Abstract
Rationale:
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are 2 rare but life-threatening diseases characterized by detachment of epidermis, bullous skin lesions, and mucous membrane erosions. Drugs are highly suspected to be the causative agents. We report a case of SJS/TEN induced by oseltamivir, which is a very rare event.
Patient concerns:
A 9-year-old girl with upper respiratory tract infections presented with generalized maculopapular rash the second day after taking oseltamivir.
Diagnosis:
The diagnosis of SJS/TEN was made based on cytotoxic skin lesions and mucous membrane involvement.
Interventions:
After discontinuing of the drug and combination therapy of corticosteroid and human immunoglobulin initiation, the lesions were improved. Human leukocyte antigen (HLA) gene sequencing was done.
Outcomes:
The girl was followed-up for 1 year. The skin and mucous membranes symptoms were relieved.
Lessons:
We report this case to attract attention to the rare but serious side effect of this antiviral drug.
Keywords: human leukocyte antigen, oseltamivir, Stevens–Johnson syndrome, toxic epidermal necrolysis
1. Introduction
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are known as a delayed hypersensitivity reaction to medicines. Their bullous mucocutaneous reactions (cutaneous and mucous membrane lesions including ocular, oral, and genital) are characterized by extensive necrosis and detachment of epidermis. SJS and TEN are defined according to their degree of skin detachment. SJS, which is less severe, showed as a skin detachment <10%. SJS, whose skin involvement is >30%, is much more severe.[1] SJS/TEN can lead to multi-organ to implicate[2–5] which not only involved cutaneous and mucous membranes but also several internal organs. Thus, it is necessary to take a multidisciplinary treatment strategy. The first step is to withdrawal the potentially causative drugs immediately. Then the patients should be referred to hospital for treatment. The commonly used regimens for SJS/TEN are systemic corticosteroids, immunoglobulins, and cyclosporine A.
Drugs are the most common cause of SJS/TEN. Drugs at a high risk of SJS/TEN include anti-epileptic drugs, anti-infective sulfonamides, non-steroidal anti-inflammatory drugs (oxicam type), allopurinol, nevirapine, and chlormezanone. Except for these conventional medications, some new biologics and herbal remedies are also considered as related drugs. Oseltamivir, a prodrug of the neuraminidase inhibitor [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid (Ro 64–0802), is widely used as an antiviral agent for prevention and treatment of influenza. Despite this drug seems to be very well tolerated to most patients, there have been reports of some less common side effects with the increased use of oseltamivir, such as neuropsychiatric events[6,7] and serious skin/hypersensitivity reactions.[8,9] Through literature researches, we found that oseltamivir-induced SJS/TEN was rarely reported. Studies have reported that increased risk of SJS/TEN to certain drugs might related to specific human leukocyte antigen (HLA).[10] However, many factors contributing to these hypersensitivity reactions still have to be identified, such as drug-specific T cell-mediated cytotoxicity, genetic linkage with non-HLA-genes, TCR restriction, as well as virus-induced and autoimmune forms of epidermal necrolysis not related to drugs. Here, we report a rare case of oseltamivir-induced SJS/TEN and give a review of the relevant literature. Furthermore, HLA gene sequencing was performed in this case and the potential significance is analyzed.
