Abstract
Over the past three decades, transcatheter arterial embolization has become the first-line therapy for the management of acute nonvariceal upper gastrointestinal bleeding refractory to endoscopic hemostasis. Overall, transcatheter arterial interventions have high technical and clinical success rates. This review will focus on patient presentation and technical considerations as predictors of complications from transcatheter arterial embolization in the management of acute upper gastrointestinal hemorrhage.
Keywords: upper gastrointestinal hemorrhage, angiography, transcatheter arterial embolization, complication rates, bowel ischemia, interventional radiology
Acute nonvariceal upper gastrointestinal hemorrhage is associated with significant morbidity and mortality. Aggressive treatment with early endoscopic hemostasis is essential for a favorable outcome. However, severe bleeding despite endoscopic intervention occurs in 5 to 10% of patients. Over the past three decades, transcatheter arterial embolization has become the first-line therapy for the management of acute nonvariceal upper gastrointestinal bleeding (UGIB) refractory to endoscopic hemostasis. Overall, transcatheter arterial interventions have high technical and clinical success rates ranging from 69 to 100% and 63 to 97%, respectively, 1 and complication rates ranging from 4 to 20%. 2 This review will focus on patient presentation and technical considerations as predictors of complications from transcatheter arterial embolization.
Case Presentation
A 60-year-old male with acute myeloid leukemia (AML) status post stem cell transplant presented with persistent anemia and dark stools. The patient was taken to the gastroenterology suite for upper endoscopy. Preprocedural laboratories were significant for hemoglobin of 7.3 g/dL and platelet level of 99 × 10 3 /µL. On endoscopy, the patient was found to have multiple pyloric and duodenal ulcers, one of which started massively bleeding during the procedure leading to significant hemodynamic instability. The patient was rapidly intubated, started on phenylephrine for blood pressure support, and placed on a resuscitation protocol with intravenous fluid and packed red blood cell (PRBC) replacement. Interventional radiology (IR) was emergently consulted for transcatheter arterial embolization, and the patient was transported to the IR suite with anesthesia support.
Initial celiac and selective gastroduodenal artery (GDA) angiograms ( Fig. 1a , b ) were grossly unremarkable but given the patients hemodynamic instability and endoscopy findings, the decision was made to empirically coil embolize the GDA. During coil embolization of the GDA, there was a sudden increase in the amount of blood being suctioned from the nasogastric tube and repeat angiogram demonstrated massive extravasation from branches of the proximal GDA ( Fig. 1c ). Additional units of PRBCs were administered by the anesthesiologist, bringing the total units of PRBCs that the patient received since the resuscitation began to five. Embolization was continued with Gelfoam slurry “sandwiched” between distal and proximal coils. Post embolization angiogram with the microcatheter tip in the common hepatic artery demonstrated no flow in the main GDA and superior pancreaticoduodenal artery and no active extravasation ( Fig. 1d ).
Fig. 1.

A 60-year-old man with massive upper gastrointestinal hemorrhage. ( a ) Initial celiac angiogram with no evidence of active extravasation. ( b ) Selective angiogram of the gastroduodenal artery (GDA) with no evidence of active extravasation. ( c ) Repeat selective angiogram, after placement of backdoor coils in the right gastroepiploic artery, demonstrating a significant amount of active extravasation into the duodenum from branches from the proximal GDA. ( d ) Common hepatic angiogram after additional embolization with Gelfoam and coil embolization of the GDA and superior pancreaticoduodenal artery; no active bleeding identified.
Selective angiography of the superior mesenteric artery (SMA) demonstrated continued extravasation from branches of the inferior pancreaticoduodenal artery (IPDA; Fig. 2a ); additional coils were placed in branches of the IPDA for hemostasis ( Fig. 2b ). A total of 1,500 mL of blood had been suctioned from the nasogastric tube over the course of the procedure, so a stat CBC was sent by anesthesia. At this point, the patient was hemodynamically improving, and the phenylephrine dose was titrated lower. Additional angiography of the celiac artery demonstrated a small amount of continued active extravasation into the duodenum from small collateral vessels from the dorsal pancreatic artery ( Fig. 3 ).
Fig. 2.

(a) Angiogram of the superior mesenteric artery demonstrating persistent active extravasation from collateral flow in the inferior pancreaticoduodenal artery ( arrow ). ( b ) Superior mesenteric angiogram status post coil embolization of branches of the inferior pancreaticoduodenal artery with no evidence of persistent extravasation.
