Supplemental Digital Content is available in the text
Keywords: clinical observation, MAP, meta-analysis, osteosarcoma, survival
Abstract
Background:
We designed the study to investigate whether methotrexate, doxorubicin, and cisplatinum (MAP) chemotherapy strategy was still the preferred option for the survival of osteosarcoma patients.
Method:
We collected some trials of osteosarcoma to make a meta-analysis first. Then, we retrospectively collected data from 115 patients with osteosarcoma and performed further analysis to verify the impact of MAP regimen on the survival of patients.
Results:
Seven studies including 3433 participants met the preliminary inclusion criteria. Meta-analysis of the 3-year disease-free survival (odds ratio [OR] = 1.06, 95% confidence interval [CI]: 0.88–1.28; P = .52) and overall survival (OR = 1.21, 95% CI: 0.70–2.11; P = .54), 5-year disease-free survival (OR = 1.07, 95% CI: 0.87–1.30; P = .54) and overall survival (OR = 0.86, 95% CI: 0.65–1.12; P = .26), and mortality rate (OR = 0.90, 95% CI: 0.70–1.17; P = .44), showed no statistically significant differences. The most common grade 3/4 adverse events were neutropenia (498 [85.9%] patients in MAP vs 533 [93.3%] in MAP plus ifosfamide and etoposide, or other adjuvant therapy drugs [MAP+]). MAP was associated with less frequent toxicities than MAP+ group with statistical significance in thrombocytopenia, febrile neutropenia, anemia, and hypophosphatemia. The same phenomenon could also be seen in the analysis of clinical data.
Conclusion:
MAP regimen remains the preferred option for osteosarcoma chemotherapy.
1. Introduction
In recent years, many studies have shown that the development and prognosis of osteosarcoma is a pathological process involving multiple genes and factors. Although basic research on tumor markers has progressed rapidly in the field of osteosarcoma, treatment methods have changed little in clinical work.[1–5] Surgery, chemotherapy and selective radiotherapy are still the main treatments for patients with osteosarcoma.[6–12] It was reported that neoadjuvant chemotherapy including doxorubicin, methotrexate, and cisplatin with intercalated surgery is the standard of care for resectable osteosarcoma patients in those younger than 40 years.[11] The prognosis for osteosarcoma patients presenting with advanced or recurrent disease, or among those older than 40 years are generally poor. Overall prognosis has improved little for all patients with osteosarcoma, and new treatment combinations or methods are needed.[11]
As chemotherapy is the most commonly used treatment, in the field of osteosarcoma treatment, the choice of chemotherapy drugs and chemotherapy regimens or the choice of chemotherapy density are still controversial. The combination of surgical removal of the tumor and systemic multidrug chemotherapy mainly consisting of methotrexate, adriamycin, and cisplatin with or without ifosfamide is the standard strategy to treat conventional osteosarcoma.[13] Postoperative adjuvant chemotherapy definitely improves disease-free and overall survival (OS) in patients with osteosarcoma,[13] while there was no advantage in event-free survival (EFS) for patients given presurgical chemotherapy.[14] After the failure of first-line treatment, second-line chemotherapy was often given to patients with osteosarcoma who were in good condition. Gemcitabine-based combined chemotherapy predicted a good clinical application prospect as the second-line treatment for osteosarcoma patients, whether it was combined with docetaxel or sirolimus,[15,16] while dose intensification with high-dose chemotherapy did not increase the probability of survival.[17]
In other words, there were still different voices for the optimal treatment options for the treatment of osteosarcoma patients throughout the course of the disease. As more and more phase III clinical studies on chemotherapy are completed, more and more chemotherapy options are available.[18–24] So which is the best chemotherapy option for the entire course of osteosarcoma patients? In order to answer this question, we conducted a meta-analysis of the osteosarcoma chemotherapy regimen and then collected many clinical cases from 4 clinical hospitals to verify whether the meta-analysis results were consistent with the clinical observations.
2. Materials and methods
2.1. Data collection
2.1.1. Search strategy and selection criteria
We took the method called the Preferred Reporting Items for Systematic Reviews and Meta-Analyses to search materials.[25] Original articles, related to results of prospective clinical trials were verified by a Pubmed search. During the process of searching, we mainly selected English studies ranged from January 1, 2000 to August 30, 2018 (Keywords: “Osteosarcoma,” “osteoma,” “chemotherapy,” “doxorubicin,” “methotrexate,” “cisplatin,” “prognosis,” “radiotherapy,” “chemoradiotherapy,” “death,” “mortality”). In the process of searching data, we only focused on Phase III clinical trials. We adopted the studies just in human beings which were presented in full text, abstract, or poster form. Some ongoing clinical trials were checked from American or European clinical trial centers, while other data of interest were selected from information seeking on the internet and manual access of bibliographies. We appointed 4 staff members to take charge of confirming their eligibility, and then get agreement together.
