Abstract
Objective:
The aim of this study was to explore the benefits of in vitro fertilization (IVF) for patients and hospitals under different protocols and if IVF treatment should be incorporated into health care.
Perspective:
The government should consider including IVF treatment in health insurance. Hospitals and patients could obtain the best benefit by following the hospital's recommended protocol.
Setting:
This retrospective study was conducted from January 2014 to August 2017 at an academic hospital.
Methods:
A total of 7440 patients used gonadotropin-releasing hormone agonists (GnRHa) protocol, 2619 patients used, gonadotropin-releasing hormone antagonists (GnRHant) protocol, and 1514 patients used GnRHa ultra-long protocol. Primary outcomes were live birth rate (LBR), cost-effectiveness, hospital revenue, and government investment.
Results:
The cycle times for the GnRHa protocol and the GnRHa ultra-long protocol were significantly higher than the GnRHant protocol. Patients who were ≤29 years chose the GnRHant protocol. The cost of a successful cycle was 67,579.39 ± 9,917.55 ¥ and LBR was 29.25%. Patients who were >30 years had the GnRHa protocol as the dominant strategy, as it was more effective at lower costs and higher LBR. When patients were >30 to ≤34 years, the cost of a successful cycle was 66,556.7 ± 8,448.08 ¥ and the LBR was 31.05%. When patients were >35 years, the cost of a successful cycle was 83,297.92 ± 10,918.05 ¥ and the LBR was 25.07%. The government reimbursement for a cycle ranged between 11,372.12 ± 2,147.71 ¥ and 12,753.67 ± 1,905.02 ¥.
Conclusions:
The government should consider including IVF treatment in health insurance. Hospitals recommend the GnRHant protocol for patients <29 years old and the GnRHa protocol for patients >30 years old, to obtain the best benefits. Patients could obtain the best benefit by using the protocol recommended by the hospital.
Keywords: cost-effectiveness, decision tree models, health insurance, hospital service efficiency, return on investment
1. Introduction
China's healthcare reform is presently performing as a hierarchical medical system.[1] The primary healthcare system is unable to take care of the basic work of one-fifth of the world's population for a variety of reasons.[2] Patients are more likely to choose a tertiary hospital for their treatment.[1] According to the National Bureau of Statistics of the People's Republic of China, the hospital bed utilization rate for tertiary hospitals was 95.8% in the first quarter of 2018, although the community health service center's bed utilization rate was 55%.[3] Medical resources at tertiary hospitals are limited in China. The public hospitals also use profit as their operating target, where 90% of the income is dependent on providing services to patients.[1] Therefore, while improving the cure rate of patients, it is also important for hospitals to improve the efficiency of medical resources to increase profits.
And 1 in 6 couples worldwide will experience at least one infertility problem during their reproductive years.[4] Infertility causes enormous psychological stress to women, leading to pain, depression, and discrimination.[5,6] The development of Chinese culture is influenced by Taoism, Confucianism, and Buddhism. Under this unique environment, infertility seriously reduces the family happiness index.[7] Although the majority of infertility would benefit from assisted reproductive technology (ART), high prices hinder the promotion of this technology.[8] Previous research states that developing countries should incorporate ART technology into health insurance and prioritize the cost.[7] The United States, Britain, and other countries have ART technology in health care. However, patients in China who use this technology must pay the full cost of their own. Each in vitro fertilization (IVF) cycle has a large chance of failure. These fertility treatment failure will seriously affect the patient's well-being and increase the medical expenses.[9] In the hope of giving birth to a healthy baby, the patient also hopes to maximize the benefits, meaning that the success rate should be balanced with the cost, which is particularly important for families with less economic resources.
For the government, the sixth census shows that China has become an aging society. The one-child policy in China has accelerated this phenomenon. The one-child policy has also led to sex imbalance and a reduction in the working-age population. China decided in 2015 to open up the two-child policy.[10] Young people generally postpone reproducing because of the pressure of life, additional education,[11] or worry that their careers would be affected.[12] This delay of the childbearing age has caused more people to not be able to conceive naturally, thus increasing the number of people who require ART technology. The government should consider incorporating ART technology into health care and encourage fertility among those who have difficulty conceiving. This is not only conducive to implement national policies, but also reflects the government's concern for infertile people, particularly in China's special cultural environment.
China is undergoing medical reforms and hopes to achieve universal health care coverage by 2020.[1] Providing cost-effective information for health services in different environments is key to designing high-quality health care while achieving universal coverage of the healthcare system. We analyzed the benefits of governments, hospitals, and patients using large sample data that included complete and accurate IVF costs and patient care information. The results could provide policy-makers, hospital decision-makers, and patients with accurate cost-effectiveness information, allowing decision-makers to incorporate economic evidence into the decision-making process.
The propose of the study is to analyze the effectiveness of gonadotropin-releasing hormone agonists (GnRHa), gonadotropin-releasing hormone antagonists (GnRHant), and long-term GnRHa protocol, to explore the benefits of the government's inclusion of IVF technology in medical insurance, to analyze the patient's cost-effectiveness, and the time-cost effectiveness. A decision model was obtained, as this is how hospitals recommend IVF protocol to patients.
