Abstract
Depression is one of the most common mental health problems which affects more than 10% of the global population. The prevalence of this disorder is higher in fibromyalgia patients. However, the influence of the combination of depression and fibromyalgia in the brain processing is poorly understood.
To explore the modifications of EEG power spectrum in women with fibromyalgia when depressive feelings are elicited.
Twenty eight women with fibromyalgia participated in this cross-sectional study. They were classified as women with depression or women without depression according to the score in the Geriatric Depression Scale. This questionnaire was used to elicit depression symptoms during the EEG recording. Analyses were performed with the standardized LOw Resolution Electric Tomography (sLORETA) software. Power spectrum were compared in the following frequency bands: delta, theta, alpha-1, alpha-2, beta-1, beta-2, and beta-3.
Fibromyalgia patients with untreated depression showed a hypoactivation of the left hemisphere when compared with fibromyalgia patients without depression. In addition, when compared fibromyalgia patients without depression and women with both fibromyalgia and depression who were taking antidepressant medications, differences in EEG power spectrum in the studied frequency bands were not found.
The current study contributes to the understanding on the influence of the combination of fibromyalgia and depression in the brain activity patterns. Patients with untreated depression showed a hypoactivation of the left hemisphere while eliciting depression symptoms. However, further research is needed, antidepressant medication might reduce the differences between patients with depression and those who do not suffer from depression symptoms.
Keywords: antidepressant, depression, EEG, fibromyalgia, left hemisphere, mood congruent recall, sLORETA
1. Introduction
Depression is one of the most common mental health problems which affects more than 10% of the global population.[1] It has been associated with altered brain processing patterns in the default mode network (DMN) and task-positive network (TPN) in patients with depression.[2–5] Whereas TPN have been related to subserve active cognitive processing[6] (working memory or executive control), DMN has been proposed to be related with self-relational processing[7] (autobiographical recall). Furthermore, differences in medial prefrontal, medial temporal, and occipital regions have been reported in patients with depression when compared with healthy controls during an autobiographical memory task.[8]
There is growing interest in the understanding of the impact of depression on brain activity patterns when processing emotions,[9,10] recalling fears,[11] or during autobiographical memory.[11] These brain functioning changes influence the behavior and cognitive functions of people with depression. Thus, the mood congruent recall emerged as a characteristic of autobiographical memory in depression,[12] referring to a preference for remembering negative[12] or distressing memories.[8]
Fibromyalgia (FM) is characterized by chronic widespread pain which leads to somatic (fatigue or stiffness), psychiatric and psychological symptoms (sleep disturbance, depression, and cognitive impairment).[13,14] In patients with FM, the prevalence of depression is higher in comparison with people without FM,[15] and a bidirectional association between depression and FM has been observed.[16] Regarding brain functioning, patients with FM showed alterations in the functional connectivity[17] and gray matter atrophy in the DMN regions.[18] In addition, FM patients showed an altered brain activity when observing negative emotions, such as facial expression of pain.[19] Despite the scientific literature about changes in brain activity of patients with depression performing different mental tasks is rich, the influence of the combination of depression and FM is poorly understood.
The objective of the present study was to explore the modifications of scalp EEG power spectrum in women with FM when depressive feelings are elicited. This study also aims to compare the EEG power spectrum (in delta, theta, alpha-1, alpha-2, beta-1, beta-2, and beta-3 frequency bands) between fibromyalgia patients with and without depression. Furthermore, the study aims to compare the EEG power spectrum (in delta, theta, alpha-1, alpha-2, beta-1, beta-2, and beta-3 frequency bands) of patients with depression who were or not taking antidepressant medication. To do that, brain electrical activity was recorded while patients with FM answered the geriatric depression scale (GDS), which address depressive symptoms with a simple yes/no format.[20]
2. Methods
2.1. Participants
The Extremadura Association of Fibromyalgia (AFIBROEX) in Cáceres (Spain) called and invited all female members to participate in the study. Consecutive patients who agreed to participate and fulfilled the inclusion and exclusion criteria were included. Twenty eight women with FM (54.96 ± 10.43 years), diagnosed according to the American College of Rheumatology's criteria,[13] participated in this cross-sectional observational study. All participants underwent the procedure between May and June, 2017.
The sample was divided into 3 groups:
-
1.
FM patients with depression (GDS score > 5) who were taking antidepressant medication (n = 9, 52.33 ± 9.90 years);
-
2.
FM patients with depression (GDS score > 5) who were not taking antidepressant medication (n = 7, 53.43 ± 11.43 years); and
-
3.
FM patients without depression (GDS score ≤ 5) and who were not taking antidepressants (n = 12, 57.83 ± 10.42 years).
The antidepressant medication for all 9 participants was selective serotonin reuptake inhibitors (SSRI). Characteristics of each group can be observed in Table 1. Participants with neurological diseases, psychiatric diagnose (i.e., schizophrenia or substance abuse), or neurodegenerative diseases were excluded. Participants were verbally informed about the details of the study and gave written informed consent to participate in the study. All procedures were approved by the University research ethics committee and were carried out in accordance with the updated Declaration of Helsinki (approval number: 62/2017).