The criteria for the selected data:
-
(1)
available data in cases and controls provided;
-
(2)
self-reported results and risk assessment and/or displayed data necessary for evaluating odds ratio (OR) with 95% confidence interval (CI) or other evaluable indicators such as risk ratio (RR), hazard ratios (HR), and so on;
-
(3)
subjects were diagnosed with osteosarcoma by typical imaging changes or pathological biopsy;
-
(4)
phase-III randomized controlled trials or comparative studies;
-
(5)
the chemotherapy regimens involved in the clinical data in the article are in line with the chemotherapy regimen recommended by the National Comprehensive Cancer Network (NCCN) guidelines.
Exclusion criteria:
-
(1)
studies that crossing with others or reported with data from the same authors;
-
(2)
studies involved neonates or patients older than 65 years;
-
(3)
serious complications not related to the purpose of the study;
-
(4)
sarcoma but not osteosarcoma;
-
(5)
studies with incomplete results or data;
-
(6)
study type of letters, case reports, editorials, or reviews.
2.1.2. Data extraction and validity assessment
The extraction of the data was put into practice according to the criteria recommended by the Cochrane Collaboration.[26] Treatment groups were confirmed as patients who had received chemotherapy. We summarized necessary data of the study involving first author, year of publication, the number of patients, research design, population, chemotherapy protocol name, chemotherapy cycles, dosage and toxic side effects of chemotherapy drugs, the survival status of malignant tumor patients. If no useful data was found, we would try to get in touch with the corresponding author for more information.
2.1.3. Assessment of bias risk
We selected 4 clinicians to evaluate the extracted data separately. The quality of study was evaluated by the Newcastle–Ottawa scale as proposed by the Cochrane Collaboration.[27] We would check up HR, RR, OR, and 95% CI which were calculated with random effect (RE) or fixed effect (FE) models according to the actual situation of the data. P ≤ .05 was considered as statistically significant.
2.1.4. Main outcome measures
The primary aims were EFS, OS, and mortality rate, while secondary outcomes were chemotherapy toxicities. Targeted toxicities included neutropenia, febrile neutropenia, fever without neutropenia, thrombocytopenia, hypophosphatemia, mucositis, cardiac dysfunction, renal dysfunction, anemia, and so on.
We designated 2 clinicians to check up dosage and toxic side effects of chemotherapy drugs, the survival status of tumor patients. Chemotherapy efficacy evaluation, according to the standard of the response evaluation criteria in solid tumors, could be diagnosed by computed tomography scan. Survival status comprised mortality rate of patients and complications affecting the prognosis. The general condition of patients was evaluated according to the Eastern Cooperative Oncology Group score. The collection of survival time would be accurately calculated to the day. Some unclear data was gotten from the study authors. If some useful details were unavailable, the study would be deleted from the analysis. The corresponding author of the article would be responsible for disagreements and have the right to make a final decision.
2.2. Statistical analysis
All the data were collected with OR by using the Comprehensive Meta-Analysis software according to whether or not they were presented with a comparison group. The OR was considered as a much more conservative estimate and might be more likely to detect a safety signal, as the method by which an OR is calculated provided a point estimate farther from unity than that provided by a HR. A RE model was taken to check most of treatment effects which are different among all studies,[28] while a FE model was used occasionally for some analysis when the treatment effects were deemed to be the same and that differences in results were just due to random probability. Cochrane Q statistic and the I2 statistic were used to check the heterogeneity among studies just as recommended by Higgins et al.[29] Harbord test was taken to evaluate publication bias for studies; P ≤ .05 was considered as publication bias. We would summarize all survival information of patients including long-term follow-up data. All data consolidation and analyses were calculated by Review Manager 5.3. Statistical tests were all 2-sided.
For clinical data survival analysis, all risk evaluation processes would be easily finished with online analysis tools, SPSS 19.0 software.[30] The HR is the RR of the terms stated by 2 levels of risk groups. Survival rate was plotted using Kaplan–Meier method and analyzed by using Log-rank test method. The frequencies of categorical variables were compared using Pearson χ2 or Fisher exact test, when appropriate. A value of P ≤ .05 was deemed to be statistical significant.
3. Results
Among all the citations identified by our electronic and manual searches, 7 studies including 3433 participants met the inclusion criteria.[18–24] All the articles were imported into EndNote X8 and checked. The characteristics of them were listed in Table 1. All the funnel plots were provided in Supplemental Figures 1 and 2.
Table 1.
Characteristics of all the enrolled data.