Fig. 3.
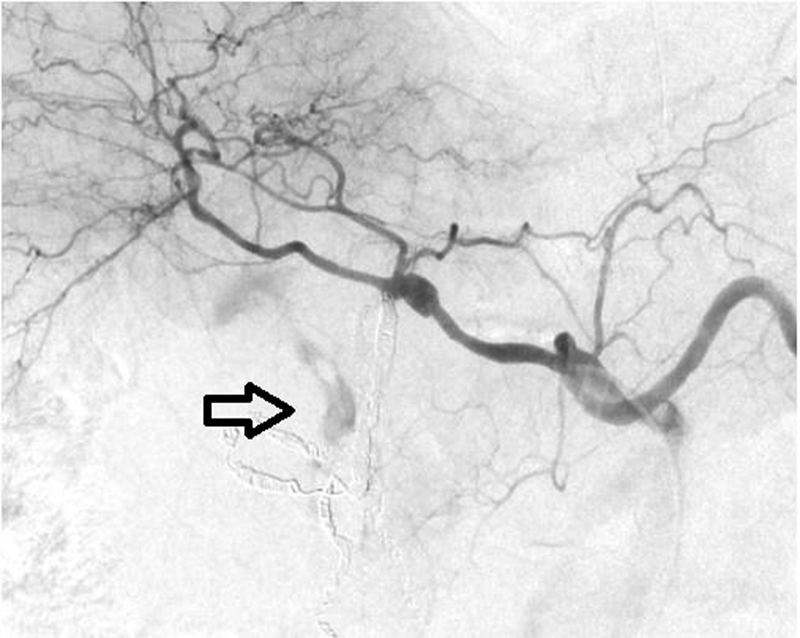
Repeat celiac angiogram demonstrates persistent active extravasation from small collateral branches from the dorsal pancreatic artery ( arrow ).
Although there was still a small amount of active bleeding, and having performed extensive embolization, the patient was stabilizing from a hemodynamic standpoint. The platelet level returned at 46 × 10 3 /µL, so the decision was made to transport the patient to the intensive care unit (ICU) for additional resuscitation, platelet transfusions, and monitoring. The hope was the small amount of bleeding would stop with appropriate platelet replenishment, as the risk of intestinal ischemia would be significantly increased with additional embolization.
The patient remained hemodynamically stable in the ICU over the next 72 hours, but he continued to have a slow downward trend in his hemoglobin. On repeat endoscopy, he was found to have diffuse mild oozing and ischemic changes in the first and second portions of the duodenum ( Fig. 4 ). Since there was no evidence of massive bleeding, the patient was transported back to the ICU for further monitoring and transfusions. The next morning the patient complained of abdominal pain. A chest radiograph revealed free air under the diaphragm ( Fig. 5a ). CT of the abdomen confirmed free air likely from a perforated duodenum ( Fig. 5b, c ). After discussing the goals of care with the patient and his family, the decision was made to initiate comfort care and the patient died 2 days later.
Fig. 4.

( a ) Representative endoscopy image from initial presentation demonstrating ulceration in the duodenum with minimal bleeding. These images were captured just prior to the start of the massive bleeding episode which was not able to be controlled with endoscopic techniques. This bleeding event prompted the consult to interventional radiology for emergent transcatheter arterial embolization. ( b ) Representative endoscopy image from 3 days post transcatheter arterial embolization demonstrating ischemic changes of the mucosa in the duodenum with mild diffuse oozing.
Fig. 5.

( a ) Upright chest radiograph demonstrating a large amount of free air under the right hemidiaphragm ( arrow ). ( b ) Axial abdominal CT image in soft-tissue windows demonstrating the large amount of free air in the abdomen ( asterisk ) with no fluid collection. ( c ) Axial abdominal CT image in lung windows demonstrating the large amount of free air in the abdomen with small foci of air in the peritoneal fat tracking to the duodenum suggesting this as the site of perforation.
Background
UGIB is defined as bleeding proximal to the ligament of Treitz, originating from the distal esophagus, stomach, or duodenum. 3 Typically, patients with UGIB will present with hematemesis or melena but can also report hematochezia if bleeding is brisk. UGIB remains a significant cause of morbidity and mortality, with 100 episodes per 10,000 admissions annually in the United States and mortality rates as high as 14%. 3 4 The most common cause of UGIB is peptic ulcer disease, but differential considerations would include tumors, gastritis, ischemia, arteriovenous malformations, Mallory Weiss tears, trauma, arterioenteric fistula, and iatrogenic causes. Varices are also an important differential consideration for UGIB; however, because variceal bleeding involves the diagnosis and management of portal hypertension, it will not be included in this discussion.