Table 1.
Sample size, age, antidepressant medication, and GDS score of the 3 groups of patients with fibromyalgia.

2.2. Instruments
EEG data was collected using the Enobio device, a wireless electrode system (Neuroelectrics, Cambridge, MA, USA).[21] The reliability of this instrument was demonstrated, even using dry electrodes.[22] In order to elicit depressive feelings, the 15-items Geriatric Depression Scale (GDS-15)[20] in the Spanish version[23] was administered by personal interview with one of the members of the research group. This questionnaire allows researchers to classify which participants are suffering from depression. The selected cut-off was 5 in order to enhance the specificity, sensibility, and both the predictive- positive and negative values of the depression diagnosis.[20,24] Although, this questionnaire was originally designed for geriatrics patients, it has been used in patients with FM[25,26] since it has less focus on somatic symptoms and might therefore be a more accurate assessment of depression symptoms in patients with FM independently of their health complaints.[26]
2.3. Procedure
EEG data was continuously registered while a researcher asked the GDS items. Participants were encouraged to response only with a yes or no, keeping in silence if more time was needed to make a decision. The experimental room was calm, and light and temperature were continuously regulated.
2.4. EEG procedure and preprocessing
EEG was recorded from 19 scalp locations, according to the International 10 to 20 system such as: frontal (Fz, Fp1, Fp2, F3, F4, F7, and F8), central (Cz, C3, and C4), temporal (T3, T4, T5, and T6), parietal (Pz, P3, and P4), and occipital (O1 and O2).
Electrodes placed in the mastoids served as references and impedance was kept below 10 KΩ. EEG was recorded with a sampling rate of 500 Hz, 50 Hz notch filter, and bandpass filtering (1–40 Hz) was employed. EEGLAB toolbox (MATLAB) was applied for pre-processing and data analysis. Rough artifacts were manually removed from EEG signals and eye movement artifacts were corrected using independent component analysis (ICA).[27] Data were divided in 5 s epochs from the cleaned dataset.
2.5. Electrical source imaging (ESI)
To compute the intracerebral electrical sources underlying EEG activity recorded at the scalp we used the sLORETA/eLORETA software package (http://www.uzh.ch/keyinst/loreta.htm).[28] This tool represents the cortex as a collection of volume elements (6239 voxels, size 5 × 5 × 5 mm). sLORETA is restricted to cortical gray matter, hippocampus, and amygdala in the digitized Montreal Neurological Institute (MNI) coordinates corrected to the Talairach coordinates. Neuronal activity is computed as current density (μA/mm2) without assuming a predefined number of active sources.[28,29] Scalp electrode coordinates on the MNI brain were based on the international 5% system.[30] The sLORETA algorithm solves the inverse problem by assuming related orientations and strengths of neighboring neuronal sources (represented by adjacent voxels). sLORETA has proved to be an efficient tool for functional mapping because it is consistent with physiology and is capable of correct localization.[28] Additionally, the sLORETA localization properties have been independently validated.[31,32]
Anatomical labels as Brodmann areas are also reported using MNI space. We calculated sLORETA images corresponding to the estimated neuronal generators of brain activity within each band.[33] The ranges of the frequency bands were as follows: delta, 1.5 to 6 Hz; theta, 6.5 to 8 Hz; alpha-1, 8.5 to 10 Hz; alpha-2, 10.5 to 12 Hz; 8.5 to 12 Hz; beta-1, 12.5 to 18 Hz; beta-2, 18.5 to 21 Hz; beta-3, 21.5 to 30 Hz.
2.6. Statistical analysis
A Kruskall–Wallis and Chi-Squared tests, using the SPSS statistical package (version 20.0; SPSS, Inc., Chicago, Ill.), were conducted to assess differences between groups in age, GDS total score and medication.
Nonparametric randomization statistic was performed using sLORETA non-Parametric Mapping tool.[34] This tool includes a correction for multiple comparisons and does not require any assumption of Gaussianity.[35] In addition, a second level of non-parametric analysis, the exceedance proportion tests evaluated the significance of activity based on its spatial extent, obtaining clusters of supra-threshold voxels. In this line, in order to control Type I error cluster size were calculated[34] within a hemisphere for single Broadmann Area (BA).
The sLORETA non-Parametric mapping tool was used to perform between-group comparisons. It allows us to obtain LORETA images (see Figs. 1–3), representing the electrical activity of each voxel in the neuroanatomic MNI space as amplitude of the computed current source density (μA/mm2) for each frequency band[33] (delta, theta, alpha-1, alpha-2, beta-1, beta-2, beta-3) as well as the color bar scale which represents the log-F-ratio value for each voxel. In more detail, the sLORETA current density distribution were performed using a statistical analysis based on voxel-by-voxel log of F ratio test with 5000 randomizations. The results corresponded, for each band, to maps of log-F-ratio statistics for each voxel, for corrected P < .05. Significant activations at the exceedance proportion tests with a P value < .05, F value over 2 z-score and a minimum cluster of voxels major than calculated for each BA within a hemisphere.
Figure 1.
