Abstract
Concomitant influenza and cryptococcal infections are rare. Herein, we describe an unusual case of an avian influenza A (H7N9) infection with several severe mixed bacterial infections and systemic super-infection with Cryptococcus neoformans presenting as ventilator-associated pneumonia (VAP) and bloodstream infection in a previously immunocompetent man during hospitalization.
A 58-year-old man was admitted to our hospital complaining of hyperpyrexia, dyspnoea, cough, and phlegm with blood. A chest computed tomography scan revealed multiple ground-glass opacities and consolidation in both lungs with right pleural effusion. An initial sputum test was positive for influenza A (H7N9) virus. After antiviral treatment and other supportive measures, the patient's condition improved. However, the patient's condition deteriorated again approximately 2 weeks after admission, and bronchoalveolar lavage fluid (BALF) and blood cultures were positive for C. neoformans. Therapy with intravenous liposomal amphotericin B and fluconazole was started. After a 2-week antifungal treatment, BALF and blood cultures were negative for C. neoformans. However, the patient had persistent lung infiltrates with severe pulmonary fibrosis with a prolonged course of disease. On hospital day 40, BALF and blood cultures were both positive for multidrug-resistant Stenotrophomonas maltophilia. Finally, the patient developed septic shock, disseminated intravascular coagulation and multi-organ failure and succumbed to treatment failure.
Cryptococcal infection can occur in patients with severe influenza during hospitalization with a more severe condition, and the clinician should be aware of this infection.
Keywords: avian, Cryptococcus neoformans, H7N9, influenza virus, ventilator-associated pneumonia
1. Background
Infections due to Cryptococcus species occur globally and can affect both immunocompromised (IC) and non-IC hosts.[1] To date, many cases of cryptococcal infection have been reported,[2–5] but only 3 cases of concomitant severe influenza and cryptococcal infections have been mentioned in the English and Chinese literature.[6–8] Herein, we describe an unusual case of an avian influenza A (H7N9) infection with several severe mixed bacterial infections and systemic super-infection with Cryptococcus neoformans presenting as ventilator-associated pneumonia (VAP) and bloodstream infection in a previously immunocompetent man during hospitalization. There is no report in the literature of concomitant infections of H7N9-influenza and disseminated cryptococcosis.
2. Methods
We describe the data of the case and extensively discuss the relation between severe influenza and cryptococcal infections during hospitalization. This study was approved by the ethics committee of Fuzhou Pulmonary Hospital of Fujian. Informed consent was obtained from a relative of the patient in the study.
3. Case presentation
On January 15, 2016, a 58-year-old man was admitted to our respiratory intensive care unit (RICU) complaining of hyperpyrexia for 7 days and dyspnoea, cough and phlegm with blood for 2 days. The patient had a history of hypertension for 2 years. His travel history revealed that he bought a chicken from a local live poultry market (LPM) and had poultry-related exposure 7 days before his symptom onset. The patient was diagnosed with community-acquired pneumonia (CAP) and was given antibacterial therapy alone for several days in a local community hospital; however, his condition failed to improve. A week after symptom onset, a chest computed tomography (CT) scan was obtained and revealed multiple ground-glass opacities and consolidation in both lungs with right pleural effusion (Fig. 1). Then, he was transferred to our hospital. The laboratory results showed increased C-reactive protein (CRP, >200 mg/L) and procalcitonin (PCT, 9.9 ng/mL) levels. Leukopenia and lymphopenia with significantly reduced T lymphocyte subgroups including CD3, CD4, and CD8 T cells in peripheral blood were also noted upon admission. A human immunodeficiency virus (HIV) antibody test was negative. Arterial blood gas analysis showed a pH of 7.46, a partial pressure of carbon dioxide (PaCO2) of 24.5 mm Hg, and a partial pressure of oxygen (PaO2) of 46.1 mm Hg while receiving 10 L/min inspired oxygen with a partial rebreathing mask. A bedside chest X-ray revealed multiple infiltrates in both lungs (Fig. 2). Severe acute respiratory distress syndrome (ARDS) led to endotracheal intubation and mechanical ventilation on the first day of hospitalization (Table 1), and initial antibiotic treatment with moxifloxacin and cefoperazone-sulbactam along with corticosteroids and continuous renal replacement therapy (CRRT) were also initiated. Then, the result of an initial sputum test obtained upon admission, which was performed as a qualitative assay and confirmed by the Fuzhou City Centre for Disease Control and Prevention, was positive for influenza A (H7N9) virus and negative for other prevalent strains of influenza by reverse transcription polymerase chain reaction (RT-PCR). Conversely, bacterial and fungal cultures of the patient's sputum and bronchoalveolar lavage fluid (BALF) were negative. He was diagnosed with avian influenza A (H7N9), and antiviral therapy with oseltamivir (150 mg twice daily) was immediately started. However, ARDS still progressively developed despite the treatment of lung recruitment and prone-position ventilation with a need for high pressure and FiO2 ventilation (Table 1), and veno-venous extracorporeal membrane oxygenation (ECMO) was required and started on hospital day 4 (11 days after symptom onset) because of the failure of invasive mechanical ventilation. Meanwhile, blood culture yielded Staphylococcus haemolyticus (MRSH) with a significant rise in PCT (85.0 ng/mL), suggesting bloodstream infection with MRSH, and vancomycin was started. Due to severe mixed infection and the use of ECMO, he was also administered imipenem/cilastatin and caspofungin at the same time. During this period, the BALF also yielded Pseudomonas aeruginosa. After this treatment, the serum levels of CRP and PCT gradually improved and decreased to normal levels, and a chest X-ray revealed an improvement of infiltrates in both lungs (Fig. 3). However, subcutaneous and mediastinal emphysemas emerged on hospital day 10; then, the ventilator parameters were decreased (Table 1), and the emphysemas were significantly relieved. BALF was retested on hospital day 14 (21 days after symptom onset), but the result was still positive for influenza A (H7N9) virus. On hospital day 16, the patient's condition deteriorated again. A chest X-ray revealed the rapid progression of infiltrative lesions in both lungs (Fig. 4). Both a 1,3-β-d-glucan (G) test and a galactomannan (GM) test were negative. The patient was considered to have new bacterial co-infections, and several strong antibacterial drugs were successively administered for treatment of the aggravated infection without improvement. On hospital day 23, BALF and blood cultures were positive for C. neoformans (Fig. 5A, B, C, and D). The C. neoformans strain is sensitive to flucytosine, fluconazole, voriconazole, amphotericin B, and itraconazole. A serum cryptococcal capsular polysaccharide antigen test by lateral flow assay (LFA), which was performed as a qualitative assay, was positive. The patient was diagnosed with disseminated cryptococcal infection involving the lungs and bloodstream. Caspofungin was ceased, and intravenous liposomal amphotericin B (1 mg/kg of body weight) and fluconazole were started. Meanwhile, examinations of possible exogenous sources of cryptococcal infection, including various examination and treatment equipment (fibre bronchoscope, breathing machine, etc), indoor air and respiratory tract specimens from the other inpatients in the same period in RICU, were performed, but the results were negative for Cryptococcus species. On hospital days 19 and 20 (26 and 27 days after symptom onset, respectively), BALF specimens were tested again, and the results were negative for influenza A (H7N9) virus. After a 2-week antifungal treatment, BALF and blood cultures were negative for C. neoformans. However, the patient had persistent lung infiltrates with progressive pulmonary fibrosis, and ventilator monitoring data showed a constant decrease in tidal volume (Table 1). On hospital day 40, BALF and blood cultures were both positive for multidrug-resistant Stenotrophomonas maltophilia (SMA). The patient's condition deteriorated, despite strong anti-infective therapy, with progressively increasing PCT from 1.7 to 20.7 ng/mL due to the new aggravated infection with SMA involving the lungs and bloodstream. Finally, the patient developed septic shock, disseminated intravascular coagulation (DIC) and multi-organ failure (MOF) and died on hospital day 47 (54 days after symptom onset).
