Abstract
To investigate the epidemiology trend and characteristics of sepsis-related hospitalizations in Taiwan, and to compare the differences among different severity levels of sepsis.
This study is a retrospective national claim database analysis. Hospitalized adult patients with sepsis between 2010 and 2014 were identified from the Two-Million-Sample Longitudinal Health and Welfare Database (LHWD) by the International Classification of Diseases 9th Edition Clinical Modification (ICD-9-CM). The patients were divided into 3 severity groups based on their medical records during hospitalization.
The study results showed that in Taiwan, there were 643 new cases of sepsis in 100,000 Taiwanese. The mortality of all septic patients in Taiwan was 287 per 100,000 people, and the case fatality was 29.2%. It was found that the mortality and incidence of sepsis in Taiwan have increased year by year, but there has been no significant change over time. In addition, demographic variation exists in the epidemiology of sepsis. In all the rates investigated, the men's were higher than the women's and the elderly's were higher than the youths’. The analysis results also showed that the respiratory system was the most common site of organ failure in septic patients.
The incidence and mortality of any severity level of sepsis were 643, and 287 per 100,000 people in Taiwan, respectively, and the average case fatality was 29.2% during the study period (2010–2014). The respiratory system was the major infected site and site of organ dysfunction, especially in the more severe levels.
Keywords: epidemiology, sepsis, septic shock, severe sepsis, Taiwan
1. Introduction
Sepsis is a common, life-threatening clinical condition in most hospitals worldwide and a major cause of mortality for critically ill patients. Despite advances in treatment, sepsis remains a huge burden for many countries. There were 31.5 million sepsis cases, 19.4 million severe sepsis cases, and up to 5.3 million deaths in the world annually.[1] In the US, the prevalence of all septic patient in 2003 and 2009 was 359 and 535 per 100,000 people, respectively, and the prevalence had increased 6.9% per year.[2] Additional studies’ findings indicated that the prevalence of severe sepsis has increased year by year in the US by a rate of 3.0% to 16.5% annually.[3–5] Unlike prevalence, however, case fatality was found to decrease annually.[3,6]
Age and gender have been found to be associated with sepsis. Several studies reported that there were more male than female septic patients.[3–5] In 2007, the prevalence of severe sepsis in males and females was 308 and 292 per 100,000 people, respectively, in the US.[6] In addition, it was reported that the mean age of septic patients was 68 years old,[5] and the proportion of the elderly in septic patients was over 60%.[4,6]
The epidemiology of sepsis changes over time and varies among countries. In one of the few studies of sepsis in Taiwan, Shen et al used the longitudinal National Health Insurance Research Database (NHIRD) to investigate severe sepsis in Taiwan from 1997 to 2006[27] and found that the incidence increased from 135 to 217 per 100,000 people.[27] More recently, Liu et al used the National Health Insurance Research Database (NHIRD) to investigate the epidemiology of sepsis in Taiwan in 2013, and the study results showed that the hospital mortality rate was 19.3% and that the most common site of organ dysfunction was in the respiratory system.[7] However, there is no study that has investigated the temporal change of sepsis after 2006 or compared different severity levels of sepsis in Taiwan. As such, in this study, we updated the temporal change and the epidemiology information of sepsis in Taiwan in order to fill this information gap and to provide a reference to decision makers for the development of health care policy and disease prevention. Moreover, this is the first study that has investigated 3 severity levels of sepsis in Taiwan simultaneously, and the first one that has included septic shock in the analysis.
2. Methods
This is a retrospective claim database study on the epidemiology of sepsis in Taiwan. Hospitalized adult patients with sepsis between 2010 and 2014 were identified from the Health and Welfare Database by the International Classification of Diseases 9th Edition Clinical Modification (ICD-9-CM) codes. These patients were further divided into 3 levels of severity based on their medical records during hospitalization.
