Supplemental Digital Content is available in the text
Keywords: brain education-based meditation, hypertension, inflammatory gene, low-density lipoprotein cholesterol, type 2 diabetes
Abstract
Background:
Hypertension and type 2 diabetes are chronic diseases, which generally require lifetime care. Meditation and yoga can be complementary to pharmacological therapies according to the scientific evidences so far. Brain education-based meditation (BEM) is a technique, which has been known to change brain structure, psychology, and physiology of healthy adult participants. This randomized, nonblinded pilot trial aimed to examine whether BEM affects the conditions of patients with hypertension and/or type 2 diabetes compared with health education classes.
Methods:
We randomly allocated 48 patients with hypertension and/or type 2 diabetes to BEM (n = 24) or health education (n = 24) classes in the Ulsan Junggu Public Health Center in Korea, where the classes were run during the same period and explored the impact of 8-week practice on the serum glutamic-oxaloacetic transaminase, serum glutamic pyruvic transaminase, gamma glutamyl transpeptidase, creatinine, high-density lipoprotein cholesterol, and low-density lipoprotein (LDL) cholesterol. Total RNA was extracted to examine inflammatory gene expressions from the whole blood using PAXgene blood RNA System. In addition, self-reports on mental/physical health were evaluated. The Student's t test, chi-squared test, and analysis of covariance were used for statistical analysis.
Results:
The number of people who participated until the completion of the study was 14 in the control and 21 in the BEM group. After 8 weeks, LDL cholesterol level was significantly decreased in the BEM group after the intervention (13.82 mg/dL reduction, P < .05), while it was not significantly altered in the control group. The expression of inflammatory genes was significantly reduced after 8 weeks of the BEM training (0.3-, 0.5-, and 0.2-fold change for NFKB2, RELA, and IL1B, respectively, all P < .05). In the item analysis of mental/physical health self-reports, a significant improvement was confirmed as follows: increases in focus, confidence, relaxation, and happiness; decreases in fatigue, anger, and loneliness (all P < .05). There were no important adverse events or side-effects by BEM intervention.
Conclusion:
Compared to health education, BEM helps lower LDL cholesterol level and the inflammatory gene expression in the patients with hypertension and/or type 2 diabetes. Moreover, BEM induces positive effects on the self-reported mental/physical states, warranting further study.
1. Introduction
There were 1.13 billion people worldwide with high blood pressure in 2015[1] and 422 million people with diabetes in 2014,[2] respectively. Hypertension and type 2 diabetes can cause other fatal complications. For example, pooled data from 54 countries show that at least 80% of cases of end-stage renal disease are caused by diabetes, hypertension, or a combination of the 2.[3] Furthermore, adults with diabetes have a 2 or 3 times higher rate of cardiovascular disease than adults without diabetes.[4]
Many evidences have shown that stress affects the risk of the hypertension. Temporary increase in blood pressure appears as part of the immediate response to stress; exposure to stress for long period may contribute to the development of persistent hypertension.[5] Indeed, stress increases the sympathetic nervous activity, for example, elevation of blood pressure.[6,7] In addition, stress affects the risks of type 2 diabetes. Activation of the physiologic stress response from chronic exposure to stressors, low socioeconomic status, severe mental health problems, or aggressive behavior increases the risk of type 2 diabetes.[8] Exposure to chronic stressors lead to health-adverse behaviors and physiologic stress response, resulting in chronic subclinical inflammation, followed by central obesity, insulin resistance, dyslipidemia, hypertension, and depression, eventually causing type 2 diabetes or cardiovascular diseases.[8]
Low-density lipoprotein (LDL) cholesterol contributes to fat accumulation in arteries; this narrows the arteries, induces hypertension, increases the risk of cardiovascular diseases; LDL cholesterol-lowering therapy reduces the risk of cardiovascular disease.[9] In the action of drugs for type 2 diabetes, LDL was found to be included in the mechanism, suggesting the close relations between type 2 diabetes and LDL.[10] Moreover interestingly, LDL seems also affected by higher function of human brain. Chronic work stress elevates serum LDL cholesterol in an occupationally homogeneous group of healthy middle-aged men.[11] Moreover, higher LDL cholesterol was found in participants with higher self-perceived stress scores.[12] These studies imply the relationship between stress and LDL cholesterol.
In inflammation, nuclear factor kappa B subunit (NF-κB) family is activated by canonical and noncanonical signaling pathways. The noncanonical NF-κB pathway, which includes NFKB2 and RELB, has been revealed to be involved with metabolic diseases such as obesity and type 2 diabetes.[13–15] Psychological stress contributes to chronic activation of acute-phase inflammation.[16] As mentioned above, stress increases the risk of hypertension/type 2 diabetes. Inflammation has been shown to play an important role in the pathogenesis of type 2 diabetes[17] and hypertension.[18]
Brain education-based meditation (BEM) is a training system applying 5 steps of brain education (BE), which is a modernized method of traditional mind–body training in Korea[19] and has been also known as brain wave vibration meditation.[20–31] Since BEM has both the characteristics of yoga and meditation, it is involved in the improvement of both physical and mental features of participants. Its effects have been investigated in various settings such as randomized controlled trials for novice or cross-sectional studies for long-term meditators; off/online training; healthy individuals, and breast cancer patients. They include psychological improvements such as decreases in stress,[24] anxiety,[28] depression,[23] fatigue[28] and increases in positive effect,[24] quality of life[28]; behavioral improvements such as increases in sleep quality,[22,23] problem solving[31]; physiological effects such as increases in plasma nitric oxide,[21] dopamine,[24] and interleukin 10[30]; brain structural changes such as thickening of prefrontal cortex area[27] and alterations in conectivities between insula and several brain regions.[20] Findings in certain psychological improvements indicate its association with epigenetic changes.[26,29]
Since mental health significantly affects hypertension and type 2 diabetes,[8] BEM, which improves mental health, may have beneficial effects on the physiology of the diseases. People with hypertension and/or type 2 diabetes should control the body condition throughout their lifetime, and nonpharmacological interventions can be used as complementary treatments. This suggests that mind–body interventions such as BEM may be beneficial. However, there have been no studies of BEM on patients with hypertension and/or type 2 diabetes. Herein, we performed a randomized controlled pilot trial to investigate the effectiveness of BEM on physiological and psychological changes in people with hypertension and/or type 2 diabetes under medication.
