Supplemental Digital Content is available in the text
Keywords: immune regulation, morphine, systemic or neuraxial route
Abstract
In the study, we tried to evaluate the effects of morphine injected through the systemic or neuraxial route on immune cell function and cytokine production in healthy women.
In total, 29 paired samples of fresh peripheral blood were collected from healthy women who had been administered morphine for anesthetic analgesia through intravenous (IV), epidural, or spinal route postpartum. Their isolated peripheral blood mononuclear cells were mitogen-activated and stained with fluorochrome-conjugated anti-CD4, anti-CD8, anti-interleukin (IL)-2, and anti-interferon (IFN)-γ antibodies for flow cytometry, and the plasma levels of cytokines, including IL-6, IFN-α2, IL-10, IL-8, GM-CSF, and monocyte chemoattractant protein (MCP)-1, were measured through enzyme-linked immunosorbent assay.
The results demonstrated that regardless of the administration route, morphine delivery slightly reduced IL-2 expression in CD4+ cells after activation, and the same effect was not noted for CD8+ cells. Intravenous or epidural morphine tended to reduce IFN-γ expression in CD8+ cells. Spinal and IV morphine substantially increased IL-6 production, whereas epidural morphine hindered IL-10 and GM-CSF production. IV morphine injection reduced MCP-1 production in plasma. Compared with spinal morphine, IV or epidural morphine may more effectively inhibit the expression of various cytokines and thus affect immune response.
All 3 routes of morphine injection tended to decrease IL-2 production by CD4+ cells, whereas IV or epidural morphine injection showed lower IFN-γ production by CD8+ cells. However, additional large-scale studies with longer follow-up durations are warranted.
1. Introduction
Opioids, such as morphine, are widely used to relieve pain during various surgeries or in cancer patients; however, their side effects include nausea, vomiting, and depression of respiration. Recent studies have demonstrated a relationship between morphine and immunosuppression potentially affecting both innate and adaptive immune function and lead to opportunistic infections.[1,2] Furthermore, pregnant women undergoing cesarean section under epidural injection of morphine might be at a high risk of recurrent herpes infection.[3,4] Many experimental results from human and animal studies have revealed that morphine can inhibit antibody production, activate natural killer cells, stimulate cytokine secretion, and cause phagocytosis of immune cells.[5] In addition, morphine may repress the cellular immune system and accelerate the deterioration of patients with cancer.[1] Therefore, increased understanding of the mechanism underlying immunosuppression by morphine may aid doctors in determining the optimal method of morphine administration to relieve various types of pain and prevent immunodeficiency due to long-term morphine use.
Morphine, the most common opioid for anesthetic analgesia, is delivered via the intravenous (IV), epidural, or spinal route to inhibit sensory neurons. Morphine-induced side effects include nausea, vomiting, and dyspnea. Accumulating evidence suggests that morphine induces alterations in immune function through inhibition of antibody production, immune cell activation, and cytokine production; all these inhibition types can increase opportunistic infection, herpes viral reactivation, and tumorigenesis risks. Transduced signals secreted by cells after morphine stimulation affect cytokines in peripheral blood (e.g., increase in interleukin (IL)-6 concentration).[6–8] This evidence suggests that interaction between morphine stimulation in neural cells and peripheral tissue affects immune responses. This study evaluated the effects of various routes of morphine administration for pain relief during anesthesia on immune function.
2. Method
This study was approved by the Ethics Committee of National Taiwan University Hospital, Hsinchu branch (Institutional Review Board: 104-092-F), and all participating patients provided informed consent prior to this study. In this study, we recruited those healthy pregnant women (after they provided verbal and written informed consent) undergoing the same surgery (Cesarean Section) with the same anesthetic method (spinal anesthesia) and anesthetic drug (bupivacaine 10–12 mg, according to patients’ height). Intravenous, epidural, or intrathecal with morphine were our routine clinical practice for postpartum analgesia regimens. We just chose 10 patients from Group 1 formula, 9 from Group 2 formula, and 10 from Group 3 formula, respectively. Group 1 received IVPCA (intravenous patient-controlled analgesia) setting with 1 mg morphine in each bolus; Group 2 received 2 mg of epidural morphine diluted with 10 mL of normal saline administered every 12 h, with first dose administered immediately after Cesarean Section; and Group 3 received 0.3 mg of intrathecal morphine combined with bupivacaine.