2.1. Data source
The Taiwanese government provides compulsory national health insurance that covers over 99% of residents in Taiwan[8,9]. This system is now operated by the Ministry of Health and Welfare. The database “Two-Million-Sample Longitudinal Health and Welfare Database (LHWD) of 2010” used in this study was provided by the Health and Welfare Data Science Center. The LHWD 2010 stratified the total population in Taiwan according to the Household Registration File of the Ministry of the Interior by gender, age, and household registration in 2010. Stratified random sampling was then conducted proportional to the individuals in each stratum, and a drawing of a total of 2 million people, or 8.6% of the total population, was done.[10,11] The LHWD 2010 provided the claim records and other medical information about the sampled individuals back to 2000 (2000∼2009) and also followed them for 6 years (2010∼2015). Four files in the LHWD 2010 dataset were used in this study: Ambulatory Care Expenditures by Visits, Inpatient Expenditures by Admissions, Registry for Beneficiaries, and Cause of Death Data. These 4 files can be linked with each other to provide complete information about patients.
This study was approved by the Taipei Medical University Joint Institutional Review Board (TMU-JIRB-N201708040). Informed consent was not required since all data were encrypted.
2.2. Patients
To be selected for analysis, a patient needed to be hospitalized between 2010 and 2014. Other inclusion criteria were being an adult (i.e., ≥20 years old on the admission date) and suffering from any severity of sepsis during the hospitalization. In addition, a patient was excluded if he or she was hospitalized without a discharge record or with missing sex or age information. Multiple hospital admissions of a single patient were included in the analysis as long as they met the selection criteria. However, 2 admissions were regarded as the same admission if the patient was re-hospitalized within 2 days of discharge. It is also important to note that throughout the study we used “sepsis-related” to mean that sepsis was documented at some point in a hospitalization even though it may not have been the main reason for admission.
Due to a lack of laboratory data in the study database, ICD-9-CM codes were used to identify patients with sepsis:
-
(1)
“038.xx” or
-
(2)
“995.91” in combination with an infection code (Supplementary Table 1).[12]
According to the ICD-9-CM official Guidelines, the codes “038.xx” and “995.91” are used for sepsis whereas “995.92” and “758.52” are used for severe sepsis and septic shock, respectively.[13] These codes have been used in previous studies.[14–16] However, we did not use “995.92” and “758.52” to identify patients with severe sepsis and septic shock because these codes were not found in the study database. Instead, following the study by Angus et al,[17] patients who had both an infection and an acute organ dysfunction (Supplementary Table 2)[18] were regarded as having severe sepsis, which definition was used in previous studies of sepsis.[6,12,17,18] In addition, those patients with sepsis or severe sepsis identified who were administered norepinephrine or experienced a shock (i.e., ICD9 code of “785.50”, “785.52”, or “785.59”) during hospitalization were considered to have a septic shock.[19,20] If a hospitalization admission met the definitions of more than 1 severity level of sepsis, it was assigned to the most severe group.
Furthermore, in order to investigate temporal change, we divided all sepsis-related hospitalizations into 5 one-year periods (2010–2014) based on admission date. Patients were also divided into 14 age groups in intervals of 5 years (i.e., 20∼24, 25∼30…, 75∼84, 85+) to assess outcome variation by age.
2.3. Outcomes
Crude rates were calculated for all septic patients and the 3 severity groups, including incidence, mortality, and case fatality. Incidence was the proportion of new cases of sepsis without diagnosis of any severity level of sepsis in the previous year in the total adult population in Taiwan. The proportion of septic patients that died within 3 days after discharge in the total adult population was calculated as mortality, and case fatality was defined as the proportion of septic patients who died within 3 days after discharge.
In addition, the temporal change and demographic (age and gender) variation of these rates were examined. If a patient had multiple hospital admissions involving different severity levels of sepsis in a year, all of the admissions were included in the analysis. However, if a patient had multiple hospital admissions involving the same severity level of sepsis in a year, 1 admission was randomly selected for the analysis in that particular year. Moreover, the characteristics of sepsis-related admissions examined included infection site (Supplementary Table 3)[18] and organ dysfunction. Infection sites were divided into 7 sites: respiratory, genitourinary, intra-abdominal, skin/soft tissue, central nervous system, endocarditis, and others. In addition, dysfunctional organs were divided into 7 types: cardiovascular, respiratory, renal, hepatic, neurologic, hematologic, and metabolic.