Figure 1.
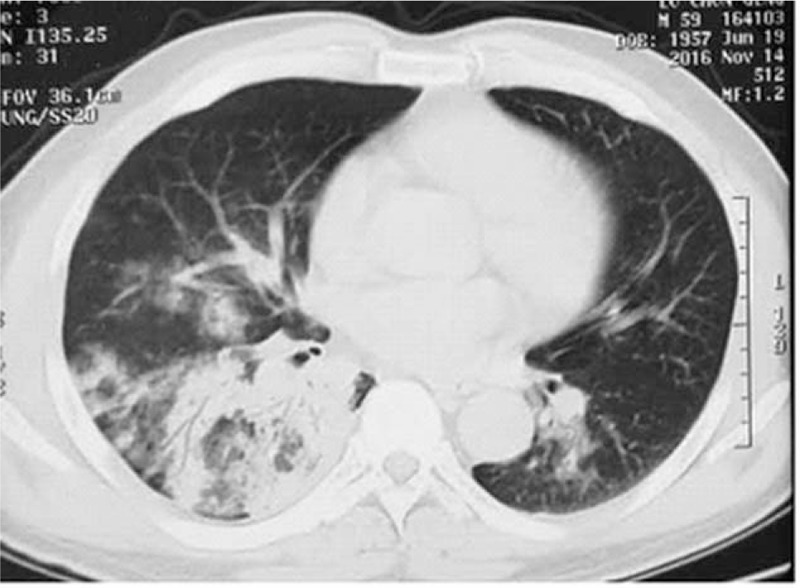
Chest computed tomography (CT) scan revealing multiple ground-glass opacities and consolidation in both lungs with right pleural effusion.
Figure 2.
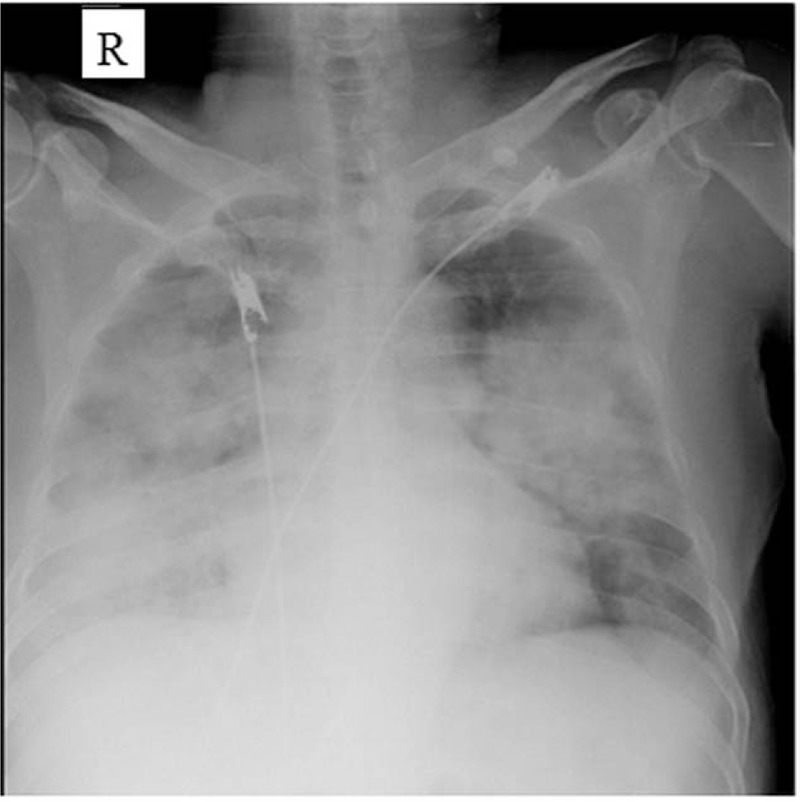
Chest X-ray revealing multiple infiltrates in both lungs.
Table 1.
Major ventilator settings and monitoring data and serial changes during RICU stay and ventilator support.
