lead to shortening of the anterior chest wall and could cause changes to the resting alignment of shoulder girdle, limited range of motion (ROM) of the shoulder, and rotator cuff injury.[7,8] One study with serial follow-up of breast cancer survivor reported postoperative pectoralis tightness.[3] In this study, the definition of pectoralis tightness was based only on limited shoulder motion, and direct measurement of the length of the pectoralis muscle was not attempted. Moreover, the authors evaluated pectoralis tightness on the time after breast cancer surgery and therefore they could not compare pectoralis tightness before and after surgery.
A previous study reported that the length of the pectoralis minor muscle is reliable measure of chest tightness that indicates postural abnormality and muscle imbalance in the upper extremity, which cause many shoulder pathologies such as impingement syndrome, rotator cuff injury, and adhesive capsulitis of the shoulder.[9] The resting length of the pectoralis minor is a simple measure of chest tightness that could demonstrate altered scapular kinematics with acceptable inter- and intrarater reliability.[10,11] To our best knowledge, there is no study about chest tightness using pectoralis minor length before and after breast cancer surgery or radiation therapy. The objective of this study was to investigate the longitudinal change in anterior chest wall tightness measured using pectoralis minor muscle length during the postoperative periods in breast cancer. We also tried to identify whether the change in pectoralis minor muscle length is correlated with upper limb dysfunction after breast cancer treatment.
2. Materials and methods
2.1. Participants
From April 2014 to February 2015, a total of 83 women who underwent breast cancer surgery were enrolled for our study at university hospital. All patients for this study were provided written informed consent. Patients were excluded if they had a history of chest, shoulder surgery, or sustained fractures. In addition, patients were excluded if they had recurrent or bilateral breast cancer surgery. This study was exempted from institutional review board requirements according to the guidelines of Korea University Guro Hospital.
2.2. Follow-up evaluation
We evaluated the status of patients before and 2 weeks, 3 months, and 9 months after surgery. We evaluated the patients’ general information, pectoralis minor length, and upper-limb function including shoulder ROM and K-DASH (Korean version of the Disabilities of the Arm, Shoulder and Hand questionnaire) before surgery.[12] The preoperative assessment was conducted in the ward 1 day before breast surgery. The follow-up evaluation was conducted postoperatively in the outpatient clinic at 2 weeks (including pectoralis minor length and shoulder ROM), 3 months, and 9 months (including pectoralis minor length, shoulder ROM, and K-DASH) after surgery.
2.3. Chest tightness using pectoralis minor length
Chest tightness was evaluated using pectoralis minor length. The pectoralis minor muscle length was measured using 2 methods. The one method involved measuring the length from the coracoid process to the anterior inferior edge of the fourth rib 1-finger apart from lateral to the sternum. The patients were in the supine position with a normal, relaxed posture during data collection. The caliper arms were placed at the landmarks to measure the distance between them (Fig. 1).[10]
Figure 1.
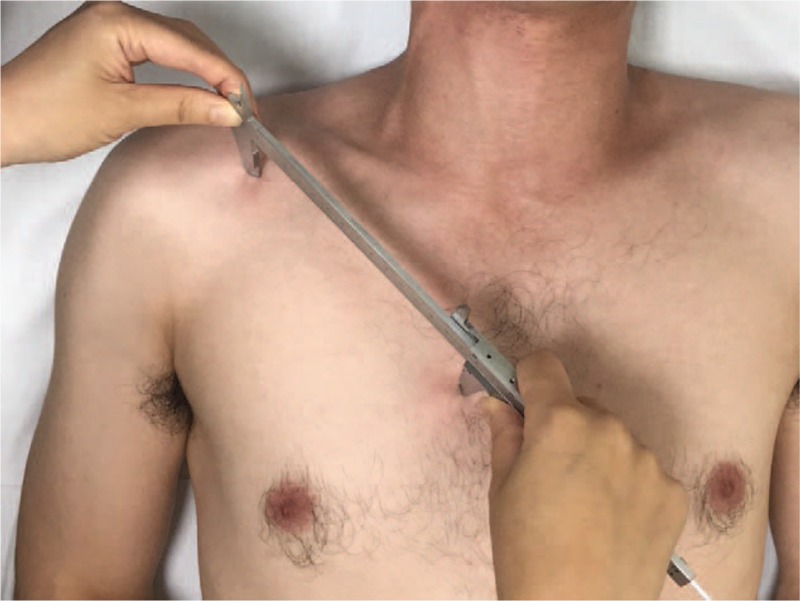
Measurement of pectoralis minor muscle length, the length from the coracoid process to the anterior inferior edge of the fourth rib 1-finger apart from lateral to the sternum with vernier calliper.
