Abstract
Low testosterone has been inversely associated with hypertension. Our objective was to determine the associations between total testosterone (TT), free testosterone (FT), bioavailable testosterone (BioT), sex hormone–binding globulin (SHBG), and hypertension. Two hundred fifty-three men were enrolled in this study. TT and SHBG were measured by chemiluminescent immunoassay, and FT and BioT were calculated. Hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg and/or diastolic blood pressure (DBP) ≥90 mm Hg. Our results showed that hypertensive men had higher SHBG levels, and lower FT and BioT, compared to normotensive men. FT and BioT were inversely associated with SBP and DBP after adjusting for covariates (age, smoking, alcohol consumption, and physical activity). Furthermore, there was a significant decrease in the odds ratios for hypertension in the third and fourth quartiles of BioT and FT, compared to the lowest quartile before and after adjusting for covariates. In contrast, the OR for hypertension in the third quartile of SHBG was lower than the highest quartile. Our data show that FT and BioT are inversely correlated with SBP, DBP, and hypertension in men.
Keywords: bioavailable testosterone, free testosterone, hypertension, sex hormone–binding globulin
1. Introduction
Hypertension is a leading risk factor for cardiovascular diseases, such as stroke, coronary heart disease, and arteriosclerosis, and contributes to about half of all cardiovascular deaths.[1,2] Androgens, synthesized and produced by Leydig cells of the testes, play a crucial role in the development and maintenance of male reproductive and sexual functions. In addition, several reports have documented that testosterone, which has a vasodilating effect, has beneficial effects on hypertension,[3,4] and testosterone replacement therapy may decrease blood pressure in rats with hypertension.[5]
The relationships between total testosterone (TT), sex hormone–binding globulin (SHBG), and hypertension are mostly based on studies involving metabolic syndrome (MetS), and those relationships have been inconsistent. MetS clinical components include abdominal obesity, dyslipidemia, hyperglycemia, and hypertension. It is reported that lower TT and free testosterone (FT) levels are associated with hypertension.[6] However, some investigators have reported that TT and FT are not correlated with hypertension.[7,8] It has also been reported that lower SHBG is associated with hypertension.[8] In contrast, 1 article reports that SHBG shows no association with hypertension.[6] According to the American Heart Association, hypertension among MetS is defined as systolic blood pressure (SBP) ≥135 mm Hg and/or diastolic blood pressure (DBP) ≥85 mm Hg, which is also used in the above articles.[9] This diagnostic criteria is different from the definition of hypertension according to the Seventh Report of the Joint National Committee (JNC7) guideline (SBP ≥140 mm Hg and/or DBP ≥90 mm Hg), which is widely used around the world.[10] In recent decades, only 2 articles have addressed the association of TT and hypertension using the JNC7 guidelines. One study shows that TT concentrations are inversely correlated with incident hypertension.[11] Another study suggests that men with hypertension have lower levels of TT, FT, and SHBG.[12] Therefore, in this study, we analyze the associations between TT, FT, bioavailable testosterone (BioT), SHBG and blood pressure (SBP and DPB), and hypertension using the JNC 7 guidelines.
2. Materials and methods
2.1. Subjects
We conducted a study in the Second Affiliated Hospital of Shantou University Medical College. The detailed description of this study has been reported previously.[13,14] In total, 253 male participants between the ages of 40 and 79 years who underwent a health checkup between August 2015 and May 2016 were recruited. This study was approved by the Ethical Committee of Shantou University Medical College and by the Ethics Committee of the Second Affiliated Hospital of Shantou University Medical College. All participants provided written informed consent. Trial registration number is SUMC-2015-26.