2.4. Statistical analysis
Incidence and mortality rates were presented as per 100,000 people while case fatality was presented as percentages. Temporal changes were assessed by linear regression. Moreover, continuous variables were presented as both mean with standard deviation (SD) and median with interquartile range (IQR), while discrete variables were presented as counts or percentages. The Chi-square test was used to assess the difference in epidemiological rates among different gender and severity groups. Data analysis was performed using SAS for Windows version 9.4 and a 2-tailed P <.05 was considered significant.
3. Results
There were 2,000,164 people, including 1.5 to 1.6 million adults, in the LHWD in the 5-year observation period. In total, 62,517 patients with 97,790 sepsis-related hospitalizations were identified and included in the analyses. In 2014, the mean (±SD) and median (IQR) age of the patients included were 71.0 (±16.5) and 75 (61–84) years old, respectively. In addition, the majority of the patients were male (54.2%) and elderly (68.2%).
The incidence of all severity levels of sepsis increased from 623 per 100,000 people in 2010 to 647 per 100,000 in 2014, but there was no significant temporal trend (P = .72, Fig. 1). The mortality increased from 264 to 297 per 100,000 people during the same 5-year period (Fig. 1). Although more and more people suffered from sepsis and died over time, the changes in mortality were not significant (P = .10). When stratified by gender, there was a lack of temporal change in incidence and mortality between men and women, but the rates were consistently higher in men than in women (Fig. 2). For example, the incidence rate in males and in females was 718 and 578 per 100,000 people, respectively (P <.0001) in 2014. The mortality in men and women was 361 and 235 per 100,000 people, respectively (P <.0001). When stratified by age, incidence, and mortality of the elderly with sepsis were higher than in the youth (Fig. 3).
Figure 1.
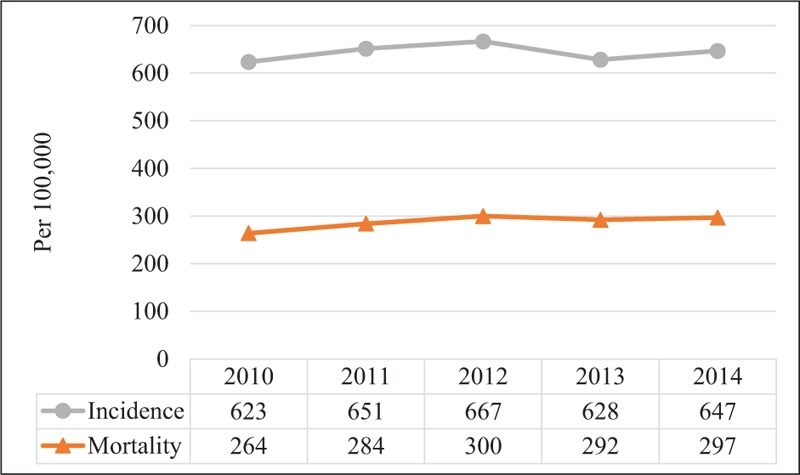
Temporal change in incidence and mortality rates of patients with any severity level of sepsis.
Figure 2.
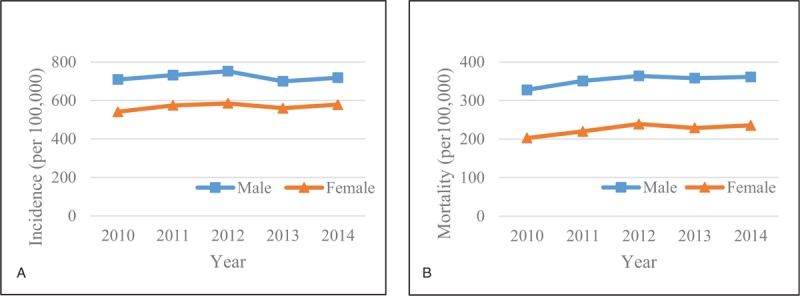
Temporal change in (A) incidence and (B) mortality rates of patients with any severity level of sepsis by gender.
Figure 3.
