Abstract
Serum concentrations of bilirubin, albumin, and uric acid (UA) play important roles in controlling oxidative stress. Until now, there are few researches related to the relationship between oxidative stress and Crohn's disease (CD); furthermore, no such study has been reported from China. Our aim was to evaluate serum bilirubin, albumin, and UA levels in CD patients and relate them to disease activity.
Seventy-one patients diagnosed with CD and 125 sex- and age-matched healthy individuals were retrospectively analyzed during the same period. Clinical characteristics and laboratory parameters were analyzed in CD patients and healthy control groups.
Serum levels of bilirubin, albumin, and UA in patients with CD were significantly lower than those in the healthy control group. Correlation analysis demonstrated that serum concentrations of total bilirubin, direct bilirubin, indirect bilirubin, albumin, and UA were negatively related to disease activity in patients with CD (r = −0.620, P < .001; r = −0.304, P < .05; r = −0.623, P < .001; r = −0.408, P < .01; and r = −0.296, P < .05; respectively).
Serum bilirubin, albumin and UA levels were significantly lower in CD patients, suggesting potential correlations between serum bilirubin, albumin, and UA levels and disease activity in CD patients. In addition, the noninvasive biochemical index may be potential markers for assessing the disease activity of patients with CD.
Keywords: albumin, anti-inflammatory, antioxidants, bilirubin, Crohn's disease, uric acid
1. Introduction
Inflammatory bowel disease, a chronic nonspecific inflammatory disease involving the intestine, is comprised of 2 major disorders, Crohn's disease (CD) and ulcerative colitis.[1] CD is a chronic inflammatory condition of the gastrointestinal tract characterized by inflammation at any point from the mouth to the rectum.[2] Currently, the annual incidence of CD is the highest in North America (20.2 per 100,000 person-years)[3]; whereas, the annual incidence of CD in China is obviously lower than that in western countries.[4,5] The annual incidence has considerable variation on CD since different geographic regions, environmental exposure, genetic susceptibility, and lifestyle.[6] With the increasing incidence of inflammatory bowel diseases, CD has become one of the most challenging diseases in both diagnosis and treatment of gastroenterology.[7] The etiologies of CD have not been fully elucidated, and there is no complete cure for CD to date. Therefore, the purpose of treatment is to turn CD disease activity into remission. Recently, multiple laboratory indices were applied to diagnose CD and assess the disease activity in CD. Some laboratory indices such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), red cell distribution width, tumor necrosis factor, and fecal calprotectin were correlated with CD.[8–12] However, such indices are not only a sign of disease activity, but also a combination of bacterial infection. Thus, considering the cost and the compliance of patients, there is a need for some low-cost and noninvasive biological indices to assess CD disease activity.
Bilirubin, the final decomposition product of heme metabolism, belongs to important potent endogenous antioxidants.[13] It has long been suggested that bilirubin as a cytotoxic waste product has potential toxicity, whereas bilirubin is considered to have strong antioxidant, anti-inflammatory, and immunosuppressive properties in recent years.[13,14] The antioxidant properties of bilirubin have been demonstrated in patients with neuromyelitis optica,[15] myasthenia gravis,[16] systemic lupus erythematosus,[17] and polymyositis.[18] Uric acid (UA), the end product of purine catabolism, has long been regarded as a metabolic waste. It is well known that UA is related to gout and urinary tract stones.[19] However, evidence suggested that UA plays an important role in antioxidation and can clear more than half the free radicals in human blood.[20] Besides, its antioxidant capacity is much higher than vitamin C and vitamin E.[21] Some studies have shown that the antioxidant properties of UA are closely associated with neuromyelitis optica,[15] myasthenia gravis,[16] and acute ischaemic stroke.[22] Moreover, previous studies have illustrated that serum albumin exerts important antioxidant activities when against oxidative damage.[15,16] These researches have indicated the properties of bilirubin, albumin, and UA in varied inflammation-related diseases, while few researches related to CD. To the best of our knowledge, no such study has been reported from China. Therefore, our aim was to assess the correlations between serum bilirubin, albumin, and UA levels and disease activity in CD patients from China.
