Abstract
Recently, apatinib has been shown to be effective in treating sarcoma. This study aimed to assess the safety and efficacy of apatinib in the treatment of patients with osteosarcoma after failed of standard multimodal therapy and to compare the therapeutic effects of apatinib on osteosarcoma between high-dose group and low-dose group.
A total of 27 patients with osteosarcoma who received apatinib between January 2016 and August 2017 were retrospectively reviewed. Among the 27 patients, the objective response rate (ORR) and the disease control rate (DCR) were 25.93% and 66.67%, respectively. The median of progression-free survival (m-PFS) was 3.5 months (95% confidence interval [CI], 2.5–4.8 months), and the median of overall survival (m-OS) was 9.5 months (95% CI, 7.8–10.5 months). There was no statistically significant difference in ORR (36.36% vs 18.75%), DCR (63.64% vs 68.75%), m-PFS (4.3 months [95% CI, 1.8–7 months) vs 3.35 months (95% CI, 1.8–4 months]), and m-OS (9.5 months [95% CI, 7.8–10.5 months] vs 9.4 months [95% CI, 7.8–10.8 months]) (P > .05) between the high-dose group (the average dose was 659 mg/qd) and the low-dose group (the average dose was 516 mg/qd). Most of the adverse events (AEs) were in grade 1 or grade 2. The main AEs in grade 3 were hypertension, rash, weight loss, hand-foot syndrome, and diarrhea.
Apatinib is safe and effective in the treatment of advanced osteosarcoma. We recommend that the initial dose of apatinib should be 500 mg/qd in the treatment of osteosarcoma.
Keywords: apatinib, low-dose administration, osteosarcoma, tyrosine-kinase inhibitor
1. Introduction
Osteosarcomas are the most common primary malignant bone tumors. This disease is rare, with an incidence of about 3 per million each year. About 75% of osteosarcomas occur in children, adolescents, and young adult.[1] Most osteosarcomas originate in the bones of the extremities and can metastasize through the blood. Majority of the metastases occur in the lungs, followed by the other bones.[2,3] Growth of osteosarcoma in the primary sites only leads to impaired limb function of the patients, and metastasis leads to death. In the recent 40 years, neoadjuvant chemotherapy and surgery have been the standard treatments for osteosarcoma. Each cycle of chemotherapy includes four drugs: doxorubicin, cisplatin, methotrexate, and isocyclophosphamide.[1] Two cycles of chemotherapy are generally performed before surgery, and four cycles of chemotherapy are performed after surgery. Surgery involves the removal of the primary lesion and the reconstruction of the limb function. The 5-year survival rate of initially diagnosed nonmetastatic osteosarcoma after standard treatment is 60%–70%.[1–5] Approximately 30% of initially diagnosed nonmetastatic osteosarcomas has disease recurrence and distant metastasis within 2 years after surgery. Radiotherapy is not effective in the treatment of osteosarcoma. Patients with these metastases have no choice but to continue undergoing standard chemotherapy. If chemotherapy fails, or if the patient cannot tolerate continued chemotherapy, the metastatic lesions can grow freely. Therefore, the 5-year survival rate of metastatic osteosarcoma is less than 10%.[2,4] The treatment of these patients with metastatic osteosarcoma has not progressed much over the years. The invention of targeted drugs provides a new method for the treatment of metastatic osteosarcoma.[1,6–9] One of the targeted drugs is “apatinib,’, which is the first generation of oral antiangiogenesis drug invented in China.
Apatinib is a small molecule tyrosine kinase inhibitor (TKI) that highly and selectively targets vascular endothelial growth factor receptor 2 (VEGFR-2), leading to the inhibition of VEGF-mediated endothelial cell migration and proliferation and decrease in tumor microvascular density. Additionally, it had been approved in China for the treatment of advanced or metastatic gastric cancer.[10] Currently, some studies have confirmed that apatinib is effective in the treatment of bone and soft tissue sarcomas. For instance, Li et al reported that apatinib exhibited objective efficacy and manageable toxicity in stage IV sarcoma patients who failed in chemotherapy.[11] Study of Zhu et al suggested that apatinib showed promising efficacy and acceptable safety profile in metastatic or recurrent sarcoma.[9] A multiple institutions’ off-label use of apatinib showed that apatinib might be effective, with a high objective response rate (ORR), in patients with previously treated advanced or metastatic soft tissue sarcoma. The duration of response was consistent with the previous reports in different subtypes of sarcomas.[6]
Because other targeted drugs that have been proven to be effective in treating sarcoma, for example, sorafenib and pazopanib, have not been officially available on the market in China, patients with advanced sarcomas are typically orally administered with apatinib in China. Based on the abovementioned conditions, and in order to further confirm the therapeutic effects of apatinib in treating osteosarcoma, in this study, the clinical data of 27 patients with osteosarcoma who off-label used apatinib were reviewed, and the efficacy and complications were summarized and analyzed.