3.1. Meta-analysis of 3-year event-free and OS rates
Five studies (n = 2891) reported methotrexate, doxorubicin, and cisplatinum (MAP) (n = 1153) versus MAP plus ifosfamide and etoposide, or other adjuvant therapy drugs (MAP+) strategies (n = 1738) with the related data of 3 year EFS,[18,20,21,22,24] while only 3 studies of them were shown with the information of 3 year OS rates.[18,20,21] We took a RE model of meta-analysis to deal with all the data. Meta-analysis of 3-year event-free survival rate showed that there was no difference between the 2 groups (OR = 1.06, 95% CI: 0.88–1.28, Tau2 = 0.02; Chi2 = 8.87; df = 6 [P = .18]; I2 = 32%, Z = 0.64 [P = .52]; Fig. 1A).[18,20,21,22,24] Similar results could also be seen in the results of 3-year OS rates analysis (OR = 1.21, 95% CI: 0.70–2.11, Tau2 = 0.18; Chi2 = 8.37; df = 2 [P = .02]; I2 = 76%, Z = 0.68 [P = .49]; Fig. 1B).[18,20,21] We did not take the fixed model to make further analysis for the existence of heterogeneity (I2 = 32%). The funnel plots could be seen in Supplemental Figure 1A and B.
Figure 1.
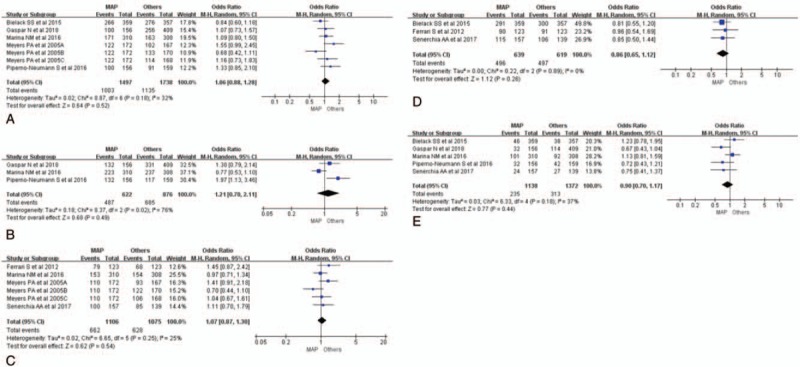
Meta-analysis of survival rate between MAP and other chemotherapy strategies. (A) Forest plot (RE) of 3-year EFS rates between MAP and other chemotherapy strategies. (B) Forest plot (RE) of 3-year OS rates between MAP and other chemotherapy strategies. (C) Forest plot (RE) of 5-year EFS rates between MAP and other chemotherapy strategies. (D) Forest plot (RE) of 5-year OS rates between MAP and other chemotherapy strategies. (E) Forest plot (RE) of mortality rates between MAP and other chemotherapy strategies. EFS = event-free survival, FE = fixed effect, MAP+ = MAP plus ifosfamide and etoposide, or other adjuvant therapy drugs, MAP = methotrexate + doxorubicin + cisplatinum, OR = odds ratio, OS = overall survival, RE = random effect, RR = risk ratio.
3.2. Meta-analysis of 5-year event-free and OS rates
The 5-year EFS rate and OS rate were also taken into account.[19,20,22,23,24] For 4 included studies, the data of 5-year EFS rates were reported. The 5-year EFS rate was 59.9% (662/1106) in the MAP group versus 58.4% (628/1075) in the other chemotherapy group.[19,20,23,24] No difference could be found between the 2 groups (OR = 1.07, 95% CI: 0.87–1.30, Tau2 = 0.02; Chi2 = 6.65; df = 5 [P = .25]; I2 = 25%, Z = 0.62 [P = .54]; Fig. 1C).[19,20,23,24] Heterogeneity could be found (I2 = 25%), so the FE model of meta-analysis was deserted.
Three studies with 5-year OS rate data were taken to make further analysis.[19,22,23] The RE model was tried to deal with the raw data. The forest plot could be seen in Figure 1D. The overall outcome of the analysis were summarized at the bottom of it (OR = 0.86, 95% CI: 0.65–1.12, Tau2 = 0.00; Chi2 = 0.22; df = 2 [P = .89]; I2 = 0, Z = 1.12 [P = .26]; Fig. 1D).[19,22,23] Harbord test statistic did not suggest obvious publication bias in funnel plots (Supplemental Fig. 1C and D).
3.3. Meta-analysis of mortality rates
For 5 studies, the data of mortality rate to chemotherapy was reported.[18–22] The rate of mortality was 20.7% (235/1138) in the MAP group versus 22.8% (313/1372) in other group. No statistically significant difference was also seen between the 2 chemotherapy treatments (OR = 0.90, 95% CI: 0.70–1.17, Tau2 = 0.03; Chi2 = 6.33; df = 4 [P = .18]; I2 = 37%, Z = 0.77 [P = .44]; Fig. 1E), suggesting that the MAP strategies did not decrease mortality rate of tumor to the chemotherapy which was highly correlated with longer survival.[18–22] FE model of meta-analysis is not suitable for dealing with the data of mortality rate for the existence of heterogeneity (I2 = 37%). The funnel plot was shown in Supplemental Figure 1E.