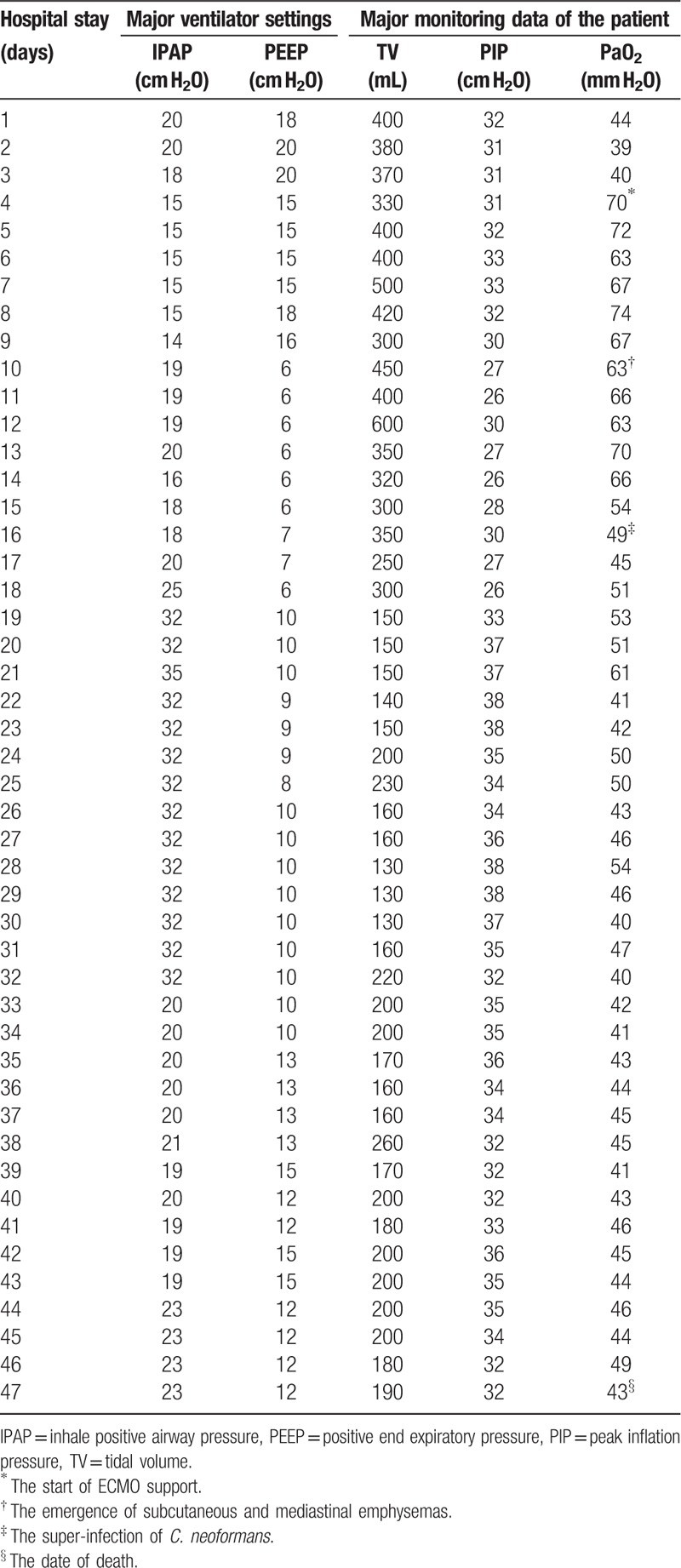
Figure 3.
Chest X-ray revealing an improvement of infiltrates in both lungs.
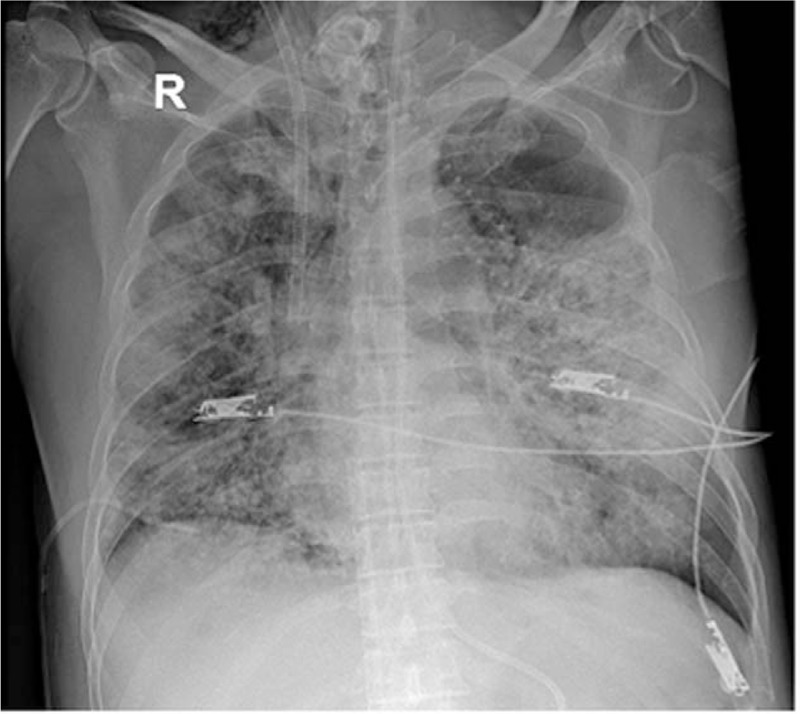
Figure 4.
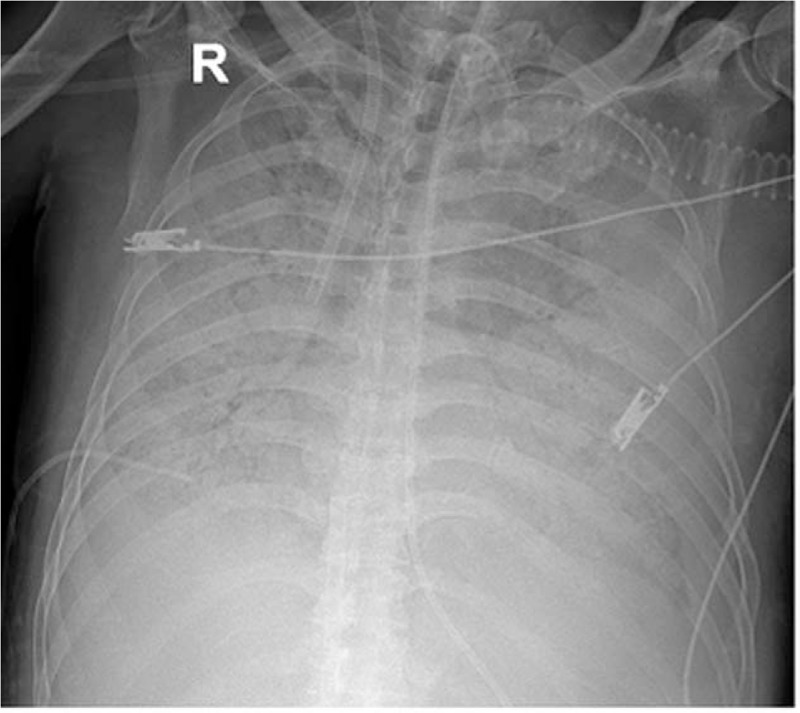
Chest X-ray revealing a rapid progression of infiltrative lesions in both lungs.