2. Case presentation
A 9-year-old female attended a clinic in Shandong province of China. This girl developed unexplained fever the day before and self-medication with ibuprofen suspension in the setting of the fever. She was diagnosed with upper respiratory infection based on clinical routine biochemistry test and was given oseltamivir (60 mg, bid) for treatment. On the second day after taking oseltamivir, the patient not only showed repeated attack of fever but also appeared with red, raised, pressure of fade rashes around the forehead. A cutaneous drug reaction was suspected. The patient stopped taking oseltamivir immediately on their own. The rashes had rapidly spread toward her face, body, arms, and legs (Fig. 1A, B). Then multiple oral ulcers, mucosa congestion, and myricarubra tongue were also noted. She was brought to their local hospital that day and admitted under the presumed diagnosis of Kawasaki disease. She was managed with intravenous antibiotics for the treatment of pneumonia at the same time. It is worth noting that ibuprofen suspension, which has been taken many times before, was continued to use for fever-lowering. Laboratory investigations showed leukocytosis (WBC, 2.83 × 109), neutrophile granulocyte percentage (71.9%), T lymphocyte percentage (19.1%), Serum amyloid A (SAA, 37.9 mg/L) and elevated C-reactive protein (CRP, 5.27 mg/mL).Cervical lymph node enlargement was found inphysical examination. On the second hospital day, the rash had worsened with a red, confluent maculopapular exanthema, and bullae and denudation of the epidermis in large patches of the back were now observed (Fig. 1C, D). More than that, the patient showed a similar appearance to that of a large burned. A diagnosis of TEN was made. Then she was transferred to an intensive care unit and stabilized with intravenous immunoglobulin (IvIg), methylprednisolone 100 mg qd, tobramycin eye drops, recombinant bovine alkaline fibroblast growth factor and mupirocin ointment. The patient was moved out of intensive care on the twentieth day. After methylprednisolone and other recommended supportive care (eye symptomatic treatment) for another 20 days, the patient improved and was discharged in a good general condition. After obtaining informed consent, we took the pictures and carried out gene sequencing of HLA. The sequencing results were compared with the known genetic loci related to SIS/TEN. We found that HLA-A∗0206 was positive in our case. The sequencing results were discussed in the next section.
Figure 1.
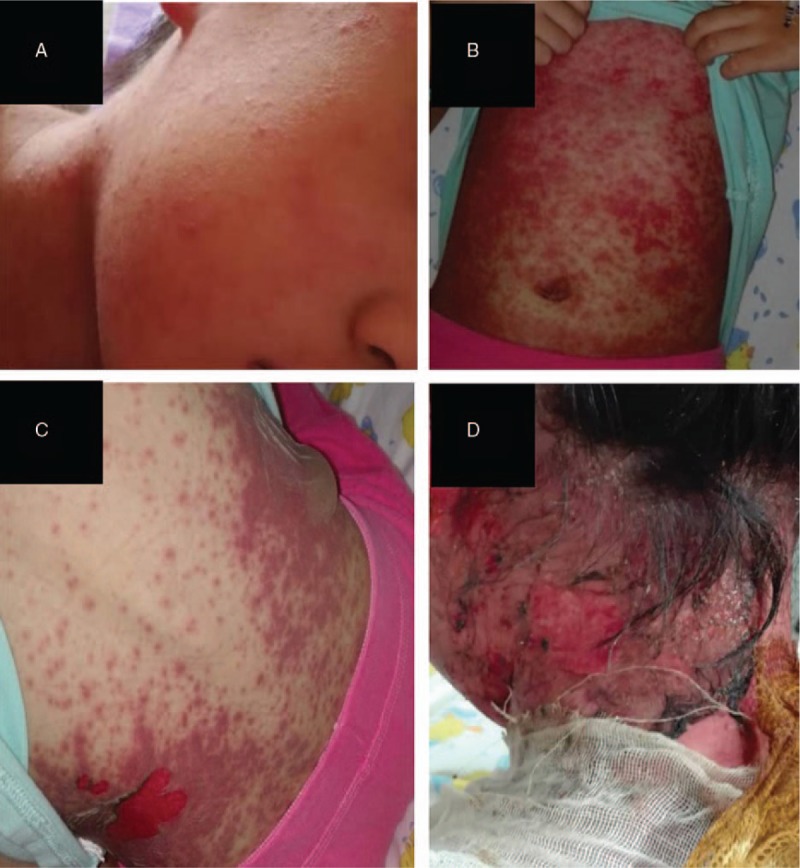
The progression of skin involvement in our patient. (A), (B) Rapidly spread of rashes over the face and body; (C), (D) Denudation of the epidermis and formation of blisters of the back.