2. Patients and methods
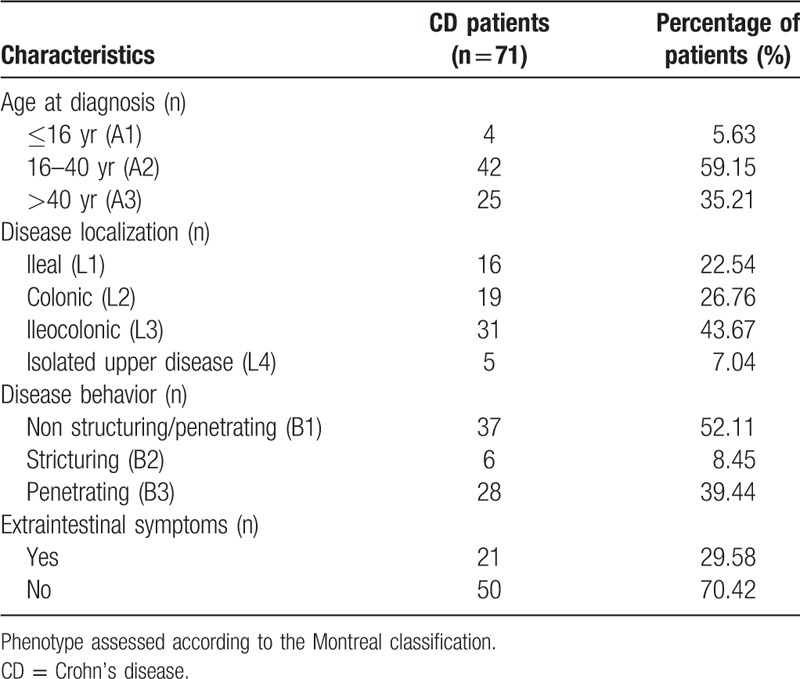
Patients diagnosed with CD at the First Affiliated Hospital of Guangxi Medical University (Guangxi, China) from July 2012 to June 2017 were retrospectively analyzed, and 71 patients newly diagnosed with CD who did not receive any treatment on admission were included. During the same period, 125 sex- and age-matched healthy individuals who underwent routine physical examinations in our hospital were considered as controls. Clinical characteristics and laboratory parameters of patients were retrieved from the database of the center. Diagnosis of CD was based on the comprehensive analysis of medical history, clinical manifestations, endoscopic and histopathology, imaging, as well as laboratory tests.[1] Disease activity in CD was evaluated by the CD activity index (CDAI) score.[23] Demographic and clinical characteristics of CD patients were assessed according to the Montreal classification[24] (Table 1).
Table 1.
Demographic and clinical characteristics of CD patients.
The following patients were excluded: hepatic or renal insufficiency, biliary disease, diabetes, hypertension, smoking, excessive drinking (the level of alcohol in the blood ≥0.08 g/dL), cardiovascular disease, infection, hematological disorder, and cancer. In addition, patients with other gastrointestinal diseases and autoimmune diseases were also excluded in this study. We used the exclusion criteria in order to avoid interference from other diseases, and excluded patients included hepatic or renal insufficiency (N = 2), biliary disease (N = 1), diabetes (N = 2), hypertension (N = 3), smoking (N = 6), excessive drinking (N = 4), cardiovascular disease (N = 1), infection (N = 3), cancer (N = 1), other gastrointestinal diseases or autoimmune diseases (N = 2), and treatment with anti-inflammatory medications or analgesic in recent month (N = 3). Among these, smoking + excessive drinking (N = 1), hypertension + diabetes (N = 1), smoking + infection (N = 1). Additionally, 9 CD patients who had incomplete clinical data were also excluded. Finally, 34 CD patients were excluded and 71 GBS patients were included in the study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University.
Serum concentrations of total bilirubin (Tbil), direct bilirubin (Dbil), indirect bilirubin (Ibil), gamma-glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, albumin, UA, and CRP were tested by using automatic biochemical Analyzer 7600-120 (Hitachi High Technologies, Japan). ESR was measured using automatic Analyzer Minitor-100 (Electa Lab S.r.l; Forli, Italy).