The other method involved measuring the linear distance from the table to the posterior acromion of the patients in supine position with the arms by the side and elbows in flexed and rested on the wall of abdomen. We measured the linear distance in millimeters by using a rigid standard plastic transparent right angle. Without adding any pressure on the table, the base of the protractor was placed on the bed and the vertical side was placed posterior to the acromion (Fig. 2).[11]
Figure 2.
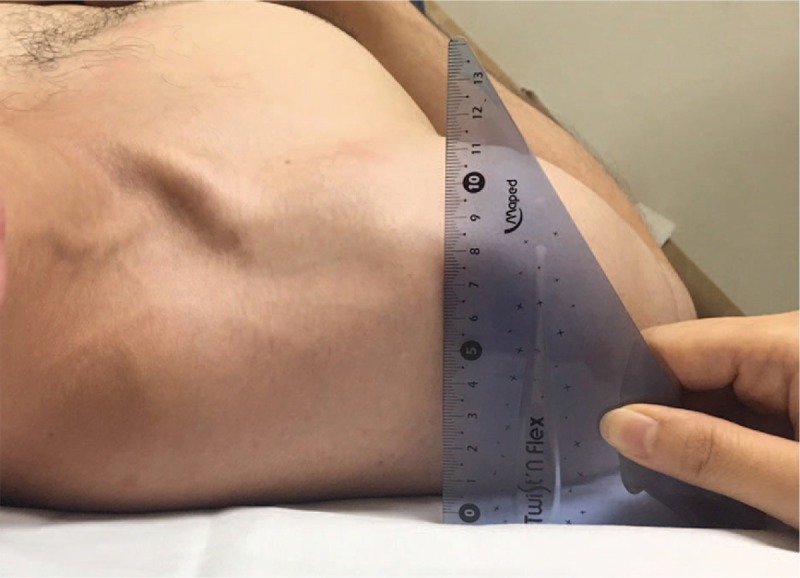
Measurement of pectoralis minor muscle length, the linear distance from the table to the posterior acromion in supine position.
2.4. Statistical analysis
All statistical analyses were performed with SAS version 9.2 software (SAS Institute Inc, Cary, NC). To analyze the results, generalized linear models were used. The pectoralis minor length, shoulder ROM, K-DASH were compared according to time, breast surgery, axillary surgery, radiation therapy on axillary area. Correlation between pectoralis minor length, shoulder ROM, and K-DASH was evaluated after adjustment for age, sex, body mass index. P < .05 was considered statistically significant.
3. Results
3.1. General characteristics
Eighty-three women were included in the study. The mean age was 52.7 ± 10.1 years (range, 28–76 years). The mean body mass index was 25.7 ± 10.1. Ten out of 83 patients underwent modified radical mastectomy (MRM) and the remaining patients underwent breast conserving surgery (BCS) (46 right breast and 37 left breast). With respect to axillary lymph node surgery, 28 out of 83 participants underwent axillary lymph node dissection and the rest of them underwent sentinel lymph node biopsy without further lymph node surgery. A total of 77 patients received radiotherapy of the breast or chest wall after surgery and 22 out of 83 patients received radiotherapy of the axilla and supraclavicular fossa.
The preoperative K-DASH score was 3.312+5.71 (0–27.5). The pectoralis minor length measured by the distance from the coracoid process to anterior inferior edge of the fourth rib was 15.6 ± 1.5 cm and the length from posterior acromion to the table was 5.0 ± 1.3 cm.
3.2. Longitudinal changes in shoulder ROM
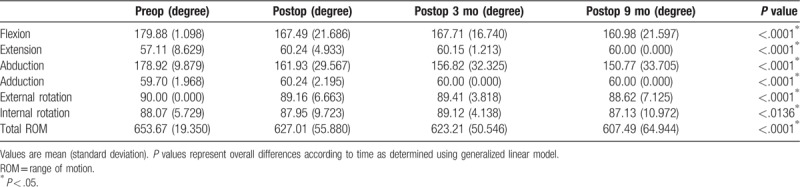
Table 1 shows changes in shoulder ROM over time, reported as mean and standard deviation for follow-up and preoperative baseline values. Patients who underwent breast cancer surgery showed significant reduction in shoulder ROM over time (P < .05). The reduction in ROM was observed in all directions of shoulder motion (flexion, extension, abduction, adduction, external rotation, and internal rotation). The reduction in shoulder flexion and abduction movement were greater in patients who underwent MRM than BCS (P < .05). The reduction in postoperative shoulder ROM became more severe as age increased.