Information concerning blood pressure and albumin were recorded at the Second Affiliated Hospital of Shantou University Medical College. Blood pressure was measured after 10 minutes of rest in a sitting position using an automatic sphygmomanometer (OMRON, BP-203RVIIIC, Kentaro, Japan). Information on lifestyle factors (smoking, alcohol consumption, and physical activity) was assessed with questionnaires. The participants were asked about their average frequency of physical activity: rarely/never, 1 to 3 times per month, 1 to 2 times per week, and more than 3 times per week.[15] Smoking status was recorded as never, former, or current.[15] Alcohol drinking status was classified as never and current drinker.[16] Hypertension was defined as an SBP ≥140 mm Hg and/or DBP ≥90 mm Hg, according to the JNC7 guidelines.[17]
2.2. Serum measurements of sex hormones
Testosterone mainly exists in 3 forms in the blood circulation. About half of circulating testosterone is bound to SHBG, and another half to albumin, and only 0.5% to 3% of the testosterone remains in the free, non-protein-bound form, and is referred to as FT.[18] FT and albumin-binding testosterone can be readily used by the tissues and are denoted as BioT.
Fasting blood samples were collected in the morning (before 10 am). Blood samples were centrifuged at 3000 g for 10 minutes. All aliquots of serum were stored at −80°C until the time of assay. Serum TT and SHBG levels were measured using a Beckman DXI 800 Analysis System (Fullerton, CA). The levels of FT and BioT were calculated from known values of the TT, SHBG, and albumin by using a simple formula and a calculator widely available on the web (http://www.issam.ch/freetesto.htm).[19]
2.3. Statistical analysis
Differences in means between normotensive and hypertensive subjects were tested by the independent samples t test or χ2 test. The t test was used for continuous variables, and χ2 test for categorical variables. Contributions of TT, FT, BioT, and SHBG to SBP and DBP were analyzed by multivariable linear analysis. SBP or DBP was used as dependent variables, and TT, FT, BioT, SHBG, age, and lifestyle factors (smoking, alcohol consumption, and physical activity) were used as independent variables. The associations of TT, FT, BioT, SHBG or their quartiles and hypertension were also analyzed using univariate logistic regression. TT, FT, BioT, and SHBG were categorized into quartiles. The lowest quartiles of TT, FT, and BioT were used as the reference category, while the highest quartile was used as the reference category for SHBG. For this regression analyses, 3 models were evaluated: model 1 was crude odd; model 2 was adjusted for age and lifestyle factors.
A confidence interval (CI) of 95% was used, and P < .05 was regarded as statistically significant. Values were presented as mean ± standard deviation. SPSS 20.0 statistical package software (SPSS Inc, Chicago, IL) was used for statistical analysis.
3. Results
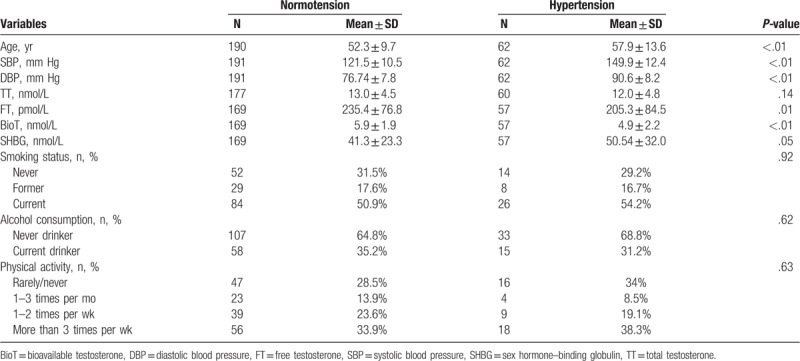
Table 1 presents the variable characteristics of the study population between hypertension and normotension. The numbers of hypertensive and normotensive men were 62 and 191, respectively. Compared to normotensive men, the hypertensive men had significantly higher mean age, SBP, DBP, and SHBG level, and lower FT and BioT levels. However, TT levels between the 2 groups were not significantly different.
Table 1.
Comparison of characteristics between normotensive and hypertensive men.
3.1. Association between TT, FT, BioT, SHBG, and SBP and DBP
Table 2 shows results of multiple regression analyses without or with adjustment for age and lifestyle factors (smoking, alcohol consumption, and physical activity). TT was inversely correlated with SBP before and after adjustment for age and lifestyle factors, but was not correlated with DBP. FT was inversely correlated with SBP with and without adjustment, and was inversely correlated with DBP when adjusting for covariates (models 2). BioT was inversely correlated with SBP and DBP with and without adjustments. SHBG was positively correlated with SBP without adjustment (models 1), but was not correlated with DBP. Additionally, Figure 1 showed that SBP was inversely associated with TT, BioT, and FT after adjusting for age and lifestyle factors. Therefore, TT, FT, BioT, and SHBG showed significant associations with SBP, and FT and BioT showed the significant associations with both SBP and DBP.