Figure 5.
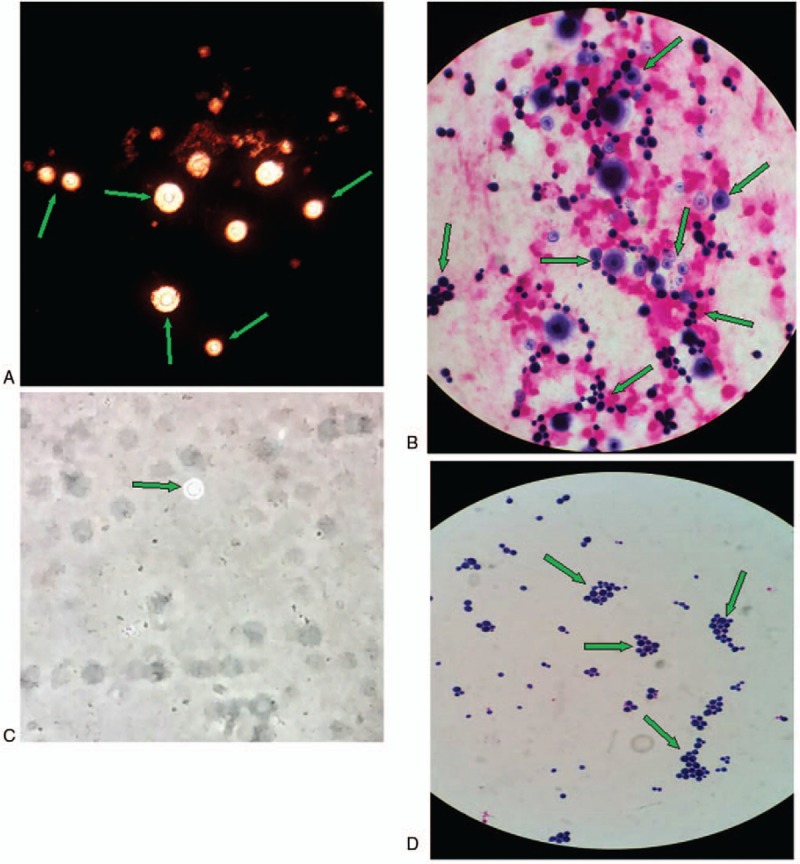
The findings for bronchoalveolar lavage fluid (BALF) culture specimens and blood culture specimens under oil immersion lenses. A: India ink examination of a BALF specimen demonstrating typical transparent thick capsule Cryptococcus spores (green arrows); B: Gram's stain smear of a BALF sample showing the yeast form of C. neoformans and that the capsules could be stained and found in some of them (green arrows). C: India ink examination of a blood specimen demonstrating the typical transparent thick capsule Cryptococcus spore (green arrow); D: Gram's stain smear of pure fungal colonies cultured from a blood sample showing C. neoformans without staining of the capsule (green arrows).
4. Conclusion
In this study, the patient was diagnosed with H7N9-influenza first and then had complications with several severe bacterial mixed infections and disseminated cryptococcosis presenting as VAP and bloodstream infection with severe ARDS. This report showed that cryptococcal infection can occur in a patient with severe influenza during hospitalization with a severe condition, with the exception of the common emergence of bacterial co-infection.
5. Discussion and literature review
Generally, the fungal pathogens that cause super-infections are predominantly Candida spp. and Aspergillus spp., which are most commonly isolated in patients with influenza infection,[9–16] but C. neoformans has rarely been reported in such patients. Cryptococcosis is an invasive fungal infection that is more common in immunocompromised (IC) hosts, while the principal predisposing factor is HIV infection.[17] The other susceptibility factors are the presence of long-term immunosuppressants or corticosteroid therapy, hematologic malignancies, severe diabetes mellitus, and other conditions that impair T cell mediated immunity.[18,19] In our case, there were several risk factors for cryptococcal infection after H7N9 virus infection. First, the BALF specimens were continuously positive for influenza A (H7N9) virus for more than 3 weeks after symptom onset despite antiviral treatment. A lengthy course of avian influenza virus infection may result in immunologic defects, impairment of normal ciliary function and leukopenia.[20] Usually, host defence against cryptococcal infection mainly depends on the host immune function and defensive response to infection,[21] and the cellular immune response is the main immune mechanism of cryptococcal infection control.[22,23] Previous reports have found that a prominent clinical feature of atypical influenza, including H7N9, H5N1, and H1N1, is lymphopenia,[24–26] which is similar to what occurred in our patient. Additionally, many studies of influenza infection have also revealed that the number of T lymphocyte subgroups, including CD3 cells, CD4 cells, CD8 cells, regulatory T (Treg) cells, helper T (Th) 17 cells, etc, declined dramatically,[27–30] along with alterations in the ratio of T cell subgroups, such as a decrease in CD4/CD8.[27,28] It has also been reported that the T lymphocyte subgroups including CD3, CD4, and CD8 T cells in peripheral blood and lung tissue were significantly decreased,[31] as well as peripheral blood monocytes,[32] natural killer (NK) cells, and NKT cells[28] being significantly reduced, suggesting a weakened immune status in patients with influenza infection. Furthermore, some antimicrobial cytokines such as interleukin (IL)-17 also decreased in influenza patients.[30] All the above studies indicated that obvious cellular immune dysfunction was primarily driven by viral-induced apoptosis of T lymphocytes,[31] which greatly increased the risk of cryptococcal infection and occurred in influenza patients, especially severe influenza patients. Second, in the early course, the patient's condition deteriorated progressively, and he developed severe ARDS requiring treatment with systemic steroids. Corticosteroid therapy should also result in an IC state and significantly increase the risk of fungal infection.[7,13,14] Finally, the use of several broad-spectrum antibiotics for the treatment of bacterial co-infection could cause bodily dysbacteriosis, which might result in secondary invasive fungal infection. Additionally, some studies described earlier found that Cryptococcus sp. invasion into the human body, including immunocompetent and immunosuppressive hosts, might also lead to Thl/Th2 cytokine imbalance; this imbalance presents as a decrease in Th1 cytokines, such as interferon gamma (IFN-γ), and enhancement of Th2 cytokines, such as IL-10, which is called “Th1/Th2 balance drift,” with the domination of a Th2 cytokine response resulting in immune inhibition in the body, thus aggravating cryptococcal infection.[22,23,33] Due to all the risk factors mentioned above, a great potential possibility for the development of C. neoformans infection may occur in previously immunocompetent patients fatally infected with H7N9. Further studies are needed to clarify the complicated mechanisms of IC status after co-infection of atypical influenza and C. neoformans in the future.