In patients presenting with UGIB, aggressive volume resuscitation and maintenance of hemodynamic stability are the first priorities. 3 Except for patients who have a contraindication, endoscopy is the best initial method for both the diagnosis and treatment of UGIB with the source of bleeding being identified in 95% of cases and endoscopic treatment being effective in ∼90% of cases. 5 Severe bleeding despite endoscopic intervention occurs in 5 to 10% of patients. 1 Historically, these patients underwent surgical intervention, which was associated with high mortality rates. Transcatheter arterial embolization emerged as an alternative to surgery in the 1970s. 6 Many studies have been published confirming the feasibility of transcatheter arterial interventions citing high technical and clinical success rates ranging from 69 to 100% and 63 to 97%, respectively. 1 Although originally only offered to poor surgical candidates, transcatheter arterial embolization has been shown to be equally effective at controlling bleeding, with lower overall complication rates and trends toward lower 30-day mortality rates compared with surgical intervention. 7 8 In a comparative study of 70 patients, mortality rates and recurrent bleeding rates were similar in the surgery and transcatheter arterial embolization groups despite an older patient population with more comorbidities in the embolization group. 8 Over the past three decades, transcatheter arterial embolization has emerged as the first-line therapy for massive UGIB refractory to endoscopic treatment.
Vascular Anatomy of the UGI Tract
The blood supply to the upper gastrointestinal tract is redundant. The lesser curvature of the stomach is supplied by the left gastric artery which anastomoses with the right gastric artery. Additionally, there can also be collateral supply from the short gastric arteries and the left inferior phrenic artery. The greater curvature of the stomach is supplied by the gastroepiploic arcade which is composed of the right gastroepiploic artery, the terminal branch of the GDA, and the left gastroepiploic artery, originating from the distal splenic artery. The duodenum is supplied by the pancreaticoduodenal arcade, made up of superior and inferior pancreaticoduodenal arteries arising from the gastroduodenal and superior mesenteric arteries, respectively. The likelihood of successful embolization is improved with prior knowledge of the location of the bleed. 2 The redundant vascular supply to the stomach and duodenum can make successful embolization challenging. However, this rich blood supply also decreases the incidence of postembolization bowel ischemia. 9
Indications for Transcatheter Arterial Embolization
Transcatheter arterial embolization has become a useful diagnostic and therapeutic tool in selected patient populations. Typically, patients fall into one of the following four categories: (1) prior endoscopy reveals a clear arterial bleeding source, but endoscopic techniques were not able to control the bleeding; (2) endoscopy confirms upper gastrointestinal bleeding (i.e., blood in the gastrointestinal lumen) without identifying a clear source to treat; (3) the endoscopy was negative, but there remains high clinical suspicion for upper gastrointestinal bleed; and (4) upper gastrointestinal bleeding highly suspected but endoscopy contraindicated because of prior surgery or trauma. 5 Ten percent to 15% of patients treated with endoscopy experience rebleeding requiring transcatheter arterial embolization or surgery. 7 When there is acute overt hemorrhage in an unstable patient, angiography should be performed on an emergent basis. 10 In patients who suffer from postsurgical UGIB, it may not be feasible to have endoscopic interventions secondary to concern for postsurgical perforation. In this clinical context, primary angiography or CTA is the preferred method for evaluation. 11 Angiography has been shown to positively identify the cause of postoperative GI hemorrhage in 81% of postsurgical patients, with transcatheter arterial embolization being a safe intervention to manage the postsurgical bleeding. 12
Transcatheter arterial embolization has been shown to be effective in controlling bleeding related to peptic ulcer disease, 8 13 14 gastric adenocarcinoma, 15 nitinol stent placement, 16 and life-threatening hemorrhagic pancreatitis. 17 Patients on hemodialysis have been shown to have a higher rate of UGIB compared with the general population and transcatheter arterial embolization has been shown to be effective in controlling bleeding in this patient population as well. 18 Additionally, angiography and transcatheter arterial embolization have been shown to be effective in the treatment of hemobilia, a rare cause of UGIB. Causes of hemobilia include blunt hepatic trauma, laparoscopic cholecystectomy, iatrogenic from hepatobiliary intervention, vascular malformations, and hepatic artery pseudoaneurysm. 19