3. Statistical analysis
All data were analyzed using the SPSS statistical software (version 22.0, Chicago, IL), and P < .05 was considered statistically significant. We used the Kolmogorov–Smirnov test to identify data for normality. Continuous variables with normal distribution were analyzed by independent Student t test; if not, data were compared using Mann–Whitney U test. The differences in proportions between groups were analyzed by Chi-square test. According to disease location of CD patients, serum levels of bilirubin, albumin, and UA have been analyzed by 1-way analysis of variance. According to the CDAI score, differences of Tbil, Dbil, Ibil, albumin, UA, and CRP concentrations at different disease activity of CD patients were compared using 1-way analysis of variance. Correlations between serum CRP and bilirubin, albumin, and UA levels were assessed with the Pearson correlation test. Meanwhile, the potential associations between disease activity of CD and serum bilirubin, albumin, and UA levels were assessed with the Spearman correlation test.
4. Results
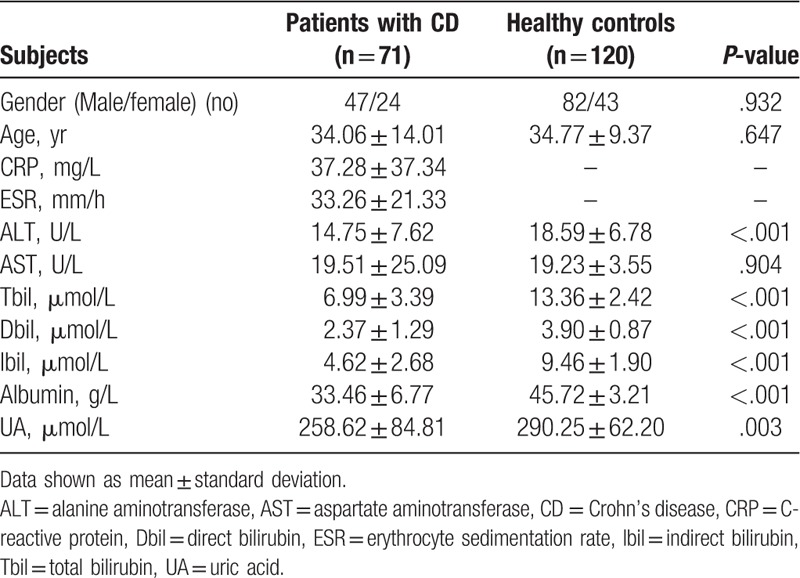
Compared with the healthy control group, serum Tbil, Dbil, Ibil, albumin, and UA levels were significantly lower in CD patients (P < .001, P < .001, P < .001, P < .001, and P < .01; respectively) (Table 2). Since there is enterohepatic circulation, we stratified CD patients according to disease location. Among the 4 groups, serum levels of bilirubin, albumin, and UA had no significant difference.
Table 2.
Demographic characteristics and laboratory parameters of CD patients and healthy controls.
Moreover, to better elucidate the relation between concentrations of disease activity of CD and serum Tbil, Dbil, Ibil, albumin, UA and CRP, patients with CD were divided into 4 subgroups according to CDAI score: inactive group, mild group, moderate group, and severe group (Table 3). There was no significant difference in serum UA levels between subgroups of disease activity. However, we found that the relative decrease in serum concentrations of Tbil, Dbil, Ibil, and albumin associated with the degree of disease progression; whereas serum CRP level increased related to the degree of disease progression in CD patients (Table 3 and Figure 1A–E).
Table 3.
Serum bilirubin, UA, albumin and CRP levels in CD patients according to the disease activity.
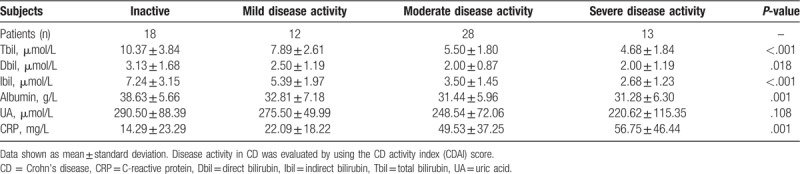
Figure 1.