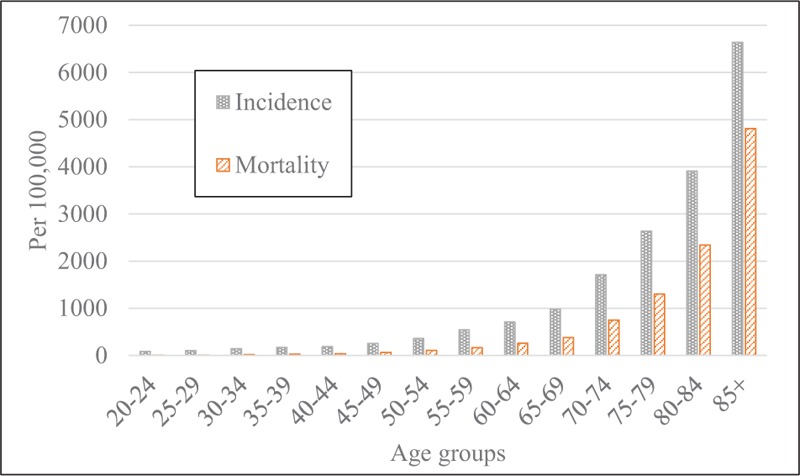
Incidence and mortality rates of patients with any severity level of sepsis by age group, in 2014.
The case fatality of all severity levels of sepsis was 29.4% in 2014, and it had no significant change over the 5-year observation period (mean = 29.2%, P = .08) (Supplementary Fig. 1). However, case fatality in males was significantly higher than in females (32.1% vs 26.1% in 2014, P <.0001) (Supplementary Fig. 1), and older patients’ case fatality was higher than younger ones’, with an estimated 5.3% case fatality in the youngest group (20–24 years old) and 41.7% in the oldest (over 85 years old) in 2014 (Supplementary Fig. 2).
Moreover, we also divided the patients into 3 sepsis severity groups. There was no significant temporal change in mortality or case fatality in any of the severity groups other than the increased mortality in the septic shock group (P = .01) (Fig. 4A and B). Among the 3 groups, septic shock had the highest mortality and case fatality consistently throughout the observation period. Specifically, the mortality of sepsis, severe sepsis, and septic shock was 18, 115, 164 per 100,000 people, respectively, while case fatality was 6.6%, 21.3%, and 56.7%, respectively, in 2014. Moreover, the mortality and case fatality in males were consistently higher than those in females for severe sepsis (all P <.05) and septic shock (all P <.05), but there was no significant difference in these values between males and females (P = .68, and .90, respectively).
Figure 4.
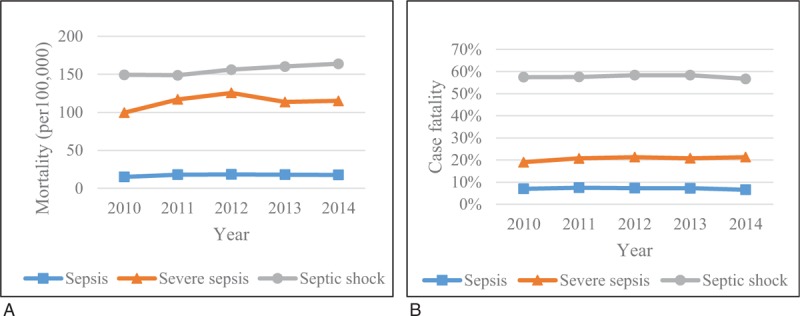
Temporal change in (A) mortality and (B) case fatality for 3 severity groups.
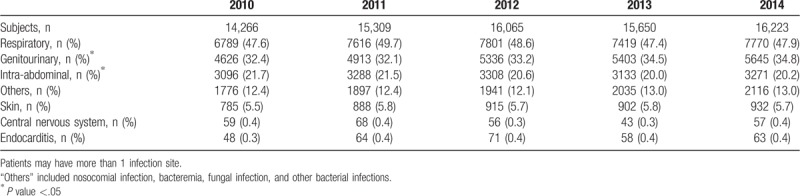
The most common infection site in patients was the respiratory system (47.9% in 2014), followed by the genitourinary system (34.8% in 2014) (Table 1). There was significant temporal change in the sites of the genitourinary system and intra-abdomen, with the proportion in the genitourinary system increasing (P = .02) and the proportion in the intra-abdomen decreasing (P = .02). When stratified by severity, the respiratory system was the second most common infection site in the sepsis groups, which was different from the other groups (Supplementary Fig. 3).
Table 1.
Infection sites by proportion.
