Supplemental Digital Content is available in the text
Keywords: direct-acting antiviral, genotype 6, hepatitis C, hepatitis C virus
Abstract
Background:
Because of the heterogeneity of hepatitis C virus (HCV) distribution of different genotypes, large-scale clinical trials on direct-acting antiviral (DAA) mainly included patients with genotype 1 and genotype 3 infection. Data on the efficacy of direct-acting antiviral agents in patients with chronic genotype 6 HCV infection are limited.
Methods:
The PubMed, Embase, and the Cochrane Libraries were searched comprehensively. All published clinical trials assessing the efficacy of DAA therapy for patients with chronic genotype 6 HCV infection were included. Sustained virological response (SVR) and rapid virological response (RVR) were pooled. Additional meta-analyses were also performed to compare the efficacy of DAA therapy in HCV-6 versus HCV-1 or HCV-3 patients.
Results:
Seventeen studies met the inclusion criteria and were included in our meta-analysis. The pooled SVR of all single arms was 95% [95% confidence interval (CI): 0.90–0.97]. The pooled RVR of all single arms was 97% (95% CI: 0.95–0.99). The SVR and RVR were both similar between HCV-6 and HCV-1 or HCV-3. Adverse events were common but rarely caused treatment interruption.
Conclusion:
Based on the available data, our results indicate that DAA treatment is effective and safe for patients with genotype 6 HCV infection, and the efficacy was similar compared to patients with genotype 1 HCV or genotype 3 HCV infection.
1. Introduction
Hepatitis C virus (HCV) infection remains a major public health concern, with 1.75 million people worldwide receiving a new diagnosis for HCV each year.[1] HCV is also one of the leading causes of liver cirrhosis and hepatocellular carcinoma.[2] There are 7 genotypes and 67 subtypes of HCV distributed in different regions of the world. Among them, genotypes 1 and 3 are the 2 most common genotypes, accounting for 46.2% and 30.1%, respectively. Genotype 6 is the least distributed genotype accounting for <1% of cases.[2] The genotype 6 HCV is mainly concentrated in Southeast Asia, such as China, Vietnam, Thailand, and Myanmar, with a prevalence of approximately 30% to 40%.[3] Among all the subtypes of HCV-6, genotype 6a is the most geographically restricted, mainly in Vietnam, Macau, and Hong Kong.[4]
Before the direct-acting antiviral (DAA), the main treatment for HCV was composed of interferon, peg interferon, and ribavirin. The cure rate of these regimens is only 40% to 65%.[5] And these regimens are poorly tolerated due to adverse events such as flulike symptoms and depression.[6] So far, the Food and Drug Administration has approved >10 DAAs for the treatment of HCV infection, which can achieve a sustained viral response (serum HCV RNA < 15 IU/mL at least 12 weeks after treatment has ended) rate of >90%. Due to the heterogeneity of HCV distribution of different genotypes, large-scale clinical trials on DAA were mainly included patients with genotype 1 and genotype 3, whereas data on DAA therapy for genotype 6 HCV infection are lacking and the conclusions are inconsistent. In addition, currently, no comprehensive meta-analyses of clinical trial data were reported. Therefore, the aim of the present study was to conduct a meta-analysis of trials to assess the efficacy and safety of DAA treatment in chronic HCV-6 patients.
2. Materials and methods
2.1. Ethics statement
As all the data were from previously published studies, no ethical approval or patient consent was required.
2.2. Search strategy
Relevant studies regarding the therapy with DAA for patients with chronic genotype 6 HCV infection were identified by searching the PubMed, Embase, and the Cochrane Libraries using the following keywords “sofosbuvir,” “simeprevir,” “grazoprevir,” “elbasvir,” “ombitasvir,” “paritaprevir,” “ritonavir,” “dasabuvir,” “daclatasvir,” “asunaprevir,” “direct acting antiviral,” “DAA,” “DAAs,” “HCV,” “hepatitis C,” and “genotype.” The specific search strategy for each database was presented in Table S1. The search was restricted to “human.” The reference lists of all the retrieved documents were manually searched for potentially relevant reports missed by the intelligent retrieval systems mentioned above. The search was carried out in December 2018. Titles and abstracts of potentially eligible publications were screened independently by 3 investigators (A.L., X.J., and Q.L.). Conflicting opinions were resolved with the arbitration of the corresponding author (Q.L.) where necessary.
2.3. Selection criteria
Published articles or abstracts had met the following inclusion criteria: study design: randomized controlled trials (RCTs), retrospective and prospective cohort study designs (each group sample size >10); subjects: patients with chronic genotype 6 HCV infection; treatment strategy: including a DAA plus interferon or ribavirin combination therapy, or DAAs combination therapy group; and outcome: including information on a primary outcome of interest clearly defined as sustained virological response (SVR), which was defined as undetectable HCV RNA at least 12 weeks after the end of treatment, and a second outcome of rapid virological response (RVR) defined as undetectable HCV RNA at the fourth week during treatment. The exclusion criteria were as follows: duplicated data; including <10 patients infected with genotype 6 HCV; autoimmune hepatitis, alcoholic liver disease, primary biliary cirrhosis, Wilson disease, hepatocellular carcinoma, and so on; and any report without available outcome measures.