2. Materials and methods
2.1. Study design
This study was conducted from January 2014 to August 2017 at the Reproductive Medicine Center of Tongji Hospital, PR China. Patient's treatment and cost data were gathered retrospectively. A total of 11,573 infertile women were included, 7,440 infertile women were stimulated with the GnRHa protocol, 2,619 infertile women were stimulated with the GnRHant protocol, and 1,514 infertile women were stimulated with the GnRHa ultra-long protocol. Patients who underwent IVF/ICSI therapy used their own oocytes. The guidelines about IVF were ovarian, tubal, and male factors. All data acquisition, management, and analyses were performed by the Data Analysis Center of Tongji Hospital. This research was approved by the Institutional Review Board (IRB). And the data set was available.
2.2. Study procedures
Ovarian stimulation was performed with the GnRHa protocol that was subcutaneously injected with triptorelin 0.1 mg/d beginning from the midluteal phase of the last menstrual cycle. Nondiameter follicular cysts >10 mm were defined as achieving a reduction criterion. Patients were subsequently administered to the patient until the day of human chorionic gonadotrophin (HCG). The patients were administered 75 IU-150 IU/day follicle-stimulating hormone (FSH) or human menopausal gonadotropin (HMG). At the same time, triptorelin was reduced to 0.05 mg/d. HCG 10,000 IU was administered when a leading follicle reached 18 mm. Oocyte retrieval was performed after HCG administration. Embryo transfer was performed on day 2 or 3 after oocyte retrieval. The standard of a positive pregnancy test was a beta HCG level of ≥5 mIU/mL. Women were monitored by fetal ultrasound after 3 weeks.
Ovarian stimulation was performed with the GnRHant protocol. HMG or FSH was administered to the patient on the first 2 to 3 days of the menstrual cycle. The dosage was adjusted according to the ovarian response. The cetrorelix was subcutaneously injected at 0.25 mg/d on the fifth day of the menstrual cycle. Both drugs were used until the follicle reached the oocyte retrieval criterion. The rest of the procedure was consistent with the GnRHa protocol.
GnRHa ultra-long 3.75 mg was used on the first day of the menstrual cycle. When the reduction criterion was achieved, the gonadotropin was administered to the patient. The quantity of gonadotropins (Gn) was adjusted according to the follicular growth and the serum hormone levels until the follicle reached the oocyte retrieval criterion. The rest of procedure was consistent with the GnRHa protocol. Figure 1 shows the treatment procedures of each protocol.
Figure 1.
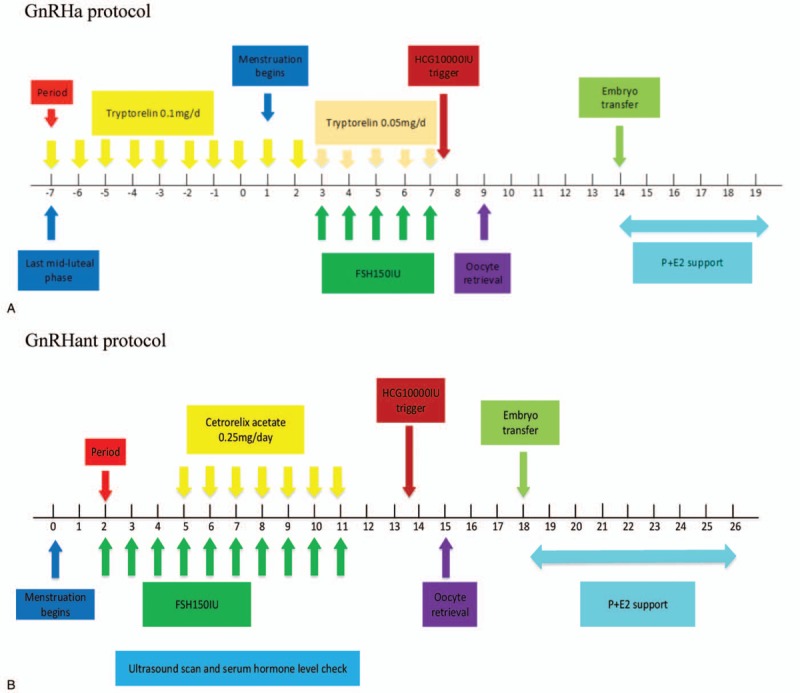
Treatment procedures. E2 = estradiol, FSH = follicle-stimulating hormone; HCG: human chorionic gonadotrophin, GnRHa = gonadotropin-releasing hormone agonist, GnRHant = gonadotropin-releasing hormone antagonist, P = progesterone.
2.3. Decision tree models
We adopted the classic decision tree model in management to select the 3 protocols. The decision-making process included the embryo transfer rate, the pregnancy rate, the miscarriage rate, the pregnancy termination rate, and the live birth rate (LBR) (Fig. 2). The LBR was defined as the number of deliveries that resulted in at least one live birth, expressed per 100 cycle attempts.[13] We combined cost-effectiveness and LBRs to ensure the best decisions were achieved.
Figure 1 (Continued).