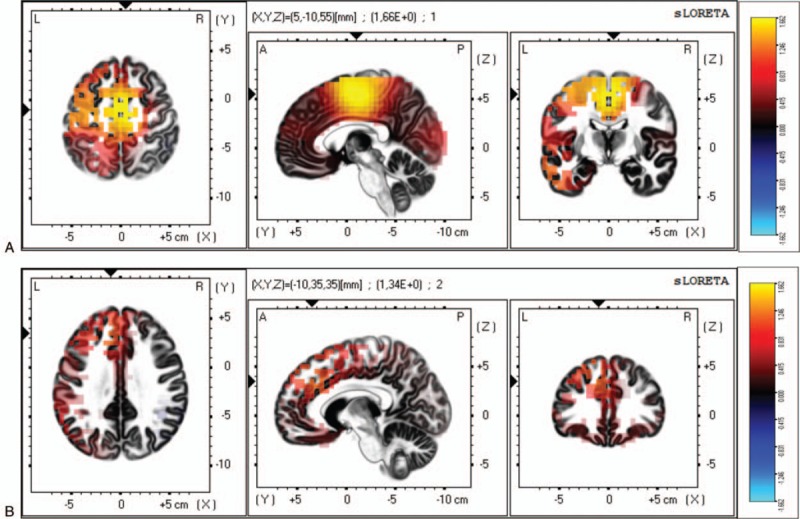
Differences between patients with FM with depression and patients with FM without depression on the mean current source density analyzed by sLORETA. (A) For the delta frequency, the maximal difference across patients with FM appeared in the medial frontal gyrus (BA 6, MNI coordinates: x = 5, y = −10, z = 55). (B) For the theta frequency, the maximal difference across participant was detected in the medial frontal gyrus (BA 9, MNI coordinates: x = −10, y = −35, z = 35). The color bar shows sLORETA activation which represents the log-F-ratio value for each voxel.
Figure 3.
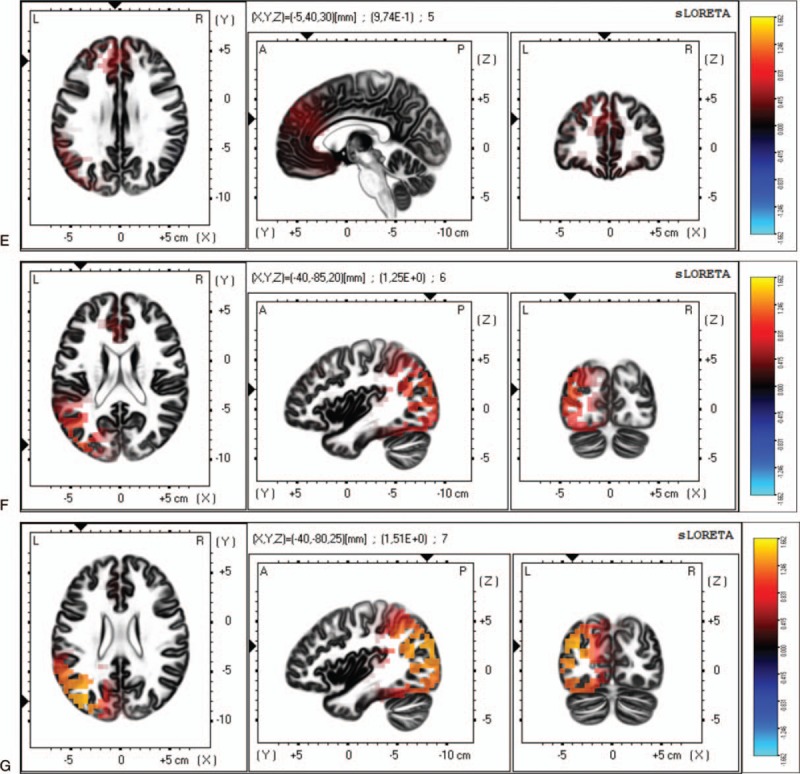
Differences between patients with FM with depression and patients with FM without depression on the mean current source density analyzed by sLORETA. (E) For the beta-1 frequency, the maximal difference across patients with FM appeared in the medial frontal gyrus (BA 9, MNI coordinates: x = −5, y = 40, z = 30). (F) For the beta-2 frequency, the maximal difference across participant was detected in the middle temporal gyrus (BA 19, MNI coordinates: x = −40, y = −85, z = 20). (G) For the beta-3 frequency, the maximal difference across participant was detected in the medial frontal gyrus (BA 19, MNI coordinates: x = −40, y = −80, z = 25). The color bar shows sLORETA activation which represents the log-F-ratio value for each voxel.
All possible comparisons between groups were performed using the procedures showed above.
3. Results
The Kruskall–Wallis test did not reveal any significant difference between groups in age (P = .407). Moreover, Kruskall–Wallis and Chi-Squared showed significant differences between FM without depression and FM unmedicated and medicated groups (Table 1).