Table 2.
Multiple regression analyses with SBP and DBP as dependent variables.
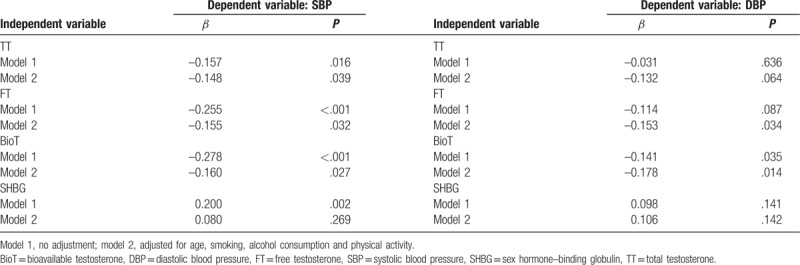
Figure 1.
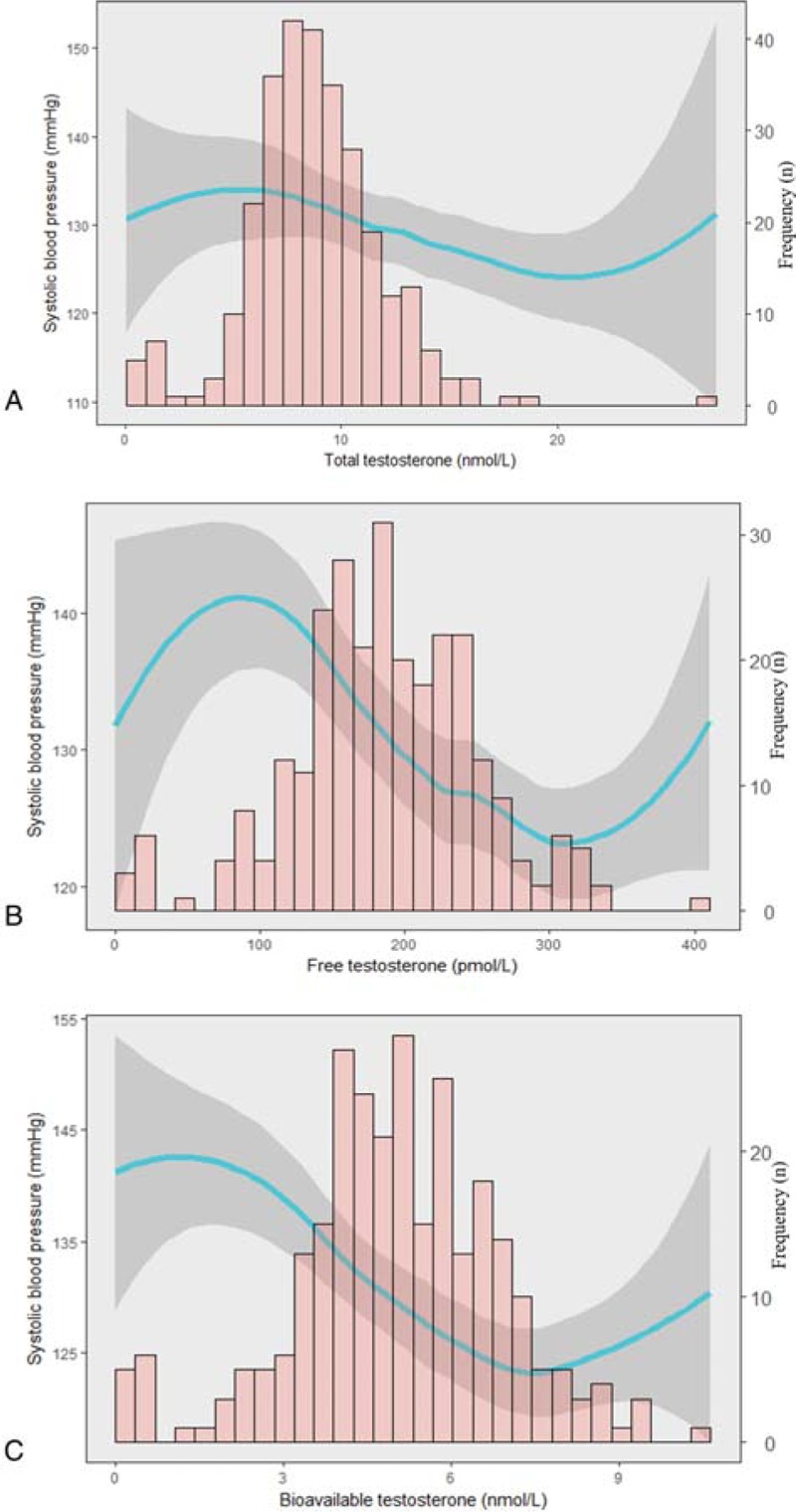
Shown is the cubic spline regression plot of the associations between TT (A), FT (B), BioT (C), and SBP adjusted for age, smoking, alcohol consumption, and physical activity. Predicted values are shown as a solid line, and 95% CI is shown as a black area. BioT = bioavailable, testosterone, CI = confidence interval, FT = free testosterone, SBP = systolic blood pressure, TT = total testosterone.
3.2. Associations between TT, FT, BioT, SHBG, and hypertension
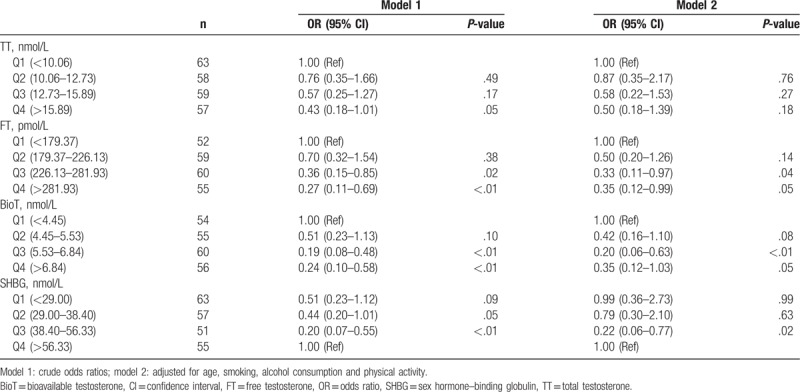
The associations between quartiles of TT, FT, BioT, SHBG, and hypertension as determined by univariate logistic regression analysis are shown in Table 3. The odds ratio (OR) for hypertension in the fourth quartile of TT (0.43 [95% CI: 0.18–1.01]) was lower than in the lowest quartile, but no significant association was observed after adjusting for covariates (models 2). Compared with the lowest quartile, the ORs for hypertension in the third and fourth quartiles (0.36 [95% CI: 0.15–0.85] and 0.27 [95% CI: 0.11–0.69], respectively) of FT were significantly lower. When adjusting for covariates (models 2), inverse associations were still significant. There was a significant decrease in the ORs for hypertension in the third and fourth quartiles of BioT with and without adjustment, compared to the lowest quartile. In contrast, SHBG showed a positive association with hypertension, and the OR for hypertension in the third quartile was lower than the highest quartile with and without adjustment. These results revealed that FT and BioT were inversely associated with hypertension, and SHBG was positively associated with hypertension.
Table 3.
Odds ratios of hypertension according to quartiles for TT, FT, BioT, and SHBG.
4. Discussion
As mentioned above, the majority of plasma testosterone is bound to either SHBG or albumin and only 0.5% to 3% of TT is free and non-protein bound, and is referred to as FT. Free and albumin-bound testosterones can be readily available to the tissues and are defined as BioT.[18] Because testosterone bound to SHBG is biologically inactive,[20] the concentration of TT may not always reflect the true androgen status. Therefore, FT and BioT may be better reflections of biological activity than TT alone.[21] Therefore, we study the associations between TT, FT, BioT, SHBG, and hypertension.