2. Methods
2.1. Participants
The research was approved by the Ethics Committee of the University of Brain Education (IRB201709-01) and informed consents were obtained for experimentation with all the participants. The participants with hypertension and/or type 2 diabetes under regular medication, aged 57 to 87 years, were recruited among registered patients in the Hypertension/Diabetes Registration Education Center of the Ulsan Junggu Public Health Center in Republic of Korea through advertisements over 1 month (September 2017). Excluded were people who have other diseases except hypertension/type 2 diabetes. Forty-nine participants were recruited and 1 person declined to participate because of the schedule. They were simply randomized into 2 groups by a computer-generated list of random numbers, each including 24 people. Thirty-five participants (BEM: 21; control: 14) completed the study, with 13 dropouts (BEM: 3; control: 10). The dropouts said their schedules do not fit the experimental schedule (2 controls) or they do not have interests (BEM: 3; control: 8) (Supplemental Content 1).
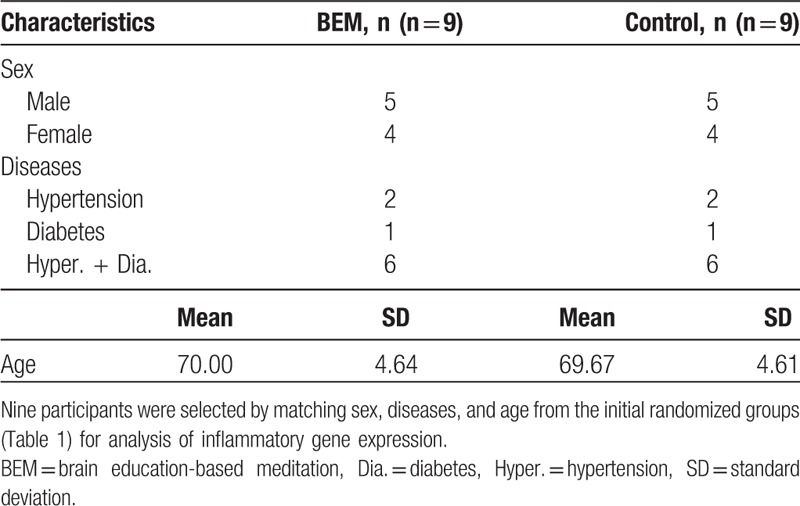
For inflammatory gene expression analysis, a matched group design was used: 9 people were equally selected from the above-randomized groups in terms of sex, diseases, and age (Table 5). Fourteen participants (BEM: 8; control: 6) completed the study, with 4 dropouts (BEM: 1; control: 3). The dropouts said they do not have interests.
Table 5.
Baseline characteristics of sex/disease/age-matched participants for inflammatory gene expression analysis.
Random allocation sequence was generated by the researchers. Staffs of the Ulsan Junggu Public Health Center, who do not know the detail of the research, enrolled participants and assigned participants to interventions.
2.2. Interventions
The participants allocated to the BEM group attended BEM classes, designed to improve the conditions of patients of hypertension and/or type 2 diabetes, 16 times during 8 weeks (twice per week, from October 10, 2017 to December 1, 2017), while the participants in the control group participated in the health education class provided by the Ulsan Junggu Public Health Center. The BEM classes were taught by a certified BRAIN TRAINER (Korean Government Certification 2016-14), who is a trained instructor for BEM. The BEM classes basically followed as described previously[23] with slight modifications; each week has an individual topic following BE 5 steps (Supplemental Content 3). The attendance of participants was taken care of by the education center during the study. Full protocols are available from the authors on request.
2.3. Blood preparations and biochemical measurements
Venous blood was collected by phlebotomy with butterfly needle from subjects with their consent in the Ulsan Junggu Public Health Center. Excessive exercise, herbal medicine, and fasting were prohibited for 1 week before the blood collection, as well as during the experimental period. Sampling was performed after 8 hours of fasting. Blood was collected into serum separating tube (5 mL) and PAXgene tube (2.5 mL blood + 6.9 mL buffer). All the samples were inverted 10 times immediately after the collection. Serum separating tubes underwent centrifugation and serum was collected to measure serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), γ-glutamyl transferase (GGT), creatinine, high-density lipoprotein (HDL) cholesterol, and LDL cholesterol. The biochemical measurements were performed on the same day in the pathology laboratory of the Ulsan Junggu Public Health Center by COBAS INTEGRA 400 PLUS/800 analyzers. PAXgene RNA tubes were transferred in room temperature to the University of Brain Education on the same day and stored at −80°C upon arrival until RNA isolation.
2.4. Total RNA extraction, cDNA synthesis, and real-time quantitative polymerase chain reaction
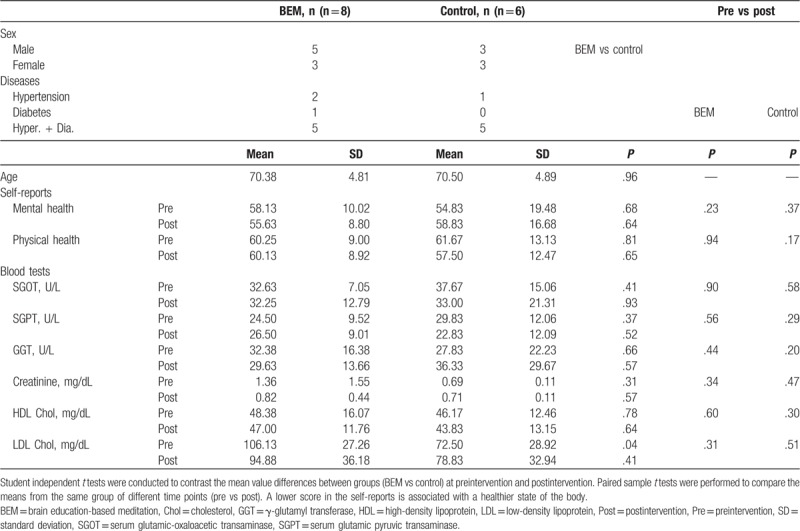
For this purpose, initially 9 participants per group were selected by matching sex, diseases, and age (Table 5). Because of the high expense of the experiment, not all the participants were included for RNA extraction, followed by cDNA synthesis and qPCR. Among them, 6 and 8 participants in the control and BEM groups, respectively, completed the designed study (Table 6). For RNA isolation, the frozen blood samples were returned to room temperature, and procedures followed the “Purification of Total RNA from human whole Blood Collected into PAXgene Blood RNA Tubes” protocol in the PAXgene Blood RNA Kit Handbook (PreAnalytiX GmbH, 08/2005, REF: 762174), including a DNAse treatment. cDNA was synthesized from 0.5 μg total RNA using the Super Script IV Reverse Transcriptase (Invitrogen, Cat No. 18090010, Carlsbad, California) following the manufacturer's guidelines with Oligo-dT primer. For qPCR, each reaction comprised of 7.5 μL 2× PowerUp SYBR Green Master Mix (A25780, Applied biosystems, Waltham, MA), 0.9 μL 10 μM primer mix, 1.6 μL ultrapure water, and 5 μL cDNA (1:10). Samples were then amplified on an ABI StepOnePlus. Following specific primer sets were used for each gene: NFKB2 (forward, 5′-CCATTGTGGAACCCAAGGAG-3′; reverse, 5′-GGATAGGTCTTTCGGCCCTT-3′), RELA (forward, 5′-GATACCACCAAGACCCACCC-3′; reverse, 5′-GCTCAGCCTCATAGAAGCCAT-3′), RELB (forward, 5′-CTCGTGGGGAAAGACTGCAC-3′; reverse, 5′-TCCTGATGGTTCTTCAGGGAC-3′), interleukin 1 beta (IL1B) (forward, 5′-GGG ACA GGA TAT GGA GCA AC-3′; reverse, 5′-CGCTTTTCCATCTTCTTCTTTGG-3′), and ACTB (forward, 5′-CATCGAGCACGG CATCGTCA-3′; reverse, 5′-TAGCACAGCCTGGATAGCAAC-3′). ACTB was used as a reference gene. All the reactions were carried out in triplicate.