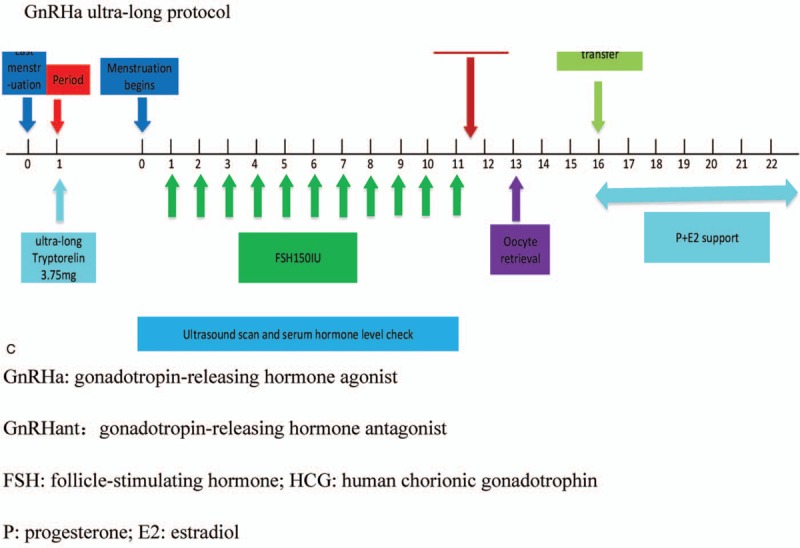
Treatment procedures. E2 = estradiol, FSH = follicle-stimulating hormone; HCG: human chorionic gonadotrophin, GnRHa = gonadotropin-releasing hormone agonist, GnRHant = gonadotropin-releasing hormone antagonist, P = progesterone.
The decision tree is a decision-making method that simulates a group of patients following a predefined approach with relevant probability, cost, and result.[14] The model begins with the selection of a protocol, where each circle represents a state node and a triangle represents the resulting node. The model contains all important state nodes and clearly reflects the probability of each important node.
2.4. Study outcomes
The patient's cost included economic costs and noneconomic costs. The economic costs primarily included the cost of the examination before the patient underwent the infertility treatment and the costs of IVF during the entire cycle (the GnRH analogues were used until the embryo transfer was counted as a cycle, not including the freezing costs of embryos and blastocysts). The noneconomic costs were mainly time consumption.
The primary outcomes were LBRs and cost-effectiveness, medical resource utilization efficiency and hospital revenue, government investment, and return on investment (ROI). The cost-effectiveness analysis was an economic evaluation that compared the relative costs and the consequences of the different processes, where the consequences were measured by the results of the clinical effectiveness.[14] The cost-effectiveness of a patient was defined as the cost per successful cycle. The successful cycle is defined as the patient completing the IVF cycle and giving birth to a living baby. Medical resource utilization efficiency was defined as the number of IVF cycles that a hospital could provide a year. Government investment was defined as the IVF cycle of reimbursement expenses. The government's ROI was defined as the ratio of the individual's lifetime value that is the government's investment in a live birth. We calculated the lifetime economic value (LEV) of a baby with per capita Gross Domestic Product (GDP).[15] The formula for the LEV per cycle was: per capita GDP × the average life expectancy of the population × LBR. Other results included embryo transfer rate, pregnancy rate, and time-cost effectiveness.
2.5. Statistical methods
Using a multidisciplinary method, a quantitative analysis of the data was made using the decision tree model in management and cost-effectiveness in economics. Continuous variables were expressed as mean and standard deviation (SD), and categorical variables were expressed as percentages. Among the group comparisons, parameters tests were used for the normal distribution variables, and nonparametric tests were used for the non-normal distribution variables. The χ2 test was used to evaluate the significance of the proportion of the categorical variables. One-way ANOVA was performed to evaluate the statistical relationship between the groups. A P < .05 was considered statistically significant. All statistical analyses were performed with SPSS.
3. Results
As shown in Figure 2, the mean costs per cycle were 20,468.41 ¥ for the GnRHa protocol, 19,396.41 ¥ for the GnRHant protocol, and 20,881.06 ¥ for the GnRHa ultra-long protocol. The mean costs for a successful cycle were 67,798.64 ¥ for GnRHa protocol, 94,940.82 ¥ for GnRHant protocol, and 55,757.17 ¥ for the GnRHa ultra-long protocol. When patients consider the mean costs for a successful cycle or the LBR as the criteria for selecting the protocols, the majority of patients should choose the GnRHa ultra-long protocol. Out of the 11,573 treatment cycles eligible for analysis, 64.29%, that is, 7,740 treatments, were used in the GnRHa protocol, 2,619 treatments were used in the GnRHant protocol, and 1,514 treatments were used in the GnRHa ultra-long protocol (Table 1). This indicated that considering the LBR or the mean costs for a successful cycle is not enough to explain why most patients do not chose the GnRHa ultra-long protocol, but instead chose the GnRHa protocol.
Table 1.
Decision parameters for different roles.
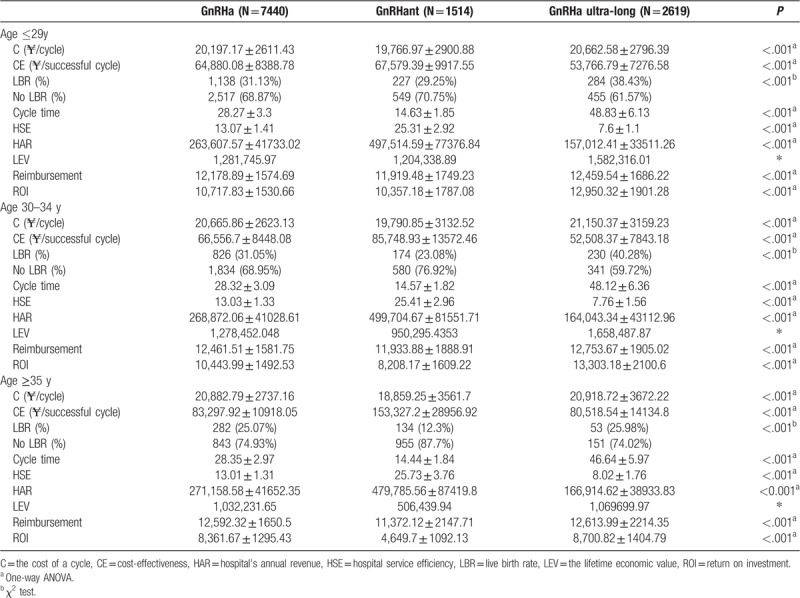
Figure 3B shows the cost-effectiveness and the incremental cost-effectiveness (ICE) in the protocols. We divided the patients into 3 groups by age, which significantly affected the fertility.[16] When women were <29 years old, there was a significant narrow the lessening in the ICE for the GnRHant protocol and the GnRHa protocol, which was 219.26 ¥ for a successful cycle. When not divided groups by age, the ICE for the GnRHant protocol was approximately 40,000 ¥ for a successful cycle. When women are <29 years, the ICE between the GnRHant protocol and the GnRHa ultra-long protocol was about 10,000 ¥ for a successful cycle. The ICEs between groups dropped significantly by age grouping, showing that these groupings are meaningful (Fig. 3B).