Before and 24 hours after morphine injection, peripheral blood was drawn, and peripheral blood mononuclear cells (PBMCs) were separated by centrifuging blood in a concentration gradient composed of Ficoll-Paque PLUS (an aqueous solution with density of 1.077 + 0.001 g/mL containing 5.7 g Ficoll 400 and 9 g sodium diatrizoate and with 0.0231 g calcium disodium ethylenediaminetetraacetic acid in every 100 mL) as supplement 1.
2.1. Enumeration of cytokine-secreting cells through flow cytometry
Assay antigen-specific T cells were analyzed using interferon-γ (IFN-γ) and IL-2 secretion assays (Miltenyi Biotec, Bergisch Gladbach, Germany). After 18 and 72 hours of culture, the cells were incubated for 5 minutes on ice with IFN-γ and IL-2 catch reagents (Miltenyi Biotech; amounts were determined according to the manufacturer's instructions). The cells (105 cells/mL) were then diluted by adding warm (37°C) CM and incubated for 45 minutes under slow continuous rotation at 37°C in a 5% CO2 atmosphere. The cells were then further incubated for 10 minutes with anti-IFN-γ mAb (mouse IgG1) fluorescein-isothiocyanate-conjugated and anti-IL-2 mAb (mouse IgG1) PE-conjugated detection antibodies (Miltenyi Biotech; amounts were determined according to the manufacturer's instructions). In addition, the cells were incubated with mAbs against CD4 (PE-Cy7 conjugated, clone SK3, mouse IgG1) and CD8 (APC-Cy7 conjugated, clone SK1, mouse IgG1). Flow cytometry was performed using a FACSCanto flow cytometer (Becton Dickinson, San Jose, CA); 50,000 viable cells and data were analyzed on BDFACS Diva. The percentages of IFN-γ+, IL-2+, and IFN-γ+/IL-2+ (double positive) events were calculated using quadrant statistics on CD4+ and CD8+-gated cell populations.
2.2. ELISA for plasma cytokine levels
The concentrations of IL-8, IFNα2, and GM-CSF in plasma samples were measured using commercially available ELISA kits (BDOptEIA; BD Biosciences, Rockville, MD) according to the manufacturer's instructions. The concentration of cytokines or chemokines was calculated based on standard curves provided with the kits, and the results were expressed in picogram per milliliter. IL-6, IL-10, and monocyte chemoattractant protein (MCP)-1 concentrations were measured using Multiplex Analyte Profiling Technology (xMAP) and HSCYTOMAG-60 K lit (Luminex, Millipore, Billerica, MA) read on a MAGPIX (MILLIPLEX, Millipore, MA). The assay kits included a standard curve and 2 controls (high and low concentrations) for each cytokine. For ELISA and xMAP, all samples were tested in duplicate; the average values were used for further analysis.
2.3. Statistical analysis
For statistical analysis, the control group comprised 29 healthy subjects. A comparison of intracellular expression of IL-2 and IFN-γ in CD4+ and CD8+ cells before and after morphine injection at various degrees of influence (epidural, spinal, and IV) on the immune system was performed using paired t tests. Multiple comparisons of morphine injection through various routes (epidural, spinal, and IV) on their effects on the immune system were conducted using an analysis of variance (ANOVA), A P of < .05 was considered significant.