2.4. Outcome measures
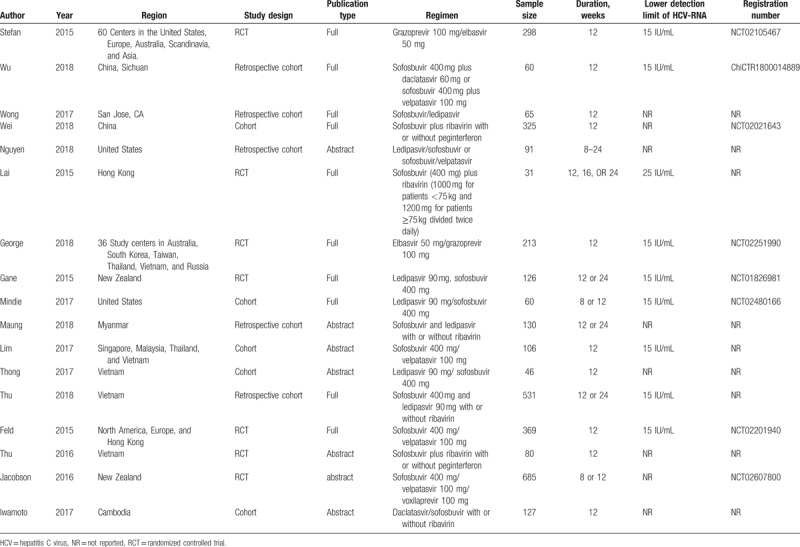
Endpoints were defined before the initiation of the study. To estimate the efficacy of DAA treatment in the selected trials, the SVR rate (proportion of patients with undetectable HCV RNA at least 12 weeks after the end of treatment) was defined as the primary outcome. The secondary outcomes were the rates of RVR (undetectable HCV RNA at week 4) and adverse events. Differences in the limits of HCV RNA detectability among the studies are presented in Table 1.
Table 1.
Characteristics of the included trials in this meta-analysis.
2.5. Data extraction
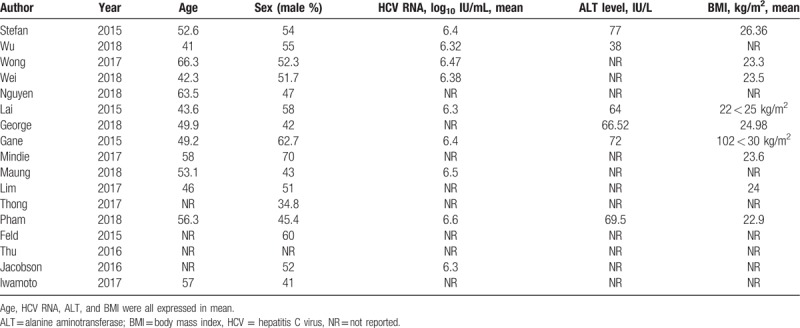
Three reviewers (A.L., X.J., and Q.L.) independently used inclusion criteria, selected the studies, and extracted data and outcomes. The following data were extracted from each study: study characteristics (author, year of publication, region, study design, publication type, regimen, sample size, duration of follow-up, lower detection limit of HCV-RNA, and registration number); patient demographics (age, sex, body mass index) and baseline characteristics (HCV RNA, Alanine aminotransferase level); and the study outcomes (SVR, RVR, and adverse events) after treatment. Any disagreement between the reviewers was resolved by the third party (Q.L.).
2.6. Statistical analysis
All the statistical analyses were conducted with Review Manager Software 5.3 (Cochrane Collaboration, Oxford, UK) and R (version 3.5.1) software. For the dichotomous outcomes we extracted, the results were presented as the odds ratio (OR) with a 95% confidence interval (95% CI). The statistical heterogeneity was evaluated by using chi-square and I2 tests with significance set at P < .1. If the I2 value exceeded 50%, then the random effect model was used on combined results. Otherwise, the fixed effect model was used. A sensitivity analysis was then performed through the sequential omission of individual studies to investigate the effect of each study on the heterogeneity. The possible publication bias was assessed by Egger tests. All the P values were 2 sided. Apart from Cochran's Q-test, the significance level was 0.05.