2. Materials and methods
2.1. Patients
From January, 2016 to August, 2017, 27 patients received apatinib for the treatment of osteosarcoma. All patients met the following criteria: first, histologically confirmed osteosarcoma; second, presence of multiple metastases after the performance of standard multimodal therapy; third, presence of multiple metastatic lesions that could not be cured by local therapy; fourth, absence of treatments received with other targeted drugs; fifth, acceptable hepatic, hematologic, and renal function; sixth, an Eastern Cooperative Oncology Group performance status (ECOG) score of grade 0 or grade 1;[12] and seventh, measurable lesions according to the Response Evaluation Criteria in Solid Tumors (RECIST).[13]
This study was approved by the Institutional Review Board of the Ethics Committee for Clinical Investigation of The Affiliated Cancer Hospital of Zhengzhou University (Zhengzhou, Henan Province, China). All patients or children's legal parent had signed informed consent for data collection and research purposes.
2.2. Drug treatment
During the first stage, patients were classified as the high-dose (HD) group. Patients with body surface area (BSA) greater than 1.5 m2 orally received 750 mg/qd of apatinib (Jiangsu Hengrui Medicine, Lianyungang, China) once daily, and patients with BSA less than 1.5 m2 received 500 mg/qd of apatinib daily. Administered dose of apatinib was reduced to 500 mg/qd if BSA was greater than 1.5 m2 and 250 mg/qd if BSA was less than 1.5 m2 for patients who cannot tolerate the AEs.
During the second stage, patients were classified as the low-dose (LD) group. Patients with BSA greater than 1.5 m2 received 500 mg/qd of apatinib, and patients with BSA less than 1.5 m2 received 250 mg/qd of apatinib. Administered dose of apatinib was reduced to 250 mg/qd if BSA was greater than1.5 m2 and 125 mg/qd if BSA was less than 1.5 m2 for patients who cannot tolerate the AEs. The administered dose of apatinib for patients who experienced progressive disease (PD) increased to 750 mg/qd if BSA was greater than 1.5 m2 and 500 mg/qd if BSA was less than 1.5 m2.
2.3. Evaluation
Tumor response was assessed every 1 or 2 months by using computed tomography or magnetic resonance imaging, and was categorized as complete response (CR), partial response (PR), stable disease (SD), and PD according to RECIST criteria. the main concern was the differences including the ORR, the disease control rate (DCR), the median progression free survival (m-PFS), and the median overall survival (m-OS) between the HD group and LD group. PFS was defined as the time from the initiation of apatinib to the occurrence of PD or death, whichever occurred first. OS was defined as the duration from the time of treatment administration to the time of death of any cause or the last follow-up. All adverse events (AEs) were classified and graded based on the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.[14]
2.4. Statistical analysis
All the statistical analyses were performed using SPSS 21.0 software for Windows (IBM, Armonk, NY). PFS and OS were estimated using the Kaplan–Meier method, with 95% confidence interval (CI). Group-wise comparison used Fisher's exact test and Wilcoxon rank sum test with continuity correction. Quantitative variables were presented as median (range) or number of patients (percentage). All statistical analyses were two-sided, and P value less than .05 was considered statistically significant. The database was locked for statistical analysis in July 2018, and this is a descriptive analysis.
3. Results
3.1. Patients’ characteristics
A total of 27 patients with advanced osteosarcoma after failed standard multimodal therapy underwent apatinib treatment from January 2016 to August 2017. All the patients underwent complete standard chemotherapy for osteosarcoma before apatinib administration. The chemotherapy regimen included the following drugs: methotrexate, cisplatin, doxorubicin, and ifosfamide.[5] Patients without metastasis before the operation underwent limb salvage or amputation. The remaining patients did not undergo surgical resection of the primary tumor.