3. Results
In total, 29 healthy pregnant women were recruited and divided into 3 groups: 10, 9, and 10 patients in Groups 1, 2, and 3, respectively. The demographic data are shown in Table 1; no significant differences were observed in the age, body weight, or gestational age among the 3 groups (Table 1). During the 24 hours of this study, IV, epidural, and spinal morphine dosage was 25 to 30, 2 to 4, and 0.3 mg, respectively. Changes in intracellular IL-2, IFN-γ, and IL-2+IFN-γ expression in CD4+ and CD8+ cells before and after epidural, spinal, and IV morphine injection, based on paired t test results, are listed in Table 2. No statistical differences (before and after morphine administration) were noted among the 3 groups except for the change in IFN-γ expression in the CD8+ cells in Group 2. The results of multiple comparisons (Tukey test) of the effects of morphine administration through epidural, spinal, and IV routes on intracellular cytokines (ANOVA) are listed in Table 3. No significant differences among the 3 groups in terms of intracellular cytokine expression (before and after morphine injection) were observed, except for significant IL-2+IFN-γ expression in CD4+ cells in the epidural and spinal groups (P =.024). The trend of individualized IL-2 expression in CD4+ and CD8+ cells after morphine injection via the systemic (IV) or neuraxial (spinal or epidural) route is shown in Fig. 1. Fifteen patients exhibited reduced IL-2 expression in CD4+ cells immediately after morphine injection. Of these 15 patients, 5, 4, and 6 were in Groups 1, 2, and 3, respectively. The change in IL-2 expression in CD8+ cells was minimal, without any significant difference (Fig. 1). The trend of individualized IFN-γ expression in CD4+ or CD8+ cells after morphine systemic (IV) or neuraxial (spinal or epidural) injection is presented in Fig. 2. The effects of IFN-γ expression on CD4+ cells were slightly reduced in all 3 groups after morphine injection, and the degree of reduction was higher in Groups 1 and 3; however, no significant difference was observed (Fig. 2). The trend of individualized expression of IL-2+IFN-γ in CD4+ and CD8+ cells after morphine injection through the systemic (IV) or neuraxial (spinal or epidural) route is shown in Fig. 3. For CD4+ and CD8+ cells with simultaneous IL-2 and IFN-γ expression, the concentration was twice as high after morphine injection in 3 patients in Group 1; however, all other Group 1 patients exhibited reduced concentrations. IL-2+IFN-γ levels slightly decreased in most Group 2 patients but remained relatively high in more than half of Group 3 patients (Fig. 3).
Table 1.
Demographic and clinical measurement.
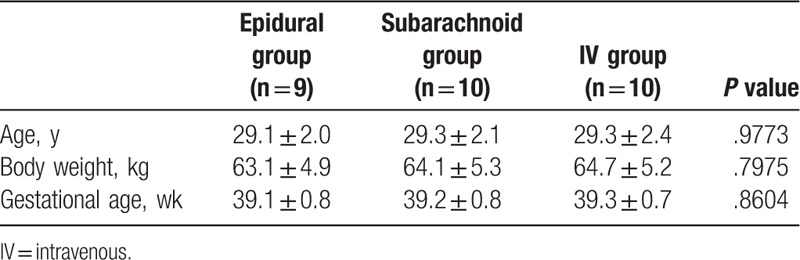
Table 2.
Intracellular expression of IL-2, IFN-γ, IL-2+ IFN-γ in CD4-cell or CD8-cell before and after morphine injection via different routes (Epidural, Spinal, IV) on immune system.
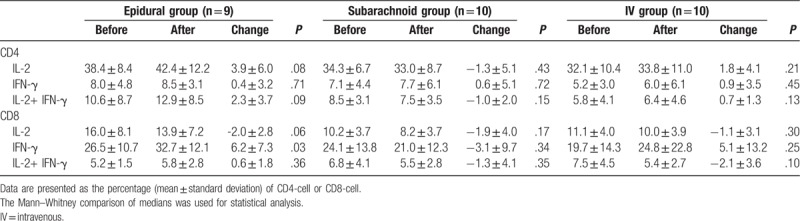
Table 3.
