Abstract
The study was designed to reveal the relationship of toll-like receptor 4 (TLR4, rs1927914 and rs1927907) polymorphisms with risk of age-related macular degeneration (AMD), as well as the adjustment of this association by some environmental and lifestyle factors in Chinese Han population.
TLR4 polymorphisms were genotyped by polymerase chain reaction-restricted fragment length polymorphisms and direct sequencing method in 138 AMD patients and 146 healthy controls. Genotype distribution in the control group was checked with Hardy–Weinberg equilibrium. Association of TLR4 polymorphisms and AMD risk was evaluated by χ2 test and adjusted by age and sex, smoking and drinking. Odds ratio (OR) with 95% confidence interval (95% CI) was used to represent the association strength. Logistic regressive analysis was used to calculate the adjusted OR values.
CC genotype of rs1927914 had significantly lower frequency in AMD patients (P = .010), indicated a negative association with AMD risk (crude: OR = 0.358, 95% CI = 0.162–0.791; adjusted: OR = 0.355, 95% CI = 0.160–0.789). C allele of rs1927914 might decrease the susceptibility of AMD (crude: OR = 0.698, 95% CI = 0.497–0.982; adjusted: OR = 0.698, 95% CI = 0.495–0.984). No significant association has been discovered between TLR4 rs1927907 polymorphism and AMD susceptibility. Strong linkage disequilibrium existed between rs1927914 and rs1927907 polymorphisms. C-C haplotype was negatively associated with AMD risk (OR = 0.242, 95% CI = 0.121–0.485; OR = 0.242, 95% CI = 0.120–0.488).
CC genotype and C allele of rs1927914 were significantly associated with the decreased AMD susceptibility.
Keywords: age-related macular degeneration, haplotypes, polymorphisms, TLR4
1. Introduction
Age-related macular degeneration (AMD) is a prevalent multifactorial disorder of the central retina and is the leading cause of vision loss in individuals over the age of 50.[1,2] AMD is divided into 2 basic clinical classification: early (visual symptoms are inconspicuous) and late (severe loss of vision is usual) 2 stages.[3] Based on the pathological features, AMD is classified into dry (nonexudative) and wet (exudative) 2 forms. Dry AMD is characterized by geographic atrophy of retinal pigment epithelium (RPE); wet AMD is characterized by abnormal choroidal neovascularization in choriocapillaris.[3] Although the etiology of AMD remains unknown, it is well known that the occurrence of AMD is a complex multistep and multiple-factor process, various factors have been discovered involving in AMD development.[4,5] Some studies suggested that finding AMD-related genetic factors may be an effective way to identify the high-risk AMD population.[6,7]
Toll-like receptors (TLRs) belong to a family of pattern recognition receptor, which are involved in innate immunity and pathogen recognition.[8,9] Toll-like receptor 4 (TLR4) serves as a surface receptor for lipopolysaccharides, which is highly expressed on lymphocytes, monocytes, and neutrophils.[10,11] TLR4 can recognize proteins as their ligands. It has been reported that TLR4 level showed a significant alteration in AMD patients.[12,13] However, the genetic basis and molecular mechanism of TLR4 in the occurrence and progression of AMD were unclear. Chen et al demonstrated that TLR4 signaling pathway is a possible mechanism that is involved with stimulation of inflammatory and angiogenic factors in RPE cells.[14] Another study has indicated that the mRNA levels of TLR4 were elevated in mice with retinal degeneration.[15] Several single nucleotide polymorphisms (SNPs) of TLR4 gene have been discovered in different diseases.[16,17] All these data indicated that the reactivity of TLR4 may play crucial roles in the development and progression of AMD. However, the effects of TLR4 SNPs for AMD susceptibility were still unclear.
In present study, we selected rs1927914 and rs1927907 SNPs in TLR4 gene to detect the association of TLR4 gene with the susceptibility of AMD. Besides, the association has been adjusted by confounding factors in a Chinese Han population.