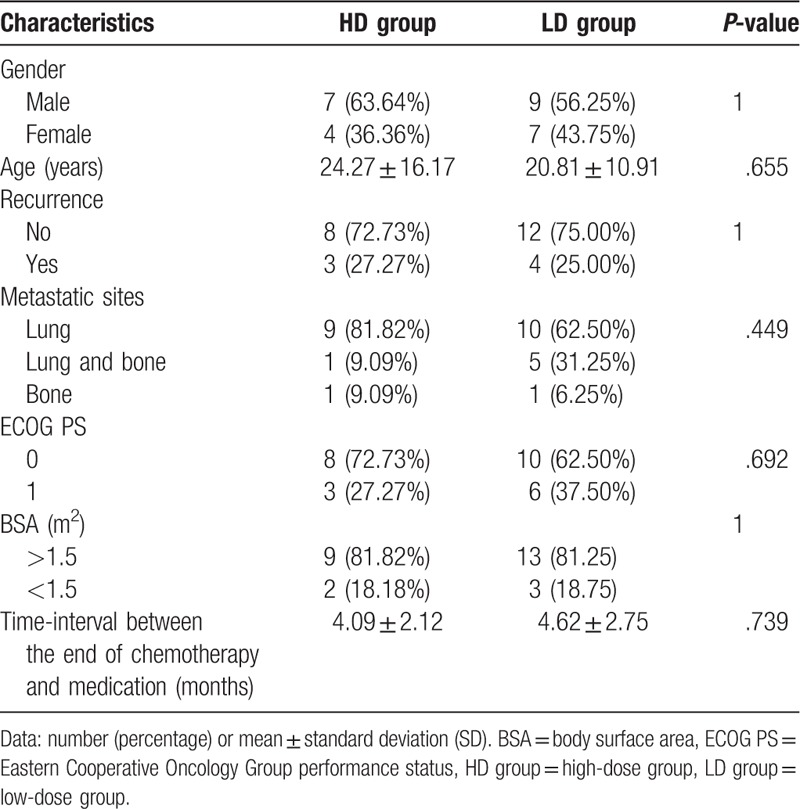
There were 11 patients in the HD group, including 7 males (63.64%) and 4 females (36.34%). The average age of the patients was 24.27 ± 16.17 years. Three patients (27.27%) suffered from disease recurrence in the primary sites. Most of the metastases were in the lungs (90.91%), and only one patient (9.09%) had metastasis in the other bones. The ECOG performance status in eight patients (72.73%) was grade 0, and the remaining was grade 1. The average time interval from the end of chemotherapy to the start of oral administration of apatinib was 4.09 ± 2.12 months.
There were 16 cases in the LD group, including 9 males (56.25%) and 7 females (43.75%). The average age of patients was 20.81 ± 10.91 years. Four patients (25.00%) developed disease recurrence in the primary sites. Most of the metastases were in the lungs (93.75%), and only one patient (6.25%) had metastasis in the other bones. The ECOG performance status in 10 patients (62.50%) was grade 0, and the remaining was grade 1. The average time interval from the end of chemotherapy to the start of oral administration of apatinib was 4.62 ± 2.75 months. Comparison of the basic characteristics of patients between the HD group and LD group is shown in Table 1. Comparison of various characteristics revealed that there was no statistically significant difference between the two groups.
Table 1.
Comparing the basic characteristics of patients.
3.2. Efficacy of therapy
Among the 27 patients, although no patient achieved CR, 7 (25.93%) patients had PR, and 11 patients (40.74%) had SD. These resulted in an ORR of 25.93% and a DCR of 66.67%. m-PFS was 3.5 months (95% CI, 2.5–4.8 months) and m-OS was 9.5 months (95% CI, 7.8–10.5 months).
There was a difference in efficacy between the HD group and LD group (Table 2 and Fig. 1). In the HD group, ORR was 36.36%, DCR was 63.64%, m-PFS was 4.3 months (95% CI, 1.8–7 months), and m-OS was 9.5 months (95% CI, 3.8–12 months). Additionally, in the LD group, ORR was 18.75%, DCR was 68.75%, m-PFS was 3.35 months (95% CI, 1.8–4 months), and m-OS was 9.4 months (95% CI, 7.8–10.8 m). There were no statistically significant differences between patients with different characteristics in these two groups (Table 2).