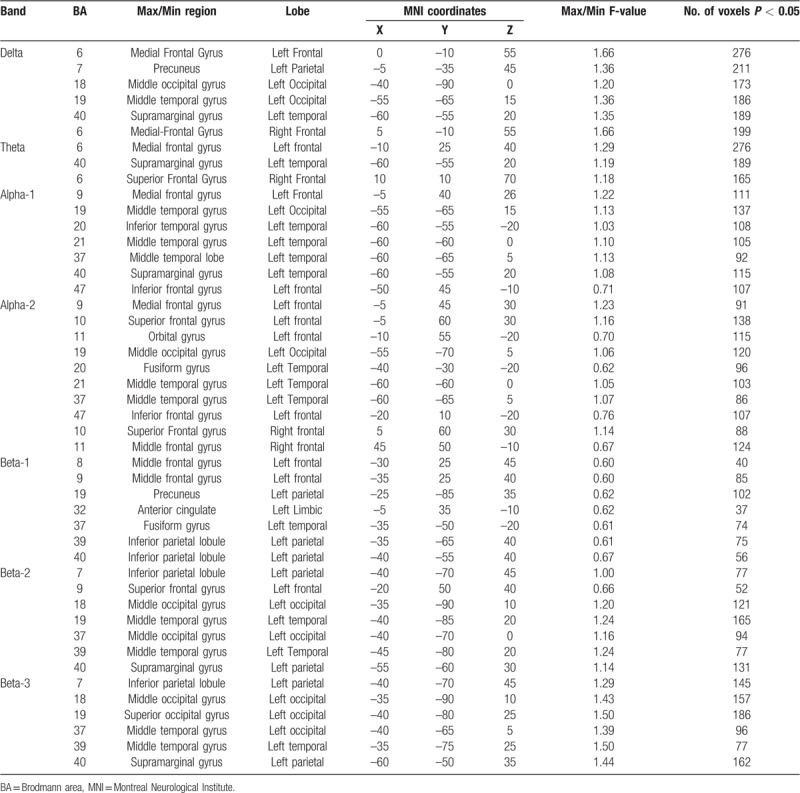
However, exceedance proportion test revealed significant differences between FM patients with depression who were not taking antidepressant medication and FM patients without depression (log-F-ratio threshold = 0.664, P value = .043). Specifically, the unmedicated group with depression showed reduced power in delta, theta, alpha-1, alpha-2, beta-1, beta-2, and beta-3 while answering the GDS (Table 2 and Fig. 1). These differences are located predominantly in the left hemisphere.
Table 2.
Statistical non-parametric comparisons between FM patients without depression and FM patients who have depression and were not taking antidepressants using sLORETA. The table only shows significant cluster for the delta, theta, alpha-1, alpha-2, beta-1-beta-2, and beta-3 activity.
The sLORETA non-Parametric mapping tool allows us to obtain images (see Figs. 1–3), representing the electrical activity of each voxel in the neuroanatomic MNI space as well as a color scale bar which represents the log-F-ratio for each voxel. Figure 1a represents the EEG delta power spectrum. It shows that the maximal difference is located in the medial frontal gyrus (BA 6, MNI coordinates: x = 5, y = −10, z = 55). Figure 1b represents the EEG theta power spectrum. We found the maximal differences in the medial frontal gyrus (BA 9, MNI coordinates: x = −10, y = −35, z = 35) (see Fig. 1b). Figure 2c and 2d represents the EEG in the alpha-1 and alpha-2 power spectrums respectively. Maximal differences appeared in the cingulated gyrus (BA 32, MNI coordinates: x = −5, y = 35, z = 30) for alpha-1 and in the medial frontal gyrus (BA 9, MNI coordinates: x = −5, y = −45, z = 30) for alpha-2. Figure 3 represents the EEG power spectrum in beta-1, beta-2, and beta-3. In the beta-1, maximal differences were found in the medial frontal gyrus (BA 9, MNI coordinates: x = −5, y = 40, z = 30) (see Fig. 3e). In the beta-3 the maximal differences were obtained in the middle temporal gyrus (BA 19, MNI coordinates: x = −40, y = −85, z = 20) (see Fig. 3f). In the beta-3 the maximal difference across participant was detected in the medial frontal gyrus (BA 19, MNI coordinates: x = −40, y = −80, z = 25) (see Fig. 3g). Each of the previous figure is accompanied by a color bar which represents the log-F-ratio value for each voxel.
Figure 2.