2. Materials and methods
2.1. Subjects
A total of 138 patients with AMD and 146 healthy individuals were enrolled in this case–control study. They were all recruited from Aerospace Center Hospital. They were diagnosed by 2 ophthalmologists. AMD patients were diagnosed by optical coherent tomography, fluorescence fundus angiography, and other ophthalmologic examinations conforming to previous guideline.[18] Patients would be excluded if suffered from other eye diseases, inflammatory or immune diseases. Controls were also recruited from the same hospital and had no obvious evidence of eye disease. Age and sex were frequency-matched between the control and case groups.
This research obtained the support from Ethics Committee of Aerospace Center Hospital. Every subject was informed the objective of this study. All subjects signed written informed consents before collecting blood sample.
2.2. Sample collection
Five-millilitre peripheral blood samples were collected from every subject and put into ethylene diamine tetraacetic acid tubes in the morning. Immediately after collection, whole blood was stored at −20°C until use. Genomic DNA was extracted from whole blood using Roche DNA purification kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions. The isolated DNA samples were stored at −20°C refrigerator.
2.3. Genotyping
Polymerase chain reaction-restricted fragment length polymorphisms were used to conduct the genotyping of TLR4 rs1927914; direct sequencing method was used to genotype rs1927907 SNP, according to previous studies.[19,20]
2.4. Statistical analysis
In present study, all statistical tests were performed using PASW Statistics 18.0. Continuous variables were shown as mean ± standard deviation. Genotype and allele frequencies of TLR4 gene rs1927914 and rs1927911 polymorphisms were estimated by direct counting. Genotype distributions whether conformed to Hardy–Weinberg equilibrium (HWE) in the control group was checked by chi-squared test. Association of TLR4 polymorphisms with AMD risk was evaluated by χ2 test. Odds ratio (OR) with the corresponding 95% confidence interval (95% CI) was used to express the association intensity. Age, gender, smoking, and drinking were used as the confounding factors to adjust the association through logistic regressive analysis. P-value less than .05 was considered as statistically significant.
3. Results
3.1. Demographic characteristic
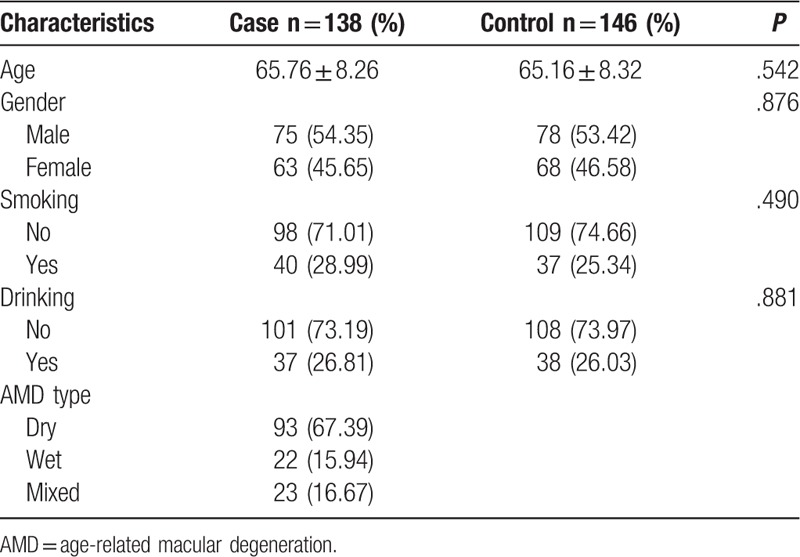
AMD patients were classified into dry AMD (93 cases), wet AMD (22 cases), and mixed AMD (23 cases). AMD patients included 75 males and 63 females, with the mean age of 65.76 ± 8.26. There were 78 males and 68 females in the control group, mean age of them was 65.16 ± 8.32 years old. No significant difference was discovered in the age and gender distributions between case and control groups (Table 1, P > .05). It indicated that the controls were well matched with cases. Meanwhile, the smoking and drinking status also had no significant difference between cases and controls (Table 1, P > .05).
Table 1.
Demographic characteristics of subjects.