Views on the relationship between SBP, DBP, and testosterone are conflicting. In the Tromsø Study, among 1548 men aged 25 to 84 years, TT and SHBG were inversely correlated with SBP and DBP after adjusting for age and body mass index.[12] In the Study of Health in Pomerania, among adults 20 to 79 years of age, significant inverse associations between TT and SBP and DBP were also found.[11] In prior study showed that in 24- to 45-year-old Finnish men, TT, FT, and SHBG were inversely correlated with SBP and DBP when no adjustments were made. After adjusting for covariates, TT and FT were still correlated inversely with SBP, but TT, FT, and SHBG did not show significant association with DBP.[22] In the Rancho Bernardo study, conducted on 1132 men between 30 and 79 years old, showed TT was inversely correlated with SBP and DBP, but SHBG showed no relationship with blood pressure.[23] Our results are in general consistent with the previous reports. Our study shows that, without adjustments, TT, FT, and BioT are inversely associated with SBP, and BioT is also inversely associated with DBP (Table 2). After adjustment for covariates, those associations remain significant. Therefore, our results provide new insight that BioT shows an inverse association with SBP and DBP before and after adjustment, and FT shows an inverse association with SBP and DBP after adjustment.
As for the relationship between testosterone and hypertension, most studies investigate the relationships between testosterone and MetS, as mentioned above. The relationships between TT, SHBG, and hypertension are conflicting among those studies. In non-diabetic men older than 40, TT levels have been shown not to be associated with hypertension.[7] In a cross-sectional study of 2502 community-dwelling men 70 years old or older, lower SHBG was found to be associated with hypertension.[8] In contrast, 1 study of adult Korean men, showed that TT and FT were associated with hypertension, and SHBG was not.[6] It was also reported that TT concentrations were significantly lower in men with hypertension, and that lower TT concentrations carried an increased risk of incident hypertension.[11] In postmenopausal women, TT and BioT have been shown to be positively and SHBG inversely associated with risk of hypertension.[24] Furthermore, men with hypertension have lower levels of TT, FT, and SHBG.[12] These inconsistencies may relate to differences in study design, study sample, characteristics of the study population such as age and ethics, covariates adjusted for, or methodologies used. In our study, the hypertensive men have a higher mean SHBG level, and lower FT and BioT than normotensive men (Table 1). However, TT levels between the 2 groups were not significantly different, (Table 1). Therefore, we demonstrate that men with hypertension have higher FT and BioT and lower SHBG.
To our knowledge, only 1 article showed the relationship between TT quartiles and hypertension, and showed TT concentrations in the lowest quartile increase the risk of incident hypertension compared to the highest quartile, independent of age, waist circumference, physical activity, smoking, and alcohol consumption.[11] In our study, Table 3 showed the associations between the quartiles of TT, FT, BioT, SHBG, and hypertension. The OR for hypertension in the fourth quartile of TT were lower than the lowest quartile, but no significant difference was found after adjusting for covariates. The ORs for hypertension in the third and fourth quartiles of FT were also lower, compared with the lowest quartile before and after adjusting for covariates. The ORs for hypertension in the third, and fourth quartiles of BioT were also lower, compared to the lowest quartile. In contrast, the OR for hypertension in the third quartile of SHBG were lower than the highest quartile. Those results confirm that FT and BioT are inversely and SHBG positively associated with hypertension
There could be several possible explanations for the mechanisms of the association between testosterone and blood pressure. Testosterone receptors have been identified in endothelial cells and vascular smooth muscle cells.[25] Testosterone may exert antihypertensive effects by inducing the relaxation of isolated rabbit and rat aorta.[26,27] Acute intracoronary administration of testosterone can cause coronary artery dilation and increase coronary blood flow.[28] In addition, TT replacement therapy had beneficial effects on blood pressure,[5,29] but androgen suppressive therapy increased the blood pressure.[30] Our results also show that BioT and TT are inversely associated with SBP, DBP, and hypertension in men. Therefore, testosterone replacement therapy may represent potential beneficial vascular effects.