Figure 2.
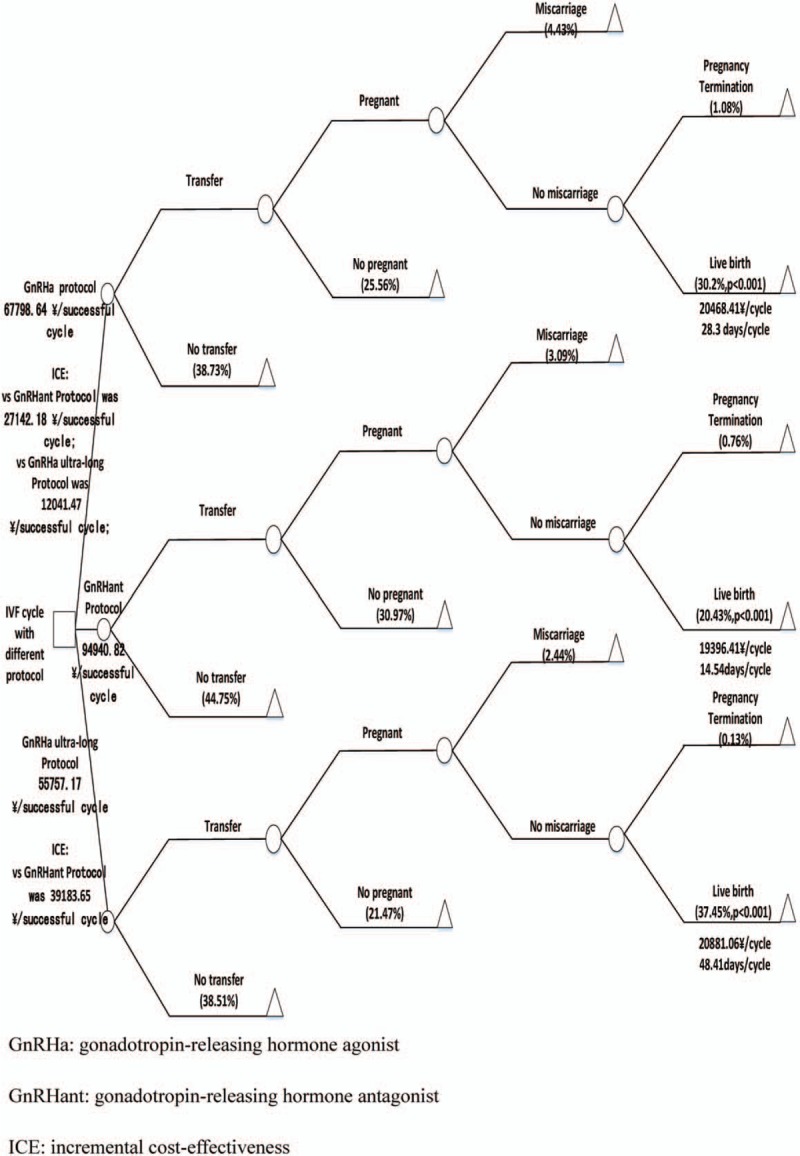
Decision tree models include transplantation outcomes, probability, and cost parameters. GnRHa = gonadotropin-releasing hormone agonist, GnRHant = gonadotropin-releasing hormone antagonist, ICE = incremental cost-effectiveness.
Figure 3.