3.2. Association of TLR4 polymorphisms with AMD susceptibility
Genotype and allele frequencies of TLR4 gene rs1927914 and rs1927907 polymorphisms in control group were confirmed with the HWE test (Table 2, P > .05), indicated that present study subjects could represent the general population.
Table 2.
Association of TLR4 polymorphisms with AMD susceptibility.
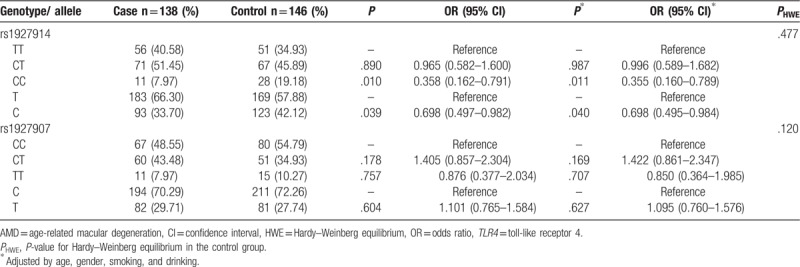
TT, CT, and CC genotype frequencies of rs1927914 SNP were respectively 40.58%, 51.45%, and 7.97% in AMD patients, 34.93%, 45.89%, and 19.18% in controls. The rs1927914 CT and CC genotypes had higher frequencies in AMD patients than in controls; however, only CC frequency had significant difference (Table 2, P = .010). It suggested that rs1927914 CC genotype was negatively correlated with AMD risk (OR = 0.358, 95% CI = 0.162–0.791). When adjusted by confounding factors, the association was still significant (P = .011, OR = 0.355, 95% CI = 0.160–0.789). Frequencies of rs1927914 T and C alleles were 66.30%, 33.70% in AMD patients and 57.88%, 42.12% in controls. In comparison with T allele, the C allele was distinctly correlated with reduced AMD susceptibility (P = .039, OR = 0.698, 95% CI = 0.497–0.982). Negative association also have been discovered between rs1927914 C allele and AMD risk, in the adjustment (OR = 0.698, 95% CI = 0.495–0.984).
Frequencies of rs1927907 CC, CT, and TT genotypes were 48.55%, 43.48%, 7.97% in AMD patients, and 54.79%, 34.93%, 10.27% in controls respectively. Although the CT and TT genotypes had higher frequencies in AMD patients than controls, the differences had no statistical significance (P > .05). C and T alleles of rs1927907 SNP were 70.29%, 29.71% in case group, 72.26%, 27.74% in control group. Both of the genotype and allele of rs1927907 SNP had no significant association with AMD risk respectively in crude and adjusted analysis.
3.3. Haplotype analysis of TLR4 polymorphisms in AMD patients
Strong linkage disequilibrium (D′ = 1.0, r2 = 0.521) existed between rs1927914 and rs1927907 SNPs, they formed 3 haplotypes: T-C, C-T, and C-C (Table 3). When compared with T-C haplotype, the C-T haplotype had no significant association with AMD susceptibility, while, the C-C haplotype was significantly correlated with decreased AMD susceptibility (OR = 0.242, 95% CI = 0.121–0.485). When adjusted by confounding factors, C-C haplotype was also negatively correlated AMD risk (OR = 0.242, 95% CI = 0.120–0.488).
Table 3.
Haplotype analysis of TLR4 polymorphisms in AMD patients.

4. Discussion
AMD is a multiple factor disease, which is characterized by the development of drusen in Bruch's membrane, the degeneration of RPE and neovascularization.[17,21] Accumulating evidence demonstrates that inflammation seems to play a key role in the development of AMD.[22–24] TLRs play principal roles in immune defense.[25] Human TLR4 gene is located on chromosome 9q33.1, consisting of 4 exons.[10] Previous studies indicated that TLR4 was identified to be involved in the ocular inflammation both in iris pigment epithelial cells and RPE.[26]TLR4 gene polymorphisms have been reported to be related to ocular diseases, such as AMD.[17,27,28]
TLR4 rs1927914 and rs1927907 polymorphisms were respectively located in the 5′-untranslated region (UTR) and intron of TLR4 gene. In atherosclerotic patients, A allele of rs1927914 SNP could downregulated the expression of TLR4 gene.[29] TLR4 expression level was higher in the asthma patients with AA genotype than that carrying GG genotype.[30]TLR4 gene rs1927914 and rs1927907 polymorphisms have been researched in other diseases, and might contribute to the AMD susceptibility.