3. Results
3.1. Search results and study characteristics
The search strategy resulted in the identification of 1185 records in total. One hundred ten duplicates were excluded. A total of 1026 records were excluded after scanning titles and abstracts. As a result, 49 full-text articles were subjected to detailed evaluation, of which, in one study, patients were coinfected with HIV[7]; 4 papers were review articles[8–11]; 2 studies did not include patients infected with genotype 6 HCV[12,13]; 1 study had a smaller sample size than the other study from the same region with the same topic[14]; 10 studies did not have relevant outcomes[13,15–23]; 6 studies were repeat reports[24–28]; 8 studies included <10 patients infected with genotype 6 HCV.[29–35] Finally, 7 randomized-controlled trials and 10 cohorts were chosen for inclusion in the meta-analysis, which comprised a total of 3343 patients. Figure 1 shows the study selection process. The basic characteristics of the 12 studies and the included patients are listed in Tables 1 and 2. Among the 17 eligible trials, 10 were published as full-texts, whereas 7 were abstracts. The included studies were published between 2015 and 2018. The sample size of patients with genotype 6 HCV infection for each study ranged from 31 to 685. The mean age ranged from 41 to 66.3 years. The duration of treatment ranged from 8 to 24 weeks. The percentage of males ranged from 34.8% to 62.7%.
Figure 1.
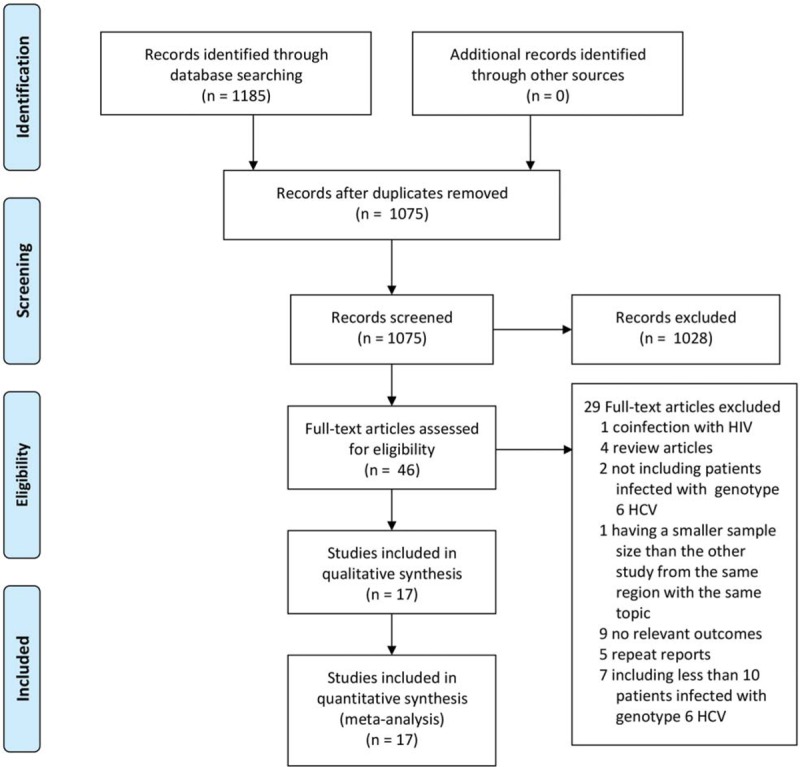
Study selection process.
Table 2.
Characteristics of the included patients in this meta-analysis.
3.2. Pooling of sustained viral response rates and rapid response rates
All 17 trials reported SVR data.[28,34,36–50] The SVR for patients with genotype 6 HCV infection ranged from 63% to 100% in these trials. As shown in Figure 2, the pooled SVR across all study arms was 95% (95% CI: 0.90–0.97, I2 = 79%). The 6 included studies involving 415 patients reported RVR data.[36,37,39,41,45,47] As shown in Figure 3, The RVR for patients with genotype 6 HCV infection ranged from 95% to 100%, with pooled rate of 97% (95% CI: 0.95–0.99, I2 = 0%). Based on an asymmetrical funnel plot (Fig. S1) and Egger tests (P = .0002), some evidence of publication bias was identified.
Figure 2.
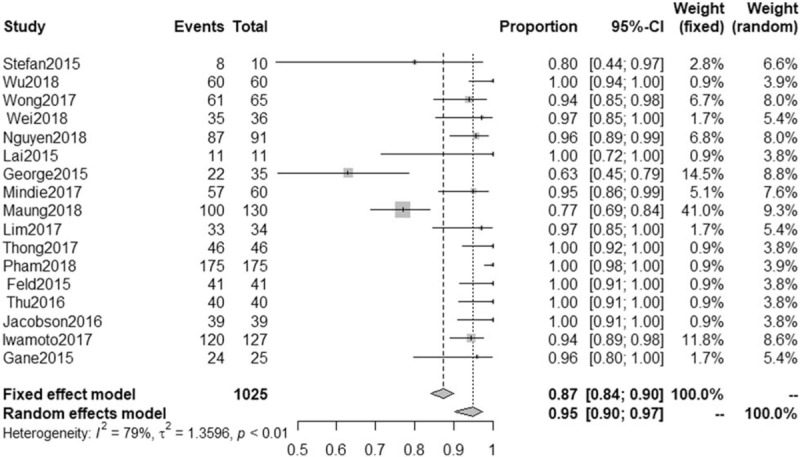
Proportion meta-analysis of sustained virological response (SVR) in all eligible study arms of patients with chronic genotype 6 hepatitis C virus (HCV) infection. CI = confidence interval.
