Abstract
Thyroidectomy or hemithyroidectomy may be performed as treatment for papillary thyroid microcarcinoma (PTMC). However, in cases of bilateral PTMCs, only thyroidectomies should be recommended. Sometimes bilateral PTMC may be undetected in presurgical evaluations, so reoperation might be necessary after a partial thyroid resection. The aim of this study was to assess the occurrence of and predictive factors for the multifocality and bilaterality of PTMCs.
We performed a retrospective review of 4716 consecutive patients with thyroid tumors. Of these patients, 434 (9.2%) had thyroid malignancies. All patients underwent thyroidectomies with central and/or lateral lymph node dissection between January 2008 and December 2017. PTMC was identified in 177 (3.75%) individuals.
Solitary PTMC was observed in 114 (64.4%) patients, multifocal PTMC was seen in 48 (27.1%) patients, and bilateral PTMC was detected in 15 (8.5%) patients. The occurrence of solitary PTMC increased from 11.1% in 2008 to 61.9% in 2017. The occurrence of multifocal tumors significantly decreased from 77.8% in 2008 to 6.3% to 18.4% in 2013 to 2016 (P < .05). The occurrence of bilateral tumors, with respect to all PTMC cases, did not change during the 10-year period. We observed significantly higher rates of hypoechogenicity, more microcalcifications, more irregular margins, larger tumor sizes, and higher vascularity in the patients with multifocal and bilateral tumors than in the patients with solitary tumors (P < .0001 for all).
The occurrence of bilateral PTMC is not very common. In patients with PTMC, thyroidectomy should be considered when microcalcifications, an irregular tumor shape, unclear margins, hypoechogenicity, high vascularity, and a large tumor size are observed. These clinicopathological features are prognostic factors for multifocal and bilateral PTMC.
Keywords: bilaterality, multifocality, papillary thyroid microcarcinoma
1. Introduction
The incidence of papillary thyroid cancer (PTC) is increasing, and PTC is the most common type of thyroid malignancy.[1] However, lesions being 1.0 cm or less in the largest dimension known as papillary thyroid microcarcinomas (PTMCs), are responsible for a progressively higher rate of thyroid cancer during the last few decades.[1,2] According to our own experience,[3] the prognosis of PTMC is excellent; however, in cases of locoregional recurrences, worse oncological outcomes might be observed.
Multifocality and bilaterality are common features in PTMC and are considered risk factors for disease recurrence.[4,5] According to the observations from Lu et al,[6] multifocality may arise from intrathyroidal metastases from a single cancer cell clone or from multiple independent origins. This latter hypothesis was investigated by some modern molecular techniques, which confirmed that PTMCs are multiple synchronous primary tumors arising from autonomic clones.[7,8] This finding makes diagnosing the multifocality and bilaterality of PTMC extremely difficult with early presurgical diagnostics.
The prevalence of multifocal PTC, in which multifocality is one of its most characteristic features, depends on epidemiological and methodological factors and ranges from 18% to 87%.[9] However, regardless of its prevalence, the multifocality and bilaterality of PTC might easily go undetected by presurgical evaluations, such as ultrasonography and subsequently ultrasound guided-fine needle aspiration biopsy (UG-FNAB); thus, partial thyroid resections for cases of bilateral PTC may lead to the necessity of reoperation.[10] So et al[10] highlighted that additional PTMC foci are very often too small to be diagnosed before surgery, especially under the current guidelines on ultrasound screening evaluations for PTC. He also noticed that multifocality might be treated as a risk factor for bilateral PTMC, which requires more extensive surgery and more thorough follow-up. However, multifocality is the histopathological feature that may be ultimately evaluated after surgical resection. Some authors confirm that multifocal PTMC is very often associated with poor prognosis and more aggressiveness.[4] Multifocal PTMC has been associated with increased risks of lymph nodes and distant metastases, persistent local disease after primary surgery and locoregional recurrence.[10] If the PTMC presents multifocality and bilaterality, a thyroidectomy should be performed, but more extensive surgery should also be introduced.[11] Total thyroidectomy in cases of PTMC is an acceptable treatment method. However, the 2017 American Thyroid Association (ATA) guidelines usually recommend thyroidectomy clinically for patients with 2 lobes involved.[12] Therefore, the role of total thyroidectomy in patients with PTMC localized in one lobe remains controversial. These doubts are further emphasized because of the limited survival benefits and potential postsurgical complications after more radical procedures, such as injury of the recurrent laryngeal nerve and hypoparathyroidism. However, in many cases, multifocality and bilaterality are very often found in postsurgical histopathological examinations. When contralateral PTMC foci are initially diagnosed, not after total surgery, the diagnosis may lead to insufficient treatment rather than radical treatment.