In this case–control study, we observed a close association between TLR4 gene polymorphisms (rs1927914 and rs1927907) and AMD susceptibility. We found that CC genotype of rs1927914 polymorphism was more frequently discovered in AMD patients than in healthy controls. It suggested that rs1927914 CC genotype was significantly associated with 0.358 times reduced AMD risk. When adjusted by age, gender, smoking, and drinking, the association strength was becoming to 0.355 times. Significantly higher frequency of rs1927914 C allele polymorphism in AMD patients indicated a negative association with 0.698 fold AMD risk. Adjustment analysis showed that the association strength had no alteration. Gu et al suggested that rs1927914 SNP impacted the inflammatory response in ischemic stroke (IS) patients, and C allele of rs1927914 was a protective factor against IS.[16] Negative association also has been discovered between rs1927914 G allele and Henoch-Schönlein purpura (HSP) susceptibility.[31] Besides, Zhao et al also found that TLR4 gene might contribute to the risk of developing Parkinson's disease (PD) in Han Chinese and rs1927914 polymorphism may be a protective factor for sporadic PD, male PD, and early onset PD.[32] However, Singh et al indicated that CC genotype of rs1927914 SNP was positively correlated with the risk of diabetic foot ulcer.[19] These differences might be caused by the difference of pathology.
We have also examined the relationship between the rs1927907 polymorphisms in the TLR4. The data showed that genotype or allele frequencies of rs1927907 polymorphisms did not differ between groups. Genotypes and alleles of rs1927907 SNP had no significant association with AMD susceptibility. Present results were confirmed with the study reported by Zhou et al.[33] Yu et al demonstrated that chronic periodontitis patients carrying rs1927907 SNP had higher susceptibility for chronic obstructive pulmonary disease.[34]
Strong linkage disequilibrium was discovered between rs1927914 and rs1927907 SNPs in CHB population and formed 3 haplotypes. In comparison with T-C haplotype, the C-C haplotype was distinctly correlated with 0.242 times reduced AMD risk both in crude and adjusted results. Xu et al indicated that G-C haplotype was negatively correlated with the susceptibility of HSP.[31] Taken together, the mutations of TLR4 gene might influence individual susceptibility to AMD. However, the study carried out by Despriet et al demonstrated that there was no significant association between TLR4 D299G and T399I polymorphisms and risk of AMD.[13] The differences might be caused by the different studied region of TLR4 gene and different polymorphisms, as well as the heterogeneous genetic background of the study population. In their study, the genetic alterations in exon4 of the TLR4 gene were selected. While, the SNPs selected in our study were located in the 5′-UTR and intron of TLR4 gene, respectively. The genetic alterations in exon of gene could result in the amino acid changes, thus influencing the function and structure of the protein. Meanwhile, the genetic alterations in the noncoding region might have the ability to influence transcriptional activity of the gene, thus modifying the levels of protein. The different studied regions of TLR4 gene might be responsible for the differences between our results and the study of Despriet et al.[13]
In addition, in their study, the results were obtained based on the Caucasian population, and the Chinese Han population was studied in our study. Therefore, we hypothesized that the function of TLR4 genetic alterations in AMD was limited by the alteration region and study population.
In summary, TLR4 gene rs1927914 and rs1927907 SNPs might be identified as preventive factor for AMD. Although we obtained this meaningful result, limitations should be recognized in this study. First of all, sample size was not large enough to obtain high statistical power. Second, SNP distribution was different between populations; however, only 1 population was studied in this study. This might limit the application range. Third, various factors participate in the AMD development, interactions between these factors were neglected in this study. Therefore, further studies with large sample size and other factors should be conducted in future for verification. Besides, the functions of TLR4 gene polymorphism in AMD development should be explored in in-vitro studies.