Notably, C. neoformans usually causes a community-acquired infection; however, cryptococcal infection involving the lungs bilaterally and the bloodstream in our case occurred approximately 2 weeks after admission when the patient was treated by invasive mechanical ventilation with tracheotomy. There was no initial evidence of cryptococcal infection in our case, and the secondary fungal infection occurred more than 48 h after invasive mechanical ventilation during hospitalization, which was confirmed by positive cultures of C. neoformans from BALF and blood samples and positive results of serum cryptococcal antigen testing. There is no doubt that the present case was diagnosed as hospital-acquired cryptococcal infection, and cryptococcal pneumonia was considered as VAP according to the diagnostic criteria of VAP,[34] which was similar to what was observed in a previous report.[6] To date, a review of the medical literature revealed only 3 reported cases regarding concomitant hospital-acquired cryptococcal infection and severe influenza.[6–8] Adding our case to the review, the four cases are summarized in Table 2. All cases occurred in patients with severe influenza virus infection (2 cases with H1N1 and 2 cases with H7N9) from 7 to 25 days after admission. During the four patients’ courses, there were 3 cases with cryptococcal pneumonia, 2 cases with cryptococcal fungemia, 1 case with cryptococcal pleurisy and 2 cases with cryptococcal meningitis, and 3 of them presented with disseminated cryptococcal infection. In the 3 cases with cryptococcal pneumonia, 1 case was diagnosed as hospital-acquired pneumonia (HAP), and 2 cases were VAP according to the diagnostic criteria of HAP and VAP.[34,35] As recognized, the pathogenic organisms of HAP and VAP are derived from either an endogenous or exogenous source.[35,36] Endogenous infection is the most frequent cause of HAP and VAP, and it can occur with either community-acquired or hospital-acquired pathogens that colonize the host.[37] Initial colonization of the respiratory tract with pathogens, especially colonization of the oropharynx, occurs most commonly.[37] Since C. neoformans is a ubiquitous organism that has a pulmonary portal of entry[38] and can persist in the human oropharynx on occasion, the pathogen may be an endogenous source of lung (aspiration) infections.[39] In our case, when cryptococcal infection occurred, examinations of possible exogenous sources of cryptococcosis in the RICU were performed, but the results were negative for Cryptococcus sp. There was no evidence for the pathogen from exogenous sources, such as medical equipment or other then-hospitalized patients. Therefore, the source of C. neoformans in our patient was considered to be from initial colonization of the respiratory tract before hospitalization. In general, C. neoformans is only conditionally pathogenic in healthy human hosts, but it causes important opportunistic infection in immunosuppressed hosts, as seen in the present patient.
Table 2.
Literature review: concomitant severe influenza and cryptococcal infections.
In the early phase of the clinical course, due to severe mixed infection and the use of ECMO, the patient was treated with strong antibacterial drugs, while caspofungin was also used empirically for the treatment and prevention of Candida spp. infection, which is the most common invasive fungal infection.[40] Unfortunately, although caspofungin is an echinocandin antifungal agent that exhibits potent activity against most species of Candida and Aspergillus, C. neoformans is initially resistant to this antifungal drug.[41] Hence, the present antifungal therapy with an echinocandin drug for our patient failed to play a role in the timely and effective treatment of the subsequent C. neoformans infection, which was similar to what was observed in a previous report by Chen et al.[6] Due to the lack of awareness of hospital-acquired cryptococcal infection with delayed anti-Cryptococcus treatment, the cryptococcal infection in our case rapidly progressed and caused further injury to the lung tissue. Notably, in the patient, LFA was detected and positive for cryptococcal antigen after the detection of C. neoformans in fungal cultures of blood and BALF. Actually, it has been reported that the serum cryptococcal antigen LFA has a very high accuracy for the diagnosis of cryptococcosis with a high level of agreement with latex agglutination (LA).[42] Thus, in such critically ill patients clinically suspected for invasive fungal infection, in addition to fungal culture and the conventional G and GM tests,[43] the cryptococcal capsular antigen test, which is conducive to early detection of cryptococcal infection and avoidance of delays in diagnosis and treatment, should be considered.
As observed in our patient, apart from systemic super-infection with C. neoformans, which obviously worsened the clinical condition, several mixed bacterial infections also occurred and had an important effect on the patient's prognosis. Generally, the most common pathogens of co-infection in patients with influenza infection are bacteria,[44] and super-infection with fungi is relatively less common. Many reports have shown that gram-positive cocci, which are predominantly Staphylococcus aureus, including methicillin-resistant S aureus (MRSA), Streptococcus pneumoniae, and Streptococcus pyogenes, were most commonly isolated in patients with influenza infection,[12,44–48] and gram-negative cocci, which were predominantly Acinetobacter baumannii, P aeruginosa, Haemophilus influenzae, and Klebsiella pneumoniae, were also commonly observed in influenza patients.[9,25,49,50] In our case, the pathogens causing bacterial co-infection were similar to those observed in the literature. MRSH was isolated at the early stage of influenza infection, and the subsequent isolated bacterial strains were P aeruginosa and SMA during prolonged invasive ventilator and ECMO support. The severe systemic infection of multidrug-resistant SMA occurred in the late course and obviously worsened the patient's condition, which was the main factor of death.