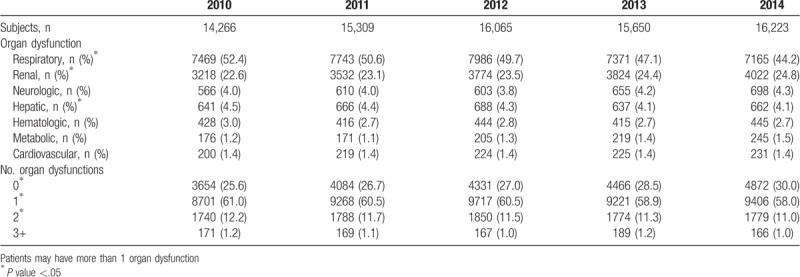
In this study, the most common organ dysfunction observed in septic-related hospitalizations was respiratory and renal failure, with the proportion of renal dysfunction significantly increasing over time (P <.01). Conversely, respiratory and hepatic dysfunction decreased (P = .01, .01) (Table 2). When the patients were divided into 3 severity groups, respiratory dysfunction was still the most common site of organ failure in severe sepsis and septic shock (Supplementary Fig. 4). Furthermore, in 2014, 70% of septic patients were found to have some organ dysfunction, with 12% of patients experiencing multi-organ dysfunction (Table 2). However, the proportion of patients with dysfunction significantly decreased over time (P <.01).
Table 2.
Organ dysfunction by proportion.
4. Discussion
In this study, we found that the average incidence and mortality of any severity level of sepsis in Taiwan was 643, and 287 per 100,000 people, respectively, and the average case fatality was 29.2% during the study period (2010–2014). The mortality of sepsis was higher than those of diabetes and hypertension (42.1 and 23.3 per 100,000 people, respectively)[21] in Taiwan. In addition, the incidence and mortality of sepsis were higher than those of cancer, which has an incidence and mortality of 440.2 and 196.7 per 100,000 people respectively.[22] Despite the high mortality of sepsis in Taiwan, relatively little research has been done on it. Our findings call for more attention and interventions devoted to this life-threatening condition from both the government and researchers.
Moreover, it was found that the case fatality of any severity level of sepsis was higher in our study than in the US (17.2%–23.8%),[2,5,16] which finding could be partially explained by the higher mean age of our study sample (71.0 years old) than that those in previous research (e.g., 65.2 years old in the study by Jones et al[16] and 68.0 years old in the study by Stoller et al[5]). Age is indeed an influencing factor for case fatality, and our study found that the case fatality of the elderly was higher than that of the youth. In addition to closer investigation of age, further research is needed to examine whether and how causes of sepsis and differences in health care contributed to the differences in sepsis burden among countries.
In the present study, approximately half of the sepsis-related hospital admissions were related to severe sepsis. Conflicting results were reported in previous studies that used the ICD-10 and ICD-10-AM to identify and classify sepsis patients, where only approximately one-third of the patients had severe sepsis.[23,24] In addition to the differences in classifications due to different versions of ICD codes, 1 explanation for the relatively high proportion of patients with severe sepsis observed in the present study could be the Case Mix Index used in Taiwan, where hospitals can apply for a higher amount of reimbursement if patients are coded with more severe conditions. As such, the proportion of patients with less than severe sepsis could have been underestimated.
The most common infection sites observed in the present study were the respiratory and genitourinary systems, which was consistent with previous studies’ findings.[25–27] In addition, the proportion of respiratory system infections was higher in patients with severe sepsis or septic shock. These findings could be explained by the fact that critically ill patients usually require endotracheal intubation and mechanical ventilation or placement of a Foley catheter, which invasive interventions increase the risk of infection. In addition, respiratory and urinary tract infections are common nosocomial infections; therefore, it is possible for patients who require invasive interventions to acquire an infection during their hospital stay that can lead to sepsis even if they were not admitted because of infections or sepsis. Nevertheless, due to the limitation of the study database, we were unable to differentiate the cause of infection.
The present study found that the epidemiological crude rates of sepsis were higher in the elderly than in the youth, which indicates that we need to further investigate the effects of an aging population on the epidemiology of sepsis. We also ought to investigate or at least be sensitive to the way we identify and classify septic patients with a goal of increasing the comparability of study results, especially in light of changes like the use of the ICD-10-CM instead of the ICD-9-CM since 2016.