Table 2.
Clinical efficacy of the two groups.
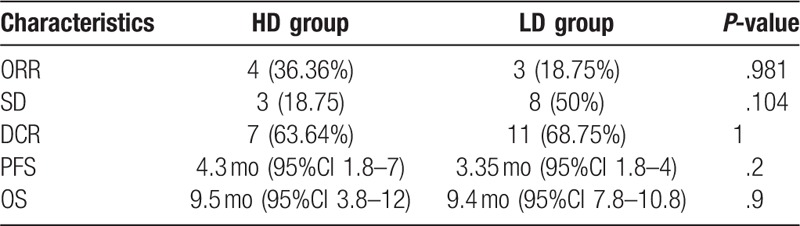
Figure 1.
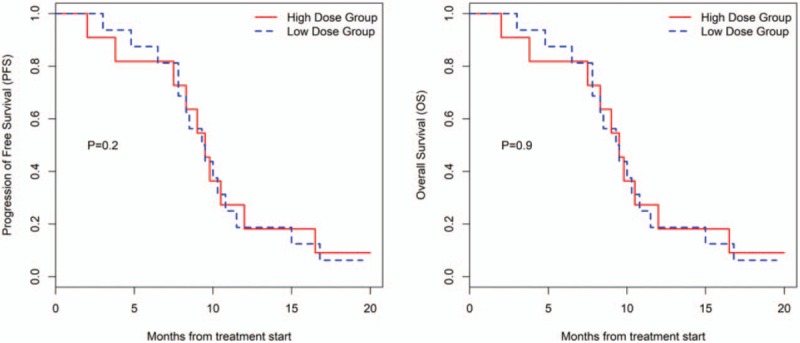
Kaplan–Meier estimates of progression free survival and overall survival for different groups.
3.3. Toxicity and safety
In the HD group, the dose was reduced to 500 mg/qd for patients who cannot tolerate AEs. Additionally, two patients required temporary treatment discontinuation for toxicity management. The average dose administered was 659 mg/qd. In the LD group, four patients required temporary treatment discontinuation for toxicity management. In this group, none of the patients required dose reduction, and even four patients required an increased dose of 500 or 750 mg/qd due to the occurrence of PD. The average dose administered was 516 mg/qd in this group.
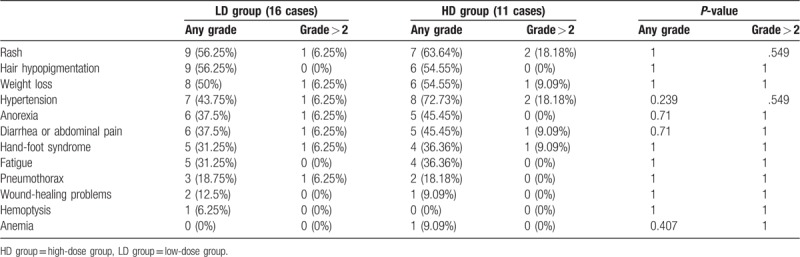
Moreover, AEs occurred in 21 (77.8%) patients. Majority of the patients experienced grade 1 or grade 2 AEs, only a few patients experienced grade 3 AEs, and none of the patients experienced grade 4 AEs or drug-related death. AEs include fatigue, hypertension, hand-foot syndrome, diarrhea, weight loss, hair hypopigmentation, anorexia, rash or desquamation, mucositis, pneumothorax, and wound-healing problems (Table 3). There were no statistically significant differences between patients in AEs between patients in the two groups (Table 3).
Table 3.
Comparison of complications.