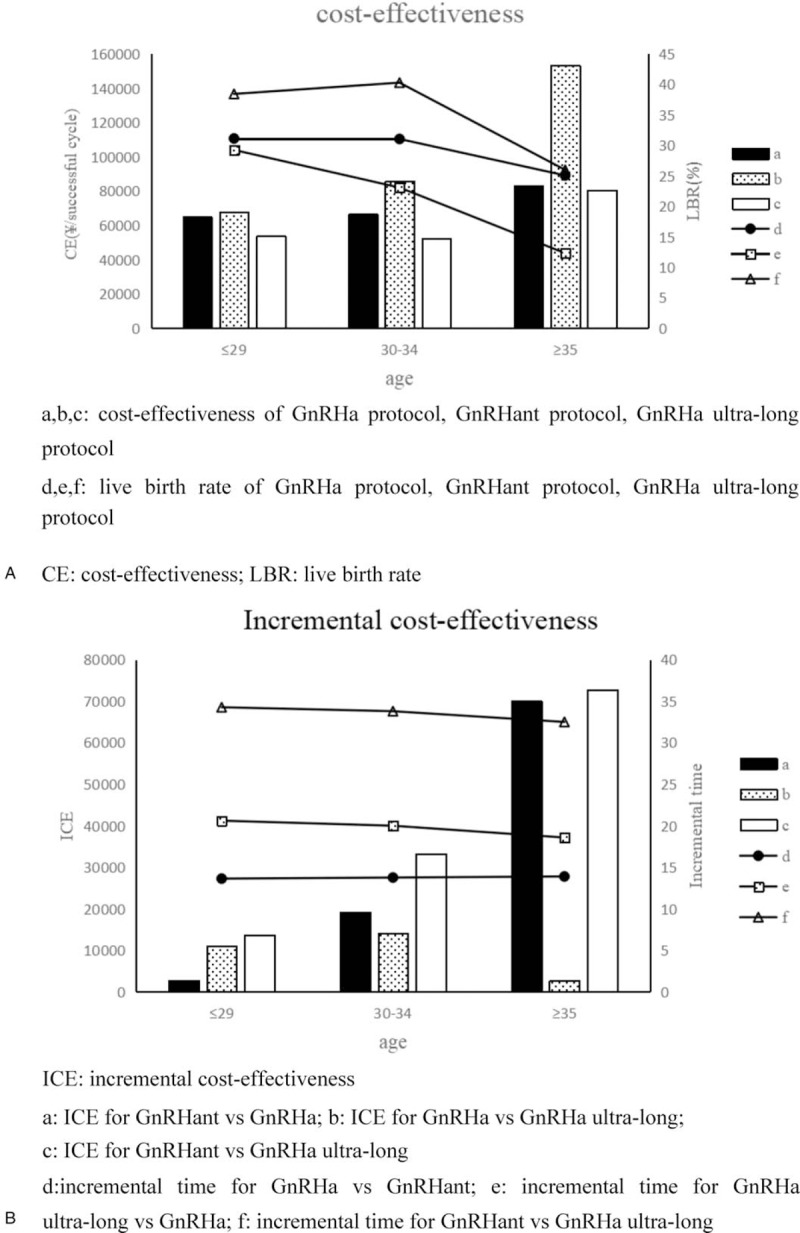
Cost-effectiveness and incremental cost-effectiveness. (A) a, b, c = cost-effectiveness of GnRHa protocol, GnRHant protocol, GnRHa ultra-long protocol, CE = cost-effectiveness, LBR = live birth rate, d, e, f = live birth rate of GnRHa protocol, GnRHant protocol, GnRHa ultra-long protocol. (B) ICE = incremental cost-effectiveness, a = ICE for GnRHant vs GnRHa, b = ICE for GnRHa vs GnRHa ultra-long, c = ICE for GnRHant vs GnRHa ultra-long, d = incremental time for GnRHa vs GnRHant, e = incremental time for GnRHa ultra-long vs GnRHa, f = incremental time for GnRHant vs GnRHa ultra-long.
Figure 3A shows that women's age significantly affected cost-effectiveness, particularly when >35 years. When women were >35 years, the average cost-effectiveness of the protocol significantly increased. The LBR of the GnRHant protocol was particularly sensitive to age. The cost-effectiveness change in the GnRHant protocol was greater than other protocols. ICE for individuals aged >35 years was 70,000 ¥ more than <35 years for a successful cycle (Fig. 3A). Although the average cost-effectiveness gap was reduced between the protocols by dividing patients into 3 groups, the GnRHa ultra-long protocol remained the most cost-effectiveness in all the subgroups. It was not enough to consider the cost-effectiveness. We believe that we cannot ignore the obvious differences in time consumption among the 3 protocols. We know the value of time is different for everyone. The sum of noneconomic costs and economic costs is the total cost of patients for the IVF treatment. In this paper, we assumed that the main impact on noneconomic costs was the time cost.
The GnRH analogues were used until the embryo transfer was counted as a cycle time. The average cycle times of the GnRHa protocol, the GnRHant protocol, and the GnRHa ultra-long protocol were 28.3, 14.54, and 48.41 days (Fig. 2). We assumed that the total cost-effectiveness of the protocols was equal. The incremental cost-effectiveness between different protocols was used to measure the time-cost effectiveness. The time-cost effectiveness for the GnRHant protocol was 27,142.18 ¥ more for a successful cycle than the GnRHa protocol and 39,183.65 ¥ more for a successful cycle than the GnRHa ultra-long protocol. The time-cost effectiveness for the GnRHa protocol was 12,041.47 ¥ more for a successful cycle than the GnRHa ultra-long protocol (Fig. 2).
Figure 3B shows the ICE for each protocol and the incremental time for each protocol cycle. The incremental time for each protocol cycle did not change significantly between the different age groups. The ICE of the protocol gradually increased among the different age groups. The older patients were more sensitive to the time cost.
When women were <29 years, the difference in time-cost effectiveness between the GnRHa protocol and the GnRHant protocol was small, 2699.31 ¥ for a successful cycle. In the same group, the time-cost effectiveness for the long cycle time of the GnRHa ultra-long protocol was over 10,000 ¥ for a successful cycle. When women were >30 years and <34 years, the cycle time increased from 28.34 to 48.34 days, and the time-cost effectiveness was 14,048.35 ¥ for a successful cycle. The cycle time increased from 14.57 to 28.34 days and 48.34 days and the time-cost effectiveness was 19,192.2 ¥ and 33,240.55 ¥ for a successful cycle. When women were >35 years, the patients that chose GnRHa protocol were insensitive to the time cost because the ICE for the GnRHa protocol was 2779.38 ¥ more for a successful cycle than the GnRHa ultra-long protocol. For those who were willing to choose the GnRHant protocol for age ≥35 years old, the time cost was large. If they gave up the GnRHant protocol and chose the GnRHa protocol or the GnRHa ultra-long protocol, their time-cost effectiveness was >70,000 ¥ for a successful cycle (Fig. 3B).