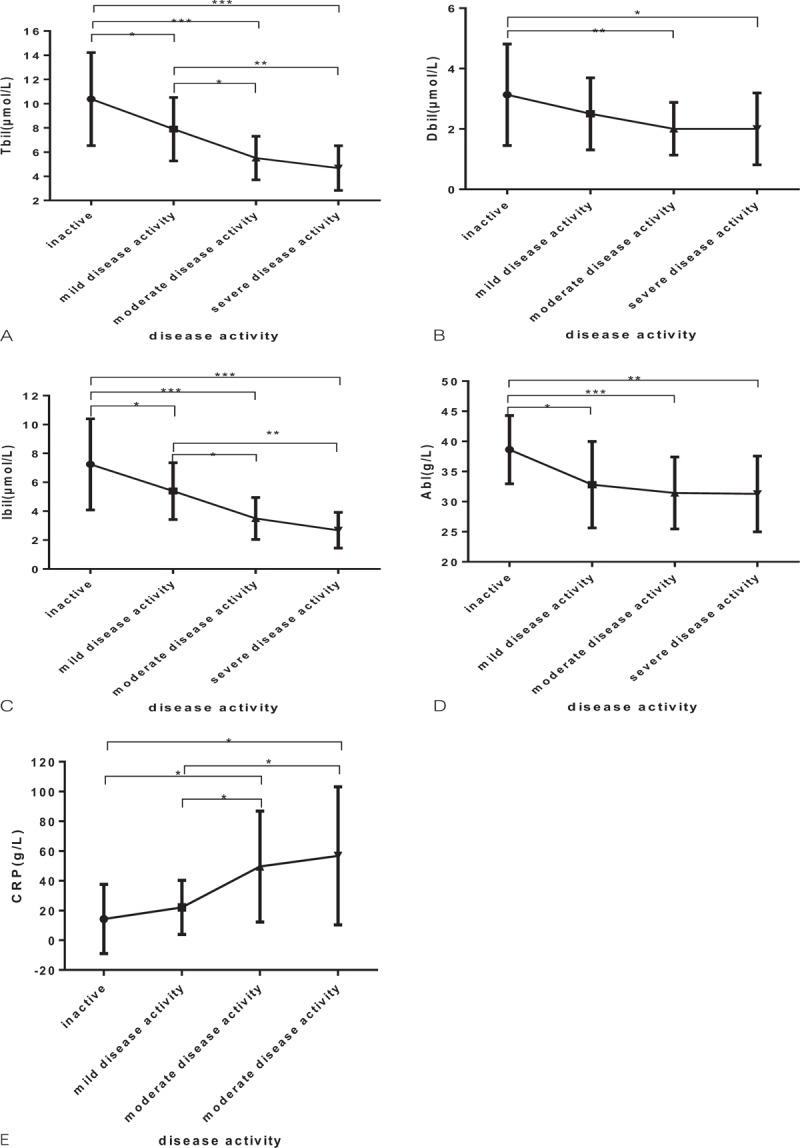
Serum bilirubin, albumin, and CRP levels in 4 disease activity according to the CDAI score (∗P < .05, ∗∗P < .01, and ∗∗∗P < .001): (A) total bilirubin; (B) direct bilirubin; (C) indirect bilirubin; (D) albumin; (E) C-reactive protein. CDAI = Crohn's disease activity index, CRP = C-reactive protein.
Eliminating the effect of gender, age, and liver function, correlation analysis demonstrated that serum Tbil, Ibil, albumin, and UA levels were negatively correlated with CRP in patients with CD (r = −0.364, P < .01; r = −0.373, P < .01; r = −0.337, P < .01; and r = −0.335, P < .01; respectively). Furthermore, serum Tbil, Dibl, Ibil, albumin, and UA levels were negatively related to disease activity in patients with CD (r = −0.620, P < .001; r = −0.304, P < .05; r = −0.623, P < .001; r = −0.408, P < .01; and r = −0.296, P < .05; respectively). However, serum concentrations of CRP were positively correlated with disease activity in CD patients (r = 0.577, P < .001).