Figure 3.
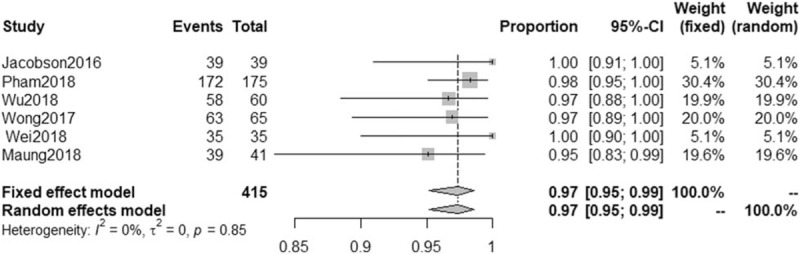
Proportion meta-analysis of rapid virological response (RVR) in all eligible study arms of patients with chronic genotype 6 hepatitis C virus (HCV) infection. CI = confidence interval.
3.3. Comparison of SVR in HCV genotype 6 group versus HCV genotype 1 or genotype 3 group
The 7 included studies involving 1415 patients reported the rate of SVR in patients with genotype 6 HCV infection versus patients with genotype 1 HCV infection.[34,36,40,41,43,49,50] As the heterogeneity among these studies was significant (I2 = 69%), the random-effect method was applied to calculate the overall effects. The SVR was similar between the HCV-6 group and the HCV-1 group (OR = 0.47, 95% CI: 0.10–2.15, P = .003; Fig. 4). In addition, the sensitivity analysis was performed through the sequential omission of every study; the results showed that the significance of the ORs was not influenced excessively.
Figure 4.
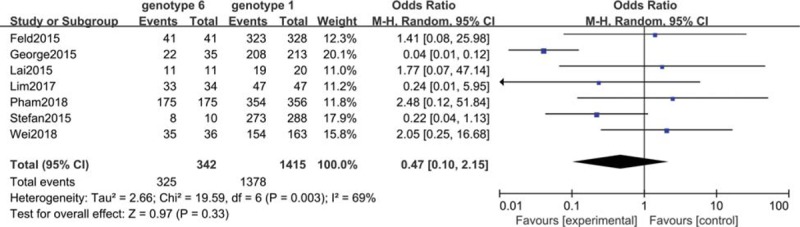
Meta-analysis of sustained virological Response (SVR) in patients with chronic genotype 6 hepatitis C virus (HCV) infection versus patients with chronic genotype 1 HCV infection. CI = confidence interval.
The 3 included studies involving 252 patients reported the rate of SVR in HCV-6 versus HCV-3 patients.[28,36,43] The between-study heterogeneity was not significant when the 3 studies were pooled into a meta-analysis (I2 = 0%), the fixed-effect method was applied to calculate the overall effects. The rate of SVR was similar between the patients with genotype 6 HCV infection and the patients with genotype 3 HCV infection (OR = 3.27, 95% CI: 0.92–11.61, P = .07; Fig. S2).
3.4. Comparison of RVR in HCV genotype 6 group versus HCV genotype 1 or genotype 3 group
The 2 included studies involving 821 patients reported the RVR of HCV in patients with genotype 6 HCV infection versus patients with genotype 1 HCV infection.[41,47] As the heterogeneity among these studies was not significant (I2 = 0%), the fixed-effect method was applied to calculate the overall effects. The rate of RVR was similar between the HCV-6 group and the HCV-1 group (OR = 1.30, 95% CI: 0.38–4.41, P = .67; Fig. S3).
The 2 included studies involving 307 patients reported the rate of RVR in patients with genotype 6 HCV infection versus patients with genotype 3 HCV infection.[36,47] As the heterogeneity among these studies was not significant (I2 = 0%), the fixed-effect method was applied to calculate the overall effects. The rate of RVR was similar between the HCV-6 group and the HCV-3 group (OR = 1.17, 95% CI: 0.13–10.47, P = .89; Fig. S4).
3.5. Adverse events
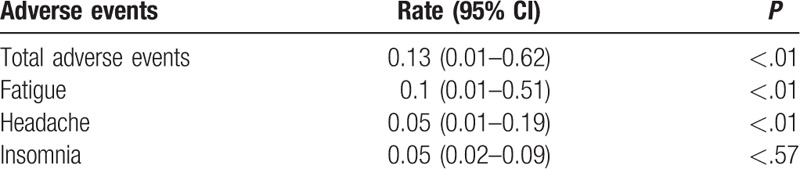
The meta-analysis results of adverse events are presented in Table 3. The pooled proportion by random effects model of adverse events was 0.13 (95% CI: 0.01–0.62, I2 = 96%, P < .01). The most frequent adverse events included fatigue (the pooled proportion = 0.1, 95% CI: 0.01–0.51, I2 = 95%, P < .01), headache (the pooled proportion = 0.05, 95% CI: 0.01–0.19, I2 = 79%, P < .01), and insomnia (the pooled proportion = 0.05, 95% CI: 0.02–0.09, I2 = 0%, P < .57).