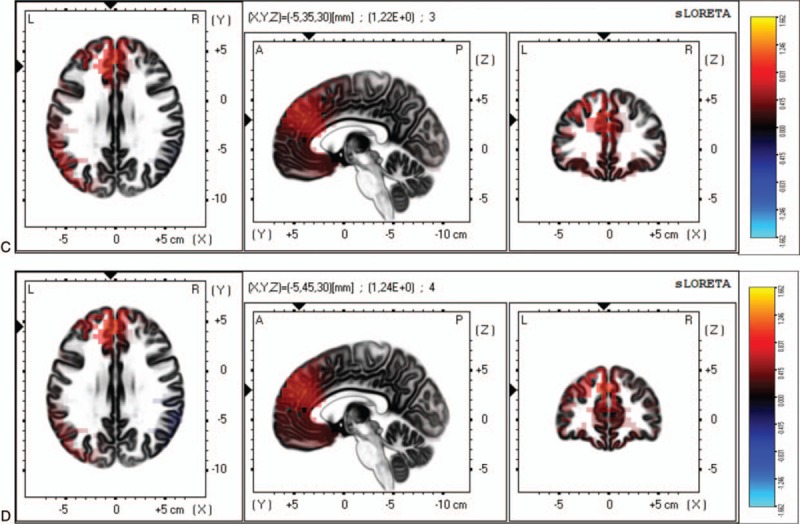
Differences between patients with FM with depression and patients with FM without depression on the mean current source density analyzed by sLORETA. (C) For the alpha-1 frequency, the maximal difference across patients with FM appeared in the cingulate gyrus (BA 32, MNI coordinates: x = −5, y = 35, z = 30). (D) For the alpha-2 frequency, the maximal difference across participant was detected in the medial frontal gyrus (BA 9, MNI coordinates: x = −5, y = −45, z = 30). The color bar shows sLORETA activation which represents the log-F-ratio value for each voxel.
In delta power, the clusters of supra-threshold were located in left BA 6, 7, 19, 40 and right BA 6 (Table 2). Moreover, in theta power the clusters of supra-threshold were located in left and right BA 6 and in left BA 40 (see Table 2). In alpha-1 and alpha-2 were located in BA 9, 19 and 37, whereas in alpha-2 were also identified in right BA 10 (Table 2). Furthermore, beta-1 power significant clusters of supra-threshold were located in left BA 9, 40, 32 and 37, while in beta-2 significant cluster of supra-threshold were identified in left BA 9, 19, 40, and 37 (Table 2). Lastly, in beta-3 were also identified significant cluster of supra-threshold in left BA 39, 7, and 19 (Table 2).
Non-parametric analyses using sLORETA showed that the FM patients who were taking antidepressant medication did not statistically differ from the FM patients who were not being treated with this medication in terms of significant voxel or clusters of supra-threshold voxels at delta, theta, alpha, or beta frequency bands. Furthermore, comparison between FM patients with antidepressant treatment and FM women without depression was also performed. Non-significant voxel or cluster of supra- threshold voxels at delta, theta, alpha, or beta frequency bands were located.
4. Discussion
The principal aim of this study was to explore the modification of EEG power spectra induced by eliciting depressive feelings in patients with FM and to compare EEG power spectrum (in delta, theta, alpha-1, alpha-2, beta-1, beta-2, and beta-3 frequency bands) between fibromyalgia patients with and without depression, taking the antidepressant medication into account.
The main finding was that women with FM and untreated depression obtained reduced power in delta, theta, alpha-1, alpha-2, beta-1, beta-2, and beta-3 while answering GDS-15 questionnaire. Differences were found predominantly in the left hemisphere. Regarding delta power differences were located in left frontal, parieto-temporal, and occipital areas. Differences in theta power were located in frontal and parietal areas. In relation with alpha-1 and alpha-2 power, differences were located in frontal, temporal, and occipital areas. Beta-1, beta-2, and beta-3 showed differences in frontal, parieto-temporal, and occipital. However, differences were not found between patients with FM with and without depression symptoms.
Previous studies in clinical populations with affective disorders[36,37] have showed that left hemisphere is important for regulation of negative emotions.[38] In patients with depression, a hypoactivity in the left hemisphere has been observed,[37] thus the alterations in left hemisphere may be related with depression symptoms, such as reduced motivation and activity[39] or apathy.[40] In line with these studies, Stewart and colleagues[10] reported frontal EEG asymmetry during facial emotion task. This frontal EEG asymmetry is considered by authors as a risk major for major depression disorder. These abnormalities are consistent with our results where left hemisphere hypoactivity in power spectrum has been observed in patients with FM and depression while depressive feelings were elicited.
Answering the GDS is other alternative to elicit depressive feelings and provide emotional stimulus and according to a previous study[41] there is a decreased activation in prefrontal areas, hippocampus, and caudate in patients with depression when the emotional stimulus is based on viewing images of faces expressing negative emotional state, suggesting that functional impairment of fronto-limbic and fronto-subcortical brain regions may be implicated in depression. According to the mood congruent memory, these patients are more likely to remember negative information[42] or create distressing memories.[8] Regarding FM, patients have shown alterations in the functional connectivity[17] and gray matter atrophy[18] in the DMN. Interestingly, patients with FM have also reported altered brain activity viewing facial expression of pain.[19] These alterations in the DMN have been also observed in patients with depression[43] during emotion processing.
According to Bertolucci and de Oliveira,[44] patients with FM have phonetic and semantic verbal fluency impairments, which may be correlated with irritability or anxiety.[45] In this regard, voxel-based morphometric studies have shown reduced performance in non-verbal working memory and non-verbal long-term memory in a free recall condition, which is associated with changes in structural gray matter in the left dorsolateral prefrontal cortex and in the supplementary motor cortex.[46] This may be in line with our results where we found left hemisphere hypoactivation while answering the GDS questionnaire, which may lead to a mentally recall of depressive feelings. In this regard, lesions in the left dorsolateral prefrontal cortex are related to increased depression symptoms, demonstrating a direct neurological contribution to depression symptoms after left hemisphere stroke.[47] Thus, the morphology and functioning of the left hemisphere may be of great interest to investigate potential differences in brain functioning between patients with FM and pain-free subjects.