With respect to the infection chain of H7N9 influenza in the present case, a history of direct contact with live poultry in a local LPM before illness onset was considered to be the source of H7N9 influenza virus. As recognized, a majority of infected persons with the new H7N9 influenza A virus have live poultry exposure history before showing symptoms.[51] LPMs seem to have served as the main places where the H7N9 virus originally mutated, spread and thus infected human beings,[51,52] as observed in our patient. Since patients with influenza infection can deteriorate with ARDS in a short time,[26] it is necessary to provide effective therapy including antiviral drugs and other necessary medical measures as soon as possible to laboratory-confirmed influenza patients and highly suspected influenza patients, especially those with direct exposure to poultry. Thus far, the most vital therapeutic of atypical influenza infection is antiviral treatment with neuraminidase inhibitors, such as oseltamivir and peramivir, within 48 h after the onset of illness.[53,54] Severely ill patients with influenza infection still need to be treated with antiviral drugs even more than 48 h after onset.[53] Notably, a novel possible treatment method of influenza infection with a mammalian target of rapamycin (mTOR) inhibitor has been reported in recent years.[55,56] Wang et al found that oseltamivir combined with early adjuvant treatment with corticosteroids and an mTOR inhibitor (sirolimus) was associated with decreased viral titre and improvement in respiratory function in patients with severe H1N1 pneumonia.[55] Jia et al also reported that combined oseltamivir treatment with sirolimus as an adjuvant may be a promising immunomodulatory strategy for managing severe influenza by inhibiting lung immunopathologic injury.[56] However, some studies with different results showed that sirolimus treatment increased disease severity after influenza infection due to impairing virus clearance, suppressing T cell immunity and worsening lung inflammation.[57,58] Indeed, irrespective of whether an immunoregulator is being used or not, timely treatment with effective etiotropic antiviral drugs such as oseltamivir to depress viral load and reduce virus-induced cell death is always necessary during treatment of influenza infection.[56] It is well known that the use of immunosuppressant drugs such as sirolimus must be conducive to achieving homeostasis of the immune response, which not only inhibits an excessive immune response but also does not impede cellular and humoural responses to promote virus clearance.[56] Therefore, treatment of influenza with rapamycin inhibitor must be based on sufficient antiviral treatment, and we think it would be the best to administer a joint antiviral therapy such as oseltamivir in combination with one of the other direct-acting antiviral drugs that is proven safe and more effective than the use of a single drug such as oseltamivir monotherapy; moreover, the clinician must try to choose an appropriate window when the inflammatory response is particularly strong to administer the immunosuppressive medicine. Nonetheless, more data from animal and human studies of severe influenza infection are clearly needed before a rapamycin inhibitor (sirolimus or everolimus) can be recommended for the treatment of patients with severe influenza.[59]
Additionally, the emergence of prolonged viral replication despite antiviral treatment with oseltamivir after admission was another remarkable phenomenon in our patient. Previous studies have shown that prolonged viral infection can occur in patients with severe avian influenza A (H7N9) despite antiviral treatment,[60–63] and this effect might occur for up to 30 days,[63] which is similar to what was observed in our case. A recent multi-centre study suggested that corticosteroid therapy and delayed antiviral treatment were associated with prolonged influenza A (H7N9) RNA shedding,[63] as observed in our patient. Wang et al reported that influenza A (H7N9) RNA shedding was shorter in survivors than in patients who died[63] and that there was an increase in the mortality hazard rate with each day of delay in initiation of treatment up to day 5 when compared with treatment initiated within 2 days of symptom onset.[64] In the present patient, the resulting continuous viral replication with ARDS as well as prolonged mechanical ventilation with ventilator-induced lung injury (VILI) caused severe pulmonary fibrosis and significantly prolonged the course of the disease.[61] During this period, the emergence of fatal fungal and bacterial co-infections might also have contributed to diffuse lung injury and result in progressive deterioration of the patient's condition. Although the treatment of secondary disseminated cryptococcal infection was considered successful, in the late course, the patient still succumbed to fatal multidrug-resistant bacterial mixed infection with severe septic shock, which was considered to be the only independent risk factor for mortality in H7N9 patients.[65] Due to the importance of early antiviral treatment, it is vital for clinicians to recognize the disease induced by avian influenza A (H7N9) virus and provide effective antiviral treatment early on. The early control of viral infection can be beneficial to shorten the course of the disease and to avoid mixed infection of highly resistant bacteria and fungi, which are associated with an increased risk of death.[62] Additionally, corticosteroids should be avoided except in clinical situations where they have proven benefit.[63]
In conclusion, cryptococcal infection can occur not only in the community but also in IC patients during hospitalization, especially in patients with severe influenza virus infection. Since patients with hospital-acquired cryptococcal infection (especially VAP and disseminated cryptococcosis) usually have a severe condition with high mortality,[6,7] the clinician should be aware of this infection and try to avoid a missed diagnosis. Unfortunately, in our case, successful therapy for the cryptococcal infection did not reverse the increased predisposition to secondary fatal bacterial co-infection due to progressive pulmonary fibrosis with a prolonged course of the disease. Finally, the patient succumbed to severe septic shock, DIC and MOF.
Acknowledgments
Not applicable.
Author contributions
Conceptualization: Heng Weng.
Data curation: Jinbao Huang, Hongyan Li, Changqing Lan, Shenghua Zou, Hongying Zhang, Xinhang Wang.
Writing – original draft: Jinbao Huang, Hongyan Li, Changqing Lan.
Writing – review & editing: Heng Weng.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, BALF = bronchoalveolar lavage fluid, CAP = community-acquired pneumonia, CRP = C-reactive protein, CRRT = continuous renal replacement therapy, CT = computed tomography, DIC = disseminated intravascular coagulation, ECMO = extracorporeal membrane oxygenation, G = glucan, GM = galactomannan, HAP = hospital-acquired pneumonia, HIV = human immunodeficiency virus, IC = immunocompromised, IFN-γ = interferon gamma, IL = interleukin, LA = latex agglutination, LFA = lateral flow assay, LPM = live poultry market, MOF = multi-organ failure, MRSA = methicillin-resistant Staphylococcus aureus, MRSH = Staphylococcus haemolyticus, mTOR = mammalian target of rapamycin, NK = natural killer, PaCO2 = pressure of carbon dioxide, PaO2 = pressure of oxygen, PCT = procalcitonin, RICU = respiratory intensive care unit, RT-PCR = reverse transcription polymerase chain reaction, SMA = Stenotrophomonas maltophilia, Th = helper T, VAP = ventilator-associated pneumonia, VILI = ventilator-induced lung injury.
JH, HL, and CL contributed equally to this work.
Funding: The work was sponsored by the fund of the Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C. (2018-145) and the Clinical Medicine Center Construction Program of Fuzhou, Fujian, P.R.C. (2018080305).
Conflict of interest: The authors declare that they have no conflicts of interest.