This study has several strengths. Fasting blood samples were collected between 08:00 and 10:00 hours. Samples were preferably drawn in the morning because of a diurnal variation of TT concentrations with a decline in the afternoon.[31] Samples were drawn between 0800 and 1600 hours in some studies.[11,12] In addition, we measured individual albumin to calculate values of FT and BioT, even though it was accurate and reliable to use a fixed mean albumin for this calculation.[32] However, some potential limitations should also be mentioned. Our results were based on a single blood sample, which might have resulted in a measurement error in testosterone. Nevertheless, a single measure was reported to be adequate in middle-aged and elderly men.[33] Furthermore, TT and SHBG were measured with a chemiluminescent immunoassay instead of mass spectrometry. Although it was suggested that the best results were achieved by mass-spectrometry, the chemiluminescent immunoassay was technically simple, rapid, and was used widely in hospitals in China. Additionally, our sample size was small compared to much larger studies.[8,11,12]
5. Conclusion
Our study showed that FT and BioT were inversely associated with SBP, and BioT was also inversely associated with DBP with and without adjusting for covariates (age, smoking, alcohol consumption, and physical activity). In addition, hypertensive men had significantly higher SHBG level, and lower FT and BioT, compared to normotensive men. The ORs for hypertension in the third and fourth quartiles of FT were lower, compared with the lowest quartile before and after adjusting for covariates. The ORs for hypertension in the third and fourth quartiles of BioT were also lower, compared to the lowest quartile. In contrast, the OR for hypertension in the third quartile of SHBG was lower than the highest quartile. Taken together, our data show that FT and BioT are inversely associated with SBP, DBP, and hypertension in men.
Author contributions
Data curation: Liang Lu.
Funding acquisition: Xuxia Sui.
Investigation: Zhenjie Li, Wencai Li, Haoqiang Wu, Yiyii Zhuang.
Resources: Qingtao Yang.
Software: Kusheng Wu.
Writing – original draft: Qingtao Yang, Xuxia Sui.
Writing – review and editing: Kusheng Wu, Xuxia Sui.
Footnotes
Abbreviations: BioT = bioavailable testosterone, DBP = diastolic blood pressure, FT = free testosterone, JNC 7 = the Seventh Report of the Joint National Committee, MetS = metabolic syndrome, ORs = odds ratios, SBP = systolic blood pressure, SHBG = sex hormone–binding globulin, TT = total testosterone.
This work was supported by the Scientific Research Foundation of Shantou University Medical College (50010203) and Innovative Experimental Project of University Students, Guangdong Province, China (201510560025).
The authors report no conflicts of interest.
References
- [1].Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001;345:1291–7. [DOI] [PubMed] [Google Scholar]
- [2].Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- [3].Perusquia M. Androgen-induced vasorelaxation: a potential vascular protective effect. Exp Clin Endocrinol Diabetes 2003;111:55–9. [DOI] [PubMed] [Google Scholar]
- [4].Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol 2013;217:R47–71. [DOI] [PubMed] [Google Scholar]
- [5].Perusquia M, Herrera N, Ferrer M, et al. Antihypertensive effects of androgens in conscious, spontaneously hypertensive rats. J Steroid Biochem Mol Biol 2017;167:106–14. [DOI] [PubMed] [Google Scholar]
- [6].Moon H, Choi I, Kim S, et al. Cross-sectional association between testosterone, sex hormone-binding globulin and metabolic syndrome: the Healthy Twin Study. Clin Endocrinol 2017;87:523–31. [DOI] [PubMed] [Google Scholar]
- [7].Blaya R, Thomaz LD, Guilhermano F, et al. Total testosterone levels are correlated to metabolic syndrome components. Aging Male 2016;19:85–9. [DOI] [PubMed] [Google Scholar]
- [8].Chubb SA, Hyde Z, Almeida OP, et al. Lower sex hormone-binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study. Eur J Endocrinol 2008;158:785–92. [DOI] [PubMed] [Google Scholar]