According to our results, comparing between women with FM and depression treated with SSRI and those without depression, significant differences were not found. Pharmacological treatment is widely used in order to normalize amygdala over-activation in response to negative stimuli.[48] In this regard, in patients suffering from major depressive disorders, SSRI treatment is associated with favorable changes in brain electrical activity.[49] In line with this study, Fales and colleagues[50] reported that antidepressant medication increased pre-frontal cortex activity during emotional tasks.
In addition, a non-significant statistical tendency showed higher power spectrum in FM patients who were taking antidepressants when compared with those with untreated depression. Future research should study the effects of antidepressant treatment on EEG power spectrum in patients with FM when depression feelings are elicited. Furthermore, EEG neurofeedback is an alternative to other treatments such as medication,[51] increasing the relative right frontal alpha power, and reporting positive effects on emotion, cognition, and performance in the executive function.[52] Also, aerobic training may have benefits in alpha power in patients with depression,[53] supporting the effect of aerobic training on alpha activity and on depression symptoms in elderly patients. Exercise facilitates the treatment of depressive elderly adults, leading to clinical and physical improvement and protecting against a decrease in cortical activity.[53]
One potential limitation of our study was the number of scalp EEG recordings electrodes (n = 19). Spatial resolution increases with the number of EEG sources reflected by sLORETA software. However, a large number of studies have been developed with the same or less number of scalp EEG recordings,[54–56] even the reliability of sLORETA and the original validation use the original 19 electrodes.[57–59] Lastly, the sample size (n = 28) was relatively small that might have caused that only great differences have reached the statistical significance level.
To summarize, the current study contributes to the understanding on the influence of the combination of FM and depression in the EEG power spectrum. Differences in EEG power spectrum were observed between FM patients with and without depression, showing in patients with untreated depression a hypoactivation of the left hemisphere compared with non-depressed FM women. Antidepressant medication seems to reduce the differences between patients with depression and those who do not suffer from depression symptoms. However, according to our results, there is no enough evidence to provide conclusions about brain dynamics changes as a consequence of the medication intake.
Author contributions
Conceptualization: Santos Villafaina, Juan P. Fuentes-García, Narcis Gusi.
Data curation: Santos Villafaina.
Formal analysis: Santos Villafaina, Carolina Sitges, Daniel Collado-Mateo.
Investigation: Santos Villafaina, Juan P. Fuentes-García, Narcis Gusi.
Methodology: Santos Villafaina, Carolina Sitges, Narcis Gusi.
Project administration: Narcis Gusi.
Resources: Narcis Gusi.
Software: Santos Villafaina, Carolina Sitges.
Supervision: Carolina Sitges, Daniel Collado-Mateo, Juan P. Fuentes-García, Narcis Gusi.
Validation: Carolina Sitges, Daniel Collado-Mateo, Juan P. Fuentes-García, Narcis Gusi.
Visualization: Narcis Gusi.
Writing – original draft: Santos Villafaina, Daniel Collado-Mateo.
Writing – review & editing: Carolina Sitges, Daniel Collado-Mateo, Juan P. Fuentes-García, Narcis Gusi.
Daniel Collado-Mateo orcid: 0000-0002-5140-465X.
Footnotes
Abbreviations: BA = Broadmann area, DMN = default mode network, FM = fibromyalgia, GDS = Geriatric Depression Scale, GDS-15 = 15 - items Geriatric Depression Scale, ICA = independent component analysis, MNI = Montreal Neurological Institute, SSRI = selective serotonin reuptake inhibitors, TPN = task – positive network.
This study was co-funded by the Spanish Ministry of Economy and Competitiveness (reference no DEP2015–70356) in the framework of the Spanish National R+D+i Plan. This study was also supported by the Biomedical Research Networking Center on Frailty and Healthy Aging (CIBERFES) and by FEDER funds from the European Union (CB16/10/00477) a way of doing Europe. This study was also funded by the Research Grant for Groups (GR18155) funded by Junta de Extremadura (Regional Government of Extremadura) and European Regional Development Fund (ERDF/FEDER) ‘a way of doing Europe’. The author SV was supported by a grant from the regional department of economy and infrastructure of the Government of Extremadura and the European Social Fund (PD16008). The funders played no role in the study design, the data collection and analysis, the decision to publish, or the preparation of the manuscript.
The authors have no conflicts of interests to disclose.