Table 3.
Proportion meta-analysis of adverse events by random effects model.
4. Discussion
With the advent of direct-acting antiviral drugs (DAAs) that can target various enzymes in HCV replication, the prospects for HCV therapy have changed dramatically.[51] Due to the heterogeneity of distribution of different HCV genotype, large-scale clinical trials on DAA are now mainly included patients with genotype 1 and genotype 3 HCV infection, whereas data on DAA therapy for genotype 6 HCV infection are lacking. The first study for treatment of HCV GT6 using an all oral fixed-dose DAA combination tablet was with the use of ledipasvir and sofosbuvir (LDV/SOF). The results were quite favorable for HCV GT6 treatment-naïve patients who received 12 weeks of LDV/SOF (n = 25/26; SVR12 = 96%).[28] Other studies focusing on the treatment of HCV GT6 patients with DAA for 12 weeks in both treatment naïve and patients with compensated cirrhosis also had favorable results.[10] However, the sample size of these studies were small. Furthermore, relevant experiments have been continuously published in recent years. It is necessary to conduct a meta-analysis to summarize the data. The present meta-analysis was performed by carefully reviewing 7 individual RCT studies and 10 cohort studies.
In the present study, we assessed efficacy in terms of virological outcomes such as SVR and RVR. As for the adverse events, common adverse events such as fatigue, headache, and insomnia were examined as well. For a more comprehensive understanding of the efficacy of this treatment strategy in patients with genotype 6 HCV infection, we also evaluated SVR and RVR for patients with genotype 6 HCV infection versus patients with genotype 1 or genotype 3 HCV infection in some head-to head comparison trials. Our analyses demonstrated that DAA-based therapy was effective in the majority of HCV-6 patients, and efficacy was similar between genotype 6 and genotype 1 or 3. Adverse events were common, but rarely caused treatment discontinuation.
Before the DAA, the main treatment for HCV-6 was composed of interferon, peg interferon, and ribavirin. A meta-analysis of the efficacy and safety of interferon and ribavirin in the treatment of hepatitis C indicated that the pooled SVR and the pooled RVR for Peg-IFN plus weight-based RBV were 75% (95% CI: 0.68–0.81) and 70% (95% CI: 0.60–0.79), respectively.[52] The present study, however showed that the pooled SVR and the pooled RVR for DAA were 95%(95% CI: 0.90–0.97) and 97% (95% CI: 0.95–0.99), respectively. The Wang study also showed that the SVR in patients with chronic genotype 6 HCV infection was significantly higher than that in HCV-1 patients, with a relative risk of 1.35 (95% CI: 1.16–1.57, P = .001). Our results, however, showed that the SVR and RVR were both similar between patients with genotype 6 infection and patients with genotype 1 (OR = 0.47, 95% CI: 0.10–2.15; OR = 1.30, 95% CI: 0.38–4.41, P = .67) or genotype 3 HCV infection (OR = 3.27, 95% CI: 0.92–11.61; OR = 1.17, 95% CI: 0.13–10.47). The above results indicated that on the one hand, DAAs were more efficacious than interferon-based treatment for HCV-6 infection; on the other hand, the interferon-based treatment was more genotype-selective than DAAs.
Several limitations in our meta-analysis should be considered. First, 7 RCTs and 10 cohorts were included, so not all of the included studies were RCTs. Second, detailed information on individual patients was not enough to evaluate the treatment effects in the different subgroups. Third, 7 included trials were only available as abstracts. These studies, however, met all the inclusion criteria, and could provide data on the relevant outcomes. Therefore, we included these studies in our meta-analysis here. Fourth, the studies were not identical in the types of DAA administered, or the courses of treatment. Fifth, the important limitation was publication bias, which may be related to the inclusion of meeting abstracts. But with the official publication of these studies, we can update this study to avoid publication bias. Therefore, more high-quality, well-designed, randomized controlled, multicenter studies that are adequately powered will clearly be needed to guide evolving standards of care for treating patients with patients with chronic genotype 6 HCV infection. Despite of the limitations, currently, the present study is the first meta-analysis which summarized the world's newest data of DAA treatment for genotype 6 HCV infection.
In conclusion, based on the available data, our results indicate that DAA is effective and safe for patients with HCV-6, and the efficacy was similar compared to patients with genotype 1 or genotype 3 HCV infection.
Author contributions
Methodology: Qiang Luo.
Supervision: Hong Ren.
Validation: Pan Xu, Jin Wang, Zuli Li, Shunli Wang, Xiaoyan Jiang.
Writing – original draft: Aoran Luo.