5. Discussion
To the best of our knowledge, this is the first paper to investigate the association between serum levels of bilirubin, albumin, UA levels, and CD patients from China. In this study, we observed that serum levels of bilirubin, albumin, and UA are significantly lower in patients with CD than those in healthy controls. Notably, serum bilirubin, albumin, and UA levels were negatively associated with disease activity in CD patients, whereas serum CRP levels were positively related to disease activity in CD patients.
The typical clinical manifestations of CD, which is marked by episodes of relapse and remission,[2,7] abdominal pain, diarrhea, weight loss and associated with abdominal masses, intestinal obstruction, and fistula. Because of episodes of relapse in CD patients, the goals of the therapy are control of symptoms, induction of clinical remission, and maintenance of remission with minimal adverse effects.[25] During chronic inflammation, serum CRP level is an important laboratory index to evaluate the disease activity and the risk of recurrence for CD patients.[10,26] Serum level of CRP is increased at the early stage of CD, and decreased rapidly after remission. Indeed, CRP is a nonspecific marker of inflammation, and it rises dramatically during acute trauma and infection. During inflammatory process, a cascade of inflammatory response occurs at the intestinal level, which activated neutrophils produce reactive oxygen species (ROS). ROS may result in further oxidative damage, so oxidative stress is thought to be one of the important pathogenic factors in the progression of CD.[27]
Recently, some literature[28–30] have been reported that the antioxidant capacity of CD patients is significantly decreased. To date, there are not yet such reports from China. Oxidative stress and antioxidant deficiency may play key roles in the pathogenesis of CD-associated gastrointestinal injury.[28] As Lenicek et al reported that each 1 mmol/L decreased in serum bilirubin was related to a 13% increase in the risk of CD manifestation.[31] In our study, we also found that serum levels of bilirubin, albumin, and UA were significantly lower in CD patients than those in healthy individuals. In addition, serum concentrations of bilirubin, albumin, and UA reversely correlated with the degree of disease progression in CD patients. These results may attribute to chronic inflammation in the gastrointestinal tract of CD patients, and suggested that there may be an association between inflammatory processes and increased oxidative stress in CD patients.
It has been well known that the imbalance of oxidation and antioxidation is associated with the aging process or varieties of inflammatory conditions, which results in oxidation of cellular components such as proteins, DNA, carbohydrates, and lipids.[32] Recently, there is mounting evidence that bilirubin, albumin, and UA exert important antioxidant activities against oxidative damage.[14,19,20,33] Bilirubin contains a reactive hydrogen atom and conjugated double bonds, thus it could possess antioxidant properties.[14] Since bilirubin is important to the systemic antioxidant capacity by efficiently scavenging peroxyl radicals, it may protect linoleic acid and vitamin A from oxidative destruction in the intestinal tract.[14] As the main extracellular molecule responsible for maintaining the plasma redox state, the specific antioxidant properties of albumin are due to its multiple ligand-binding capacities and free radical trapping properties and are closely related to the structure and the redox state of the molecule.[33] Besides, albumin plays a decisive role in redox species distribution of thiols in plasma, acting by oxidation and albumin-dependent thiol/disulfide (SH/SS) exchange reactions.[34] Additionally, UA is oxidized to urea in the process that scavenge hydroxyl radicals, singlet molecular oxygen, oxo-haem oxidants, and lipid hydroperoxide radicals to inhibit lipid peroxidation.[19,20] UA can protect linoleic acid stability and erythrocyte membrane integrity.[19,20] Furthermore, UA and vitamin C have synergistic effect on antioxidant, which inhibits ascorbate oxidation by forming stable complexes with transition-metal ions.[35] ROS may promote the release of inflammatory cytokines, which may weaken antioxidant defenses and lead to oxidative stress. Thus, we suppose that bilirubin, albumin, and UA as endogenous antioxidants may be destroyed by chronic systemic inflammation. On the other hand, antioxidant molecules have been shown to inhibit the production of proinflammatory cytokine.[27] During the inflammatory process, levels of bilirubin, albumin, and UA decreased in CD patients may be due to overconsumption and destruction of bilirubin, albumin, and UA. With the progress of disease activity, serum Tbil, Dibl, Ibil, albumin, and UA levels were significantly decreased in CD patients.