Table 6.
Pre and post mean, SD, t tests for self-reports and blood tests of participants of inflammatory gene expression analysis.
2.5. Mental/physical health self-reports
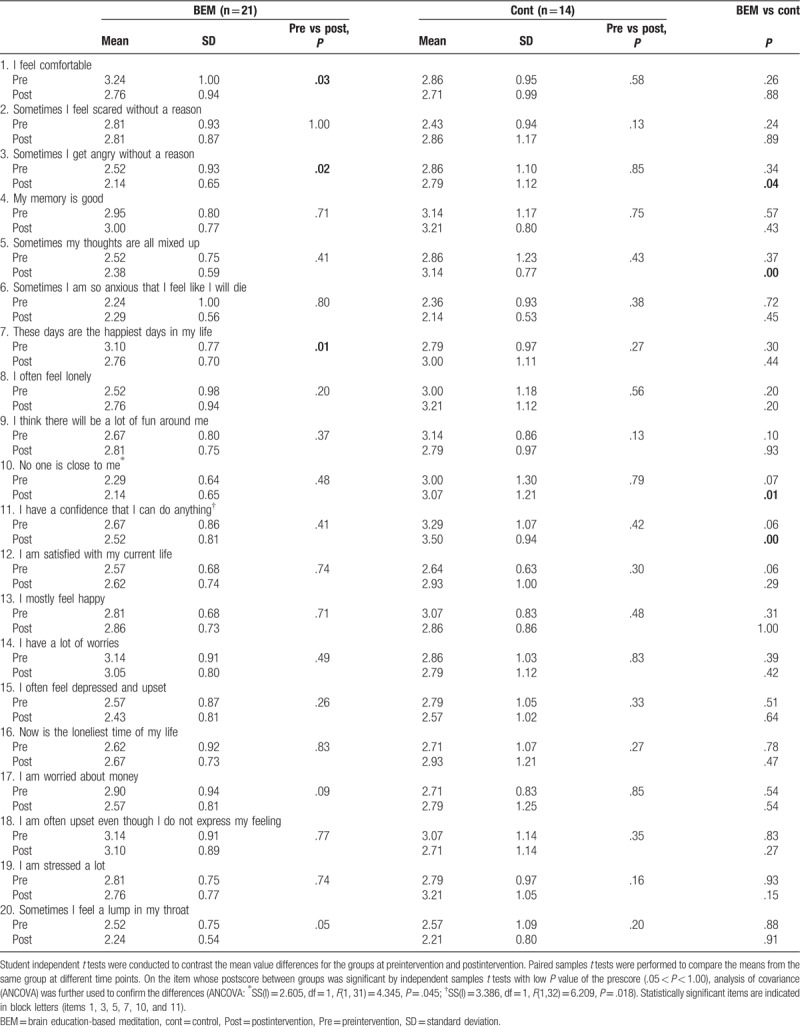
Self-report questionnaires were used in this study to assess mental and physical health. The questionnaires were reconstructed on the basis of the Personality Assessment Inventory[32] and Minnesota Multiphasic Personality Inventory.[33] In particular, the scales were validated for the elderly, based on the dynamic aspects of everyday life and the static aspects of the individual life cycle. Both mental and physical health self-reports are composed of 20 items, using a 5-point Likert-type scale ranging from “1 = strongly disagree” to “5 = strongly agree.” In the scoring calculation, positive items (Table 3: Item no. 1, 3, 4, 5, 6, 7, 8, 11, 12, 14, 17, 18, 20; Table 4: Item no. 1, 4, 7, 9, 11, 12, 13) were reversed scored so that lower scores reflect a healthier state and they can be included in the total scale scores. The internal consistency for the scales in the study was acceptable with Cronbach alpha values 0.93 (mental health) and 0.86 (physical health).
Table 3.
Pre and post mean, SD, t tests for physical health score.
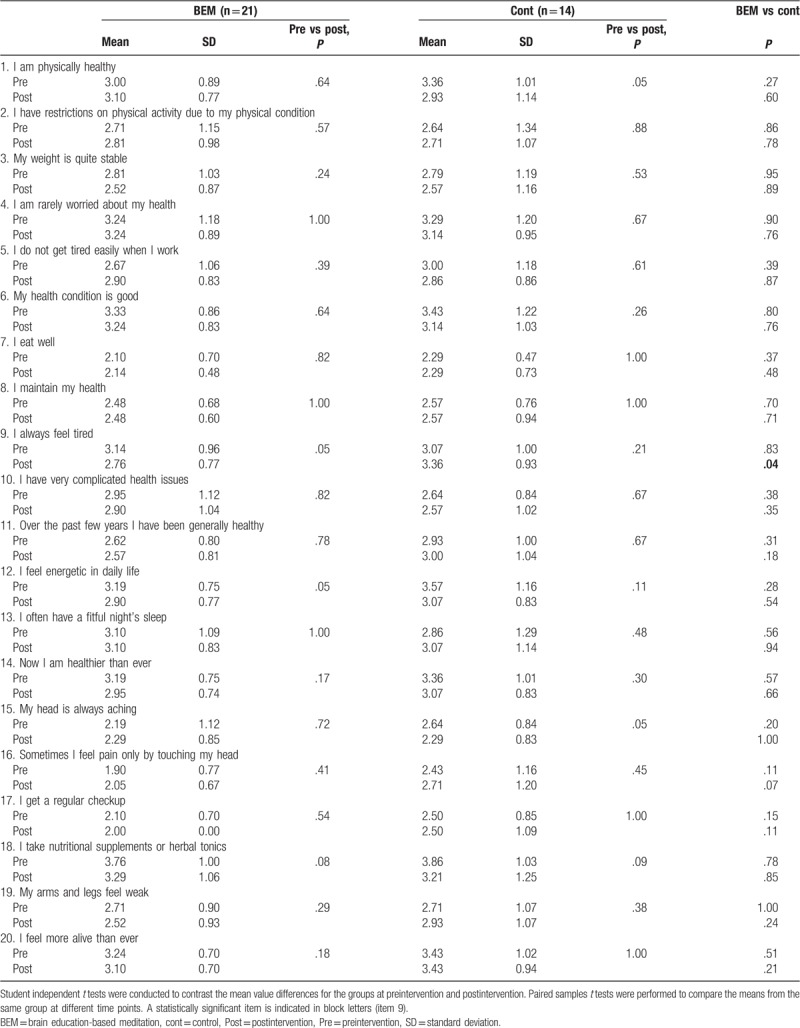
Table 4.