Multiple comparisons of morphine injection through different routes (Epidural, Spinal, IV) on intracellular cytokines.
Figure 1.
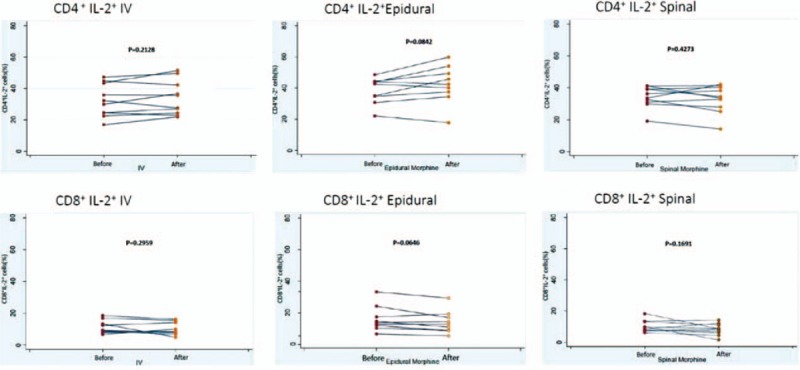
The change of IL-2 expression of CD4+ or CD8+ after morphine injection. A, The IL-2 expression of CD4+ after intravenous injection. B, The IL-2 expression of CD4+ after epidural injection. C, The IL-2 expression of CD4+ after spinal injection. D, The IL-2 expression of CD8+ after intravenous injection. E, The IL-2 expression of CD8+ after epidural injection. F, The IL-2 expression of CD8+ after spinal injection. IL = interleukin.
Figure 2.
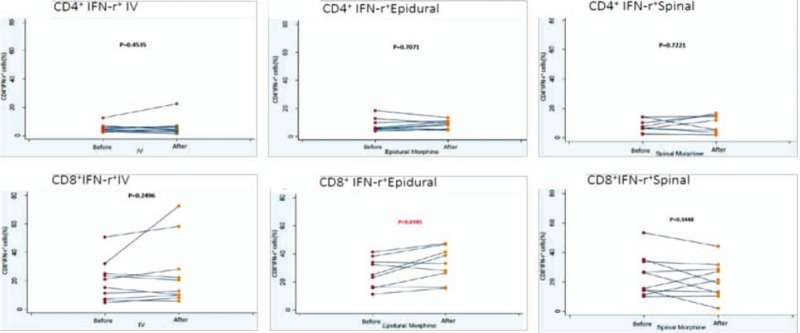
The expression of IFN-γ in CD4+ or CD8+ cells after morphine injection through systemic (IV) or neuraxial route (spinal or epidural). A, The IFN-γ expression of CD4+ after intravenous injection. B, The IFN-γ expression of CD4+ after epidural injection. C, The IFN-γ expression of CD4+ after spinal injection. D, The IFN-γ expression of CD8+ after intravenous injection. E, The IFN-γ expression of CD8+ after epidural injection. F, The IFN-γ expression of CD8+ after spinal injection. IFN = interferon.
Figure 3.
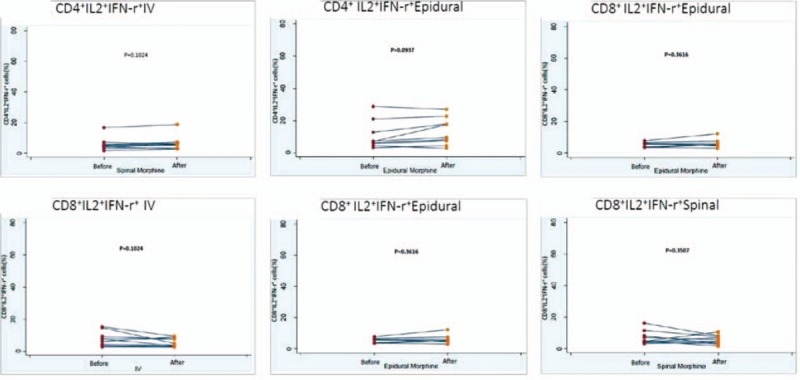
The change of IL-2 + IFN-γ expression of CD4+ or CD8+ after morphine injection. A, The IL-2 + IFN-γ expression of CD4+ after intravenous injection. B, The IL-2 + IFN-γ expression of CD4+ after epidural injection. C, The IL-2 + IFN-γ expression of CD4+ after spinal injection. D, The IL-2 + IFN-γ expression of CD8+ after intravenous injection. E, The IL-2 + IFN-γ expression of CD8+ after epidural injection. F, The IL-2 + IFN-γ expression of CD8+ after spinal injection. IFN = interferon, IL = interleukin.