Table 1.
Longitudinal change of shoulder range of motion.
3.3. Longitudinal changes in pectoralis minor length and K-DASH score
Postoperative chest tightness increased over time in both 2 pectoralis minor length measurement methods, (P < .05) (Fig. 3). There was no significant difference in the increase of chest tightness in terms of surgery type, axillary lymph node surgery, and presence or absence of radiotherapy.
Figure 3.
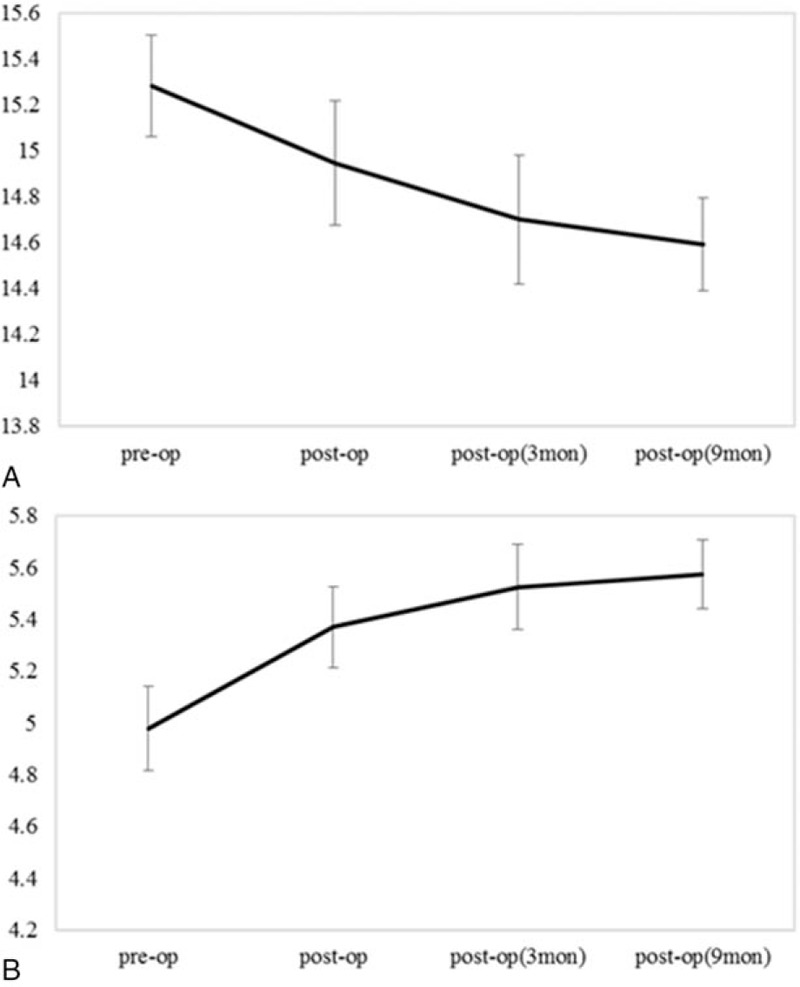
Longitudinal changes of pectoralis minor length. A, The length from the coracoid process to the anterior inferior edge of the fourth rib 1-finger apart from lateral to the sternum. B, Distance from the table to the posterior acromion. Values are estimated mean and standard error (cm).
K-DASH score rose significantly over time after surgery (P < .05) (Fig. 4). There was no significant difference depending on the type of breast surgery (MRM vs BCS), lymph node surgery (axillary lymph node dissection vs selective lymph node biopsy), and whether the patients received radiotherapy or not.
Figure 4.