- [9].Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- [10].Lenfant C, Chobanian AV, Jones DW, et al. Joint National Committee on the Prevention DE, Treatment of High Blood P. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension 2003;41:1178–9. [DOI] [PubMed] [Google Scholar]
- [11].Torkler S, Wallaschofski H, Baumeister SE, et al. Inverse association between total testosterone concentrations, incident hypertension and blood pressure. Aging Male 2011;14:176–82. [DOI] [PubMed] [Google Scholar]
- [12].Svartberg J, von Muhlen D, Schirmer H, et al. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso Study. Eur J Endocrinol 2004;150:65–71. [DOI] [PubMed] [Google Scholar]
- [13].Yang Q, Wu K, Zhuang Y, et al. Association of total testosterone, free testosterone, bioavailable testosterone and sex hormone-binding globulin with hepatic steatosis and the ratio of aspartate aminotransferase to alanine aminotransferase. Endocr J 2018;65:915–21. [DOI] [PubMed] [Google Scholar]
- [14].Yang QT, Wu KS, Li ZJ, et al. Risk factors for late-onset hypogonadism. Andrologia 2018;50:e13016. [DOI] [PubMed] [Google Scholar]
- [15].Goto A, Morita A, Goto M, et al. Associations of sex hormone-binding globulin and testosterone with diabetes among men and women (the Saku Diabetes study): a case control study. Cardiovasc Diabetol 2012;11:130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chin KY, Ima-Nirwana S, Mohamed IN, et al. Total testosterone and sex hormone-binding globulin are significantly associated with metabolic syndrome in middle-aged and elderly men. Exp Clin Endocrinol Diabetes 2013;121:407–12. [DOI] [PubMed] [Google Scholar]
- [17].Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- [18].Belchetz PE, Barth JH, Kaufman JM. Biochemical endocrinology of the hypogonadal male. Ann Clin Biochem 2010;47:503–15. [DOI] [PubMed] [Google Scholar]
- [19].Decaroli MC, Rochira V. Aging and sex hormones in males. Virulence 2017;8:545–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol 1974;3:69–96. [DOI] [PubMed] [Google Scholar]
- [21].Diver MJ. Clinical Scince Reviews Committee of the Association for Clinical B. Analytical and physiological factors affecting the interpretation of serum testosterone concentration in men. Ann Clin Biochem 2006;43:3–12. [DOI] [PubMed] [Google Scholar]
- [22].Firtser S, Juonala M, Magnussen CG, et al. Relation of total and free testosterone and sex hormone-binding globulin with cardiovascular risk factors in men aged 24-45 years. The Cardiovascular Risk in Young Finns Study. Atherosclerosis 2012;222:257–62. [DOI] [PubMed] [Google Scholar]
- [23].Khaw KT, Barrett-Connor E. Blood pressure and endogenous testosterone in men: an inverse relationship. J Hypertens 1988;6:329–32. [PubMed] [Google Scholar]
- [24].Wang L, Szklo M, Folsom AR, et al. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2012;224:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 2004;286:R233–249. [DOI] [PubMed] [Google Scholar]
- [26].Yue P, Chatterjee K, Beale C, et al. Testosterone relaxes rabbit coronary arteries and aorta. Circulation 1995;91:1154–60. [DOI] [PubMed] [Google Scholar]
- [27].Costarella CE, Stallone JN, Rutecki GW, et al. Testosterone causes direct relaxation of rat thoracic aorta. J Pharmacol Exp Ther 1996;277:34–9. [PubMed] [Google Scholar]
- [28].Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev 2003;24:183–217. [DOI] [PubMed] [Google Scholar]
- [29].Anderson FH, Francis RM, Faulkner K. Androgen supplementation in eugonadal men with osteoporosis-effects of 6 months of treatment on bone mineral density and cardiovascular risk factors. Bone 1996;18:171–7. [DOI] [PubMed] [Google Scholar]
- [30].Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 2001;86:4261–7. [DOI] [PubMed] [Google Scholar]
- [31].Diver MJ, Imtiaz KE, Ahmad AM, et al. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol 2003;58:710–7. [DOI] [PubMed] [Google Scholar]
- [32].Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72. [DOI] [PubMed] [Google Scholar]
- [33].Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long term hormonal milieu in men. J Clin Endocrinol Metab 1992;74:939–42. [DOI] [PubMed] [Google Scholar]