Pre and post mean, SD, t tests for mental health scores.
2.6. Statistical data analysis
All data analyses were done in blind manner without knowing the group information. Analyses for self-reports and blood tests were conducted with the SPSS 18.0 software package (IBM, Seoul). Chi-squared (for gender and disease) and independent samples t tests (for age, self-reports, biochemical analysis, and inflammatory gene expression analysis) were performed to assess the success of randomization and examine pretest differences between groups. The statistical significance of pre–post differences within each group and between groups was verified by paired samples t tests and independent samples t tests. Statistical values of the Welch–Aspin test were used when Levene's equivariant was not assumed in the independent samples t test. When the postscores between groups were statistically significant by independent samples t tests, with low P value of the prescore (.05 < P < 1.00), the difference between groups was finally analyzed by analysis of covariance (ANCOVA), which controls prescores.
3. Results
3.1. Comparison of blood biochemical characteristics between meditation and control group
In this pilot study, to find out the complementary effects of BEM on the health state of the patients, subjects who have diagnosed with hypertension and/or type 2 diabetes under medication participated in either BEM or health education class, both of which were run by the public healthcare center during the same period. The subjects were randomly allocated into 2 groups (Table 1, Supplemental Content 1). The numbers of people who participated in the premeasurement were 24 (BEM) and 22 (control), and there were no significant differences in the ratio of sex (50% each), disease (P = .42, chi-squared test), and age (P = .36, independent samples t test) between them. The period after the initial diagnosis of type 2 diabetes and hypertension was 8.84 ± 2.94 and 8.94 ± 2.39 years, respectively, for BEM; 5.02 ± 2.01 and 8.00 ± 2.59 years, respectively, for control (average ± standard error of the mean, Supplemental Content 2). There were no significant differences in the baseline scores of the groups for mental and physical health scores, SGOT, SGPT, GGT, creatinine, HDL, and LDL cholesterol, as examined by independent samples t test (Table 1). There were 3 (BEM) and 8 (control) dropouts, resulting in 21 (BEM) and 14 (control) participants for the final analysis (Table 2). The BEM group with high attendance (BEM [Att ≥ 5]) was analyzed additionally. There were no significant differences in the premeasurement of the above-mentioned parameters between the control and BEM group (or BEM [Att ≥ 5]) (Table 2).
Table 1.
Baseline characteristics of randomized participants.
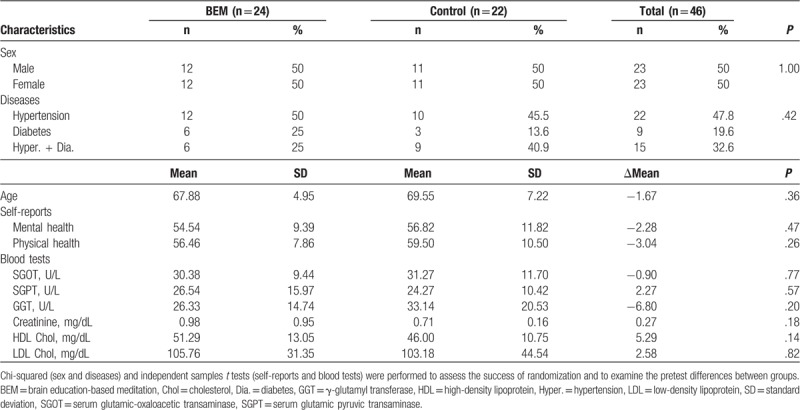
Table 2.
Pre and post mean, SD, t test for self-reports and blood tests.
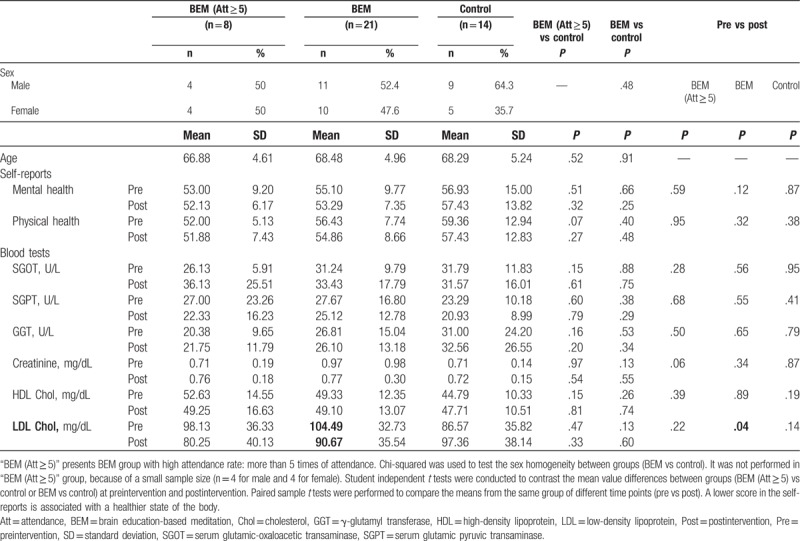
The results showed no significant changes between BEM and control, as well as between preintervention and postintervention of each group in SGOT, SGPT, GGT, creatinine, and HDL cholesterol (Table 2). However, LDL level in the total BEM group was significantly reduced after the intervention (13.82 mg/dL reduction, #P = .04, paired samples t test, n = 21) (Table 2, Fig. 1H). The Δ LDL cholesterol level (=LDL [post] − LDL [pre]) was significantly lower in the BEM (Att ≥ 5) (∗P = .04, independent samples t test, Fig. 1H) as well as the total BEM group (∗P = .01, independent samples t test, Fig. 1H) than the control group, while other markers did not show significant changes (Fig. 1A–G).
Figure 1.