Author contributions
Conceptualization: Fei Xiong.
Data curation: Yu Ling, Fei Xiong.
Formal analysis: Yu Ling, Fei Xiong.
Funding acquisition: Fei Xiong.
Investigation: Fei Xiong.
Methodology: Fei Xiong.
Project administration: Fei Xiong.
Resources: Fei Xiong.
Software: Fei Xiong.
Supervision: Fei Xiong.
Validation: Fei Xiong.
Visualization: Fei Xiong.
Writing – original draft: Yu Ling, Fei Xiong.
Writing – review and editing: Yu Ling, Fei Xiong.
Footnotes
Abbreviations: AMD = age-related macular degeneration, CI = confidence interval, HSP = Henoch-Schönlein purpura, HWE = Hardy–Weinberg equilibrium, IS = ischemic stroke, OR = odds ratio, PD = Parkinson's disease, RPE = retinal pigment epithelium, SNP = single nucleotide polymorphism, TLR4 = toll-like receptor 4, TLRs = toll-like receptors, UTR = untranslated region.
The authors declare that they have no conflict of interest.
References
- [1].Lana TP, da Silva Costa SM, Ananina G, et al. Association of HTRA1 rs11200638 with age-related macular degeneration (AMD) in Brazilian patients. Ophthalmic Genet 2017;39:46–50. [DOI] [PubMed] [Google Scholar]
- [2].Liutkeviciene R, Sungailiene R, Vilkeviciute A, et al. Associations between CYP2C8 rs10509681 and rs11572080 gene polymorphisms and age-related macular degeneration. Acta Med Litu 2017;24:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang K, Zhong Q, Chen S, et al. An epidemiological investigation of age-related macular degeneration in aged population in China: the Hainan study. Int Ophthalmol 2017;38:1659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ma B, Dang G, Yang S, et al. CX3CR1 polymorphisms and the risk of age-related macular degeneration. Int J Clin Exp Pathol 2015;8:9592–6. [PMC free article] [PubMed] [Google Scholar]
- [5].Garcia-Layana A, Cabrera-Lopez F, Garcia-Arumi J, et al. Early and intermediate age-related macular degeneration: update and clinical review. Clin Interv Aging 2017;12:1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A 2010;107:7401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mitchell P, Liew G, Gopinath B, et al. Age-related macular degeneration. Lancet 2018;392:1147–59. [DOI] [PubMed] [Google Scholar]
- [8].Gata V, Florin Laurentiu I. The role of Toll-like receptors in ovarian cancer. J BUON 2017;22:1092–6. [PubMed] [Google Scholar]
- [9].Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol 2004;5:975–9. [DOI] [PubMed] [Google Scholar]
- [10].Sarli A, Skalidakis I, Velissari A, et al. Investigation of associations of ARMS2, CD14, and TLR4 gene polymorphisms with wet age-related macular degeneration in a Greek population. Clin Ophthalmol 2017;11:1347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Robinson E, Durrer C, Simtchouk S, et al. Short-term high-intensity interval and moderate-intensity continuous training reduce leukocyte TLR4 in inactive adults at elevated risk of type 2 diabetes. J Appl Physiol 2015;119:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen C, Guo D, Lu G. Wogonin protects human retinal pigment epithelium cells from LPS-induced barrier dysfunction and inflammatory responses by regulating the TLR4/NF-kappaB signaling pathway. Mol Med Rep 2017;15:2289–95. [DOI] [PubMed] [Google Scholar]
- [13].Despriet DD, Bergen AA, Merriam JE, et al. Comprehensive analysis of the candidate genes CCL2, CCR2, and TLR4 in age-related macular degeneration. Invest Ophthalmol Vis Sci 2008;49:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen L, Bai Y, Zhao M, et al. TLR4 inhibitor attenuates amyloid-beta-induced angiogenic and inflammatory factors in ARPE-19 cells: implications for age-related macular degeneration. Mol Med Rep 2016;13:3249–56. [DOI] [PubMed] [Google Scholar]