There are several limitations to this study. First, there are no laboratory data in the study database; therefore, we could not assess types of pathogens. Only Huang et al have examined this subject in an ICU in Northern Taiwan, and such information in Taiwan remains limited.[28] Second, as only 5 diagnostic codes were able to be recorded for hospital admissions in the database, the severity level of sepsis and the type of organ failure may have been misclassified. Third, as the study database did not provide information about diagnosis time or drug administration time, we did not know the onset date of sepsis. Therefore, we could not be certain about the causal relationship between infection and organ dysfunction. As a result, the crude rates could have been overestimated. Fourth, the study by Page et al indicates that community-acquired severe sepsis only accounts for 62.8% of severe sepsis cases,[29] which indicates that the proportion of sepsis patients with nosocomial infections is high. Nevertheless, we were unable to identify these patients or assess their proportion as a result of the database limitations. Fifth, due to the restriction of the study database, we had to exclude patients without a discharge record. As a result, sepsis-related rates in more recent years could have been underestimated. Lastly, as all of the analyses were performed in the same cohort of patients and re-admissions were common in patients with sepsis, the study samples of the 5 study years (2010–2014) were not independent of each other.
5. Conclusion
In this study, we found that there was no significant change in the incidence or mortality of all severity levels of sepsis between 2010 and 2014 in Taiwan. Furthermore, when stratified by severity levels, the case fatality of all severity levels has remained about the same. We also found that all epidemiological crude rates of sepsis were higher in males than in females, and higher in the elderly than in the youth. Moreover, case fatality was found to increase with sepsis severity. Additional study findings showed that the most common infection sites were respiratory and genitourinary, while the most common organ dysfunctions were respiratory and renal.
Acknowledgments
We would like to thank Dr Hsiu-Nien Shen for providing us with the ICD-9 codes of infection sites.
Author contributions
Conceptualization: Yen-Jung Chen, Fu-Lun Chen, Man-Tzu Marcie Wu, Yu Ko.
Data curation: Jin-Hua Chen.
Formal analysis: Yen-Jung Chen, Jin-Hua Chen.
Funding acquisition: Yen-Ling Chen, Du-Shieng Chien, Yu Ko.
Investigation: Yu Ko.
Methodology: Fu-Lun Chen, Man-Tzu Marcie Wu, Yu Ko.
Project administration: Yen-Jung Chen.
Resources: Fu-Lun Chen, Man-Tzu Marcie Wu, Yen-Ling Chen, Du-Shieng Chien.
Software: Jin-Hua Chen.
Supervision: Yu Ko.
Writing – original draft: Yen-Jung Chen.
Writing – review & editing: Fu-Lun Chen, Jin-Hua Chen, Man-Tzu Marcie Wu, Yen-Ling Chen, Du-Shieng Chien, Yu Ko.
Footnotes
Abbreviations: ICD-9-CM = International Classification of Diseases 9th Edition Clinical Modification, IQR = interquartile range, LHWD = Longitudinal Health and Welfare Database, SD = standard deviation.
This study was sponsored by TaiRx Inc., Taipei, Taiwan.
The authors have no conflicts of interest to disclose.