3.4. Meta-analysis of toxicity incidence rates
Seven kinds of chemotherapy toxicities, including neutropenia, thrombocytopenia, febrile neutropenia, cardiac toxicity, anemia, hypophosphatemia, mucositis, infection, were collected for evaluation (Fig. 2). Compared with other combination chemotherapy regimens, MAP chemotherapy regimen showed lower incidence rates of chemotherapy toxicities, especially in thrombocytopenia, febrile neutropenia, anemia, hypophosphatemia (Fig. 2B, C, E, and F). There was obvious heterogeneity among most of enrolled studies for the differences of chemotherapy drugs in each group (Fig. 2A–G). Though Harbord test statistic did not suggest obvious publication bias in funnel plot, the heterogeneity is moderate among studies (Supplemental Fig. 2A–H). So, we just took RE model of meta-analysis to deal with all the data (Fig. 2A–H).
Figure 2.
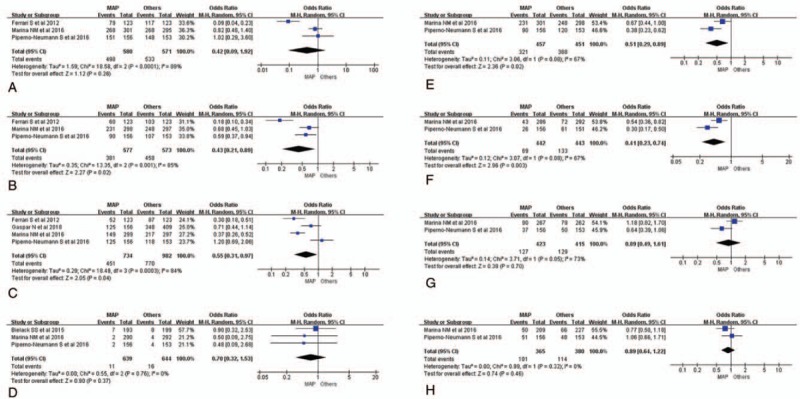
Meta-analysis of all kinds of toxicity rates among chemotherapy strategies. (A) Forest plot (RE) of grade 3/4 neutropenia incidence rates between MAP and other chemotherapy strategies. (B) Forest plot (RE) of grade 3/4 thrombocytopenia incidence rates between MAP and other chemotherapy strategies. (C) Forest plot (RE) of grade 3/4 febrile neutropenia incidence rates between MAP and other chemotherapy strategies. (D) Forest plot (RE) of cardiac toxicity incidence rates between MAP and other chemotherapy strategies. (E) Forest plot (RE) of grade 3/4 anemia incidence rates between MAP and other chemotherapy strategies. (F) Forest plot (RE) of grade 3/4 hypophosphatemia incidence rates between MAP and other chemotherapy strategies. (G) Forest plot (RE) of grade 3/4 mucositis incidence rates between MAP and other chemotherapy strategies. (H) Forest plot (RE) of grade 3/4 infection incidence rates between MAP and other chemotherapy strategies. EFS = event-free survival, FE = fixed effect, MAP+ = MAP plus ifosfamide and etoposide, or other adjuvant therapy drugs, MAP = Methotrexate + doxorubicin + cisplatinum, OR = odds ratio, OS = overall survival, RE = random effect, RR = risk ratio.
Neutropenia was still the most common chemotherapy side effect, the incidence rates of 2 groups were 85.9% (498/580) and 93.3% (533/571).[20,21,23] The forest plot could be seen in Figure 3A, and the overall outcome of the analysis were summarized at the bottom of it (OR = 0.42, 95% CI: 0.09–1.42, Tau2 = 1.59; Chi2 = 18.58; df = 2 [P < .0001]; I2 = 89%, Z = 1.12 [P = .26]; Fig. 2A). The neutropenia difference of OR value is much more pronounced in the synchronized chemotherapy group (OR = 0.09, 95% CI: 0.04–0.23; Ferrari et al, 2012) than continuous chemotherapy (OR = 0.82, 95% CI: 0.48–1.20; Marina et al, 2016) or chemotherapy combined with other drugs (OR = 1.02, 95% CI: 0.29–3.60; Piperno et al, 2016). However, the overall outcome of analysis about neutropenia was of no statistical significance. Similar analysis results were also displayed as forest plots in Figure 2D, G, and H.[20,21,22]
Figure 3.