Identification of the occurrence and the risk factors for multifocality and bilaterality could help surgeons evaluate the clinical status of individuals with PTMC and determine whether hemithyroidectomy is a sufficient surgical option or if total thyroidectomy is required.
In our study, we performed clinical analyses to identify some predictive factors for multifocality and bilaterality in patients with PTMC who underwent total thyroidectomy and central/lateral lymphadenectomy.
2. Materials and methods
The study protocol was approved by the Bioethics Committee of Wroclaw Medical University (Reference number: KB-783/2017). The data were analyzed anonymously and retrospectively on the basis of medical records. The authors did not have access to any identifying patient information and did not have any direct access to the study participants.
2.1. Patients selection
The case records of 4716 patients with thyroid tumors who were treated consecutively at the Department of General, Gastroenterological and Endocrine Surgery of Wroclaw Medical University (Poland) in 2008 to 2017 were retrospectively analyzed. Of these patients, 434 (9.2%) individuals showed thyroid malignancy. The inclusion criteria were as follows: solitary, multifocal or bilateral PTMC confirmed in histopathology, clearly defined ultrasound features of thyroid nodule/nodules performed minimum 2 times by 2 independent radiologists before surgery, UG-FNAB performed before surgery with cytology result confirmed by 2 independent experienced cytologists. All patients had ultrasound examination and UG-FNAB performed by 1 endocrine clinicians team experienced in thyroid diseases. All analyzed FNAB results were reported using the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) classification.[13] Only the specimens obtained from UG-FNAB of the thyroid nodules from patients operated in 2008 (study period: 2008–2017) were retrospectively reanalyzed and assigned to adequate categories according to TBSRTC because this classification was formed and finally recommended in 2009.[13] All individuals were operated on by one endocrine surgical team trained in thyroid surgery. The histopathological specimens were analyzed by 2 pathologists experienced in thyroid cancers. The exclusion criteria included nodules with diameter larger than 1.0 cm, not clearly defined preoperative ultrasound features of thyroid nodule/nodules, thyroid autoimmunity, previous neck and head radiotherapy or surgery, family history of thyroid cancer and patients with locally advanced PTC with no radical treatment (n = 9). One hundred seventy seven (3.75%) patients were diagnosed with PTMC and met inclusion criteria. There were 144 (81.4%) women and 33 (18.6%) men. The mean age of the patients with PTMC was 50.2 ± 10.9 years. All patients with PTMC diagnosed in UG-FNAB before surgery underwent total thyroidectomy and elective central lymph node dissection. Only the individuals with ultrasound pathologically changed and cytologically confirmed metastatic lateral lymph nodes underwent selective lateral lymph node dissection. The steps for patient selection are presented in Fig. 1.
Figure 1.
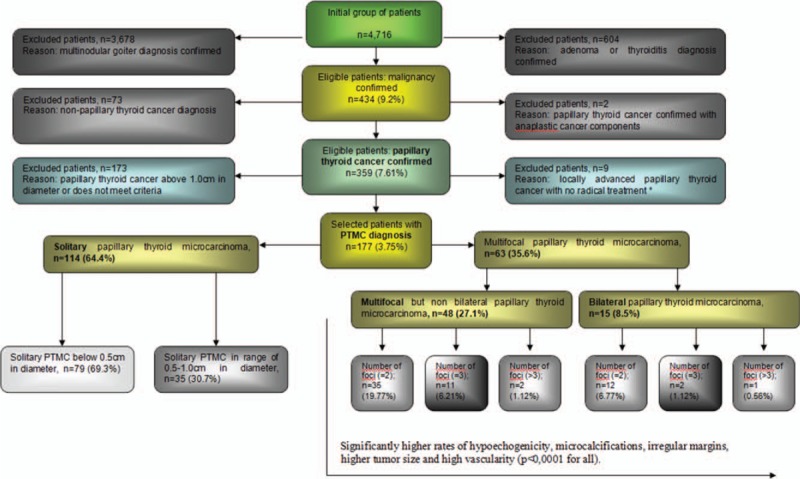
Selection of the study group from 4716 individuals referred for surgery in one center from 2008 to 2017. All participants underwent UG-FNAB. Histopathological verification was conducted for all participants. ∗: In 8 cases, we performed only surgical biopsy due to advanced malignant processes, and 1 individual did not undergo surgery due to an extremely advanced malignant process and extremely poor general condition (ASA 4)—that patient was excluded.