References
- [1].Pappas PG. Cryptococcal infections in non-HIV-infected patients. Trans Am Clin Climatol Assoc 2013;124:61–79. [PMC free article] [PubMed] [Google Scholar]
- [2].Wang BQ, Zhang HZ, Fan BJ, et al. Meta-analysis of clinical manifestations of pulmonary cryptococcosis in China Mainland. Chin J Clin Med 2013;20:351–4. [Google Scholar]
- [3].Kohno S, Kakeya H, Izumikawa K, et al. Clinical features of pulmonary cryptococcosis in non-HIV patients in Japan. J Infect Chemother 2015;21:23–30. [DOI] [PubMed] [Google Scholar]
- [4].Lan CQ, Weng H, Li HY, et al. Retrospective analysis of 117 cases of pulmonary cryptococcosis. Chin J Tuberc Respir Dis 2016;39:862–5. [DOI] [PubMed] [Google Scholar]
- [5].Lomes NR, Melhem MS, Szeszs MW, et al. Cryptococcosis in non-HIV/non-transplant patients: a Brazilian case series. Med Mycol 2016;54:669–76. [DOI] [PubMed] [Google Scholar]
- [6].Chen XH, Wang Q, Ni FY, et al. A case of severe avian influenza A (H7N9) complicated by pulmonary cryptococcal disease. Chin J Tuberc Respir Dis 2016;39:558–61. [Google Scholar]
- [7].Hosseinnezhad A, Rapose A. Cryptococccal meningoencephalitis after H1N1 influenza. BMJ Case Rep 2012;2012: pii: bcr1120115224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gupta A, Capoor MR, Gupta S, et al. Concomitant infections of influenza A H1N1 and disseminated Cryptococcosis in an HIV seropositive patient. J Lab Physicians 2015;7:134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tan W, Dai B, Sun LF, et al. Clinical analysis of survival and death cases in 289 patients with novel influenza A (H1N1). Int J Respir 2011;31:411–4. [Google Scholar]
- [10].Wang XJ, Jiang RM, Xu YL, et al. The analysis of the clinical features between survivors and non-survivors with the severe form of new influenza A (H1N1) viral infection. Chin J Tuberc Respir Dis 2010;33:406–10. [PubMed] [Google Scholar]
- [11].Huang H, Pan XJ, Zhang WX, et al. Clinical characteristics and treatment of 17 patients with avian influenza A H7N9 virus infection. Chin J Infect Dis 2014;32:735–9. [Google Scholar]
- [12].Martin-Loeches IJ, Schultz M, Vincent JL, et al. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med 2017;43:48–58. [DOI] [PubMed] [Google Scholar]
- [13].Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 2018;6:782–92. [DOI] [PubMed] [Google Scholar]
- [14].Wauters J, Baar I, Meersseman P, et al. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med 2012;38:1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vanderbeke L, Spriet I, Breynaert C, et al. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis 2018;31:471–80. [DOI] [PubMed] [Google Scholar]
- [16].Crum-Cianflone NF. Invasive Aspergillosis associated with severe influenza infections. Open Forum Infect Dis 2016;3: ofw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jarvis JN, Dromer F, Harrison TS, et al. Managing cryptococcosis in the immunocompromised host. Curr Opin Infect Dis 2008;21:596–603. [DOI] [PubMed] [Google Scholar]
- [18].Kishi K, Homma S, Kurosaki A, et al. Clinical features and high-resolution CT findings of pulmonary cryptococcosis in non-AIDS patients. Respir Med 2006;100:807–12. [DOI] [PubMed] [Google Scholar]
- [19].Kim YS, Lee IH, Kim HS, et al. Pulmonary cryptococcosis mimicking primary lung cancer with multiple lung metastases. Tuberc Respir Dis (Seoul) 2012;73:182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lewis DE, Gilbert BE, Knight V. Influenza virus infection induces functional alterations in peripheral blood lymphocytes. J Immunol 1986;137:3777–81. [PubMed] [Google Scholar]
- [21].Xie J, Ge Y, Qiu ZF, et al. Immunophenotype of peripheral lymphocytes in HIV-negative patients with cryptococcal meningitis. Chin J Mycol 2017;12:262–7. [Google Scholar]
- [22].Wang JL, Li SY, Luo YF, et al. Changes of Th1/Th2 cytokines in immunocompetent patients with pulmonary cryptococcosis. Genet Mol Res 2013;12:5733–42. [DOI] [PubMed] [Google Scholar]
- [23].Jiang L, Li W, Lin N, et al. Effect of VADl mRNA expression on the immune balance of cerebrospinal fluid Th1/Th2 in patients with cryptococcal meningitis. Chin J Microbiol Immunol 2012;32:716–7. [Google Scholar]
- [24].Wang C, Yu H, Horby PW, et al. Comparison of patients hospitalized with influenza A subtypes H7N9, H5N1, and 2009 pandemic H1N1. Clin Infect Dis 2014;58:1095–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 2013;368:2277–85. [DOI] [PubMed] [Google Scholar]
- [26].Li H, Weng H, Lan C, et al. Comparison of patients with avian influenza A (H7N9) and influenza A (H1N1) complicated by acute respiratory distress syndrome. Medicine (Baltimore) 2018;97:e0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu J, Yan XB, Zhou PP, et al. Lymphocyte subpopulation and CD4+CD25+ Treg signature analysis of patients with severe influenza A (H1N1) virus infection. Chin J Exp Chin Dis (Electron Ed) 2011;5:27–35. [Google Scholar]
- [28].Chen WW, Xie YX, Zhang YH, et al. Changes and analysis of peripheral white blood cells and lymphocyte subsets for patients with pandemic influenza A virus (H1N1 infection. Chin J Exp Clin Virol 2010;24:331–3. [PubMed] [Google Scholar]
- [29].Chen YU, Wang J, Wen H, et al. Clinical significance of peripheral blood T-lymphocyte subsets changes in pandemic influenza (H1N1) patients. J Chin Med Univ 2011;40:140–3. [Google Scholar]
- [30].Fang MT, Chong WT, Yang GL, et al. The effect of Th17 cells on A (H1N1) influenza virus clearance. Chin J Infect Dis 2010;28:593–6. [Google Scholar]
- [31].Feng Y, Hu L, Lu S, et al. Molecular pathology analyses of two fatal human infections of avian influenza A(H7N9) virus. J Clin Pathol 2015;68:57–63. [DOI] [PubMed] [Google Scholar]
- [32].Wu W, Shi Y, Gao H, et al. Immune derangement occurs in patients with H7N9 avian influenza. Crit Care 2014;18:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang L, Chen MK, Shi L, et al. Increased expression of microRNA-31 in peripheral blood mononuclear cells from patients with cryptococcal meningitis. J Mod Lab Med 2014;29:16–21. [Google Scholar]