References
- [1].Ballenger JC, Davidson JRT, Lecrubier Y, et al. Consensus statement on the primary care management of depression from the international consensus group on depression and anxiety. J Clin Psychiatry 1999;60:54–61. [PubMed] [Google Scholar]
- [2].Broyd SJ, Demanuele C, Debener S, et al. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev 2009;33:279–96. [DOI] [PubMed] [Google Scholar]
- [3].Marchetti I, Koster EHW, Sonuga-Barke EJS, et al. The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev 2012;22:229–51. [DOI] [PubMed] [Google Scholar]
- [4].Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiol Dis 2013;52:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011;15:483–506. [DOI] [PubMed] [Google Scholar]
- [6].Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102:9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cognit Neurosci 2009;21:489–510. [DOI] [PubMed] [Google Scholar]
- [8].Whalley MG, Rugg MD, Brewin CR. Autobiographical memory in depression: An fMRI study. Psychiatry Res 2012;201:98–106. [DOI] [PubMed] [Google Scholar]
- [9].Bocharov AV, Knyazev GG, Savostyanov AN. Depression and implicit emotion processing: An EEG study. Neurophysiol Clin 2017;47:225–30. [DOI] [PubMed] [Google Scholar]
- [10].Stewart JL, Coan JA, Towers DN, et al. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. J Affect Disord 2011;129:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mueller EM, Panitz C, Hermann C, et al. Prefrontal oscillations during recall of conditioned and extinguished fear in humans. J Neurosci 2014;34:7059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Elliott R, Rubinsztein JS, Sahakian BJ, et al. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry 2002;59:597–604. [DOI] [PubMed] [Google Scholar]
- [13].Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American college of rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- [14].Buskila D, Cohen H. Comorbidity of fibromyalgia and psychiatric disorders. Curr Pain Headache Rep 2007;11:333–8. [DOI] [PubMed] [Google Scholar]
- [15].Berger A, Dukes E, Martin S, et al. Characteristics and healthcare costs of patients with fibromyalgia syndrome. Int J Clin Pract 2007;61:1498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chang M-H, Hsu J-W, Huang K-L, et al. Bidirectional association between depression and fibromyalgia syndrome: a nationwide longitudinal study. J Pain 2015;16:895–902. [DOI] [PubMed] [Google Scholar]
- [17].Fallon N, Chiu Y, Nurmikko T, et al. Functional connectivity with the default mode network is altered in fibromyalgia patients. PloS One 2016;11:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin C, Lee S-H, Weng H-H. Gray matter atrophy within the default mode network of fibromyalgia: a meta-analysis of voxel-based morphometry studies. Biomed Res Int 2016;1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gonzalez-Roldan AM, Munoz MA, Cifre I, et al. Altered psychophysiological responses to the view of others’ pain and anger faces in fibromyalgia patients. J Pain 2013;14:709–19. [DOI] [PubMed] [Google Scholar]
- [20].Yesavage JA, Sheikh JI. Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin Gerontol 1986;5:165–73. [Google Scholar]
- [21].Ruffini G, Dunne S, Farres E, et al. ENOBIO dry electrophysiology electrode; first human trial plus wireless electrode system. Conf Proc IEEE Eng Med Biol Soc 2007. [DOI] [PubMed] [Google Scholar]
- [22].Collado-Mateo D, Adsuar JC, Olivares PR, et al. Using a dry electrode EEG device during balance tasks in healthy young-adult males: test-retest reliability analysis. Somatosens Motor Res 2015;32:219–26. [DOI] [PubMed] [Google Scholar]
- [23].Martínez de La Iglesia J, Onís-Vilches M, Dueñas-Herrero R, et al. Versión española del cuestionario de Yesavage abreviado (GDS) para el despistaje de depresión en mayores de 65 años: adaptación y validación. Medifam 2002;12:26–40. [Google Scholar]
- [24].Martí D, Miralles R, Llorach I, et al. Trastornos depresivos en una unidad de convalecencia: experiencia y validación de una versión española de 15 preguntas de la escala de depresión geriátrica de Yesavage. Revista Española de Geriatría y Gerontología 2000;35:7–14. [Google Scholar]
- [25].Villafaina S, Collado-Mateo D, Domínguez-Muñoz FJ, et al. Impact of adding a cognitive task while performing physical fitness tests in women with fibromyalgia: a cross-sectional descriptive study. Medicine 2018;97:e13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Park DC, Glass JM, Minear M, et al. Cognitive function in fibromyalgia patients. Arthritis Rheumat 2001;44:2125–33. [DOI] [PubMed] [Google Scholar]
- [27].Jung TP, Makeig S, Westerfield M, et al. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clin Neurophysiol 2000;111:1745–58. [DOI] [PubMed] [Google Scholar]
- [28].Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol 2002;24:5–12. [PubMed] [Google Scholar]
- [29].Fuchs M, Kastner J, Wagner M, et al. A standardized boundary element method volume conductor model. Clin Neurophysiol 2002;113:702–12. [DOI] [PubMed] [Google Scholar]
- [30].Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. Neuroimage 2007;34:1600–11. [DOI] [PubMed] [Google Scholar]