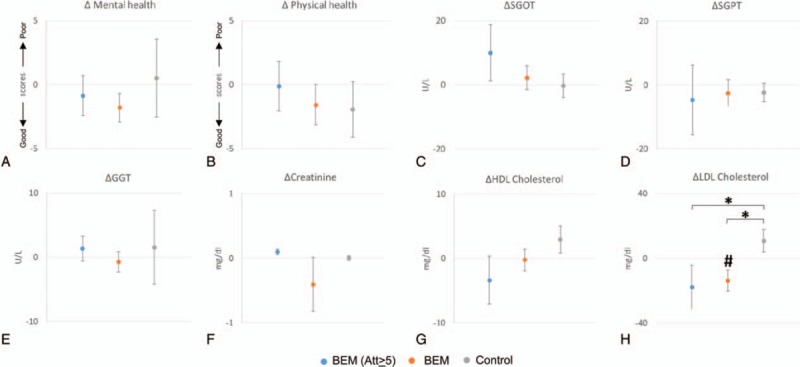
Alterations in values of self-reports and blood markers between preintervention and postintervention. “BEM (Att ≥ 5)” presents brain education-based meditation (BEM) group with high attendance rate: more than 5 times of attendance. The indicated values stand for the subtraction of values at preintervention from values at postintervention. Dots at the midline (y = 0) mean no changes between the values of preintervention and postintervention. The values of following self-reports and blood markers are shown: (A) mental health; (B) physical health; (C) serum glutamic-oxaloacetic transaminase (SGOT); (D) serum glutamic pyruvic transaminase (SGPT); (E) γ-glutamyl transferase (GGT); (F) creatinine; (G) high-density lipoprotein (HDL) cholesterol; (H) low-density lipoprotein (LDL) cholesterol. ∗P = .04, an independent samples t test between BEM (Att ≥ 5) and control; ∗P = .01, an independent samples t test between BEM and control; #P = .04, a paired samples t test from the same group at preintervention and postintervention; n = 8 (BEM (Att ≥ 5)), 21 (BEM), and 14 (control). Dots and error bars indicate the mean values and standard of errors, respectively. Att = attendance, GGT = γ-Glutamyl transferase, HDL = high-density lipoprotein, LDL = low-density lipoprotein, SGOT = serum glutamic-oxaloacetic transaminase, SGPT = serum glutamic pyruvic transaminase.
3.2. Comparison of self-report scores between meditation and control group
To check whether the BEM program affects the mental and physical health state, the subjects were asked to complete self-report questionnaires. In the physical health self-report, the score of 1 item “I always feel tired” showed significant reductions (better physical health) after the intervention (BEM = 2.76 ± 0.77 [n = 21], Cont = 3.36 ± 0.93 [n = 14], P = .04, independent samples t test), while other items did not show significant changes (Table 3). When the mental health scores were compared between preintervention and postintervention, there were significant reductions in scores (meaning better mental health) only in the BEM group for the following items (Table 4): “I feel comfortable” (pre = 3.24 ± 1.00, post = 2.76 ± 0.94, P = .03, paired samples t test, n = 21), “Sometimes I get angry without a reason” (pre = 2.52 ± 0.93, post = 2.14 ± 0.65, P = .02, paired sample t test, n = 21), and “These days are the happiest days in my life” (pre = 3.10 ± 0.77, post = 2.76 ± 0.70, P = .01, paired samples t test, n = 21). When the mental health scores of postintervention between BEM and control were compared, there were significant reductions (thus, better mental health) in the BEM group than in the control group in the following items: “Sometimes I get angry without a reason” (BEM = 2.14 ± 0.65 [n = 21], Cont = 2.79 ± 1.12 [n = 14], P = .04, independent samples t test), “Sometimes my thoughts are all mixed up” (BEM = 2.38 ± 0.59 [n = 21], Cont = 3.14 ± 0.77 [n = 14], P < .01, independent samples t test), “No one is close to me” (BEM = 2.14 ± 0.65 [n = 21], Cont = 3.07 ± 1.21 [n = 14], P = .01, independent samples t test), and “I have a confidence that I can do anything” (BEM = 2.52 ± 0.81 [n = 21], Cont = 3.50 ± 0.94 [n = 14], P < .01, independent samples t test). ANCOVA was performed for following 2 items with low P value (.05 < P < .10) in the prescores between groups: “No one is close to me” (ANCOVA SS(I) = 2.60, df = 1, F = 4.34, P = .04) and “I have a confidence that I can do anything” (ANCOVA SS(I) = 3.38, df = 1, F = 6.20, P = .01). The results indicate that BEM has significant reductions in mental scores (thus, better mental health) in both items. To summarize, BEM intervention was beneficial for physical health in terms of fatigue relief (item no. 9). Moreover, it was beneficial for mental health in the following aspects: increases in relaxation (item no. 1), focus (item no. 5), happiness (item no. 7), and confidence (item no. 11); reductions in anger (item no. 3); and loneliness (item no. 10).
3.3. Comparison of inflammatory gene expression between meditation and control group
Inflammatory conditions are associated with type 2 diabetes,[34] as well as hypertension,[35] which suggest that inflammation participates in the pathogenesis of the diseases. To know whether inflammatory markers are changed by the intervention, we performed qPCR on cDNA synthesized from RNA derived from the whole peripheral blood at preintervention and postintervention. Nine participants for gene expression analysis were selected by matching sex, disease, and age from each group. Baseline characteristics of sex, diseases, and age between groups were comparable (Table 5). Among them, 8 participants for BEM and 6 participants for control completed the study. They did not show significant differences in mental/physical health self-report scores as well as blood biochemical analysis (Table 6). However, there were significant changes in inflammatory gene expression level between preintervention and postintervention by meditation training (Fig. 2). We investigated NFKB2 and RELB for noncanonical NF-κB pathway, RELA for canonical NF-κB pathway,[36] and IL1B which is a risk factor for type 2 diabetes.[37] Because of the changes in temperature between preintervention and postintervention which may affect the gene expression, we normalized the ACTB-normalized gene expression of BEM group by the correspondence of control group of the same time point. As a result, the expressions of NFKB2, RELA, and IL1B were significantly reduced by meditation training (Fig. 2A, B, D: NFKB2, 0.3-fold change, ∗P = .01; RELA, 0.5-fold change, ∗P = .03; RELB, P = .34; IL1B, 0.2-fold change, ∗∗P < .01 [BEM, n = 8; control, n = 6], paired samples t test). The expression of RELB showed a reduction by meditation training without a significant difference (Fig. 2C). To summarize, 8-week BEM training reduces the inflammatory gene expressions; NFKB2, RELA, and IL1B.
Figure 2.