- [15].Kohno H, Chen Y, Kevany BM, et al. Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J Biol Chem 2013;288:15326–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gu L, Huang J, Liang B, et al. TLR4 polymorphisms affect stroke risk and inflammatory response in Chinese ischemic stroke patients. Neurol Sci 2017;39:127–33. [DOI] [PubMed] [Google Scholar]
- [17].Guven M, Batar B, Mutlu T, et al. Toll-like receptors 2 and 4 polymorphisms in age-related macular degeneration. Curr Eye Res 2016;41:856–61. [DOI] [PubMed] [Google Scholar]
- [18].Seddon JM, Sharma S, Adelman RA. Evaluation of the clinical age-related maculopathy staging system. Ophthalmology 2006;113:260–6. [DOI] [PubMed] [Google Scholar]
- [19].Singh K, Singh VK, Agrawal NK, et al. Association of Toll-like receptor 4 polymorphisms with diabetic foot ulcers and application of artificial neural network in DFU risk assessment in type 2 diabetes patients. BioMed Res Int 2013;2013:318686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang H, Wei Y, Zeng Y, et al. The association of polymorphisms of TLR4 and CD14 genes with susceptibility to sepsis in a Chinese population. BMC Med Genet 2014;15:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen Y, Bedell M, Zhang K. Age-related macular degeneration: genetic and environmental factors of disease. Mol Interv 2010;10:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fritsche LG, Fariss RN, Stambolian D, et al. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet 2014;15:151–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nussenblatt RB, Liu B, Li Z. Age-related macular degeneration: an immunologically driven disease. Curr Opin Investig Drugs 2009;10:434–42. [PubMed] [Google Scholar]
- [24].Kauppinen A, Paterno JJ, Blasiak J, et al. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci 2016;73:1765–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ozato K, Tsujimura H, Tamura T. Toll-like receptor signaling and regulation of cytokine gene expression in the immune system. BioTechniques 2002;Suppl:66–8. [PubMed] [Google Scholar]
- [26].Mai K, Chui JJ, Di Girolamo N, et al. Role of toll-like receptors in human iris pigment epithelial cells and their response to pathogen-associated molecular patterns. J Inflamm (Lond) 2014;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zareparsi S, Buraczynska M, Branham KE, et al. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet 2005;14:1449–55. [DOI] [PubMed] [Google Scholar]
- [28].Cho Y, Wang JJ, Chew EY, et al. Toll-like receptor polymorphisms and age-related macular degeneration: replication in three case-control samples. Invest Ophthalmol Vis Sci 2009;50:5614–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ferronato S, Gomez-Lira M, Menegazzi M, et al. Polymorphism -2604G>A variants in TLR4 promoter are associated with different gene expression level in peripheral blood of atherosclerotic patients. J Hum Genet 2013;58:812–4. [DOI] [PubMed] [Google Scholar]
- [30].Zhang L, Xu AG, Zhao W, et al. A toll-like receptor 4 (TLR4) variant is associated with asthma severity. Int J Clin Exp Med 2015;8:7849–54. [PMC free article] [PubMed] [Google Scholar]
- [31].Xu H, Jiang G, Shen H, et al. Association of TLR4 gene polymorphisms with childhood Henoch-Schonlein purpura in a Chinese population. Rheumatol Int 2017;37:1909–15. [DOI] [PubMed] [Google Scholar]
- [32].Zhao J, Han X, Xue L, et al. Association of TLR4 gene polymorphisms with sporadic Parkinson's disease in a Han Chinese population. Neurol Sci 2015;36:1659–65. [DOI] [PubMed] [Google Scholar]
- [33].Zhou L, Zheng D, Wang S, et al. Genetic association of Toll-like receptor 4 gene and coronary artery disease in a Chinese Han population. SpringerPlus 2016;5:1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yu H, Lin M, Wang X, et al. Toll-like receptor 4 polymorphism is associated with increased susceptibility to chronic obstructive pulmonary disease in Han Chinese patients with chronic periodontitis. J Oral Sci 2016;58:555–60. [DOI] [PubMed] [Google Scholar]