4. Discussion
The multi-drug chemotherapy, including cisplatin, doxorubicin, methotrexate, and ifosfamide that has been popularized since the 1980s has increased the 5-year survival rate of osteosarcoma from 20% to about 70%. However, in the recent 20 years, different scholars have universally worked on further improving the efficacy of osteosarcoma, in which minimal achievements have been obtained.[1,15] With the increased of application of antiangiogenic therapy, there is a new treatment method for patients with advanced osteosarcoma after failed standard multimodal therapy. One of the drugs that has definite effects in treating osteosarcoma is second-generation broad-spectrum VEGF-receptor TKIs.[15–17]
Extensive studies established that: first, VEGF is a key driver of sprouting angiogenesis, second, VEGF is overexpressed in most solid malignant tumors, and third, inhibition of VEGF can suppress tumor growth in animal models. This has led to the development of pharmacological agents for antiangiogenesis therapy through the blockade of VEGF/VEGF receptor signaling to disrupt the vascular supply and starve the tumor of oxygen and nutrients. TKI is one of the pharmacological agents. Multiple TKIs have been approved by a single therapy in specific indications based on improvement of OS or PFS in phase III clinical trials. These include sorafenib, sunitinib, axitinib, regorafenib, pazopanib, vandetanib, cabozantinib, and lenvatinib.[17] Drugs that have been proven to be effective in treating osteosarcoma include sorafenib, sunitinib, regorafenib, and pazopanib.[7,8,18,19] Drugs that have been proven to be effective on osteosarcoma include sorafenib, sunitinib, and pazopanib.[8,15,20,21] In 2012, Grignani et al reported a phase II clinical trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy. The results showed that ORR was 14%, DCR was 48%, and m-PFS was 4 months.[8] This is a milestone because no other antiangiogenic drug has been able to achieve such a high level of efficacy. Several case reports demonstrated the response of patients with osteosarcoma to sunitinib and pazopanib. Penel-Page et al treated five patients with advanced osteosarcoma by sunitinib. The results showed that the treatment was effective for two patients (one patient obtained PR and one patient obtained SD). Median duration of response was 3.4 months.[20] Safwat et al reported that three patients with advanced osteosarcoma obtained SD after the oral administration of pazopanib.[22]
Same with the abovementioned three targeted drugs that have effects on osteosarcoma, apatinib is one of the TKIs. Relevant in vitro studies revealed that apatinib was a highly selectively inhibitor of the following: VEGFR2 (50% inhibitory concentration [IC50], 2 nmol/L), VEGFR1 (IC50, 70 nmol/L), platelet-derived growth factor receptor beta (537 nmol/L), c-Kit (IC50, 429 nmol/L), c-Src (530 nmol/L), and RET (13 nmol/L) tyrosine kinase.[23,24] In December 2014, apatinib was approved to be available on the market in China to treat advanced gastric cancer. Thereafter, a large number of studies have confirmed that apatinib is effective in several solid tumors (Table 4).[6,9,11,25–38] The doses of apatinib widely vary for different malignant tumors, which were treated by different researchers. Currently, it has been proven that the effective dose of apatinib is generally between 250 and 850 mg/d (Table 4).
Table 4.
Previous studies about apatinib on different malignant tumors.
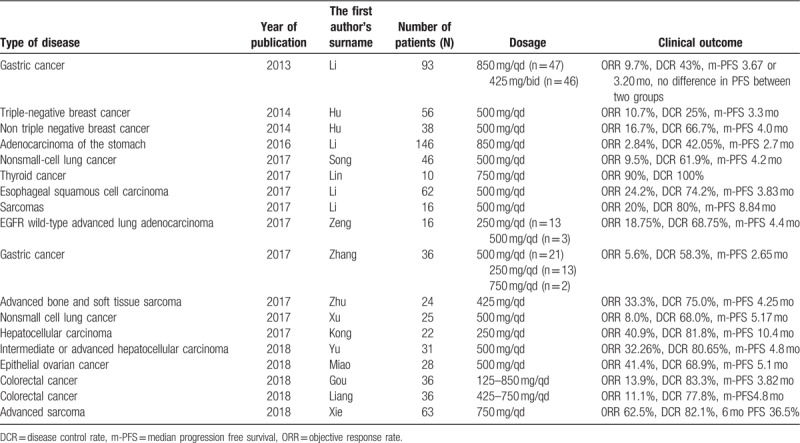
In addition to the clinical application of apatinib in bone and soft tissue sarcomas as listed in Table 4, some basic studies suggested that apatinib plays a key role in osteosarcoma cells in vitro. Liu et al's study revealed that apatinib promoted autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma.[39] Zheng et al believed that apatinib inhibited tumor migration and invasion, as well as PD-L1 expression in osteosarcoma by targeting STAT3.[40] Based on the abovementioned studies, our treatment center performed oral administration of apatinib for patients with advanced osteosarcoma after failed standard therapy. To determine the standard dose of apatinib and to obtain better therapeutic effects, patients in the first stage of our study were orally received apatinib at a higher dose (750 mg/qd for patients with BSA > 1.5 m2 and 500 mg/qd for patients with BSA < 1.5 m2). With the accumulation of cases, we observed that the side effects were serious when the dose of apatinib was 750 mg/qd. Thus the administered dose was reduced for many patients. Several recent studies reported that the dose of 500 mg/qd can also result in better clinical effects (Table 4). Thus, the administered dose in the second stage was adjusted (500 mg/qd for patients with BSA > 1.5 m2 and 250 mg/qd for patients with BSA < 1.5 m2). Moreover, the dose should be increased to 750 or 500 mg/qd when PD occurred. The results confirmed that apatinib was effective for osteosarcoma at the two doses. In the results, ORR and DCR significantly increased compared to the results of phase II clinical trial of sorafenib in the treatment of osteosarcoma as previously reported by Grignani et al suggesting that apatinib had a stronger effect in treating osteosarcoma than sorafenib.[8] However, the level of evidence in this study was low. Further phase II multi-center studies should be conducted to determine whether apatinib is superior to sorafenib in the treatment of osteosarcoma.