3. Discussion
Based on extensive research of literature, we first summed up the genetics linked to SJS/TEN. Carbamazepine (CBZ), an aromatic antiepileptic, is one of the first drugs that could be linked SJS/TEN. Many researches demonstrated that there is a strong relationship between HLA-B∗1502 and CBZ-induced SJS/TEN.[11,12] Due to the high allele frequency found not only in Han Chinese but also in other Asian ethnics, the US FDA recommends HLA-B∗1502 genotyping in all Asian patients before administering CBZ.[13] Other studies further showed there was a significant correlation between antiepileptics-associated SJS/TEN and HLA-A∗3101,[13] HLA-A∗1101[14] or HLA-A∗2402.[15] HLA-B∗1502 was also proved to be related to other antiepileptics such as oxcarbazepine, lamotrigine, and phenytoin.[16,17] Allopurinol as a major cause of SJS/TEN was studied extensively in terms of genetic linkage. A relevant association of HLA-B∗5801 and allopurinol-associated SJS/TEN could also be found in variety studies.[18] HLA-B∗5901 was found to strongly link to methazolamide-associated SJS/TEN in both Han Chinese and Koreans.[19,20] HLA-A∗0206 is known to be strongly associated with cold medicine-associated SJS/TEN.[21]
Further compared with our sequencing result, we found that apart from HLA-A∗0206, all the alleles mentioned above were negative. With the limitation of the small sample size, we could not jump to conclusions with HLA-A∗0206 and oseltamivir-associated SJS/TEN. The factors contributing to it still have to be identified with further studies.
TEN is refined as a severe reaction of exfoliation leading to an epidermal detachment of greater than 30% body surface area (BSA). Another spectrum is SJS, which is a term used to describe an epidermal detachment of less than 10% BSA. For 10% to 30% BSA is termed as an SJS/TEN overlap. SJS/TEN is a very rare event, with an estimated prevalence of 1 to 7 ppm (parts per million) each year and 0.4 to 1.5 ppm each year, respectively. Mortality rate are 1% to 3% and 30% to 50%, respectively.[22]
Although many mechanisms may involve in SJS/TEN, including reactive drug-mediated cytotoxicity, pharmacogenomics, infections, and so on, no convincing theory has appeared until now. Among them, most SJS/TEN are induced by drugs. By now, more than 100 drugs were considered to be associated with SJS/TEN, including sulfonamides (metazolamide and acetazolamide), anticonvulsants (phenytoin, phenobarbital, carbamazepine, and lamotrigine), nonsteroidal anti-inflammatory drugs (NSAIDs), xanthineoxidase inhibitors (allopurinol), and nevirapine (antiretroviral drug).[23,24]
In this case, the patient had not taken oseltamivir previously and had no allergies or family history of allergies. Before the rash appeared, he took only 2 medicines: ibuprofen suspension and oseltamivir. On 1 hand there is medical history of ibuprofen suspension, on the other hand, the patient continuously taking this medicine for easing fever at the time of hospitalization. Thus we ruled out the contribution of ibuprofen suspension. Although oseltamivir usage resulting in SJS/TEN has been rarely reported, the adverse drug reaction probability scale indicated a possible relation between oseltamivir and the skin manifestations. First, there has been 1 published case report associated with oseltamivir related (in Spanish) cutaneous adverse reactions,[8] and serious skin/hypersensitivity reactions such as SJS/TEN have been mentioned and warned in prescribing information of oseltamivir.[9] Second, the skin manifestations appeared shortly after oseltamivir was administered. Therefore we inferred that oseltamivir was the contributor to SJS/TEN in this case.
For the restriction of medical condition in their local hospital, histopathological investigation and genetic tests were not done at the time of hospitalization. The diagnosis of TEN is based on clinical manifestations with acute onset of rapidly expanding targetoid erythematous macules, necrosis, and detachment of the epidermis along with erythema, erosions and crusting of 2 or more mucosal surfaces. The mortality of TEN has been estimated at 30%, and intravenous immunoglobulins and steroids pulse may be efficacious.[25,26] Although the use of steroids is quite controversial in SJS/TEN patients, immunoglobulins and steroids have contributed to the resolution of the TEN like lesions in this case.