- [31].Wagner M, Fuchs M, Kastner J. Evaluation of sLORETA in the presence of noise and multiple sources. Brain Topography 2004;16:277–80. [DOI] [PubMed] [Google Scholar]
- [32].Sekihara K, Sahani M, Nagarajan SS. Localization bias and spatial resolution of adaptive and non-adaptive spatial filters for MEG source reconstruction. Neuroimage 2005;25:1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Frei E, Gamma A, Pascual-Marqui R, et al. Localization of MDMA-induced brain activity in healthy volunteers using low resolution brain electromagnetic tomography (LORETA). Hum Brain Mapp 2001;14:152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 2002;15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Diener C, Kuehner C, Flor H. Loss of control during instrumental learning: a source localization study. NeuroImage 2010;50:717–26. [DOI] [PubMed] [Google Scholar]
- [36].Prasko J, Horacek J, Zalesky R, et al. The change of regional brain metabolism (18FDG PET) in panic disorder during the treatment with cognitive behavioral therapy or antidepressants. Neuro Endocrinol Lett 2004;25:340–8. [PubMed] [Google Scholar]
- [37].Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnormal Psychol 1991;100:535–45. [DOI] [PubMed] [Google Scholar]
- [38].Shobe ER. Independent and collaborative contributions of the cerebral hemispheres to emotional processing. Front Hum Neurosci 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovas Dis 2013;35:23–39. [DOI] [PubMed] [Google Scholar]
- [40].Onoda K, Kuroda Y, Yamamoto Y, et al. Post-stroke apathy and hypoperfusion in basal ganglia: SPECT study. Cerebrovas Dis 2011;31:6–11. [DOI] [PubMed] [Google Scholar]
- [41].Lee BT, Seok JH, Lee BC, et al. Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Progr Neuro-Psychopharmacol Biol Psychiatry 2008;32:778–85. [DOI] [PubMed] [Google Scholar]
- [42].Barry ES, Naus MJ, Rehm LP. Depression and implicit memory: understanding mood congruent memory bias. Cognit Therapy Res 2004;28:387–414. [Google Scholar]
- [43].Grimm S, Boesiger P, Beck J, et al. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology 2009;34:932–43. [DOI] [PubMed] [Google Scholar]
- [44].Bertolucci PH, de Oliveira FF. Cognitive impairment in fibromyalgia. Curr Pain Headache Rep 2013;17:344. [DOI] [PubMed] [Google Scholar]
- [45].de Melo LF, Da-Silva SL. Neuropsychological assessment of cognitive disorders in patients with fibromyalgia, rheumatoid arthritis, and systemic lupus erythematosus. Revista brasileira de reumatologia 2012;52:181–8. [PubMed] [Google Scholar]
- [46].Gracely RH, Ambrose KR. Neuroimaging of fibromyalgia. Best Pract Res Clin Rheumatol 2011;25:271–84. [DOI] [PubMed] [Google Scholar]
- [47].Grajny K, Pyata H, Spiegel K, et al. Depression Symptoms in Chronic Left Hemisphere Stroke Are Related to Dorsolateral Prefrontal Cortex Damage. J Neuropsychiatry Clin Neurosci 2016;28:292–8. [DOI] [PubMed] [Google Scholar]
- [48].Sheline YI, Barch DM, Donnelly JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 2001;50:651–8. [DOI] [PubMed] [Google Scholar]
- [49].Haghighi M, Ludyga S, Rahimi B, et al. In patients suffering from major depressive disorders, quantitative EEG showed favorable changes in left and right prefrontal cortex. Psychiatry Res 2017;251:137–41. [DOI] [PubMed] [Google Scholar]
- [50].Fales CL, Barch DM, Rundle MA, et al. Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord 2009;112:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hammond DC. Neurofeedback treatment of depression and anxiety. J Adult Develop 2005;12:131–7. [Google Scholar]
- [52].Quaedflieg CWEM, Smulders FTY, Meyer T, et al. The validity of individual frontal alpha asymmetry EEG neurofeedback. Soc Cogn Affect Neurosci 2016;11:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Deslandes AC, Moraes H, Alves H, et al. Effect of aerobic training on EEG alpha asymmetry and depressive symptoms in the elderly: a 1-year follow-up study. Braz J Med Biol Res 2010;43:585–92. [DOI] [PubMed] [Google Scholar]
- [54].Steinberg B, Blum K, McLaughlin T, et al. Low-resolution electromagnetic tomography (LORETA) of changed brain function provoked by pro-dopamine regulator (KB220z) in one adult ADHD case. Open J Clin Med Case Rep 2016;2: [PMC free article] [PubMed] [Google Scholar]
- [55].Pascual-Marqui RD, Lehmann D, Koenig T, et al. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res 1999;90:169–79. [DOI] [PubMed] [Google Scholar]
- [56].Eugene AR, Masiak J. Electrophysiological neuroimaging using sLORETA comparing 100 Schizophrenia Patients to 48 Patients with Major Depression. Brain 2014;5:16–25. [PMC free article] [PubMed] [Google Scholar]
- [57].Cannon RL, Baldwin DR, Shaw TL, et al. Reliability of quantitative EEG (qEEG) measures and LORETA current source density at 30 days. Neurosci Lett 2012;518:27–31. [DOI] [PubMed] [Google Scholar]
- [58].Thatcher RW, North D, Biver C. Evaluation and validity of a LORETA normative EEG database. Clin Eeg Neurosci 2005;36:116–22. [DOI] [PubMed] [Google Scholar]
- [59].Pascual-Marqui RD, Esslen M, Kochi K, et al. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods Find Exp Clin Pharmacol 2002;24 Suppl C:91–5. [PubMed] [Google Scholar]