Kaplan–Meier curve of overall survival for 115 patients. (A) Kaplan–Meier curve of overall survival by metastases status at registration. (P1 = .000 [without metastasis vs lung metastasis]; P2 = .000 [without metastasis vs brain metastasis] P3 = .000 [without metastasis vs other metastasis]). (B) Kaplan–Meier curve of overall survival by treatment regimens. (PST = palliative supportive treatment; P1 = .151 [MAP vs MAP+]; P2 = .000 [MAP vs PST]; P3 = .000 [MAP+ vs PST]). EFS = event-free survival, FE = fixed effect, MAP+ = MAP plus ifosfamide and etoposide, or other adjuvant therapy drugs, MAP = methotrexate + doxorubicin + cisplatinum, OR = odds ratio, OS = overall survival, PST = palliative supportive treatment, RE = random effect, RR = risk ratio.
Three studies related to the information of thrombocytopenia were put into practice of meta-analysis.[20,21,23] The detail of the result was shown in Figure 2B. It illustrated that there was statistical significant difference between the 2 chemotherapy treatments (OR = 0.43, 95% CI: 0.21–0.89, Tau2 = 0.35; Chi2 = 13.35; df = 2 [P = .001]; I2 = 85%, Z = 2.27 [P = .02]; Fig. 2B), suggesting that the controlled chemotherapy strategies increased thrombocytopenia incidence rate of patients which might be correlated with survival time and quality of life. Similar analysis results of statistical significance could also be seen in febrile neutropenia, anemia, and hypophosphatemia.[18,20,21,23] The forest plots could be seen in Figure 2C, E, and F, and the results of the analysis were gathered at the bottom of them.
3.5. Clinical data observation and analysis
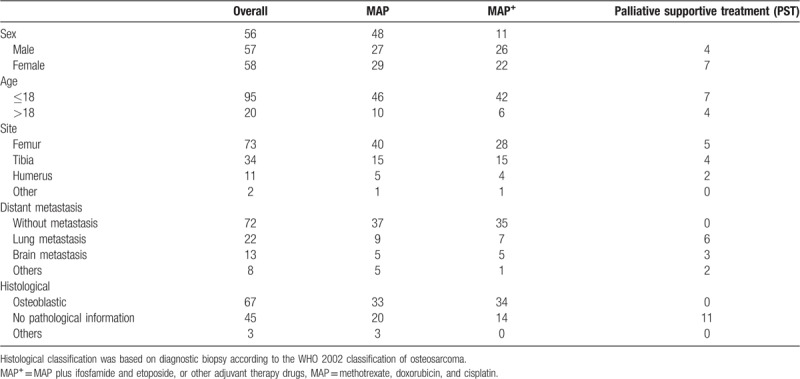
Between April 14, 2008, and June 30, 2013, 115 patients were collected from 4 different hospitals in Shandong province of China (MAP, n = 56; MAP+, n = 48; PST, n = 11). All the OS time of patients could be collected from corresponding hospital or relatives of the patient. Baseline characteristics of all enrolled patients were gathered in Table 2. Fifty-eight patients were female and 57 were male. The survival analysis of 115 patients was put into practice and the details were displayed in Figure 3A and B. Metastasis remains the most significant factor in the prognosis of patients, especially for brain metastasis (Fig. 3A). The Kaplan–Meier survival curves were obviously separated from each other, which P value was of statistical significance (Fig. 3A).
Table 2.
Baseline characteristics of all enrolled patients.
Patients receiving chemotherapy (MAP or MAP+) had a better prognosis than patients who had just received palliative supportive treatment (Fig. 3B), while the difference between MAP and MAP+ treatment approaches, compared in pairs, was of no obvious significance (P = .151).
4. Discussion
As is known to all, osteosarcoma is the most common bone malignant tumour in children and adolescents and is associated with high mortality. Surgery, chemotherapy, and selective radiotherapy are still the main treatments for patients with osteosarcoma.[6–12] The 3-year EFS for high-grade osteosarcoma with multidrug chemotherapy and resection was reported to be 60% to 70%.[22,24,31,32] Chemotherapy combination, named MAP, including cisplatin, doxorubicin, and high-dose methotrexate, was mainly used for the therapy of osteosarcoma,[31,33–39] while MAP+, used for the treatment of patients with metastatic disease, seemed to improve EFS.[40–42] However, the conclusion was supported in individual clinical trials and was still controversial in many randomized controlled trials.[22,23,24,43] So, we performed this analysis and clinical observation to verify whether MAP and MAP+ have significant differences on the survival of patients.