2.2. Statistical analysis
Statistical analysis was performed using Statistica 13.1 software (StatSoft, Tulsa, OK). Categorical variables were presented as the number of observations and percentages, and continuous variables were expressed as means ± standard deviation. Categorical variables were analyzed by chi-square tests or Fisher exact tests. The continuous variable of “age” had a normal distribution (Shapiro-Wilk normality test) and equality of variance (Levene test) and was tested by analysis of variance.
Univariate and multivariate logistic regression analyses were used to confirm the results obtained for independent predictive factors that were associated with the risk of multifocal and bilateral tumors.
The diagnostic potential of independent variables was determined by receiver operating characteristic (ROC) analyses and was expressed in terms of the area under the ROC curve (AUC). The accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratio of positive results (LR(+)), and likelihood ratio of negative results (LR(–)), and Youden index were also calculated.
All values of α ≤ 0.05 were considered statistically significant.
3. Results
3.1. Demographic characteristics of PTMC patients
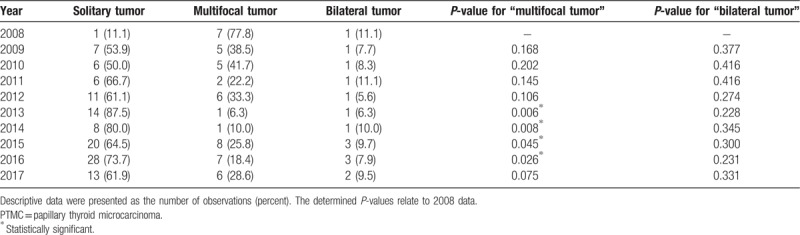
The results of the prevalence of solitary, multifocal, and bilateral tumors in PTMC patients from 2008 to 2017 are presented in Table 1. The prevalence of solitary PTMCs increased from 11.1% in 2008 to 61.9% in 2017. The prevalence of multifocal tumors significantly decreased from 77.8% in 2008 to 6.3% to 18.4% in 2013 to 2016 (P < .05). The prevalence of bilateral tumors, with respect to all PTMC cases, did not change during the 10-year period.
Table 1.
The prevalence of solitary, multifocal, and bilateral tumors in PTMC patients in 2008 to 2017.
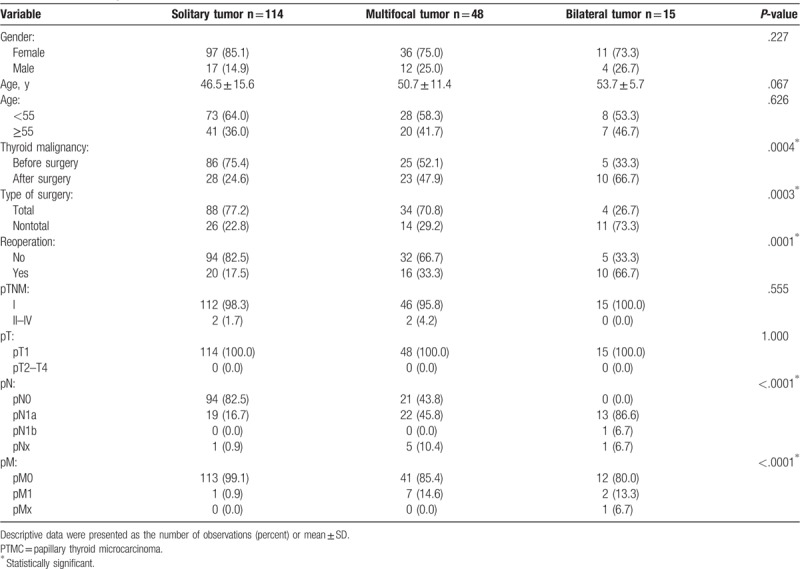
Among the 177 PTMC patients, solitary thyroid tumors were observed in 114 (64.4%) patients, multifocal tumors were observed in 48 (27.1%) patients, and bilateral tumors were detected in 15 (8.5%) patients. As demonstrated in Table 2, there were no significant differences in age and sex between the 3 subgroups of PTMC patients.