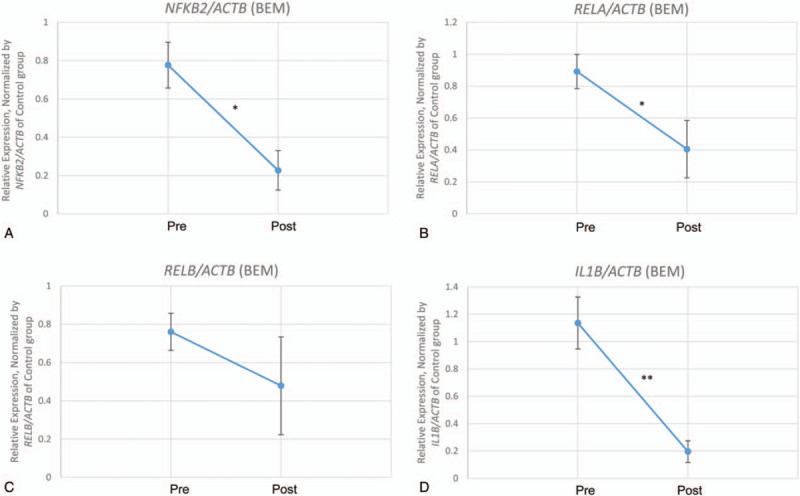
Relative inflammatory gene expressions of the meditation group at preintervention and postintervention. Expression of inflammatory genes was all initially normalized by ACTB expression and the normalized values were used for further analysis. Second normalizations for values of brain education-based meditation (BEM) group at preintervention and postintervention were done by using values of control group of the same time point; this was performed due to the gene expression changes by the temperature at preintervention and postintervention. Dots indicate the mean values of the following relative gene expressions of BEM group, which are normalized by the value of the control group: (A) Nuclear Factor kappa B subunit 2 (NF-κB2), ∗P = .01; (B) RELA, ∗P = .03; (C) RELB, P = .34; (D) interleukin 1 beta (IL1B), ∗∗P < .01, a paired samples t test (n = 6 and 8 for control and BEM, respectively). Error bars indicate standard of errors.
4. Discussion
Type 2 diabetes and hypertension are diseases which demand continuous lifetime care because of their notorious complications.[38,39] Since they require lifetime care, nondrug approaches are pursued to manage long-term health care. This study is the first randomized controlled trial using BEM, whose subjects are patients of hypertension and/or type 2 diabetes. The purpose of the study is to investigate the effectiveness of 8-week BEM intervention, as a complementary care to a conventional drug treatment. Our results showed that the use of BEM with drug therapy is significantly more effective than drug-only therapy in certain aspects.
Plasma lipids and lipoproteins such as LDL are among the most important risk factors for cardiovascular diseases.[40] In the meta analyses of data from 170,000 participants in 26 randomized trials, the reduction of major vascular event is directly proportional to the absolute LDL reduction, even if LDL cholesterol is lower than 2.0 mmol/L (=77.34 mg/dL).[41] If there is a nonpharmacological way to lower LDL cholesterol, it can be a useful complementary method for patients with chronic hypertension/diabetes to maintain their health state and prevent potential complications. BEM program significantly reduced the concentration of LDL cholesterol in the serum of the subjects (Table 2, Fig. 1). Although the mechanism is unknown, the current beneficial effect of BEM on cardiovascular diseases is consistent with a previous finding; the amount of plasma nitric oxide is twice higher in the BEM group than in the control group.[21] Since established cardiovascular risk factors such as hypertension and diabetes mellitus decrease endothelial NO production,[42] its increase in plasma level suggests beneficial effects of NO on potential prevention of cardiovascular diseases.
According to a recent systemic review,[43] 7 of 8 randomized controlled trials (total 838 participants) using yoga-based programs reported significant beneficial changes in serum lipids, including a significant decline in LDL, compared to standard care, group education, or a moderate intensity exercise program. However, LDL level is not significantly changed in several meditation studies, including 12-week Buddhist walking meditation,[44] and a community-based mind–body meditative Tai Chi program.[45] Sometimes yoga-based program includes more physical activity than static regular meditation. BEM has combinatory features, which include dynamic physical activities as well as mental meditative characters.[23] Considering the above-mentioned yoga, meditative techniques, and BEM, it is interesting to predict that the reduction of LDL cholesterol may be associated with techniques that use more dynamic physical activities than relatively static meditation, if the subjects are novice meditators, which is the case for most randomized controlled studies. This is because novice meditators may have difficulties in reaching the level of experienced meditators who can quickly change their metabolism by simple breathing or walking mediation alone. On the contrary, novice meditators can relatively easily follow dynamic physical activities, which may induce physiological changes on the limited training period. Subjects in the current pilot study did not have previous experiences on meditation; the characteristics of BEM containing both dynamic and static activities seem to contribute to induce the alterations in physiology-lowering LDL cholesterol level—during the limited training period.
Data have suggested that low-grade inflammations play a role in the development and progression of hypertension and end-organ damage.[18] Moreover, the role of inflammation in the pathogenesis of type 2 diabetes and associated complications is now well understood.[34] Clinical studies are using anti-inflammatory approaches to treat patients with type 2 diabetes. Its mechanisms of action include the inhibition of NF-κB pathway and IL-1β.[34] The reductions in the expression level of NFKB2, RELA, and IL1B by BEM practice (Fig. 2) suggest BEM may reverse low-grade inflammation occurring in patients with hypertension and/or type 2 diabetes. The other blood biochemical markers did not show significant changes in these patients (Table 6). This may be derived from the different sensitivity of the detection method: biochemical analysis using serum (Table 6) and real-time PCR using cDNA synthesized from blood RNA (Fig. 2). The current result of inhibitory effects of NF-κB family expression by BEM (Fig. 2) is also consistent with a previous BEM study which showed the increase of plasma NO,[21] which inhibits NF-κB-mediated gene expression.[46,47] The reduced expression of pro-inflammatory IL1B by BEM training is also consistent with the previous finding which showed the increased expression of anti-inflammatory IL10 by BEM,[30] which suggests that BEM helps reduce inflammation. Our results suggest that BEM training reduces low-grade inflammation by reducing the expression of NF-κB, RELA, and IL1B.
Studies have shown that people with type 2 diabetes have poorer general mental health,[48] and are more likely to be alcohol dependent,[49] and have more cognitive decline and dementia,[50] compared with those who do not have type 2 diabetes. Reversely, Joseph and Golden proposed that psychological stress contributes to the development of type 2 diabetes through alterations in the hypothalamic–pituitary–adrenal (HPA) axis, autonomic nervous system, and immune system.[51] Depression and depressive symptoms elevate the risk for progressive insulin resistance[52] and diabetes.[53] Moreover, it is clear that hypertension increases one's risk of cognitive decline and dementia.[54] These suggest a correlation between mental health and diabetes/hypertension. In the current study, mental health self-report score suggests that BEM significantly improves relaxation, focus, happiness, and confidence; decreases anger, and loneliness in patient with hypertension and/or diabetes (Table 4). This is consistent with previous findings of the beneficial effects of BEM on mental health of healthy individuals: reductions in stress,[23,24,26,31] depression,[21,23] anxiety,[21,28] neuroticism[29]; increases in extraversion and openness to experience,[29] positive effect,[24] quality of life,[28] problem solving, emotional intelligence, and resilience.[31] The positive effects of BEM on mental health indicate its beneficial use in patients with diabetes to prevent diabetes-related complications related with cognitive decline. In the physical health self-report score, BEM significantly relieves the feeling of fatigue (Table 3). This is consistent with a result from the previous research performed on breast cancer patients.[28] Improvement in sleep quality by BEM may contribute to the relief of fatigue.[22,23]
When the brain perceives the environment as stress, HPA axis and sympathetic nervous system are activated. If it becomes chronic, various psychological symptoms, changes in metabolism, immune system are led to development of diseases[55] (Supplemental Content 4A). In the current research, BEM training reduces the chronic activation through reducing stress itself by changing the way of thinking. Moreover, it induces balanced recovery of automatic nervous system by activating parasympathetic nervous system through breathing. The indicated main observations of the current study are supposed to be led by those mechanisms (Supplemental Content 4B).