- [34].Chinese Medical Association Severe Medicine Branch. Guidelines for the diagnosis, prevention and treatment of ventilator-associated pneumonia. Chin J Intern Med 2013;52:524–43. [Google Scholar]
- [35].Infectious Group of Chinese Medical Association Respiratory Branch. Guidelines for the diagnosis and treatment of Chinese adults with hospital-acquired and ventilator-associated pneumonia. Chin J Tuberc Respir Dis 2018;41:255–80. [Google Scholar]
- [36].Young PJ, Ridley SA. Ventilator-associated pneumonia. Diagnosis, pathogenesis and prevention. Anaesthesia 1999;54:1183–97. [DOI] [PubMed] [Google Scholar]
- [37].Rotstein C, Evans G, Born A, et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol 2008;19:19–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Severo CB, Bruno RM, Oliveira FM, et al. A case of miliary pulmonary cryptococcosis and review of literature. Mycopathologia 2015;179:313–5. [DOI] [PubMed] [Google Scholar]
- [39].Salkowski CA, Bartizal KF, Balish MJ, et al. Colonization and pathogenesis of Cryptococcus neoformans in gnotobiotic mice. Infect Immun 1987;55:2000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Drafted by a Scientific Working Group. The diagnosis and treatment of Candidiasis: the expert consensus. Chin J Infect Chemother 2011;11:81–95. [Google Scholar]
- [41].Stan CD, Tuchiluş C, Stan CI. Echinocandins—new antifungal agents. Rev Med Chir Soc Med Nat Iasi 2014;118:528–36. [PubMed] [Google Scholar]
- [42].Huang HR, Fan LC, Rajbanshi B, et al. Evaluation of a new cryptococcal antigen lateral flow immunoassay in serum, cerebrospinal fluid and urine for the diagnosis of cryptococcosis: a meta-analysis and systematic review. PLoS One 2015;10:e0127117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shi Y. The re-recognition of the diagnosis and treatment of invasive pneumomycosis. Chin J Tuberc Respir Dis 2011;34:83–5. [Google Scholar]
- [44].Kash JC, Taubenberger JK. The role of viral, host, and secondary bacterial factors in influenza pathogenesis. Am J Pathol 2015;185:1528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA 2013;309:275e282. [DOI] [PubMed] [Google Scholar]
- [46].Centers for Disease Control and Prevention (CDC). Bacterial coinfections in lung tissue specimens from fatal eases of 2009 pandemic influenza A(H1N1)-United States, May-August 2009. MMWR Morb Mortal Wkly Rep 2009;58:1071–4. [PubMed] [Google Scholar]
- [47].Pfister R, Kochanek M, Leygeber T, et al. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: a prospective cohort study, systematic review and individual patient data meta-analysis. Crit Care 2014;18:R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yang M, Gao H, Chen J, et al. Bacterial coinfection is associated with severity of avian influenza A(H7N9), and procalcitonin is a useful marker for early diagnosis. Diagn Microbiol Infect Dis 2016;84:165–9. [DOI] [PubMed] [Google Scholar]
- [49].Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PloS One 2009;4:e8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yu L, Wang Z, Chen Y, et al. Clinical, virological, and histopathological manifestations of fatal human infections by avian influenza A(H7N9) virus. Clin Infect Dis 2013;57:1449–57. [DOI] [PubMed] [Google Scholar]
- [51].Han DD, Han CX, Li LY, et al. Epidemiology of human infection with avian influenza A(H7N9) virus in China, 2013-2017. Zhonghua Liu Xing Bing Xue Za Zhi 2018;39:44–6. [DOI] [PubMed] [Google Scholar]
- [52].Liu T, Zhu GH, Zhang B, et al. The effects of closure to live poultry markets on Avian influenza A (H7N9) epidemics in China. Zhonghua Liu Xing Bing Xue Za Zhi 2017;38:1716–8. [DOI] [PubMed] [Google Scholar]
- [53].Chinese Ministry of Health. The diagnosis and treatment protocol for human infections with influenza A (H1N1) (2010). Int J Respir 2011;31:81–4. [Google Scholar]
- [54].National Health and Family Planning Commission (NHFPC). The diagnosis and treatment protocol for human infections with avian influenza A (H7N9) (2014). Chin J Clin Infect Dis 2014;7:1–3. [Google Scholar]
- [55].Wang CH, Chung FT, Lin SM, et al. Adjuvant treatment with a mammalian target of rapamycin inhibitor, sirolimus, and steroids improves outcomes in patients with severe H1N1 pneumonia and acute respiratory failure. Crit Care Med 2014;42:313–21. [DOI] [PubMed] [Google Scholar]
- [56].Jia X, Liu B, Bao L, et al. Delayed oseltamivir plus sirolimus treatment attenuates H1N1 virus-induced severe lung injury correlated with repressed NLRP3 inflammasome activation and inflammatory cell infiltration. PLoS Pathog 2018;14:e1007428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Huang CT, Hung CY, Chen TC, et al. Rapamycin adjuvant and exacerbation of severe influenza in an experimental mouse model. Sci Rep 2017;7:4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Alsuwaidi AR, George JA, Almarzooqi S, et al. Sirolimus alters lung pathology and viral load following influenza A virus infection. Respir Res 2017;18:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ison MG. Adjuvant immunosuppression in the management of severe influenza: friend or foe? Crit Care Med 2014;42:457–9. [DOI] [PubMed] [Google Scholar]
- [60].Chen Y, Liang W, Yang S, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 2013;381:1916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Huang JB, Li HY, Liu JF, et al. Histopathological findings in a critically ill patient with avian influenza A (H7N9). J Thorac Dis 2015;7:E672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yu L, Wang Z, Chen Y, et al. Clinical virological and histopathological manifestations of fatal human infections by avian influenza A(H7N9) virus. Clin Infect Dis 2013;57:1449–57. [DOI] [PubMed] [Google Scholar]
- [63].Wang Y, Guo Q, Yan Z, et al. Factors associated with prolonged viral shedding in patients with avian influenza A (H7N9) virus infection. J Infect Dis 2018;217:1708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2014;2:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang Y, Guo F, Zhao W, et al. Novel avian-origin influenza A (H7N9) in critically ill patients in China. Crit Care Med 2015;43:339–45. [DOI] [PubMed] [Google Scholar]