Angiographic Considerations
Since endoscopy is usually the first intervention for patients with upper gastrointestinal bleeding, the site, and often source, of bleeding is known at the time of angiography. Therefore, angiography is most often performed only as a precursor to transcatheter arterial embolization based on known vascular supply in the area of endoscopically identified abnormality. A positive angiographic examination is typically described as extravasation of contrast into the bowel lumen or false aneurysm-like lesion. Additionally, an abnormal blush of the mucosa is indicative of an inflammatory process (gastritis or duodenitis) which, when correlated with endoscopy findings, could also be considered a positive angiographic finding for bleeding. Angiography can identify a bleeding source in up to 80% of cases. 20 21 22 Bleeding into the gastrointestinal tract can be detected with visceral angiography at rates as low as 0.5 mL/min. 23 Marking the site of bleeding with metallic clips during endoscopy has been shown to aid in the accurate localization of a bleeding source. 24 There are limitations of angiography that can result in false-negative studies. If the bleed is intermittent, it can be missed on angiogram. 25 26 Additionally, the cause of bleeding may not be determined if there is variant vascular anatomy or if the contrast resolution is not adequate. 25 Even in the absence of active extravasation on angiogram, empiric embolization of the vascular territory corresponding to the bleeding location noted on endoscopy can be performed safely and successfully. Long-term clinical success has been demonstrated in patients undergoing transcatheter arterial embolization for UGIB irrespective of whether active contrast extravasation is observed at the time of angiography or not. 20 27 28 29 30
Choice of Embolic Agent
Once the decision has been made to embolize a target vessel, either empirically or based on active angiographic extravasation, the interventional radiologist must choose an appropriate embolic agent to complete the therapy. The role of transcatheter arterial embolization is to selectively reduce blood supply to the source of bleeding while maintaining enough collateral blood flow to maintain intestinal viability. The choice of best embolic agent is a matter of debate and includes coils, cyanoacrylate glue, particles, and gelatin sponge. 1
Coils alone inserted into the GDA or superselectively into the pancreaticoduodenal arteries have been used with success by several investigators. 31 32 33 When used in this setting, coils are usually used to occlude or reduce flow in a major vessel. The main advantage of using coils is that they can be delivered in a precise fashion and carry a low risk of infarction because of the preservation of the distal microvasculature. The main drawback of coils is that they rely on intrinsic blood clotting and several studies have demonstrated an association between the use of coils alone and the incidence of bleeding recurrence, especially in patients with coagulopathy. 1 20 For this reason, coils are often used in combination with Gelfoam.
Gelfoam is a commonly used temporary embolic agent. Flow is generally restored within days to weeks after Gelfoam embolization. Additionally, it is cheap and unlikely to cause ischemia. The main disadvantage is that it can take time to prepare and the rate of recanalization is unpredictable. Several studies have shown lower clinical success rates and high rebleeding rates when Gelfoam is used as the only embolic agent. 34 35 Use of calibrated particles, such as polyvinyl alcohol (PVA) or microspheres, may be advantageous when attempting to exclude diffuse tumor vascularization from the main arterial supply. These agents should be injected through a microcatheter at a site distal to major vessels. 36 Only larger particles (>500 µm) should be used to decrease the risk of ischemia from normal tissue devascularization.
Recently, high clinical success rates have been reported using cyanoacrylate glue. Studies have also shown shorter procedure times when using glue, which can be advantages when dealing with hemodynamically unstable patients. 36 Other studies investigating the role of cyanoacrylate in the treatment of UGIB demonstrate much higher success in coagulopathic patients, as this agent theoretically does not rely on the patient's coagulation system to ensure success. One report demonstrated clinical success in 83% of coagulopathic patients undergoing transcatheter arterial embolization for UGIB, using cyanoacrylate as the embolic agent. 37 However, there is a higher risk of bowel infarction and glue reflux into other vessels leading to potential complications related to nontarget embolization.