Writing – review and editing: Aoran Luo, Pan Xu, Jin Wang, Zuli Li, Shunli Wang, Xiaoyan Jiang, Hong Ren, Qiang Luo.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, DAA = direct-acting antiviral, HCV = hepatitis C virus, OR = odds ratio, RCT = randomized controlled trial, RVR = rapid virological response, SVR = sustained virological response.
Supplemental Digital Content is available for this article.
The authors report no conflicts of interest.
References
- [1].Wantuck JM, Ahmed A, Nguyen MH. Review article: the epidemiology and therapy of chronic hepatitis C genotypes 4, 5 and 6. Aliment Pharmacol Ther 2014;39:137–47. [DOI] [PubMed] [Google Scholar]
- [2].Jane PM, Isla H, Abraham F, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology (Baltimore, MD) 2015;61:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nguyen LH, Nguyen MH. Systematic review: Asian patients with chronic hepatitis C infection. Aliment Pharmacol Ther 2013;37:921–36. [DOI] [PubMed] [Google Scholar]
- [4].Mellor J, Walsh EA, Prescott LE, et al. Survey of type 6 group variants of hepatitis C virus in Southeast Asia by using a core-based genotyping assay. J Clin Microbiol 1996;34:417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chao DT, Abe K, Nguyen MH. Systematic review: epidemiology of hepatitis C genotype 6 and its management. Aliment Pharmacol Ther 2011;34:286–96. [DOI] [PubMed] [Google Scholar]
- [6].Slim J, Afridi MS. Managing adverse effects of interferon-alfa and ribavirin in combination therapy for HCV. Infect Dis Clin North Am 2012;26:917–29. [DOI] [PubMed] [Google Scholar]
- [7].Barr E, Rockstroh JK, Nelson M, et al. High efficacy of elbasvir/grazoprevir (EBR/GZR) in HCV genotype 1, 4, and 6-infected patients with HIV coinfection: SVR24 data from the phase 3 C-EDGE coinfection study. J Gastroenterol Hepatol 2016;31:66. [Google Scholar]
- [8].Chahine EB, Kelley D, Childs-Kean LM. Sofosbuvir/velpatasvir/voxilaprevir: a pan-genotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother 2018;52:352–63. [DOI] [PubMed] [Google Scholar]
- [9].He T, Lopez-Olivo MA, Hur C, et al. Systematic review: cost-effectiveness of direct-acting antivirals for treatment of hepatitis C genotypes 2-6. Aliment Pharmacol Ther 2017;46:711–21. [DOI] [PubMed] [Google Scholar]
- [10].Nguyen NH, Nguyen MH. Current treatment options in patients with hepatitis C virus genotype 6. Gastroenterol Clin North Am 2015;44:871–81. [DOI] [PubMed] [Google Scholar]
- [11].Papastergiou V, Karatapanis S. Current status and emerging challenges in the treatment of hepatitis C virus genotypes 4 to 6. World J Clin Cases 2015;3:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Landis CS, Sulkowski MS, Reau N, et al. Safety and efficacy of velpatasvir and sofosbuvir-based regimens for the treatment of HCV genotype 1-6: Results of the HCV-target study. Hepatology 2017;66:587A. [Google Scholar]
- [13].Su F, Beste LA, Green PK, et al. Direct-acting antivirals are effective for chronic hepatitis C treatment in elderly patients: a real-world study of 17 487 patients. Eur J Gastroenterol Hepatol 2017;29:686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Van Nguyen K, Pham P, Lam Y, et al. Sofosbuvir + ribavirin for 24 weeks or sofosbuvir + pegylatedinterferon + ribavirin for 12 weeks in genotype 1 or genotype 6 HCV-infected patients: results from a phase 3 study in Vietnam. Hepatol Int 2017;11:S104. [Google Scholar]
- [15].Ku KS, Chodavarapu RK, Martin R, et al. Sequencing analysis of NS3/4A, NS5A, and NS5B genes from patients infected with hepatitis C virus genotypes 5 and 6. J Clin Microbiol 2016;54:1835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cheng W, Sobhonslidsuk A, Pham TTT, et al. Impact of a 12-week oral regimen of elbasvir/grazoprevir (EBR/GZR) on health-related quality of life (HRQOL) and fatigue in treatment-naïve patients with chronic hepatitis C virus (HCV) genotype (GT) 1, 4, or 6 infection: Data from the C-CORAL study. Hepatol Int 2017;11:S298–9. [Google Scholar]
- [17].Lawitz E, Gane EJ, Feld JJ, et al. C-BREEZE 2: efficacy and safety of a two-drug direct-acting antiviral agent (DAA) regimen ruzasvir 180 mg and uprifosbuvir 450 mg for 12 weeks in adults with chronic hepatitis C virus (HCV) genotype (GT) 1, 2, 3, 4, or 6. Hepatology 2017;66:34A–5A. [Google Scholar]