We took the evaluation of survival time of patients with osteosarcoma as the primary evaluation index. Seven randomized trials including 3433 participants met the inclusion criteria.[18–24] The characteristics of them were listed in Table 1. As each clinical trial was collected from a different research center, the heterogeneity among the data was inevitable. So, we took a REs model to deal with the data first. The addition of mifamurtide to the MAP chemotherapy regimen for patients with localized osteosarcoma resulted in improved OS.[24] A meta-analysis of the extracted data revealed that there was no statistically significant difference in 3EFS, 3OS, 5EFS, 5OS, and mortality rates among osteosarcoma patients when MAP was compared with MAP+ regimen (Fig. 1A–E). Funnel plots were provided in Supplemental Figure 1A–E. The value of I2 in Figure 1A–D displayed the existence of heterogeneity. Though Harbords test statistic did not suggest obvious publication bias in funnel plot, the heterogeneity is moderate among studies (Supplemental Fig. 1A–D). So, we just took RE model to deal with all the data (Fig. 1A–E). The FE model is not suitable for most of the data. The results of the comprehensive meta-analysis were consistent with the results obtained in each clinical trial.[18–24]
Compared with other combination chemotherapy regimens (MAP+), MAP chemotherapy regimen showed lower incidence rates of chemotherapy toxicities, especially in thrombocytopenia, febrile neutropenia, anemia, hypophosphatemia (Fig. 2B, C, E, and F) with statistical significant differences.[18–24] It meant that there was no significant difference in the prognosis survival of osteosarcoma between the MAP and MAP+ regimens, while the incidence of chemotherapy toxicities in MAP regimen was lower. The similar conclusion of OS time of patients, treated by MAP and MAP+ respectively, could be verified by the analysis of 115 patient's survival data (Fig. 3B). The data used for this meta-analysis represented a wide variety of sample sizes. It is the first time that 3-EFS, 3-OS, 5-EFS, 5-OS, mortality rates, and chemotherapy toxicities were evaluated together.
In recent years, many studies have shown that the development and prognosis of osteosarcoma is a pathological process involving multiple genes and factors. Although basic research on tumor markers has progressed rapidly in the field of osteosarcoma, treatment methods have changed little in clinical work.[1–5] Although various clinical trials for the treatment of osteosarcoma have been carried out gradually, there has been no significant improvement in the prognosis of patients with osteosarcoma.[18–24,44] The treatment of osteosarcoma seems to reach an apparent plateau. The development of strategies to better understand the pathological biology of this tumor might aid in the identification of drug targets.[45] In view of the success of PD-1 targeted drugs in the treatment of malignant melanoma,[46–48] we also hope for the emergence of new targeted drugs for osteosarcoma treatment.
5. Conclusions
Compared with other chemotherapy combination, MAP regimen remained the preferred option for osteosarcoma chemotherapy, which was verified by clinical observation.
5.1. Ethical approval
We declared that all procedures involved in this study about human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. The ethical issues involved in the article have been filed and approved by the corresponding hospital (Ethics Committee of Qianfoshan Hospital of Shandong Province). The chemotherapy regimens involved in the clinical data in the article are in line with the chemotherapy regimen recommended by the NCCN guidelines.
Author contributions
The corresponding author (Yuan Tian) had full access to all data in the study and all authors had final responsibility for the decision to submit for publication. Dapeng Yu, Shuisheng Zhang, Deguo Xu, and Alei Feng had the full data of the paper. Dapeng Yu, Alei Feng, and Yantao Mao were responsible for the collection of clinical data. Qingshan Zhu did the literature search, data gathering, and writing of the report. Yi Zhao, Yajuan Lv, Cuiping Han, and Rujun Liu helped with study design, data collection and checked the references.
Conceptualization: Yajuan Lv.
Data curation: Dapeng Yu, Shuisheng Zhang, Alei Feng, Deguo Xu, Qingshan Zhu, Yantao Mao, Yajuan Lv, Cuiping Han, Rujun Liu.
Formal analysis: Shuisheng Zhang, Qingshan Zhu, Yantao Mao, Rujun Liu.
Investigation: Shuisheng Zhang.
Methodology: Yajuan Lv.
Resources: Shuisheng Zhang, Alei Feng, Yajuan Lv, Cuiping Han.
Writing – original draft: Dapeng Yu, Yuan Tian.
Writing – review and editing: Yi Zhao, Yuan Tian.
Yuan Tian orcid: 0000-0002-2296-1246.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, FE = fixed effect, HR = hazard ratios, MAP = methotrexate, doxorubicin, and cisplatin, MAP+ = MAP plus ifosfamide and etoposide, or other adjuvant therapy drugs, NCCN = National Comprehensive Cancer Network, OR = odds ratio, RE = random effect, RR = risk ratio.
DY and SZ contributed equally to this work.