Procedural Outcomes and Complications
Many studies have been published confirming the feasibility of transcatheter arterial interventions citing high technical and clinical success rates ranging from 69 to 100% and 63 to 97%, respectively. 1 A literature review published by Loffroy et al summarized the data on factors predicting embolization failures and complications from transcatheter arterial treatment. 1 The review identified 15 studies, for a combined 819 patients, looking at outcomes after endovascular treatment for uncontrolled nonvariceal UGIB. Endovascular embolization was technically successful in 93% of patients; 54% of patients had active extravasation at the time of angiography and 46% underwent bland embolization. Of the subgroup that had technically successful embolization, 67% had clinical cessation of bleeding. Thirty-three percent of patients continued to bleed, and almost half of those patients clinically responded to repeat embolization. Factors that were believed to contribute to clinical failure were the presence of multiple or large duodenal ulcers, gastritis, coagulopathy, or multiorgan failure.
Reviewing the 15 studies cited by Loffroy et al, the combined complication rate was 9% (range: 4–26%) and the 30-day mortality of 28% (range: 4–46%). 1 Major and minor embolization-related complications included access site complications, dissection of the target vessel, and liver or spleen infarction. Arterial embolization in the upper gastrointestinal tract is generally considered very safe because of the rich collateral supply to the stomach and duodenum. However, the risk of significant ischemia after embolization is increased in patients with prior surgery in the same area, 2 38 or with the use of deeply penetrating embolic agents such as cyanoacrylate or very small particles. 2 While there have been some cases of ischemia presenting in the acute phase, like the one mentioned earlier, postembolization ischemia usually presents as duodenal stenosis in the chronic phase. The risk of duodenal stenosis was shown to be higher in patients treated with cyanoacrylate embolization. 39
Unsurprisingly, studies have shown that transcatheter arterial embolization for UGIB in patients with coagulopathy portends a greater risk of clinical failure. Coagulopathy, in these studies, has been defined as prothrombin ratio >1.3, partial thromboplastin time >40 ×, or platelet count < 80 × 10 3 /µL. Encarnacion et al found that embolization was 2.9 times more likely to fail in patients with coagulopathy, and death from bleeding after embolization was 9.6 times more likely. 35 Although every effort should be made to correct coagulopathy before, during, or immediately after embolization, coagulopathy should not exclude patients from treatment in the setting of life-threatening arterial UGI hemorrhage. Other clinical variables found to be predictive of rebleeding include peptic ulcer disease, longer time from onset of shock to treatment with angiography, having more than two comorbid conditions, previous surgery for bleeding, transfusion of more than 6 units of packed red blood cells before the procedure, and corticosteroid use. 1
The patient in the case presentation above had several clinical factors that could have predicted the poor outcome: coagulopathy, prior steroid use, hypotension requiring pressor support, and diffuse ulcer disease. Although the platelet level was acceptable prior to endoscopy, the massive bleeding rapidly depleted the patients supply, making him coagulopathic with a platelet level around 46 × 10 3 /µL at the time of embolization. Despite aggressive platelet repletion, his platelet count remained low, ranging from 59 to 173 × 10 3 /µL, in the days following the procedure. While cyanoacrylate could have been considered for the embolic agent to improve the chance of clinical success given his thrombocytopenia, this must be weighed against the increased risk of bowel ischemia or nontarget embolization with this agent. Additionally, the underlying graft versus host disease (GVHD) and history of extensive steroid use for immunosuppression made intervention less likely to be clinically successful. Surgical literature has demonstrated that surgical intervention provides, at best, temporarily cessation of hemorrhage in patients with UGIB related to GVHD. 40 41 42 While gastrointestinal hemorrhage following bone marrow transplantation is relatively low, 10%, 43 44 acute UGIB from GVHD has reported mortality rates as high as 30 to 60%. 43 45 While UGIB can be effectively controlled with transcatheter arterial embolization, patients with bleeding related to GVHD should be approached with treatment strategies that take into consideration the diffuse nature of this disease and the near universal presence of severe refractory thrombocytopenia.
Conclusion
Managing massive UGIB often requires a multidisciplinary approach with endoscopists, intensive care specialists, surgeons, and interventional radiologists all having a role. Due to the demonstrated safety and efficacy of transcatheter arterial embolization in the treatment of nonvariceal gastrointestinal bleeding, it has become the treatment of choice for endoscopy refractory patients. Embolization can be effective even in gravely ill patients for whom surgery is not an option. Clinical success is similar in patients where active extravasation is present at the time of embolization and in patients who undergo empiric embolization based on the site of bleeding noted during endoscopy. Several clinical and technical factors must be known by interventional radiologists as they can influence the technical and clinical success of the procedure. Every effort should be made to perform embolization early after the onset of bleeding and to correct coagulation disorders. In addition, careful selection of embolic agents may play a role in successful outcome.