Table 2.
The baseline demographics and tumor characteristics of patients with solitary, multifocal, and bilateral PTMC tumors.
3.2. Relationship between multifocal and bilateral tumors and other clinicopathological characteristics
The rate of predicting thyroid malignancy before surgery was significantly higher in the patients with solitary tumors than in patients with multifocal or bilateral tumors (P = .0004) (Table 2). The rate of total thyroid resection and lack of necessity for reoperation were also significantly higher in PTMC patients with solitary tumors than in patients with multifocal or bilateral tumors (P = .0003 and P = .0001, respectively).
The histopathological results presented in Table 2 show that there were no significant differences in pTNM and pT stages between study groups (P > .05 for both). However, significantly more patients with lymph node metastasis (pN1) or distant metastasis (pM1) had PTMC with multifocal or bilateral tumors (P < .0001).
3.3. Selected ultrasound features as independent predictors of multifocal and bilateral PTMC
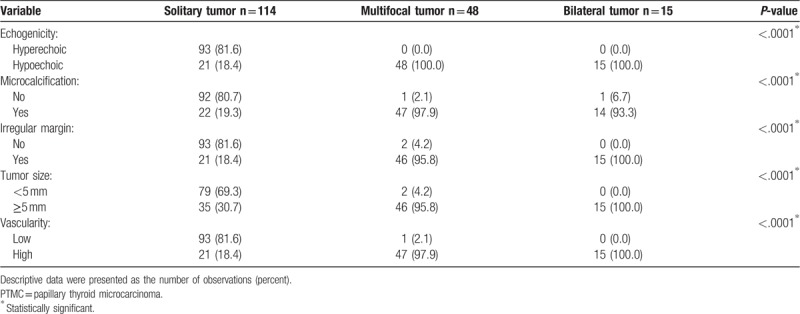
The preoperative ultrasound features of the 3 subgroups of patients with PTMC are shown in Table 3. We observed a significantly higher rate of hypoechogenicity, significantly more microcalcifications, significantly more irregular margins of the dominant tumor, significantly larger tumor size (≥0.5 cm), and significantly higher vascularity in the patients with multifocal or bilateral tumors than in the patients with solitary PTMCs (P < 0.0001 for all). Additionally, univariate and multivariate logistic regression analyses confirmed a significantly high to total correlation between the abovementioned ultrasound factors and the risk of multifocal and bilateral PTMC (data not shown).
Table 3.
Selected ultrasound features of patients diagnosed with PTMC.
The diagnostic potential of preoperative ultrasound features to discriminate multifocal or bilateral tumors from solitary tumors was evaluated by ROC analysis. The results presented in Tables 4 and 5 show that all selected ultrasound features were significant predictors of the presence of multifocal and bilateral tumors (P < .0001 for all).
Table 4.
Diagnostic potential of the ultrasound features as indicators of the presence of multifocal tumors in PTMC.
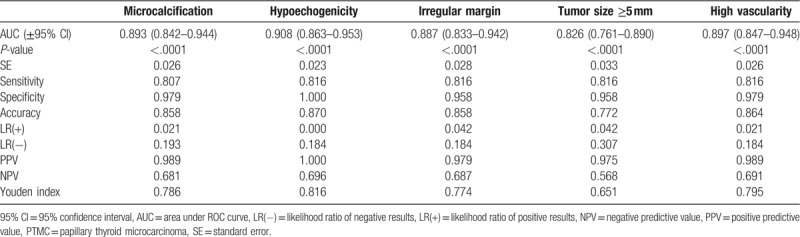
Table 5.
Diagnostic potential of ultrasound features as indicators of the presence of bilateral tumors in PTMC.