Pharmacogenetic studies have been advanced by leaps and bounds over the past several decades. It has been supposed that there is a relationship between HLA-related genetic factors (not the only role) and the susceptibility to develop SJS/TEN in response to various drugs.[27] Considering there is no definite genetic marker of oseltamivir induced SJS/TEN, a gene sequencing of HLA was done. Compared with the existing research results by others, we found that the alleles that proved to be related to other drugs (antiepileptics or methazolamide)-associated SJS/TEN were negative in this case. However, the HLA-A∗0206 was found to be positive in our case. A study out of Japan found that there was a strong relationship between HLA-A∗0206 and cold medicine-associated SJS/TEN.
In conclusion, we have described the first case of oseltamivir-induced SJS in China. Whether HLA-A∗0206 exert a role in oseltamivir-associated SJS/TEN remains unclear for findings are limited to a single case. It will be necessary for us to recruit more patients to clarify the pathomechanisms and genetic markers of oseltamivir-induced SJS/TEN.
Author contributions
Conceptualization: Bo Zhang.
Data curation: Wei Zuo.
Formal analysis: Wei Zuo.
Funding acquisition: Wei Zuo.
Investigation: Wei Zuo.
Methodology: Jun Li.
Resources: Liping Wen.
Supervision: Jun Li.
Validation: Dan Mei.
Writing – original draft: Wei Zuo.
Writing – review & editing: Liping Wen, Qiang Fu, Bo Zhang.
Footnotes
Abbreviations: BSA = body surface area, CBZ = Carbamazepine, HLA = human leukocyte antigen, SJS = Stevens–Johnson syndrome, TEN = toxic epidermal necrolysis.
WZ and L-PW contributed equally to this work.
Informed written consent was obtained from the patient for publication of this case report and accompanying images.
All methods concerned in this article were carried out in accordance with the approved guidelines. Here, we confirm that all case information was approved by Peking Union Medical College Hospital. The patient has provided informed consent for publication of the case.
This work was supported by funding from by National Natural Science Foundation of China Grants No.81601033, Beijing Natural Science Foundation No.7174342, and CAMS Innovation Fund for Medical Science (CAMS-2017-I2M-1–011).
The authors have no conflicts of interest to disclose.
References
- [1].Marianne L, Carlo M, Benedetta TB, et al. Current perspectives on Stevens–Johnson syndrome and toxic epidermal necrolysis. Clinic Rev AllergImmunol 2017. [Google Scholar]
- [2].Chantaphakul H, Sanon T, Klaewsongkram J. Clinical characteristics and treatment outcome of Stevens-Johnson syndrome and toxic epidermal necrolysis. Exp Ther Med 2015;10:519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang L, Mei XL. Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in 88 Chinese patients. Chin Med J 2017;130:1062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lim VM, Do A, Berger TG, et al. A decade of burn unit experience with Stevens-Johnson syndrome/toxic epidermal necrolysis: clinical pathological diagnosis and risk factor awareness. Burns 2016;42:836–43. [DOI] [PubMed] [Google Scholar]
- [5].McCullough M, Burg M, Lin E, et al. Steven Johnson syndrome and toxic epidermal necrolysis in a burn unit: a 15-year experience. Burns 2017;43:200–5. [DOI] [PubMed] [Google Scholar]
- [6].Toovey S, Prinssen EP, Rayner CR, et al. Post-marketing assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir: an updated review. Adv Ther 2012;29:826–48. [DOI] [PubMed] [Google Scholar]
- [7].Hoffman KB, Demakas A, Erdman CB, et al. Neuropsychiatric adverse effects of oseltamivir in the FDA adverse event reporting system, 1999–2012. BMJ 2013;347:4656. [DOI] [PubMed] [Google Scholar]