Table 1 shows that age had a significant effect on the LBR using the GnRHant protocol. Doctors should recommend that women >30 years of age, particularly those over 35, should consider the other protocols if possible.
Different protocols have different cycle time. Hospital service efficiency (HSE) is defined as the number of cycles that the reproductive center could provide a year. We assume that the reproductive center performed one cycle at a time and that the next cycle does not begin until the current cycle ends. Its formula is HSE = 365/a cycle time. The hospital's annual revenue (HAR) was defined as the cost of a cycle multiplied by the number of cycles that the hospital could provide a year. When the hospital provides only a certain scheme, the calculation formula for the annual revenue is HAR = HSE × the cost of a cycle. Table 1 shows that approximately 13 cycles could be completed within 1 year, if the GnRHa protocol was the only option provided by the hospital. If the reproductive center provides the GnRHant protocol, it would be able to complete approximately 25 cycles within 1 year, approximately 2 times as many as the GnRHa protocol. If the reproductive center provides the GnRHa ultra-long protocol, only 7.5 cycles could be completed within 1 year. Due to the difference in the number of completed cycles, the hospital's annual income would also be different. Table 1 shows that hospitals only provided the GnRHant protocol obtained the maximum benefit, and the GnRHa ultra-long protocol obtained the minimum benefit. Although the hospitals considered profit, the biggest responsibility is providing the best medical service for the patient (in this case, the medical service was expressed by LBR). A good medical service would bring a better reputation to the hospital. The hospitals should develop a medical treatment plan for patients by considering comprehensive benefits (including annual revenue and reputation) and the actual conditions of the patients.
Measuring the value of life in terms of money may seem unsavory, although we believe this approach is necessary to provide important information to the policy-makers. We calculated the LEV of a baby with per capita GDP.[15] The per capita GDP and the average life expectancy of the population were based on the latest data that were released by the National Bureau of Statistics of the People's Republic of China, which was 53,935 ¥/y and 76.34 years old.[17] The proportion of Medicare reimbursement was 60.3%, which was the proportion of medical expenses paid by the government for patients.[18] The formula for the LEV per cycle was: per capita GDP × the average life expectancy of the population × LBR. The government's ROI formula was: LEV/(cost of a cycle × Medicare reimbursement ratio) × 100%. The government reimbursement for a cycle ranged between 11,372.12 ± 2,147.71 and 12,753.67 ± 1,905.02 ¥, and the government's ROI ranged between 4649.7 ± 1092.13% and 13303.18 ± 2100.6%.
4. Discussion
We recommend the GnRHa ultra-long protocol for patients who are insensitive to the time cost, as this option could ensure patients achieve the best economic cost-effectiveness. For patients who are extremely sensitive to the cost of time, we recommend the GnRHa protocol regardless of their age. For patients who can withstand the cost of time and are <29 years old, we recommend the GnRHant protocol. For patients who are >30 years and <34 years, we believe that the GnRHa protocol is the best choice, as it has the best balance between economic cost-effectiveness and time cost. For patients who are >35 years, the GnRHa protocol is the best choose.
When the comprehensive benefit and the LBR are 2 indicators, the recommendation to the hospital was consistent with the patient's decision to pay a certain amount of time for the treatment. When women were <29 years, patients were advised to choose the GnRHant protocol. When women were >30 years, patients were advised to choose the GnRHa protocol. These recommendations not only enable the hospital to obtain better benefits, but also will result in the hospital having a better reputation and fulfilling its social moral obligations. The government should consider incorporating IVF into health insurance and determine the appropriate reimbursement ratio because the economic benefits are substantial.
Previous studies have found that the cost of a short-term GNRHa protocol cycle is lower than the GNRHant protocol cycle, but the cost of a pregnancy is higher than the GNRHant protocol and the pregnancy rate is lower.[19] IVF becomes a more cost-effectiveness alternative to the intrauterine insemination because of the perceived value of transplanting fewer embryos.[20] It is more cost-effectiveness to stimulate ovaries with rFSH than high purity HMG.[21] From a cost-benefit perspective of controlling ovarian stimulation, the combination of rFSH + HMG is more economical and offers a better quality of life than the combination of rFSH + rLH.[22] Although there are many studies on the cost-effectiveness of IVF treatments, the conclusions reached are not the same. The different treatment options lead to significant cost heterogeneity.[15,23]
The cost-effectiveness study of reproduction is important not only for individuals and families, but also for healthcare systems and society.[24] The value of willingness to pay for reproductive health depends on the age, race, income, and type of treatment.[25] The family income is closely related to seeking infertility treatment.[26,27] It is more difficult for women to obtain ART technology who are in a socioeconomically disadvantaged situation than women with socioeconomic advantages.[28] German reproductive experts and policy-makers believe that moderate copayment ART technology is acceptable,[29] and private insurance alone is not enough.[27] Although China provides partial subsidies, the cost of ART technology remains a catastrophic cost for poor families.[30] To improve the availability, the affordability, and the acceptability of global ART technologies, low-cost IVFs need to be encouraged, or many infertile couples must accept catastrophic payments to use IVFs.[31]
In this study, hospital and patient benefits for all 3 treatments were considered by calculating the incremental time among different protocols. Patients not only knew the cost-effectiveness of each protocol, but also took the treatment time into consideration. Our findings could help patients to achieve better outcomes and hospitals to gain maximum benefits.