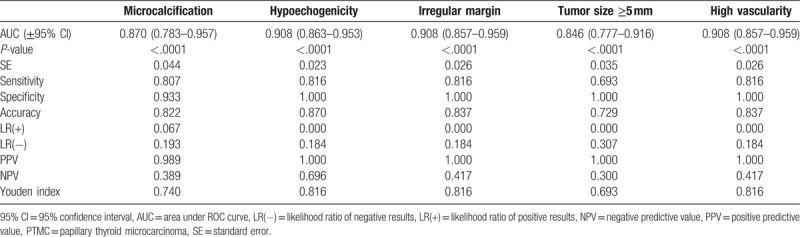
4. Discussion
Multifocality and bilaterality are common pathological features of PTMC. Although PTMC is generally thought to be a tumor with excellent prognosis, some studies have described patients with very aggressive or fatal cases of multifocal PTC.[14] Genpeng et al[4] confirmed the multifocality of PTC as a risk factor for disease recurrence and poorer prognosis. They also suggest a higher incidence rate of lymph node metastasis than does unifocal PTC. In their study, 70.5% of multifocal cases were diagnosed. The sensitivity of detecting multifocal and bilateral PTMCs by UG-FNAB is relatively unsatisfactory. Predictive factors for multifocality and bilaterality might be useful for clinicians to evaluate the clinicopathological status of both thyroid lobes in individuals with PTMC and, subsequently, to help surgeons determine whether total thyroidectomy or hemithyroidectomy is required.
Although PTMC may be treated by hemithyroidectomy, if multifocality or bilaterality are found, more aggressive treatment is recommended. Several studies have reported clinicopathological factors associated with multifocality and bilaterality, but few of studies evaluated these characteristics with respect to the recommended surgical procedure. This is a common clinical situation where an additional small malignant nodule in multifocal PTMC is not diagnosed preoperatively but is recognized in histopathological examination.
In our study, we tried to distinguish the clinical risk factors of pathologically positive multifocal and bilateral PTMCs and the characteristics of unilateral tumors. The present study showed that 27.1% and 8.5% of the 177 patients with PTMC had multifocality and bilaterality, respectively. Hypoechogenicity, microcalcifications, irregular margins of the dominant tumor, tumor sizes of 0.5 to 1.0 cm, unclear margins, irregular tumor shape, and high vascularity were classified as risk factors for multifocality and bilaterality; however, small tumor size <0.5, not hypoechoic structure and round tumor shapes were protective factors against the presence of multifocality and bilaterality. Younger age (<55) was not classified as a prognostic factor for solitary tumors because, in our study, almost all patients (97.7%) under 55 years old were in stage I of the disease. The positive correlation between microcalcifications with high vascularity and multifocality with bilaterality was statistically significant. The ultrasound features, such as irregular tumor shape, unclear margins, and hypoechogenicity, were known as characteristic factors of increased risk of multifocality and bilaterality.
We decided to undertake this study because in our clinic, the value of total thyroidectomy in patients with unilateral and non-multifocal PTMC still remains controversial. Until now, there has been limited evidence showing that total thyroidectomy in such cases could improve disease-free survival and reduce locoregional recurrence. Additionally, some studies have shown that total thyroidectomy exhibits no improvement in the long-term outcomes of patients, while increasing the possibility of morbidity through adverse events such as recurrent laryngeal nerve palsy and iatrogenic parathyroid gland injury. On the other hand, the authors of this study suggest that total thyroidectomy without an increase in morbidity may be achieved by experienced surgeons.[3] Some authors suggest more aggressive treatment for multifocal PTC. Those authors confirm that multifocality is significantly correlated with extrathyroid extension and lymph node metastasis compared with unilaterality of PTMC.[10]
In our study, the diagnostic potential of ultrasound features as indicators of the multifocality and bilaterality of PTMC were estimated on the basis of the evaluation of the dominant tumor. We state that this approach is pragmatic and clinically valuable. Secondary tumors have a significantly smaller size than primary tumors and are very difficult to diagnose preoperatively by ultrasonography and UG-FNAB. Additionally, according to ATA guidelines, such small tumors are not recommended for further evaluation. Fortunately, preoperatively undiagnosed secondary foci of multifocal PTMCs found after surgery do not significantly change the management strategy if these foci were localized unilaterally, and minimum lobectomy with or without isthmectomy should be performed. However, if secondary tumors are found contralaterally in the postsurgical histopathology of patients who underwent nonradical surgery, then remnant malignant tissue may appear after the procedure[10]; this may lead to the necessity of a secondary surgical treatment method or, if reoperation is not performed, to locoregional and distant metastases. Some authors have highlighted that multifocality is a risk factor for bilaterality, which increases the incidence of bilaterality to 30%.[15] This increased risk is why other researchers recommend total thyroidectomy in cases of preoperatively diagnosed PTMC with smaller multifocal tumors and complete thyroidectomy after lobectomy when multifocal PTMC is diagnosed incidentally in postsurgical histopathology. The probability of contralateral foci and poor prognosis in such cases is rather high. Some authors allow performing only lobectomy in some cases of multifocal PTMC; however, thorough follow-up is recommended.