Footnotes
Conflict of Interest None.
References
- 1.Loffroy R, Rao P, Ota S, De Lin M, Kwak B K, Geschwind J F. Embolization of acute nonvariceal upper gastrointestinal hemorrhage resistant to endoscopic treatment: results and predictors of recurrent bleeding. Cardiovasc Intervent Radiol. 2010;33(06):1088–1100. doi: 10.1007/s00270-010-9829-7. [DOI] [PubMed] [Google Scholar]
- 2.Loffroy R, Favelier S, Pottecher Pet al. Transcatheter arterial embolization for acute nonvariceal upper gastrointestinal bleeding: Indications, techniques and outcomes Diagn Interv Imaging 201596(7-8):731–744. [DOI] [PubMed] [Google Scholar]
- 3.Feinman M, Haut E R. Upper gastrointestinal bleeding. Surg Clin North Am. 2014;94(01):43–53. doi: 10.1016/j.suc.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Barkun A N, Bardou M, Kuipers E J et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(02):101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 5.Millward S F. ACR Appropriateness Criteria on treatment of acute nonvariceal gastrointestinal tract bleeding. J Am Coll Radiol. 2008;5(04):550–554. doi: 10.1016/j.jacr.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Rösch J, Dotter C T, Brown M J. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1972;102(02):303–306. doi: 10.1148/102.2.303. [DOI] [PubMed] [Google Scholar]
- 7.Jairath V, Kahan B C, Logan R F et al. National audit of the use of surgery and radiological embolization after failed endoscopic haemostasis for non-variceal upper gastrointestinal bleeding. Br J Surg. 2012;99(12):1672–1680. doi: 10.1002/bjs.8932. [DOI] [PubMed] [Google Scholar]
- 8.Ripoll C, Bañares R, Beceiro I et al. Comparison of transcatheter arterial embolization and surgery for treatment of bleeding peptic ulcer after endoscopic treatment failure. J Vasc Interv Radiol. 2004;15(05):447–450. doi: 10.1097/01.rvi.0000126813.89981.b6. [DOI] [PubMed] [Google Scholar]
- 9.Frisoli J K, Sze D Y, Kee S. Transcatheter embolization for the treatment of upper gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7(03):136–142. doi: 10.1053/j.tvir.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Gerson L B, Fidler J L, Cave D R, Leighton J A.ACG Clinical Guideline: diagnosis and management of small bowel bleeding Am J Gastroenterol 2015110091265–1287., quiz 1288 [DOI] [PubMed] [Google Scholar]
- 11.Rebibo L, Fuks D, Blot C et al. Gastrointestinal bleeding complication of gastric fistula after sleeve gastrectomy: consider pseudoaneurysms. Surg Endosc. 2013;27(08):2849–2855. doi: 10.1007/s00464-013-2833-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhou C G, Shi H B, Liu S et al. Transarterial embolization for massive gastrointestinal hemorrhage following abdominal surgery. World J Gastroenterol. 2013;19(40):6869–6875. doi: 10.3748/wjg.v19.i40.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Wispelaere J F, De Ronde T, Trigaux J P, de Cannière L, De Geeter T. Duodenal ulcer hemorrhage treated by embolization: results in 28 patients. Acta Gastroenterol Belg. 2002;65(01):6–11. [PubMed] [Google Scholar]
- 14.Holme J B, Nielsen D T, Funch-Jensen P, Mortensen F V. Transcatheter arterial embolization in patients with bleeding duodenal ulcer: an alternative to surgery. Acta Radiol. 2006;47(03):244–247. doi: 10.1080/02841850600550690. [DOI] [PubMed] [Google Scholar]
- 15.Meehan T, Stecker M S, Kalva S P, Oklu R, Walker T G, Ganguli S. Outcomes of transcatheter arterial embolization for acute hemorrhage originating from gastric adenocarcinoma. J Vasc Interv Radiol. 2014;25(06):847–851. doi: 10.1016/j.jvir.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Oh S J, Song H Y, Nam D H et al. Bleeding after expandable nitinol stent placement in patients with esophageal and upper gastrointestinal obstruction: incidence, management, and predictors. Acta Radiol. 2014;55(09):1069–1075. doi: 10.1177/0284185113511080. [DOI] [PubMed] [Google Scholar]