ELISAs for IL-6, IFN-α2, IL-10, IL-8, GM-CSF, and MCP-1 were performed in 18 samples (7, 3, and 8 in Groups 1, 2, and 3 patients, respectively). IV and spinal, but not epidural, morphine significantly increased IL-6 levels (Fig. 4). A slight decrease in IFN-α2 expression was observed but no significant difference was in all group patients. A significant decrease in IL-10 expression was observed in Group 2, but no such significant difference was noted in the other 2 groups (Fig. 4). IL-8 concentration slightly increased in Group 3 and slightly decreased in Groups 1 and 2 after morphine injection, but no significant changes were noted (Fig. 5). GM-CSF concentration significantly decreased after morphine injection in Group 2, but no such significant difference was noted in Groups 1 and 3. MCP-1 concentration significantly decreased after morphine injection in Group 1, but no significant change was found in Groups 2 and 3 (Fig. 5).
Figure 4.
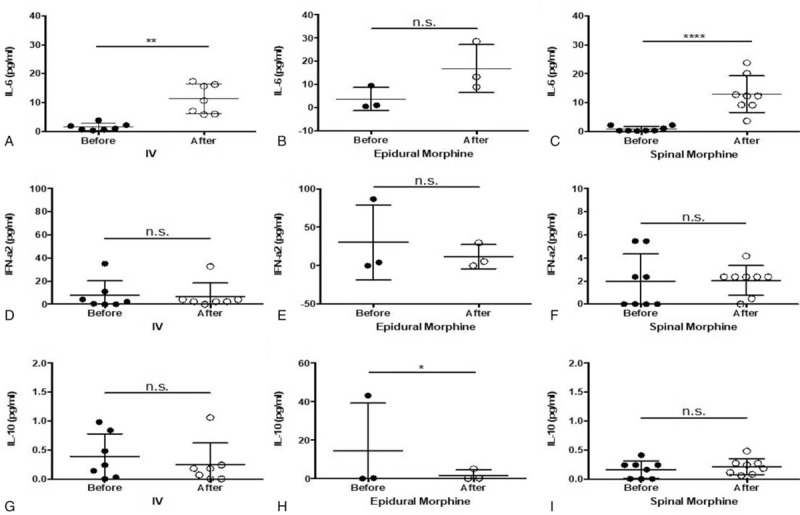
A–C, ELISA analysis of IL-6 expression in blood after morphine injection through 3 different routes. D–F, ELISA analysis of IFN-α2 cytokines in blood after morphine injection through 3 different routes. G–I, ELISA analysis of IL-10 cytokines in blood after morphine injection through 3 different routes. ∗P < .05; ∗∗ P < .01. ELISA = enzyme-linked immunosorbent assay, IFN = interferon, IL = interleukin.
Figure 5.