Because of the low sensitivity and specificity of UG-FNAB in detecting multifocal and bilateral PTMC, which have unknown prognostic significance, we believe that the evaluation of presurgical predictors for multifocal and bilateral PTMC should be analyzed.[16] Some authors suggest that multifocality is a strong significant factor for bilateral PTMC. Unfortunately, as we previously stated, this clinicopathological feature is often evaluated after surgery, which means this characteristic cannot contribute to planning extension surgeries. We did not observe any significant correlation between the number of PTMC foci in the dominant lobe and bilaterality. However, some authors have confirmed that the number of tumors is the most significant clinicopathological predictor for bilateral PTMCs.[10] These authors added that well-established predictive factors for multifocal PTMC can decrease the risk of reoperation. The percentage of preoperatively undiagnosed malignant tumors was the highest in the bilateral PTMC subgroup. However, some of these diagnostic procedures for patients with multifocal and bilateral PTMCs who were surgically treated in our clinic were performed outside of our department. However, when the dominant tumor was ≥0.5 cm in diameter, the chance for multifocality and bilaterality was higher. We confirmed a significant correlation between these clinicopathological features and multifocality and bilaterality.
There are several limitations in this study. We did not use some other modern diagnostic techniques such elastography to describe ultrasound thyroid nodules characteristics more accurately. However we wanted to analyze only this method, which is used routinely in clinical practice. We did not performed elective lateral lymph node dissection in all patients with PTMC. Only patients with cytologically confirmed lateral lymph node metastasis had selective lateral lymphadenectomy performed. Because of potential complications and ethical issues associated with prophylactic lateral lymph node dissection, we avoided this procedure if was not necessary. Regarding excellent prognosis of PTMC, this procedure cannot be recommended for every case of PTMC. Our study is also limited by its retrospective design and limited number of multifocal and bilateral PTMC. However, besides of the fact, that the one of the most common pathological features of PTC is multifocality, the bilateral tumors are not very often observed. The next limitation of our study is its single center analysis. Thus, multicenter and large cohorts study should be performed to identify the risk factors for multifocal and bilateral PTMC. It may reduce the number of thyroid cancer reoperations.
5. Conclusion
In our study, we identified the risk factors of multifocality and bilaterality in PTMC patients. The presence of a larger size (0.5–1.0 cm), hypoechogenicity, unclear margins, irregular tumor shape, high vascularity, and microcalcifications could help surgeons evaluate the thyroid lobe status of patients with PTMC and further analyze the necessity of total thyroidectomy individually for each patient. Total thyroidectomy might be avoided when the risk factors of multifocality and bilaterality are excluded.
Author contributions
Conceptualization: Krzysztof Kaliszewski.
Data curation: Krzysztof Kaliszewski, Beata Wojtczak, Jakub Migoń, Agata Kasprzyk.
Formal analysis: Krzysztof Kaliszewski, Dorota Diakowska.
Funding acquisition: Krzysztof Kaliszewski.
Investigation: Krzysztof Kaliszewski.
Methodology: Krzysztof Kaliszewski, Dorota Diakowska.
Project administration: Krzysztof Kaliszewski.
Resources: Krzysztof Kaliszewski, Beata Wojtczak, Jakub Migoń, Agata Kasprzyk.
Software: Krzysztof Kaliszewski.
Supervision: Krzysztof Kaliszewski, Dorota Diakowska, Jerzy Rudnicki.
Validation: Krzysztof Kaliszewski, Dorota Diakowska.