Simply economic evaluation cannot fully explain the impact that a child has on parents and society.[32] In the absence of data, we were unable to evaluate the psychological stress of the patient after IVF treatment or the sense of well-being after obtaining a baby. Moreover, we could not specifically assess the impact that a healthy child has on alleviating ageing and balancing the sex ratio of the population. This study made some necessary simplification assumptions about the analysis. For example, we assumed that the total cost-effectiveness of the 3 treatments were equal when assessing the value of time for heterogeneous people. When we evaluated an individual's LEV, the economic growth that the individual output value would increase was not considered. The time value of money and the difference in individual economic value were not considered. More than 70 years of time would cause dramatic changes in the economic situation, so the discount rate could not eliminate the impact that time had on the economy and money. Some previous literature state that the inclusion of medical projects about health insurance needs to consider both cost-effectiveness and government affordability.[33] The subsequent articles should continue to explore the affordability for the inclusion of IVF in medical insurance. China's health insurance system is not perfect, and its development is not diversified, making it a good choice to learn from other countries’ ways of solving financial problems by cooperating with the private sector.[1,34] In addition, infertility should be considered as a general disease. If we consider infertility to be covered in medical insurance, we should investigate the national prevalence of infertility.
5. Conclusions
This study provided up-to-date information on the cost-effectiveness of IVF technology. These results were conservative because of the lack of data regarding indirect costs. The results remain important reference values for patients, hospitals, and governments. If the IVF treatment is included in medical insurance, the government could obtain a considerable return. The government should consider covering IVF treatment in health insurance. We not only considered hospital benefits from the perspective of service efficiency, but also provided a scientific basis for improving the overall benefit of the hospital. Hospitals recommend the GnRHant protocol for patients <29 years old and the GnRHa protocol for patients >30 years old, where patients can get the best benefit by adopting the hospital recommended protocol.
Acknowledgment
We gratefully acknowledge all staff of the Reproductive Medicine Center of Tongji Hospital for their support and cooperation.
Author contributions
WP, HT, LJ, and SJL took part in study design; YHL, RJW, WMH, and SJL collected and analyzed data; CH and SJL interpreted results; WP, HT, LJ, and SJL wrote the manuscript. Revision of the manuscript and the final approval of the version to be published: all authors.
Data curation: Haiting Tu, Cheng Hu, Yuehan Li, Renjie Wang.
Funding acquisition: Shujie Liao.
Methodology: Haiting Tu, Cheng Hu, Yuehan Li.
Project administration: Lei Jin, Shujie Liao.
Software: Weiming Huang.
Writing – original draft: Wei Pan, Haiting Tu, Shujie Liao.
Writing – review and editing: Lei Jin, Shujie Liao.
Footnotes
Abbreviations: ART = assisted reproductive technology, E2 = estradiol, FSH = follicle-stimulating hormone, GDP = gross domestic product, Gn = gonadotropins, GnRHa = gonadotropin-releasing hormone agonist, GnRHant = gonadotropin-releasing hormone antagonist, HAR = hospital's annual revenue, HCG = human chorionic gonadotropin, HMG = human menopausal gonadotropin, HSE = hospital service efficiency, ICE = incremental cost-effectiveness, IVF = in vitro fertilization, LBR = live birth rate, P = progesterone, ROI = return on investment.
WP, HT, and LJ equally contributed to this work.
Approval for the study was obtained from the ethics committees of Reproductive Medical Center, Tongji Hospital, Tongji College of Medicine, Huazhong University of Science and Technology (ref. no. 20180902).
This study is supported by the National Nature Science Foundation of China (NSFC) (Grant no. 81672085, 71373188, 81372804, U1333115, 30901586). The Chinese medical association of clinical medicine special funds for scientific research projects (17020400709).
The authors have no conflicts of interest to disclose.