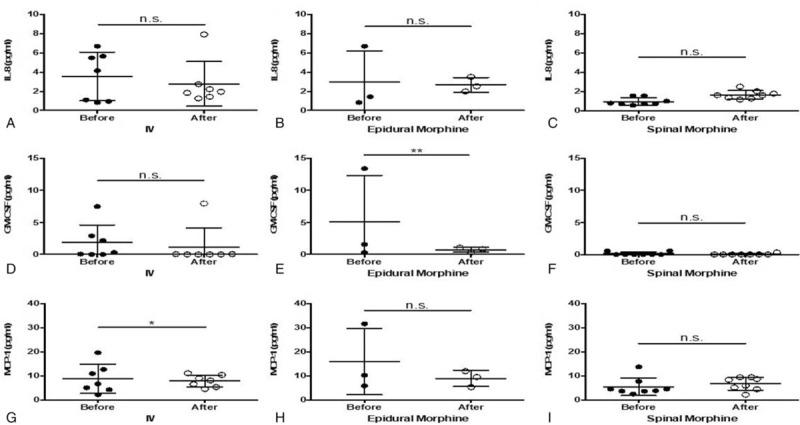
A–C, ELISA analysis of IL-8 expression in blood after morphine injection through 3 different routes. D–F, ELISA analysis of GM-CSF cytokines in blood after morphine injection through 3 different routes. G–I, ELISA analysis of MCP-1 cytokines in blood after morphine. ∗P < .05; ∗∗ P < .01. ELISA = enzyme-linked immunosorbent assay, GM-CSF = granulocyte-macrophage colony-stimulating factor, IL = interleukin, MCP-1 = monocyte chemoattractant protein 1.
4. Discussion
Opioid drugs, such as morphine, affect the signal transportation of activated T cells, thereby inhibiting T-cell activation.[9,10] IL-6, IL-8, IFN-γ, and TNF-α are key in activating acute or chronic phases of inflammation, including B-cell differentiation and B- and T-cell activation.[11,12] Moreover, as an anti-inflammatory cytokine, IL-10 suppresses the activation of immune cell response.[13–15] Our study showed that IV or epidural injection of morphine inhibits production of GM-CSF and MCP-1, whereas IL-10 expression decreased in the epidural group. Therefore, the IV or epidural morphine likely inhibits the activation of immune cells and inflammatory responses but IL-10 which anti-inflammatory cytokine modulate simultaneously.
IL-2 and IFN-γ are key cytokines for the activation of CD4+ and CD8+ cells. Roy et al[16] reported that chronic morphine treatment in vivo could increase the Th2 differentiation of CD4+ T cells. However, in contrast to that of CD4+ cells, acute morphine suppresses CD8+ cell activation during cellular immune responses.[17] An animal study demonstrated that morphine hinders the abilities of macrophage migration to infected sites and bacteria eradication.[18,19] In the present study, no significant differences among the 3 groups in terms of intracellular cytokine expression (before and after morphine injection) were observed, except for significant IL-2+IFN-γ expression in CD4+ cells in the epidural and spinal groups. The explanation why there existed significant change in combined IL-2 + IFN-γ expression in CD4+ cells, but individual IL-2 and IFN-γ expressions were not changed could be as the following: IL-12 and IFN-γ are the critical cytokines initiating the downstream signaling cascade to develop Th1 cells (a subset of CD4+ cells). The IL12, in turn, induces natural killer cells to produce IFN-γ. IL4 and IL2 are critical for Th2 (another subset of CD4+ cells) differentiation. Intercellular communication between anergic human T cells and agonist peptide-loaded APCs (antigen-presenting cells) was not a null event, since it triggered secretion of T-cell IFN-γ but not IL-2. Furthermore, IV or epidural morphine injection was relatively likely to hinder IFN-γ production by CD8+ cells. IFN-γ is a key moderator of cell-mediated immunity with mainly pro-inflammatory actions on immunocytes and target tissue and it may enhance antitumor and antiviral effects of CD8 T cells. Therefore, we might speculate that IV or epidural morphine is more likely than spinal morphine to affect the proliferation of neutrophils and macrophages, which reduce inflammatory reactions to bacterial infections and negatively influence the ability for antitumor and antiviral response.