The results of the study by Bacci et al revealed that the ORR of standard chemotherapy for initially metastatic osteosarcoma was 61%,[41] significantly higher than that of apatinib (26%) in this study and that of sorafenib (14%) in the treatment of osteosarcoma as previously reported by Grignani et al.[8] This indicates that the effects of these multiple TKIs in treating osteosarcoma are far lower than that of standard first-line chemotherapy. These drugs can only be used as second- or more line treatment when used alone in treating osteosarcomas.
In this study, ORR in the HD group was higher compared to the LD group (4 [36.36%] vs 3 [18.75%]), as shown in Table 2. Although there was no statistically significant difference (P = .391) between the two groups, it can be seen that ORR seemed to be positively correlated with doses when apatinib was used in the treatment of osteosarcoma. Other relevant reports also showed that ORR was associated with dose when apatinib was used to treat sarcomas. Study of Xie et al showed that ORR was up to 62.5% on bone and soft-tissue sarcomas when the administered dose of apatinib was 750 mg/qd.[6] Zhu et al's research showed that ORR was 33.3% on bone and soft-tissue sarcomas when the administered dose of apatinib was 425 mg/qd.[9] In this study, after PD occurred in some patients in the LD group, SD can be observed when the dose of apatinib was increased. Although PFS in these cases was short, OS was not shortened accordingly, leading to a smaller difference in OS compared to the PFS between the two groups (Table 2). Results of this study suggested that the therapeutic effects of the initial dose of 500 mg/qd were not statistically significant compared to the dose of 750 mg/qd when apatinib was used in the treatment of osteosarcoma (Table 2, Fig. 1). It is obvious that the cost-effect ratio at low-level dose was lower than that of high-level dose, which was more cost-efficient for patients.
Same with other targeted drugs, the main AEs of apatinib include hypertension, rash, hair hypopigmentation, weight loss, anorexia, diarrhea or abdominal pain, hand-foot syndrome, fatigue, pneumothorax, and wound-healing problems, etc.[6–8,15,18,25] The incidence rates of complications were different in various dose-based groups. Results of this study showed that the most common AEs were hypertension (with the incidence rate of 72.73%) in the HD group (659 mg/qd) and rash (56.25%) in the LD group (516 mg/qd) (Table 3). We also found that the incidence rate of various AEs increased at high dose. Although the incidence rates of AEs were not statistically significant, we cannot deny that AEs were positively correlated with dose.[10] Report of Zhu et al showed that the incidence rates of the three most common AEs were fatigue (54.8%), hypertension (45.2%), and hand-foot syndrome (38.7%), respectively, when the administered dose was 425 mg/qd.[9] Xie et al reported that the incidence rates of the three most common AEs were hypertension (77.8%), rash or desquamation (57.8%), and hair hypopigmentation (55.6%), respectively, when the administered dose increased to 750 mg/qd.[6] These results suggested that the AEs of apatinib were positively correlated with dose. In some patients in the LD group, the dose of apatinib was increased when PD occurred. However, we did not observe a significant increase in AEs. This may be related to drug tolerance.
This study was a single-center retrospectively analysis without blank control and its sample size was small, decreasing the level of evidence. A phase II multicenter clinical study of apatinib in the treatment of bone and soft tissue sarcoma should be performed in the near future. And studies comparing apatinib with other similar drugs should also be conducted.