- 17.Fitzpatrick J, Bhat R, Young J A. Angiographic embolization is an effective treatment of severe hemorrhage in pancreatitis. Pancreas. 2014;43(03):436–439. doi: 10.1097/MPA.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 18.Banshodani M, Kawanishi H, Moriishi M, Shintaku S, Sato T, Tsuchiya S. Efficacy of intra-arterial treatment for massive gastrointestinal bleeding in hemodialysis patients. Ther Apher Dial. 2014;18(01):24–30. doi: 10.1111/1744-9987.12062. [DOI] [PubMed] [Google Scholar]
- 19.Murugesan S D, Sathyanesan J, Lakshmanan A et al. Massive hemobilia: a diagnostic and therapeutic challenge. World J Surg. 2014;38(07):1755–1762. doi: 10.1007/s00268-013-2435-5. [DOI] [PubMed] [Google Scholar]
- 20.Aina R, Oliva V L, Therasse E et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: outcome assessment. J Vasc Interv Radiol. 2001;12(02):195–200. doi: 10.1016/s1051-0443(07)61825-9. [DOI] [PubMed] [Google Scholar]
- 21.Schenker M P, Duszak R, Jr, Soulen M C et al. Upper gastrointestinal hemorrhage and transcatheter embolotherapy: clinical and technical factors impacting success and survival. J Vasc Interv Radiol. 2001;12(11):1263–1271. doi: 10.1016/s1051-0443(07)61549-8. [DOI] [PubMed] [Google Scholar]
- 22.Nanavati S M. What if endoscopic hemostasis fails? Alternative treatment strategies: interventional radiology. Gastroenterol Clin North Am. 2014;43(04):739–752. doi: 10.1016/j.gtc.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Aal A K, Bag A K, Saddekni S, Hamed M F, Ahmed F Y. Endovascular management of nonvariceal upper gastrointestinal hemorrhage. Eur J Gastroenterol Hepatol. 2013;25(07):755–763. doi: 10.1097/MEG.0b013e32835fb9a9. [DOI] [PubMed] [Google Scholar]
- 24.Song J S, Kwak H S, Chung G H. Nonvariceal upper gastrointestinal bleeding: the usefulness of rotational angiography after endoscopic marking with a metallic clip. Korean J Radiol. 2011;12(04):473–480. doi: 10.3348/kjr.2011.12.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sos T A, Lee J G, Wixson D, Sniderman K W. Intermittent bleeding from minute to minute in acute massive gastrointestinal hemorrhage: arteriographic demonstration. AJR Am J Roentgenol. 1978;131(06):1015–1017. doi: 10.2214/ajr.131.6.1015. [DOI] [PubMed] [Google Scholar]
- 26.Geffroy Y, Rodallec M H, Boulay-Coletta I, Jullès M C, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31(03):E35–E46. doi: 10.1148/rg.313105206. [DOI] [PubMed] [Google Scholar]
- 27.Dixon S, Chan V, Shrivastava V, Anthony S, Uberoi R, Bratby M. Is there a role for empiric gastroduodenal artery embolization in the management of patients with active upper GI hemorrhage? Cardiovasc Intervent Radiol. 2013;36(04):970–977. doi: 10.1007/s00270-012-0511-0. [DOI] [PubMed] [Google Scholar]
- 28.Ichiro I, Shushi H, Akihiko I, Yasuhiko I, Yasuyuki Y. Empiric transcatheter arterial embolization for massive bleeding from duodenal ulcers: efficacy and complications. J Vasc Interv Radiol. 2011;22(07):911–916. doi: 10.1016/j.jvir.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Padia S A, Geisinger M A, Newman J S, Pierce G, Obuchowski N A, Sands M J. Effectiveness of coil embolization in angiographically detectable versus non-detectable sources of upper gastrointestinal hemorrhage. J Vasc Interv Radiol. 2009;20(04):461–466. doi: 10.1016/j.jvir.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Tandberg D J, Smith T P, Suhocki P V et al. Early outcomes of empiric embolization of tumor-related gastrointestinal hemorrhage in patients with advanced malignancy. J Vasc Interv Radiol. 2012;23(11):1445–1452. doi: 10.1016/j.jvir.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Defreyne L, Vanlangenhove P, Decruyenaere J et al. Outcome of acute nonvariceal gastrointestinal haemorrhage after nontherapeutic arteriography compared with embolization. Eur Radiol. 2003;13(12):2604–2614. doi: 10.1007/s00330-003-1882-z. [DOI] [PubMed] [Google Scholar]