Wang et al[20] reported that chronic morphine treatment had significantly inhibited the synthesis of some inflammatory cytokines (TNF-α, IL-1, and IL-6 concentrations) when measured 4 hours after infection. However, other studies have indicated that maternal IL-6 and TNF-α concentrations during vaginal birth are significantly higher than those during birth through cesarean section.[21,22] Thus, our observed effect of the increase in plasma IL-6 concentration in patients was not only due to morphine treatment but also a consequence of such medication being administered during birth through cesarean section. Future examinations could exert greater control by recruiting women who have given vaginal birth both with and without morphine treatment. Makimura et al[23] attempted to detect markers predicting resistance to morphine treatment by examining the plasma concentrations of 26 cytokines before and after morphine treatment in 44 patients with metastatic cancer. The researchers concluded that no significant changes in any cytokine concentration had occurred after 8 days of morphine treatment in previously opioid-naive patients.[23] By contrast, Palm et al[24] demonstrated that IL-2 synthesis and secretion by lymphocytes significantly increased after 4 weeks of morphine treatment in 10 patients with chronic pain. Moreover, the effects of acute opioid exposure (over several days) on the immune system differ from those of chronic exposure (over several weeks). Nevertheless, the effect of morphine (injected via various routes) on immune function (parameters) has seldom been elucidated in human in vivo studies.
This study has some limitations. First, the sample size was relatively small. Second, the effect of morphine was evaluated only within 24 hours. Third, this study recruited only healthy women. The findings of this research are valuable because this was the first study to reveal that morphine potentially influences immune function in healthy individuals. Long-term monitoring of adverse morphine-induced effects on immune-function-related diseases and development of effective morphine-based pain relief strategies for anesthetic analgesia in cancer patients should be considered extensively in the future.
In conclusion, this study was the first to use the selected immunoassays to detect the effect of morphine injection through multiple routes (epidural, spinal, and IV) on the immune system in a human (in vivo) study. This study yielded similar findings to those of our previous animal study regarding the effect of morphine on immune function. All 3 routes of morphine injection appeared to minimally hinder IL-2 production by CD4+ cells, whereas IV or epidural morphine injection was more likely to hinder IFN-γ production by CD8+ cells.
Acknowledgments
The authors thank the staff of the Biotechnology R&D Center at the National Taiwan University Hospital (Hsin-Chu branch) for their assistance in the statistical analyses.
This manuscript was edited by Wallace Academic Editing.
Author contributions
Li-Kuei Chen contributed to the study design.
Yi-Ping Wang and Shiou-Sheng Chen contributed to data collection and analyses.
Shin-Hong Chen and Li-Kuei Chen interpreted the results and drafted the manuscript.
All authors read and approved the final manuscript and take full responsibility for all aspects of the study.
Data curation: Shiou-Sheng Chen, Yi-Ping Wang.
Formal analysis: Shiou-Sheng Chen.
Supervision: Li-Kuei Chen.
Writing – original draft: Shih-Hong Chen.
Writing – review & editing: Li-Kuei Chen.
Supplementary Material
Footnotes
Abbreviations: ELISA = enzyme-linked immunosorbent assay, GM-CSF = granulocyte-macrophage colony-stimulating factor, IFN = interferon, IL = interleukin, MAP = multiplex analyte profiling, MCP-1 = monocyte chemoattractant protein 1, PBMCs = peripheral blood mononuclear cells.