- [18].Rao H, Song G, Li G, et al. Safety and efficacy of coblopasvir and sofosbuvir in genotype 1, 2, 3, and 6 HCV infected patients with compensated cirrhosis or without cirrhosis. Hepatology 2018;68:582A–3A. [Google Scholar]
- [19].Thompson A, Manns MP, Bourlière M, et al. Sofosbuvir/velpatasvir/voxilaprevir for 12 weeks is a safe and effective salvage regimen for NS5A inhibitor-experienced patients with genotype 1-6 hepatitis C virus infection: an integrated analysis of phase 2 and phase 3 studies. J Gastroenterol Hepatol 2018;33:42. [Google Scholar]
- [20].Li Z, Liu Y, Zhang Y, et al. Naturally occurring resistance-associated variants to hepatitis C virus direct-acting antiviral agents in treatment-naive HCV genotype 6a-infected patients. Biomed Res Int 2017;2017:9849823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Curry M, Bacon B, Dieterich DT, et al. Sofosbuvir/velpatasvir in genotype 2-6 HCV: real-world experience from the trio network. Gastroenterology 2017;152:S1061–2. [Google Scholar]
- [22].Everson GT, Towner WJ, Davis MN, et al. Sofosbuvir with velpatasvir in treatment-naive noncirrhotic patients with genotype 1 to 6 hepatitis c virus infection. Ann Intern Med 2015;163:818–26. [DOI] [PubMed] [Google Scholar]
- [23].Younossi ZM, Stepanova M, Feld J, et al. The use of sofosbuvir and velpatasvir is associated with high efficacy and improvement in patient-reported outcomes in patients with genotype 1, 2, 4, 5 and 6 chronic hepatitis C: results from the ASTRAL-1 clinical trial. Gastroenterology 2016;150:S1267–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Feld JJ, Agarwal K, Hezode C, et al. A phase 3 double-blind placebo-controlled evaluation of sofosbuvir/velpatasvir fixed dose combination for 12 weeks in naïve and experienced genotype 1, 2, 4, 5, 6 HCV infected patients with and without cirrhosis: results of the astral-1 study. Hepatology 2015;62:1379A–80A. [Google Scholar]
- [25].Nguyen MH, Trinh H, Do S, et al. Ledipasvir/sofosbuvir fixed-dose combination (LDV/SOF FDC) for 8 weeks for treatment-Naïve, non-cirrhotic hepatitis C genotype 6 (HCV-6) and 12 weeks in those with cirrhosis and/or prior treatment failure: a multicenter open-labelled clinical trial. Hepatol Int 2017;11:S105. [Google Scholar]
- [26].Wong RJ, Nguyen MT, Trinh HN, et al. Community-based real world outcomes of sofosbuvir/ledipasvir without ribavirin in the treatment of Asians with chronic hepatitis C virus genotype 6 in the United States. Hepatology 2016;64:467A–8A. [Google Scholar]
- [27].Wei L, Xie Q, Hou J, et al. Sofosbuvir + ribavirin-pegylated-interferon in genotype 1, 2, 3 or 6 HCV-infected patients: results from a phase 3 study in China. Hepatol Int 2017;11:S157–8. [Google Scholar]
- [28].Gane EJ, Hyland RH, An D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology 2015;149:1454–61. [DOI] [PubMed] [Google Scholar]
- [29].Gane EJ, Kowdley KV, Pound D, et al. Efficacy of sofosbuvir, velpatasvir, and GS-9857 in patients with hepatitis C virus genotype 2, 3, 4, or 6 infections in an open-label, phase 2 trial. Gastroenterology 2016;151:902–9. [DOI] [PubMed] [Google Scholar]
- [30].Kowdley KV, Lawitz E, Crespo I, et al. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet 2013;381:2100–7. [DOI] [PubMed] [Google Scholar]
- [31].Lawitz E, Buti M, Vierling JM, et al. Safety and efficacy of a fixed-dose combination regimen of grazoprevir, ruzasvir, and uprifosbuvir with or without ribavirin in participants with and without cirrhosis with chronic hepatitis C virus genotype 1, 2, or 3 infection (C-CREST-1 and C-CREST-2, part B): two randomised, phase 2, open-label trials. Lancet Gastroenterol Hepatol 2017;2:814–23. [DOI] [PubMed] [Google Scholar]
- [32].Thompson A, Jacobson I, Lawitz E, et al. An integrated analysis of 402 compensated cirrhotic patients with HCV genotype (GT) 1, 4 or 6 infection treated with elbasvir/grazoprevir. J Gastroenterol Hepatol 2016;31:75. [Google Scholar]
- [33].Brown A, Hézode C, Zuckerman E, et al. Efficacy and safety of 12 weeks of elbasvir ± grazoprevir ± ribavirin in participants with hepatitis C virus genotype 2, 4, 5 or 6 infection: The C-SCAPE study. J Viral Hepat 2018;25:457–64. [DOI] [PubMed] [Google Scholar]