- 32.Loffroy R, Guiu B, Mezzetta L et al. Short- and long-term results of transcatheter embolization for massive arterial hemorrhage from gastroduodenal ulcers not controlled by endoscopic hemostasis. Can J Gastroenterol. 2009;23(02):115–120. doi: 10.1155/2009/795460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loffroy R, Guiu B, Cercueil J P et al. Refractory bleeding from gastroduodenal ulcers: arterial embolization in high-operative-risk patients. J Clin Gastroenterol. 2008;42(04):361–367. doi: 10.1097/MCG.0b013e3180319177. [DOI] [PubMed] [Google Scholar]
- 34.Lang E V, Picus D, Marx M V, Hicks M E. Massive arterial hemorrhage from the stomach and lower esophagus: impact of embolotherapy on survival. Radiology. 1990;177(01):249–252. doi: 10.1148/radiology.177.1.2399325. [DOI] [PubMed] [Google Scholar]
- 35.Encarnacion C E, Kadir S, Beam C A, Payne C S. Gastrointestinal bleeding: treatment with gastrointestinal arterial embolization. Radiology. 1992;183(02):505–508. doi: 10.1148/radiology.183.2.1561358. [DOI] [PubMed] [Google Scholar]
- 36.Toyoda H, Nakano S, Kumada T et al. Estimation of usefulness of N-butyl-2-cyanoacrylate-lipiodol mixture in transcatheter arterial embolization for urgent control of life-threatening massive bleeding from gastric or duodenal ulcer. J Gastroenterol Hepatol. 1996;11(03):252–258. doi: 10.1111/j.1440-1746.1996.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 37.Jae H J, Chung J W, Jung A Y, Lee W, Park J H. Transcatheter arterial embolization of nonvariceal upper gastrointestinal bleeding with N-butyl cyanoacrylate. Korean J Radiol. 2007;8(01):48–56. doi: 10.3348/kjr.2007.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh R M, Anain P, Geisinger Met al. Role of angiography and embolization for massive gastroduodenal hemorrhage J Gastrointest Surg 199930161–65., discussion 66 [DOI] [PubMed] [Google Scholar]
- 39.Lang E K. Transcatheter embolization in management of hemorrhage from duodenal ulcer: long-term results and complications. Radiology. 1992;182(03):703–707. doi: 10.1148/radiology.182.3.1535883. [DOI] [PubMed] [Google Scholar]
- 40.Irani J L, Cutler C S, Whang E Eet al. Severe acute gastrointestinal graft-vs-host disease: an emerging surgical dilemma in contemporary cancer care Arch Surg 2008143111041–1045., discussion 1046 [DOI] [PubMed] [Google Scholar]
- 41.Shabahang M, Pasquale M D, Bitterman P, Cirenza E, Spitzer T, Evans S R. Massive hematochezia secondary to graft-versus-host disease and cytomegalovirus. Am J Gastroenterol. 1994;89(04):632–633. [PubMed] [Google Scholar]
- 42.Jones A D, Maziarz R, Gilster J, Domreis J, Deveney C W, Sheppard B C. Surgical complications of bone marrow transplantation. Am J Surg. 2003;185(05):481–484. doi: 10.1016/s0002-9610(03)00055-2. [DOI] [PubMed] [Google Scholar]
- 43.Chen R Z, Zhao G, Jin N, Chen B A, Ding J H. Superselective arterial embolization of the superior mesenteric artery for the treatment of gastrointestinal hemorrhage following allogeneic hematopoietic stem cell transplantation. Patient Prefer Adherence. 2014;8:1581–1585. doi: 10.2147/PPA.S72875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuroiwa Y, Suzuki N, Mizue N et al. Gastric antral vascular ectasia in 2-yr-old girl undergoing unrelated cord blood stem cell transplantation. Pediatr Transplant. 2005;9(06):788–791. doi: 10.1111/j.1399-3046.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 45.Caronna R, Cardi M, Arcese W et al. Gastrointestinal surgical emergencies in patients treated for hematological malignancies. Suppl Tumori. 2005;4(03):S141–S145. [PubMed] [Google Scholar]