This research received no external funding.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Sacerdote P. Opioids and the immune system. Palliat Med 2006;20suppl 1:s9–15. [PubMed] [Google Scholar]
- [2].Sacerdote P, Franchi S, Panerai AE. Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced immunodepression. Curr Pharm Des 2012;18:6034–42. [DOI] [PubMed] [Google Scholar]
- [3].Crone LA, Conly JM, Storgard C, et al. Herpes labialis in parturients receiving epidural morphine following cesarean section. Anesthesiology 1990;73:208–13. [DOI] [PubMed] [Google Scholar]
- [4].James CF. Recurrence of herpes simplex virus blepharitis after cesarean section and epidural morphine. Anesth Analg 1996;82:1094–6. [DOI] [PubMed] [Google Scholar]
- [5].Roy S, Wang J, Kelschenbach J, et al. Modulation of immune function by morphine: implications for susceptibility to infection. J Neuroimmune Pharmacol 2006;1:77–89. [DOI] [PubMed] [Google Scholar]
- [6].Mellon RD, Bayer BM. Role of central opioid receptor subtypes in morphine-induced alterations in peripheral lymphocyte activity. Brain Res 1998;789:56–67. [DOI] [PubMed] [Google Scholar]
- [7].Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol 1998;83:63–9. [DOI] [PubMed] [Google Scholar]
- [8].Houghtling RA, Bayer BM. Rapid elevation of plasma interleukin-6 by morphine is dependent on autonomic stimulation of adrenal gland. J Pharmacol Exp Ther 2002;300:213–9. [DOI] [PubMed] [Google Scholar]
- [9].Börner C, Warnick B, Smida M, et al. Mechanisim of opioid-mediated inhibition of human T cell receptor signaling. J Immunol 2009;183:882–9. [DOI] [PubMed] [Google Scholar]
- [10].Bastami S, Norling C, Trinks C, et al. Inhibitory effect of opiates on LPS mediated release of TNF and IL-8. Acta Oncol 2013;52:1022–33. [DOI] [PubMed] [Google Scholar]
- [11].Cem G. Interleukin-6 and chronic inflammation. Arthritis Res Ther 2006;8suppl 2:s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harada A, Sekido N, Akahoshi SN, et al. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 1994;56:559–64. [PubMed] [Google Scholar]
- [13].Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol 2008;180:5771–7. [DOI] [PubMed] [Google Scholar]
- [14].Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 2012;32:23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ng TH, Hill BG, Verhagen EV, et al. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol 2013;4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Roy S, Wang J, Charboneau R, et al. Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway. J Immunol 2005;175:6361–7. [DOI] [PubMed] [Google Scholar]
- [17].Mojadadi S, Khansarinejad JA, Soleimanjahi B, et al. Acute morphine administration reduces cell-mediated immunity and induces reactivation of latent herpes simplex virus type 1 in BALB/c mice. Cell Mol Immunol 2009;6:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Malik AARN, Radhakrishnan N, Reddy K, et al. Morphine-induced macrophage apoptosis modulates migration of macrophages: use of in vitro model of urinary tract infection. J Endourol 2002;16:605–10. [DOI] [PubMed] [Google Scholar]
- [19].Bhaskaran M, Reddy K, Sharma S, et al. Morphine-induced degradation of the host defense barrier: role of macrophage injury. J Infect Dis 2001;184:1524–31. [DOI] [PubMed] [Google Scholar]
- [20].Wang J, Ma J, Charboneau R, et al. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol 2005;174:426–34. [DOI] [PubMed] [Google Scholar]
- [21].Duncombe G, Veldhuizen RA, Gratton RJ, et al. IL-6 and TNFalpha across the umbilical circulation in term pregnancies: relationship with labour events. Early Hum Dev 2010;86:113–7. [DOI] [PubMed] [Google Scholar]
- [22].Haghshenas Mojaveri M, Mohammadzadeh I, Al-Sadat Bouzari Z, et al. The comparison of serum interleukin-6 of mothers in vaginal and elective cesarean delivery-. Caspian J Intern Med 2014;5:223–6. [PMC free article] [PubMed] [Google Scholar]
- [23].Makimura C, Arao T, Matsuoka H, et al. Prospective study evaluating the plasma concentrations of twenty-six cytokines and response to morphine treatment in cancer patients. Anticancer Res 2011;31:4561–8. [PubMed] [Google Scholar]
- [24].Palm S, Lehzen S, Mignat C, et al. Does prolonged oral treatment with sustained-release morphine tablets influence immune function? Anesth Analg 1998;86:166–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