References
- [1].Yip W, Hsiao W. Harnessing the privatisation of China's fragmented health-care delivery. Lancet 2014;384:805–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li X, Lu J, Hu S, et al. The primary health-care system in China. Lancet 2017;390:2584–94. [DOI] [PubMed] [Google Scholar]
- [3].National Bureau of Statistics of the People's Republic of China. National Medical Service Situation in January-March 2018 [EB/OL]. Available at: http://www.moh.gov.cn/mohwsbwstjxxzx/s7967/201805/6903c497e1f34499bc25d26977539929.shtml Accessed June 10, 2018. [Google Scholar]
- [4].Gameiro S, Boivin J, Dancet E, et al. ESHRE guideline: routine psychosocial care in infertility and medically assisted reproduction—a guide for fertility staff. Hum Reprod 2015;30:2476–85. [DOI] [PubMed] [Google Scholar]
- [5].Bilinski A, Neumann P, Cohen J, et al. When cost-effective interventions are unaffordable: Integrating cost-effectiveness and budget impact in priority setting for global health programs. PLoS Med 2017;14:e1002397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Montagu D, Goodman C. Prohibit, constrain, encourage, or purchase: how should we engage with the private health-care sector? Lancet 2016;388:613–21. [DOI] [PubMed] [Google Scholar]
- [7].Ombelet W, Cooke I, Dyer S, et al. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update 2008;14:605–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Connolly MP, Hoorens S, Chambers GM. The costs and consequences of assisted reproductive technology: an economic perspective. Hum Reprod Update 2010;16:603–13. [DOI] [PubMed] [Google Scholar]
- [9].Gameiro S, Finnigan A. Long-term adjustment to unmet parenthood goals following ART: a systematic review and meta-analysis. Hum Reprod Update 2017;23:322–37. [DOI] [PubMed] [Google Scholar]
- [10].Zeng Y, Hesketh T. The effects of China's universal two-child policy. Lancet 2016;388:1930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jensen RE, Martins N, Parks MM. Public perception of female fertility: initial fertility, peak fertility, and age-related infertility among U.S. adults. Arch Sex Behav 2018;47:1507–16. [DOI] [PubMed] [Google Scholar]
- [12].Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med 2010;85:640–6. [DOI] [PubMed] [Google Scholar]
- [13].Zegershochschild F, Adamson GD, Dyer S, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril 2017;108:393–406. [DOI] [PubMed] [Google Scholar]
- [14].Payne K, Gavan SP, Wright SJ, et al. Cost-effectiveness analyses of genetic and genomic diagnostic tests. Nat Rev Genet 2018;19:235. [DOI] [PubMed] [Google Scholar]
- [15].Crosignani PG, Baird DT, Barri PN, et al. Economic aspects of infertility care: a challenge for researchers and clinicians. Hum Reprod 2015;30:2243–8. [DOI] [PubMed] [Google Scholar]
- [16].Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].National Bureau of Statistics of the People's Republic of China. National economic accounting [EB/OL]. Available at: http://data.stats.gov.cn/easyquery.htm?cn=C01 June 10, 2018. [Google Scholar]
- [18].Dieleman JL, Campbell M, Chapin A, et al. Future and potential spending on health 2015–40: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet 2017;389:1980–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maldonado LG, Franco JG, Setti AS, et al. Cost-effectiveness comparison between pituitary down-regulation with a gonadotropin-releasing hormone agonist short regimen on alternate days and an antagonist protocol for assisted fertilization treatments. Fertil Steril 2013;99:1615–22. [DOI] [PubMed] [Google Scholar]
- [20].van Rumste MME, Custers IM, van Wely M, et al. IVF with planned single-embryo transfer versus IUI with ovarian stimulation in couples with unexplained subfertility: an economic analysis. Reprod Biomed Online 2014;28:336–42. [DOI] [PubMed] [Google Scholar]
- [21].Elkalyoubi M, Garg N, Farag TE, et al. The cost-effectiveness of IVF treatments Gonal-F® versus HP-HMG in the United Arab Emirates (UAE). Value Health 2017;20:A522. [Google Scholar]
- [22].Mennini FS, Marcellusi A, Bini C, et al. Probabilistic cost-utility analysis of pergoveris in women patients undergoing IVF. Value Health 2017;20:A522. [Google Scholar]
- [23].Tjon-Kon-Fat RI, Bensdorp AJ, Bossuyt PM, et al. Is IVF-served two different ways-more cost-effective than IUI with controlled ovarian hyperstimulation? Hum Reprod 2015;30:2331–9. [DOI] [PubMed] [Google Scholar]
- [24].Cohlen B, Bijkerk A, Van der Poel S, et al. IUI: review and systematic assessment of the evidence that supports global recommendations. Hum Reprod Update 2018;24:300–19. [DOI] [PubMed] [Google Scholar]
- [25].Huppelschoten AG, Verkerk EW, Appleby J, et al. The monetary value of patient-centred care: results from a discrete choice experiment in Dutch fertility care. Hum Reprod 2014;29:1712–20. [DOI] [PubMed] [Google Scholar]
- [26].Kessler LM, Craig BM, Plosker SM, et al. Infertility evaluation and treatment among women in the United States. Fertil Steril 2013;100:1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Staniec JFO, Webb NJ. Utilization of infertility services: how much does money matter? Health Serv Res 2010;42:971–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Harris K, Burley H, Mclachlan R, et al. Socio-economic disparities in access to assisted reproductive technologies in Australia. Reprod Biomed Online 2016;33:575–84. [DOI] [PubMed] [Google Scholar]
- [29].Rauprich O, Berns E, Vollmann J. Who should pay for assisted reproductive techniques? Answers from patients, professionals and the general public in Germany. Hum Reprod 2010;25:1225–33. [DOI] [PubMed] [Google Scholar]
- [30].Dyer SJ, Sherwood K, Mcintyre D, et al. Catastrophic payment for assisted reproduction techniques with conventional ovarian stimulation in the public health sector of South Africa: frequency and coping strategies. Hum Reprod 2013;28:2755–64. [DOI] [PubMed] [Google Scholar]
- [31].Campbell HE, Kurinczuk JJ, Heazell AEP, et al. Healthcare and wider societal implications of stillbirth: a population-based cost of illness study. BJOG 2018;125:108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–26. [DOI] [PubMed] [Google Scholar]
- [33].Organization WH. Mother or nothing: the agony of infertility. Bull World Health Organ 2010;88:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chachamovich JR, Chachamovich E, Ezer H, et al. Investigating quality of life and health-related quality of life in infertility: a systematic review. J Psychosom Obstet Gynaecol 2010;31:101–10. [DOI] [PubMed] [Google Scholar]