- [34].Feld JJ, Jacobson IM, Hezode C, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015;373:2599–607. [DOI] [PubMed] [Google Scholar]
- [35].Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 2015;373:2618–28. [DOI] [PubMed] [Google Scholar]
- [36].Wei L, Xie Q, Hou JL, et al. Sofosbuvir plus ribavirin with or without peginterferon for the treatment of hepatitis C virus: results from a phase 3b study in China. J Gastroenterol Hepatol 2018;33:1168–76. [DOI] [PubMed] [Google Scholar]
- [37].Wu DB, Jiang W, Wang YH, et al. Safety and efficacy of sofosbuvir-based direct-acting antiviral regimens for hepatitis C virus genotype 6 in southwest China: real-world experience of a retrospective study. J Viral Hepat 2019;26:316–22. [DOI] [PubMed] [Google Scholar]
- [38].Nguyen E, Trinh S, Trinh H, et al. Sustained virologic response rates (SVR-12) for chronic hepatitis C genotype 6 patients treated with ledipasvir/sofosbuvir or sofosbuvir/velpatasvir. J Hepatol 2018;68:S284–5. [DOI] [PubMed] [Google Scholar]
- [39].Maung ST, Win KM, Bwa AH, et al. Efficacy of fixed dose combination of sofosbuvir and ledipasvir (SOF/LDV) A ± ribavirin in patients (n = 130) infected with HCV genotype 6 (REAL world Myanmar experience). Hepatol Int 2018;12:S304. [Google Scholar]
- [40].George J, Burnevich E, Sheen IS, et al. Elbasvir/grazoprevir in Asia-Pacific/Russian participants with chronic hepatitis C virus genotype 1, 4, or 6 infection. Hepatol Commun 2018;2:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thu TPT, Bunchorntavakul C, Tan DH, et al. Sofosbuvir-ledipasvir with or without ribavirin for chronic hepatitis C genotype-1 and 6: real-world experience in Vietnam. Antivir Ther 2018;23:415–23. [DOI] [PubMed] [Google Scholar]
- [42].Nguyen MH, Trinh H, Do S, et al. Open label study of 8 vs. 12 weeks of ledipasvir/sofosbuvir in genotype 6 treatment naive or experienced patients. Am J Gastroenterol 2017;112:1824–31. [DOI] [PubMed] [Google Scholar]
- [43].Lim SG, Mohamed R, Le P, et al. Safety and efficacy of sofosbuvir/velpatasvir in a genotype 1-6 HCV infected population from Singapore, Malaysia, Thailand, and Vietnam: Results from a phase 3, clinical trial. Hepatology 2017;66:586A. [Google Scholar]
- [44].Thong VD. Efficacy and safety of ledipasvir/sofosbuvir in treatment-naïve and-experienced patients with hepatitis C virus genotype 6 infection. Hepatology 2017;66:604A–5A. [Google Scholar]
- [45].Wong RJ, Nguyen MT, Trinh HN, et al. Community-based real-world treatment outcomes of sofosbuvir/ledipasvir in Asians with chronic hepatitis C virus genotype 6 in the United States. J Viral Hepat 2017;24:17–21. [DOI] [PubMed] [Google Scholar]
- [46].Iwamoto M, Sonderup MW, Sann K, et al. Real-world effectiveness and safety of daclatasvir/sofosbuvir with or without ribavirin among genotype 5 and 6 hepatitis c virus patients. Hepatology 2017;66:1264A–5A. [Google Scholar]
- [47].Jacobson IM, Asselah T, Nahass R, et al. A randomized phase 3 trial of sofosbuvir/velpatasvir/voxilaprevir for 8 weeks compared to sofosbuvir/velpatasvir for 12 weeks in DAA-naïve genotype 1-6 HCV-infected patients: the POLARIS-2 study. Hepatology 2016;64:1126A. [Google Scholar]
- [48].Thi TTP, Ho TD. The efficacy and safety of sofosbuvir plus ribavirin with or without peg-interferon in treatment of naïve and experienced Vietnamese patients with chronic genotype 1 and 6 HCV infection. Hepatology 2016;64:954A.27388553 [Google Scholar]
- [49].Lai CL, Wong VWS, Yuen MF, et al. Sofosbuvir plus ribavirin for the treatment of patients with chronic genotype 1 or 6 hepatitis C virus infection in Hong Kong. Aliment Pharmacol Ther 2016;43:96–101. [DOI] [PubMed] [Google Scholar]
- [50].Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-Elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann Intern Med 2015;163:1–3. [DOI] [PubMed] [Google Scholar]
- [51].Hcv PGOE, Halota W, Flisiak R, et al. Recommendations for the treatment of hepatitis C in 2017. Clin Exp Hepatol 2017;3:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wang X, Liu F, Wei F, et al. Efficacy and safety of pegylated interferon plus ribavirin therapy for chronic hepatitis C genotype 6: a meta-analysis. PLoS One 2014;9:e100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