The present study has following limitations. Since this is a pilot study, the number of subjects is relatively small. To validate the current results, a follow-up study is needed with a larger sample size as well as measurements of other related components such as body mass index and hemoglobin A1c. BEM includes static meditation as well as dynamic elements. To know which element contributed to the current results, not only education control but also element-corresponding controls will be needed. The drug type was not controlled since the subject registration was performed in the public healthcare center not in the clinic where each subject was prescribed for his/her disease. Therefore, there is a possibility that the drug types might affect the results, with different molecular mechanisms. The subjects include both hypertension and type 2 diabetes. Although their correlation is well known, it is desirable to investigate each condition separately in the future study with a larger number of subjects to see the specific effects of BEM on each disease.
5. Conclusions
In this pilot randomized controlled trial, our results showed that 8-week-BEM training reduces LDL cholesterol and inflammatory gene expression and improves some elements of the investigated physical/mental health of patients with type 2 diabetes/hypertension, which suggest that BEM induces beneficial changes in physical/mental health of the patients. Since type 2 diabetes and hypertension are chronic, slowly progressing and systemic diseases, patients need to take care of their body for long term to prevent complications. In this aspect, noninvasive and nonpharmacologic methods such as BEM are very useful for a long-term complementary care of hypertension/type 2 diabetes.
Acknowledgments
This work was supported by University of Brain Education (2017-03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have declared that no competing interests exist. The authors thank the patients who gave their consents to participate in this study, and all staffs in the Hypertension/Diabetes Registration Education Center of the Ulsan Junggu Public Health Center, for their support of the study. The authors also thank Enago for the English language review.
Author contributions
Conceptualization: Hyun-Jeong Yang, Seung-Ho Lee, Sun-Mi Hwang, Do-Hyung Kang
Data curation: Hyun-Jeong Yang, Seung-Ho Lee, Sun-Mi Hwang, Do-Hyung Kang
Formal analysis: Seung-Ho Lee
Funding acquisition: Hyun-Jeong Yang
Investigation: Hyun-Jeong Yang
Methodology: Hyun-Jeong Yang, Seung-Ho Lee, Sun-Mi Hwang, Do-Hyung Kang
Project administration: Hyun-Jeong Yang, Seung-Ho Lee, Sun-Mi Hwang, Do-Hyung Kang
Resources: Hyun-Jeong Yang, Seung-Ho Lee, Sun-Mi Hwang
Software: Seung-Ho Lee, Sun-Mi Hwang
Supervision: Hyun-Jeong Yang, Do-Hyung Kang
Validation: Hyun-Jeong Yang, Seung-Ho Lee, Sun-Mi Hwang, Do-Hyung Kang
Visualization: Hyun-Jeong Yang
Writing – original draft: Hyun-Jeong Yang
Writing – review & editing: Hyun-Jeong Yang, Do-Hyung Kang
Supplementary Material
Footnotes
Abbreviations: ANCOVA = analysis of covariance, BEM = brain education-based meditation, GGT = γ-glutamyl transferase, HDL = high-density lipoprotein, HPA = hypothalamic–pituitary–adrenal, IL1B = interleukin 1 beta, LDL = low-density lipoprotein, NF-κB = nuclear factor kappa B subunit, SGOT = serum glutamic-oxaloacetic transaminase, SGPT = serum glutamic pyruvic transaminase.
S-HL and S-MH have contributed equally to this work.
All authors declare that we carefully prepared our manuscript following the Ethics in publishing and Ethical guidelines for journal publication.
This work has not been published previously and is not under consideration for publication elsewhere. This publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out. If accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].NCD Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017;389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].System USRD. 2014 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. [Google Scholar]
- [4].Sarwar N, Gao P, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sparrenberger F, Cichelero FT, Ascoli AM, et al. Does psychosocial stress cause hypertension? A systematic review of observational studies. J Hum Hypertens 2009;23:12–9. [DOI] [PubMed] [Google Scholar]
- [6].Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension 2009;54:690–7. [DOI] [PubMed] [Google Scholar]
- [7].Steptoe A, Kivimaki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health 2013;34:337–54. [DOI] [PubMed] [Google Scholar]
- [8].Kelly SJ, Ismail M. Stress and type 2 diabetes: a review of how stress contributes to the development of type 2 diabetes. Annu Rev Public Health 2015;36:441–62. [DOI] [PubMed] [Google Scholar]
- [9].Fulcher J, O’Connell R, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397–405. [DOI] [PubMed] [Google Scholar]
- [10].Xu T, Brandmaier S, Messias AC, et al. Effects of metformin on metabolite profiles and LDL cholesterol in patients with type 2 diabetes. Diabetes Care 2015;38:1858–67. [DOI] [PubMed] [Google Scholar]
- [11].Siegrist J, Peter R, Cremer P, et al. Chronic work stress is associated with atherogenic lipids and elevated fibrinogen in middle-aged men. J Intern Med 1997;242:149–56. [DOI] [PubMed] [Google Scholar]
- [12].Gerber M, Borjesson M, Ljung T, et al. Fitness moderates the relationship between stress and cardiovascular risk factors. Med Sci Sports Exerc 2016;48:2075–81. [DOI] [PubMed] [Google Scholar]
- [13].Choudhary S, Sinha S, Zhao Y, et al. NF-kappaB-inducing kinase (NIK) mediates skeletal muscle insulin resistance: blockade by adiponectin. Endocrinology 2011;152:3622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sheng L, Zhou Y, Chen Z, et al. NF-kappaB-inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nat Med 2012;18:943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Starkey JM, Haidacher SJ, LeJeune WS, et al. Diabetes-induced activation of canonical and noncanonical nuclear factor-kappaB pathways in renal cortex. Diabetes 2006;55:1252–9. [DOI] [PubMed] [Google Scholar]
- [16].Black PH, Garbutt LD. Stress, inflammation and cardiovascular disease. J Psychosom Res 2002;52:1–23. [DOI] [PubMed] [Google Scholar]
- [17].Esser N, Legrand-Poels S, Piette J, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014;105:141–50. [DOI] [PubMed] [Google Scholar]
- [18].Caillon A, Schiffrin EL. Role of inflammation and immunity in hypertension: recent epidemiological, laboratory, and clinical evidence. Curr Hypertens Rep 2016;18:21. [DOI] [PubMed] [Google Scholar]
- [19].Lee I. The power brain: five steps to upgrading your brain operating system. Best Life Media 2016. 88–92. [Google Scholar]