However, there were some limitations in the present study. First, this was a retrospective study of patients with CD, and the small sample size prevents us from drawing conclusions about the correlation between the bilirubin, albumin, UA, and CD. Second, the correlations between some antioxidant enzymes (SOD, CAT, and GSH-Px) and disease activity of CD patients have not been assessed in our study. Finally, serum bilirubin, albumin, and UA levels were not evaluated in treated patients with CD. Thus, a large-scale prospective study is needed for further confirmation.
6. Conclusion
The study revealed that serum bilirubin, albumin, and UA levels were significantly lower in CD patients, suggesting negative correlations between serum bilirubin, albumin, and UA levels and disease activity in CD patients. Therefore, this noninvasive biochemical method may be a potential marker for assessing the disease activity of patients with CD. The use of serum bilirubin, albumin, and UA levels is needed further investigating to help the assessment of disease activity and treatment in CD patients.
Acknowledgments
The authors would like to thank all the personnel of Department of Clinical Laboratory, First Affiliated Hospital of Guangxi Medical University.
Author contributions
Conceptualization: Qisheng Su.
Data curation: Zheng Yang.
Formal analysis: Qisheng Su, Wuning Mo, Zheng Yang.
Investigation: Qisheng Su, Zheng Yang.
Methodology: Qisheng Su, Zheng Yang.
Project administration: Wuning Mo.
Resources: Qisheng Su, Wuning Mo.
Software: Xiaohong Li.
Supervision: Xiaohong Li, Zheng Yang.
Validation: Xiaohong Li, Wuning Mo.
Writing – original draft: Qisheng Su, Xiaohong Li.
Writing – review and editing: Qisheng Su, Wuning Mo, Zheng Yang.
Footnotes
Abbreviations: CD = Crohn's disease, CDAI = CD activity index, CRP = C-reactive protein, Dbil = direct bilirubin, ESR = erythrocyte sedimentation rate, Ibil = indirect bilirubin, ROS = reactive oxygen species, Tbil = total bilirubin, UA = uric acid.
The authors have no conflicts of interest to disclose.
References
- [1].Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014;8:443–68. [DOI] [PubMed] [Google Scholar]
- [2].Wilkins T, Jarvis K, Patel J. Diagnosis and management of Crohn's disease. Am Fam Physician 2011;84:1365–75. [PubMed] [Google Scholar]
- [3].Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46–54.e42. [DOI] [PubMed] [Google Scholar]
- [4].Zeng Z, Zhu Z, Yang Y, et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol 2013;28:1148–53. [DOI] [PubMed] [Google Scholar]
- [5].Zhao J, Ng SC, Lei Y, et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of “western” disease. Inflamm Bowel Dis 2013;19:1839–45. [DOI] [PubMed] [Google Scholar]
- [6].Yang H, Li Y, Wu W, et al. The incidence of inflammatory bowel disease in Northern China: a prospective population-based study. PloS One 2014;9:e101296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011;140:1785–94. [DOI] [PubMed] [Google Scholar]
- [8].Yesil A, Senates E, Bayoglu IV, et al. Red cell distribution width: a novel marker of activity in inflammatory bowel disease. Gut Liver 2011;5:460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eder P, Stawczyk-Eder K, Lykowska-Szuber L, et al. Association between disease duration and usefulness of fecal calprotectin measurement in patients with Crohn's disease. Pol Arch Med Wewn 2014;124:51–7. [PubMed] [Google Scholar]
- [10].Reinisch W, Wang Y, Oddens BJ, et al. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther 2012;35:568–76. [DOI] [PubMed] [Google Scholar]
- [11].Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 2006;55:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ananthakrishnan AN, Cheng SC, Cai T, et al. Serum inflammatory markers and risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014;12:1342–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jangi S, Otterbein L, Robson S. The molecular basis for the immunomodulatory activities of unconjugated bilirubin. Int J Biochem Cell Biol 2013;45:2843–51. [DOI] [PubMed] [Google Scholar]