In summary, apatinib is effective for the treatment of osteosarcoma. In this study, ORR, DCR, PFS, and OS were not statistically significant with the following administered doses of apatinib: 500 and 750 mg/qd. The advantage of initial low-dose administration of apatinib was that it can reduce treatment cost; therefore, we suggested that the initial dose of apatinib should be 500 mg/qd in the treatment of osteosarcoma.
Author contributions
Conceptualization: Jiaqiang Wang.
Data curation: Zhiyuan Gu, Jiaqiang Wang, Qiqing Cai.
Formal analysis: Zhiyuan Gu, Jiaqiang Wang, Qiqing Cai.
Funding acquisition: Qiqing Cai.
Investigation: Zhiyong Liu.
Methodology: Xin Wang, Zhiyong Liu.
Project administration: Xin Wang, Zhiyong Liu.
Resources: Xin Wang.
Supervision: Weitao Yao.
Validation: Weitao Yao.
Visualization: Weitao Yao, Peng Zhang.
Writing – original draft: Zhichao Tian, Peng Zhang.
Writing – review & editing: Hong Ge.
Footnotes
Abbreviations: AEs = adverse effects, BSA = body surface area, CI = confidence interval, CR = complete response, CTCAE = the Common Terminology Criteria for Adverse Events, DCR = disease control rate, ECOG = Eastern Cooperative Oncology Group performance status, HD = high-dose, LD = low-dose, ORR = objective response rate, OS = overall survival, PD = progressive disease, PFS = progression free survival, PR = partial response, RECIST = response evaluation criteria in solid tumor, SD = stable disease, TKI = tyrosine kinase inhibitor, VEGFR-2 = vascular endothelia growth factor receptor-2.
The study was approved by the ethics committee of the Ethics Committee of The Affiliated Cancer Hospital of Zhengzhou University (Changsha, China).
The authors declare that they have no competing interests.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Harrison DJ, Geller DS, Gill JD, et al. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther 2018;18:39–50. [DOI] [PubMed] [Google Scholar]
- [2].Ferrari S, Briccoli A, Mercuri M, et al. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J Clin Oncol 2003;21:710–5. [DOI] [PubMed] [Google Scholar]
- [3].Bhattasali O, Vo AT, Roth M, et al. Variability in the reported management of pulmonary metastases in osteosarcoma. Cancer Med 2015;4:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kleinerman E. Maximum benefit of chemotherapy for osteosarcoma achieved-what are the next steps? Lancet Oncol 2016;17:1340–2. [DOI] [PubMed] [Google Scholar]
- [5].Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol 2005;23:8845–52. [DOI] [PubMed] [Google Scholar]
- [6].Xie L, Guo W, Wang Y, et al. Apatinib for advanced sarcoma: results from multiple institutions’ off-label use in China. BMC Cancer 2018;18:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86. [DOI] [PubMed] [Google Scholar]
- [8].Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol 2012;23:508–16. [DOI] [PubMed] [Google Scholar]
- [9].Zhu B, Li J, Xie Q, et al. Efficacy and safety of apatinib monotherapy in advanced bone and soft tissue sarcoma: an observational study. Cancer Biol Ther 2018;19:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Scott LJ. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs 2018;78:747–58. [DOI] [PubMed] [Google Scholar]
- [11].Li F, Liao Z, Zhao J, et al. Efficacy and safety of Apatinib in stage IV sarcomas: experience of a major sarcoma center in China. Oncotarget 2017;8:64471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Park CM, Koh Y, Jeon K, et al. Impact of Eastern Cooperative Oncology Group Performance Status on hospital mortality in critically ill patients. J Crit Care 2014;29:409–13. [DOI] [PubMed] [Google Scholar]
- [13].Schwartz LH, Seymour L, Litière S, et al. RECIST 1.1—standardisation and disease-specific adaptations: perspectives from the RECIST Working Group. Eur J Cancer 2016;62:138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang S, Liang F, Tannock I. Use and misuse of common terminology criteria for adverse events in cancer clinical trials. BMC Cancer 2016;16:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xie L, Ji T, Guo W. Anti-angiogenesis target therapy for advanced osteosarcoma (review). Oncol Rep 2017;38:625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Itatani Y, Kawada K, Yamamoto T, et al. Resistance to anti-angiogenic therapy in cancer-alterations to anti-VEGF pathway. Int J Mol Sci 2018;19:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zirlik K, Duyster J. Anti-angiogenics: current situation and future perspectives. Oncol Res Treat 2018;41:166–71. [DOI] [PubMed] [Google Scholar]