This study was funded by the Natural Science Foundation of Shandong Province (ZR2015HL078 and ZR2015CL010), Medical and Health Technology Development Plan Project of Shandong Province (2014WS0357), and the National Natural Science Foundation of China (No. 81502508), which were in charged by Yuan Tian (ZR2015HL078), Deguo Xu (ZR2015CL010, 2014WS0357), and Alei Feng (No. 81502508).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Li B, Wang Z, Wu H, et al. Epigenetic regulation of CXCL12 plays a critical role in mediating tumor progression and the immune response in osteosarcoma. Cancer Res 2018;78:3938–53. [DOI] [PubMed] [Google Scholar]
- [2].Sakthikumar S, Elvers I, Kim J, et al. SETD2 is recurrently mutated in whole-exome sequenced canine osteosarcoma. Cancer Res 2018;78:3421–31. [DOI] [PubMed] [Google Scholar]
- [3].Del Mare S, Husanie H, Iancu O, et al. WWOX and p53 dysregulation synergize to drive the development of osteosarcoma. Cancer Res 2016;76:6107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Flynn RL, Cox KE, Jeitany M, et al. Alternative lengthening of telomeres renders cancer cells hypersensitive to ATR inhibitors. Science 2015;347:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Garnett MJ, Edelman EJ, Heidorn SJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 2012;483:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jacob JA. Researchers turn to canine clinical trials to advance cancer therapies. JAMA 2016;315:1550–2. [DOI] [PubMed] [Google Scholar]
- [7].Gill M, McCarthy M, Murrells T, et al. Chemotherapy for the primary treatment of osteosarcoma: population effectiveness over 20 years. Lancet 1988;1:689–92. [DOI] [PubMed] [Google Scholar]
- [8].Blum RH. Simplified vs complex adjuvant chemotherapy schedule for osteosarcoma. Lancet 1997;350:900–1. [DOI] [PubMed] [Google Scholar]
- [9].Souhami RL, Craft AW, Van der Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet 1997;350:911–7. [DOI] [PubMed] [Google Scholar]
- [10].Takahashi Y, Yasui T, Tamari K, et al. Radiation enhanced the local and distant anti-tumor efficacy in dual immune checkpoint blockade therapy in osteosarcoma. PLoS One 2017;12:e0189697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Whelan JS, Davis LE. Osteosarcoma, chondrosarcoma, and chordoma. J Clin Oncol 2018;36:188–93. [DOI] [PubMed] [Google Scholar]
- [12].Claude L, Rousmans S, Carrie C, et al. Fédération nationale des centres de lutte contre le cancer (FNCLCC); Fédération hospitalière de France (FHF); Fédération nationale de cancérologie des CHRU (FNCCHRU); Fédération française de cancérologie des CHG (FFCCHG); centres régionaux de lutte contre le cancer (CRLCC); Société française de lutte contre les cancers de l’enfant et de l’adolescent (SFCE). Standards and options for the use of radiation therapy in the management of patients with osteosarcoma. Update 2004. Cancer Radiother 2005;9:104–21.15880886 [Google Scholar]
- [13].Eilber F, Giuliano A, Eckardt J, et al. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol 1987;5:21–6. [DOI] [PubMed] [Google Scholar]
- [14].Goorin AM, Schwartzentruber DJ, Devidas M, et al. Pediatric Oncology Group. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol 2003;21:1574–80. [DOI] [PubMed] [Google Scholar]
- [15].He A, Qi W, Huang Y, et al. Comparison of pirarubicin-based versus gemcitabine-docetaxel chemotherapy for relapsed and refractory osteosarcoma: a single institution experience. Int J Clin Oncol 2013;18:498–505. [DOI] [PubMed] [Google Scholar]
- [16].Martin-Broto J, Redondo A, Valverde C, et al. Gemcitabine plus sirolimus for relapsed and progressing osteosarcoma patients after standard chemotherapy: a multicenter, single-arm phase II trial of Spanish Group for Research on Sarcoma (GEIS). Ann Oncol 2017;28:2994–9. [DOI] [PubMed] [Google Scholar]
- [17].Boye K, Del Prever AB, Eriksson M, et al. High-dose chemotherapy with stem cell rescue in the primary treatment of metastatic and pelvic osteosarcoma: final results of the ISG/SSG II study. Pediatr Blood Cancer 2014;61:840–5. [DOI] [PubMed] [Google Scholar]
- [18].Gaspar N, Occean BV, Pacquement H, et al. SFCE (Société Française des Cancers de l’Enfant et l’adolescent); GSF-GETO (Groupe Sarcome Français); UNICANCER sarcoma group. Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study. Eur J Cancer 2018;88:57–66. [DOI] [PubMed] [Google Scholar]
- [19].Senerchia AA, Macedo CR, Ferman S, et al. Results of a randomized, prospective clinical trial evaluating metronomic chemotherapy in nonmetastatic patients with high-grade, operable osteosarcomas of the extremities: a report from the Latin American Group of Osteosarcoma Treatment. Cancer 2017;123:1003–10. [DOI] [PubMed] [Google Scholar]