- [14].Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–6. [DOI] [PubMed] [Google Scholar]
- [15].Peng F, Yang Y, Liu J, et al. Low antioxidant status of serum uric acid, bilirubin and albumin in patients with neuromyelitis optica. Eur J Neurol 2012;19:277–83. [DOI] [PubMed] [Google Scholar]
- [16].Yang D, Su Z, Wu S, et al. Low antioxidant status of serum bilirubin, uric acid, albumin and creatinine in patients with myasthenia gravis. Int J Neurosci 2016;126:1120–6. [DOI] [PubMed] [Google Scholar]
- [17].dos Santos BH, de RACM, Skare TL. Systemic lupus erythematosus activity and serum bilirubins. Acta Reumatol Port 2013;38:242–6. [PubMed] [Google Scholar]
- [18].Peng YF, Zhang L, Pan GG, et al. A potential clinical usefulness of measuring serum bilirubin levels in patients with polymyositis. Eur Rev Med Pharmacol Sci 2016;20:631–5. [PubMed] [Google Scholar]
- [19].Hediger MA. Physiology and biochemistry of uric acid. Ther Umsch 2004;61:541–5. [DOI] [PubMed] [Google Scholar]
- [20].Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 1981;78:6858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ekaidem IS, Usoh IF, Akpanabiatu MI, et al. Urate synthesis and oxidative stress in phenytoin hepatotoxicity: the role of antioxidant vitamins. Pak J Biol Sci 2014;17:1179–84. [DOI] [PubMed] [Google Scholar]
- [22].Chamorro A, Amaro S, Castellanos M, et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol 2014;13:453–60. [DOI] [PubMed] [Google Scholar]
- [23].Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- [24].Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19Suppl A:5A–36A. [DOI] [PubMed] [Google Scholar]
- [25].Baumgart DC, Sandborn WJ. Crohn's disease. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- [26].Sostegni R, Daperno M, Scaglione N, et al. Review article: Crohn's disease: monitoring disease activity. Aliment Pharmacol Ther 2003;17Suppl 2:11–7. [DOI] [PubMed] [Google Scholar]
- [27].Maor I, Rainis T, Lanir A, et al. Oxidative stress, inflammation and neutrophil superoxide release in patients with Crohn's disease: distinction between active and non-active disease. Dig Dis Sci 2008;53:2208–14. [DOI] [PubMed] [Google Scholar]
- [28].Koutroubakis IE, Malliaraki N, Dimoulios PD, et al. Decreased total and corrected antioxidant capacity in patients with inflammatory bowel disease. Dig Dis Sci 2004;49:1433–7. [DOI] [PubMed] [Google Scholar]
- [29].D’Odorico A, Bortolan S, Cardin R, et al. Reduced plasma antioxidant concentrations and increased oxidative DNA damage in inflammatory bowel disease. Scand J Gastroenterol 2001;36:1289–94. [DOI] [PubMed] [Google Scholar]
- [30].Buffinton GD, Doe WF. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic Biol Med 1995;19:911–8. [DOI] [PubMed] [Google Scholar]
- [31].Lenicek M, Duricova D, Hradsky O, et al. The relationship between serum bilirubin and Crohn's disease. Inflamm Bowel Dis 2014;20:481–7. [DOI] [PubMed] [Google Scholar]
- [32].Grimsrud PA, Xie H, Griffin TJ, et al. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem 2008;283:21837–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Taverna M, Marie AL, Mira JP, et al. Specific antioxidant properties of human serum albumin. Ann Intensive Care 2013;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Di Giuseppe D, Ulivelli M, Bartalini S, et al. Regulation of redox forms of plasma thiols by albumin in multiple sclerosis after fasting and methionine loading test. Amino Acids 2010;38:1461–71. [DOI] [PubMed] [Google Scholar]
- [35].Davies KJ, Sevanian A, Muakkassah-Kelly SF, et al. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J 1986;235:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