Visualization: Krzysztof Kaliszewski, Jerzy Rudnicki.
Writing – original draft: Krzysztof Kaliszewski, Dorota Diakowska.
Writing – review & editing: Krzysztof Kaliszewski.
Krzysztof Kaliszewski orcid: 0000-0002-3291-5294.
Footnotes
Abbreviations: PTC = papillary thyroid cancer, PTMC = papillary thyroid microcarcinoma, TBSRTC = the Bethesda System for Reporting Thyroid Cytopathology, UG-FNAB = ultrasound guided-fine needle aspiration biopsy.
This work was not funded.
The authors declare that they have no conflict of interest.
References
- [1].Shen Y, Liu M, He J, et al. Differences in treatment outcomes between ultrasound-guided percutaneous microwave ablation and endoscopic thyroidectomy for patients with papillary thyroid microcarcinoma: protocol for a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ma B, Wang Y, Yang S, et al. Predictive factors for central lymph node metastasis in patients with cN0 papillary thyroid carcinoma: a systematic review and meta-analysis. Int J Surg 2016;28:153–61. [DOI] [PubMed] [Google Scholar]
- [3].Kaliszewski K, Wojtczak B, Grzegrzółka J, et al. Nontoxic multinodular goitre and incidental thyroid cancer: what is the best surgical strategy? A retrospective study of 2032 patients. Int J Endocrinol 2018;2018:4735436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Genpeng L, Jianyong L, Jiaying Y, et al. Independent predictors and lymph node metastasis characteristics of multifocal papillary thyroid cancer. Medicine (Baltimore) 2018;97:e9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ng SC, Kuo SF, Chen ST, et al. Therapeutic outcomes of patients with multifocal papillary thyroid microcarcinomas and larger tumors. Int J Endocrinol 2017;2017:4208178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lu Z, Sheng J, Zhang Y, et al. Clonality analysis of multifocal papillary thyroid carcinoma by using genetic profiles. J Pathol 2016;239:72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Park SY, Park YJ, Lee YJ, et al. Analysis of differential BRAF(V600E) mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer 2006;107:1831–8. [DOI] [PubMed] [Google Scholar]
- [8].Giannini R, Ugolini C, Lupi C, et al. The heterogeneous distribution of BRAF mutation supports the independent clonal origin of distinct tumor foci in multifocal papillary thyroid carcinoma. J Clin Endocrinol Metab 2007;92:3511–6. [DOI] [PubMed] [Google Scholar]
- [9].Tam AA, Özdemir D, Çuhaci N, et al. Association of multifocality, tumor number, and total tumor diameter with clinicopathological features in papillary thyroid cancer. Endocrine 2016;53:774–83. [DOI] [PubMed] [Google Scholar]
- [10].So YK, Kim MW, Son YI. Multifocality and bilaterality of papillary thyroid microcarcinoma. Clin Exp Otorhinolaryngol 2015;8:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Iacobone M, Jansson S, Barczynski M, et al. Multifocal papillary thyroid carcinoma: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg 2014;399:141–54. [DOI] [PubMed] [Google Scholar]
- [12].Sawka AM, Carty SE, Haugen BR, et al. American Thyroid Association Guidelines and statements: past, present, and future. Thyroid 2018;28:692–706. [DOI] [PubMed] [Google Scholar]
- [13].Cibas ES, Ali SZ. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol 2009;132:658–65. [DOI] [PubMed] [Google Scholar]
- [14].Marcy PY, Thariat J, Peyrottes I, et al. Fulminant lethal spread of occult papillary microcarcinoma of the thyroid. Thyroid 2010;20:445–8. [DOI] [PubMed] [Google Scholar]
- [15].Connor MP, Wells D, Schmalbach CE. Variables predictive of bilateral occult papillary microcarcinoma following total thyroidectomy. Otolaryngol Head Neck Surg 2011;144:210–5. [DOI] [PubMed] [Google Scholar]
- [16].Kaliszewski K, Diakowska D, Wojtczak B, et al. Fine-needle aspiration biopsy as a preoperative procedure in patients with malignancy in solitary and multiple thyroid nodules. PLoS One 2016;11:e0146883. [DOI] [PMC free article] [PubMed] [Google Scholar]