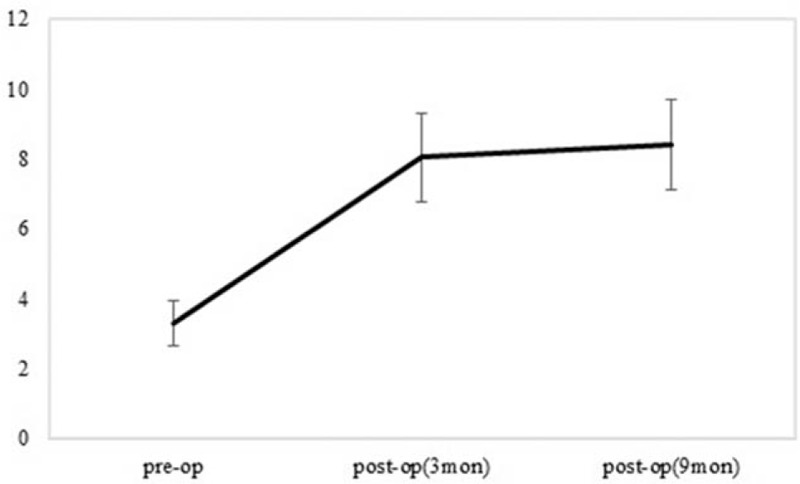
Longitudinal changes of Korean version of Disability Arm and Shoulder Questionnaire. Values are estimated mean and standard error.
3.4. Relationship among pectoralis minor length, shoulder ROM, and K-DASH score
The increase in chest tightness had significant correlation with reduction in shoulder ROM. Increase in chest tightness that was measured by pectoralis minor muscle length (the distance from coracoid process to the anterior inferior edge of the fourth rib) was significantly correlated with decrease in range of shoulder extension and internal rotation movement (P < .05). Pectoralis minor length, the distance from the table to the posterior acromion was significantly correlated with decrease in range of shoulder adduction and internal rotation movement (P < .05). In our results, there was no direct correlation between increase in chest tightness and K-DASH score. However, decrease in total shoulder ROM was correlated with increase of K-DASH score, (P < .05).
4. Discussion
This study verified that chest tightness increased in patients with breast cancer after surgery. Also, chest tightness was associated with reduction of shoulder ROM that was correlated with upper limb dysfunction.
Nesvold et al[13] reported that arm/shoulder problem in breast cancer survivors was associated with having had mastectomy, longer follow-up time, minimal physical activity. In our study, decrease in shoulder ROM after surgery was more prominent in patients who underwent mastectomy than in those who underwent BCS. Moreover, ROM limitation was significantly correlated with K-DASH score. Thus our findings are consistent with previous findings that mastectomy caused arm shoulder problem more than breast conserving surgery. However, surgery type (mastectomy vs breast conserving surgery) did not affect degree of postoperative chest tightness in our study. Therefore, chest tightness can be thought of as an independent factor causing limited shoulder ROM that affects shoulder pain. Also, reduction of shoulder ROM was more prominent in older patients. This is an unreported finding in previous studies of Nesvold et al.[13] They reported that postoperative longer follow-up time was an independent factor affecting shoulder pain. Special attention to prevent shoulder dysfunction is needed during long-term follow-up after surgery in patients with older age and undergoing MRM.
One previous study about shoulder and spinal kinematics after mastectomy reported that patients showed altered scapular kinematics on upper-limb movement.[14] The authors reported that patients who underwent mastectomy demonstrated more considerable changes in scapular kinematics in the lesion side when rotated upward than controls. They analyzed the scapular movement while performing arm elevation after mastectomy. However, they could not find any correlation between kinematic variables and arm disability using DASH score. Our results increase in chest tightness after breast cancer surgery was measured in the resting state (supine posture). Therefore, we cannot verify previous result of increasing scapular upward rotation is not directly associated with resting chest wall tightness. However, resting chest tightness with decreased pectoralis minor length attributed slightly resting scapular posture on downward posture and it might contribute increasing extent of upward movement during elevation of arm.
Increase in chest tightness is correlated with decrease in range of shoulder internal rotation motion in 2 measurement methods of pectoralis minor length. Increase in chest tightness causes an increase in a scapula internally rotated. This suggests that the internal rotation of the shoulder joint is reduced because the scapula has already been internally rotated. This is also true for study of healthy subjects without shoulder pain. The scapula was internally rotated in person with short pectoralis minor length compared with a person with long pectoralis minor length.[15]
This study is valuable for the first study to investigate chest tightness after breast cancer. However, several limitations in the present study were acknowledged. First, the number of enrolled patients who underwent BCS and MRM is different. Also, patients who underwent selective lymph node biopsy account for a larger number than axillary lymph node dissection in this analysis. This difference in the number of patients may have affected the results of the relationship between upper limb dysfunction and the type of breast or lymph node surgery. Second, although K-DASH is a reliable and valid measure of upper limb dysfunction, it is not an objective evaluation tool but a self-reported questionnaire.[12] Therefore, upper limb dysfunction might not be evaluated objectively. Finally, we followed up the patients until 9 months postoperatively. The effect of chest tightness on upper limb dysfunction could not be fully evaluated because breast cancer survivors generally have been living for a long time and upper extremity abnormality was often reported later time.[16]
5. Conclusion
The findings of this study indicate that chest tightness and upper limb dysfunction increased on breast cancer survivor after surgery. The reduction of shoulder ROM is observed after breast cancer surgery, especially in mastectomy patients than BCS. As the chest tightness after surgery is associated with upper limb dysfunction careful attention is required for prevention.