- [20].Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol 2016;17:1396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Piperno-Neumann S, Le Deley MC, Rédini F, et al. Sarcoma Group of UNICANCER; French Society of Pediatric Oncology (SFCE); French Sarcoma Group (GSF-GETO). Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 2016;17:1070–80. [DOI] [PubMed] [Google Scholar]
- [22].Bielack SS, Smeland S, Whelan JS, et al. EURAMOS-1 Investigators. Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon Alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol 2015;33:2279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ferrari S, Ruggieri P, Cefalo G, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian sarcoma group trial ISG/OS-1. J Clin Oncol 2012;30:2112–8. [DOI] [PubMed] [Google Scholar]
- [24].Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005;23:2004–11. [DOI] [PubMed] [Google Scholar]
- [25].Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [26].Higgins J, Green R. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. 2009. Available at: http://handbook.cochrane.orgaccessed [Accessed on Jan 3, 2013]. [Google Scholar]
- [27].Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-analyses. 2009. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspaccessed [Accessed on July 6, 2012]. [Google Scholar]
- [28].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [29].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aguirre-Gamboa R, Gomez-Rueda H, Martínez-Ledesma E, et al. SurvExpress: an online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS One 2013;8:e74250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Smeland S, Bruland OS, Hjorth L. Results of the Scandinavian Sarcoma Group XIV protocol for classical osteosarcoma: 63 patients with a minimum follow-up of 4 years. Acta Orthop 2011;82:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lewis IJ, Nooij MA, Whelan J. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst 2007;99:112–28. [DOI] [PubMed] [Google Scholar]
- [33].Baum ES, Gaynon P, Greenberg L, et al. Phase II trail cisplatin in refractory childhood cancer: children's cancer study group report. Cancer Treat Rep 1981;65:815–22. [PubMed] [Google Scholar]
- [34].Gasparini M, Rouesse J, van Oosterom A. Phase II study of cisplatin in advanced osteogenic sarcoma. European Organization for Research on Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Cancer Treat Rep 1985;69:211–3. [PubMed] [Google Scholar]
- [35].Ochs JJ, Freeman AI, Douglass HO, et al. Cis-dichlorodiammineplatinum (II) in advanced osteogenic sarcoma. Cancer Treat Rep 1978;62:239–45. [PubMed] [Google Scholar]
- [36].Pratt CB, Roberts D, Shanks EC, et al. Clinical trials and pharmacokinetics of intermittent high-dose methotrexate-“leucovorin rescue” for children with malignant tumors. Cancer Res 1974;34:3326–31. [PubMed] [Google Scholar]
- [37].Cortes EP, Holland JF, Wang JJ. Amputation and adriamycin in primary osteosarcoma. N Engl J Med 1974;291:998–1000. [DOI] [PubMed] [Google Scholar]
- [38].Jaffe N, Frei E, Traggis D, et al. Adjuvant methotrexate and citrovorum-factor treatment of osteogenic sarcoma. N Engl J Med 1974;291:994–7. [DOI] [PubMed] [Google Scholar]
- [39].Pratt CB, Howarth C, Ransom JL. High-dose methotrexate used alone and in combination for measurable primary or metastatic osteosarcoma. Cancer Treat Rep 1980;64:11–20. [PubMed] [Google Scholar]
- [40].Kung FH, Pratt CB, Vega RA. Ifosfamide/etoposide combination in the treatment of recurrent malignant solid tumors of childhood. A Pediatric Oncology Group phase II study. Cancer 1993;71:1898–903. [DOI] [PubMed] [Google Scholar]
- [41].Gasparini M. High-dose ifosfamide alone and in combination for solid malignancies in childhood. Cancer Chemother Pharmacol 1986;18Suppl 2:S18. [DOI] [PubMed] [Google Scholar]
- [42].Goorin AM, Harris MB, Bernstein M. Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: a pediatric oncology group trial. J Clin Oncol 2002;20:426–33. [DOI] [PubMed] [Google Scholar]
- [43].Ferrari S, Mercuri M, Picci P. Nonmetastatic osteosarcoma of the extremity: results of a neoadjuvant chemotherapy protocol (IOR/OS-3) with high-dose methotrexate, intraarterial or intravenous cisplatin, doxorubicin, and salvage chemotherapy based on histologic tumor response. Tumori 1999;85:458–64. [DOI] [PubMed] [Google Scholar]
- [44].Marina N, Bielack S, Whelan J. International collaboration is feasible in trials for rare conditions: the EURAMOS experience. Cancer Treat Res 2009;152:339–53. [DOI] [PubMed] [Google Scholar]
- [45].Glover J, Krailo M, Tello T. A summary of the osteosarcoma banking efforts: a report from the Children's Oncology Group and the QuadW Foundation. Pediatr Blood Cancer 2015;62:450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016;315:1600–9. [DOI] [PubMed] [Google Scholar]
- [47].Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547:217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017;390:1853–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