- [20].Jang JH, Kim JH, Yun JY, et al. Differences in functional connectivity of the insula between brain wave vibration in meditators and non-meditators. Mindfulness 2018;9:1857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee DH, Park HY, Lee US, et al. The effects of brain wave vibration on oxidative stress response and psychological symptoms. Compr Psychiatry 2015;60:99–104. [DOI] [PubMed] [Google Scholar]
- [22].Bowden DE, McLennan D, Gruzelier J. A randomised controlled trial of the effects of brain wave vibration training on mood and well-being. J Complement Integr Med 2014;11:223–32. [DOI] [PubMed] [Google Scholar]
- [23].Bowden D, Gaudry C, An SC, et al. A comparative randomised controlled trial of the effects of brain wave vibration training, Iyengar yoga, and mindfulness on mood, well-being, and salivary cortisol. Evid Based Complement Alternat Med 2012;2012:234713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jung YH, Kang DH, Jang JH, et al. The effects of mind–body training on stress reduction, positive effect, and plasma catecholamines. Neurosci Lett 2010;479:138–42. [DOI] [PubMed] [Google Scholar]
- [25].Jang JH, Jung WH, Kang DH, et al. Increased default mode network connectivity associated with meditation. Neurosci Lett 2011;487:358–62. [DOI] [PubMed] [Google Scholar]
- [26].Jung YH, Kang DH, Byun MS, et al. Influence of brain-derived neurotrophic factor and catechol O-methyl transferase polymorphisms on effects of meditation on plasma catecholamines and stress. Stress 2012;15:97–104. [DOI] [PubMed] [Google Scholar]
- [27].Kang DH, Jo HJ, Jung WH, et al. The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Soc Cogn Affect Neurosci 2013;8:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim YH, Kim HJ, Ahn SD, et al. Effects of meditation on anxiety, depression, fatigue, and quality of life of women undergoing radiation therapy for breast cancer. Complement Ther Med 2013;21:379–87. [DOI] [PubMed] [Google Scholar]
- [29].Jung YH, Lee US, Jang JH, et al. Effects of mind-body training on personality and behavioral activation and inhibition system according to BDNF Val66Met polymorphism. Psychiatry Investig 2016;13:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jang JH, Park HY, Lee US, et al. Effects of mind-body training on cytokines and their interactions with catecholamines. Psychiatry Investig 2017;14:483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jung YH, Ha TM, Oh CY, et al. The effects of an online mind-body training program on stress, coping strategies, emotional intelligence, resilience and psychological state. PLoS ONE 2016;11:e0159841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim YH, Oh SW, Hong SH, et al. Clinical Interpretation of PAI. Seoul: Hakjisa; 2011. [Google Scholar]
- [33].Camara WJ, Nathan JS, Puente AE. Psychological test usage: implications in professional psychology. Prof Psychol Res Pr 2000;31:141–54. [Google Scholar]
- [34].Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107. [DOI] [PubMed] [Google Scholar]
- [35].Bang CN, Okin PM, Kober L, et al. Psoriasis is associated with subsequent atrial fibrillation in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention for Endpoint study. J Hypertens 2014;32:667–72. [DOI] [PubMed] [Google Scholar]
- [36].Sun SC. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol 2017;17:545–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Spranger J, Kroke A, Mohlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003;52:812–7. [DOI] [PubMed] [Google Scholar]
- [38].Sun Z. Aging, arterial stiffness, and hypertension. Hypertension 2015;65:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zoungas S, Woodward M, Li Q, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014;57:2465–74. [DOI] [PubMed] [Google Scholar]
- [40].Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat Rev Genet 2009;10:109–21. [DOI] [PubMed] [Google Scholar]
- [41].Baigent C, Blackwell L, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Forstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res 2017;120:713–35. [DOI] [PubMed] [Google Scholar]
- [43].Innes KE, Selfe TK. Yoga for adults with type 2 diabetes: a systematic review of controlled trials. J Diabetes Res 2016;2016:6979370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gainey A, Himathongkam T, Tanaka H, et al. Effects of Buddhist walking meditation on glycemic control and vascular function in patients with type 2 diabetes. Complement Ther Med 2016;26:92–7. [DOI] [PubMed] [Google Scholar]
- [45].Sun J, Buys N. Community-based mind-body meditative Tai Chi program and its effects on improvement of blood pressure, weight, renal function, serum lipoprotein, and quality of life in Chinese adults with hypertension. Am J Cardiol 2015;116:1076–81. [DOI] [PubMed] [Google Scholar]
- [46].Marui N, Offermann MK, Swerlick R, et al. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest 1993;92:1866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Weber C, Erl W, Pietsch A, et al. Antioxidants inhibit monocyte adhesion by suppressing nuclear factor-kappa B mobilization and induction of vascular cell adhesion molecule-1 in endothelial cells stimulated to generate radicals. Arterioscler Thromb 1994;14:1665–73. [DOI] [PubMed] [Google Scholar]
- [48].Agardh EE, Ahlbom A, Andersson T, et al. Explanations of socioeconomic differences in excess risk of type 2 diabetes in Swedish men and women. Diabetes Care 2004;27:716–21. [DOI] [PubMed] [Google Scholar]
- [49].Jiang L, Beals J, Whitesell NR, et al. Association between diabetes and mental disorders in two American Indian reservation communities. Diabetes Care 2007;30:2228–9. [DOI] [PubMed] [Google Scholar]
- [50].Biessels GJ, Strachan MW, Visseren FL, et al. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol 2014;2:246–55. [DOI] [PubMed] [Google Scholar]
- [51].Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci 2017;1391:20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shomaker LB, Tanofsky-Kraff M, Stern EA, et al. Longitudinal study of depressive symptoms and progression of insulin resistance in youth at risk for adult obesity. Diabetes Care 2011;34:2458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hasan SS, Clavarino AM, Mamun AA, et al. Incidence and risk of diabetes mellitus associated with depressive symptoms in adults: evidence from longitudinal studies. Diabetes Metab Syndr 2014;8:82–7. [DOI] [PubMed] [Google Scholar]
- [54].Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr Hypertens Rep 2017;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun 2013;31:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