Author contributions
Conceptualization: Woo Young Kim, Seung Nam Yang.
Data curation: Chung Ho Lee, Seong Yun Chung
Supervision: Woo Young Kim, Seung Nam Yang.
Writing – original draft: Chung Ho Lee.
Writing – review & editing: Seung Nam Yang.
Footnotes
Abbreviations: K-DASH = Korean version of Disability Arm and Shoulder Questionnaire, ROM = range of motion.
The authors have no conflicts of interest to disclose.
References
- [1].McCredie MR, Dite GS, Porter L, et al. Prevalence of self-reported arm morbidity following treatment for breast cancer in the Australian Breast Cancer Family Study. Breast 2001;10:515–22. [DOI] [PubMed] [Google Scholar]
- [2].Stubblefield MD, Custodio CM. Upper-extremity pain disorders in breast cancer. Arch Phys Med Rehabil 2006;87:S96–9. [DOI] [PubMed] [Google Scholar]
- [3].Yang EJ, Park WB, Seo KS, et al. Longitudinal change of treatment-related upper limb dysfunction and its impact on late dysfunction in breast cancer survivors: a prospective cohort study. J Surg Oncol 2010;101:84–91. [DOI] [PubMed] [Google Scholar]
- [4].Gho SA, Steele JR, Jones SC, et al. Self-reported side effects of breast cancer treatment: a cross-sectional study of incidence, associations, and the influence of exercise. Cancer Causes Control 2013;24:517–28. [DOI] [PubMed] [Google Scholar]
- [5].Lee TS, Kilbreath SL, Refshauge KM, et al. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res Treat 2008;110:19–37. [DOI] [PubMed] [Google Scholar]
- [6].Rietman JS, Dijkstra PU, Hoekstra HJ, et al. Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: a systematic review. Eur J Surg Oncol 2003;29:229–38. [DOI] [PubMed] [Google Scholar]
- [7].Ebaugh D, Spinelli B, Schmitz KH. Shoulder impairments and their association with symptomatic rotator cuff disease in breast cancer survivors. Med Hypotheses 2011;77:481–7. [DOI] [PubMed] [Google Scholar]
- [8].Shamley D, Srinaganathan R, Oskrochi R, et al. Three-dimensional scapulothoracic motion following treatment for breast cancer. Breast Cancer Res Treat 2009;118:315–22. [DOI] [PubMed] [Google Scholar]
- [9].Borstad JD. Resting position variables at the shoulder: evidence to support a posture-impairment association. Phys Ther 2006;86:549–57. [PubMed] [Google Scholar]
- [10].Borstad JD. Measurement of pectoralis minor muscle length: validation and clinical application. J Orthop Sports Phys Ther 2008;38:169–74. [DOI] [PubMed] [Google Scholar]
- [11].Lewis JS, Valentine RE. The pectoralis minor length test: a study of the intra-rater reliability and diagnostic accuracy in subjects with and without shoulder symptoms. BMC Musculoskelet Disord 2007;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lim JY, Lee HY, Song JH, et al. Evaluation of the reliability, construct validity, and responsiveness of the Korean version of the DASH. J Korean Soc Surg Hand 2005;10:192–8. [Google Scholar]
- [13].Nesvold IL, Fossa SD, Holm I, et al. Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol 2010;49:347–53. [DOI] [PubMed] [Google Scholar]
- [14].Crosbie J, Kilbreath SL, Dylke E, et al. Effects of mastectomy on shoulder and spinal kinematics during bilateral upper-limb movement. Phys Ther 2010;90:679–92. [DOI] [PubMed] [Google Scholar]
- [15].Borstad JD, Ludewig PM. The effect of long versus short pectoralis minor resting length on scapular kinematics in healthy individuals. J Orthop Sports Phys Ther 2005;35:227–38. [DOI] [PubMed] [Google Scholar]
- [16].Harrington S, Michener LA, Kendig T, et al. Patient-reported upper extremity outcome measures used in breast cancer survivors: a systematic review. Arch Phys Med Rehabil 2014;95:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