- [8].Luna P, Zuazaga M, Chede C, et al. Toxic epidermal necrolysis after treatment with oseltamivir. Case report. Arch Argent Pediatr 2010;108:e76–8. [DOI] [PubMed] [Google Scholar]
- [9].Tamiflu (oseltamivir phosphate) prescribing information. Available at: www.gene.com/download/pdf/tamiflu_prescribing.pdf [access date January 02, 2013]. [Google Scholar]
- [10].Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature 2004;428:486. [DOI] [PubMed] [Google Scholar]
- [11].Khor AH, Lim KS, Tan CT, et al. HLA-A∗31: 01 and HLA-B∗15:02 association with Stevens-Johnson syndrome and toxic epidermal necrolysis to carbamazepine in a multiethnic Malaysian population. Pharmacogenet Genomics 2007;27:275–8. [DOI] [PubMed] [Google Scholar]
- [12].Mehta TY, Prajapati LM, Mittal B, et al. Association of HLA-B∗1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol 2009;75:579–82. [DOI] [PubMed] [Google Scholar]
- [13].Ferrell PB, Jr, McLeod HL. Carbamazepine, HLA-B∗1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics 2008;9:1543–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ramirez E, Bellon T, Tong HY, et al. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol Res 2017;115:168–78. [DOI] [PubMed] [Google Scholar]
- [15].Shi YW, Min FL, Zhou D, et al. HLA-A∗24:02 as a common risk factor for antiepileptic drug-induced cutaneous adverse reactions. Neurology 2017;88:2183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hung SI, Chung WH, Liu ZS, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics 2010;11:349–56. [DOI] [PubMed] [Google Scholar]
- [17].Chen CB, Hsiao YH, Wu T, et al. Taiwan severe cutaneous adverse reaction consortium. Risk and association of HLA with oxcarbazepine-induced cutaneous adverse reactions in Asians. Neurology 2017;88:78–86. [DOI] [PubMed] [Google Scholar]
- [18].Niihara H, Kaneko S, Ito T, et al. HLA-B∗58:01 strongly associates with allopurinol-induced adverse drug reactions in a Japanese sample population. J Dermatol Sci 2013;71:150–2. [DOI] [PubMed] [Google Scholar]
- [19].Yang F, Xuan J, Chen J, et al. HLA-B∗59:01: a marker for Stevens-Johnson syndrome/toxic epidermal necrolysis caused by methazolamide in Han Chinese. Pharmacogenomics J 2016;16:83–7. [DOI] [PubMed] [Google Scholar]
- [20].Kim SH, Kim M, Lee KW, et al. HLA-B∗5901 is strongly associated with methazolamide induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Pharmacogenomics 2010;11:879–84. [DOI] [PubMed] [Google Scholar]
- [21].Ueta M, Sawai H, Shingaki R, et al. Genome-wide association study using the ethnicity-specific Japonica array: identification of new susceptibility loci for cold medicine-related Stevens-Johnson syndrome with severe ocular complications. J Hum Genet 2017;62:485–9. [DOI] [PubMed] [Google Scholar]
- [22].Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens-Johnson syndrome: a review. Crit Care Med 2011;39:1521–32. [DOI] [PubMed] [Google Scholar]
- [23].Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994;331:1272–85. [DOI] [PubMed] [Google Scholar]
- [24].Wiffen PJ, Derry S, Moore RA, et al. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev 2011;2011:5451–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Barron SJ, Del Vecchio MT, Aronoff SC. Intravenous immunoglobulin in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a meta-analysis with meta-regression of observational studies. Int J Dermatol 2015;54:108–15. [DOI] [PubMed] [Google Scholar]
- [26].Mahar PD, Wasiak J, Hii B, et al. A systematic review of the management and outcome of toxic epidermal necrolysis treated in burns centres. Burns 2014;40:1245–54. [DOI] [PubMed] [Google Scholar]
- [27].Rufini S, Ciccacci C, Politi C, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: an update on pharmacogenetics studies in drug-induced severe skin reaction. Pharmacogenomics 2015;16:1989–2002. [DOI] [PubMed] [Google Scholar]