- [18].Mahmood ST, Agresta S, Vigil CE, et al. Phase II study of sunitinib malate, a multitargeted tyrosine kinase inhibitor in patients with relapsed or refractory soft tissue sarcomas. Focus on three prevalent histologies: leiomyosarcoma, liposarcoma and malignant fibrous histiocytoma. Int J Cancer 2011;129:1963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Berry V, Basson L, Bogart E, et al. REGOSARC: regorafenib versus placebo in doxorubicin-refractory soft-tissue sarcoma—a quality-adjusted time without symptoms of progression or toxicity analysis. Cancer 2017;123:2294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Penel-Page M, Ray-Coquard I, Larcade J, et al. Off-label use of targeted therapies in osteosarcomas: data from the French registry OUTC'S (Observatoire de l’Utilisation des Therapies Ciblees dans les Sarcomes). BMC Cancer 2015;15:854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Umeda K, Kato I, Saida S, et al. Pazopanib for second recurrence of osteosarcoma in pediatric patients. Pediatr Int 2017;59:937–8. [DOI] [PubMed] [Google Scholar]
- [22].Safwat A, Boysen A, Lucke A, et al. Pazopanib in metastatic osteosarcoma: significant clinical response in three consecutive patients. Acta Oncol 2014;53:1451–4. [DOI] [PubMed] [Google Scholar]
- [23].Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Roviello G, Ravelli A, Polom K, et al. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 2016;372:187–91. [DOI] [PubMed] [Google Scholar]
- [25].Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961–9. [DOI] [PubMed] [Google Scholar]
- [26].Miao M, Deng G, Luo S, et al. A phase II study of apatinib in patients with recurrent epithelial ovarian cancer. Gynecol Oncol 2018;148:286–90. [DOI] [PubMed] [Google Scholar]
- [27].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [28].Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [30].Lin Y, Wang C, Gao W, et al. Overwhelming rapid metabolic and structural response to apatinib in radioiodine refractory differentiated thyroid cancer. Oncotarget 2017;8:42252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Song Z, Yu X, Lou G, et al. Salvage treatment with apatinib for advanced non-small-cell lung cancer. Onco Targets Ther 2017;10:1821–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li J, Wang L. Efficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinoma. Onco Targets Ther 2017;10:3965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zeng DX, Wang CG, Lei W, et al. Efficiency of low dosage apatinib in post-first-line treatment of advanced lung adenocarcinoma. Oncotarget 2017;8:66248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang Y, Han C, Li J, et al. Efficacy and safety for Apatinib treatment in advanced gastric cancer: a real world study. Sci Rep 2017;7:13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xu J, Liu X, Yang S, et al. Clinical response to apatinib monotherapy in advanced non-small cell lung cancer. Asia Pac J Clin Oncol 2018;14:264–9. [DOI] [PubMed] [Google Scholar]
- [36].Kong Y, Sun L, Hou Z, et al. Apatinib is effective for treatment of advanced hepatocellular carcinoma. Oncotarget 2017;8:105596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gou M, Si H, Zhang Y, et al. Efficacy and safety of apatinib in patients with previously treated metastatic colorectal cancer: a real-world retrospective study. Sci Rep 2018;8:4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liang L, Wang L, Zhu P, et al. A pilot study of apatinib as third-line treatment in patients with heavily treated metastatic colorectal cancer. Clin Colorectal Cancer 2018;17:e443–9. [DOI] [PubMed] [Google Scholar]
- [39].Liu K, Ren T, Huang Y, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis 2017;8:e3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zheng B, Ren T, Huang Y, et al. Apatinib inhibits migration and invasion as well as PD-L1 expression in osteosarcoma by targeting STAT3. Biochem Biophys Res Commun 2018;495:1695–701. [DOI] [PubMed] [Google Scholar]
- [41].Bacci G, Briccoli A, Rocca M, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol 2003;14:1126–34. [DOI] [PubMed] [Google Scholar]