References
- [1].Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated Sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016;193:259–72. [DOI] [PubMed] [Google Scholar]
- [2].Walkey AJ, Lagu T, Lindenauer PK. Trends in sepsis and infection sources in the United States. A population-based study. Ann Am Thorac Soc 2015;12:216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35:1244–50. [DOI] [PubMed] [Google Scholar]
- [4].Kumar G, Kumar N, Taneja A, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 2011;140:1223–31. [DOI] [PubMed] [Google Scholar]
- [5].Stoller J, Halpin L, Weis M, et al. Epidemiology of severe sepsis: 2008–2012. J Crit Care 2016;31:58–62. [DOI] [PubMed] [Google Scholar]
- [6].Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012;40:754–61. [DOI] [PubMed] [Google Scholar]
- [7].Liu TW, Chu CM, Chien WC, et al. Epidemiological characteristics and related factors analysis for hospital mortality of sepsis using Taiwan 2013 data as example. J Taiwan Assoc Med Info 2017;26:35–50. [Google Scholar]
- [8].Central Health Insurance Agency, Ministry of Health and Welfare (2018) National Health Insurance coverage rate. Available at: https://www.gender.ey.gov.tw/gecdb/Stat_Statistics_DetailData.aspx?sn=u4ceyDJ9iGzBYUGlJC0z7w%3d%3d&d=194q2o4%2botzoYO%2b8OAMYew%3d%3d Accessed March 12, 2018. [Google Scholar]
- [9].National Health Insurance Administration, Ministry of Health and Welfare (2018) 2017-2018 National Health Insurance Annual Report. Available at: https://www.nhi.gov.tw/Content_List.aspx?n=9223A12B5B31CB37&topn=FB01D469347C76A7 Accessed February 21, 2018. [Google Scholar]
- [10].Ministry of Health and Welfare (2015) Introduction of Two-Million-Sample Longitudinal Health and Welfare Database. Available at: https://dep.mohw.gov.tw/DOS/lp-2506-113.html Accessed April 26, 2018. [Google Scholar]
- [11].Ministry of the Interior Department of Statistics (2018) Population by Age (Statistical Yearbook of Interior). Available at: https://www.moi.gov.tw/files/site_stuff/321/2/year/year_en.html Accessed June 16, 2018. [Google Scholar]
- [12].Weycker D, Akhras KS, Edelsberg J, et al. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med 2003;31:2316–23. [DOI] [PubMed] [Google Scholar]
- [13].Centers for Disease Control and Prevention (2011) Official ICD-9-CM Guidelines for Coding and Reporting. Available at: https://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf Accessed June 17, 2018. [Google Scholar]
- [14].Hall MJ, et al. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief 2011;1–8. [PubMed] [Google Scholar]
- [15].Eber MR, Laxminarayan R, Perencevich EN, et al. Clinical and economic outcomes attributable to health care-associated sepsis and pneumonia. Arch Intern Med 2010;170:347–53. [DOI] [PubMed] [Google Scholar]
- [16].Jones SL, Ashton CM, Kiehne LB, et al. Outcomes and resource use of sepsis-associated stays by presence on admission, severity, and hospital type. Med Care 2016;54:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. [DOI] [PubMed] [Google Scholar]
- [18].Shen HN, Lu CL, Yang HH. Epidemiologic trend of severe sepsis in Taiwan from 1997 through 2006. Chest 2010;138:298–304. [DOI] [PubMed] [Google Scholar]
- [19].Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- [20].Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486–552. [DOI] [PubMed] [Google Scholar]
- [21].Ministry of Health and Welfare (2015) Taiwan Statistics of Causes of Death 2014. Available at: https://dep.mohw.gov.tw/DOS/lp-1779-113.html Accessed July 15, 2018. [Google Scholar]
- [22].Health Promotion Administration, Ministry of health and welfare (2017) Cancer registry annual report, 2014 Taiwan. Available at: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=7330 Accessed July 14, 2018. [Google Scholar]
- [23].Flaatten H. Epidemiology of sepsis in Norway in 1999. Crit Care 2004;8:R180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sundararajan V, Macisaac CM, Presneill JJ, et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med 2005;33:71–80. [DOI] [PubMed] [Google Scholar]
- [25].Lagu T, Rothberg MB, Nathanson BH, et al. The relationship between hospital spending and mortality in patients with sepsis. Arch Intern Med 2011;171:292–9. [DOI] [PubMed] [Google Scholar]
- [26].Barreto MF, Dellaroza MS, Kerbauy G, et al. Sepsis in a university hospital: a prospective study for the cost analysis of patients’ hospitalization. Rev Esc Enferm USP 2016;50:302–8. [DOI] [PubMed] [Google Scholar]
- [27].Henriksen DP, Laursen CB, Jensen TG, et al. Incidence rate of community-acquired sepsis among hospitalized acute medical patients-a population-based survey. Crit Care Med 2015;43:13–21. [DOI] [PubMed] [Google Scholar]
- [28].Huang CT, Tsai YJ, Tsai PR, et al. Epidemiology and outcome of severe sepsis and septic shock in surgical intensive care units in Northern Taiwan. Medicine (Baltimore) 2015;94:e2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Page DB, Donnelly JP, Wang HE. Community-, healthcare-, and hospital-acquired severe sepsis hospitalizations in the university healthsystem consortium. Crit Care Med 2015;43:1945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


